Abstract
Accumulating data indicate that G12 subfamily (Gα12/13)-mediated signaling pathways play pivotal roles in a variety of physiological processes, while aberrant regulation of this pathway has been identified in various human diseases. It has been demonstrated that Gα12/13-mediated signals form networks with other signaling proteins at various levels, from cell surface receptors to transcription factors, to regulate cellular responses. Gα12/13 have slow rates of nucleotide exchange and GTP hydrolysis, and specifically target RhoGEFs containing an amino-terminal RGS homology domain (RH-RhoGEFs), which uniquely function both as a GAP and an effector for Gα12/13. In this review, we will focus on the mechanisms regulating the Gα12/13 signaling system, particularly the Gα12/13-RH-RhoGEF-Rho pathway, which can regulate a wide variety of cellular functions from migration to transformation.
Key Words: Regulation of G12/13-mediated signaling pathways, Gα12/13-RH-RhoGEF-Rho pathway, Regulation of G12/13, Regulation of effectors by G12, Crosstalk between G12/13-mediated and other signaling pathways, Cell proliferation and transformation, Cell morphology and motility, The cardiovascular system, The immune system, The neuronal system
Introduction
The α subunits of heterotrimeric G proteins are classified into four subfamilies based on the homology of their amino acid sequences: Gs, Gi, Gq, and G12 [1]. The G12 subfamily is composed of Gα12 and Gα13 [2]. Accumulating evidence indicates that G12/13-mediated signaling pathways are involved in a variety of physiological processes, including embryonic development, cell growth, cell polarity and migration, angiogenesis, platelet activation, the immune response, apoptosis, and neuronal responses [3,4,5,6,7,8,9,10,11,12,13,14,15]. In the nervous system, this pathway plays an important role in neuronal migration, axonal guidance, formation of cerebellar and cerebral cortices, and neurotransmitter release [11,16,17,18,19,20]. In addition, abnormal regulation of this pathway has been found in disease conditions such as leukemia, cell transformation, tumor cell invasion and metastasis, hypertension, and ataxia [14, 18,21,22,23,24,25,26,27]. However, significant differences between Gα12 and Gα13 signaling have been demonstrated from knockout mouse studies [28, 29]. Crosstalk between Gα12/13 signaling and other G protein signals, especially from Gαq, have also been reported [3, 11, 28,30,31,32,33,34,35,36]. These results suggest that Gα12/13-mediated signaling pathways will play a critical role in many important biological responses. In this review, we will focus on the mechanisms by which the Gα12/13 signaling system regulates a wide variety of cellular functions.
Regulation of G12/13-Mediated Signaling Pathways
Heterotrimeric G proteins serve as key molecular switches to transduce signals from hormones, neurotransmitters, and other stimuli from cell surface receptors into cells by actively alternating their conformations between GDP-bound inactive and GTP-bound active forms (fig. 1). The transient protein-protein interactions induced by guanine nucleotide-dependent conformational changes of G proteins play central roles in these signaling pathways. In the current model, the ligand-activated G protein-coupled receptors (GPCRs) catalyze the exchange of GDP for GTP on Gα subunits [37]. Upon activation, three switch regions in the Gα subunit undergo significant conformational changes, followed by dissociation of the GTP-bound Gα subunit from the Gβγ subunits. Both Gα-GTP and free Gβγ interact with diverse downstream effectors, such as enzymes or ion channels, to transmit intracellular signals [38]. The Gα subunit hydrolyzes bound GTP to GDP by its intrinsic GTPase activity and this deactivation process is further accelerated by GTPaseactivating proteins (GAPs) such as regulator of G protein signaling (RGS) proteins [39, 40]. Gα-GDP dissociates from effectors and reassociates with Gβγ to terminate the signal. Thus, regulation of G protein cycles of activation and deactivation profoundly affect the cellular responses and the amplitude and duration of the signal response are determined by the amount of GTP-bound Gα subunit.
Fig. 1.
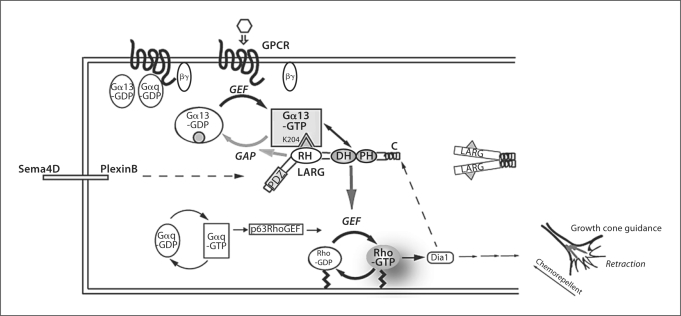
The G12/13-mediated signaling pathway interacts with diverse proteins at various levels. Upon ligand binding, GPCRs catalyze the exchange of GDP for GTP on Gα 13 subunits resulting in activation of the Gα subunit and release of Gβγ. Both Gα-GTP and free Gβγ interact with diverse downstream effectors. LARG is an effector for Gα13, which is a GEF for the monomeric GTPase RhoA. Upon binding of Gα13-GTP to LARG, the DH/PH domains activate RhoA by facilitating the exchange of GDP for GTP. LARG, in turn, stimulates the intrinsic GTPase activity of Gα13 through its N-terminal RH domain. Gα-GDP dissociates from effectors and reassociates with Gβγ to terminate the signal. Thus, regulation of the G protein cycle of activation and deactivation profoundly affects the cellular response. The amplitude and duration of the signal response are determined by the amount of GTPbound Gα subunit. A dotted line indicates evidence for direct activation of LARG without activated Gα13. Domains: RH = RGS (regulator of G protein signaling) homology; DH = Dbl homology; PH = pleckstrin homology; PDZ = PSD-95/SA90-Discs-large-ZO-1. See the text for details.
Regulation of G12/13
Activation of G12/13 by GPCRs
More than 30 GPCRs are reported to couple to either G12, G13 or both, based on direct or indirect methods of evaluating G protein activation; see details in Riobo and Manning's review [41]. It is believed that the binding of a ligand changes the conformation of critical regions of the seven-transmembrane helix pocket of the GPCR, which in turn causes conformational changes in the intracellular loops and COOH terminus. However, the precise activation mechanism of G proteins by agonist-activated GPCRs remains unknown.
In general, interaction between the C-termini of Gα and GPCRs is considered to be responsible for Gα activation. Although the exact interface between Gα12/13 and GPCRs remains unclear, antibodies against the C-termini of Gα12 or Gα13 subunits or peptides corresponding to 10–50 residues of the C-termini specifically attenuate the agonist-activated angiotensin (AT1), thrombin, and sphingosine-1-phosphate (S1P2 and S1P3) receptor signals in cells [25, 42, 43]. In addition, the N-terminal short sequences of α subunits of the G12 family, where Gα12 and Gα13 have low amino acid sequence homology, are reported to determine the selectivity of coupling to receptors [25,42,43,44]. The use of chimeric Gα12 and Gα13 proteins, in which the N-terminal short sequences are replaced with each other, demonstrated that thrombin and lysophosphatidic acid (LPA) selectively activated Gα12 or Gα13 expressed in HEK 293 cells. From studies of other Gα subfamilies, membrane-proximal regions of the second and third intracellular loops and the cytoplasmic tail of the receptor are generally believed to have an important role in Gα activation [45, 46].
Many GPCRs are reported to simultaneously couple to and activate more than one G protein subfamily member [45]. Studies suggest that most receptors coupling to the G12 subfamily could couple to both G12 and G13, except for the 5-hydroxytryptamine 4 receptor, which only couples to G13 [41, 47]. Furthermore, most receptors coupling to Gα12 and/or Gα13 couple to other G proteins, especially to Gαq [41, 45, 48]. These facts make evaluating the specificity of the signaling through G12 and G13 complicated. To analyze signaling through a specific receptor-G protein pair, Zhang et al. [48] evaluated Gα activation directly and in 1:1 stoichiometry using thromboxane A2 receptor (TPα)-Gα12 or -Gα13 fusion proteins inSpodoptera frugiperda (Sf9) cells. Interestingly, the results show that TPα-Gα12 responded to agonists with slow GTPγS binding, whereas the TPα-Gα13 response was fast. These results contrast with the case of the purified Gα proteins in vitro: Gα12 and Gα13 do not show any differences in GTPγS binding kinetics. Ligand binding may induce a specific conformational change in TPα to influence the coupling efficiency to different Gα subunits. In turn, it is also possible that binding with specific Gα-GDP subunits may change the conformation of the receptor to define the affinity of the ligand for the receptor. Alternatively, the selectivity might be enhanced in collaboration with associated proteins in the cell. Future structural analysis of a complex of a GPCR with heterotrimeric Gα12/13 proteins will provide information critical for answering these questions.
Deactivation of Gα12/13 by RGS Proteins
Like all other heterotrimeric G protein α subunits, Gα12/13 cycle between GDP- (inactive) and GTP-bound (active) states and possess an intrinsic ability to hydrolyze GTP to GDP. In vitro analysis has demonstrated that both recombinant Gα12 and Gα13 proteins have relatively slow rates of nucleotide exchange and GTP hydrolysis (Gα12: kon, GTPγS = 0.01 min–1, kcat = 0.1–0.2 min–1, Gα13: koff, GDP = 0.01 min–1, kcat = >0.2 min–1) [49, 50]. This deactivation process is accelerated by the GAP activity of RGS proteins. p115RhoGEF and leukemia-associated RhoGEF (LARG) have been shown to act as specific GAPs for Gα12 and Gα13 in vitro [51,52,53]. p115RhoGEF, LARG, and PDZ-RhoGEF/GTRAP48 are the known members of the mammalian RhoGEF family, which contain an amino-terminal RGS homology (RH, also called rgRGS) domain (RH-RhoGEFs) that recognizes activated Gα12/13 [54,55,56,57] (fig. 2). In addition, RH-RhoGEFs contain central DH/PH (Dbl homology/pleckstrin homology) domains characteristic of GEFs for Rho family GTPases. In vitro, p115RhoGEF and LARG act as specific GAPs for Gα12 and Gα13, while the RGS domain of PDZ-RhoGEF lacks detectable GAP activity for these Gα subunits [51, 52, 58]. At the same time, Gα12 and Gα13 subunits regulate the activity of Rho through RH-RhoGEFs [51,52,53, 55, 58]. RH-RhoGEFs directly link the activation of GPCRs by extracellular ligands to the regulation of Rho activity in cells. RH-RhoGEFs combine GAP and effector activity into a single molecule to regulate signaling from Gα12/13.
Fig. 2.
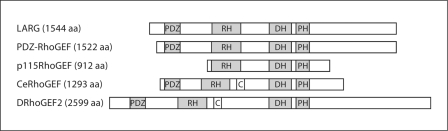
A schematic representation of domain structures of RHRhoGEFs. LARG, PDZ-RhoGEF and p115RhoGEF are the three known human RH-RhoGEFs. All RH-RhoGEFs contain RH domains followed by tandem DH/PH domains. The N-terminal region of PDZ-RhoGEF and LARG each contain a PDZ domain. For comparison, CeRhoGEF and DRhoGEF2, RGS-RhoGEFs from C. elegans and D. melanogaster, respectively, are depicted as well. Unlike human RGS-RhoGEFs, CeRhoGEF and DRhoGEF2 each contain one C1 homology domain. Domains: RH = RGS homology domain; DH = Dbl homology; PH = pleckstrin homology; PDZ = PSD-95/SA90-Discs-large-ZO-1; C = C1 homology domain.
Although the sequence identity between the RGS domains from RH-RhoGEFs and other RGS family members is low (between 10 and 15%) [51], the high resolution crystal structures of the RGS domains from both PDZ-RhoGEF and p115RhoGEF have demonstrated that these domains share a similar tertiary fold composed of an all-alpha helical bundle [59, 60]. One structural divergence between these RGS domains and RGS4 is the extended C-terminus found in the RH-RhoGEFs, which forms α helices tightly associated with the core RGS domain through a large hydrophobic surface.
Site-directed mutagenesis of both Gα13 and p115RhoGEF has provided important insights into the mechanism of the acceleration of GTPase activity by the RH domain of p115RhoGEF. In Gα13, lysine 204, located in its switch I region, has been shown to be important for interaction with the RH domains of both p115RhoGEF and LARG [61, 62]. Residues 1–252 of p115RhoGEF stimulate the GTPase activity of Gα13 similarly to the full-length protein [51, 53]. It has been demonstrated that residues at the N-terminus of the RH domain are required for RH-RhoGEFs to exert their GAP activity [53, 63]. In particular, residues 27–31 within a negatively charged cluster of residues are crucial for p115RhoGEF's GTPase activity. Mutations in this acidic patch reduce both binding to Gα13 and stimulated GTP hydrolysis [63, 64]. Furthermore, a structural study of a complex between p115RhoGEF's RH domain and a Gα13/i1 chimera has demonstrated that the interface is different from that of the RGS domain of RGS4 and Gαi1 [54]. The N-terminal small element within the RH domain, which is required for GAP activity towards Gα13, contacts the switch regions and the helical domain of Gα13/i1. The core module of the RH domain binds to the switch II region and the α3 helix of Gα13/i1 chimera, which is used for effector binding in other Gα subunits. In spite of these structural studies, the molecular mechanism of GAP and GEF activation of RH-RhoGEFs upon Gα12/13 binding has not yet been elucidated.
Post-Translational Modification of Gα12/13
Lipid modifications affect the subcellular localization of Gα subunits and the interactions of these subunits with other proteins. Gα subunits are subjected to N-myristoylation, which is an irreversible, co-translational attachment of 14-carbon myristrate to glycine at the N-terminus through an amide bond, and/or palmitoylation, which is a reversible, post-translational attachment of 16-carbon palmitate to cysteine residues near the N-terminus through thioester bonds. Both Gα12 and 13 lack a glycine residue at the second site for myristoylation and thus are modified by only palmitoylation near their N-termini [65,66,67]. Although palmitoylation has no clear consensus sequence, one site (C12) in Gα12 [65] and two sites (C14, C18) in Gα13 [66] have been defined as sites of palmitate incorporation using mutants in which serine replaces these cysteine residues.
It has been demonstrated that palmitoylation of Gα13 is critical for its association with the plasma membrane, translocation of its effector p115RhoGEF, and its ability to signal through RhoA [68]. It has also been reported that palmitoylation of Gα12 but not Gα13 is related to localization in lipid rafts [69]. A constitutively active mutant of Gα12 which cannot be palmitoylated inhibits its transforming activity in NIH-3T3 cells [65]. It is interesting to note that palmitoylation of another Gα subunit, Gαz, decreases its affinity for Gz GAP and its rate of GTP hydrolysis. However, this may not be the case with Gα12/13 [70].
Phosphorylation of Gα subunits is another important modification which regulates the amplitude and duration of G protein signals. Gα12, as well as Gαz, is a substrate for phoshporylation by protein kinase C (PKC) [71,72,73,74]. In vitro, Gα12 is phosphorylated by PKCα, -δ, -∊, and -ζ, and approximately 1 mol of phosphate was incorporated per mol subunit with PKCα [72]. Gα12 stably expressed in NIH-3T3 cells is phosphorylated following treatment of the cells with PMA (phorbol 12-myristate 13-acetate) [72], and endogenous Gα12 in human platelets is phosphorylated in response to PMA, thrombin, and the thromboxane A2 receptor agonist U46619 [75]. Although the phosphorylation site has not been mapped, the N-terminal 50 amino acid residues comprise one possible region [72]. It is possible that Ser38 corresponding to Ser16 defined in Gαz may be the phosphorylation site. As to phosphorylation of Gα13, there is a discrepancy between in vitro and cell-based experiments. In vitro experiments demonstrate purified Gα13 is not a substrate for PKCα, -δ, -∊, and -ζ [72]. However, studies with intact cells show that Gα13 in platelets is phosphorylated in response to PMA, and Gα13 expressed in COS cells is effectively phosphorylated by PKCβ, -δ, and -∊ [75]. This discrepancy might indicate that additional cellular factors are required for phosphorylation of Gα13 by PKC.
It has been demonstrated that phosphorylation blocks the interaction of the Gα12 subunit with Gβγ, and Gβγ reciprocally blocks the phosphorylation of Gα12 by PKC [72]. This appears to be consistent with the fact that Gβγ binds the N-terminal helix of Gα. Gβγ binding to the N-terminus of Gα may hinder PKC from approaching sterically, or reciprocally, phosphorylation of the N-terminus may block Gβγ binding. Furthermore, Kozasa and Gilman[37] demonstrated that the rate of GTPγS binding to phosphorylated Gα12 is not inhibited by Gβγ whereas Gβγ inhibits GTPγS binding to non-phosphorylated Gα12. It is noteworthy that phosphorylation inhibits interaction of Gαz, another substrate for PKC, with the RGS protein RGSZ1, while it is unknown if phosphorylation of Gα12 produces a similar effect [76, 77]. Interestingly, a genetic screening using Caenorhabditis elegans suggested that the novel calcium-independent PKCθ/δ is a potential downstream target of Gα12 [78]. Dhanasekaran et al. [79] have reported that Na+/H+ exchange activity stimulated by Gα12 is lost after prolonged exposure of cells to PMA. Once activated upon binding of ligand to a GPCR, Gα12 is phosphorylated by PKC, which could be activated downstream of Gα12 itself. The phosphorylated, activated, Gα12 may have reduced interaction with Gβγ, and be less susceptible to the GAP activity of RGS protein, prolonging the duration of signaling. The system would eventually desensitize because of a lack of reassociation between GDP-bound Gα with Gβγ required for receptor-mediated reactivation. PKC likely attenuates the activity of Gα12 in a negative feedback loop, while the mechanism of PKC activation by Gα12 is unknown. Tyrosine phosphorylation of Gα12 has not been reported.
Regulation of Effectors by G12
As mentioned above, Gα12 and Gα13 subunits directly activate RH-RhoGEFs to regulate the activity of the GTPase Rho [51,52,53, 55, 58]. p115RhoGEF was first identified as a direct downstream effector of the G12 subfamily 10 years ago. Since then, studies including the yeast two-hybrid system, have revealed more than 20 diverse proteins that directly interact with the G12 subfamily as well as RH-RhoGEFs; see recent reviews for further details [80, 81]. A well-established downstream effector of G12/13-mediated signaling is the monomeric GTPase RhoA, which is a regulator of a variety of intracellular processes including formation of actin stress fibers and assembly of focal adhesions, gene transcription, and control of cell growth [78, 82].
Regulation of RH-RhoGEFs by Gα12/13
Biochemical evidence using reconstitution systems with purified proteins has clearly demonstrated that the GEF activity of p115RhoGEF and LARG can be directly stimulated by Gα13 [52, 58]. As mentioned above, RH-RhoGEFs combine GAP activity and effector activity into a single molecule. While it has been well demonstrated that p115RhoGEF and LARG serve as specific GAPs for Gα12/13 through their RH domains, the molecular mechanism of RH-RhoGEF activation upon Gα13 binding remains unclear. Some recent studies have provided information about the interface between Gα13 and RH-RhoGEFs required for RH-RhoGEF activation.
A structural study using p115RhoGEF's RH domain and a Gα13/i1 chimera has demonstrated that the core module of p115RhoGEF's RH domain binds to the region of Gα13/i1 which is conventionally used for effector binding [54]. This result suggests roles for the RH domain in the stimulation of GEF activity by Gα13 in addition to GAP activity. Several studies have indicated that regions outside of RH domain of RH-RhoGEFs, particularly the DH/PH domains, interact directly with activated Gα13 [53, 58, 62]. Wells et al. [53] demonstrated that AlF4–-activated Gα13 was able to bind directly to a deletion mutant of p115RhoGEF consisting of the DH and PH domains, although it was unable to stimulate the GEF activity of the fragment in vitro. Interaction through multiple interfaces, including the RH domains and DH/PH domains of RH-RhoGEFs, and Gα13 might play an important role in stimulating GEF activity. Kinetic and thermodynamic analysis of the interaction between Gα13 and LARG using surface plasmon resonance has demonstrated that the simultaneous binding of the RH domain and DH/PH domains with Gα13 facilitates formation of the high affinity active Gα13-LARG complex [145].
In addition to analysis of the Gα13-binding surfaces on RH-RhoGEFs, the surfaces of Gα13 necessary for binding to RH-RhoGEFs have also been characterized. One study, utilizing chimeras of Gα13 and Gαi2, revealed that not only the switch regions of Gα13, but also a large portion of the Ras-like domain of Gα13 are required for efficient Rho activation in cell-based assays [83]. Another study utilizing chimeras of Gα12 and Gα13 further identified that the C-terminal 100 amino acid residues of Gα13 are required for activation of the GEF activity of p115RhoGEF and LARG, whereas the N-terminal α helical and switch regions of Gα12 and Gα13 are responsible for their differential GAP responses to the RH domain [84]. This result demonstrates that p115RhoGEF and LARG interact with distinct surfaces on Gα13 for GAP activity or GEF activity regulation. Furthermore, we have demonstrated that the interaction of Gα13 with LARG through the RH domain (a GAP interface) and the DH/PH domains (an effector interface) could coordinate together to stimulate the RhoGEF activity of LARG [145]. In summary, the spatial and kinetic connection of GAP and GEF activities within RH-RhoGEFs may help to regulate the amplification of G protein signaling, provide higher temporal resolution of the response, and increase the specificity of the signal output. Since Gα12/13 have characteristically slow rates of nucleotide exchange and GTP hydrolysis, this mechanism could be a rational system for Gα12/13 signaling to regulate multiple important cellular functions with fast responses as well as long-term processes.
On the other hand, binding of the activated Gα12 does not activate the GEF activity of either p115RhoGEF or LARG in vitro. LARG activation requires not only binding to activated Gα12 but also phosphorylation by a non-receptor tyrosine kinase [52, 58].
Phosphorylation
One mechanism regulating RH-RhoGEF activity is post-translational modification in the form of phosphorylation. Chikumi et al. [85] have demonstrated that the non-receptor tyrosine kinase FAK (focal adhesion kinase) phosphorylates both LARG and PDZ-RhoGEF, but not p115RhoGEF. By measuring the Rho activity of HEK293 cells overexpressing FAK and PDZ-RhoGEF, they have concluded that FAK enhances RhoGEF activity even in the absence of Gα12 or Gα13. They also observed that FAK can be activated by thrombin, Gα12, Gα13, and Gαq through both Rho-dependent and -independent mechanisms, and proposed the existence of positive feedback regulation between Rho and FAK [85]. Another study using reconstitution and cell-based assays has demonstrated that Gα12 can stimulate the RhoGEF activity of tyrosine-phosphorylated LARG, but not non-phosphorylated LARG [52]. The direct phosphorylation of LARG by a non-receptor tyrosine kinase, Tec, in vitro greatly enhances the RhoGEF activity of LARG in response to Gα12, while it does not affect its basal RhoGEF activity. Although binding of Tec to Gα12 in cells was also demonstrated, the mechanism by which Tec is phosphorylated downstream of Gα12 remains unclear. It is interesting to note that thrombin, which can activate the Gα12/13 pathway, can also activate Tec [86] and that Gα12 promotes the kinase activity of Bruton's tyrosine kinase (BTK), another member of the Tec family [87]. Furthermore, overexpression of Gα12 and Gα13 stimulates auto- and transphosphorylation of Tec in NIH-3T3 cells [88]. One possibility is that activated Gα12 may recruit Tec to LARG and facilitate its phosphorylation.
The role of tyrosine kinases involved in the Gα12/13-Rho pathway is controversial. In vitro, the RhoGEF activity of non-phosphorylated LARG was stimulated by Gα13 but not Gα12, suggesting that the Gα13-LARG-Rho pathway does not require tyrosine kinases. However, in cell-based assays, the presence of Tec further potentiates the RhoGEF activity of LARG stimulated by Gα13 in NIH-3T3 or HeLa cells [52, 88]. In the context of cells, Gα13 and tyrosine kinases cooperatively and efficiently activate Rho. In contrast to the study by Suzuki et al., early studies of Gα12/13-mediated cytoskeletal reorganization suggested that tyrosine kinases might play a role in regulating Rho activation downstream of Gα13, but not Gα12 in PC12 cells or Swiss 3T3 cells [11, 89]. Tyrosine phosphorylation of PDZ-RhoGEF and LARG is an important post-translational modification regulating RhoGEF activity downstream of Gα12 and Gα13.
C-Terminal Regions and Oligomerization
Several studies have reported that RH-RhoGEFs oligomerize via their C-terminal regions [90,91,92]. It has been shown that RH-RhoGEF deletion mutants lacking their C-termini have an increased ability to stimulate Rho activation as compared to the full-length protein, and that the deletion of the C-terminal region alone is sufficient to increase the RhoGEF's transforming potential in cells [90, 93]. These results suggest that the RH-RhoGEF's activity may be negatively regulated in vivothrough the C-terminus itself or by interaction of regulatory factors with this region. First, to identify regulatory proteins interacting with the C-terminus of RH-RhoGEFs, Eisenhaure et al. [91] utilized yeast two-hybrid screening with the C-terminus of the murine ortholog of p115RhoGEF, Lsc. Surprisingly, the only protein identified was a C-terminal fragment of Lsc itself, suggesting that it homo-oligomerizes via its C-terminal region. Using mutagenesis experiments, they further showed that homo-oligomerization and negative regulation of Lsc activity are distinct functions of its C-terminus. Disruption of a putative coiled-coil domain within the C-terminus impairs oligomerization, but does not result in enhanced Rho activation in cells. A subsequent study confirmed the homo-oligomerization of p115RhoGEF, LARG, and PDZ-RhoGEF via their C-terminal regions by co-immunoprecipitation using cells overexpressing the RH-RhoGEFs [90] (fig. 2). LARG and PDZ-RhoGEF have also been shown to possibly form hetero-oligomers with each other, but not with p115RhoGEF [90].
Interestingly, deletion of the C-terminus of PDZ-RhoGEF or p115RhoGEF does not affect or even reduces the RhoGEF activity in vitro, while it dramatically enhances the RhoGEF activity in cells [90, 93]. This discrepancy suggests that ancillary factors in cellular milieus might release the inhibition of the RH-RhoGEF's activity through the C-terminal region. A possible mechanism is that an activating protein releases the inhibition through the C-terminus to induce the active conformation of RH-RhoGEFs. One potential activating protein might be the active form of Gα13, since activation-dependent binding between the C-terminus of LARG and Gα13 has been observed [145]. Interestingly, the Rho effector Dia1 also binds to the C-terminus of LARG to potentiate its GEF activity, and its activation constitutes a positive feedback loop between LARG, RhoA, and Dia1 [94]. Another mechanism is post-translational modification at the C-terminal region. It is interesting to note that PDZ-RhoGEF is tyrosine-phosphorylated by FAK at its C-terminus [85].
A recent study has suggested that oligomerization of overexpressed LARG may regulate its intracellular localization. Oligomerization functions to prevent nucleocytoplasmic shuttling and to retain LARG in the cytoplasm, while the mechanism for regulation of LARG oligomerization and the function of LARG localized in the nucleus are unknown [92]. Further study is required to understand the physiological significance of oligomerization of endogenous RH-RhoGEFs.
Subcellular Localization
Translocation of RH-RhoGEFs to the plasma membrane where their target, Rho, is enriched seems to be another important mechanism for regulating RhoGEF activity. Intracellular localization of p115RhoGEF has been intensively studied. However, it should be noted that the distribution of endogenous and overexpressed RH-RhoGEFs might differ. Indeed, it has been demonstrated by subcellular fractionation of NIH-3T3 cells that endogenous p115RhoGEF is found mainly in the cytosolic fraction with approximately 10–20% of the protein localized to the membrane fraction, while overexpressed p115RhoGEF was distributed abundantly between both the membrane and cytosolic fractions [93]. Both immunocytochemical and cellular fractionation analyses have demonstrated that co-expression of the constitutively active form of Gα12 as well as Gα13 or stimulation of the thromboxane A2 receptor, which couples with Gα12/13, induces redistribution of endogenous and overexpressed p115RhoGEF to the plasma membrane in HEK293 cells [68, 95]. Using overexpressed p115RhoGEF deletion mutants, these studies have identified the RH domain and the PH domain as essential for targeting of p115RhoGEF to the plasma membrane by Gα13. However, the RhoGEF activity of these mutants in the presence of Gα13 was not tested in cells. Furthermore, Bhattacharyya and Wedegaertner [64] tested the role of the acidic-rich region N-terminal to the RGS domain of p115RhoGEF in Gα13-dependent plasma membrane recruitment in cell-based assays, based on the finding that this region is important for interacting with Gα13 [54, 63]. Two point mutations in this region, Glu27Ala and Glu29Ala, impair the ability of p115RhoGEF to bind to Gα13 but did not affect its ability to localize to the plasma membrane [64].
Compared with p115RhoGEF, the mechanisms for regulating the subcellular distribution of PDZ-RhoGEF and LARG are less well understood. One immunohistochemistry study using polyclonal antibodies has shown that Gα12, Gα13, PDZ-RhoGEF and LARG proteins are distributed widely in the mouse nervous system, but localize to distinct morphological compartments within neurons. While LARG and Gα12 were mainly found in the somata of neurons, PDZ-RhoGEF and Gα13 were predominantly localized in the neuropil of central neurons [96]. An immunocytochemical analysis has demonstrated that endogenous PDZ-RhoGEF in Neuro2a cells is localized in the nucleus, cell body, and neurites, and upon stimulation of the LPA receptor, which is coupled to Gα12/13, PDZ-RhoGEF translocates to the tips of neurites, where Rho is enriched and cortical actin reorganization is induced [97]. Furthermore, analyses by Togashi et al. [97] revealed that a proline-rich motif C-terminally adjacent to DH/PH domains is essential for plasma membrane localization of PDZ-RhoGEF and cortical actin reorganization followed by cell rounding. However, the effects of mutating in this region on the interaction with Gα13 was not investigated. It has also been reported that PDZ-RhoGEF overexpressed in HEK293T, Cos7, and Neuro2a cells is partially localized at or near the plasma membrane and co-localizes with cortical actin [98]. Immunoprecipitation and F-actin co-sedimentation assays demonstrated that PDZ-RhoGEF binds to actin. Mutants that fail to interact with the actin cytoskeleton display enhanced Rho activation compared with wild type PDZ-RhoGEF. Togashi et al. [97] also have reported that PDZ-RhoGEF appears to co-localize with microtubules. It has been proposed that not only Gα12/13 but also its downstream target myosin II may be involved in regulating PDZ-RhoGEF localization and activation, although the biochemical mechanism remains unknown [99]. These results imply that the interaction with actin, myosin, and microtubules might regulate PDZ-RhoGEF signaling.
In contrast to PDZ-RhoGEF, endogenous LARG in Cos7 cells exhibits a predominantly cytoplasmic distribution [92]. In MDCKII cells, endogenous LARG was reported to be localized at the lateral membranes and slightly in the cytoplasm [100]. Overexpressed LARG seems to be distributed throughout the cytoplasm and does not co-localize with actin [98].
The co-localization of PDZ-RhoGEF and LARG with receptors at the plasma membrane via their PDZ domains is clearly distinct from p115RhoGEF, which does not contain a PDZ domain (fig. 1, 2). Several studies using biochemical and cell-based analyses have demonstrated that plexin B1, which is a transmembrane receptor that mediates the repulsive cues of semaphorin 4D to initiate collapse of neurite growth cones in mammals, directly interacts through its C-terminus with the PDZ domains of PDZ-RhoGEF and LARG [101,102,103,104]. Taya et al. [100] have reported that the C-terminus of non-phosphorylated insulin-like growth factor-1 (IGF-1) receptor also directly interacts with LARG and that IGF-1 stimulation in MDCKII cells induces Rho activation.
Crosstalk between G12/13-Mediated Signaling and Other Signaling Pathways
The G12/13-mediated signaling pathway engages in crosstalk with other pathways at various levels such as GPCRs (described above), G proteins, and downstream effectors (fig. 1). At the G protein level, Gα12 and Gα13 interact directly and in an activation-dependent manner with the cytoplasmic tails of cadherins [105, 106]. Binding of activated Gα12 to the cytoplasmic tail of E-cadherin triggers the release of the transcriptional activator β-catenin attenuates the extracellular adhesive function of E-cadherin, and promotes cell migration [105, 106]. Gα12 may integrate the LARG-Rho signaling pathway controlling cytoskeletal rearrangement and the cadherin-β-catenin signaling pathway regulating cell-cell adhesion to govern cell migration in response to extracellular stimulation. It has also been reported that Gα12 interacts with and activates a member of the Tec family of non-receptor tyrosine kinases, BTK, while Gα13 binds and stimulates PYK2, another non-receptor tyrosine kinase [87, 107].
RH-RhoGEFs are also capable of interacting with various other cellular proteins. In particular, PDZ-RhoGEF and LARG are well known to interact with multiple cell surface receptors through their PDZ domain as previously described. One of the binding partners for the PDZ domain is plexin B1. Multiple studies have demonstrated that binding of the C-terminal PDZ-binding motif of plexin B1 with both PDZ-RhoGEF and LARG activates RhoA and promotes growth cone collapse [101,102,103,104]. This pathway is also known to be involved in angiogenesis [108]. Additionally, the direct binding of the C-terminus of the IGF-1 receptor to LARG induces RhoA activation [100]. It is noteworthy that in these two cases, binding of plexin B or IGF-1 receptor activates the GEF activity of RH-RhoGEFs independently of Gα12/13 activation. Direct interaction between the PDZ domain of LARG and CD44 in human head and neck squamous carcinoma cells has also been reported [109]. This CD44-LARG complex interacts with the EGF receptor and activates the EGFR receptor kinase. The C-terminus of the LPA receptor also interacts with the PDZ domains of LARG and PDZ-RhoGEF [110]. p115RhoGEF has been reported to interact with the C-terminus of HIV-1's transmembrane protein gp41 [111]. This interaction inhibits the ability of p115RhoGEF to initiate Rho-dependent stress fiber formation and gene transcription. Interestingly, mutations in gp41 that block the interaction with p115RhoGEF inhibit the ability of the HIV-1 virus to produce infectious particles in certain cell types. This finding suggests that the interaction between gp41 and p115RhoGEF modulates the ability of the virus to replicate. As described above, the Rho effector Dia1 binds to the C-terminus of LARG, stimulates its GEF activity, and may constitute a positive feedback loop between LARG, RhoA, and Dia1 [94].
A deficiency of Gα13 in mice is embryonic lethal, while mice lacking Gα12 develop normally and do not exhibit any overt morphological or behavioral defects. This fact clearly shows that Gα13 and Gα12 mediate distinct signaling pathways. The studies of intercrosses of Gα12-deficient mice, Gα13-deficient mice, and Gαq-deficient mice indicate that the Gα12-mediated signaling pathway functionally interacts not only with the Gα13- but also with the Gαq-mediated signaling systems [28]. Coordinated action from the two signals is known at the level of transcriptional factors [32] and protein kinases such as protein kinase D and PYK2 [36, 112]. The signals from Gα12/13 and Gαq have been known to converge on Rho, which is a target for Gα12/13 [35, 113, 114]. Recently, p63RhoGEF, together with the related Dbl-family member Trio, has been identified as a direct effector of Gαq and a guanine nucleotide exchange factor for RhoA, and the crystal structure of the Gαq-p63RhoGEF-RhoA complex has also been determined [31, 33]. The crosstalk between Gα12/13- and Gαq-mediated signals has been noted for smooth muscle contraction [30] and platelet activation [115].
Dissection of G12/13-Mediated Signaling Pathways Evoked by Specific Gα-Effector Interactions
Accumulating evidence supports the idea that GPCR signals are amplified and integrated into the intracellular signaling network at the level of G proteins [116]. The Gα subunit acts as the core of the signaling complex at the membrane, which is formed through transient protein-protein interactions between multiple signaling components.
Recently, some groups have tried to regulate the specific signal induced by the Gα-effector interaction. Using substitution mutants of Gα12 at the specific interface for the Gα12-effector interaction, Meigs et al. [117] have successfully identified a variant of Gα12 that is selectively uncoupled from one signaling pathway while retaining signaling capacity through a separate pathway: it has impaired binding to RH-RhoGEFs and is unable to activate Rho, but retains coupling to the effector cadherin and the ability to trigger β-catenin release from the cytoplasmic domain of cadherin. A study using small interfering RNAs to eliminate specific RH-RhoGEF expression in kidney and prostate cells has demonstrated that specific G12-coupled receptors require specific RH-RhoGEFs for Rho activation [118]. Thrombin-mediated stimulation of Rho requires LARG, while the LPA-stimulated Rho response requires PDZ-RhoGEF. These efforts to evaluate the specific signals mediated by G12/13 could lead to development of specific modulators for biological responses induced by divergent effectors, and eventually drugs with fewer side effects.
Physiological Function and Pathophysiological Significance of G12/13-Mediated Signaling Pathways
Advances in animal studies such as conditional knockout mice and genetic analysis in Caenorhabditis elegans and Drosophila melanogaster have imparted new biological significance to the G12 subfamily. Conservation of a Gα12/13-RH-RhoGEF-Rho signaling pathway through the course of evolution from the model organisms C. elegans and D. melanogaster to mammals is supported by genetic evidence. In a mutational screen to identify Rho signaling pathway components in Drosophila, Barrett et al. [9] identified the DRhoGEF2 gene, which encodes a protein containing the tandem DH/PH domains characteristic of RhoGEFs, as an upstream Rho1 activator. Embryos lacking functional DRhoGEF2 show similar defects in the cell shape changes associated with gastrulation to embryos without functional Concertina, the single DrosophilaGα12/13 ortholog, suggesting that Concertina may propagate signals from an upstream ligand to Rho1 via DRhoGEF2 [9, 10]. Subsequently, the N-terminus of DRhoGEF2 was shown to have sequence homology with other putative RhoGEFs containing RH domains in their N-terminus [51]. Another study has demonstrated that in C. elegans, GPA-12, the ortholog of Gα12, and a RH-RhoGEF, CeRhoGEF, can interact in an activation-dependent manner and are co-expressed in some ventral cord motor neurons [119]. In the same study, silencing of either GPA-12 or CeRhoGEF using RNA interference (RNAi) results in a similar phenotype, namely defects in egg laying and embryonic lethality, further suggesting these two proteins function in the same pathway. Additional studies in C. elegans support the notion that one of the pathways acting upstream of Rho1 in acetylcholine-releasing motor neurons, where Rho1 stimulates the release of acetylcholine [120], depends on GPA-12, which acts via the single RH-RhoGEF ortholog, RHGF-1 [16]. It has been reported that Gq also acts via the UNC-73 RhoGEF, an ortholog of mammalian Trio, to increase Rho activity in neurons [121].
The first identified Gα12/13 function was the ability to induce oncogenic transformation [12,13,14,15]. Since then, many studies of the biological functions of G12/13 have concentrated on their roles in cell proliferation, cell migration, and morphological changes. Accumulating evidence indicates that the G12/13-mediated signaling pathway is involved in a variety of physiological and pathophysiological processes as mentioned in the introduction. Furthermore, some evidence indicates that Gα12 and Gα13 have partially overlapping but distinct cellular and biological functions [28, 29], and that G12 or G13-mediated signals crosstalk with those from other G proteins, especially Gq to produce a biological response [3, 11, 28,30,31,32,33,34,35,36].
Cell Proliferation and Transformation – Neoplastic Disorders
Notably, Gα12 and Gα13 are the only heterotrimeric Gα subunits that have potent transforming capabilities when overexpressed as the wild-type forms. These tumorigenic and cell-proliferating effects of G12/13 seem to be mainly mediated by RhoA activation. Martin et al. [27] have demonstrated that overexpression of the thrombin protease-activated receptor-1 (PAR-1) promotes transformation and growth in NIH-3T3 cells through Gα12/13. PAR-1 stimulates the activity of the serum response factor and NF-κB transcription factors, which are also effectors of RhoA. Furthermore, PAR-1 transforming activity is partially blocked by co-expression of dominant negative RhoA. Other studies also suggest that GPCRs upstream of Gα12 and Gα13 may promote tumorigenesis and tumor cell growth [25, 32, 122, 123]. Indeed, recent studies demonstrated that Gα12 protein levels are upregulated in human breast or prostate adenocarcinoma tissues [22, 23]. Interestingly, in contrast to the previous studies using non-transformed cells [12–15], Gα12 and Gα13 did not promote, and in some cases even inhibited, in vitroorin vivo proliferation of human breast or prostate cancer cell lines [22, 23]. Therefore, Gα12 and Gα13 may be important for promoting normal cell proliferation. In addition, it has been reported that Gα13-induced transformation uses a Rho-independent pathway via radixin, a member of the ERM family of proteins [124, 125].
It is noteworthy that unlike Ras, activating mutations in Rho have not been found in human cancers [126,127,128]. On the other hand, the regulators of Rho activation, RhoGEFs have been isolated in screens for transforming genes. LARG was one of the very few RhoGEFs that have been found mutated in human cancers. LARG was originally identified as a novel protein fused to the MLL (mixed lineage leukemia) gene in a patient with acute myeloid leukemia [21]. It is interesting that in the MLL-LARG fusion, not only the DH/PH domains responsible for Rho activation but also the RH domain and a nuclear localization signal are retained. The MLL-LARG rearrangement is expressed as an in-frame fusion from the MLL promotor. A study has indeed shown that LARG is abundant in mouse hematopoietic stem cells [129]. Furthermore, a recent analysis of bone marrow samples from patients with the preleukemic disorder Shwachman-Diamond syndrome demonstrated that in these patients LARG expression is dramatically increased [130]. However, further studies will be necessary to confirm the roles of LARG and MLL-LARG fusion protein in leukemic disease.
Cell Morphology and Motility – Failure in Gastrulation and Tumor Cell Invasion
Some aspects of the biological effects mediated by the Gα12/13 signaling pathway are contributed by the capability of their downstream effector, RhoA, to regulate cell morphology and motility. RhoA induces the assembly of contractile actin and myosin filaments (stress fibers), and is involved in cell contraction as well as in moving the body and tail of the cell behind the leading edge [128, 131, 132]. Cadherin, acting downstream of Gα12, also affects cell migration independently of Rho activation [105]. Gastrulation, which is regulated by the Gα12/13 ortholog and a RH-RhoGEF in D. melanogaster[9, 10], and tumor cell invasion, are related to these shape changes and movement.
Tumor Cell Invasion
Although Gα12 and Gα13 did not promote proliferation of human breast or prostate cancer cell lines, they promoted invasion by both types of cancer cells in vitro[22, 23]. Mouse mammary carcinoma cells implanted in the mammary fat pad of a mouse, which grow and metastasize in a manner similar to that of human breast cancer, has demonstrated that inhibition of Gα12/13 signaling by stable expression of the RH domain of p115RhoGEF in carcinoma cells reduced the rate of metastatic dissemination [22]. These studies suggest that Rho activation through Gα12/13 signaling is critical for promoting invasion. However, when seeded directly into the bloodstream, inhibition of G12/13 signaling by the RH domain had no effect on the ability of the cells to metastasize. Signaling via the G12-Rho pathway may coordinate with signals from cadherin, which influences cell-cell contacts, to promote invasion away from the primary tumor. However, it is possible that invasion stimulated by Gα12 and Gα13 may be cell type-specific [80].
The Cardiovascular System – Heart and Vascular Disease and Angiogenesis
A deficiency of Gα13 in mice impairs the development of the vascular system and is embryonic lethal [6]. Embryonic fibroblasts cultured from these mice show impaired motility in response to thrombin and LPA. Although vasculogenic blood vessel formation through the differentiation of progenitor cells into endothelial cells was not affected, angiogenesis, which includes sprouting, growth, migration and remodeling of existing endothelial cells, was severely disturbed [6, 29]. Endothelial-specific Gα13 knockout embryos also showed a similar phenotype to that of Gα13 null animals. However, restoration of Gα13 expression in endothelial cells in Gα13 conventional knockouts fails to completely rescue the phenotype, suggesting that Gα13 expression in other cell types is necessary during embryonic development [7]. In contrast to the phenotype observed in Gα13 null mice, mice lacking Gα12 develop normally and do not exhibit any obvious morphological or behavioral defects. A double knockout of Gα12 and Gα13 produces developmental defects in the headfold, somites, and neural tube and these embryos arrest earlier than Gα13 null embryos, suggesting that the function of Gα12 is not completely redundant to that of Gα13 during embryonic development [28]. In addition, a study suggests that semaphorin 4D/plexin B1-mediated angiogenic responses require Rho-mediated signaling via PDZ-RhoGEF or LARG [108]. As described above, binding of the C-terminal PDZ-binding motif of plexin B with both PDZ-RhoGEF and LARG activates RhoA.
Platelets
Studies using mice lacking Gα13 in platelets revealed that this α subunit is involved in normal hemostasis and thrombosis. Platelets lacking Gα13, but not Gα12, have impaired shape changes and aggregation in response to multiple platelet activators in vitro, and fail to form stable thrombi ex vivo. The mice exhibit a large increase in tail-bleeding times [5].
Smooth Muscle
It is believed that upon binding with vasoconstrictors, receptors coupling to both Gq/11 and G12/13 stimulate phosphorylation of myosin light chain (MLC) via the Ca2+/MLC kinase- and Rho/Rho kinase-mediated signaling pathways, respectively, to regulate vascular smooth muscle tone [30]. Recently, mice with conditional Gα12/13 double deficiencies in smooth muscle cells have been developed [24]. Aortic segments from these mice show impaired contractile responses to the vasoconstrictors angiotensin II, thromboxane A2, and endothelin I. Furthermore, these mice are almost completely protected from salt-induced hypertension, while their basal blood pressure is unaffected. Similar phenotypes were observed in mice lacking LARG in smooth muscle cells. These findings suggest that the Gα12/13-LARG pathway is a key regulator of vascular smooth muscle tone in the context of hypertension.
Heart
In cardiomyocytes, c-Jun NH2-terminal kinase (JNK) activation triggers hypertrophic responses [133]. It has been reported thatα1-adrenergic receptor-induced hypertrophic responses are mediated in part by a Gα12/13-Rho-JNK pathway, and in part by a Gq/11-JNK pathway that is Rho independent [134].
The Immune System
Several studies have suggested that Gα12/13 signaling pathways play a pivotal role in regulating chemotaxis. Defects in Lsc (p115RhoGEF) null mice are primarily found in the immune system, which is reasonable as p115RhoGEF/Lsc is strongly expressed in hematopoietic tissue [4,135,136,137,138]. These mice have reduced T-cell populations in their spleen and lymph nodes, as well as reduced numbers of marginal zone B cells in the spleen. Lsc null mice also display defects in lymphocyte migration, pseudopod formation, integrin-mediated adhesion and immune responses, suggesting that it is required for normal B- and T-lymphocyte function [4, 139].
The Neuronal System – Neurologic Disorders
The Rho family, which includes Rho, Rac, and Cdc42, has an important role in regulating actin cytoskeletal dynamics and has been implicated in growth cone guidance [140]. Rac and Cdc42 activation promote the formation of cell protrusions and adhesions at the leading edge, whereas Rho activation is thought to induce retraction through actinomyosin contraction at the trailing edge. It has been demonstrated that in neuronal cell lines, Gα12 and Gα13 specifically activate RhoA and cause Rho-dependent neurite retraction [11, 17]. Katoh et al. also have shown that Gαq collaborates with Gα12 and Gα13 to regulate neurite retraction in a Rho activation-dependent manner. Furthermore, LPA and S1P have been shown to induce neurite retraction through GPCR and RhoA activation [17, 141].
Recently, Moers et al. [18] have developed mice with conditional ablation of the genes encoding both Gα12 and Gα13 in the nervous system. They crossed Gα12-deficient mice which were homozygous for a floxed Gα13 allele with a transgenic mouse line expressing Cre under the control of the neuron-specific enhancer of the nestin promoter and the NEX promotor, which restricts recombination to neuronal and glial precursor cells starting at E10.5 and to principal neurons of the forebrain excluding glial cells and interneurons, respectively. The mice showed neuronal ectopia of cerebral and cerebellar cortices due to overmigration of cortical plate neurons and cerebellar Purkinje cells, respectively. Embryonic cortical neurons and Purkinje cells isolated from these mice were unable to retract their neurites in response to LPA and S1P. This result indicates that the Gα12/Gα13 signaling pathway is involved in the proper positioning of migrating cortical neurons.
The semaphorin4/plexin B signaling pathway also may affect axon guidance through the G12/13-RH-RhoGEF-RhoA signaling pathway. Sema4D stimulates RhoA activation through PDZRhoGEF and LARG as described above, while plexin B may suppress Rac function by competing with other Rac downstream targets, such as p21-activated kinase (PAK) [142]. A study suggests that the G12/13-RH-RhoGEF-RhoA signaling pathway is involved in Sonic hedgehog/Smoothened-mediated cellular responses, including stimulation of target gene promoters and inhibition of neurite outgrowth in neuroblastoma cells [143]. In addition, a study has demonstrated that morphological changes can also occur in glial cells in addition to neurons in response to Gα12/Gα13 activation [144]. This study also suggests that stimulation of the thromboxane A2 receptor causes astrocyte proliferation mainly through a Gα12/Gα13 signaling pathway.
Future Directions
In this review, we have focused on studies highlighting the physiological significance of G12/13-mediated signaling and the regulatory mechanisms controlling this pathway. As focus on G12/13-mediated signaling has increased, it is becoming clear that these pathways participate in a variety of disease processes. In order to develop drugs to specifically regulate biological functions induced by G12/13-mediated signaling, it is essential to define the interfaces for protein-protein interactions in this signaling system. In the coming years, it will be important to analyze the molecular dynamics of these protein-protein interactions in conjunction with determining the structures of these complexes using high resolution X-ray crystallography.
Acknowledgement
This work was supported by a grant for Translational Systems Biology and Medicine Initiative (TSBMI) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and NIH grant GM61454.
References
- 1.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 2.Strathmann MP, Simon MI. G alpha 12 and G alpha 13 subunits define a fourth class of G protein alpha subunits. Proc Natl Acad Sci USA. 1991;88:5582–5586. doi: 10.1073/pnas.88.13.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Althoefer H, Eversole-Cire P, Simon MI. Constitutively active Galphaq and Galpha13 trigger apoptosis through different pathways. J Biol Chem. 1997;272:24380–24386. doi: 10.1074/jbc.272.39.24380. [DOI] [PubMed] [Google Scholar]
- 4.Girkontaite I, Missy K, Sakk V, Harenberg A, Tedford K, Potzel T, Pfeffer K, Fischer KD. Lsc is required for marginal zone B cells, regulation of lymphocyte motility and immune responses. Nat Immunol. 2001;2:855–862. doi: 10.1038/ni0901-855. [DOI] [PubMed] [Google Scholar]
- 5.Moers A, Nieswandt B, Massberg S, Wettschureck N, Gruner S, Konrad I, Schulte V, Aktas B, Gratacap MP, Simon MI, Gawaz M, Offermanns S. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med. 2003;9:1418–1422. doi: 10.1038/nm943. [DOI] [PubMed] [Google Scholar]
- 6.Offermanns S, Mancino V, Revel JP, Simon MI. Vascular system defects and impaired cell chemokinesis as a result of Galpha13 deficiency. Science. 1997;275:533–536. doi: 10.1126/science.275.5299.533. [DOI] [PubMed] [Google Scholar]
- 7.Ruppel KM, Willison D, Kataoka H, Wang A, Zheng YW, Cornelissen I, Yin L, Xu SM, Coughlin SR. Essential role for Galpha13 in endothelial cells during embryonic development. Proc Natl Acad Sci USA. 2005;102:8281–8286. doi: 10.1073/pnas.0503326102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 9.Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 1997;91:905–915. doi: 10.1016/s0092-8674(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 10.Parks S, Wieschaus E. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell. 1991;64:447–458. doi: 10.1016/0092-8674(91)90652-f. [DOI] [PubMed] [Google Scholar]
- 11.Katoh H, Aoki J, Yamaguchi Y, Kitano Y, Ichikawa A, Negishi M. Constitutively active Galpha12, Galpha13, and Galphaq induce Rho-dependent neurite retraction through different signaling pathways. J Biol Chem. 1998;273:28700–28707. doi: 10.1074/jbc.273.44.28700. [DOI] [PubMed] [Google Scholar]
- 12.Chan AM, Fleming TP, McGovern ES, Chedid M, Miki T, Aaronson SA. Expression CDNA cloning of a transforming gene encoding the wild-type G alpha 12 gene product. Mol Cell Biol. 1993;13:762–768. doi: 10.1128/mcb.13.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang H, Wu D, Simon MI. The transforming activity of activated G alpha 12. FEBS Lett. 1993;330:319–322. doi: 10.1016/0014-5793(93)80896-3. [DOI] [PubMed] [Google Scholar]
- 14.Voyno-Yasenetskaya TA, Pace AM, Bourne HR. Mutant alpha subunits of G12 and G13 proteins induce neoplastic transformation of Rat-1 fibroblasts. Oncogene. 1994;9:2559–2565. [PubMed] [Google Scholar]
- 15.Xu N, Bradley L, Ambdukar I, Gutkind JS. A mutant alpha subunit of G12 potentiates the eicosanoid pathway and is highly oncogenic in NIH-3T3 cells. Proc Natl Acad Sci USA. 1993;90:6741–6745. doi: 10.1073/pnas.90.14.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiley E, McMullan R, Nurrish SJ. The Galpha12-RGS RhoGEF-RhoA signalling pathway regulates neurotransmitter release in C. elegans. EMBO J. 2006;25:5884–5895. doi: 10.1038/sj.emboj.7601458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kranenburg O, Poland M, van Horck FP, Drechsel D, Hall A, Moolenaar WH. Activation of RhoA by lysophosphatidic acid and Galpha12/13 subunits in neuronal cells: induction of neurite retraction. Mol Biol Cell. 1999;10:1851–1857. doi: 10.1091/mbc.10.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moers A, Nurnberg A, Goebbels S, Wettschureck N, Offermanns S. Galpha12/Galpha13 deficiency causes localized overmigration of neurons in the developing cerebral and cerebellar cortices. Mol Cell Biol. 2008;28:1480–1488. doi: 10.1128/MCB.00651-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jho EH, Malbon CC. Galpha12 and Galpha13 mediate differentiation of p19 mouse embryonal carcinoma cells in response to retinoic acid. J Biol Chem. 1997;272:24461–24467. doi: 10.1074/jbc.272.39.24461. [DOI] [PubMed] [Google Scholar]
- 20.Dettlaff-Swiercz DA, Wettschureck N, Moers A, Huber K, Offermanns S. Characteristic defects in neural crest cell-specific Galphaq/Galpha11- and Galpha12/Galpha13-deficient mice. Dev Biol. 2005;282:174–182. doi: 10.1016/j.ydbio.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Kourlas PJ, Strout MP, Becknell B, Veronese ML, Croce CM, Theil KS, Krahe R, Ruutu T, Knuutila S, Bloomfield CD, Caligiuri MA. Identification of a gene at 11q23 encoding a guanine nucleotide exchange factor: evidence for its fusion with MLL in acute myeloid leukemia. Proc Natl Acad Sci USA. 2000;97:2145–2150. doi: 10.1073/pnas.040569197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly P, Moeller BJ, Juneja J, Booden MA, Der CJ, Daaka Y, Dewhirst MW, Fields TA, Casey PJ. The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proc Natl Acad Sci USA. 2006;103:8173–8178. doi: 10.1073/pnas.0510254103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly P, Stemmle LN, Madden JF, Fields TA, Daaka Y, Casey PJ. A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. J Biol Chem. 2006;281:26483–26490. doi: 10.1074/jbc.M604376200. [DOI] [PubMed] [Google Scholar]
- 24.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 25.Aragay AM, Collins LR, Post GR, Watson AJ, Feramisco JR, Brown JH, Simon MI. G12 requirement for thrombin-stimulated gene expression and DNA synthesis in 1321N1 astrocytoma cells. J Biol Chem. 1995;270:20073–20077. doi: 10.1074/jbc.270.34.20073. [DOI] [PubMed] [Google Scholar]
- 26.Jho EH, Davis RJ, Malbon CC. C-jun amino-terminal kinase is regulated by Galpha12/Galpha13 and obligate for differentiation of p19 embryonal carcinoma cells by retinoic acid. J Biol Chem. 1997;272:24468–24474. doi: 10.1074/jbc.272.39.24468. [DOI] [PubMed] [Google Scholar]
- 27.Martin CB, Mahon GM, Klinger MB, Kay RJ, Symons M, Der CJ, Whitehead IP. The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways. Oncogene. 2001;20:1953–1963. doi: 10.1038/sj.onc.1204281. [DOI] [PubMed] [Google Scholar]
- 28.Gu JL, Muller S, Mancino V, Offermanns S, Simon MI. Interaction of G alpha(12) with G alpha(13) and G alpha(q) signaling pathways. Proc Natl Acad Sci USA. 2002;99:9352–9357. doi: 10.1073/pnas.102291599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Offermanns S. In vivo functions of heterotrimeric G-proteins: Studies in Galpha-deficient mice. Oncogene. 2001;20:1635–1642. doi: 10.1038/sj.onc.1204189. [DOI] [PubMed] [Google Scholar]
- 30.Gohla A, Schultz G, Offermanns S. Role for G(12)/G(13) in agonist-induced vascular smooth muscle cell contraction. Circ Res. 2000;87:221–227. doi: 10.1161/01.res.87.3.221. [DOI] [PubMed] [Google Scholar]
- 31.Lutz S, Shankaranarayanan A, Coco C, Ridilla M, Nance MR, Vettel C, Baltus D, Evelyn CR, Neubig RR, Wieland T, Tesmer JJ. Structure of Galphaq-p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRs. Science. 2007;318:1923–1927. doi: 10.1126/science.1147554. [DOI] [PubMed] [Google Scholar]
- 32.Marinissen MJ, Servitja JM, Offermanns S, Simon MI, Gutkind JS. Thrombin protease-activated receptor-1 signals through Gq- and G13-initiated MAPK cascades regulating c-Jun expression to induce cell transformation. J Biol Chem. 2003;278:46814–46825. doi: 10.1074/jbc.M305709200. [DOI] [PubMed] [Google Scholar]
- 33.Rojas RJ, Yohe ME, Gershburg S, Kawano T, Kozasa T, Sondek J. Galphaq directly activates p63RhoGEF and trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. J Biol Chem. 2007;282:29201–29210. doi: 10.1074/jbc.M703458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagi SA, Seasholtz TM, Kobiashvili M, Wilson BA, Toksoz D, Brown JH. Physical and functional interactions of Galphaq with Rho and its exchange factors. J Biol Chem. 2001;276:15445–15452. doi: 10.1074/jbc.M008961200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogt S, Grosse R, Schultz G, Offermanns S. Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of gq/G11. J Biol Chem. 2003;278:28743–28749. doi: 10.1074/jbc.M304570200. [DOI] [PubMed] [Google Scholar]
- 36.Yuan J, Slice LW, Rozengurt E. Activation of protein kinase d by signaling through Rho and the alpha subunit of the heterotrimeric G protein G13. J Biol Chem. 2001;276:38619–38627. doi: 10.1074/jbc.M105530200. [DOI] [PubMed] [Google Scholar]
- 37.Hepler JR, Gilman AG. G proteins. Trends Biochem Sci. 1992;17:383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- 38.Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 39.Ross EM, Wilkie TM. GTPaseactivating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 40.Zerangue N, Jan LY. G-protein signaling: fine-tuning signaling kinetics. Curr Biol. 1998;8:R313–R316. doi: 10.1016/s0960-9822(98)70196-4. [DOI] [PubMed] [Google Scholar]
- 41.Riobo NA, Manning DR. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol Sci. 2005;26:146–154. doi: 10.1016/j.tips.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G-protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macrez-Lepretre N, Kalkbrenner F, Morel JL, Schultz G, Mironneau J. G protein heterotrimer Galpha13beta1gamma3 couples the angiotensin at1a receptor to increases in cytoplasmic Ca2+ in rat portal vein myocytes. J Biol Chem. 1997;272:10095–10102. doi: 10.1074/jbc.272.15.10095. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi Y, Katoh H, Negishi M. N-terminal short sequences of alpha subunits of the G12 family determine selective coupling to receptors. J Biol Chem. 2003;278:14936–14939. doi: 10.1074/jbc.M301409200. [DOI] [PubMed] [Google Scholar]
- 45.Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther. 2003;99:25–44. doi: 10.1016/s0163-7258(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 46.Kuniyeda K, Okuno T, Terawaki K, Miyano M, Yokomizo T, Shimizu T. Identification of the intracellular region of the leukotriene B4 receptor type 1 that is specifically involved in Gi activation. J Biol Chem. 2007;282:3998–4006. doi: 10.1074/jbc.M610540200. [DOI] [PubMed] [Google Scholar]
- 47.Ponimaskin EG, Profirovic J, Vaiskunaite R, Richter DW, Voyno-Yasenetskaya TA. 5-Hydroxytryptamine 4(a) receptor is coupled to the Galpha subunit of heterotrimeric G13 protein. J Biol Chem. 2002;277:20812–20819. doi: 10.1074/jbc.M112216200. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, DiLizio C, Kim D, Smyth EM, Manning DR. The G12 family of G proteins as a reporter of thromboxane A2 receptor activity. Mol Pharmacol. 2006;69:1433–1440. doi: 10.1124/mol.105.019703. [DOI] [PubMed] [Google Scholar]
- 49.Kozasa T, Gilman AG. Purification of recombinant G proteins from sf9 cells by hexahistidine tagging of associated subunits. Characterization of alpha 12 and inhibition of adenylyl cyclase by alpha z. J Biol Chem. 1995;270:1734–1741. doi: 10.1074/jbc.270.4.1734. [DOI] [PubMed] [Google Scholar]
- 50.Singer WD, Miller RT, Sternweis PC. Purification and characterization of the alpha subunit of G13. J Biol Chem. 1994;269:19796–19802. [PubMed] [Google Scholar]
- 51.Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. P115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki N, Nakamura S, Mano H, Kozasa T. Galpha 12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc Natl Acad Sci USA. 2003;100:733–738. doi: 10.1073/pnas.0234057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wells CD, Liu MY, Jackson M, Gutowski S, Sternweis PM, Rothstein JD, Kozasa T, Sternweis PC. Mechanisms for reversible regulation between G13 and Rho exchange factors. J Biol Chem. 2002;277:1174–1181. doi: 10.1074/jbc.M105274200. [DOI] [PubMed] [Google Scholar]
- 54.Chen Z, Singer WD, Sternweis PC, Sprang SR. Structure of the p115RhoGEF rgRGS domain-Galpha13/i1 chimera complex suggests convergent evolution of a GTPase activator. Nat Struct Mol Biol. 2005;12:191–197. doi: 10.1038/nsmb888. [DOI] [PubMed] [Google Scholar]
- 55.Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem. 1999;274:5868–5879. doi: 10.1074/jbc.274.9.5868. [DOI] [PubMed] [Google Scholar]
- 56.Kreutz B, Yau DM, Nance MR, Tanabe S, Tesmer JJ, Kozasa T. A new approach to producing functional Galpha subunits yields the activated and deactivated structures of Galpha(12/13) proteins. Biochemistry. 2006;45:167–174. doi: 10.1021/bi051729t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane: the Galphaq-GRK2-gbetagamma complex. Science. 2005;310:1686–1690. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 58.Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 59.Chen Z, Wells CD, Sternweis PC, Sprang SR. Structure of the rgRGS domain of p115RhoGEF. Nat Struct Biol. 2001;8:805–809. doi: 10.1038/nsb0901-805. [DOI] [PubMed] [Google Scholar]
- 60.Longenecker KL, Lewis ME, Chikumi H, Gutkind JS, Derewenda ZS. Structure of the RGS-like domain from PDZ-RhoGEF: linking heterotrimeric G protein-coupled signaling to Rho GTPases. Structure. 2001;9:559–569. doi: 10.1016/s0969-2126(01)00620-7. [DOI] [PubMed] [Google Scholar]
- 61.Grabocka E, Wedegaertner PB. Functional consequences of G alpha 13 mutations that disrupt interaction with p115RhoGEF. Oncogene. 2005;24:2155–2165. doi: 10.1038/sj.onc.1208414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakamura S, Kreutz B, Tanabe S, Suzuki N, Kozasa T. Critical role of lysine 204 in switch I region of Galpha13 for regulation of p115RhoGEF and leukemia-associated RhoGEF. Mol Pharmacol. 2004;66:1029–1034. doi: 10.1124/mol.104.002287. [DOI] [PubMed] [Google Scholar]
- 63.Chen Z, Singer WD, Wells CD, Sprang SR, Sternweis PC. Mapping the Galpha13 binding interface of the rgRGS domain of p115RhoGEF. J Biol Chem. 2003;278:9912–9919. doi: 10.1074/jbc.M212695200. [DOI] [PubMed] [Google Scholar]
- 64.Bhattacharyya R, Wedegaertner PB. Mutation of an n-terminal acidic-rich region of p115-RhoGEF dissociates alpha13 binding and alpha13-promoted plasma membrane recruitment. FEBS Lett. 2003;540:211–216. doi: 10.1016/s0014-5793(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 65.Jones TL, Gutkind JS. Galpha12 requires acylation for its transforming activity. Biochemistry. 1998;37:3196–3202. doi: 10.1021/bi972253j. [DOI] [PubMed] [Google Scholar]
- 66.Ponimaskin E, Behn H, Adarichev V, Voyno-Yasenetskaya TA, Offermanns S, Schmidt MF. Acylation of Galpha(13) is important for its interaction with thrombin receptor, transforming activity and actin stress fiber formation. FEBS Lett. 2000;478:173–177. doi: 10.1016/s0014-5793(00)01845-7. [DOI] [PubMed] [Google Scholar]
- 67.Veit M, Nurnberg B, Spicher K, Harteneck C, Ponimaskin E, Schultz G, Schmidt MF. The alpha-subunits of G-proteins G12 and G13 are palmitoylated, but not amidically myristoylated. FEBS Lett. 1994;339:160–164. doi: 10.1016/0014-5793(94)80406-0. [DOI] [PubMed] [Google Scholar]
- 68.Bhattacharyya R, Wedegaertner PB. Galpha 13 requires palmitoylation for plasma membrane localization, Rho-dependent signaling, and promotion of p115-RhoGEF membrane binding. J Biol Chem. 2000;275:14992–14999. doi: 10.1074/jbc.M000415200. [DOI] [PubMed] [Google Scholar]
- 69.Waheed AA, Jones TL. Hsp90 interactions and acylation target the G protein Galpha 12 but not Galpha 13 to lipid rafts. J Biol Chem. 2002;277:32409–32412. doi: 10.1074/jbc.C200383200. [DOI] [PubMed] [Google Scholar]
- 70.Tu Y, Wang J, Ross EM. Inhibition of brain Gz gap and other RGS proteins by palmitoylation of G protein alpha subunits. Science. 1997;278:1132–1135. doi: 10.1126/science.278.5340.1132. [DOI] [PubMed] [Google Scholar]
- 71.Fields TA, Casey PJ. Phosphorylation of Gz alpha by protein kinase C blocks interaction with the beta gamma complex. J Biol Chem. 1995;270:23119–23125. doi: 10.1074/jbc.270.39.23119. [DOI] [PubMed] [Google Scholar]
- 72.Kozasa T, Gilman AG. Protein kinase C phosphorylates G12 alpha and inhibits its interaction with g beta gamma. J Biol Chem. 1996;271:12562–12567. doi: 10.1074/jbc.271.21.12562. [DOI] [PubMed] [Google Scholar]
- 73.Lounsbury KM, Casey PJ, Brass LF, Manning DR. Phosphorylation of Gz in human platelets. Selectivity and site of modification. J Biol Chem. 1991;266:22051–22056. [PubMed] [Google Scholar]
- 74.Lounsbury KM, Schlegel B, Poncz M, Brass LF, Manning DR. Analysis of Gz alpha by site-directed mutagenesis. Sites and specificity of protein kinase C-dependent phosphorylation. J Biol Chem. 1993;268:3494–3498. [PubMed] [Google Scholar]
- 75.Offermanns S, Hu YH, Simon MI. Galpha12 and Galpha13 are phosphorylated during platelet activation. J Biol Chem. 1996;271:26044–26048. doi: 10.1074/jbc.271.42.26044. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Ducret A, Tu Y, Kozasa T, Aebersold R, Ross EM. RGSz1, a Gz-selective RGS protein in brain. Structure, membrane association, regulation by Galphaz phosphorylation, and relationship to a Gz GTPaseactivating protein subfamily. J Biol Chem. 1998;273:26014–26025. doi: 10.1074/jbc.273.40.26014. [DOI] [PubMed] [Google Scholar]
- 77.Glick JL, Meigs TE, Miron A, Casey PJ. RGSz1, a Gz-selective regulator of G protein signaling whose action is sensitive to the phosphorylation state of Gz alpha. J Biol Chem. 1998;273:26008–26013. doi: 10.1074/jbc.273.40.26008. [DOI] [PubMed] [Google Scholar]
- 78.Van der Linden AM, Moorman C, Cuppen E, Korswagen HC, Plasterk RH. Hyperactivation of the G12-mediated signaling pathway in Caenorhabditis elegans induces a developmental growth arrest via protein kinase C. Curr Biol. 2003;13:516–521. doi: 10.1016/s0960-9822(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 79.Dhanasekaran N, Prasad MV, Wadsworth SJ, Dermott JM, van Rossum G. Protein kinase C-dependent and -independent activation of Na+/H+ exchanger by G alpha 12 class of G proteins. J Biol Chem. 1994;269:11802–11806. [PubMed] [Google Scholar]
- 80.Kelly P, Casey PJ, Meigs TE. Biologic functions of the G12 subfamily of heterotrimeric G proteins: growth, migration, and metastasis. Biochemistry. 2007;46:6677–6687. doi: 10.1021/bi700235f. [DOI] [PubMed] [Google Scholar]
- 81.Sternweis PC, Carter AM, Chen Z, Danesh SM, Hsiung YF, Singer WD. Regulation of Rho guanine nucleotide exchange factors by G proteins. Adv Protein Chem. 2007;74:189–228. doi: 10.1016/S0065-3233(07)74006-8. [DOI] [PubMed] [Google Scholar]
- 82.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 83.Vazquez-Prado J, Miyazaki H, Castellone MD, Teramoto H, Gutkind JS. Chimeric G alpha i2/G alpha 13 proteins reveal the structural requirements for the binding and activation of the RGS-like (RGL)-containing Rho guanine nucleotide exchange factors (GEFs) by G alpha 13. J Biol Chem. 2004;279:54283–54290. doi: 10.1074/jbc.M410594200. [DOI] [PubMed] [Google Scholar]
- 84.Kreutz B, Hajicek N, Yau DM, Nakamura S, Kozasa T. Distinct regions of Galpha13 participate in its regulatory interactions with RGS homology domain-containing RhoGEFs. Cell Signal. 2007;19:1681–1689. doi: 10.1016/j.cellsig.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Chikumi H, Fukuhara S, Gutkind JS. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: Evidence of a role for focal adhesion kinase. J Biol Chem. 2002;277:12463–12473. doi: 10.1074/jbc.M108504200. [DOI] [PubMed] [Google Scholar]
- 86.Hamazaki Y, Kojima H, Mano H, Nagata Y, Todokoro K, Abe T, Nagasawa T. Tec is involved in G-protein-coupled receptor- and integrin-mediated signalings in human blood platelets. Oncogene. 1998;16:2773–2779. doi: 10.1038/sj.onc.1201799. [DOI] [PubMed] [Google Scholar]
- 87.Jiang Y, Ma W, Wan Y, Kozasa T, Hattori S, Huang XY. The G protein G alpha12 stimulates Bruton's tyrosine kinase and a rasGAP through a conserved PH/BM domain. Nature. 1998;395:808–813. doi: 10.1038/27454. [DOI] [PubMed] [Google Scholar]
- 88.Mao J, Xie W, Yuan H, Simon MI, Mano H, Wu D. Tec/Bmx non-receptor tyrosine kinases are involved in regulation of Rho and serum response factor by Galpha12/13. EMBO J. 1998;17:5638–5646. doi: 10.1093/emboj/17.19.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gohla A, Harhammer R, Schultz G. The G-protein G13 but not G12 mediates signaling from lysophosphatidic acid receptor via epidermal growth factor receptor to Rho. J Biol Chem. 1998;273:4653–4659. doi: 10.1074/jbc.273.8.4653. [DOI] [PubMed] [Google Scholar]
- 90.Chikumi H, Barac A, Behbahani B, Gao Y, Teramoto H, Zheng Y, Gutkind JS. Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential. Oncogene. 2004;23:233–240. doi: 10.1038/sj.onc.1207012. [DOI] [PubMed] [Google Scholar]
- 91.Eisenhaure TM, Francis SA, Willison LD, Coughlin SR, Lerner DJ. The Rho guanine nucleotide exchange factor Lsc homo-oligomerizes and is negatively regulated through domains in its carboxyl terminus that are absent in novel splenic isoforms. J Biol Chem. 2003;278:30975–30984. doi: 10.1074/jbc.M303277200. [DOI] [PubMed] [Google Scholar]
- 92.Grabocka E, Wedegaertner PB. Disruption of oligomerization induces nucleocytoplasmic shuttling of leukemia-associated Rho guanine-nucleotide exchange factor. Mol Pharmacol. 2007;72:993–1002. doi: 10.1124/mol.107.035162. [DOI] [PubMed] [Google Scholar]
- 93.Wells CD, Gutowski S, Bollag G, Sternweis PC. Identification of potential mechanisms for regulation of p115 RhoGEF through analysis of endogenous and mutant forms of the exchange factor. J Biol Chem. 2001;276:28897–28905. doi: 10.1074/jbc.M102913200. [DOI] [PubMed] [Google Scholar]
- 94.Kitzing TM, Sahadevan AS, Brandt DT, Knieling H, Hannemann S, Fackler OT, Grosshans J, Grosse R. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes Dev. 2007;21:1478–1483. doi: 10.1101/gad.424807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhattacharyya R, Wedegaertner PB. Characterization of G alpha 13-dependent plasma membrane recruitment of p115RhoGEF. Biochem J. 2003;371:709–720. doi: 10.1042/BJ20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuner R, Swiercz JM, Zywietz A, Tappe A, Offermanns S. Characterization of the expression of PDZ-RhoGEF, LARG and G(alpha)12/G(alpha)13 proteins in the murine nervous system. Eur J Neurosci. 2002;16:2333–2341. doi: 10.1046/j.1460-9568.2002.02402.x. [DOI] [PubMed] [Google Scholar]
- 97.Togashi H, Nagata K, Takagishi M, Saitoh N, Inagaki M. Functions of a Rho-specific guanine nucleotide exchange factor in neurite retraction. Possible role of a proline-rich motif of KIAA0380 in localization. J Biol Chem. 2000;275:29570–29578. doi: 10.1074/jbc.M003726200. [DOI] [PubMed] [Google Scholar]
- 98.Banerjee J, Wedegaertner PB. Identification of a novel sequence in PDZ-RhoGEF that mediates interaction with the actin cytoskeleton. Mol Biol Cell. 2004;15:1760–1775. doi: 10.1091/mbc.E03-07-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wong K, Van Keymeulen A, Bourne HR. PDZ-RhoGEF and myosin II localize RhoA activity to the back of polarizing neutrophil-like cells. J Cell Biol. 2007;179:1141–1148. doi: 10.1083/jcb.200706167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taya S, Inagaki N, Sengiku H, Makino H, Iwamatsu A, Urakawa I, Nagao K, Kataoka S, Kaibuchi K. Direct interaction of insulin-like growth factor-1 receptor with leukemia-associated RhoGEF. J Cell Biol. 2001;155:809–820. doi: 10.1083/jcb.200106139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aurandt J, Vikis HG, Gutkind JS, Ahn N, Guan KL. The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc Natl Acad Sci USA. 2002;99:12085–12090. doi: 10.1073/pnas.142433199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hirotani M, Ohoka Y, Yamamoto T, Nirasawa H, Furuyama T, Kogo M, Matsuya T, Inagaki S. Interaction of plexin-B1 with PDZ domain-containing Rho guanine nucleotide exchange factors. Biochem Biophys Res Commun. 2002;297:32–37. doi: 10.1016/s0006-291x(02)02122-8. [DOI] [PubMed] [Google Scholar]
- 103.Perrot V, Vazquez-Prado J, Gutkind JS. Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. J Biol Chem. 2002;277:43115–43120. doi: 10.1074/jbc.M206005200. [DOI] [PubMed] [Google Scholar]
- 104.Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- 105.Meigs TE, Fedor-Chaiken M, Kaplan DD, Brackenbury R, Casey PJ. Galpha12 and Galpha13 negatively regulate the adhesive functions of cadherin. J Biol Chem. 2002;277:24594–24600. doi: 10.1074/jbc.M201984200. [DOI] [PubMed] [Google Scholar]
- 106.Meigs TE, Fields TA, McKee DD, Casey PJ. Interaction of Galpha 12 and Galpha 13 with the cytoplasmic domain of cadherin provides a mechanism for beta-catenin release. Proc Natl Acad Sci USA. 2001;98:519–524. doi: 10.1073/pnas.021350998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi CS, Sinnarajah S, Cho H, Kozasa T, Kehrl JH. G13alpha-mediated PYK2 activation. PYK2 is a mediator of G13alpha-induced serum response element-dependent transcription. J Biol Chem. 2000;275:24470–24476. doi: 10.1074/jbc.M908449199. [DOI] [PubMed] [Google Scholar]
- 108.Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64:5212–5224. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- 109.Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase C epsilon-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem. 2006;281:14026–14040. doi: 10.1074/jbc.M507734200. [DOI] [PubMed] [Google Scholar]
- 110.Yamada T, Ohoka Y, Kogo M, Inagaki S. Physical and functional interactions of the lysophosphatidic acid receptors with PDZ domain-containing Rho guanine nucleotide exchange factors (RhoGEFs) J Biol Chem. 2005;280:19358–19363. doi: 10.1074/jbc.M414561200. [DOI] [PubMed] [Google Scholar]
- 111.Zhang H, Wang L, Kao S, Whitehead IP, Hart MJ, Liu B, Duus K, Burridge K, Der CJ, Su L. Functional interaction between the cytoplasmic leucine-zipper domain of HIV-1 gp41 and p115-RhoGEF. Curr Biol. 1999;9:1271–1274. doi: 10.1016/s0960-9822(99)80511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shi CS, Kehrl JH. PYK2 links G/q)alpha and G(13)alpha signaling to NF-κB activation. J Biol Chem. 2001;276:31845–31850. doi: 10.1074/jbc.M101043200. [DOI] [PubMed] [Google Scholar]
- 113.Booden MA, Siderovski DP, Der CJ. Leukemia-associated Rho guanine nucleotide exchange factor promotes G alpha q-coupled activation of RhoA. Mol Cell Biol. 2002;22:4053–4061. doi: 10.1128/MCB.22.12.4053-4061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chikumi H, Vazquez-Prado J, Servitja JM, Miyazaki H, Gutkind JS. Potent activation of RhoA by Galpha q and Gq-coupled receptors. J Biol Chem. 2002;277:27130–27134. doi: 10.1074/jbc.M204715200. [DOI] [PubMed] [Google Scholar]
- 115.Klages B, Brandt U, Simon MI, Schultz G, Offermanns S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J Cell Biol. 1999;144:745–754. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ross EM. Signal sorting and amplification through G-protein-coupled receptors. Neuron. 1989;3:141–152. doi: 10.1016/0896-6273(89)90027-5. [DOI] [PubMed] [Google Scholar]
- 117.Meigs TE, Juneja J, DeMarco CT, Stemmle LN, Kaplan DD, Casey PJ. Selective uncoupling of G alpha 12 from Rho-mediated signaling. J Biol Chem. 2005;280:18049–18055. doi: 10.1074/jbc.M500445200. [DOI] [PubMed] [Google Scholar]
- 118.Wang Q, Liu M, Kozasa T, Rothstein JD, Sternweis PC, Neubig RR. Thrombin and lysophosphatidic acid receptors utilize distinct RhoGEFs in prostate cancer cells. J Biol Chem. 2004;279:28831–28834. doi: 10.1074/jbc.C400105200. [DOI] [PubMed] [Google Scholar]
- 119.Yau DM, Yokoyama N, Goshima Y, Siddiqui ZK, Siddiqui SS, Kozasa T. Identification and molecular characterization of the G alpha12-Rho guanine nucleotide exchange factor pathway in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2003;100:14748–14753. doi: 10.1073/pnas.2533143100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McMullan R, Hiley E, Morrison P, Nurrish SJ. Rho is a presynaptic activator of neurotransmitter release at pre-existing synapses in C. elegans. Genes Dev. 2006;20:65–76. doi: 10.1101/gad.359706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Williams SL, Lutz S, Charlie NK, Vettel C, Ailion M, Coco C, Tesmer JJ, Jorgensen EM, Wieland T, Miller KG. Trio's Rho-specific GEF domain is the missing Galpha q effector in C. elegans. Genes Dev. 2007;21:2731–2746. doi: 10.1101/gad.1592007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fukuhara S, Chikumi H, Gutkind JS. RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene. 2001;20:1661–1668. doi: 10.1038/sj.onc.1204182. [DOI] [PubMed] [Google Scholar]
- 123.Radhika V, Dhanasekaran N. Transforming G proteins. Oncogene. 2001;20:1607–1614. doi: 10.1038/sj.onc.1204274. [DOI] [PubMed] [Google Scholar]
- 124.Vaiskunaite R, Adarichev V, Furthmayr H, Kozasa T, Gudkov A, Voyno-Yasenetskaya TA. Conformational activation of radixin by G13 protein alpha subunit. J Biol Chem. 2000;275:26206–26212. doi: 10.1074/jbc.M001863200. [DOI] [PubMed] [Google Scholar]
- 125.Liu G, Voyno-Yasenetskaya TA. Radixin stimulates Rac1 and Ca2+/calmodulin-dependent kinase, CaMKII: cross-talk with Galpha13 signaling. J Biol Chem. 2005;280:39042–39049. doi: 10.1074/jbc.M504341200. [DOI] [PubMed] [Google Scholar]
- 126.Bos JL. The ras gene family and human carcinogenesis. Mutat Res. 1988;195:255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- 127.Boettner B, Van Aelst L. The role of Rho GTPases in disease development. Gene. 2002;286:155–174. doi: 10.1016/s0378-1119(02)00426-2. [DOI] [PubMed] [Google Scholar]
- 128.Sahai E, Marshall CJ. Rho-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 129.Zinovyeva M, Sveshnikova E, Visser J, Belyavsky A. Molecular cloning, sequence and expression pattern analysis of the mouse orthologue of the leukemia-associated guanine nucleotide exchange factor. Gene. 2004;337:181–188. doi: 10.1016/j.gene.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 130.Rujkijyanont P, Beyene J, Wei K, Khan F, Dror Y. Leukaemia-related gene expression in bone marrow cells from patients with the preleukaemic disorder Shwachman-diamond syndrome. Br J Haematol. 2007;137:537–544. doi: 10.1111/j.1365-2141.2007.06608.x. [DOI] [PubMed] [Google Scholar]
- 131.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 132.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 133.Sugden PH, Clerk A. ‘Stress-responsive’ mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ Res. 1998;83:345–352. doi: 10.1161/01.res.83.4.345. [DOI] [PubMed] [Google Scholar]
- 134.Maruyama Y, Nishida M, Sugimoto Y, Tanabe S, Turner JH, Kozasa T, Wada T, Nagao T, Kurose H. Galpha(12/13) mediates alpha(1)-adrenergic receptor-induced cardiac hypertrophy. Circ Res. 2002;91:961–969. doi: 10.1161/01.res.0000043282.39776.7c. [DOI] [PubMed] [Google Scholar]
- 135.Rubtsov A, Strauch P, Digiacomo A, Hu J, Pelanda R, Torres RM. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity. 2005;23:527–538. doi: 10.1016/j.immuni.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 136.Hart MJ, Sharma S, el Masry N, Qiu RG, McCabe P, Polakis P, Bollag G. Identification of a novel guanine nucleotide exchange factor for the Rho GTPase. J Biol Chem. 1996;271:25452–25458. doi: 10.1074/jbc.271.41.25452. [DOI] [PubMed] [Google Scholar]
- 137.Whitehead IP, Khosravi-Far R, Kirk H, Trigo-Gonzalez G, Der CJ, Kay R. Expression cloning of lsc, a novel oncogene with structural similarities to the Dbl family of guanine nucleotide exchange factors. J Biol Chem. 1996;271:18643–18650. doi: 10.1074/jbc.271.31.18643. [DOI] [PubMed] [Google Scholar]
- 138.Aasheim HC, Pedeutour F, Smeland EB. Characterization, expression and chromosomal localization of a human gene homologous to the mouse Lsc oncogene, with strongest expression in hematopoietic tissues. Oncogene. 1997;14:1747–1752. doi: 10.1038/sj.onc.1200994. [DOI] [PubMed] [Google Scholar]
- 139.Francis SA, Shen X, Young JB, Kaul P, Lerner DJ. Rho GEF Lsc is required for normal polarization, migration, and adhesion of formyl-peptide-stimulated neutrophils. Blood. 2006;107:1627–1635. doi: 10.1182/blood-2005-03-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guan KL, Rao Y. Signalling mechanisms mediating neuronal responses to guidance cues. Nat Rev Neurosci. 2003;4:941–956. doi: 10.1038/nrn1254. [DOI] [PubMed] [Google Scholar]
- 141.Postma FR, Jalink K, Hengeveld T, Moolenaar WH. Sphingosine-1-phosphate rapidly induces Rho-dependent neurite retraction: action through a specific cell surface receptor. EMBO J. 1996;15:2388–2392. [PMC free article] [PubMed] [Google Scholar]
- 142.Vikis HG, Li W, Guan KL. The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Genes Dev. 2002;16:836–845. doi: 10.1101/gad.966402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kasai K, Takahashi M, Osumi N, Sinnarajah S, Takeo T, Ikeda H, Kehrl JH, Itoh G, Arnheiter H. The G12 family of heterotrimeric G proteins and Rho GTPase mediate sonic hedgehog signalling. Genes Cells. 2004;9:49–58. doi: 10.1111/j.1356-9597.2004.00701.x. [DOI] [PubMed] [Google Scholar]
- 144.Honma S, Saika M, Ohkubo S, Kurose H, Nakahata N. Thromboxane A2 receptor-mediated G12/13-dependent glial morphological change. Eur J Pharmacol. 2006;545:100–108. doi: 10.1016/j.ejphar.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 145.Suzuki N, Tsumoto K, Hajicek N, Daigo K, Tokita R, Minami S, Kodama T, Hamakubo T, Kozasa T: Activation of Leukemia-associated RhoGEF by Galpha13 with significant conformational rearrangements in the interface. J Biol Chem 2008, in press. [DOI] [PMC free article] [PubMed]