Abstract
Beclin 1/Atg6 is an essential component of the evolutionary conserved PtdIns(3)-kinase (Vps34) protein complex that regulates macroautophagy (autophagy) in eukaryotic cells and also interacts with antiapoptotic Bcl-2 family members, Bcl-2, and Bcl-xL. To elucidate the physiological function of Beclin 1, we generated transgenic mice producing a green fluorescent Beclin 1 protein (Beclin 1-GFP) under Beclin 1 endogenous regulation. The beclin 1-GFP transgene is functional because it completely rescues early embryonic lethality in beclin 1-deficient mice. The transgenic mice appear normal, with undetected change in basal autophagy levels in different tissues, despite the additional expression of functional Beclin 1-GFP. Staining of Beclin 1-GFP shows mostly diffuse cytoplasmic distribution in various tissues. Detailed analysis of the transgene expression by flow cytometry reveals a Bcl-2-like biphasic expression pattern in developing T and B cells, as well as differential regulation of expression in mature versus immature thymocytes following in vitro stimulation. Moreover, thymocytes expressing high Beclin 1-GFP levels appear increasingly sensitive to glucocorticoid-induced apoptosis in vitro. Our results, therefore, support a role for Beclin 1 in lymphocyte development involving cross talk between autophagy and apoptosis.
Keywords: Beclin 1, autophagy, T cell, B cell, apoptosis
Most developing T and B cells die by apoptosis due to rigorous selection process, which eliminates cells bearing autoreactive antigen receptors. Despite the significant progress in elucidating survival and death mechanisms during lymphocyte development and in peripheral lymphoid organs, many aspects of this selection process are still not completely understood. Recent evidence suggests that a form of nonapoptotic cell death, termed autophagy, could play a significant role in lymphocyte homeostasis.1,2 Autophagy is an evolutionary conserved mechanism of bulk degradation of cytoplasmic material that involves a complex process of a double-membrane-bound vacuole formation (termed autophagosome). It primarily serves as a cell survival mechanism during starvation.3,4 When completely formed, autophagosome contains portions of the cytoplasm to be delivered to the lysosome for degradation.5 Autophagy can be activated by stressful conditions, such as nutrient starvation, hypoxia, overcrowding, high temperature, accumulation of damaged organelles and cytoplasmic components, and growth factor deprivation. In addition to its role in stress response, autophagy is also involved in cellular remodeling during differentiation and development,6–8 ageing,7,9 cellular defense against invading bacterial and viral pathogens,10–12 and antigen presentation.13,14
Beclin 1 was originally identified in a yeast two-hybrid screen as a 60-kD coiled-coil containing protein that interacts with antiapoptotic proteins, Bcl-2 and Bcl-XL.15 Beclin 1 or yeast homologue Atg6 is a component of the PtdIns(3)-kinase (Vps34) complex that plays a critical role in autophagy.16 Beclin 1 is ubiquitously expressed in mouse tissues15 and was shown to be primarily localized to the trans-Golgi network in cultured cells.15,17 In mice, targeted disruption of beclin 1 (beclin 1−/−) results in early embryonic lethality, suggesting an essential role of this gene in early embryonic development.18,19 In addition, beclin 1−/− embryos exhibit an exaggerated apoptosis, suggesting a possible antiapoptotic role of this protein.4,19 In accordance with the observation that some forms of tumors display low autophagy levels,20 beclin 1+/− mutant mice show high incidence of spontaneous and virus-induced tumors, including epithelial and haematopoietic malignancies, and decreased autophagy in vitro, presumably accompanied with reduced autophagosome formation during starvation.18,19 In addition, beclin 1+/− mutant mice have deregulated proliferation of B cells within germinal centers.19 These results underscore the role of Beclin 1 in the control of cell growth and proliferation, as well as cancer progression.
In this report, we used BAC-mediated fluorescent Beclin 1 (Beclin 1-GFP) transgenic mice as a reporter system to study Beclin 1 expression and function. We show that Beclin 1-GFP transgene produces a functional fusion protein with a diffuse distribution in the cytoplasm. Using this transgenic reporter system, we demonstrate that Beclin 1 is developmentally regulated in T and B cells, and that it modulates glucocorticoid-induced apoptosis in thymocytes. The expression control of Beclin 1 in lymphocytes, which parallels that of the antiapoptotic Bcl-2 protein, as well as its effect on thymocyte apoptosis, implicate the interplay between autophagy and apoptosis in lymphocyte development.
Results
Expression levels, subcellular localization of Beclin 1-GFP and basal levels of autophagy in beclin 1-GFP mice
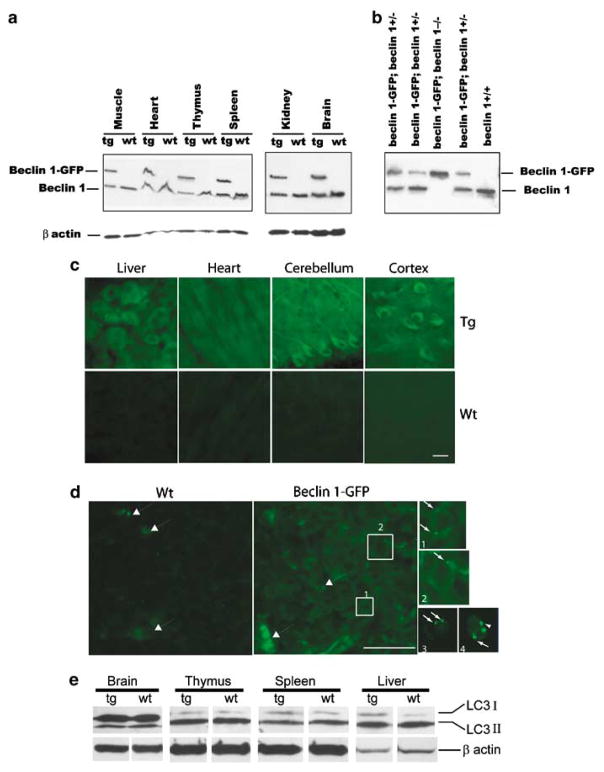
To elucidate the physiological function of beclin 1, we have developed BAC-mediated transgenic mice producing the Beclin 1-GFP fusion protein under endogenous transcriptional control. Western blot analysis using anti-Beclin 1 polyclonal antibody reveals two bands in multiple tissues examined from Beclin 1-GFP transgenic mice (Figure 1a). The lower band running at approximately 60 kD represents the endogenous Beclin 1 protein, whereas the upper band running at approximately 76 kD represents the Beclin 1-GFP fusion protein. The expression level of Beclin 1-GFP protein to the endogenous Beclin 1 is nearly 1 to 1 ratio in all tissues examined (Figure 1a), approximately corresponding to two to three copies of the transgene being present. This was also confirmed by Southern blot analysis and similar levels of the transgene expression were found in two different lines of Beclin 1-GFP mice generated so far (data not shown).
Figure 1.
Expression levels and subcellular localization of functional Beclin 1-GFP protein in Beclin 1-GFP BAC transgenic mice. (a) Total protein extracts of various tissues from Beclin 1-GFP transgenic and control wild-type mice were analyzed with anti-Beclin 1 antibody and anti-β actin antibody. (b) Western blot analysis of Beclin 1 expression in muscle protein extracts, indicating the survival of beclin 1-GFP; beclin 1−/− mice. (c) Subcellular localization of Beclin 1-GFP analyzed by immunofluorescent staining with anti-GFP antibody reveals largely diffuse Beclin 1-GFP subcellular localization in the liver (hepatocytes), heart (cardiomyocytes) and cerebellum (Purkinje cells) or cortex (projection neurons) of the CNS. Top row, transgenic (Tg) tissues; bottom row, wild-type (Wt) tissues. (d) Immunofluorescent staining with anti-GFP antibody reveals a diffuse as well as punctate localization of Beclin 1-GFP in the thymus. Boxes 1 and 2 in the transgenic thymus are also shown in separate, enlarged pictures. Large arrows, autofluorescent dots; small arrows, Beclin 1-GFP positive puncta. Small fluorescent images 3 and 4 show live transgenic thymocytes in cell suspension. Scale bar, 20 μ. (e) Western blot analysis of endogenous LC3 (I and II) protein levels in the brain, thymus, spleen and liver of Wt mice and Tg mice.
To provide evidence that Beclin 1-GFP is functional in transgenic mice, we generated mice in which Beclin 1-GFP functionally replaced the endogenous Beclin 1 (beclin 1-GFP; beclin 1−/−). We found that beclin 1-GFP; beclin 1−/− mice can survive postnatally, whereas beclin 1−/− mice die during early embryonic development.18 Indeed, the 1-year survival rate in ‘rescued’ mice (beclin 1-GFP; beclin 1−/−) is the same as in wild-type animals (data not shown). The ‘genotyping’ of mice with anti-Beclin 1 antibody immunoblot demonstrated the presence of mice beclin 1-GFP; beclin 1−/−, which lack endogenous Beclin 1 protein (Figure 1b). These results confirm that beclin 1-GFP completely rescues the early embryonic lethality in beclin 1−/− mice, and can functionally substitute for the endogenous Beclin 1 protein.
Since the subcellular localization of Beclin 1/PtdIns(3)-kinase (Vps34) may provide important clues for the membrane origin of autophagosomes, we examined the intracellular distribution of direct GFP fluorescence of the Beclin 1-GFP protein in transgenic tissues. Unexpectedly, despite the expression levels of Beclin 1-GFP similar to endogenous Beclin 1, no significant fluorescence of Beclin 1-GFP above background levels (nontransgenic control) was detected in most transgenic tissues (data not shown). This result could be due to low-expression levels of the fluorescent fusion protein or quenching of the GFP fluorescence inside the acidic environment of the Beclin 1-GFP/PtdIns(3)-kinase (Vps34) complex (which produces large quantities of PI-3(P)). Nevertheless, by using anti-GFP-specific antibody followed by fluorescent imaging, we detected significant levels of fluorescent staining in transgenic tissues, such as the brain, liver, and heart, as compared to control mice (Figure 1c). Interestingly, in contrast to the previous report showing predominant trans-Golgi localization of Beclin 1 in cultured cells,17 staining of Beclin 1-GFP in transgenic tissues appears largely diffuse and not associated with any particular cellular compartments in most of tissues examined. For example, Beclin 1-GFP staining in neurons is observed throughout the cell body and proximal processes, consistent with our previous report with anti-Beclin 1 antibody staining21 (Figure 1c). In addition, fractionation of the brain lysate shows that around half of the Beclin 1 protein is soluble and the rest is membrane-associated (data not shown).
To assess if Beclin 1-GFP expression affects constitutive autophagy levels, we performed western blot with anti-LC3 antibody to analyze the levels of LC3 protein, a well-known marker for autophagy,22 in several transgenic tissues. Anti-LC3 antibody recognizes two distinct forms of LC3, LC3 I and II. We do not observe a significant change in the protein levels of either form in any of the tissues tested (Figure 1e), which suggests that the expression of functional exogenous Beclin 1-GFP has little effect on the basal level of autophagy in our transgenic mice.
Unique Beclin 1-GFP expression during T-cell development in the thymus
Like all other tissues examined in this study, staining of the transgenic thymus with anti-GFP antibody also reveals some levels of Beclin 1-GFP expression over the control levels, as shown in the wild-type thymus (Figure 1d). Interestingly, we observe punctate Beclin 1-GFP distribution in transgenic thymocytes (Boxs 1 and 2, pucta are indicated with small arrows in enlarged images), but not in wild-type cells. It is worth mentioning that in both wild-type and transgenic thymi we noticed a number of bright autofluorescencent ‘dots’ (big arrows, Figure 1d), also observed in earlier studies,22 which can be distinguished from Beclin 1-GFP puncta. Further analysis in single-cell suspensions of live thymocytes from Beclin 1-GFP transgenic animals by either confocal (Figures 1d, 3 and 4, small arrows), or AMNIS ImageStream analysis (Supplementary Figure 1), reveals a punctate Beclin 1-GFP distribution in all major thymocyte subsets. Our results are, therefore, in agreement with earlier reports describing Beclin 1 puncta in cultured cells.23
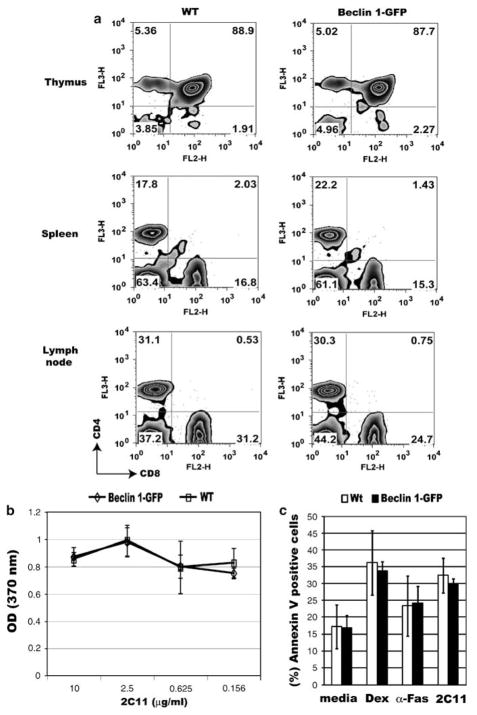
Figure 3.
Normal T-cell development, activation, and apoptosis in Beclin 1-GFP transgenic mice. (a) Single-cell suspensions of the thymus, spleen, or lymph nodes from control (left panel), or Beclin 1-GFP transgenic mice (right panel) were stained with antibodies against CD4 and CD8 and analyzed on a FACScan flow cytometer. The percentage of gated cells in each quadrant is indicated. Data are representative of three independent experiments. (b) In vitro proliferation of purified Beclin 1-GFP transgenic (Tg) and control CD4 + T cells (Wt) stimulated with different concentrations of plate-bound 2C11 antibody and soluble anti-CD28 antibody (2 μg/ml) as described in Materials and Methods. (c) Apoptosis of Beclin 1-GFP transgenic and control thymocytes in vitro. Thymocytes from transgenic (Tg) and control (Wt) mice were incubated in the presence of either media alone (spontaneous apoptosis), dexamethasone (10−7 M), anti-Fas antibody (2 μg/ml), or precoated 2C11 antibody (10 μg/ml) for 24 h, cells stained with Annexin V and 7-AAD, and analyzed using flow cytometry. Late apoptotic/necrotic cells (Annexin V +7-AAD +) were excluded. Data represent means ± S.D. from three independent experiments
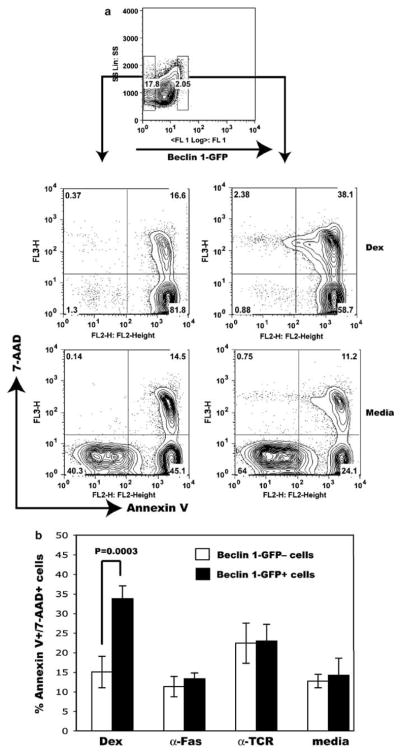
Figure 4.
Accelerated dexamethasone-induced cell death in thymocytes expressing high Beclin 1-GFP levels. (a) Thymocytes expressing high levels of Beclin 1-GFP fluorescence, as well as Beclin 1-GFP-negative cells were sorted and cultured in vitro as described in Figure 4. The positions of corresponding gates were indicated with small rectangles (top panel). After 24 h in culture, cells were washed, stained with Annexin V and 7-AAD, and analyzed by flow cytometry. Only flow cytometry profiles obtained using cells cultured in dexamethasone and media from one representative experiment are shown. (b) Percent of late apoptotic/necrotic cells (Annexin V +7-AAD +) following stimulation of sorted transgenic thymocytes using indicated apoptotic stimuli. The results represent means ± S.D. from four independent experiments. Statistical analysis was performed using Student’s t-test. P < 0.05 was considered statistically significant
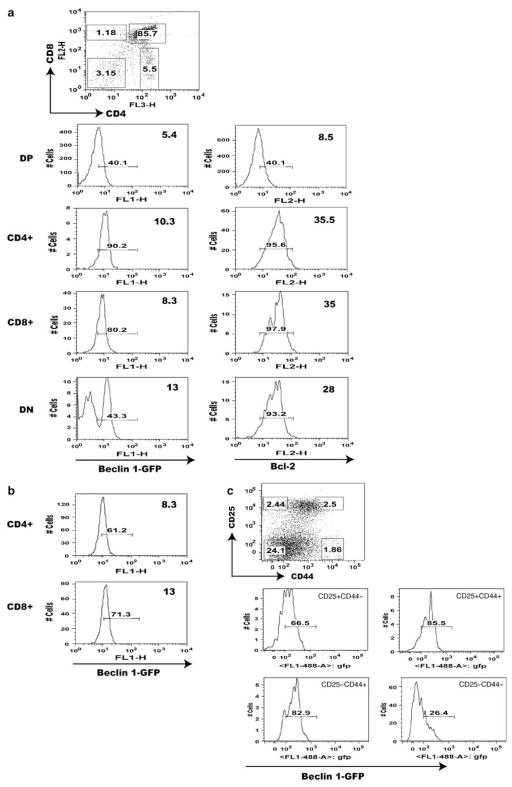
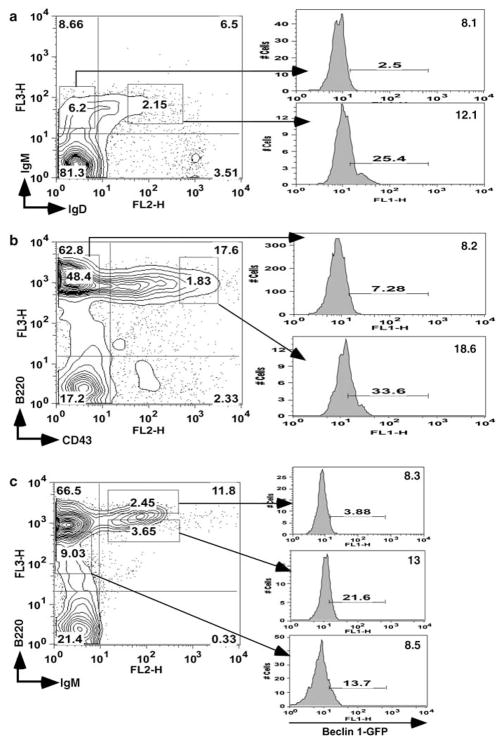
By taking advantage of this useful transgenic mouse, we performed flow cytometry analysis of Beclin 1-GFP expression in the thymus. The result shows distinctly heterogeneous pattern of Beclin 1-GFP in the thymus. We find that Beclin 1-GFP is highly expressed in a fraction (30–45%) of double-negative (DN) thymocytes, downregulated in double-positive (DP) cells, and re-induced in mature, post-selection thymocytes (Figure 2a). A comparison between mean Beclin 1-GFP and mean fluorescence intensities (MFI) in DP and SP thymocyte populations indicates higher expression in the latter (MFI = 6.1 ± 0.4 versus 9.9 ± 0.2, P = 0.0004). The transgene is also expressed in most mature T cells in the spleen (Figure 2b). Thus, our results using Beclin 1-GFP transgenic mice show a biphasic reporter gene expression very similar to Bcl-2.24,25 To confirm that Bcl-2 also exhibits biphasic expression in our transgenic mice, we performed intracellular Bcl-2 staining of transgenic thymocytes. As indicated in Figure 2a, Bcl-2 expression is high in DN thymocytes, reduced in DP cells, and re-induced at the SP stage, thus confirming that Beclin 1-GFP expression parallels Bcl-2.
Figure 2.
Flow cytometry analysis of Beclin 1-GFP expression in major populations of transgenic T cells. (a) Beclin 1-GFP and Bcl-2 expression analysis in the thymus of Beclin 1-GFP transgenic mice. The percentage of Beclin 1-GFP positive cells within each thymocyte subset defined by corresponding gates in the top dot plot is indicated with small numbers within each histogram. The numbers in the upper right corner of each histogram represent mean fluorescence intensities (MFI) for each subset within indicated histogram gates. The histograms in (b) indicate Beclin 1-GFP expression in mature spleen CD4 + and CD8 + cells. (c) The histograms represent Beclin 1-GFP + fluorescence for each group of of pregated CD3−CD4−CD8− triple-negative Beclin 1-GFP + TN transgenic thymocytes defined by the gates indicated in the CD44 versus CD25 dot plot. Data are representative of at least four independent experiments
To examine the population of DN thymocytes expressing high levels of Beclin 1-GFP in detail, pregated CD4−CD8−CD3− (triple-negative, TN) thymocytes were analyzed for the expression of Beclin-1-GFP in four major groups of TN thymocytes (Figure 2c), based on the expression of CD25 and CD44.26 The results indicate that three groups of TN thymocytes, CD25+CD44+, CD25+CD44−, and CD25−CD44+ expressed Beclin 1-GFP, whereas the expression was almost absent in more mature CD25−CD44− thymocytes.
To determine if T-cell development is affected by the expression of Beclin 1-GFP transgene, we analyzed major T-cell subpopulations in the thymus, spleen, and lymph nodes of Beclin 1-GFP transgenic animals. The major T-cell subsets in Beclin 1-GFP transgenic animals did not differ significantly from T-cell subsets in nontransgenic animals, indicating that the transgene expression has no discernable effect on T-cell development (Figure 3a). Moreover, the transgenic T cells response to in vitro stimulation with anti-TCR antibody (Figure 3b), as well as apoptosis induction with different apoptotic stimuli, such as Dexamethasone (Dex), anti-Fas and anti-TCR antibodies (Figure 3c), are not significantly different from wild-type cells.
Similar to Beclin 1-GFP transgenic mice, beclin 1+/− mice expressing reduced Beclin 1 protein levels have no obvious T- or B-cell abnormalities in the thymus and bone marrow (BM), respectively (Supplementary Table 1).
Accelerated glucocorticoid-induced apoptosis of thymocytes expressing high Beclin 1-GFP levels
Even though unfractionated thymocytes display an apoptotic response similar to nontrangenic thymocytes (Figure 3c), it is possible that any subtle differences in apoptosis may not be detectable because of moderate overexpression of Beclin 1 in the thymus (Figure 1). We, therefore, asked if cells expressing higher levels of Beclin 1-GFP (Beclin 1-GFPhigh) might have different sensitivity to apoptosis compared to thymocytes not expressing the transgene. We sorted Beclin 1-GFPhigh (the brightest 2%, Figure 4a) and Beclin -1-GFP-negative thymocytes and tested their sensitivity to apoptosis induced with dexamethasone, anti-Fas, or anti-TCR antibodies. As indicated in Figure 2, Beclin 1-GFPhigh cells mostly belong to SP and DN subsets, previously shown to have lower sensitivity to dexamethasone-induced cell death compared to DP cells.27 Interestingly, Beclin 1-GFPhigh cells treated with dexamethasone exhibited accelerated transition from early to late apoptosis, as determined by approximately twofold increase in the number of late apoptotic/necrotic Annexin V + 7-AAD + cells (33.7 ± 4% versus 14.9 ± 4.0%, P < 0.001), and reduced numbers of early apoptotic, Annexin V + 7-AAD− cells (Figures 4a and b). However, we did not detect any significant difference between Beclin 1-GFPhigh-and Beclin 1-GFP-negative cells with respect to their sensitivity to two other apoptotic stimuli, anti-Fas and anti-TCR antibodies (Figures 4a and b). The ectopic expression of the Beclin 1-GFP protein, thus, augments cell death induced by dexamethasone, with no significant impact on apoptosis induced by anti-Fas or anti-TCR antibodies.
Biphasic expression of Beclin 1-GFP during B-cell development
We have also analyzed Beclin 1-GFP expression in BM B cells using fluorescent-labeled antibodies against several characteristic B-cell markers. We first examined Beclin 1-GFP expression in immature and mature BM B cells, which can be distinguished by the presence of surface IgM and IgD. A significant fraction of mature (IgM+IgD+) B cells displayed high levels of Beclin 1-GFP, whereas the expression was lower in IgM +IgD−-immature B cells (MFI = 13.0 ± 1.2 versus 8.3 ± 0.3, P = 0.03) (Figure 5a). The analysis of Beclin 1-GFP expression in early B-cell progenitors using CD43 and B220 markers revealed that Beclin 1-GFP expression levels were higher in pro-B cells (CD43+B220+) than in pre-B cells (MFI = 20.0 ± 3.1 versus 10.5 ± 1.9, P = 0.01), and immature CD43−B220+ B cells (MFI = 20.0 ± 3.1 versus 8.3 ± 0.3, P = 0.002), indicating that Beclin 1 is downregulated during pro-B to pre-B transition (Figure 5b). Moreover, the reporter gene protein expression is higher in IgM+B220high mature and recirculating B cells than in IgM +B220low transitional cells (MFI = 14.1 ± 1.1 versus 9.7 ± 1.3, P = 0.01) (Figure 5c). The population of IgM−B220dull cells contains a small fraction of Beclin 1-GFP-positive cells. The expression of the Beclin 1-GFP protein in mature peripheral B cells from the spleen is similar to the expression in mature BM-derived B cells (data not shown). Thus, our results using BAC-mediated Beclin 1-GFP transgenic mice show a biphasic reporter gene expression similar to Bcl-2 expression in B cells.28 Further studies are necessary to understand the regulatory mechanism behind this similarity.
Figure 5.
Analysis of Beclin 1-GFP expression in bone marrow B cells. Bone marrow cells from Beclin 1-GFP transgenic mice were stained with antibodies against (a) IgM and IgD, (b) CD43 and B220, and (c) IgM and B220, and analyzed by flow cytometry. The expression of Beclin 1-GFP in gated populations is shown in histograms on the right. Gate positions were indicated with rectangles. Numbers within histograms indicate percentage of GFP-positive cells using wild-type bone marrow as a negative control. Data are representative of three independent experiments
Beclin 1-GFP expression is induced following in vitro stimulation of CD4+ T cells
To determine if T-cell activation in vitro would have an effect on Beclin 1-GFP expression, purified CD4+ T cells were incubated with plate-bound anti-TCR antibody and soluble anti-CD28 antibody. In vitro activation of CD4+ T cells increased Beclin 1-GFP expression in a significant proportion of cells compared to unstimulated cells after 24 h in culture, and this number increased after 48 h (72.5 ± 6.0% vs 10.1 ± 4.1%, P < 0.001) (Figure 6a). Interestingly, stimulation of CD4+ T cells from GFP-LC3 also resulted in upregulation of the green fluorescence level in a significant fraction of CD4+ T cells compared to unstimulated cells (41 ± 10.0% versus 8.6 ± 1.1, P = 0.001) (Figure 6b). These results suggest that Beclin 1 upregulation in a significant proportion of in vitro-activated CD4 + T cells is associated with increased autophagic activity in these cells.1 To examine the expression of Beclin 1 in thymocytes following in vitro activation, we incubated whole thymus suspensions from beclin 1-GFP transgenic mice with plate-bound anti-TCR antibody for 24 h. While in vitro activation, similar to peripheral CD4+ T cells, upregulated Beclin 1-GFP expression in SP thymocytes failed to induce significant increase in Beclin 1-GFP expression in DP thymocytes (Figure 6c). These results indicate that Beclin 1 expression appears to be differentially regulated in mature versus immature DP T cells, which is again similar to the previous observation of Bcl-2 expression in these two cell types.24
Figure 6.
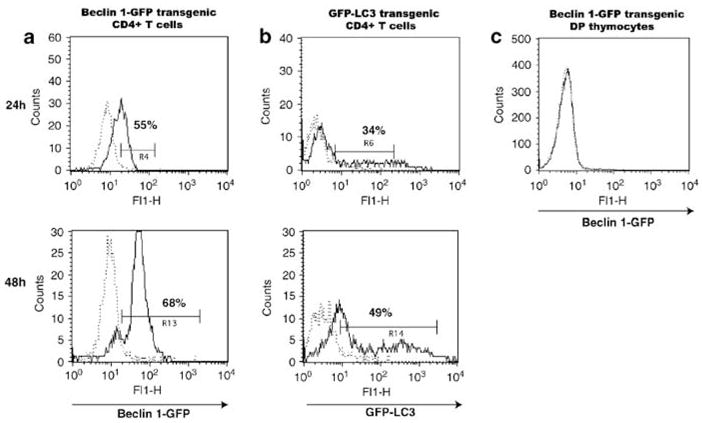
Stimulation of T cells upregulates Beclin 1-GFP and GFP-LC3 in CD4 + T cells. Purified (a) Beclin 1-GFP CD4 + T cells, (b) GFP-LC3 CD4 + T cells, or (c) Beclin 1-GFP total thymocytes were stimulated with plate-bound 2C11 antibody and soluble anti-CD28 antibody, as described in Materials and Methods (solid lines), or PBS (dotted lines) for 24 h (upper panels) or 48 h (lower panels), and analyzed for GFP expression using flow cytometry. The percentage of cells in each gate is indicated. Beclin 1-GFP fluorescence in (c) is shown for gated double-positive cells. Data are representative of three independent experiments
Discussion
In this report, we have shown that Beclin 1-GFP expression levels in the BAC-mediated transgenic mice largely mirror the endogenous Beclin 1 protein and that the fusion protein is functional, which indicates that our Beclin 1-GFP transgenic mice provide an excellent experimental tool to study Beclin 1 expression and function. The Beclin 1-GFP protein is distributed diffusely in the cytoplasm in most tissues and not restricted to any particular cellular compartment. This result is in contrast with the previous study indicating predominantly trans-Golgi localization of the Beclin 1 and may suggest that Beclin 1 function is broader than previously thought. Interestingly, Beclin 1-GFP expression in live thymocytes appears to be confined to characteristic puncta with similar distribution in all thymocyte subsets (Supplementary Figure 1). These puncta might indicate sites of the most intense Beclin 1 accumulation, whereas the rest of the protein is diffusely distributed and detectable only by anti-GFP antibody staining (Figure 1).
We find that expression of the functional fusion protein does not cause alterations in the steady-state levels of the autophagic marker LC3I/II (Figure 1e), nor does it lead to any abnormality in lymphocyte development and sensitivity to apoptosis (Figure 3). Importantly, we show a remarkable similarity between developmental controls of Beclin 1 and Bcl-2 protein expression in T and B cells. Thus, our results suggest a role for Beclin 1 in the regulation of lymphocyte development.
Since Beclin 1 is essential for autophagy, the regulated expression of the Beclin 1-GFP fusion protein in the thymus could indicate changes in autophagic activity. However, we are unable to detect a significant GFP-LC3 fluorescence in transgenic thymocytes by flow cytometry (data not shown), which is in agreement with the previous study using GFP-LC3 transgenic mice.22 In addition, only discrete abnormalities were seen in thymocytes lacking the expression of Atg5, a protein essential for autophagosome, suggesting that autophagy is not essential for T-cell development in the thymus.2 It is conceivable that Beclin 1 is involved in cell death mechanism in the thymus by interacting with Bcl-2 and/or Bcl-xL.29 Therefore, considering the significant role that Bcl-2 plays in T and B cells,30–33 and an emerging role of autophagy in this process, it is likely that Beclin 1 provides a critical link between apoptosis and autophagy in the lymphoid tissue, which has previously been demonstrated in other systems.34–36
The expression of Bcl-2 in the thymus is tightly regulated. It is induced in DN cells, extinguished in DP cells, and then reinduced in SP mature thymocytes.24,25 The biphasic pattern of Beclin 1-GFP expression in the thymus (Figure 2a) is, therefore, highly reminiscent of Bcl-2 (Figure 2a). Within the DN thymocyte population, Beclin 1-GFP is the least expressed in more mature CD25−CD44− thymocytes (Figure 2c). Since Beclin 1 is expressed in haematopoetic cells (our unpublished observation), we hypothesize that the expression of this protein in some DN cells probably represents a residual protein, again paralleling Bcl-2.24 Since Beclin 1 and Bcl-2 are both upregulated during the DP to SP transition, we further hypothesize that similar to Bcl-2,37 Beclin 1 is involved in thymocyte survival mechanisms in the thymus through interaction with Bcl-2.
A twofold Beclin 1 overexpression in Beclin 1-GFP transgenic mice is insufficient to produce any significant effects on lymphocyte development and apoptosis (Figure 2) and the same is true for lymhocyte development in beclin 1+/− heterozygous animals expressing half Beclin 1 protein levels (Supplementary Table 1) However, the ectopic expression of Beclin 1-GFP rendered thymocytes increasingly sensitive to glucocorticoid-induced apoptosis in vitro (Figure 4), indicating a direct involvement of Beclin 1 in the apoptotic process induced by this strong apoptotic stimulus. It is presently unclear, however, whether this effect is mediated through Beclin 1 interaction with Bcl-2, which has previously been shown to have a protective effect against glucocorticoid-induced apoptosis in thymocytes.38 Surprisingly, we did not observe a significant increase in cell death in thymocytes expressing high Beclin 1-GFP levels triggered by two other apoptotic stimuli, anti-Fas or anti-TCR antibody, compared to Beclin 1-GFP-negative cells (Figure 4). This might reflect the intrinsic differences in which endogenous (glucocorticoid-induced, Bcl-2 inhibitable) and exogenous (anti-Fas-induced, Bcl-2 resistant) apoptosis is triggered,39 and a differential role for Beclin 1 in these two death pathways.
The expression of Beclin 1-GFP similar to Bcl-2 is also observed in the B-cell compartment. Bcl-2 is highly expressed in pro-B cells and mature B cells but downregulated in pre-B cells and immature B cells.28 Downregulation of Bcl-2 at the transition between pro- and pre-B cells coincides with accelerated cell death. Extensive cell death at this stage reflects the elimination of those cells failing to express a pre-B cell receptor complex.40 In immature B cells, Bcl-2 down-regulation is again linked to increased apoptosis, but this time due to elimination of cells bearing autoreactive B-cell receptors.41,42 As Beclin 1 also appears to be downregulated at the same steps as Bcl-2 that involve massive cell death, we hypothesize that in B cells, and probably in T cells as well, it may be involved in the regulation of autophagy and apoptosis.
This study also demonstrates that activation of naive CD4+ cells from Beclin 1-GFP mice in vitro using anti-TCR antibody results in a marked increase in Beclin 1 expression (Figure 6). To determine if T-cell activation would increase the expression of GFP-LC3, we used purified CD4 + T cells from GFP-LC3 transgenic mice (Figure 6b). Similar to Beclin-GFP transgenic T cells, activation of GFP-LC3 CD4+ T cells produced an increase in the GFP marker expression. This increase of GFP-LC3 expression following stimulation of naïve T cells is in agreement with the earlier study describing T-cell activation-induced LC3 marker upregulation in cultured CD4+ T cells,1 suggesting that Beclin 1-GFP upregulation following stimulation correlates with increased autophagic activity in mature T cells, whereas in DP thymocytes, neither Beclin 1 nor autophagic activity is increased following TCR stimulation. Therefore, our results suggest that Beclin 1 plays different roles in mature versus immature T cells. In support of this idea, Beclin 1-GFP upregulation is observed only in mature, but not in immature T cells following in vitro stimulation (Figure 6c).
In conclusion, our report indicates that Beclin 1 is regulated during T- and B-cell development and T-cell activation, and also modulates glucocorticoid-induced thymocyte apoptosis in vitro. Additional studies are, thus, required to determine the role of this protein in the molecular network regulating cell death and survival decisions in the lymphoid system.
Materials and Methods
Animals
Generation of Beclin 1-GFP BAC transgenic mice is described elsewhere (submitted) and genotyping done by PCR with primers against the EGFP sequence (forward primer: 5′-CCTACGGCGTGCAGTGCTTCAGC-3′, reverse primer: 5′-CGGCGAGCTGCACGCTGCGTCCTC-3′).43 GFP-LC3 transgenic mice are described elsewhere.22 Both transgenic strains were housed, cared for and used in accordance with the Guide for Care and Use of Laboratory Animals (NIH publication 86–23, 1985; National Institutes for Health, Bethesda, MD). Mice of both sexes were typically used at 6–8 weeks of age.
Antibodies and flow cytometry
Fluorescent-labeled monoclonal antibodies against mouse CD4 (clone GK1.5), CD8 (clone 53–6.7), CD43 (clone IM7), CD44 (clone IM7), CD25 (clone PC61), CD3 (2C11), IgM (R6–60.2), CD43 (clone S7), and B220 (clone RA3–6B2) were purchased from BD Biosciences (San Jose, CA). PE-conjugated anti-mouse IgD (clone 11–26) was from Southern Biotech (Birmingham, AL). Single-cell suspensions were generated from the spleen, lymph nodes, and thymus and stained for 30 min on ice using saturating concentrations of directly conjugated monoclonal antibodies. Cells were washed three times with PBS containing 2% fetal bovine serum (FBS), and data acquired using either FACScan or FACS-Vantage flow cytometer (BD Biosciences). FACS-Vantage or MoFlo sorters were used in thymocyte-sorting experiments. Flow cytometry data was analyzed using either CellQuest (BD Biosciences) or FlowJo (Tree Star, Inc., Ashland, OR) software.
Multispectral imaging flow cytometry
To analyze the distribution of puncti in the different subsets in a large number of cells, we used a multispectral imaging flow cytometer (Amnis Corporation, Seattle, WA). Cells were labeled with monoclonal antibodies against murine CD8 (APC) and CD4 (Pacific Blue) and 10 000 cells were assayed using three lasers to excite the three flurochromes (Pacific Blue/405 nm, GFP/488 nm, and APC/658 nm) independently to maximize specificity. The laser settings were 18 mW (405 nm), 200 mW (488 nm) and 40 mW (658 nm). The images of individual cells were compensated using a compensation matrix derived from a single fluorochrome-labeled sample. Images of single cells were separated from duplets and artifacts by drawing a region on a two-dimensional field describing the area of the cells and the aspect ratio (length of the cell divided by the width), which is unity for a round cell. For further analysis, we choose images that were in focus based on the root mean square of the contrast between the background and the cell, as this change in pixel intensity of the bright field image in an in-focus image is higher than in a blurred image. These single and focused cells were separated in four groups according to their expression of the two surface markers, CD4 and CD8, similar to a classical flow cytometry analysis. To identify the single GFP spots, a spot-identifying mask, which can detect local maxima regardless of their intensity, was employed. The puncti per cell were counted and the distribution of cells containing different numbers of puncti was compared among the four groups.
T-cell proliferation and apoptosis assays
For T-cell stimulation, flat-bottomed 96-well plates were precoated with the indicated concentrations of 2C11 antibody (BD Biosciences) by 4-hour incubation in a tissue culture incubator at 37°C or overnight incubation at 4°C. Plates were washed three times with ice-cold PBS, and purified T cells (1–2 × 105 cells per well) or thymus suspensions (2 × 105 cells per well) incubated in RPMI media, supplemented with 10% FBS containing soluble anti-CD28 antibody (BD Biosciences) at a concentration of 2 μg/ml. Cultures were incubated in a tissue culture incubator at 37°C in a humidified atmosphere containing 5% CO2 for 24 or 48 h. Proliferation assays were performed using BrdU-based calorimetric ELISA kit from Roche (Nutley, NJ). Briefly, CD4 + T cells were incubated in RPMI media containing 10% FBS in a tissue culture incubator for 48 h in the presence of above antibodies, pulsed overnight with BrdU (10 μM), followed by incubation with peroxidase-labeled anti-BrdU antibody. Development of color following substrate (tetramethyl-benzidine) addition was monitored using an ELISA reader at 370 nm.
For apoptosis assays, thymocytes were incubated in 24-well plates with either Dexamethasone (Sigma-Aldrich, St. Louis, MO), anti-CD95 (BD Biosciences), or in 96-well plates precoated with indicated concentrations of anti-T-cell receptor ε chain antibody 2C11 (BD Biosciences) for 24 h. Following incubation, cells were washed twice in PBS, stained with Annexin V and 7-AAD (both from BD Biosciences), and analyzed using FACScan flow cytometer.
Immunocytochemistry
Wild-type or transgenic mice (6–8 week) were perfused with 4% paraformaldehyde (PFA) in PBS. Tissues were dissected, postfixed overnight in 4% PFA and cryoprotected in 30% sucrose, and tissue sections other than the thymus prepared at 25 μm on a sliding microtome. Cryosectioning of frozen, OCT-embedded thymus blocks was performed using an Ultrapro 5000 cryostat (Vibratome, St. Louis, MO). The slices were incubated overnight at 4°C with anti-GFP antibody used at dilution 1: 2000 as described,44 followed by incubation with secondary antibodies conjugated to Alexa Fluor 488 (Invitrogen, Carlsbad, CA) used at dilution 1: 500. The mounted sections were visualized by Leica DMI 6000 microscope using fluorescent optics.
Western blot
Proteins were prepared from different tissues by homogenizing the tissue in 1% Triton extraction buffer (1% Triton X-100, 50 mM Tris-HCl pH 7.5, 150 mM NaCl) or RIPA buffer (Upstate USA Inc., Charlottesville, VA) containing protease inhibitor cocktail (Roche). Tissue homogenates were centrifuged for 20 min at 12 000 rpm at 4°C and supernatant proteins separated by SDS-PAGE. Following separation, proteins were transferred to a nylon membrane, probed with polyclonal anti-Beclin 1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or polyclonal anti-LC3 antibody,44 and blots developed using an ECL kit (BioRad, Hercules, CA). Polyclonal antibody against β actin (clone AC-74), used as a loading control, was purchased from Sigma-Aldrich.
Statistical analysis
Statistical analysis was performed using two-tailed Student’s t-test. P<0.05 was considered statistically significant.
Acknowledgments
We would like to thank Noboru Mizushima for providing GFP-LC3 transgenic mice, Nina Papavasiliou for critically reading the manuscript and help with B-cell analysis, Italas George from Mount Sinai flow cytometry facility for help with flow cytometry analysis, and Yun Zhong for transgenic mice work. This work was supported by grants from The National Institutes of Health MBRS SCORE program S06GM08153-31 (to IA), PSC-CUNY (to IA), NINDS R21RNS055683, R01RNS060123 (to ZY), NIH NIAID Contract HHSN2662000500021C and Grant U19 AI06231 (to SCS) and research funding support from Department of Neurology (to ZY). GM and JC are recipients of Minority Access to Biomedical Research (MARC) fellowships from National Institutes of Health (T32GM8498-11).
Abbreviations
- BAC
bacterial artificial chromosome
- VPS
vacuolar protein sorting
- PtdIns
phosphatidylinositols
- GFP
green fluorescence protein
- TCR
T-cell receptor
- FBS
fetal bovine serum
- PBS
phosphate-buffered saline
- 7-AAD
7-Amino-actinomycin
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
References
- 1.Li C, Capan E, Zhao Y, Zhao J, Stolz D, Watkins SC, et al. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J Immunol. 2006;177:5163–5168. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- 2.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 5.Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 6.Juhasz G, Csikos G, Sinka R, Erdelyi M, Sass M. The Drosophila homolog of Aut1 is essential for autophagy and development. FEBS Lett. 2003;543:154–158. doi: 10.1016/s0014-5793(03)00431-9. [DOI] [PubMed] [Google Scholar]
- 7.Melendez A, Tallóczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B, et al. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 8.Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, et al. Autophagy and CD4(+) T lymphocyte destruction by HIV-1. Autophagy. 2007;3:32–34. doi: 10.4161/auto.3275. [DOI] [PubMed] [Google Scholar]
- 9.Hars ES, Qi H, Ryazanov AG, Jin S, Cai L, Hu C, et al. Autophagy regulates ageing in C. elegans. Autophagy. 2007;3:93–95. doi: 10.4161/auto.3636. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, et al. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 12.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 13.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 14.Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Müller M, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci USA. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stromhaug PE, Reggiori F, Guan J, Wang CW, Klionsky DJ. Atg21 is a phosphoinositide binding protein required for efficient lipidation and localization of Atg8 during uptake of aminopeptidase I by selective autophagy. Mol Biol Cell. 2004;15:3553–3566. doi: 10.1091/mbc.E04-02-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 21.Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, Heintz N, et al. A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron. 2002;35:921–933. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- 22.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- 25.Gratiot-Deans J, Merino R, Nunez G, Turka LA. Bcl-2 expression during T-cell development: early loss and late return occur at specific stages of commitment to differentiation and survival. Proc Natl Acad Sci USA. 1994;91:10685–10689. doi: 10.1073/pnas.91.22.10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3–CD4–CD8-triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 27.Berki T, Palinkas L, Boldizsar F, Nemeth P. Glucocorticoid (GC) sensitivity and GC receptor expression differ in thymocyte subpopulations. Int Immunol. 2002;14:463–469. doi: 10.1093/intimm/14.5.463. [DOI] [PubMed] [Google Scholar]
- 28.Merino R, Ding L, Veis DJ, Korsmeyer SJ, Nunez G. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 1994;13:683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Sohn SJ, Rajpal A, Winoto A. Apoptosis during lymphoid development. Curr Opin Immunol. 2003;15:209–216. doi: 10.1016/s0952-7915(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 31.Tao W, Teh SJ, Melhado I, Jirik F, Korsmeyer SJ, Teh HS, et al. The T cell receptor repertoire of CD4-8+ thymocytes is altered by overexpression of the BCL-2 protooncogene in the thymus. J Exp Med. 1994;179:145–153. doi: 10.1084/jem.179.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linette GP, Hess JL, Sentman CL, Korsmeyer SJ. Peripheral T-cell lymphoma in lckpr-bcl-2 transgenic mice. Blood. 1995;86:1255–1260. [PubMed] [Google Scholar]
- 33.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 34.Furuya D, Tsuji N, Yagihashi A, Watanabe N. Beclin 1 augmented cis-diamminedichloroplatinum induced apoptosis via enhancing caspase-9 activity. Exp Cell Res. 2005;307:26–40. doi: 10.1016/j.yexcr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Schiff M, Czymmek K, Tallóczy Z, Levine B, Dinesh-Kumar SP, et al. Autophagy regulates programmed cell death during the plant innate immune response. Cell. 2005;121:567–577. doi: 10.1016/j.cell.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Takacs-Vellai K, Vellai T, Puoti A, Passannante M, Wicky C, Streit A, et al. Inactivation of the autophagy gene bec-1 triggers apoptotic cell death in C. elegans. Curr Biol. 2005;15:1513–1517. doi: 10.1016/j.cub.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 37.Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ, et al. Bcl-2 is upregulated at the CD4+CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 38.Siegel RM, Katsumata M, Miyashita T, Louie DC, Greene MI, Reed JC, et al. Inhibition of thymocyte apoptosis and negative antigenic selection in bcl-2 transgenic mice. Proc Natl Acad Sci USA. 1992;89:7003–7007. doi: 10.1073/pnas.89.15.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dallaporta B, Pablo M, Maisse C, Daugas E, Loeffler M, Zamzami N, et al. Proteasome activation as a critical event of thymocyte apoptosis. Cell Death Differ. 2000;7:368–373. doi: 10.1038/sj.cdd.4400661. [DOI] [PubMed] [Google Scholar]
- 40.Ehlich A, Schaal S, Gu H, Kitamura D, Müller W, Rajewsky K, et al. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993;72:695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- 41.Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, et al. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 42.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 43.Heintz N. BAC to the future: the use of bac transgenic mice for neuroscience research. Nat Rev Neurosci. 2001;2:861–870. doi: 10.1038/35104049. [DOI] [PubMed] [Google Scholar]
- 44.Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, et al. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]