Abstract
Cardiovascular disease (CVD) is the number one cause of morbidity and mortality in men and women worldwide. According to the WHO, by 2015, almost 20 million people will die from CVD each year. It is well established that men and women differ not only in baseline cardiac parameters, but also in the clinical presentation, diagnosis and treatment outcomes of CVD. Women tend to develop heart disease later in life than men. This difference has been attributed to the loss of estrogen during the menopausal transition; however, the biological explanations for the sexual dimorphism in CVD are more complex and seem unlikely to be due to estrogen alone. The current controversy that has arisen regarding the effects of HRT on CVD in women is a case in point. In this review, the sex-based differences in cardiac (patho-) physiology are discussed with emphasis on the impact of sex hormones, hormone receptors and diet on heart disease.
Keywords: androgen receptor, estrogen, estrogen receptor, heart disease, phytoestrogen, sex difference, testosterone
Biological sex plays an important role in cardiac pathophysiology. The incidence of heart disease in women increases after menopause, indicating that sex hormones play a critical role in the development of heart disease. HRT and dietary interventations are commonly undertaken for the prevention and management of cardiovascular disease (CVD). In this review, we highlight the fundamental differences in cardiac function between men and women. The emphasis is on the role of sex hormones and phytoestrogens on heart disease. By drawing on a breadth of data from studies in humans as well as animal models, it is possible to identify important effects of the role of sex hormones and phytoestrogens on heart disease.
Sex differences in heart function
Sex-based differences in normal heart function have long been recognized; however, in more recent years the influence of sex on heart disease has been explored at cellular, molecular and genetic levels. Before puberty, the left ventricular (LV) mass normalized to body weight is only modestly higher in boys than in girls (mean of ~6%) [1]. In all older age strata, the LV:bodyweight ratio in men is 25–38% greater than in women [1,2]. Given that the number of human cardiac myocytes is determined within the first year of birth, when the mitotic activity of normal myocytes appears to cease, any subsequent increase in heart size is primarily due to changes in myocyte size (hypertrophy) rather than in their numbers [3]. A larger LV mass in men reflects a greater degree of hypertrophy compared with women. This LV hypertrophy has been demonstrated to be due to a symmetrical increase both in chamber dimension and in wall thickness in males, resulting in no sex difference in relative wall thickness [1].
Sex also plays a critical role in the detrimental effects of the aging process in the heart. An interesting observation in this context is the preservation of myocardial mass, myocyte number and average cell diameter and volume in women through the ages of 20–95 years. By contrast, men between the ages of 17–89 years demonstrate a gradual loss of nearly 1 g/year of the myocardium, accounting for approximately 64 million cells lost from both the left and right ventricles [4,5]. In the remaining cells, myocyte cell volume increases (hypertrophy) at a rate of 158 μm3/year in the left and 167 μm3/year in the right ventricle in males [5]. The underlying mechanism(s) for this difference between male and female hearts remains to be elucidated. A previous report using hearts from human subjects ranging from 21 to 93 years of age demonstrated that there is no correlation between age and cardiomyocyte apoptosis between the two sexes; however, in this study, cardiomyocyte apoptosis was threefold higher in men compared with women in all age groups [4]. The fact that apoptosis and age are not correlated does not rule out the possibility of other forms of cell death (i.e., necrosis) occurring in aging cardiomyocytes. The sex difference in apoptosis could in part be explained by the role of estrogen in protecting cardiomyocytes from apoptosis in women. In vitro estrogen treatment has been demonstrated to protect neonatal cardiac myocytes from angiotensin II- [6], staurosporine- [7] and H2O2-induced apoptosis [6]. In vivo long-term estrogen treatment can prevent apoptosis in cardiac myocytes in a mouse model of heart failure (HF) that induces apoptosis of cardiac myoctyes (overexpression of Gαq). The mechanism of this protection was demonstrated to be by the downregulation of Rac 1, leading to the attenuation of NAPDH oxidase activity and superoxide anion production [6]. Estrogen replacement was also shown to significantly reduce cardiac myocyte apoptosis after myocardial infarction (MI) in ovariectomized female mice by activating the prosurvival kinase Akt [8]. Whether the same mechanism holds true in postmenopausal women remains to be determined. More recently, Anversa and Kajstura suggested that the preservation of myocyte numbers in the female myocardium may result from the increased regenerative capacity of myocytes rather than from the absence of cell death [9]. Further research is warranted in order to understand the mechanisms of etiology and cell death in the aging human heart.
Calcium handling
In general, the force of contraction in a normal myocardium is comparable between males and females [10-12]. However, the durations of contraction and relaxation are usually shorter in the female myocardium, and the maximum velocities of tension development and decline are also markedly faster in the female myocardium [10,13]. An overt sex-related difference also exists in myofilament Ca2+-responsiveness that is believed to contribute to the disparity of myocardial contractile function between men and women. The female myocardium seems to possess a lower myofilament Ca2+-sensitivity than the male myocardium [13]. Myocellular Ca2+-handling is the key determinant of normal cardiac contractile function. Perturbations in Ca2+-handling lead to cardiac dysfunction and HF [14-16] characterized by attenuation in force development and impaired relaxation in the cardiac muscle [17]. Functional changes in contractility are associated with changes in the expression of key Ca2+-handling proteins [18,19]. For example, alterations in the expression of myocellular proteins such as the sodium–calcium exchanger (NCX) [20-22] and sarcoplasmic reticulum calcium ATPase (SERCA) have been well-documented, both in clinical HF and experimental models of HF [23-26]. Sex-dependent differences in the expression of these proteins exist in normal and failing myocardiums, and may play a role in how the heart responds to cardiac dysfunction in both sexes. NCX protein levels in humans are consistently higher in female hearts compared with male hearts [22]. However, previous reports indicate that in both male and female failing hearts, NCX levels are higher than in nonfailing hearts; however, this increase is proportionately greater in males [20-22]. By contrast, SERCA protein levels are downregulated in human HF in both males and females [27]. However, reports from an animal model of pressure overload hypertrophy have demonstrated that SERCA mRNA levels are depressed in males but not in females [28]. Therefore, differences in the myocardial expression of Ca2+-handling proteins that exist between the sexes should be taken into consideration when designing experimental and therapeutic strategies. The underlying mechanism of how sex hormones modulate the expression and function of these proteins remains to be determined.
Sex differences in the manifestation of cardiovascular disease
Cardiovascular disease manifests itself differently in women compared with men. Several scenarios in which sex plays a critical role in this context in humans and in animal models are discussed in this section. Table 1 describes sex differences in the manifestation of CVD in various genetically manipulated mouse models.
Table 1.
Differences in the manifestation of cardiovascular disease in male and female mice.
| Cardiovascular manipulation | Males | Females | Ref. |
|---|---|---|---|
| Chronic MI | ↑ MMP-9 activity leading to increase remodeling; ↑ incidence of rupture and increased infiltration of inflammatory cells | Better preserved cardiac function; less severe cardiac remodeling | [30,31] |
| Phospholamban overexpression | Hypertrophy at 15 months; earlier mortality by 22 months | Hypertrophy at 22 months | [42] |
| α-myosin heavy chain R403Q mutation | LV dilation; heart failure; ↑ fibrosis | Hypertrophy; preserved cardiac function | [43-46] |
| FKBP12.6 knockout | Hypertrophy | No hypertrophy; dysregulation of Ca2+-release mechanisms similar to males | [47] |
| TNF-α overexpression | Cardiac chamber dilation; low Ca2+-handling; ↑ fibrosis | Hypertrophy; normal Ca2+-handling | [62] |
| β2-adrenergic receptor overexpression | Hypercontractile; ↑ fibrosis | Hypercontractile; normal response to injury | [63] |
| Relaxin knockout | Cadiomyopathy; ↑ fibrosis | No altered phenotype | [64] |
| Guanylyl cyclase-A knockout | Cardiac hypertrophy; ↑ fibrosis | Less hypertrophy and fibrosis compared with males at 16 weeks of age | [65] |
LV: Left ventricular; MI: Myocardial infarction; MMP: Matrix metalloproteinase.
Myocardial infarction
In the USA, the median age for the first MI is 65.8 years for men and 70.4 years for women [29]. Women are more likely than men to die as a result of a MI; 38% of women die within 1 year of their first MI compared with 25% of men [29]. This could be attributed to the fact that women are more likely to be older when they have their first MI compared with men [29,30]. In addition, women are more likely to be misdiagnosed after their first MI because of their atypical symptoms [31]. Perimenopausal women rarely experience chest pain as typically expected, making a proper diagnosis more difficult. Chest pain, described by men, which often precedes MI, does not necessarily indicate MI in women. Angina in women is only predictive of coronary heart disease (CHD) in 50–60% of cases, whereas it is predictive of CHD in 80–99% of cases among men [31]. Factors that indicate MI in women with more effectiveness than in men include shoulder pain, neck pain, dyspnea and fatigue [31]. Studies using mice after a chronic MI indicate that females have a better preserved cardiac function following MI; in addition, the LV remodeling in females is less severe compared with their male counterparts [32,33]. The cardiac rupture rate following MI is significantly lower in females compared with males, consistent with less remodeling in female hearts [32-34]. This sex-specific dimorphism in response to MI could also be attributed to the increased activity of matrix metalloproteinases (MMP-9) leading to an enhanced degradation of extracellular matrix proteins in male mice [35]. In addition, hearts from female mice subjected to an MI were demonstrated to have markedly less infiltration of inflammatory cells compared with males, which could also contribute to the lower incidence of rupture in females [34]. However, the literature on cardiac rupture in humans remains controversial. Shapira et al. reported that the incidence of cardiac rupture in women aged younger than 59 years after an acute MI is rare compared with age-matched men [36]. By contrast, other reports in a clinical setting suggest that the risk of rupture is actually higher in women than in men [37,38].
Myocardial hypertrophy
Hypertrophy per se is an independent risk factor for HF and sudden death. Women demonstrated a marked increase in the incidence of LV hypertrophy after menopause [2] and HRT in postmenopausal women results in a significantly reduced LV mass relative to age-matched controls [39]. Clinically, female patients with aortic stenosis show increased hypertrophy, greater concentric remodeling and better preserved LV function compared with male patients [40-42]. Consistent with clinical observations, male but not female rats with pressure overload induced by aortic banding demonstrated an early transition to HF, LV cavity dilation, loss of concentric remodeling, elevated wall stress and diastolic dysfunction [43]. In mice with phospholamban, overexpressing male mice present an earlier onset of hypertrophy compared with females (15 months in males vs 22 months in females) [44]. The male mice also demonstrate earlier mortality compared with females (15 months in males vs 22 months in females) [44]. In a mouse model of hypertrophic cardiomyopathy, associated with an α-myosin heavy chain R403Q mutation, significant left and right ventricular hypertrophy are evident early in life in both sexes; however, the typical histological features and electrophysiological parameters associated with this disease are more pronounced in male hearts than in females. In addition, male mice develop progressive LV dilation and impaired cardiac function, whereas females demonstrate increasing hypertrophy without dilation and have preserved ventricular function [45-48]. The genetic inactivation of FKBP12.6 causes cardiac hypertrophy in male mice, but not in females [49]. FKBP12.6 is an intracellular binding protein that modulates the activity of the cardiac ryanodine receptor complex, which plays an essential role in sarcoplasmic reticulum Ca2+ release [49]. Disruption of the FKBP12.6 gene in mice results in cardiac hypertrophy in male mice, but not in females. Although male and female FKBP12.6-null mice display similar dysregulation in Ca2+ release mechanisms, female hearts continue to appear normal. Interestingly, female FKBP12.6-null mice treated with tamoxifen, an estrogen receptor (ER) antagonist, develop cardiac hypertrophy similar to that of male mice, indicating that estrogen probably plays a protective role in the hypertrophic response of the heart [49].
Arrhythmias
Sex-based differences in cardiac electrophysiology play a crucial role in the prevalence of different clinical arrhythmias. In general, women have faster resting heart rates (3–5 beats/min faster) [50] and rate-corrected QT intervals compared with men [51]. In addition, sex differences in T-wave shape have been observed [52]. The interval from S-wave offset to the peak of T-wave is also significantly prolonged in women compared with men [53]. The action potential duration in women is longer at all of the phases of an action potential compared with men [52]. Atrial fibrillation (AF) is the most common arrhythmia observed in clinical practice today, with an incidence rate that increases with age [54]. In the Framingham Heart Study, men were found to have a 1.5-fold higher risk for developing AF compared with women [55]. However, the mortality rate owing to AF is similar between men and women. Therefore, AF diminishes women’s survival advantage that is normally observed in other forms of heart disease [56]. Furthermore, women with AF are more likely than men to have embolic strokes [57]. Female gender is also an independent risk factor for both congenital and acquired long QT syndrome [54,58]. The incidence of syncope and sudden death associated with congenital long QT syndrome is also higher in women. A major threat attributed to the prolongation of QT interval is the life-threatening Torsades de Pointes (TDP), which is usually induced by antiarrhythmic drugs and is also more common in women than men [59]. The mechanisms underlying these sex differences with respect to arrhythmia are not completely understood; however, sex hormones are thought to be involved. A similar propensity towards drug-induced TDP was observed in both pre- and post-menopausal women, suggesting that estrogen does not have a protective role [60]. In addition, women taking oral contraceptives reportedly have a higher incidence of ventricular arrhythmia, suggesting that estrogen or progesterone could be arrhythmogenic [61]. It has been suggested that the actual ratio of estrogen to progesterone, rather than the absolute levels of the two hormones, might determine the propensity for women to develop TDP [62,63].
Cardiac fibrosis
Cardiac fibrosis is a hallmark of various forms of heart disease. It is characterized by an enhanced deposition of extracellular matrix proteins – most importantly collagen – leading to an impairment of diastolic and systolic function. Fibrosis develops following myocyte death, inflammation, pressure and volume overload, hypertrophy and stimulation by a variety of hormones, growth factors and cytokines. Sex differences in the progression of fibrosis have been postulated clinically. Biopsies from patients with aortic stenosis have demonstrated an increased myocardial stiffness, more common endocardial fibrosis and abnormal collagen architecture in males compared with females. Similarly, in various animal models of HF, more severe cardiac fibrosis is observed in male mice compared with females. For example, transgenic mice with overexpression of TNF-α [64], a mutant α-MHC [45,46] or β2-adrenergic receptor [65], and relaxin (a peptide hormone) [66] null mice present with increased fibrosis in males. Guanylyl cyclase-A (GC-A) knockout mice also exhibit fibrosis, which is significantly more pronounced in male mice compared with females [67].
Differential response of the two sexes to exercise
Exercise is an important factor in preventing CVD. Studies suggest that the favorable physiological responses to exercise might slow some of the pathophysiological progression of HF [68,69]. Exercise is known to activate complex interactions of central hemodynamics, peripheral blood flow, skeletal muscle, endothelium, neurohormones and the cytokine system, all of which benefit patients with HF [70-75]. The types, intensity, duration and frequency of exercise that could have positive effects on HF outcomes have still not been clarified. The role that sex plays in cardiac adaptation to exercise is also unclear. A sex-based dichotomy exists with respect to cardiac adaptation to exercise. Women tend to exhibit a relatively smaller increase in LV ejection fraction in response to exercise compared with men. One explanation for this differential ventricular response may be related to the different mechanisms by which women and men increase cardiac output during exercise. Women tend to increase their cardiac output primarily by increasing end-diastolic volume index without significantly increasing ejection fraction; this is compared with men who primarily decrease end-systolic volume index and raise ejection fraction [76]. Data from mice also suggest that exercise provokes a sex-dependent cardiac adaptation. Female mice have an increased voluntary and forced exercise capacity and increased hypertrophic response to similar amounts of exercise [77]. Chronic exercise significantly decreases the ratio of the slower β-myosin isoform relative to the α-myosin isoform in female mice while males experience no change. Taken together, these data suggest that females tend to benefit more from exercise than males. Several signaling pathways are implicated in affecting cardiac adaptation to exercise. Potential candidates for exercise-mediated cardiac hypertrophy are CaMKII, Akt and GSK-3β [77]. Cardiac adaptation of the two sexes to the stress of exercise is further complicated by modulation of the cardioprotective heat-shock proteins (HSP70) by sex hormones [78,79]. HSP70 is required for exercise-mediated cardioprotection owing to the fact that blockage of HSP70 synthesis by an antisense oligonucleotide has been demonstrated to attenuate any improvement in exercise-mediated postischemic recovery [80]. After treadmill running, male rats exhibit a twofold greater induction of HSP70 compared with female rats. Ovariectomized female rats exhibit an exercise-mediated induction of HSP70 similar to that observed in male rats; estrogen treatment in the ovariectomized females reverses this effect [80]. Furthermore, exercise markedly improves postischemic contractile parameters in male but not female rats [80]. This indicates that the exercise-mediated beneficial effect of HSP70 is influenced by estrogen.
Differential response of the two sexes to cardiovascular therapeutics
Women have been under-represented in the majority of the cardiovascular clinical trials in the past, which has left many questions unanswered in interpreting the efficacy of various drugs in women. There is evidence suggesting the existence of sex-specific differences in clearance rates, bioavailability and the efficacy obtained with the same dose of the drugs used for treating CVD [81]. Primary drug-metabolizing enzymes in the intestinal wall and liver, which are part of the cytochrome P450 family, have different activities in men and women [81]. Recent data regarding the efficacy of digoxin therapy, which is independent of age, suggest that it is associated with an increased mortality in women, but not men, and that is also associated with HF and depressed LV systolic function in women [82]. Although the digoxin dosage was standardized to BMI, women demonstrated slightly higher serum levels of the drug compared with men, indicating a difference in pharmacokinetics between the sexes [82]. A systematic meta-analysis including 15 trials indicates that statins reduce the risk of cardiovascular events in both men and women; however, women on statins may not have the reductions in mortality, MI and stroke that are observed in their male counterparts [83]. In general, women have higher serum levels of statin and the risk of adverse drug reactions owing to statins is relatively higher in women [84]. Women have a faster clearance rate, and hence lower serum levels, of certain Ca2+-channel blockers (e.g., verapamil and nifedipine) than men. Reduction in blood pressure owing to Ca2+-channel blockers is more pronounced in women [81]. Adverse drug reactions in the form of coughs while being treated with angiotensin-converting enzyme (ACE) inhibitors occur twice as frequently in women [85]. It is therefore very important to study cardiovascular drugs in both sexes.
Sex steroids & sex steroid hormone receptors in the heart
Sex steroids
Men and women both possess significant levels of estrogen and testosterone. In women, estrogen is synthesized primarily in the ovaries and, to a lesser extent, in the pituitary gland, adipose tissue and the liver, while testosterone is synthesized in the ovaries and in the adrenals. In men, estrogen is synthesized in the testes, the pituitary gland, the liver and in adipose tissue, while testosterone is synthesized mainly in the testes and, to a lesser extent, in the adrenals.
Estrogen has been suggested to be cardioprotective in many different settings [86-88]. There have been many proposed mechanisms for the cardioprotective effects of estrogen. First, estrogen has been demonstrated to induce mitochondrial biogenesis [89]. Second, many cardioprotective genes are upregulated either directly or indirectly by estrogen, including PGC-1α [90], MCIP1 [91] and HSP72 [92]. Estrogen also activates nitric oxide synthase and Akt through the PI3 kinase pathway, which has been shown to confer protective effects on both cardiomyocytes and endothelial cells [93,94]. Finally, estrogen has been shown to upregulate the production of cardiac natriuretic hormones, which have several beneficial effects on cardiac function, including reducing blood pressure and improving endothelial function [95]. Testosterone has been far less studied and so little is known about its underlying mechanism(s) of action in the heart. Testosterone has been demonstrated to induce hypertrophy and fibrosis in the murine heart [67]. There is evidence suggesting that the prohypertrophic effect of testosterone is mediated through the mTOR axis [96]. Testosterone has also been shown to be anti-inflammatory [97] and is involved in regulating antioxidant levels [98]. Furthermore, testosterone has been demonstrated to be important in maintaining the normal vascular tone in males through its conversion to estrogen in the vasculature by the enzyme aromatase [99].
Sex steroid hormone receptors
The biological effects of sex steroids are mediated by sex steroid hormone receptors (SSHRs). Sex steroids act as ligands and activate their cognate receptors; estrogen activates ERs (i.e., ERα and ERβ) while androgens activate androgen receptors (ARs). Upon ligand binding, the SSHRs dimerize and bind to specific DNA response elements, and engage the general transcriptional apparatus. These are defined as the genomic actions of SSHRs. SSHRs also elicit their effects in the target tissues by recruiting secondary messengers and kinase signaling cascades [100,101]. These responses occur too rapidly to involve changes in target-gene transcription and are therefore regarded as nongenomic [102]. Both of these modes of action of SSHRs have been previously reviewed in detail [100-104]. Most research is focused on the actions of ERs in cardiac pathophysiology but very little is known regarding ARs. In fact, all the SSHRs are expressed in both male and female cardiac myocytes, suggesting their importance in cardiac physiology. Levels of ERα (both mRNA and protein) are equivalent in the hearts of both men and women. Levels of ERβ mRNA, on the other hand, are higher in male than in female hearts [105]. Both ERα and ERβ are upregulated during human aortic stenosis [106]. Similarly, myocardium from patients with end-stage HF demonstrates elevated expression of ERα mRNA [105]. AR mRNA is expressed in cardiac tissues from both men and women [107]. Rat myocytes respond to androgens by eliciting a hypertrophic response [107]. Hence functional SSHRs are expressed in the heart and play important roles in cardiac pathophysiology. A better understanding of these receptors in the heart could elucidate the sexual dichotomy observed in cardiac function to a considerable extent.
Genetic studies on ER & AR
The receptors ERα, ERβ and AR are expressed at equivalent levels in male and female mice hearts [108]. ERα, ERβ and AR knockout mice have been used extensively to investigate the effects of hormones on pathological cardiac remodeling. The hearts of ERα knockout (αERKO) mice do not exhibit any baseline cardiovascular differences [109]. However, after short-term ischemia-reperfusion (I/R), αERKO females display reduced function and increased apoptotic signaling compared with wild-type controls. Interestingly, αERKO males do not exhibit a differential phenotype compared with wild-type mice post-I/R [110]. Similarly, compared with wild-type controls, female βERKO mice have increased expression of caspase 3 and 8, two proapoptotic markers, post-I/R. Interestingly, male βERKO knockout mice display decreased apoptotic signaling post-I/R compared to wild-type controls, implying a sex-dependent function of ERβ [111]. The available data suggest that both ERα and ERβ are protective in females, but not males, after short-term I/R.
By contrast with αERKO mice, both male and female βERKO mice display hypertension and increased heart mass prior to cardiovascular manipulation [112]. βERKO female mice subjected to ovariectomy followed by estrogen supplementation display an increased expression of atrial natriuretic peptide and increased mortality compared with wild-type controls post-MI [113]. By contrast, another study using the same methods as described previously reported reduced mortality in βERKO females post-MI compared with wild-type controls [114].
Endoplasmic reticulum-α knockout and βERKO mice have also been used to investigate the hormonal effects on pathological cardiac hypertrophy in response to pressure overload. In response to transverse aortic constriction (TAC), which induces pressure overload, estrogen attenuates the development of cardiac hypertrophy [115]. However, this protective antihypertrophic effect of estrogen is abolished in female βERKO mice, but not in female αERKO mice, suggesting that ERβ is responsible for the antihypertrophic properties of estrogen. Interestingly, this effect was not observed in male βERKO mice [114,116].
Male AR-knockout (ARKO) mice display reduced baseline heart weight and reduced myocyte cross-sectional area, suggesting a prohypertrophic effect of testosterone [117]. Interestingly, after angiotensin II stimulation, ARKO males display increased arterial medial thickness and perivascular fibrosis compared with wild-type controls, implying an important protective role for testosterone in the vasculature [118]. The GC-A knockout (GC-AKO) mice exhibit cardiac hypertrophy and fibrosis, which is significantly more pronounced in males compared with females at 16 weeks of age [67]. Interestingly, either castration at 10 weeks of age or flutamide, an AR antagonist, markedly attenuates cardiac hypertrophy and fibrosis in male GC-AKO mice, implicating the role of androgens in hypertrophy mediated through AR [67]. More research is warranted in order to better understand the effects of testosterone on the heart.
The majority of the experimental data point to ERα and ERβ playing a protective role, especially in females; however, this hypothesis is far from proven. Importantly, all of the previous studies were on global nulls of ERs. Studies using conditional cardiac-specific knockouts would help to clarify whether ER activity is directly beneficial or detrimental to cardiac function. More could be learned regarding the cardiovascular effects of estrogen from the aromatase-knockout mouse. These mice are lacking an essential enzyme for synthesizing estrogen. The aromatase-knockout mice are reported to have a 5% lower blood pressure and a 46% lower baroreflex sensitivity compared with wild-type controls [119].
Gonadectomy & hormone replacement in mice
Gonadectomy and hormone replacement can also alter the cardiac response to pathological stimuli (Table 2). Ovariectomized females subjected to volume overload develop greater cardiac hypertrophy, dilation and pulmonary edema than intact females [86]. Ovariectomized mice treated with a high physiological dosage of estrogen and subjected to pressure overload develop less severe cardiac remodeling than placebo treated mice [87]. Patten et al. compared the effects of estrogen supplementation on ovariectomized female mice in two different models of cardiac dysfunction, TAC and MI. A stimulus-specific effect of supraphysiological estrogen supplementation on cardiac function was observed, with estrogen being beneficial in pressure overload hypertrophy but not in MI [120]. Supplementing ovariectomized females with supraphysiological levels of testosterone worsened cardiac function post-MI. Gonadectomized male mice display reduced mortality and improved cardiac function post-MI. Supplementing the gonadectomized males with estrogen did not improve cardiac function when compared with placebo treatment [32]. However, in another study, intact male rats treated with testosterone were shown to have decreased pathological remodeling and mortality post-MI compared with placebo [97]. Thus, both estrogen and testosterone have distinct roles in cardiac pathophysiology. In females, estrogen is protective in volume and pressure overload, while testosterone may be detrimental post-MI.
Table 2.
The cardiovascular effects of gonadectomy and hormone supplementation in mice.
| Stimulus | Treatment | Conclusions | Ref. |
|---|---|---|---|
| Aortocaval fistula | Ovariectomized female rats and intact controls subjected to volume overload | The loss of ovarian hormones resulted in more severe pathologic remodeling, suggesting that estrogen is protective in this setting | [84] |
| Transverse aortic constriction | Ovariectomized female mice treated with estrogen or placebo subjected to pressure overload | Estrogen treatment improved cardiac function and reduced cardiac dilation | [117] |
| Transverse aortic constriction | Ovariectomized female mice treated with estrogen or placebo subjected to pressure overload | Estrogen treatment improved cardiac function compared with placebo | [85] |
| MI | Intact and gonadectomized male mice treated with estrogen or placebo subjected to MI; intact and gonadectomized female mice treated with testosterone or placebo subjected to MI | Intact males displayed increased mortality and cardiac dilation compared with intact females post-MI; both estrogen treatment of intact males and gonadectomy improved function and decreased mortality post-MI; testosterone treatment of intact and gonadectomized females increased mortality and decreased cardiac function post-MI; this supports the hypothesis that estrogen is protective and testosterone is detrimental in post-MI | [30] |
| MI | Ovariectomized female mice treated with estrogen or placebo subjected to MI | Estrogen did not modify mortality, cardiac function or cardiac mass post-MI | [117] |
| MI | Intact male rats treated with testosterone or placebo | Testosterone reduced mortality and pathologic cardiac remodeling post-MI suggesting that testosterone may be beneficial in males | [94] |
MI: Myocardial infarction.
There are several caveats to consider when interpreting data in the literature. The effects of hormone replacement or removal experiments on the heart depend greatly on the stimulus, duration and the dose of hormone treatment, and the sex of the mice. Another important caveat is that measured hormone levels in intact wild-type mice vary dramatically depending on the laboratory and the method employed. Measurements of estrogen levels in adult female mice reported in the literature vary from 2.9 to 99 pg/ml [113,121]. By contrast, testosterone levels in adult male mice vary from 35 to 1500 ng/dl [122,123]. Therefore, it is essential that any study reporting hormone supplementation at a physiological level must measure wild-type mice, gonadectomized mice treated with placebo and gonadectomized mice supplemented with hormones. As a final caveat, most studies have focused on the effects of hormones on cardiomyocytes, and it is important to consider that the heart consists of a myriad of cell types besides cardiomyocytes, including cardiac fibroblasts and endothelial cells. The heart is 90% myocyte by mass, but by cell number, cardiomyocytes make up only 25–35% of the total number of cells. Cardiac fibroblasts constitute 90% of nonmyocyte cells [124]. Recently, the presumption that apoptosis in the myocardium primarily occurs in myocytes was challenged by a study using a monkey model of HF. This study found that the majority of apoptosis occurred in nonmyocytes [125]. Nonmyocyte cell types in the heart, such as endothelial cells, smooth muscle cells and fibroblasts, all express ERα, ERβ and AR. Therefore, the hormonal effects of estrogen and testosterone on nonmyocytes should also be considered when drawing experimental conclusions [126,127]. It should also be noted that results obtained using rodent models may not necessarily be extrapolated to humans owing to differences in cardiovascular biology, such as heart rate and molecular composition.
HRT in women
As mentioned previously, incidence of heart disease is sexually dimorphic in men and women younger than 65 years of age (CDC). However, when comparing men and women over 65 years of age, this sexual dimorphism disappears and mortality is higher in postmenopausal women compared with age-matched men [201]. This led to the hypothesis that estrogen is a cardioprotective hormone in women and that the reduction in estrogen levels in postmenopausal women causes this increase in the incidence of heart disease.
Many observational and randomized clinical trials have been conducted to investigate the relationship between menopause with or without HRT and heart disease. Observational studies have provided evidence that menopause is associated with an increased risk of heart disease. Age-matched women who undergo natural menopause prior to 50 years of age have a 1.5-fold higher rate of CHD than those who undergo menopause after 50 years [128]. In age-matched women who undergo surgical menopause, the rate of CHD is approximately twofold higher than hormonally intact women [129]. Based on these studies, postmenopausal women were prescribed estrogen for the primary purpose of preventing heart disease. A meta-analysis of 25 observational studies demonstrated a decreased risk of CHD with estrogen treatment alone. The hazard ratio, defined as the ratio of CHD occurrence of hormone users to nonhormone users, was 0.70. By contrast, in women receiving estrogen plus progesterone treatment, the hazard ratio was 0.66 [130]. However, it is important to note that these trials were not randomized, which may be a confounding factor.
The randomized Heart and Estrogen/Progestin Replacement Study (HERS) trial was designed to study the effects of hormone replacement in postmenopausal women with established coronary disease and an intact uterus. In the HERS trial, 2763 postmenopausal women were given either 0.625 mg/day of conjugated equine estrogen (CEE) plus 2.5 mg/day of medroxyprogesterone acetate (MPA) or a placebo. Women who received CEE plus MPA did not show a reduced occurrence of MI or CHD death with a hazard ratio of 0.99 [131]. Compared with placebo, hormone treatment was not beneficial in women with established CHD.
The Women’s Health Initiative (WHI) involved 16,000 women and was the first major randomized trial to test the hypothesis that replacing the endogenous estrogen lost owing to menopause with exogenous estrogen would protect women from developing heart disease. The results from the WHI study proved less conclusive than previous observational studies. In women with an intact uterus receiving CEE plus MPA, hormonal treatment increased CHD risk with a hazard ratio of 1.29 [132]. In women with prior hysterectomy treated with CEE alone, hormone treatment did not change CHD risk [133]. Secondary analysis of data from the WHI trial suggested that initiating hormone treatment soon after menopause might reduce CHD events, with a nonsignificant hazard ratio of 0.76 for women who started hormone replacement within 10 years of menopause compared with women who started hormone replacement more than 10 years postmenopause [134]. The idea that initiating hormone treatment soon after menopause might protect from CHD is termed the timing hypothesis. However, further analysis of the WHI trial, including data from both observational and randomized trials, did not demonstrate a reduction in CHD events in women who started hormonal therapy closer to menopause [135]. Thus, the cardiac benefits of HRT remain unresolved. Moreover, it should be noted that in the CEE plus MPA arm of the WHI trial, HRT significantly increased the risk of breast cancer, stroke and pulmonary embolism, and caused the trial to be stopped early. On the other hand, HRT reduces some risk factors for CVD. One such risk factor for which prevalence is reduced by HRT is central obesity, a strong predictor of CVD occurrence [136,137].
Phytoestrogens & cardiovascular health
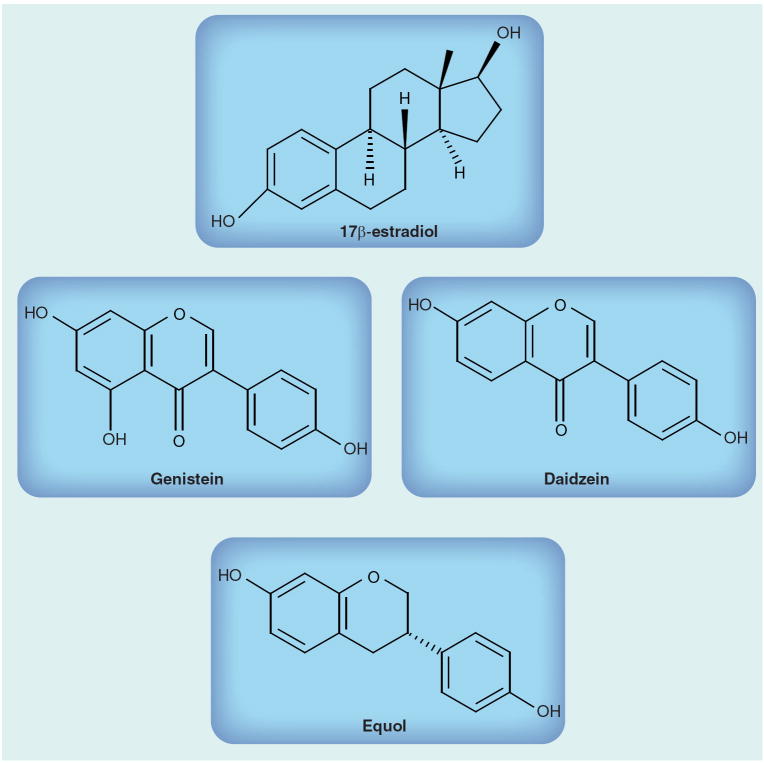
Given the risks associated with conventional HRT, there is growing scientific interest focused on alternative therapies for postmenopausal women with a predisposition to CVD. Phytoestrogens are interesting candidates in this regard since they are structurally similar to estrogens (Figure 1). They act as both estrogenic agonists and antagonists [186].
Figure 1.
Structural similarities between 17β-estradiol and phytoestrogens.
The phytoestrogens daidzein and genistein are naturally occurring isoflavones that are found in numerous edible plants, especially soybeans. Individuals consuming soy on a regular basis (~50 mg/day) could have plasma isoflavone concentrations of 50–800 ng/ml, which is 100-times higher than endogenous estradiol levels (concentrations in men and women ranging from 40 to 80 pg/ml) [138]. In infants fed on soy formula, these concentrations are even higher, reaching 9–10 μM [138]. Phytoestrogens bind both ERα and ERβ, but they bind ERβ with greater affinity (Table 3) [139]. The relative potency of phytoestrogens in activating transcription of estrogen-responsive genes is 1000- to 10,000-fold less compared with 17β-estradiol, indicating that by comparison with 17β-estradiol, genistein and daidzein have weak genomic estrogen effects (Table 4) [140].
Table 3.
Relative estrogen potencies of 17β-estradiol and phytoestrogens.
| Compound | Estrogen receptor-α | Estrogen receptor-β |
|---|---|---|
| 17β-estradiol | 100.00 | 100.00 |
| Genistein | 3.10 | 18.13 |
| Daidzein | 0.25 | 0.79 |
| Equol | 0.28 | 1.87 |
The table illustrates the relative genomic estrogenic activity of the phytoestrogens in comparison with 17β-estradiol, arbitrarily set at 100.00.
Adapted from [184].
Table 4.
Relative binding affinities of 17β-estradiol and phytoestrogens for estrogen receptors α and β.
| Compound | Genomic estrogenic activity |
|---|---|
| 17β-estradiol | 100.00 |
| Genistein | 0.08 |
| Daidzein | 0.013 |
| Equol | 0.06 |
The relative binding affinity of 17β-estradiol was arbitrarily set at 100.00.
Adapted from [185].
Isoflavones in soy foods and supplements occur as highly water-soluble glucosides, which undergo hydrolysis by intestinal β-glucosidases to release bioactive aglycons (i.e., daidzein and genistein) prior to absorption by the small and large intestines. After absorption, the isoflavone aglycons are reconjugated with glucuronic acid and, to a smaller extent, with sulfate by the phase II enzymes UDP-glucuronosyltransferases and sulfotransferases in the liver. Similar to endogenous estrogens, these conjugates are excreted via both urine and bile. Only 7–30% of the ingested amount of daidzein and genistein can be recovered in urine and less than 10% in the feces [141,142]. This low recovery could be explained by further metabolism of both genistein and daidzein by the hepatic cytochrome p450. Daidzein undergoes further metabolism by means of the gut microflora [143] to obtain a biologically active metabolite, equol, which is more estrogenic than daidzein (Table 4) [144]. Equol also has higher affinity for both ERα and ERβ than its precursor daidzein (Table 3) [145,146]. Among humans, approximately 30–50% of the population are equol-producers. By contrast, mice, rats, hamsters, cows, pigs, sheep, dogs, monkeys and chimpanzees all have the ability to produce equol [147-151]. Diet and host genetics may contribute to inter-individual variations in equol production in humans. However, the reasons for such differences in harboring the equol-producing bacteria remain essentially unknown. The specific bateria or bacterium responsible for equol production in the human gut is also yet to be identified. According to Setchell et al., individuals with plasma equol concentrations of less than 40 nmol/l (10 μg/l) can be classified as nonequol producers, and concentrations less than 83 nmol/l (20 μg/l) can be used to define equol producers [138]. Based on plasma isoflavone concentration, there are no conclusive age- or sex-based differences in the bioavailabilities of genistein and daidzein [152]. However, Lu et al. suggested that women, but not men, who are initially unable to metabolize daidzein to equol could develop this ability by long-term ingestion of soy [153].
The clinical effectiveness of soy protein in cardiovascular health is equivocal. In 2006, the American Heart Association reversed its endorsement of soy products by stating that it found no effect of soy proteins or plant estrogens on blood pressure, or menopausal changes [154]. By contrast, other human studies indicate that soy could have potential beneficial impacts on cardiovascular risk factors, including body mass index [155], lipid profiles [156,157], glucose and insulin homeostasis [158,159] and hypertension [157]. In the majority of 22 randomized trials, isolated soy protein decreased LDL-cholesterol concentrations by an average effect of approximately 3%. This reduction is very small relative to the large amount of soy protein tested in these studies, averaging 50 g, which is approximately half the usual total daily protein intake [160]. Isoflavones have been shown to induce vasodilation by stimulating the activity of the endothelial NO synthase (NOS)3. Genistein and daidzein decrease monocyte chemoattractant protein-1 and collagen-induced platelet aggregation in a dose-dependent manner, indicating that they could act as antithrombotic and antiatherogenic agents [161].
Phytoestrogens also exert a direct effect on cardiac myocyte contractility, albeit in opposing ways; genistein exerts a positive inotropic effect on isolated ventricular guinea pig myocytes, whereas equol exerts a negative inotropic effect [162]. This suggests that both of these soy components might possess distinct mechanisms of action, and the cumulative effect of these phytoestrogens on cardiac function might be the result of specific dominating actions of either genistein or equol.
There is evidence in the literature that the hormonal effects of phytoestrogens can affect fertility in humans and animals of both sexes. Genistein causes increased cAMP stimulation, leading to acrosomal loss and an increased rate of capacitation in human sperm populations, resulting in an inability of the sperm to fertilize an ovum [163]. When genistein and daidzein are tested in combination, their adverse effects on human sperm are more pronounced [163]. Male rats have decreased sperm production [164] and female mice produce fewer offspring [165] when exposed to high levels of phytoestrogens that are similar to those found in soy-based rodent chow. In order to lower reproduction during drought, quails have evolved to eat high-phytoestrogen-containing plants [166]. In addition, cheetahs in captivity were infertile until soy was reduced in the diet [167]. Phytoestrogens may also exert other detrimental hormonal effects; genistein has been demonstrated to increase glucose sensitivity of pancreatic islets in a dose-dependent manner (10–100 μM), and this effect was independent of ER. Furthermore, genistein has also been indicated to cause goiter in humans and animals by inhibiting thyroid peroxidase, which is also independent of ER [168]. Hooper et al. reported that isoflavone-rich soy products decrease follicle-stimulating hormone and lutenizing hormones in premenopausal women and may increase estradiol in postmenopausal women [169]. The clinical implication of these hormonal changes remain to be determined.
In addition to being an ER agonist, genistein in the μM range is also a potent tyrosine kinase inhibitor (TKI), whereas daidzein is inactive in this respect. Recent data indicate that several small molecule TKIs (e.g., anthracyclines, cyclophosphamide and trastuzumab) used to treat various cancers, are associated with severe cardiotoxicity, which might affect survival more than the malignancy itself [170,171]. The exact mechanism by which these TKIs induce cardiotoxicity remains under investigation. The relevance of genistein as a TKI has been most extensively studied with respect to ischemia. In rat hearts, ischemic preconditioning, which offers cardioprotection, is completely abolished by the TK inhibitory action of genistein, by preventing the enhancement of phospholipase D activity [172]. In addition, preconditioning of the rat heart stimulates protein kinase C, MAPK, and MAPKAP kinase 2 activities, which are also inhibited by genistein [172]. A similar abolition of the protective antiarrhythmic effect of preconditioning was also observed in hearts perfused in vitro with genistein 100 μM [173]. Genistein also inhibits the activation of Akt, which is a prohypertrophic signaling molecule [174]. TK inhibition by genistein has been shown to induce apoptosis in a variety of cell types in vitro [175-177]. In 3T3-L1 adipocytes, 100 μM genistein was demonstrated to induce apoptosis [178]. It was demonstrated that an in vivo dose of approximately 150 mg/kg/day, resulting in a serum concentration of 3.8 μM of genistein in ovariectomized female mice, causes adipose tissue apoptosis [178]. This indicates that genistein could elicit a proapoptotic response in the absence of estrogen, even at a physiological concentration in mice.
There are conflicting data regarding the beneficial or adverse effects of phytoestrogens, depending on experimental conditions (in vivo vs in vitro studies; dose vs concentration; and time of exposure). Phytoestrogens also exert sex-specific effects in humans and animals. A recent study indicated that habitual intake of soy caused a moderately reduced risk of metabolic syndrome in middle-aged and elderly Chinese women, but elevated the risk in men [179]. The median level of soy protein intake was 7.82 g/day (7.64 g/day in men and 8.02 g/day in women). Soy significantly increased the risk for hyperglycemia but reduced the risk for hypertension in these men [179]. Nagata et al. found an inverse correlation between soy intake and diastolic blood pressure in Japanese men, and a marginally positive association in peri- and post-menopausal women [180]. Recent data from our laboratory indicate that phytoestrogen supplementation augmented cardiac growth in males, but not females, in a mouse model of hypertrophic cardiomyopathy [45]. Taken together, these data suggest that there is a complex interaction between phytoestrogen intake and the endogenous hormonal milieu in humans and animals.
The physiological effects of phytoestrogens are further confounded by the equol producing status of humans. There is some evidence in the literature suggesting that the equol-producing status of individuals might affect their response to dietary interventions using soy. A 2-year study on lipids in normo- and hypercholesterolemic postmenopausal women found that soy had the greatest hypocholes-terolemic effect in equol-producers compared with nonproducers [181]. Hence, it might prove informative to measure serum equol levels of bacteriotype individuals for their equol-producing ability before dietary interventions with phytoestrogens are undertaken.
Conclusion & future perspective
Sex by itself is an independent variable affecting every aspect of healthy and failing heart function. Why do women before the age of 65 years have a lower incidence of heart disease compared with men? There are compelling clinical and experimental data supporting the cardioprotective role of estrogen in conferring the female advantage in heart disease. However, the interaction between sex and CVD is affected by a plethora of extrinsic and intrinsic factors, and so cannot be attributed to a single factor such as estrogen. A clinical trial that was designed to test the benefit of treating men with a predisposition to heart attack with large doses of estrogen resulted in gynecomastia, impotence and venous thromboembolic disease [182]. Recently, men with higher levels of circulating estrogen were found to be associated with the highest level of cardiovascular risk, as they demonstrated high levels of LDL-cholesterol and low HDL-cholesterol [183]. Why natural, endogenous estrogens that are generally seen as cardioprotective in women increase cardiovascular risk in men remains to be explained. The role testosterone plays in CVD, and how it interacts with sex, age, blood pressure and other potential confounding factors, is also unclear. Furthermore, a better understanding of the interaction of sex hormones with their cognate receptors, and their modulation during heart disease, will broaden our understanding of these hormones and the role they play in the cardiovascular sexual dimorphism.
A growing interest in alternative therapies to HRT for the treatment of HF and post-menopausal symptoms has led to a spike in the use of environmental agents that mimic endogenous hormones (i.e., phytoestrogens). Several confounding factors, such as lifestyle and demography, should be considered before we draw conclusions regarding the beneficial effects of phytoestrogens. Van der Schouw et al. considered age, BMI, smoking, physical activity, mean arterial pressure, time since menopause, energy intake, fiber intake, fruit intake, vegetable intake, alcohol intake, blood glucose and HDL-cholesterol as potential confounding factors [184]. Further research is warranted in order to ascertain the possible beneficial CVD outcomes via phytoestrogen intake. Prospective studies evaluating the effects of phytoestrogens on cardiovascular morbidity and mortality should also be carried out. Dose–response trials should be conducted to determine optimal doses for the cardiovascular system. Further research should be undertaken in order to distinguish the effects of supplemental doses of phytoestrogens from doses normally consumed in the diet. If phytoestrogens are to be considered an alternative to traditional HRT, then clinical trials with CVD event outcomes must be undertaken.
Executive summary.
Sex differences in heart
After puberty, the left ventricular (LV) mass:body weight ratio is 25–38% greater in men compared with women. The increase in LV mass is primarily due to changes in myocyte size (hypertrophy) rather than their numbers.
The myocardial mass, myocyte number and average cell diameter and volume in women are preserved through the ages of 20–95 years. By contrast, men between the ages of 17–89 years show a gradual loss of nearly 1 g/year of the myocardium, accounting for approximately 64 million cells lost from both the left and right ventricles. In the remaining cells in males, myocyte cell volume increases at a rate of 158 μm3/year in the left and 167 μm3/year in the right ventricle.
There is no correlation between age and cardiomyocyte apoptosis between the two sexes. However, at any given age, cardiomyocyte apoptosis is threefold higher in men compared with women.
The force of contraction in a normal myocardium is comparable between males and females. However, the durations of contraction and relaxation are usually shorter, and the maximum velocities of tension development and decline are markedly faster in the female myocardium. The female myocardium seems to possess a lower myofilament Ca2+-sensitivity than the male myocardium.
Sex-based differences in the expression of myocardial Ca2+-handling proteins (the Na+–Ca2+ exchanger and sarcoplasmic reticulum calcium ATPase) have a direct impact on the functional changes in contractility.
Sex differences in the manifestation of cardiovascular disease
Cardiovascular disease manifests itself differently in women compared with men. Myocardial infarction, hypertrophy, arrhythmias and fibrosis demonstrate sexual dimorphism in their manifestation and prognosis.
Differential response of the two sexes to exercise
A sex-based dichotomy exists with respect to cardiac adaptation to exercise. Women tend to exhibit a relatively smaller increase in LV ejection fraction in response to exercise compared with men.
Women tend to increase their cardiac output primarily by increasing end-diastolic volume index without significantly increasing ejection fraction, compared with men who primarily decrease end-systolic volume index and raise ejection fraction.
Data from mice also suggest that exercise provokes a sex-dependent cardiac adaptation. Females tend to benefit more from exercise compared with males.
Potential candidates that mediate exercise-induced cardiac hypertrophy include CaMKII, Akt and GSK-3β.
The exercise-mediated beneficial effect of HSP 70 is influenced by estrogen.
Differential response of the two sexes to cardiovascular therapeutics
Women have been under-represented in the majority of the cardiovascular clinical trials in the past, which has left many questions unanswered in interpreting the efficacy of various drugs in women.
A number of cardiovascular drugs, such as digoxin, statins, Ca2+-channel blockers and acetylcholineesterase inhibitors, exhibit differential effects in men and women in terms of efficacy, pharmacokinetics or adverse drug reactions. Therefore, it is very important to study cardiovascular drugs in both sexes.
Sex steroids & sex steroid hormone receptors in the heart
Men and women both produce significant and physiologically relevant amounts of estrogen and testosterone.
Estrogen exerts cardioprotective effects via several mechanisms.
Testosterone has been demonstrated to have both protective and deleterious effects on heart.
Functional sex steroid hormone receptors; estrogen receptor (ER)α and ERβ and androgen receptors (ARs) are expressed in the heart and play important roles in cardiac pathophysiology.
ERα and ERβ play a protective role, especially in females, after various cardiac insults.
ERβ may be responsible for the antihypertrophic effects of estrogen.
Little is known regarding the androgen receptor-knockout or aromatase-knockout mice.
Estrogen is protective in volume and pressure overload in females. Testosterone is detrimental to females post-myocardial infarction.
HRT does not decrease heart disease risk, but does increase the risks of stroke, breast cancer and pulmonary embolism.
Phytoestrogens & cardiovascular health
Phytoestrogens daidzein and genistein are naturally occurring isoflavones that are found in numerous edible plants, especially soybeans. Daidzein undergoes further metabolism by means of the gut microflora to obtain a biologically active metabolite, equol.
Phytoestrogens are structurally similar to estrogens and may act as both estrogen agonists and antagonists.
Phytoestrogens can both positively and negatively influence cardiac function.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
For reprint orders, please contact: reprints@futuremedicine.com
Contributor Information
Poornima Bhupathy, Department of Molecular, Cellular & Developmental Biology, University of Colorado, Boulder, CO 80309-80347, USA, Tel.: +1 303 492 7606, Fax: +1 303 492 8907, poornima.bhupathy@colorado.edu.
Christopher Dean Haines, Department of Molecular, Cellular & Developmental Biology, University of Colorado, Boulder, CO 80309-80347, USA, Tel.: +1 303 492 7606, Fax: +1 303 492 8907, christopher.haines@colorado.edu.
Leslie Anne Leinwand, Department of Molecular, Cellular & Developmental Biology, University of Colorado, Boulder, CO 80309-80347, USA and Department of Chemistry & Biochemistry, University of Colorado, Boulder, CO 80309-80347, USA, Tel.: +1 303 492 7606, Fax: +1 303 492 8907, leslie.leinwand@colorado.edu.
Bibliography
- 1.de Simone G, Devereux RB, Daniels SR, Meyer RA. Gender differences in left ventricular growth. Hypertension. 1995;26(6 Pt 1):979–983. doi: 10.1161/01.hyp.26.6.979. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Ann Intern Med. 1988;108(1):7–13. doi: 10.7326/0003-4819-108-1-7. [DOI] [PubMed] [Google Scholar]
- 3.Zak R. Development and proliferative capacity of cardiac muscle cells. Circ Res. 1974;35(2 Suppl II):17–26. [PubMed] [Google Scholar]
- 4.Mallat Z, Fornes P, Costagliola R, et al. Age and gender effects on cardiomyocyte apoptosis in the normal human heart. J Gerontol A Biol Sci Med Sci. 2001;56(11):M719–M723. doi: 10.1093/gerona/56.11.m719. [DOI] [PubMed] [Google Scholar]
- 5.Olivetti G, Giordano G, Corradi D, et al. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26(4):1068–1079. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 6.Satoh M, Matter CM, Ogita H, et al. Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation. 2007;115(25):3197–3204. doi: 10.1161/CIRCULATIONAHA.106.657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelzer T, Schumann M, Neumann M, et al. 17β-estradiol prevents programmed cell death in cardiac myocytes. Biochem Biophys Res Commun. 2000;268(1):192–200. doi: 10.1006/bbrc.2000.2073. [DOI] [PubMed] [Google Scholar]
- 8.Patten RD, Pourati I, Aronovitz MJ, et al. 17β-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95(7):692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 9.Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83(1):1–14. doi: 10.1161/01.res.83.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Capasso JM, Remily RM, Smith RH, Sonnenblick EH. Sex differences in myocardial contractility in the rat. Basic Res Cardiol. 1983;78(2):156–171. doi: 10.1007/BF01906669. [DOI] [PubMed] [Google Scholar]
- 11.Monasky MM, Varian KD, Janssen PM. Gender comparison of contractile performance and β-adrenergic response in isolated rat cardiac trabeculae. J Comp Physiol B. 2008;178(3):307–313. doi: 10.1007/s00360-007-0223-y. [DOI] [PubMed] [Google Scholar]
- 12.Petre RE, Quaile MP, Rossman EI, et al. Sex-based differences in myocardial contractile reserve. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R810–R818. doi: 10.1152/ajpregu.00377.2006. [DOI] [PubMed] [Google Scholar]
- 13.Ren J, Ceylan-Isik AF. Diabetic cardiomyopathy: do women differ from men? Endocrine. 2004;25(2):73–83. doi: 10.1385/ENDO:25:2:073. [DOI] [PubMed] [Google Scholar]
- 14.Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32(9):1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 15.Gwathmey JK, Copelas L, MacKinnon R, et al. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61(1):70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- 16.Gwathmey JK, Slawsky MT, Hajjar RJ, Briggs GM, Morgan JP. Role of intracellular calcium handling in force-interval relationships of human ventricular myocardium. J Clin Invest. 1990;85(5):1599–1613. doi: 10.1172/JCI114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies CH, Davia K, Bennett JG, Pepper JR, Poole-Wilson PA, Harding SE. Reduced contraction and altered frequency response of isolated ventricular myocytes from patients with heart failure. Circulation. 1995;92(9):2540–2549. doi: 10.1161/01.cir.92.9.2540. [DOI] [PubMed] [Google Scholar]
- 18.de Tombe PP. Altered contractile function in heart failure. Cardiovasc Res. 1998;37(2):367–380. doi: 10.1016/s0008-6363(97)00275-7. [DOI] [PubMed] [Google Scholar]
- 19.Prestle J, Quinn FR, Smith GL. Ca2+-handling proteins and heart failure: novel molecular targets? Curr Med Chem. 2003;10(11):967–981. doi: 10.2174/0929867033457656. [DOI] [PubMed] [Google Scholar]
- 20.Litwin SE, Bridge JH. Enhanced Na+–Ca2+ exchange in the infarcted heart. Implications for excitation-contraction coupling. Circ Res. 1997;81(6):1083–1093. doi: 10.1161/01.res.81.6.1083. [DOI] [PubMed] [Google Scholar]
- 21.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999;85(11):1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 22.Pogwizd SM. Increased Na+–Ca2+ exchanger in the failing heart. Circ Res. 2000;87(8):641–643. doi: 10.1161/01.res.87.8.641. [DOI] [PubMed] [Google Scholar]
- 23.Currie S, Smith GL. Enhanced phosphorylation of phospholamban and downregulation of sarco/endoplasmic reticulum Ca2+ ATPase type 2 (SERCA 2) in cardiac sarcoplasmic reticulum from rabbits with heart failure. Cardiovasc Res. 1999;41(1):135–146. doi: 10.1016/s0008-6363(98)00241-7. [DOI] [PubMed] [Google Scholar]
- 24.Arai M, Matsui H, Periasamy M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res. 1994;74(4):555–564. doi: 10.1161/01.res.74.4.555. [DOI] [PubMed] [Google Scholar]
- 25.Mercadier JJ, Lompré AM, Duc P, et al. Altered sarcoplasmic reticulum Ca2+-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest. 1990;85(1):305–309. doi: 10.1172/JCI114429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993;72(2):463–469. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- 27.Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. J Mol Cell Cardiol. 2001;33(7):1345–1353. doi: 10.1006/jmcc.2001.1394. [DOI] [PubMed] [Google Scholar]
- 28.Weinberg EO, Thienelt CD, Katz SE, et al. Gender differences in molecular remodeling in pressure overload hypertrophy. J Am Coll Cardiol. 1999;34(1):264–273. doi: 10.1016/s0735-1097(99)00165-5. [DOI] [PubMed] [Google Scholar]
- 29.Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150(6):405–410. doi: 10.7326/0003-4819-150-6-200903170-00009. [DOI] [PubMed] [Google Scholar]
- 30.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 31.Bedinghaus J, Leshan L, Diehr S. Coronary artery disease prevention: what’s different for women? Am Fam Physician. 2001;63(7):1393–1400. 1405–1406. [PubMed] [Google Scholar]
- 32.Cavasin MA, Sankey SS, Yu AL, Menon S, Yang XP. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284(5):H1560–H1569. doi: 10.1152/ajpheart.01087.2002. [DOI] [PubMed] [Google Scholar]
- 33.Cavasin MA, Tao Z, Menon S, Yang XP. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004;75(18):2181–2192. doi: 10.1016/j.lfs.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 34.Gao XM, Xu Q, Kiriazis H, Dart AM, Du XJ. Mouse model of post-infarct ventricular rupture: time course, strain- and gender-dependency, tensile strength, and histopathology. Cardiovasc Res. 2005;65(2):469–477. doi: 10.1016/j.cardiores.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Fang L, Gao XM, Moore XL, et al. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J Mol Cell Cardiol. 2007;43(5):535–544. doi: 10.1016/j.yjmcc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Shapira I, Isakov A, Burke M, Almog C. Cardiac rupture in patients with acute myocardial infarction. Chest. 1987;92(2):219–223. doi: 10.1378/chest.92.2.219. [DOI] [PubMed] [Google Scholar]
- 37.Becker RC, Gore JM, Lambrew C, et al. A composite view of cardiac rupture in the United States National Registry of Myocardial Infarction. J Am Coll Cardiol. 1996;27(6):1321–1326. doi: 10.1016/0735-1097(96)00008-3. [DOI] [PubMed] [Google Scholar]
- 38.Hutchins KD, Skurnick J, Lavenhar M, Natarajan GA. Cardiac rupture in acute myocardial infarction: a reassessment. Am J Forensic Med Pathol. 2002;23(1):78–82. doi: 10.1097/00000433-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Lim WK, Wren B, Jepson N, Roy S, Caplan G. Effect of hormone replacement therapy on left ventricular hypertrophy. Am J Cardiol. 1999;83(7):1132–1134. A9. doi: 10.1016/s0002-9149(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 40.Aurigemma GP, Gaasch WH. Gender differences in older patients with pressure-overload hypertrophy of the left ventricle. Cardiology. 1995;86(4):310–317. doi: 10.1159/000176895. [DOI] [PubMed] [Google Scholar]
- 41.Douglas PS, Otto CM, Mickel MC, Labovitz A, Reid CL, Davis KB. Gender differences in left ventricle geometry and function in patients undergoing balloon dilatation of the aortic valve for isolated aortic stenosis. NHLBI Balloon Valvuloplasty Registry. Br Heart J. 1995;73(6):548–554. doi: 10.1136/hrt.73.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villar AV, Llano M, Cobo M, et al. Gender differences of echocardiographic and gene expression patterns in human pressure overload left ventricular hypertrophy. J Mol Cell Cardiol. 2009;46(4):526–535. doi: 10.1016/j.yjmcc.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 43.Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol. 1998;32(4):1118–1125. doi: 10.1016/s0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 44.Dash R, Schmidt AG, Pathak A, et al. Differential regulation of p38 mitogen-activated protein kinase mediates gender-dependent catecholamine-induced hypertrophy. Cardiovasc Res. 2003;57(3):704–714. doi: 10.1016/s0008-6363(02)00772-1. [DOI] [PubMed] [Google Scholar]
- 45.Stauffer BL, Konhilas JP, Luczak ED, Leinwand LA. Soy diet worsens heart disease in mice. J Clin Invest. 2006;116(1):209–216. doi: 10.1172/JCI24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vikstrom KL, Factor SM, Leinwand LA. A murine model for hypertrophic cardiomyopathy. Z Kardiol. 1995;84(Suppl 4):49–54. [PubMed] [Google Scholar]
- 47.Geisterfer-Lowrance AAT, Christe M, Conner DA, et al. A mouse model of familial hypertrophic cardiomyopathy. Science. 1996;272(5262):731–734. doi: 10.1126/science.272.5262.731. [DOI] [PubMed] [Google Scholar]
- 48.Berul CI, Christe ME, Aronovitz MJ, Seidman CE, Seidman JG, Mendelsohn ME. Electrophysiological abnormalities and arrhythmias in α-MHC mutant familial hypertrophic cardiomyopathy mice. J Clin Invest. 1997;99(4):570–576. doi: 10.1172/JCI119197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xin HB, Senbonmatsu T, Cheng DS, et al. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature. 2002;416(6878):334–338. doi: 10.1038/416334a. [DOI] [PubMed] [Google Scholar]
- 50.Liu K, Ballew C, Jacobs DR, Jr, et al. Ethnic differences in blood pressure, pulse rate, and related characteristics in young adults. The CARDIA study. Hypertension. 1989;14(2):218–226. doi: 10.1161/01.hyp.14.2.218. [DOI] [PubMed] [Google Scholar]
- 51.Merri M, Benhorin J, Alberti M, Locati E, Moss AJ. Electrocardiographic quantitation of ventricular repolarization. Circulation. 1989;80(5):1301–1308. doi: 10.1161/01.cir.80.5.1301. [DOI] [PubMed] [Google Scholar]
- 52.Pham TV. Gender differences in cardiac development: are hormones at the heart of the matter? Cardiovasc Res. 2003;57(3):591–593. doi: 10.1016/s0008-6363(03)00221-9. [DOI] [PubMed] [Google Scholar]
- 53.Bidoggia H, Maciel JP, Capalozza N, et al. Sex-dependent electrocardiographic pattern of cardiac repolarization. Am Heart J. 2000;140(3):430–436. doi: 10.1067/mhj.2000.108510. [DOI] [PubMed] [Google Scholar]
- 54.Yarnoz MJ, Curtis AB. More reasons why men and women are not the same (gender differences in electrophysiology and arrhythmias) Am J Cardiol. 2008;101(9):1291–1296. doi: 10.1016/j.amjcard.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 55.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–844. [PubMed] [Google Scholar]
- 56.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 57.Cabin HS, Clubb KS, Hall C, Perlmutter RA, Feinstein AR. Risk for systemic embolization of atrial fibrillation without mitral stenosis. Am J Cardiol. 1990;65(16):1112–1116. doi: 10.1016/0002-9149(90)90323-s. [DOI] [PubMed] [Google Scholar]
- 58.Moss AJ, Schwartz PJ, Crampton RS, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84(3):1136–1144. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 59.Verkerk AO, Wilders R, Tan HL. Gender disparities in torsade de pointes ventricular tachycardia. Neth Heart J. 2007;15(12):405–411. doi: 10.1007/BF03086040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lehmann MH, Hardy S, Archibald D, Quart B, MacNeil DJ. Sex difference in risk of torsade de pointes with d,l-sotalol. Circulation. 1996;94(10):2535–2541. doi: 10.1161/01.cir.94.10.2535. [DOI] [PubMed] [Google Scholar]
- 61.Romhilt DW, Chaffin C, Choi SC, Irby EC. Arrhythmias on ambulatory electrocardiographic monitoring in women without apparent heart disease. Am J Cardiol. 1984;54(6):582–586. doi: 10.1016/0002-9149(84)90253-4. [DOI] [PubMed] [Google Scholar]
- 62.Kawasaki R, Machado C, Reinoehl J. Increased propensity of women to develop torsades de pointes during complete heart block. J Cardiovasc Electrophysiol. 1995;6(11):1032–1038. doi: 10.1111/j.1540-8167.1995.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 63.James AF, Hancox JC. Sex, drugs and arrhythmia: are gender differences in risk of torsades de pointes simply a matter of testosterone? Cardiovasc Res. 2003;57(1):1–4. doi: 10.1016/s0008-6363(02)00740-x. [DOI] [PubMed] [Google Scholar]
- 64.Janczewski AM, Kadokami T, Lemster B, Frye CS, McTiernan CF, Feldman AM. Morphological and functional changes in cardiac myocytes isolated from mice overexpressing TNF-α. Am J Physiol Heart Circ Physiol. 2003;284(3):H960–H969. doi: 10.1152/ajpheart.0718.2001. [DOI] [PubMed] [Google Scholar]
- 65.Cross HR, Murphy E, Koch WJ, Steenbergen C. Male and female mice overexpressing the β(2)-adrenergic receptor exhibit differences in ischemia/reperfusion injury: role of nitric oxide. Cardiovasc Res. 2002;53(3):662–671. doi: 10.1016/s0008-6363(01)00528-4. [DOI] [PubMed] [Google Scholar]
- 66.Du XJ, Samuel CS, Gao XM, Zhao L, Parry LJ, Tregear GW. Increased myocardial collagen and ventricular diastolic dysfunction in relaxin deficient mice: a gender-specific phenotype. Cardiovasc Res. 2003;57(2):395–404. doi: 10.1016/s0008-6363(02)00663-6. [DOI] [PubMed] [Google Scholar]
- 67.Li Y, Kishimoto I, Saito Y, et al. Androgen contributes to gender-related cardiac hypertrophy and fibrosis in mice lacking the gene encoding guanylyl cyclase-A. Endocrinology. 2004;145(2):951–958. doi: 10.1210/en.2003-0816. [DOI] [PubMed] [Google Scholar]
- 68.Ventura-Clapier R. Exercise training, energy metabolism, and heart failure. Appl Physiol Nutr Metab. 2009;34(3):336–339. doi: 10.1139/h09-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kemi OJ, Høydal MA, Haram PM, et al. Exercise training restores aerobic capacity and energy transfer systems in heart failure treated with losartan. Cardiovasc Res. 2007;76(1):91–99. doi: 10.1016/j.cardiores.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 70.Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98(24):2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 71.Dubach P, Myers J, Dziekan G, et al. Effect of high intensity exercise training on central hemodynamic responses to exercise in men with reduced left ventricular function. J Am Coll Cardiol. 1997;29(7):1591–1598. doi: 10.1016/s0735-1097(97)82540-5. [DOI] [PubMed] [Google Scholar]
- 72.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283(23):3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 73.Tai MK, Meininger JC, Frazier LQ. A systematic review of exercise interventions in patients with heart failure. Biol Res Nurs. 2008;10(2):156–182. doi: 10.1177/1099800408323217. [DOI] [PubMed] [Google Scholar]
- 74.Kobayashi N, Tsuruya Y, Iwasawa T, et al. Exercise training in patients with chronic heart failure improves endothelial function predominantly in the trained extremities. Circ J. 2003;67(6):505–510. doi: 10.1253/circj.67.505. [DOI] [PubMed] [Google Scholar]
- 75.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111(21):2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 76.Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation. 1984;69(2):203–213. doi: 10.1161/01.cir.69.2.203. [DOI] [PubMed] [Google Scholar]
- 77.Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol. 2004;287(6):H2768–H2776. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milne KJ, Noble EG. Response of the myocardium to exercise: sex-specific regulation of hsp70. Med Sci Sports Exerc. 2008;40(4):655–663. doi: 10.1249/MSS.0b013e3181621311. [DOI] [PubMed] [Google Scholar]
- 79.Noble EG, Milne KJ, Melling CW. Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab. 2008;33(5):1050–1065. doi: 10.1139/H08-069. [DOI] [PubMed] [Google Scholar]
- 80.Paroo Z, Haist JV, Karmazyn M, Noble EG. Exercise improves postischemic cardiac function in males but not females: consequences of a novel sex-specific heat shock protein 70 response. Circ Res. 2002;90(8):911–917. doi: 10.1161/01.res.0000016963.43856.b1. [DOI] [PubMed] [Google Scholar]
- 81.Jochmann N, Stangl K, Garbe E, Baumann G, Stangl V. Female-specific aspects in the pharmacotherapy of chronic cardiovascular diseases. Eur Heart J. 2005;26(16):1585–1595. doi: 10.1093/eurheartj/ehi397. [DOI] [PubMed] [Google Scholar]
- 82.Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347(18):1403–1411. doi: 10.1056/NEJMoa021266. [DOI] [PubMed] [Google Scholar]
- 83.Dale KM, Coleman CI, Shah SA, Patel AA, Kluger J, White CM. Impact of gender on statin efficacy. Curr Med Res Opin. 2007;23(3):565–574. doi: 10.1185/030079906X167516. [DOI] [PubMed] [Google Scholar]
- 84.Gibson DM, Bron NJ, Richens A, Hounslow NJ, Sedman AJ, Whitfield LR. Effect of age and gender on pharmacokinetics of atorvastatin in humans. J Clin Pharmacol. 1996;36(3):242–246. doi: 10.1002/j.1552-4604.1996.tb04194.x. [DOI] [PubMed] [Google Scholar]
- 85.Mackay FJ, Pearce GL, Mann RD. Cough and angiotensin II receptor antagonists: cause or confounding? Br J Clin Pharmacol. 1999;47(1):111–114. doi: 10.1046/j.1365-2125.1999.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brower GL, Gardner JD, Janicki JS. Gender mediated cardiac protection from adverse ventricular remodeling is abolished by ovariectomy. Mol Cell Biochem. 2003;251(1–2):89–95. [PubMed] [Google Scholar]
- 87.Donaldson C, Eder S, Baker C, et al. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ Res. 2009;104(2):265–275. doi: 10.1161/CIRCRESAHA.108.190397. 11P following 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-β to inhibit calcineurin. Endocrinology. 2008;149(7):3361–2269. doi: 10.1210/en.2008-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klinge CM. Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem. 2008;105(6):1342–1351. doi: 10.1002/jcb.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hsieh YC, Yang S, Choudhry MA, et al. PGC-1 upregulation via estrogen receptors: a common mechanism of salutary effects of estrogen and flutamide on heart function after trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2005;289(6):H2665–H2672. doi: 10.1152/ajpheart.00682.2005. [DOI] [PubMed] [Google Scholar]
- 91.Pedram A, Razandi M, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem. 2005;280(28):26339–26348. doi: 10.1074/jbc.M414409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Voss MR, Stallone JN, Li M, Cornelussen RN, Knuefermann P, Knowlton AA. Gender differences in the expression of heat shock proteins: the effect of estrogen. Am J Physiol Heart Circ Physiol. 2003;285(2):H687–H692. doi: 10.1152/ajpheart.01000.2002. [DOI] [PubMed] [Google Scholar]
- 93.Stice JP, Knowlton AA. Estrogen, NF-κB, and the heat shock response. Mol Med. 2008;14(7–8):517–527. doi: 10.2119/2008-00026.Stice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy E, Steenbergen C. Cardioprotection in females: a role for nitric oxide and altered gene expression. Heart Fail Rev. 2007;12(3–4):293–300. doi: 10.1007/s10741-007-9035-0. [DOI] [PubMed] [Google Scholar]
- 95.Clerico A, Fontana M, Vittorini S, Emdin M. The search for a pathophysiological link between gender, cardiac endocrine function, body mass regulation and cardiac mortality: proposal for a working hypothesis. Clin Chim Acta. 2009;405(1–2):1–7. doi: 10.1016/j.cca.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 96.Altamirano F, Oyarce C, Silva P, et al. Testosterone induces cardiomyocyte hypertrophy through mammalian target of rapamycin complex 1 pathway. J Endocrinol. 2009;202(2):299–307. doi: 10.1677/JOE-09-0044. [DOI] [PubMed] [Google Scholar]
- 97.Zhang YZ, Xing XW, He B, Wang LX. Effects of testosterone on cytokines and left ventricular remodeling following heart failure. Cell Physiol Biochem. 2007;20(6):847–852. doi: 10.1159/000110444. [DOI] [PubMed] [Google Scholar]
- 98.Klapcińska B, Jagsz S, Sadowska-Krepa E, Górski J, Kempa K, Langfort J. Effects of castration and testosterone replacement on the antioxidant defense system in rat left ventricle. J Physiol Sci. 2008;58(3):173–177. doi: 10.2170/physiolsci.RP002208. [DOI] [PubMed] [Google Scholar]
- 99.Rosano GM, Vitale C, Silvestri A, Fini M. Metabolic and vascular effect of progestins in post-menopausal women. Implications for cardioprotection. Maturitas. 2003;46(Suppl 1):S17–S29. doi: 10.1016/j.maturitas.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 100.Konhilas JP, Leinwand LA. The effects of biological sex and diet on the development of heart failure. Circulation. 2007;116(23):2747–2759. doi: 10.1161/CIRCULATIONAHA.106.672006. [DOI] [PubMed] [Google Scholar]
- 101.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24(3):313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 102.Kousteni S, Bellido T, Plotkin LI, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104(5):719–730. [PubMed] [Google Scholar]
- 103.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 104.Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annu Rev Physiol. 2009;71:1–18. doi: 10.1146/annurev.physiol.010908.163156. [DOI] [PubMed] [Google Scholar]
- 105.Mahmoodzadeh S, Eder S, Nordmeyer J, et al. Estrogen receptor-α up-regulation and redistribution in human heart failure. FASEB J. 2006;20(7):926–934. doi: 10.1096/fj.05-5148com. [DOI] [PubMed] [Google Scholar]
- 106.Nordmeyer J, Eder S, Mahmoodzadeh S, et al. Upregulation of myocardial estrogen receptors in human aortic stenosis. Circulation. 2004;110(20):3270–3275. doi: 10.1161/01.CIR.0000147610.41984.E8. [DOI] [PubMed] [Google Scholar]
- 107.Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK, Green GE, Schiebinger RJ. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation. 1998;98(3):256–261. doi: 10.1161/01.cir.98.3.256. [DOI] [PubMed] [Google Scholar]
- 108.Lizotte E, Grandy SA, Tremblay A, Allen BG, Fiset C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell Physiol Biochem. 2009;23(1–3):75–86. doi: 10.1159/000204096. [DOI] [PubMed] [Google Scholar]
- 109.Otsuki M, Dahlman-Wright K, Gustafsson JA. Cardiovascular roles of estrogen receptors: insights gained from knockout models. Nucl Recept Signal. 2003;1:E003. doi: 10.1621/nrs.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-α mediates acute myocardial protection in females. Am J Physiol Heart Circ Physiol. 2006;290(6):H2204–H2209. doi: 10.1152/ajpheart.01219.2005. [DOI] [PubMed] [Google Scholar]
- 111.Wang M. Estrogen receptor-β mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R972–R978. doi: 10.1152/ajpregu.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Förster C, Kietz S, Hultenby K, Warner M, Gustafsson JA. Characterization of the ERβ-/- mouse heart. Proc Natl Acad Sci USA. 2004;101(39):14234–14239. doi: 10.1073/pnas.0405571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pelzer T, Loza PA, Hu K, et al. Increased mortality and aggravation of heart failure in estrogen receptor-β knockout mice after myocardial infarction. Circulation. 2005;111(12):1492–1498. doi: 10.1161/01.CIR.0000159262.18512.46. [DOI] [PubMed] [Google Scholar]
- 114.Babiker FA, Lips DJ, Delvaux E, et al. Oestrogen modulates cardiac ischaemic remodelling through oestrogen receptor-specific mechanisms. Acta Physiol (Oxf) 2007;189(1):23–31. doi: 10.1111/j.1748-1716.2006.01633.x. [DOI] [PubMed] [Google Scholar]
- 115.van Eickels M, Grohé C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 17β-estradiol attenuates the development of pressure-overload hypertrophy. Circulation. 2001;104(12):1419–1423. doi: 10.1161/hc3601.095577. [DOI] [PubMed] [Google Scholar]
- 116.Skavdahl M, Steenbergen C, Clark J, et al. Estrogen receptor-β mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol. 2005;288(2):H469–H476. doi: 10.1152/ajpheart.00723.2004. [DOI] [PubMed] [Google Scholar]
- 117.Ikeda Y, Aihara K, Sato T, et al. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J Biol Chem. 2005;280(33):29661–29666. doi: 10.1074/jbc.M411694200. [DOI] [PubMed] [Google Scholar]
- 118.Ikeda Y, Aihara K, Yoshida S, et al. Androgen-androgen receptor system protects against angiotensin II-induced vascular remodeling. Endocrinology. 2009;150(6):2857–2864. doi: 10.1210/en.2008-1254. [DOI] [PubMed] [Google Scholar]
- 119.Head GA, Obeyesekere VR, Jones ME, Simpson ER, Krozowski ZS. Aromatase-deficient (ArKO) mice have reduced blood pressure and baroreflex sensitivity. Endocrinology. 2004;145(9):4286–4291. doi: 10.1210/en.2004-0421. [DOI] [PubMed] [Google Scholar]
- 120.Patten RD, Pourati I, Aronovitz MJ, et al. 17β-estradiol differentially affects left ventricular and cardiomyocyte hypertrophy following myocardial infarction and pressure overload. J Card Fail. 2008;14(3):245–253. doi: 10.1016/j.cardfail.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fukuzaki E, Takuma K, Funatsu Y, et al. Ovariectomy increases neuronal amyloid-β binding alcohol dehydrogenase level in the mouse hippocampus. Neurochem Int. 2008;52(7):1358–1364. doi: 10.1016/j.neuint.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dakhova O, O’Day D, Kinet N, et al. Dickkopf-like1 regulates postpubertal spermatocyte apoptosis and testosterone production. Endocrinology. 2009;150(1):404–412. doi: 10.1210/en.2008-0673. [DOI] [PubMed] [Google Scholar]
- 123.Kawai K, Murakami S, Senba E, et al. Changes in estrogen receptors-α and -β expression in the brain of mice exposed prenatally to bisphenol A. Regul Toxicol Pharmacol. 2007;47(2):166–170. doi: 10.1016/j.yrtph.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 124.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108(11):1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 125.Park M, Shen YT, Gaussin V, et al. Apoptosis predominates in nonmyocytes in heart failure. Am J Physiol Heart Circ Physiol. 2009;297(2):H785–H791. doi: 10.1152/ajpheart.00310.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ling S, Komesaroff P, Sudhir K. Cellular mechanisms underlying the cardiovascular actions of oestrogens. Clin Sci (Lond) 2006;111(2):107–118. doi: 10.1042/CS20050084. [DOI] [PubMed] [Google Scholar]
- 127.Kimura N, Mizokami A, Oonuma T, Sasano H, Nagura H. Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. J Histochem Cytochem. 1993;41(5):671–678. doi: 10.1177/41.5.8468448. [DOI] [PubMed] [Google Scholar]
- 128.Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159(10):1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 129.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316(18):1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 130.Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55–72. doi: 10.1146/annurev.publhealth.19.1.55. [DOI] [PubMed] [Google Scholar]
- 131.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 132.Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 133.Anderson GL, Limacher M, Assaf AR, et al. Women’s Health Initiative Steering Committee: Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 134.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 135.Prentice RL, Manson JE, Langer RD, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170(1):12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav. 2009;97(2):199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lobo RA. Metabolic syndrome after menopause and the role of hormones. Maturitas. 2008;60(1):10–18. doi: 10.1016/j.maturitas.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 138.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129(3):S758–S767. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 139.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor-β. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 140.Safford B, Dickens A, Halleron N, Briggs D, Carthew P, Baker V. A model to estimate the oestrogen receptor mediated effects from exposure to soy isoflavones in food. Regul Toxicol Pharmacol. 2003;38(2):196–209. doi: 10.1016/s0273-2300(03)00091-6. [DOI] [PubMed] [Google Scholar]
- 141.Kelly GE, Nelson C, Waring MA, Joannou GE, Reeder AY. Metabolites of dietary (soya) isoflavones in human urine. Clin Chim Acta. 1993;223(1–2):9–22. doi: 10.1016/0009-8981(93)90058-c. [DOI] [PubMed] [Google Scholar]
- 142.Xu X, Wang HJ, Murphy PA, Cook L, Hendrich S. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr. 1994;124(6):825–832. doi: 10.1093/jn/124.6.825. [DOI] [PubMed] [Google Scholar]
- 143.Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S. Bioavailability of soybean isoflavones depends upon gut microflora in women. J Nutr. 1995;125(9):2307–2315. doi: 10.1093/jn/125.9.2307. [DOI] [PubMed] [Google Scholar]
- 144.Sathyamoorthy N, Wang TT. Differential effects of dietary phyto-oestrogens daidzein and equol on human breast cancer MCF-7 cells. Eur J Cancer. 1997;33(14):2384–2389. doi: 10.1016/s0959-8049(97)00303-1. [DOI] [PubMed] [Google Scholar]
- 145.Setchell KD, Brown NM. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132(12):3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 146.Muthyala RS, Ju YH, Sheng S, et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors-α and -β. Bioorg Med Chem. 2004;12(6):1559–1567. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 147.Lamartiniere CA, Wang J, Smith-Johnson M, Eltoum IE. Daidzein: bioavailability, potential for reproductive toxicity, and breast cancer chemoprevention in female rats. Toxicol Sci. 2002;65(2):228–238. doi: 10.1093/toxsci/65.2.228. [DOI] [PubMed] [Google Scholar]
- 148.Ohta A, Uehara M, Sakai K, et al. A combination of dietary fructooligosaccharides and isoflavone conjugates increases femoral bone mineral density and equol production in ovariectomized mice. J Nutr. 2002;132(7):2048–2054. doi: 10.1093/jn/132.7.2048. [DOI] [PubMed] [Google Scholar]
- 149.Lundh T. Metabolism of estrogenic isoflavones in domestic animals. Proc Soc Exp Biol Med. 1995;208(1):33–39. doi: 10.3181/00379727-208-43828. [DOI] [PubMed] [Google Scholar]
- 150.Adlercreutz H, Musey PI, Fotsis T, et al. Identification of lignans and phytoestrogens in urine of chimpanzees. Clin Chim Acta. 1986;158(2):147–154. doi: 10.1016/0009-8981(86)90230-5. [DOI] [PubMed] [Google Scholar]
- 151.Juniewicz PE, Pallante Morell S, Moser A, Ewing LL. Identification of phytoestrogens in the urine of male dogs. J Steroid Biochem. 1988;31(6):987–994. doi: 10.1016/0022-4731(88)90343-3. [DOI] [PubMed] [Google Scholar]
- 152.Cassidy A, Brown JE, Hawdon A, et al. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr. 2006;136(1):45–51. doi: 10.1093/jn/136.1.45. [DOI] [PubMed] [Google Scholar]
- 153.Lu LJ, Anderson KE. Sex and long-term soy diets affect the metabolism and excretion of soy isoflavones in humans. Am J Clin Nutr. 1998;68(Suppl 6):S1500–S1504. doi: 10.1093/ajcn/68.6.1500S. [DOI] [PubMed] [Google Scholar]
- 154.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: a summary of a statement for professionals from the american heart association nutrition committee. Arterioscler Thromb Vasc Biol. 2006;26(8):1689–1692. doi: 10.1161/01.ATV.0000227471.00284.ef. [DOI] [PubMed] [Google Scholar]
- 155.Maskarinec G, Aylward AG, Erber E, Takata Y, Kolonel LN. Soy intake is related to a lower body mass index in adult women. Eur J Nutr. 2008;47(3):138–144. doi: 10.1007/s00394-008-0707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333(5):276–282. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 157.Welty FK, Lee KS, Lew NS, Zhou JR. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch Intern Med. 2007;167(10):1060–1067. doi: 10.1001/archinte.167.10.1060. [DOI] [PubMed] [Google Scholar]
- 158.Azadbakht L, Kimiagar M, Mehrabi Y, et al. Soy inclusion in the diet improves features of the metabolic syndrome: a randomized crossover study in postmenopausal women. Am J Clin Nutr. 2007;85(3):735–741. doi: 10.1093/ajcn/85.3.735. [DOI] [PubMed] [Google Scholar]
- 159.Cheng SY, Shaw NS, Tsai KS, Chen CY. The hypoglycemic effects of soy isoflavones on postmenopausal women. J Womens Health (Larchmt) 2004;13(10):1080–1086. doi: 10.1089/jwh.2004.13.1080. [DOI] [PubMed] [Google Scholar]
- 160.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. American Heart Association Nutrition Committee: Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113(7):1034–44. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- 161.Fotsis T, Pepper MS, Montesano R, et al. Phytoestrogens and inhibition of angiogenesis. Baillieres Clin Endocrinol Metab. 1998;12(4):649–666. doi: 10.1016/s0950-351x(98)80009-8. [DOI] [PubMed] [Google Scholar]
- 162.Liew R, Williams JK, Collins P, MacLeod KT. Soy-derived isoflavones exert opposing actions on guinea pig ventricular myocytes. J Pharmacol Exp Ther. 2003;304(3):985–993. doi: 10.1124/jpet.102.042986. [DOI] [PubMed] [Google Scholar]
- 163.Fraser LR, Beyret E, Milligan SR, Adeoya-Osiguwa SA. Effects of estrogenic xenobiotics on human and mouse spermatozoa. Hum Reprod. 2006;21(5):1184–1193. doi: 10.1093/humrep/dei486. [DOI] [PubMed] [Google Scholar]
- 164.Glover A, Assinder SJ. Acute exposure of adult male rats to dietary phytoestrogens reduces fecundity and alters epididymal steroid hormone receptor expression. J Endocrinol. 2006;189(3):565–573. doi: 10.1677/joe.1.06709. [DOI] [PubMed] [Google Scholar]
- 165.Jefferson WN, Padilla-Banks E, Newbold RR. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol Reprod. 2005;73(4):798–806. doi: 10.1095/biolreprod.105.041277. [DOI] [PubMed] [Google Scholar]
- 166.Leopold AS, Erwin M, Oh J, Browning B. Phytoestrogens: adverse effects on reproduction in California quail. Science. 1976;191(4222):98–100. doi: 10.1126/science.1246602. [DOI] [PubMed] [Google Scholar]
- 167.Setchell KD, Gosselin SJ, Welsh MB, et al. Dietary estrogens – a probable cause of infertility and liver disease in captive cheetahs. Gastroenterology. 1987;93(2):225–233. doi: 10.1016/0016-5085(87)91006-7. [DOI] [PubMed] [Google Scholar]
- 168.Doerge DR, Sheehan DM. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect. 2002;110(Suppl 3):349–353. doi: 10.1289/ehp.02110s3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hooper L, Ryder JJ, Kurzer MS, et al. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15(4):423–440. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sereno M, Brunello A, Chiappori A, et al. Cardiac toxicity: old and new issues in anti-cancer drugs. Clin Transl Oncol. 2008;10(1):35–46. doi: 10.1007/s12094-008-0150-8. [DOI] [PubMed] [Google Scholar]
- 171.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7(5):332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 172.Maulik N, Watanabe M, Zu YL, et al. Ischemic preconditioning triggers the activation of MAP kinases and MAPKAP kinase 2 in rat hearts. FEBS Lett. 1996;396(2–3):233–237. doi: 10.1016/0014-5793(96)01109-x. [DOI] [PubMed] [Google Scholar]
- 173.Fatehi-Hassanabad Z, Parratt JR. Genistein, an inhibitor of tyrosine kinase, prevents the antiarrhythmic effects of preconditioning. Eur J Pharmacol. 1997;338(1):67–70. doi: 10.1016/s0014-2999(97)01299-5. [DOI] [PubMed] [Google Scholar]
- 174.Matsui T, Li L, Wu JC, Cook SA, et al. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277(25):22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 175.Kumi-Diaka J, Sanderson NA, Hall A. The mediating role of caspase-3 protease in the intracellular mechanism of genistein-induced apoptosis in human prostatic carcinoma cell lines, DU145 and LNCaP. Biol Cell. 2000;92(8–9):595–604. doi: 10.1016/s0248-4900(00)01109-6. [DOI] [PubMed] [Google Scholar]
- 176.Choi EJ, Lee BH. Evidence for genistein mediated cytotoxicity and apoptosis in rat brain. Life Sci. 2004;75(4):499–509. doi: 10.1016/j.lfs.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 177.Yoon HS, Moon SC, Kim ND, Park BS, Jeong MH, Yoo YH. Genistein induces apoptosis of RPE-J cells by opening mitochondrial PTP. Biochem Biophys Res Commun. 2000;276(1):151–156. doi: 10.1006/bbrc.2000.3445. [DOI] [PubMed] [Google Scholar]
- 178.Kim HK, Nelson-Dooley C, Della-Fera MA, et al. Genistein decreases food intake, body weight, and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice. J Nutr. 2006;136(2):409–414. doi: 10.1093/jn/136.2.409. [DOI] [PubMed] [Google Scholar]
- 179.Pan A, Franco OH, Ye J, et al. Soy protein intake has sex-specific effects on the risk of metabolic syndrome in middle-aged and elderly Chinese. J Nutr. 2008;138(12):2413–2421. doi: 10.3945/jn.108.097519. [DOI] [PubMed] [Google Scholar]
- 180.Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Association of blood pressure with intake of soy products and other food groups in Japanese men and women. Prev Med. 2003;36(6):692–697. doi: 10.1016/s0091-7435(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 181.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 182.The Coronary Drug Project. Findings leading to discontinuation of the 2.5-mg day estrogen group. The Coronary Drug Project Research Group. JAMA. 1973;226(6):652–657. [PubMed] [Google Scholar]
- 183.Tomaszewski M, Charchar FJ, Maric C, et al. Association between lipid profile and circulating concentrations of estrogens in young men. Atherosclerosis. 2009;203(1):257–262. doi: 10.1016/j.atherosclerosis.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.van der Schouw YT, de Kleijn MJ, Peeters PH, Grobbee DE. Phyto-oestrogens and cardiovascular disease risk. Nutr Metab Cardiovasc Dis. 2000;10(3):154–167. [PubMed] [Google Scholar]
- 185.Markiewicz L, Garey J, Adlercreutz H, Gurpide E. In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol. 1993;45(5):399–405. doi: 10.1016/0960-0760(93)90009-l. [DOI] [PubMed] [Google Scholar]
- 186.Hwang CS, Kwak HS, Lim HJ, et al. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J Steroid Biochem Mol Biol. 2006;101(4–5):246–253. doi: 10.1016/j.jsbmb.2006.06.020. [DOI] [PubMed] [Google Scholar]
Website
- 201.National Center for Health Statistics Website. [August, 2009]; www.cdc.gov/nchs.