Abstract
Heterotrimeric G proteins, composed of an α, β and γ subunit, represent one of the most important and dynamic families of signaling proteins. As a testament to the significance of G protein signaling, the hundreds of seven-transmembrane-spanning receptors that interact with G proteins are estimated to occupy 1–2% of the human genome. This broad diversity of receptors is echoed in the number of potential heterotrimer combinations that can arise from the 23 α subunit, 7 β subunit and 12 γ subunit isoforms that have been identified. The potential for such vast complexity implies that the receptor G protein interface is the site of much regulation. The historical model for the activation of a G protein holds that activated receptor catalyzes the exchange of GDP for GTP on the α subunit, inducing a conformational change that substantially lowers the affinity of α for βγ. This decreased affinity enables dissociation of βγ from α and receptor. The free form of βγ is thought to activate effectors, until the hydrolysis of GTP by G α (aided by RGS proteins) allows the subunits to re-associate, effectively deactivating the G protein until another interaction with activated receptor.
Key Words: G protein, Receptor, Activation, βγ Dimer, Signal transduction
Introduction
Although there are many facets of G protein signaling via the βγ dimer, this review will primarily focus on how the structure of a βγ dimer participates in the transfer of signal from activated receptor to the heterotrimeric G protein. Structural heterogeneity of βγ combinations will be discussed regarding how specificity of interactions of βγ with G α and receptor determine which βγ dimer isoforms are activated. A discussion of specific interactions of βγ dimers with effector molecules is beyond the scope of this review, but is addressed elsewhere in this issue. However, some references to interactions with effectors are included, as the activation of effectors is by definition one of the primary measures of activity of a G protein βγ dimer. Beyond the brief descriptions of the G protein activation cycle that have appeared in thousands of papers over the years, it is clear that βγ dimer ‘activity’ may not simply be synonymous with dissociation from α. Thus, an important question is whether dissociation of α from βγ is a requisite step in the activation of βγ. Also, is a βγ active if it does not properly localize with its effector molecule? Does a βγ need a receptor, or even an α subunit, to be activated? Does a βγ always exist as dimer? The record in the literature may be limited for some of these later questions, but they represent intriguing ideas that will help refine the model of G protein signaling.
G Protein βγ Heterogeneity
At least 16 α genes, 5 β genes and 12 γ genes have been identified in the human genome [1,2,3,4,5,6]. G α isoforms can be separated into four subfamilies, Gi, Gs, Gq and G12; when alternative splicing and posttranslational processing are taken into account, there are at least 23 α isoforms, which are reviewed elsewhere [5]. The first four β isoforms discovered, β1–4, are highly homologous (80–90% identical) 36-kDa proteins; G β5, a 40-kDa protein, is only 50% identical to the first four β isoforms. Several truncated splice variants of β3 have been characterized, β3s [7], β3s2 [8] and β3v [9]; a splice variant of β5 which has an N-terminal extension, β5L[10], has also been characterized. All 12 γ isoforms are between 7 and 8.5 kDa in size, but are much more divergent than the β isoforms. Since β and γ are believed to form a functional dimer in vivo, heterogeneity of βγ defined as the product of the β and γ genes is likely more diverse than G α [11] even though not every possible βγ combination can form. Posttranslational processing of β and γ further contribute to the structural diversity of these proteins [12], with γ isoforms receiving more extensive study than β isoforms to date.
Posttranslational Modifications
β Subunit
Several posttranslational modifications of G β have been characterized. The N-terminus of β1 was reported to undergo removal of the methionine at position 1, followed by N-acetlyation of serine at postion 2 [13], the functional implications of which were unclear. Phosphorylation of β has also been reported, but interestingly, at a histidine residue [14] instead of the traditional serine, threonine or tyrosine; nucleoside diphosphate kinase (NDPK) was identified as responsible for phosphorylating histidine 266 of β1[15]. This phosphorylation event was predicted as a mechanism for G protein activation, which will be discussed further below. In contrast, the reversible mono-ADP-ribosylation of arginine-129 of activated or ‘free’ G β was demonstrated to reduce activity at effectors such as type 1 adenylyl cyclase [16], phosphoinositide 3-kinase-γ and phospholipase C-β2[17]. Mono-ADP ribosylation of β increased upon activation of a variety of cell surface receptors, including the Gq-linked thrombin receptor and the Gi-linked 5-HT serotonin receptor, and thus was predicted to be a regulatory mechanism to inhibit βγ signaling. Inhibition of βγ activity by posttranslational modification has parallels to the GTPase activity of the G α subunit, and may represent an unappreciated regulatory mechanism in G protein signaling.
γ Subunit
C-Terminal Processing
The G γ subunit contains posttranslational modifications at the N- and C-terminus, and much of this processing has been demonstrated to be critical to G protein function. Most attention has focused on covalent modification at the C-terminus of γ with either of two distinct isoprenoid moieties [for review, [18]]. For γ isoforms ending in amino acid CAAX, where X is serine, glutamine or methionine, CVIS in the case of γ1, and additionally γ8, and γ11, the enzyme farnesyltransferase (FTase) covalently attaches a 15-carbon farnesyl group via a thioether bond to the cysteine in the CAAX motif [19]. If the X in the CAAX sequence is a leucine such as CAIL for γ2, or the C-terminal sequences in γ3, γ4, γ5, γ7, γ9, γ10, γ12 and γ13, the enzyme geranylgeranyltransferase type I (GGTase-I) attaches a larger 20-carbon geranylgeranyl group to the cysteine [20, 21] via the same thioether bond. After modification with either prenyl moiety, processing is similar for all γ isoforms. An endoprotease residing in the microsomal membranes cleaves the C-terminal-AAX residues [22], and the new prenylated C-terminal cysteine is carboxy methylated by a methyltransferase [23]. Both assembly of βγ dimers, which occurs in the cytosol [24], and prenylation of γ are required to target βγ to membranes [25]; there is some debate over which membranes, as βγ has been observed in endoplasmic reticulum membranes from biochemical and immunofluorescence studies of βγ expressed in mammalian cells [26]. Alternatively, live cell imaging demonstrated localization of βγ predominantly to the plasma membrane [27]. Although posttranslational processing of G γ as generalized above is well documented and well accepted, it is also evident that processing exceptions may provide insights into how G βγ functions in vivo.
For example, it is believed that the cleavage of the C-terminal-AAX amino acids is a necessary step in protein maturation prior to βγ assembly [28]. However, this is not always the case; a geranylgeranylated γ5 isoform that had not undergone cleavage of the C-terminal-AAX amino acids was characterized by mass spectrometry from a purified preparation of G protein from bovine brain [29]. This unprocessed form was discovered to predominate over the cleaved form, and be dependent on an aromatic phenylalanine residue in the CSFL C-terminal sequence of γ5[30]. A recent study noting a physical interaction between proteins containing PDZ domains, which are important in the construction of elaborate scaffolding networks, and Gγ13[31] made the story of this processing pattern more interesting. Gγ5, with its C-terminus ending in CSFL, is one of only four γ isoforms other than γ13 with a C-terminal target sequence (CT/SXX) for class I PDZ domain containing proteins. The fact that the C-terminal sequence of γ5 can remain after maturation of a βγ dimer suggests that γ5 isoforms that do not undergo C-terminal proteolytic cleavage are differentially targeted compared to other βγ isoforms. Since this form of processing appears to be unique for γ5, there may be a specific signaling role for βγ dimers containing γ5 in signaling complexes containing PDZ domains.
Unprenylated γ
The absence of prenylation in Gγ is also associated with unexpected signaling properties. After the observation that a fraction of β2γ2 could localize to the nucleus and regulate transcriptional activity [32], a study by Kino et al. [33] demonstrated that lack of prenylation of γ2, either by mutation of the C-terminal cysteine to serine or by pharmacological inhibition, resulted in increased nuclear localization of βγ and increased ability to regulate transcription. Further, non-prenylated γ5 expressed in bacteria was shown to regulate transcription by binding to the adipocyte enhancer-binding protein (AEBP1) transcriptional repressor [34]. Although prenylation of γ is not thought to be reversible, fully processed isoforms, missing a prenyl group, have been characterized by mass spectrometry in γ2 (<1% of γ2 observed) and γ7 (1–5% of γ7 observed) from G protein purified from bovine brain [12]. These exceptions in the prenylation pathway, although apparently low in occurrence, are surprising in that conventional wisdom assumes that, although prenylation is not required for assembly [28], it is a prerequisite for βγ activity. Moreover, since regulation of transcription is not a classical signaling function for βγ, the prenylation status of βγ may represent a significant point in modulation of activity of βγ dimers, with respect to identity and localization of effector targets.
N-Terminal Processing
Other regions of γ that are sites for covalent modification include the N-terminus, which were initially observed to be refractory to Edman degradation in the case of γ2, γ5 and γ7; alternatively, γ1[35], γ3[36] and γ11[37] were not found to be N-terminally blocked. Mass spectrometry was used to identify structural modifications at the N-terminus of the γ2 isoform, which were revealed to be cleavage of the N-terminal methionine followed by N-acetylation of alanine formerly at position 2 [38]. Yet another variant of γ2 was characterized by Edman degradation, in which a novel N-terminal sequence was determined to be a substrate for the N-end rule ubiquitylation pathway [39]. Modification by ubiquitin was found likely to occur at lysine residues in the C-terminal region of γ; although ubiquitylation is generally regarded as a signal for protein degradation, the authors suggest that this modification may also modulate membrane binding of βγ.
Phosphorylation
Interestingly, phosphorylation has only been observed in the γ12 isoform, in which protein kinase C was shown to phosphorylate a serine at position one in vitro [40]. Phosphorylation appeared to increase the affinity of β1γ12 for Go α [40], and increase the ability of β1γ12 to couple to receptor [41]; however, phosphorylation diminished the activity of β1γ12 at adenylyl cyclase type II, but not phospholipase C-β [41]. Phorbol 12-myristate 13-acetate (PMA) induced in vivo phosphorylation of γ12 in cultured Swiss 3T3 cells was augmented by activation of the lysophosphatidic acid receptor, and inhibited by pertussis toxin, suggesting a role for Gi proteins in the regulation of γ12 by protein kinase C [42]. The increase in γ12 phosphorylation after receptor activation is also in agreement with the observation that βγ dimer, rather than heterotrimer, is the preferred substrate for protein kinase C [40]. It is possible that two of the potential signaling effects of this modification, increased G protein cycling, and targeting of specific effectors, combine to regulate signaling pathways within a cell. These examples demonstrate that posttranslational modifications of G protein β and γ subunit isoforms, while often overlooked, can be dynamic and thus represent points of signaling modulation that could affect intracellular targeting of a βγ dimer, and regulation of interactions with G α, receptor, and effector molecules.
Structure of βγ
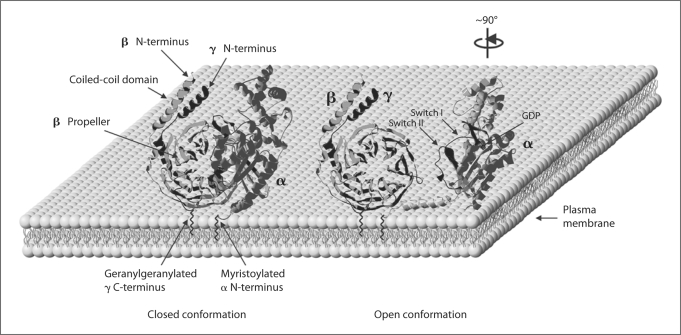
G protein β subunits belong to a family of WD40 repeat proteins, characterized by a repeating motif of 27–45 amino acids punctuated at the C-terminal end by the Trp-Asp (WD) dipeptide sequence [43]. The solutions to the crystal structures of a G protein and its constituent subunits were watershed events in the G protein field, providing for the first time a three-dimensional model of the heterotrimeric signaling molecule. This review will begin with those structures, and also discuss biochemical data that have served to complement the crystal structures of the G protein βγ dimer. The structure of the β1γ1 dimer of transducin was published by Sondek et al. [44] in 1996. At about the same time, the crystal structures of the β1γ2 dimer associated with the Gi1 α subunit, and the β1γ1 dimer associated with a chimera of the Gt α and Gi1 α subunits, were published by Wall et al. [45] and Lambright et al. [46], respectively. According to the structures, the 340-amino-acid β subunit forms a toroidal structure defined by seven propeller blades (fig. 1), with each blade comprised of a series of β-sheets. The N-terminus of the β subunit is an α-helix which interacts with the α-helical structure of the N-terminus of the approximately 70-amino-acid γ subunit to form a coiled-coil domain (fig. 1), which had been predicted from earlier biochemical studies [47]. The remainder of the γ subunit exists in an α helical structure that makes extensive contacts with the blades of the β torus (fig. 1). Crystal structures of β1γ1 and β1γ2 were highly homologous; further, a crystal structure of the more divergent β5 complexed with an RGS9 protein [48] revealed that β1 and β5 form very similar toroidal structures. This suggests that, even considering the high degree of diversity among the β and γ subunit isoforms, the overall structure of a βγ dimer is highly conserved.
Fig. 1.
Clam shell model of G protein opening after activation using the crystal structure of Wall et al. [45] . Left structure : Relationship of inactive GDP-bound form of Gi1 αβ1γ2 to the plasma membrane, with stylized lipids added to the structure. Right structure: βγ is in same conformation, but Gi1 α has been rotated approximately 90° counterclockwise; note that although lipids maintain their proximity, the face of the β propeller and switch I and II regions of Gi1 α, both occluded at left, are now sterically free to interact with effectors, and are thus ‘active’. (Although the same structure was used for simplicity, a truly active Gi1 α would have conformational changes in the switch regions, and GTPγS bound instead of GDP.)
Although the β1γ2[45] and β1γ1[46] dimers had been crystallized with G α subunits, revealing important sites of interaction, data elucidating the dynamic nature of these interactions was derived from the comparison of the crystal structures of Gt α in the inactive GDP-bound form [49] and the active GTPγS-bound form [50]. The general architecture of the G α subunit consists of a GTPase domain that is homologous to the monomeric GTP-binding proteins (such as ras), and a helical domain that is only found in heterotrimeric G protein α subunits. Two regions in the GTPase domain that interact with the γ-phosphate of GTP, coined switch I and switch II (fig. 1), stand out in the Gt α crystal structures in that they undergo a conformational change depending on the nucleotide bound. A third switch region found in the helical domain, switch III, also undergoes a conformational change, apparently dependent upon the conformational change of switch II [49]. These conformational changes, reviewed in more detail elsewhere [51], were critical to understanding the mechanism of activation of G proteins, as the crystal structures of two heterotrimeric G proteins revealed that although no contacts were observed between α and γ, many of the contact sites between α and β reside in the switch I and switch II regions (fig. 1) [46, 45]. Thus, the logical conclusion based on the crystal structures, and also predicted from earlier biochemical experiments that reported decreased affinity between activated α and βγ [52], was that nucleotide exchange in a G protein could induce conformational changes in the βγ-binding site of G α, resulting in subunit dissociation and activation of both α and βγ.
The model of G protein activation based on the crystal structures will continue to benefit from biochemical studies for a number of reasons. For one, purified G proteins could only be crystallized after removal of posttranslational farnesyl or geranylgeranyl lipid modifications [45, 46]. The three-dimensional structure of the G protein did, however, strongly suggest that the lipid modified N- and C-termini of α and γ, respectively, were proximal to one another [46]. Even after crystallization with an intact γ including the prenylated C-terminus, conformational instability of the C-terminal region precluded assignment of a static structure to this region of γ [53]. In addition, no crystal structure exists for a G protein in the empty state, that is, with no nucleotide bound; this state represents the transition between receptor-dependent release of GDP and binding GTP. Furthermore, a seven-transmembrane-spanning receptor has not been crystallized with a G protein; what is known of receptor:G protein interactions has been derived from biochemical data. Putative points of contact between G protein and receptor on the heterotrimer include the N-terminus [54] and C-terminus [55] of G α, the C-terminus of γ [56,57,58] and the C-terminus of β [59]. Thus, the mechanism of signal transfer from activated receptor in a membrane environment to G protein continues to be a point of conjecture based on empirical evidence.
Mechanism of Activation
Based largely on the crystal structures of heterotrimeric G proteins and monomeric GTP-binding proteins, several studies from Henry Bourne's laboratory, Onrust et al. [60] in 1997 and Iiri et al. [61] in 1998, sought to explain the molecular mechanism of activation of G protein by receptor through the ‘lever’ hypothesis. Such a hypothesis was necessary because the distance between the intracellular loops of a receptor and GDP were thought to be too far for direct interaction (fig. 1), thus the requirement of receptor to ‘act at a distance’ [62]. Integral to the hypothesis was the interaction between activated receptor and the β6 strand/α5 helix region of G α, which contains a loop to which GDP binds. Further, the switch I region and the β3/α2 loop of G α, in addition to containing βγ-binding sites, also form a lip that provides a secure binding site for GDP. Thus, two important events in receptor activation of G protein occur when: (1) receptor induces a conformational change in the β6 strand/α5 helix region of Gα, which would subsequently alter the GDP-binding site on the β6/α5 loop, and (2) the postulated insertion of the intracellular loops of the receptor into a crevice between α and βγ, which by employing βγ as a lever to pry α and βγ apart, could lead to an allosterically induced conformational change in the switch I/β3α2 loop ensconcing GDP, allowing release of nucleotide.
This hypothesis was tested biochemically by the creation of a mutant Gs α subunit that bound the βγ dimer in a conformation that mimicked the G protein structure described above in the absence of receptor [63]. Transfecting cells with βγ and the mutant Gs α increased the activity of the mutant Gs α at adenylyl cyclase, compared with the mutant Gs α alone, further supporting the hypotheses that βγ acts as a lever to affect GDP release in the course of G protein activation. An iteration of the lever hypothesis is the ‘gear-shift’ hypothesis [64], which holds that the N-terminus of the γ subunit acts as a gear-shift by interacting with the helical domain of the α subunit, thereby stabilizing the transitory nucleotide free empty state, and facilitating nucleotide exchange.
Studies with rhodopsin and synthetic G protein peptides suggest that the C-terminus of γ and the C-terminus of α interact with the activated receptor in a sequential, interdependent manner, and this binding facilitates the conformational change that allows release of GDP [65]. A later study refined this model using synthetic peptides and time-resolved near infrared lightscattering, indicating that the prenylated C-terminus of γ was the first point of contact with activated receptor, followed by the C-terminus of α in a brief transitory state that enabled GDP release [66]. A conformational switch in βγ, first proposed in 1995 [67], was envisioned as a mechanism for this sequence of events. Such a switch was predicted because in both detergent solubilized heterotrimeric transducin and β1γ1, the C-terminus of γ was resistant to carboxypeptidase Y, suggesting inaccessibility to other proteins; however, a 12-amino-acid C-terminal farnesylated peptide from γ1 was able to bind to and stabilize light-activated rhodopsin [67]. The details of the switch, characterized by mutational analysis and NMR structural studies, describe an unstructured C-terminus of γ that transforms into an amphipathic helix upon interaction with activated receptor [68, 69]. Three residues conserved across γ subunit isoforms, Asn62, Pro63 and Phe64, were proposed to be critical for the receptor-dependent conformational change in γ.
More recent experiments with protein in solution have begun to yield structural data on the nucleotide free empty state of a G protein activated by receptor. NMR studies with transducin and light-activated rhodopsin suggest that the empty state of transducin α is conformationally dynamic, a condition not attained in the absence of activated receptor [70]. The use of site-directed spin-labeling methods with Gi αGDP, Gi αGDPβγ and Gi αGTPγS has allowed even more precise characterization of structural changes in solution; experiments examining G protein activation by rhodopsin indicate that structural changes in Gi α are propagated via the switch I region to the αF-helix, in concert with movement of the α5 helix, which form part of the nucleotide-binding pocket [71]. While this conformational change facilitates GDP release, another interesting structural change resulting from the empty state is the formation of new contacts between α and βγ, suggested by the crystal structure. The presence of these new contacts between α and βγ in the empty state further support a receptor-dependent conformation of the empty heterotrimer distinct from Gi αGDPβγ.
Subunit Dissociation
Although receptor-dependent nucleotide exchange has been well established, the issue of subunit dissociation has been somewhat more controversial. In early experiments characterizing the biochemical nature of G proteins, purified transducin, Gi and Gs proteins activated with various combinations of aluminum, magnesium, fluoride, GDP, or GTP analogues and detergent could be dissociated into their constituent α and βγ dimers using a number of separation techniques [72,73,74]. The extrapolation that this phenomenon occurred in vivo was attractive for several reasons. For one, subunit dissociation provided a bifurcation of signal after activation of receptor; both α and βγ were free to regulate downstream effectors independently. The potential for α and βγ dimers to form new G protein combinations as a result of activation of several different receptors was yet another point where a cell could regulate signaling specificity. The molecular underpinnings to support the idea that subunit dissociation represented the mechanism of βγ activation, and reassociation of G protein served to inhibit βγ signaling, came from mutational studies that concluded that regions of βγ that activated effectors were found to overlap with G α-binding sites (fig.1) [75, 76]. These findings suggested that a βγ dimer in a heterotrimeric complex was in fact inactive, and that activation of βγ was synonymous with release from G α upon activation by receptor.
Interestingly, a study by Bonacci et al. [77] found that the region of the β subunit that interacts with the switch II region of G α could be targeted with peptides and small organic molecules to selectively disrupt interactions between βγ dimers and effectors; this strategy has been used inhibit inflammation in vivo by blocking βγ-mediated activation of PI3-kinase γ [78], and thus represents a promising area for pharmacological intervention.
Further evidence for subunit dissociation was revealed in the crystal structure of a Gq α-p63RhoGEF complex [79], in which p63RhoGEF interacted with the α2/β4 region, containing switch II, and the α3/β5 regions of Gq α. In addition, the crystal structure of activated Gs α in a complex with the catalytic domains of adenylyl cyclase also revealed effector-binding sites on Gs α to be the switch II region and the α3-β5 loop [80]. Since βγ binds the switch II region, the binding of p63RhoGEF and adenylyl cyclase to Gq α and Gs α, respectively, appears to be mutually exclusive to the binding of βγ, and supports the notion that subunit dissociation is a consequence of G protein activation. However, biochemical and kinetic arguments have been advanced to suggest that subunit dissociation is not necessary for G protein activation [81]. For example, expression of a fusion protein of G protein α and β subunits in yeast signaled as well as α and β subunits co-expressed individually [82]; since the fusion protein did not allow complete physical separation of α and β, a conformational change was proposed as a means for activation of the heterotrimer leading to signaling.
Fortunately, in the last several years sophisticated imaging techniques such as FRET (fluorescence resonance energy transfer) have emerged that allow the question of subunit dissociation to be more fully evaluated in live cells. FRET studies with receptor-dependent activation of fluorescently tagged G protein in Dictyostelium discoideum concluded that subunits did in fact dissociate upon activation [83], although a conformational change in the heterotrimer could not be completely ruled out. A similar FRET study with fluorescently tagged Gi α and β1γ2 in HEK cells concluded that the heterotrimer underwent a molecular rearrangement upon activation by receptor, but did not dissociate [84]. A comparison of heterotrimers consisting of β1γ2 and other members of the Gi subfamily using FRET found GO α appeared to dissociate from β1γ2 upon receptor stimulation, whereas Gi1,2,3 α or Gz α did not [85]; these differences in subunit dissociation were traced to several distinct regions in Gi1 α. Because the FRET signal is dependent on both proximity and orientation between two fluorophores, it became clear that the distinction between conformational change and physical dissociation of G protein subunits was not to be unequivocally resolved by FRET.
Another imaging technique called FRAP (fluorescence recovery after photobleaching) was more suited to address complete physical dissociation of G protein subunits, as fluorescence recovery is dependent on the movement of fluorescently tagged proteins into and out of bleached regions of the plasma membrane. In one FRAP study which examined G protein subunit dissociation by evaluation of the ability of fluorescently tagged, immobile GOA α, Gi3 α and Gs α subunits to constrain fluorescently tagged β1γ2[86], results were similar to the FRET studies described above. Consistent with the FRET experiments, receptor activation resulted in dissociation of GOA α from β1γ2; however, in these experiments, Gi3 α was also shown to dissociate from β1γ2. In contrast, the Gs α β1γ2 heterotrimer did not dissociate upon receptor stimulation [86]. Further evidence that subunit dissociation is not always necessary for G protein activation was provided by the discovery that the stability of complexes of effectors and heterotrimeric G proteins persisted even after activation of receptor [87]. Moreover, effector activity of Gq α was demonstrated to be augmented by βγ, independent of receptor activation [88].
If subunit dissociation does not occur, one striking paradox is how a βγ dimer can signal with effector-binding sites occluded by G α. Part of the answer is likely related to the three switch regions of G α (fig. 1) which undergo a conformational change upon exchange of GDP for GTP (GTPγS in the crystal structure) that leads to a decreased affinity of G α for βγ. However, an additional contact site suggested by the crystal structure between α and γ is the N-terminus of α and the C-terminus of γ, both modified by lipid. Biochemical evidence also supports this interaction, as myristoylated GO α has a higher affinity for βγ than GO α with an unmodified N-terminus [89]; interactions between the γ C-terminal prenyl group and a N-terminal lipid of α may also be strengthened if both modifications are inserted into the lipid membrane (fig. 1).
Thus, one model, often referred to as the ‘clam shell’, predicts that upon activation, α and βγ pull apart, perhaps with the lipid moieties inserted into the membrane serving as a hinge in the heterotrimer, enough for effector molecules to interact with the binding regions of βγ or α (fig. 1). This activation without complete subunit dissociation may be manifested in subtle conformational changes that may be difficult to detect using cellular imaging techniques. The rearrangement, but not dissociation of Gi α:βγ upon activation [84] discussed earlier may be an example of the clam shell model. Lack of, or more likely incomplete subunit dissociation implies that particular βγ dimers from heterotrimers such as Gs may not readily reassociate with other G α isoforms after activation by receptor. In a model where a receptor has unlimited access to all G proteins expressed in a cell [90], the question of preserving signaling specificity may be related to the extent to which a G protein dissociates upon activation, and subsequently, the potential to re-association with other subunit combinations.
Specificity of β/γ Interactions
Years of experimental data have led to the consensus that β and γ isoforms form a functional dimer that does not dissociate under physiological conditions. Thus, the activity of a βγ dimer is derived by the identity of both β and γ isoforms. To examine the functional diversity of βγ dimers, many experiments have aimed to discover which βγ dimers can physically form. In cell types with a restricted assortment of β and γ isoforms, such as expression of β1 and γ1 in rods and β3 and γ8 in cones, this approach was sufficient to estimate probable G protein subunit interactions. A more difficult question, especially in the context of expression of several β and γ isoforms in a single cell, is which βγ dimers actually form? Useful answers have come from studies dissecting receptor signaling pathways, discussed below; however, the most direct answer is revealed by the purification of G protein βγ isoforms from a specific tissue or cell type. Unfortunately, these are not practical experiments to undertake for the multitude of cell types and tissues that constitute an organism.
β1 and β2 Interactions with γ Isoforms
Several systems have been used to study β and γ interactions, including the yeast two-hybrid [91], in vitrotranslation [92,93,94] and transfected cell assays [95]. The conclusions generally held that for the first two β isoforms, β1 was the least restricted in its interactions with other γ isoforms, and β2 failed to interact with γ1 and γ11. The region of γ1 that inhibited dimer formation with β2 was ultimately determined to be the 3 amino acids at positions 38–40 of γ1[96]; conversely, replacement of these residues in γ2 with the analogous residues of γ1 also inhibited dimer formation with β2. More recent live cell-imaging techniques have confirmed much of the earlier literature, although in a noteworthy finding, β1 appeared to display the strongest preference for γ12[97].
β3 Interactions with γ Isoforms
Interpretation of results with β3 is more complex. Unlike dimers containing β1 or β2, dimers containing β3 are much less resistant to complete proteolysis by trypsin [7], and the β3 subunit has displayed weak or absent capacity to interact with γ subunits, including γ1 and γ2[92]. The β3 isoform does contain an additional tryptic cleavage site at lysine 177; however, in experiments with the more specific protease Arg-C, complete digestion of β3γ dimers was also observed [93]. On the other hand, β3 has been shown to co-precipitate with γ5, γ8cone or γ12[7], and a β3γ2 dimer with activity at receptor and effector has been purified using a baculovirus expression system [98]. The anomalous results obtained from studies with β3 suggest that the crystal structure of a G protein βγ dimer based on the β1 isoform may not necessarily predict subtle structural variations of βγ dimers containing other even highly homologous β isoforms.
β3 Splice Variant Interactions with γ Isoforms
Several β3 splice variants have been characterized: β3s, β3s2 and β3v. β3s, distinguished from β3 by deletion of 41 amino acids, or one entire WD-40 domain affecting blades three and four of the torus, is similar to β3 in γ-binding specificity [7]. However, purification of the β3s isoform from mammalian or insect cell expression systems has not been reported; in contrast to other βγ combinations, β3s was poorly extracted with 1% (v/v) Genapol, 1% (w/v) CHAPS, or 1% (w/v) cholate when expressed in Sf9 insect cells with either the γ2, γ5 or γ7 isoforms [98]. The β3s2 splice variant, characterized by a similar deletion that affects blades five and six, was also not found to be substantially different in γ-binding preference from β3[8]. However, the β3v splice variant, which lacks the last three blades of the β torus and has a unique C-terminal region, dimerizes with only the γ3 and γ12 isoforms [9]. The β3s and β3s2 splice variants have been found to be associated with a C825T polymorphism in the β3 gene that is associated with increased risk of hypertension [99]. Functional studies on the β3s protein suggest that the loss of 41 amino acids results in enhanced receptor-dependent G protein signaling [7], which may contribute to the etiology of hypertension.
β4 Interactions with γ Isoforms
The β4 isoform has been shown to interact similarly with all γ isoforms in precipitation experiments from in vitro expression systems [94, 100]. However, when βγ dimers were precipitated from bovine lung using specific γ antibodies, β4 clearly showed a preference for association with γ5 and γ12 over γ2 and γ3[101]. This is a good example of the difference between what βγ dimers can form in vitro and which dimers do actually form in vivo. Factors that influence such specificity in βγ formation may include chaperone proteins, which have recently been suggested to play a role in dimer formation between specific β and γ isoforms. For example, the Cytosolic Chaperonin Complex (CCT), which has been demonstrated to bind G β during βγ dimer biosynthesis, interacts most strongly with the β1 and β4 subunits, weakly with β5 and β3s, and intermediately with β2 and β3[102]. In addition, the Dopamine Receptor-interacting Protein 78 (DRiP78) has been characterized as a chaperone for G γ, and also exhibits specificity in binding with highest affinity to γ2 and γ3, compared to γ1, γ7 and γ11[103]. More detail on this emerging field as it relates to βγ dimer formation can be found in a recent review by Willardson and Howlett [104].
β5 Interactions with γ Isoforms
β5 occupies a special niche in the G β family both structurally and functionally. Early in its characterization, it was noted that dimers containing β5 and γ2[105], or any of several other γ isoforms [M.B. Jones, unpubl. observation] were highly unstable in certain detergents; this observation will be discussed further below. It was also observed that in addition to G γ subunits, the β5 isoform could bind to members of the R7 subfamily of Regulators of G Protein Signaling (RGS) proteins, and the crystal structure of a complex of β5 and RGS9 was recently solved [48]. The β5RGS literature is beyond the scope of this review, but see Berman and Gilman [4] and De Vries et al. [106] for other reviews. In terms of forming a conventional βγ dimer, β5 was shown to interact preferentially with γ4 by the yeast two-hybrid system [91], and live cell-imaging techniques suggested β5γ2 and β5γ7 are favored dimer combinations [107], although in the same system, β5 could interact with an RGS7 protein as well. The specificity observed in formation of βγ dimers with different β and γ isoforms suggests the capacity for extensive modulation of G protein signaling. Further, the fact that the β5 isoform can bind γ subunits, or alternatively members of the R7 subfamily of RGS proteins, indicates that other signaling pathways may be highly integrated into receptor-dependent βγ signaling.
Specificity of α/βγ Interactions
The G α isoform is necessary for the activation of a G βγ dimer, since specific localization of βγ to the plasma membrane has been reported to require both prenylation and heterotrimer formation [26]. Further, all three subunits are required for transfer of signal from activated receptor to G protein [108]. In addition, the identity of the Gα subunit may also determine the cellular mobility of an activated βγ dimer via its propensity to undergo subunit dissociation upon activation (discussed above). Considering the large number of α:β:γ subunit combinations that could potentially form, relatively little is known as to which heterotrimers can form, not to mention which actually do form in vivo. Limited subunit expression has provided information on probable heterotrimer combinations in different physiological systems. For example, in the visual system, Gt αβ1γ1 is likely to predominate in rods, whereas Gt αβ3γ8 is most prevalent in cones; in taste receptor cells, the most abundant isoforms are α-gustducin, β1, β3 and γ13[109].
Regulation of Heterotrimer Composition
From empirical observations, many studies characterizing various G α isoforms have used β1γ2 as the archetypal βγ dimer. The β4γ2 dimer was also observed to form a heterotrimer with GOA α [110]. Specificity has been reported in the βγ dimers associated with different GO isoforms purified from bovine brain, with the GOA heterotrimers containing much more γ7 than the GOC heterotrimers [111]; GOC α is distinguished structurally from GOA α by deamidation of Asn346 and Asn347 at the C-terminus [112]. However, βγ purified from GOA interacted equally well with the α subunits purified from GOA and GOC, as judged by γ7 immunoreactivity [113], suggesting that in this case, differences in the βγ composition observed between GOA and GOC were due to restricted expression of isoforms within cells or tissues. Transcriptional regulation is another mechanism that may regulate combinations of G α and βγ dimers; one study noted that G β4 mRNA was regulated by expression of Gs α, Gi3 α and G11 α [114]. Reduction of Golf α protein was also correlated with deletion of the G γ7 gene in mice [115]. Furthermore, genetic deletion of the γ1 gene in mice resulted in a greater than 25-fold reduction in Gt α and β1 protein levels in the retina, although interestingly, mRNA levels for Gt α and β1 were similar to wild-type mice [116]. These studies suggest that heterotrimer composition and formation are highly regulated at the levels of transcription, translation and posttranslational processing.
In one study that used immunofluorescence microscopy to compare the ability of a panel of βγ dimers to target mutant Gs α and Gq α to plasma membranes as an indication of heterotrimer formation, βγ dimers containing β1 or β2 were equally effective at interacting with either Gs or Gq α isoforms [117]; βγ dimers containing β3 did not interact well with Gs α or Gq α, and βγ dimers containing β4 were able to interact with Gs α, but not Gq α. Interactions between Gs α or Gq α and βγ dimers containing G β5 were not observed [117]. On the other hand, a study that used live cell-imaging techniques observed that both GO α and Gq α could target β5γ2 to the plasma membrane [107]. Moreover, there is also a report of Gq α from brain extract binding a β5γ2 affinity column [118]. The presence of a receptor may also facilitate interactions between specific G α and βγ isoforms. For example, in contrast to co-localization studies with only G protein subunits [117], purified β4γ2 was able to couple Gq α to the M1 muscarinic receptor [119], and β3γ2 was able to couple Gs α to the β1-adrenergic and adenosine A2A receptors [98]. Furthermore, the β5γ2 dimer has also been demonstrated to couple Gq α to the M1 muscarinic receptor [120], and weakly couple Gs α to the β1-adrenergic receptor [98].
Receptor-Dependent Translocation of βγ
The βγ dimer has been shown to translocate upon receptor stimulation. In perfused rat hearts, stimulation of the β1-adrenergic receptor induced the translocation of the β3 subunit from cytosol to membranes [121]; no such effect was observed for the β1 or β2 subunits, which were predominantly in the membrane fraction. It should be noted that the γ isoforms identified with the β1, β2 and β3 subunits were not characterized, and thus it is possible that both β and γ isoforms contributed to the translocation of β3γ.
The γ subunit is also a determinant of which βγ dimers undergo receptor-dependent translocation. Live cell-imaging experiments revealed that βγ dimers containing γ1, γ11 and γ9 translocate rapidly to the Golgi membranes upon receptor stimulation, βγ dimers containing γ5 and γ10 translocate slowly, and βγ dimers containing γ2, γ3, γ4, γ7, γ8 and γ12 do not translocate [122, 123]; the translocation was observed to be reversed upon addition of a receptor antagonist. Interestingly, although γ1, γ11 and γ9 are all farnesylated, the geranylgeranylated γ13 also translocated rapidly to the endoplasmic reticulum [123]. This behavior of γ13 reflects the study's finding that βγ translocation occurs as a reversible, diffusion-mediated process that is related to the amino acid sequence of the γ isoform, and not the identity of the prenyl group [123]. Within the family of βγ dimers that translocate, the α subunit also has influence on the rate of translocation, primarily related to the nucleotide exchange rates of the α subunits [124]. One important point to make with respect to receptor-dependent βγ translocation is that it can occur independently of G α [122], and thus represents an example of complete G protein subunit dissociation (see discussion above) determined by the nature of the γ isoform in a heterotrimer. Subsequent studies have observed that even in the absence of receptor activation, there is a basal level of heterotrimer shuttling between plasma and intracellular membranes [125]. The discovery of receptor-dependent βγ translocation is important in that it increases the complexity of the spatial dimension to βγ signaling, and suggests that cellular localization of βγ dimers after G protein activation is tightly controlled by the identity of the β and γ isoforms in a G protein.
Exceptions to the Rule
Receptor-Independent G Protein Activation
The review to this point has discussed the conventional wisdom regarding the molecular determinants that influence activation of a βγ dimer, which is usually preceded by partial or complete dissociation from G α. As discussed above, the βγ dimer is capable of dynamic translocation in the absence of G α, suggesting that activity may not be limited to the plasma membrane. Activation of a βγ dimer has been proposed to occur in the absence of receptor, or nucleotide exchange, via direct interaction by an Activator of G protein Signaling protein, AGS8 [126]; this activation was also suggested to not require subunit dissociation [127]. Another mechanism proposed for G protein activation is the absence of receptor involves phosphorylation of histidine 266 of β by NDPK; the phosphate is subsequently transferred onto the GDP bound to G α, effectively producing a GTP-bound activated α subunit without the requirement of receptor catalyzed release of GDP [15]. The discovery of G βγ in the endoplasmic reticulum (ER) of Arabidopsis led one researcher to speculate that βγ has signaling functions in the ER, such as regulation of PLC or IP3 receptors [128], independent of heterotrimer formation with G α [129]. This is an intriguing hypothesis, considering that RACK1, a G β-like scaffolding protein, has been shown to bind both βγ [130] and IP3 receptors [131]. Moreover, the ability of RACK1 to regulate βγ signaling, such as attenuation of PLC-β2 activation [132], provides a potential mechanism for G βγ signaling to occur without G α or activated receptor.
Stability of βγ Dimers: Unconventional Roles for β…γ
Deviating further still from conventional wisdom is the biological significance of instability or low affinity between particular combinations of β and γ. The model of the tightly associated βγ dimer that couples a G α subunit to an activated receptor is based largely on studies with β1γ1 or β1γ2. However, other less stable combinations such as β5γ2 and β4γ11[119] are less amenable to biochemical studies, and thus, instability could easily be interpreted as incompatibility between particular combinations of β and γ isoforms. The high degree of heterogeneity inherent in the potential combinations of βγ dimers, and the spectrum of biophysical properties suggest that unconventional signaling paradigms for β and γ exist. It may be useful in this speculative exercise to first consider examples of potential dissociation between β and γ that have been reported in the literature.
βγ Instability in vitro
Examples of low affinity of β for γ can be found with many of the β isoforms. Lower affinity of β3 for γ subunits has been reported in several systems examining βγ formation, such as in vitro translation and yeast two-hybrid [91, 92, 94], although after purification from Sf9 cells with γ2, the β3γ2 dimer was completely stable in several different detergents [105]. More evidence exists for the instability of β5 with γ subunits, and the biochemical properties of a β5 monomer. Attempts to purify the β5γ2 dimer revealed that it was sensitive to detergent, and high concentrations of CHAPS or cholate induced subunit dissociation [105]. Other β5γ dimers containing γ1, γ7, γ10 and γ12 could not be purified even at low (0.1% Genapol) detergent concentration [Miller B. Jones, doct. thesis]. Whereas the β1 subunit forms unstable high-molecular-weight aggregates in the absence of γ [133], the β5 subunit can exist as a monomer which is highly resistant tryptic cleavage [134].
Instances of βγ instability related to γ isoforms have also been reported. One early study characterizing the purification of G protein from bovine brain reported monomeric γ3 under non-denaturing conditions [36]. The γ11 isoform has also been noted for its propensity to dissociate from the β during purification [37], indicating a weak interaction. Parameters affecting G βγ11 affinity were further explored with recombinant heterotrimers consisting of Gi1 α β1γ11 and Gi1 α β4γ11, both of which were stable upon purification; however, activation of the heterotrimers with GTPγS resulted in a significant dissociation of β4 and γ11, but not β1 and γ11[119]. Immunoprecipitation experiments examining βγ dimers containing the γ13 isoform found that β1, β3 and β4 could be immunoprecipitated with a hemagglutinin tagged γ13 subunit, whereas the β2 and β5 subunits could not [135]; the authors speculated that the βγ instability was due to detergents used in the immunoprecipitation. This instability of β2γ13 and β5γ13 was not indicative of incompatibility between subunits, as these dimers were found to be effective in the activation and inhibition, respectively, of GIRK1/4 channels in transfection experiments [135]. Further, live cell-imaging techniques were used to observe that β2 and γ13 could effectively translocate together following receptor activation [123].
Potential Biological Activity of β and γ Monomers
Biochemical evidence of instability in particular combinations of βγ dimers in vitro logically leads to questions of whether monomeric β or γ subunits exist, and what they may be doing in a cell. Overexpression of two of the β3 splice variants, β3s and β3s2, was shown to markedly stimulate MAP-kinase activation [8]; interestingly, co-expression of known γ partners for these β3 splice variants had no further effect on MAP-kinase activity. These data suggest that the β3 splice variants may have biological activity in the absence of γ. Moreover, the authors of the study offer the possibility that another β3 splice variant with restricted γ-binding partners, β3v, may exist as a monomer [9]. One study established that purified β5 and γ2 monomers can be reconstituted to form a dimer with the ability to active PLC-β2 in vitro [134]; the study further demonstrated that monomeric β5 was able to functionally interact with Gi α and GO α. Another report characterized the ability γ2 expressed in Sf9 cells to bind to a GO α column, and protect the α subunit from tryptic cleavage [136]. Functional activity of a monomeric γ5 subunit was also suggested in a study that demonstrated the ability of a bacterially expressed non-prenylated γ5 subunit to regulate transcription by binding to the adipocyte enhancer-binding protein (AEBP1) transcriptional repressor [34]. Although β protein was also observed in co-immunoprecipitation studies with γ5 and AEBP1 in mammalian cells, the data support the idea of transcriptional activity of a γ5 monomer, perhaps in addition to βγ5 dimers in the nucleus. Absence of prenylation, as discussed above, may not be the only signal to direct particular βγ dimers to the nucleus; a study examining the localization of fluorescently tagged β5 co-expressed in HEK-293 cells with various fluorescently tagged γ isoforms concluded that the γ1, γ5, γ10 and γ11 isoforms targeted β5 to the nucleus, whereas γ2 and γ7 did not [107]. Since most of the γ isoforms that targeted β5 to the nucleus could not be purified as a complex with β5 even under conditions of low detergent stringency (see above), the observed differences among β5γ dimers in the context of localization suggests that dimer instability is related to βγ signaling roles.
Receptor:γ Interactions
Another potential biological role for γ subunits can be inferred from studies characterizing C-terminal γ peptides with activated receptor. A C-terminal farnesylated γ1 peptide was reported to stabilize the active form of rhodopsin [56], and a C-terminal geranylgeranylated peptide corresponding to γ5, but not γ7 or γ12, was able to inhibit M2 muscarinic receptor signaling [137]. Further receptor-binding experiments revealed that the γ5 peptide was able to stabilize a novel state of the M2 muscarinic receptor [138]. These studies suggest an interaction between the C-terminal tail of γ and receptors. However, the notion that receptor may not interact with the C-terminal tail of γ during G protein signaling was supported by reconstitution studies that found that a β1γ1 dimer engineered with a photoreactive farnesyl analogue was cross-linked to phospholipid, but not receptor, after reconstitution of transducin with rhodopsin in membranes [139]. The discrepancy between peptide and whole protein studies was also highlighted by the fact that the M2 muscarinic receptor more efficiently stimulated GTP hydrolysis in a GOαβ1γ7 heterotrimer compared to a GOαβ1γ5 heterotrimer [140]. This raises the possibility that γ peptides may have properties distinct from the analogous βγ dimer at receptor, and more importantly, begs the question regarding the biological activity of a monomeric γ subunit: If a γ peptide can functionally interact with receptor, would a γ subunit, which has also been reported to exist as a monomer in the absence of β or detergent [141], have similar binding and regulatory properties at receptor?
Receptor:β Interactions
Less complex biological systems may also inform the prospective roles of β and γ in G protein signaling. For example, in fission yeast Schizosaccharomyces pombe, the β subunit, which lacks an amino terminal coiled-coil domain, retains some activity when its γ partner is deleted [142]; further, when the C-terminal CAAX box from the γ subunit is fused onto the C-terminus of the β subunit, some activity is recovered, suggesting more of a targeting role for γ in that system. This result was mirrored in studies with mutant yeast γ subunits in Saccharomyces cerevisiae, which found that a C-terminal domain preceding the CAAX box was not required for βγ coupling to receptor in vivo [143]. Recently, another study in S. cerevisiae also found that the RACK1 ortholog Asc1 could bind and influence the nucleotide-binding properties of Gα, and essentially perform the role of a β subunit, presumably without the presence of γ [144].
There are several instances that suggest that interactions between β and γ and receptor in higher vertebrates may be more dynamic than previously thought. Expression of β3s, but not β3, was shown to be able to activate Gα subunits in the presence of mastoparan-7 in digitonin-permeabilized COS-7 cells [7]; the authors reasoned that the β3s protein dimerized with endogenous γ subunits. Such an experiment suggests the existence of monomeric β and γ subunits in vivo, because either there is biological activity of an expressed β3 splice variant, or alternatively, a pool of γ isoforms ready to dimerize with an ectopically expressed β subunit. Moreover, purification of βγ from rod outer segments of Bufo marinus yielded a small population of free β with no γ partner [145]; interestingly, the β subunit, noted for its high homology with bovine β1, was as effective as the purified βγ at stimulating GTPγS binding to Gα after reconstitution with illuminated rod outer segment disc membranes. Possibly related to the results of β1 activity in B. marinus, a γ1 knockout mouse retained some ability of rhodopsin to signal through Gt in rod outer segments with only residual amount of Gt α and β1 remaining [116]. Since no upregulation of other β or γ isoforms was observed, and no other γ isoforms were detected in immunoprecipitations of β1, one possible explanation (not advanced by the authors) is that the residual β1 may be able to substitute on some level for the β1γ1 dimer. Although examples of β coupling G α to receptor likely represent a small minority of signaling paradigms in higher vertebrates, they may shed light on the interactions of unstable βγ dimer combinations with receptors and effectors, and prove useful in providing a way of dissecting the contribution of β and γ to G protein signaling.
Receptor/βγ Interactions Determine Signaling Specificity
Specificity of receptor:G protein interactions can be influenced by the identity of each of the subunit isoforms. Thus, the assembly of a βγ dimer with an α subunit to form a heterotrimer of defined composition within a cell functions to impart a degree of specificity by limiting the number of signaling pathways in which the G protein can directly participate. The first evidence suggesting that the identity of the β and γ isoforms in a heterotrimer can influence receptor coupling were based on experiments using antisense oligonucleotides to attenuate expression of specific subunit isoforms. Inhibition of voltage-sensitive Ca2+ channels in rat GH3 cells was reported to be mediated via coupling of the GO1 αβ3γ4 heterotrimer to the muscarinic receptor and coupling of the GO2αβ1γ3 heterotrimer to the somatostatin receptor [146,147,148]. The antisense approach was further employed to suggest that in rat portal vein myocytes, the angiotensin AT1A receptor couples to G13αβ1γ3[149], and the ETA receptor couples to G11 αβ3γ5[150].
Prenyl Status
The type of prenyl moiety on the γ subunit has also been shown to be critical for receptor G protein interactions. Experiments involving rhodopsin have generated the most conflicting reports; on the one hand, the farnesyl group was observed to interact more efficiently than the geranylgeranyl group with activated rhodopsin [151, 152]. Alternatively, a mutated β1γ1 dimer that incorporated the geranylgeranyl group instead of the farnesyl group was found to have a 3-fold higher affinity for rhodopsin than wild-type β1γ1[153]. These results were mirrored in other studies that characterized β1γ1 and β1γ2 dimers that were mutated to alter the specificity of prenylation; farneslyation of β1γ2 reduced its affinity for the adenosine A1 receptor, and conversely, geranylgeranylation of β1γ1 increased its ability to couple to the same receptor [154]. Farnesylation, however, does not always result in a decreased affinity of βγ for receptor. In the case of β1γ11, which like β1γ1 is farnesylated, high receptor coupling efficiency was observed with the α2A-adrenergic receptor [155], adenosine A1 and 5-HT1A receptors [156] and the M1 muscarinic receptor [119]. These results suggest that the differences between farnesylation and geranylgeranylation of βγ dimers reflect more than degrees of hydrophobicity of a membrane anchor, and the identity of the prenyl group is intimately related to activation of a G protein, and hence βγ, by receptor.
γ Isoform Specificity
Thus, the primary sequence of γ is also critical for determining the efficiency of receptor:G protein interactions. This point was borne out in studies with chimeras of γ1 and γ2, which concluded that the C-terminal third of γ, along with the type of prenyl group, is particularly important at determining the affinity of G protein receptor interactions [58, 153]. Studies with peptides constructed with the C-terminal sequence of a geranylgeranylated γ5 subunit reached similar conclusions after these peptides were found to inhibit M2 muscarinic receptor signaling [137]. The γ5 C-terminal sequence apparently confers specificity in receptor:G protein interactions, as neither γ7 nor γ12 C-terminal peptides were able to inhibit muscarinic receptor signaling [137]. A specific signaling role for γ7 has however, been assigned to the β-adrenergic receptor using a ribozyme strategy to attenuate γ7 mRNA and protein levels [157, 158]; the β1 isoform was also suggested as the partner for γ7 in this signaling cascade, as β1 protein levels fell in response to loss of γ7 protein [158]. The ribozyme approach was also used to demonstrate that the β1 and γ7 isoforms are involved in activation of adenylyl cyclase via coupling to the D1 dopamine receptor, but not the D5 dopamine receptor [159]. The identity of γ also influences how a β isoform can interact with receptors. For example, the β1γ5 dimer couples poorly to the α2A-adrenergic receptor, whereas the β3γ5 dimer couples effectively; however, when the γ isoform is changed to γ11, the resulting β1γ11 dimer couples almost as well as β3γ5[155], suggesting that the receptor coupling efficiency of β or γ isoforms can be interrelated.
β Isoform Specificity
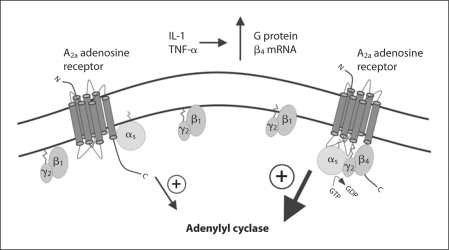
The identity of the β isoform has also been shown to influence interactions between receptor and G proteins. Attenuation of specific β isoforms using RNAi has indicated that the β2 isoform, but not β1, is involved in C5a-mediated chemotaxis in mouse macrophages [160]. In reconstitution experiments, the M2 muscarinic receptor was more efficient in catalyzing nucleotide exchange with an GO αβ4γ2 heterotrimer compared to a GO αβ1γ2 heterotrimer [161]. The β4 subunit as a dimer with γ2 was also noted to have a greater ability to couple Gs α to the adenosine A2A receptor than β1γ2[98], and shift a larger percentage of A2A receptors into the high affinity binding state than β1γ2[162]. Interestingly, the inflammatory cytokines IL-1 and TNF-α were demonstrated to upregulate G β4, but not G β1 mRNA levels in human dermal microvascular endothelialcells [163]. Figure 2 illustrates how this regulation of G β4, and the observed differences in adenosine A2A receptor coupling between β1γ2 and β4γ2 may affect second messenger levels in cells. If the β1γ2 dimer predominates over β4γ2, Gs α may couple poorly to the adenosine A2A receptor; however, an inflammatory stimulus could produce cytokines that elevate G β4 levels, thus increasing the availability of β4γ2 and enhancing the ability of Gs α to couple to the adenosine A2A receptor, activate adenylyl cyclase and raise cAMP levels.
Fig. 2.
Effect of different βγ isoforms on A2a adenosine receptor signaling. β1γ2 couples Gs α poorly to the A2a receptor, leading to lower activation of adenylyl cyclase; increasing levels of β4γ2, which couples Gs α more efficiently to the A2a receptor, increases adenylyl cyclase activation and intracellular cAMP levels.
Conclusion
In conclusion, in the scope of this review, there are multiple factors that determine the activation of a particular βγ dimer within a cell: (1) Transcription, translation and posttranslational processing of specific β and γ isoforms, together with protein:protein interactions determine the constellation and potential localization of βγ dimer combinations. (2) Expression of specific G α subunit isoforms, likely integrated to specific β and γ isoform expression, determines the identity of heterotrimer combinations, thus affecting interactions with receptors, and the degree to which a G protein may dissociate upon activation, and potentially reform with different G α isoforms. (3) Expression and activation of specific receptors, which through preferences for binding particular heterotrimer combinations, ultimately starts the signal which leads to activation of specific βγ dimers. The crystallization of a receptor G protein complex would greatly enhance our knowledge of the specificity of receptor:α:βγ interactions.
Acknowledgements
The author would like to thank Dr. James Garrison for comments helpful in the preparation of the manuscript. This study was supported in part by grants RO1-DK-19952 from the National Institutes of Health and 0535350N from the American Heart Association.
References
- 1.International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 2.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 3.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 4.Berman DM, Gilman AG. Mammalian RGS proteins: barbarians at the gate. J Biol Chem. 1998;273:1269–1272. doi: 10.1074/jbc.273.3.1269. [DOI] [PubMed] [Google Scholar]
- 5.Downes GB, Gautam N. The G protein subunit gene families. Genomics. 1999;62:544–552. doi: 10.1006/geno.1999.5992. [DOI] [PubMed] [Google Scholar]
- 6.Hurowitz EH, Melnyk JM, Chen YJ, Kouros-Mehr H, Simon MI, Shizuya H. Genomic characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes. DNA Res. 2000;7:111–120. doi: 10.1093/dnares/7.2.111. [DOI] [PubMed] [Google Scholar]
- 7.Rosskopf D, Koch K, Habich C, Geerdes J, Ludwig A, Wilhelms S, Jakobs KH, Siffert W. Interaction of Gbeta3s, a splice variant of the G-protein Gbeta3, with Ggamma. Cell Signal. 2003;15:479–488. doi: 10.1016/s0898-6568(02)00140-7. [DOI] [PubMed] [Google Scholar]
- 8.Rosskopf D, Manthey I, Habich C, Kielbik M, Eisenhardt A, Nikula C, Urban M, Kohnen S, Graf E, Ravens U, Siffert W. Identification and characterization of G beta 3s2, a novel splice variant of the G-protein beta 3 subunit. Biochem J. 2003;371:223–232. doi: 10.1042/BJ20021208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosskopf D, Kielbik M, Manthey I, Bilmen G, Eisenhardt A, Siffert W. Characterization of the splice variant Gbeta3v of the human G-protein Gbeta3 subunit. Biochim Biophys Acta. 2003;1626:33–42. doi: 10.1016/s0167-4781(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 10.Watson AJ, Aragay AM, Slepak VZ, Simon MI. A novel form of the G protein beta subunit Gbeta5 is specifically expressed in the vertebrate retina. J Biol Chem. 1996;271:28154–28160. doi: 10.1074/jbc.271.45.28154. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrandt JD. Role of subunit diversity in signaling by heterotrimeric G proteins. Biochem Pharmacol. 1997;54:325–339. doi: 10.1016/s0006-2952(97)00269-4. [DOI] [PubMed] [Google Scholar]
- 12.Cook LA, Schey KL, Wilcox MD, Dingus J, Ettling R, Nelson T, Knapp DR, Hildebrandt JD. Proteomic analysis of bovine brain G protein gamma subunit processing heterogeneity. Mol Cell Proteomics. 2006;5:671–685. doi: 10.1074/mcp.M500223-MCP200. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda T, Takao T, Shimonishi Y, Murata M, Asano T, Yoshizawa T, Fukada Y. Characterization of interactions between transducin alpha/beta gamma-subunits and lipid membranes. J Biol Chem. 1994;269:30358–30363. [PubMed] [Google Scholar]
- 14.Wieland T, Nurnberg B, Ulibarri I, Kaldenberg-Stasch S, Schultz G, Jakobs KH. Guanine nucleotide-specific phosphate transfer by guanine nucleotide-binding regulatory protein beta-subunits. Characterization of the phosphorylated amino acid. J Biol Chem. 1993;268:18111–18118. [PubMed] [Google Scholar]
- 15.Cuello F, Schulze RA, Heemeyer F, Meyer HE, Lutz S, Jakobs KH, Niroomand F, Wieland T. Activation of heterotrimeric G proteins by a high energy phosphate transfer via nucleoside diphosphate kinase (NDPK) B and Gbeta subunits. Complex formation of NDPK B with Gbeta gamma dimers and phosphorylation of His-266 IN Gbeta. J Biol Chem. 2003;278:7220–7226. doi: 10.1074/jbc.M210304200. [DOI] [PubMed] [Google Scholar]
- 16.Lupi R, Corda D, Di Girolamo M. Endogenous ADP-ribosylation of the G protein beta subunit prevents the inhibition of type 1 adenylyl cyclase. J Biol Chem. 2000;275:9418–9424. doi: 10.1074/jbc.275.13.9418. [DOI] [PubMed] [Google Scholar]
- 17.Lupi R, Dani N, Dietrich A, Marchegiani A, Turacchio S, Berrie CP, Moss J, Gierschik P, Corda D, Di Girolamo M. Endogenous mono-ADP-ribosylation of the free Gbetagamma prevents stimulation of phosphoinositide 3-kinase-gamma and phospholipase C-beta2 and is activated by G-protein-coupled receptors. Biochem J. 2002;367:825–832. doi: 10.1042/BJ20020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 19.Lai RK, Perez-Sala D, Canada FJ, Rando RR. The gamma subunit of transducin is farnesylated. Proc Natl Acad Sci USA. 1990;87:7673–7677. doi: 10.1073/pnas.87.19.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamane HK, Farnsworth CC, Xie HY, Howald W, Fung BK, Clarke S, Gelb MH, Glomset JA. Brain G protein gamma subunits contain an all-trans-geranylgeranylcysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci USA. 1990;87:5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mumby SM, Casey PJ, Gilman AG, Gutowski S, Sternweis PC. G protein gamma subunits contain a 20-carbon isoprenoid. Proc Natl Acad Sci USA. 1990;87:5873–5877. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- 23.Fukada Y, Matsuda T, Kokame K, Takao T, Shimonishi Y, Akino T, Yoshizawa T. Effects of carboxyl methylation of photoreceptor G protein gamma subunit in visual transduction. J Biol Chem. 1994;269:5163–5170. [PubMed] [Google Scholar]
- 24.Rehm A, Ploegh HL. Assembly and intracellular targeting of the betagamma subunits of heterotrimeric G proteins. J Cell Biol. 1997;137:305–317. doi: 10.1083/jcb.137.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simonds WF, Butrynski JE, Gautam N, Unson CG, Spiegel AM. G-protein beta gamma dimers. Membrane targeting requires subunit coexpression and intact gamma C-A-A-X domain. J Biol Chem. 1991;266:5363–5366. [PubMed] [Google Scholar]
- 26.Takida S, Wedegaertner PB. Heterotrimer formation, together with isoprenylation, is required for plasma membrane targeting of Gbeta gamma. J Biol Chem. 2003;278:17284–17290. doi: 10.1074/jbc.M213239200. [DOI] [PubMed] [Google Scholar]
- 27.Hynes TR, Tang L, Mervine SM, Sabo JL, Yost EA, Devreotes PN, Berlot CH. Visualization of G protein betagamma dimers using bimolecular fluorescence complementation demonstrates roles for both beta and gamma in subcellular targeting. J Biol Chem. 2004;279:30279–30286. doi: 10.1074/jbc.M401432200. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JB, Casey PJ. In vitro processing of recombinant G protein gamma subunits. Requirements for assembly of an active beta gamma complex. J Biol Chem. 1994;269:9067–9073. [PubMed] [Google Scholar]
- 29.Cook LA, Schey KL, Wilcox MD, Dingus J, Hildebrandt JD. Heterogeneous processing of a G protein gamma subunit at a site critical for protein and membrane interactions. Biochemistry. 1998;37:12280–12286. doi: 10.1021/bi980230e. [DOI] [PubMed] [Google Scholar]
- 30.Kilpatrick EL, Hildebrandt JD. Sequence dependence and differential expression of Ggamma5 subunit isoforms of the heterotrimeric G proteins variably processed after prenylation in mammalian cells. J Biol Chem. 2007;282:14038–14047. doi: 10.1074/jbc.M701338200. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Benard O, Margolskee RF. Ggamma13 interacts with PDZ domain-containing proteins. J Biol Chem. 2006;281:11066–11073. doi: 10.1074/jbc.M600113200. [DOI] [PubMed] [Google Scholar]
- 32.Kino T, Tiulpakov A, Ichijo T, Chheng L, Kozasa T, Chrousos GP. G protein beta interacts with the glucocorticoid receptor and suppresses its transcriptional activity in the nucleus. J Cell Biol. 2005;169:885–896. doi: 10.1083/jcb.200409150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kino T, Kozasa T, Chrousos GP. Statin-induced blockade of prenylation alters nucleocytoplasmic shuttling of GTP-binding proteins gamma2 and beta2 and enhances their suppressive effect on glucocorticoid receptor transcriptional activity. Eur J Clin Invest. 2005;35:508–513. doi: 10.1111/j.1365-2362.2005.01539.x. [DOI] [PubMed] [Google Scholar]
- 34.Park JG, Muise A, He GP, Kim SW, Ro HS. Transcriptional regulation by the gamma5 subunit of a heterotrimeric G protein during adipogenesis. EMBO J. 1999;18:4004–4012. doi: 10.1093/emboj/18.14.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ovchinnikov I, Lipkin VM, Telezhinskaia IN, Shuvaeva TM, Obukhov AN. Primary structure of the gamma[-]subunit of the GTP[-]binding protein from the bovine retina (in Russian) Bioorg Khim. 1985;11:1301–1314. [PubMed] [Google Scholar]
- 36.Morishita R, Kato K, Asano T. A brain-specific gamma subunit of G protein freed from the corresponding beta subunit under non-denaturing conditions. FEBS Lett. 1994;337:23–26. doi: 10.1016/0014-5793(94)80622-5. [DOI] [PubMed] [Google Scholar]
- 37.Morishita R, Ueda H, Kato K, Asano T. Identification of two forms of the gamma subunit of G protein, gamma10 and gamma11, in bovine lung and their tissue distribution in the rat. FEBS Lett. 1998;428:85–88. doi: 10.1016/s0014-5793(98)00498-0. [DOI] [PubMed] [Google Scholar]
- 38.Wilcox MD, Schey KL, Busman M, Hildebrandt JD. Determination of the complete covalent structure of the gamma 2 subunit of bovine brain G proteins by mass spectrometry. Biochem Biophys Res Commun. 1995;212:367–374. doi: 10.1006/bbrc.1995.1979. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton MH, Cook LA, McRackan TR, Schey KL, Hildebrandt JD. Gamma 2 subunit of G protein heterotrimer is an N-end rule ubiquitylation substrate. Proc Natl Acad Sci USA. 2003;100:5081–5086. doi: 10.1073/pnas.0831228100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morishita R, Nakayama H, Isobe T, Matsuda T, Hashimoto Y, Okano T, Fukada Y, Mizuno K, Ohno S, Kozawa O, et al. Primary structure of a gamma subunit of G protein, gamma 12, and its phosphorylation by protein kinase C. J Biol Chem. 1995;270:29469–29475. doi: 10.1074/jbc.270.49.29469. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda H, Lindorfer MA, Myung CS, Garrison JC. Phosphorylation of the G protein gamma12 subunit regulates effector specificity. J Biol Chem. 1998;273:21958–21965. doi: 10.1074/jbc.273.34.21958. [DOI] [PubMed] [Google Scholar]
- 42.Asano T, Morishita R, Ueda H, Asano M, Kato K. GTP-binding protein gamma12 subunit phosphorylation by protein kinase C – identification of the phosphorylation site and factors involved in cultured cells and rat tissues in vivo. Eur J Biochem. 1998;251:314–319. doi: 10.1046/j.1432-1327.1998.2510314.x. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Higuera I, Fenoglio J, Li Y, Lewis C, Panchenko MP, Reiner O, Smith TF, Neer EJ. Folding of proteins with WD-repeats: comparison of six members of the WD-repeat superfamily to the G protein beta subunit. Biochemistry. 1996;35:13985–13994. doi: 10.1021/bi9612879. [DOI] [PubMed] [Google Scholar]
- 44.Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature. 1996;379:369–374. doi: 10.1038/379369a0. published erratum appears in Nature 1996;379:847. [DOI] [PubMed] [Google Scholar]
- 45.Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. The structure of the G protein heterotrimer Gi alpha 1 beta 1 gamma 2. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 46.Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 A crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 47.Garritsen A, van Galen PJ, Simonds WF. The N-terminal coiled-coil domain of beta is essential for gamma association: a model for G-protein beta gamma subunit interaction. Proc Natl Acad Sci USA. 1993;90:7706–7710. doi: 10.1073/pnas.90.16.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheever ML, Snyder JT, Gershburg S, Siderovski DP, Harden TK, Sondek J. Crystal structure of the multifunctional Gbeta5-RGS9 complex. Nat Struct Mol Biol. 2008;15:155–162. doi: 10.1038/nsmb.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambright DG, Noel JP, Hamm HE, Sigler PB. Structural determinants for activation of the alpha-subunit of a heterotrimeric G protein. Nature. 1994;369:621–628. doi: 10.1038/369621a0. [DOI] [PubMed] [Google Scholar]
- 50.Noel JP, Hamm HE, Sigler PB. The 2.2 Å crystal structure of transducin-alpha complexed with GTP gamma S. Nature. 1993;366:654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- 51.Sprang SR. G protein mechanisms: insights from structural analysis. Annu Rev Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 52.Katada T, Bokoch GM, Northup JK, Ui M, Gilman AG. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Properties and function of the purified protein. J Biol Chem. 1984;259:3568–3577. [PubMed] [Google Scholar]
- 53.Loew A, Ho YK, Blundell T, Bax B. Phosducin induces a structural change in transducin beta gamma. Structure. 1998;6:1007–1019. doi: 10.1016/s0969-2126(98)00102-6. [DOI] [PubMed] [Google Scholar]
- 54.Hamm HE, Deretic D, Arendt A, Hargrave PA, Koenig B, Hofmann KP. Site of G protein binding to rhodopsin mapped with synthetic peptides from the alpha subunit. Science. 1988;241:832–835. doi: 10.1126/science.3136547. [DOI] [PubMed] [Google Scholar]
- 55.Martin EL, Rens-Domiano S, Schatz PJ, Hamm HE. Potent peptide analogues of a G protein receptor-binding region obtained with a combinatorial library. J Biol Chem. 1996;271:361–366. doi: 10.1074/jbc.271.1.361. [DOI] [PubMed] [Google Scholar]
- 56.Kisselev OG, Ermolaeva MV, Gautam N. A farnesylated domain in the G protein gamma subunit is a specific determinant of receptor coupling. J Biol Chem. 1994;269:21399–21402. [PubMed] [Google Scholar]
- 57.Kisselev OG, Downs MA. Rhodopsin-interacting surface of the transducin gamma subunit. Biochemistry. 2006;45:9386–9392. doi: 10.1021/bi060806x. [DOI] [PubMed] [Google Scholar]
- 58.Myung CS, Lim WK, Defilippo J, Yasuda H, Neubig R, Garrison JC. Regions in the G protein gamma subunit Important for interaction with receptors and effectors. Mol Pharmacol. 2005;69:877–887. doi: 10.1124/mol.105.018994. [DOI] [PubMed] [Google Scholar]
- 59.Taylor JM, Jacob-Mosier GG, Lawton RG, VanDort M, Neubig RR. Receptor and membrane interaction sites on Gbeta. A receptor-derived peptide binds to the carboxyl terminus. J Biol Chem. 1996;271:3336–3339. doi: 10.1074/jbc.271.7.3336. [DOI] [PubMed] [Google Scholar]
- 60.Onrust R, Herzmark P, Chi P, Garcia PD, Lichtarge O, Kingsley C, Bourne HR. Receptor and betagamma binding sites in the alpha subunit of the retinal G protein transducin. Science. 1997;275:381–384. doi: 10.1126/science.275.5298.381. published erratum appears in Science 1997;276:341. [DOI] [PubMed] [Google Scholar]
- 61.Iiri T, Farfel Z, Bourne HR. G-protein diseases furnish a model for the turn-on switch. Nature. 1998;394:35–38. doi: 10.1038/27831. [DOI] [PubMed] [Google Scholar]
- 62.Lichtarge O, Bourne HR, Cohen FE. Evolutionarily conserved Galphabetagamma binding surfaces support a model of the G protein-receptor complex. Proc Natl Acad Sci USA. 1996;93:7507–7511. doi: 10.1073/pnas.93.15.7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rondard P, Iiri T, Srinivasan S, Meng E, Fujita T, Bourne HR. Mutant G protein alpha subunit activated by Gbeta gamma: a model for receptor activation? Proc Natl Acad Sci USA. 2001;98:6150–6155. doi: 10.1073/pnas.101136198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cherfils J, Chabre M. Activation of G-protein Galpha subunits by receptors through Galpha-Gbeta and Galpha-Ggamma interactions. Trends Biochem Sci. 2003;28:13–17. doi: 10.1016/s0968-0004(02)00006-3. [DOI] [PubMed] [Google Scholar]
- 65.Kisselev OG, Meyer CK, Heck M, Ernst OP, Hofmann KP. Signal transfer from rhodopsin to the G-protein: evidence for a two-site sequential fit mechanism. Proc Natl Acad Sci USA. 1999;96:4898–4903. doi: 10.1073/pnas.96.9.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herrmann R, Heck M, Henklein P, Henklein P, Kleuss C, Hofmann KP, Ernst OP. Sequence of interactions in receptor-G protein coupling. J Biol Chem. 2004;279:24283–24290. doi: 10.1074/jbc.M311166200. [DOI] [PubMed] [Google Scholar]
- 67.Kisselev O, Pronin A, Ermolaeva M, Gautam N. Receptor-G protein coupling is established by a potential conformational switch in the beta gamma complex. Proc Natl Acad Sci USA. 1995;92:9102–9106. doi: 10.1073/pnas.92.20.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gautam N. A conformational switch regulates receptor-G protein interaction. Structure. 2003;11:359–360. doi: 10.1016/s0969-2126(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 69.Kisselev OG, Downs MA. Rhodopsin controls a conformational switch on the transducin gamma subunit. Structure (Camb) 2003;11:367–373. doi: 10.1016/s0969-2126(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 70.Abdulaev NG, Ngo T, Ramon E, Brabazon DM, Marino JP, Ridge KD. The receptor-bound ‘empty pocket’ state of the heterotrimeric G-protein alpha-subunit is conformationally dynamic. Biochemistry. 2006;45:12986–12997. doi: 10.1021/bi061088h. [DOI] [PubMed] [Google Scholar]
- 71.Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Mapping allosteric connections from the receptor to the nucleotide-binding pocket of heterotrimeric G proteins. Proc Natl Acad Sci USA. 2007;104:7927–7932. doi: 10.1073/pnas.0702623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fung BK. Characterization of transducin from bovine retinal rod outer segments. I. Separation and reconstitution of the subunits. J Biol Chem. 1983;258:10495–10502. [PubMed] [Google Scholar]
- 73.Codina J, Hildebrandt JD, Birnbaumer L, Sekura RD. Effects of guanine nucleotides and Mg on human erythrocyte Ni and Ns, the regulatory components of adenylyl cyclase. J Biol Chem. 1984;259:11408–11418. [PubMed] [Google Scholar]
- 74.Northup JK, Smigel MD, Sternweis PC, Gilman AG. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution of the activated 45,000-dalton (alpha) subunit. J Biol Chem. 1983;258:11369–11376. [PubMed] [Google Scholar]
- 75.Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shekter LR, Rosal R, Weng G, Yang CS, Iyengar R, Miller RJ, Jan LY, Lefkowitz RJ, Hamm HE. Molecular basis for interactions of G protein betagamma subunits with effectors. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 76.Li Y, Sternweis PM, Charnecki S, Smith TF, Gilman AG, Neer EJ, Kozasa T. Sites for Galpha binding on the G protein beta subunit overlap with sites for regulation of phospholipase Cbeta and adenylyl cyclase. J Biol Chem. 1998;273:16265–16272. doi: 10.1074/jbc.273.26.16265. [DOI] [PubMed] [Google Scholar]
- 77.Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science. 2006;312:443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- 78.Lehmann DM, Seneviratne AM, Smrcka AV. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol. 2008;73:410–418. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lutz S, Shankaranarayanan A, Coco C, Ridilla M, Nance MR, Vettel C, Baltus D, Evelyn CR, Neubig RR, Wieland T, Tesmer JJ. Structure of Galphaq-p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRs. Science. 2007;318:1923–1927. doi: 10.1126/science.1147554. [DOI] [PubMed] [Google Scholar]
- 80.Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha. GTPgammaS. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 81.Levitzki A, Klein S. G-protein subunit dissociation is not an integral part of G-protein action. Chembiochem. 2002;3:815–818. doi: 10.1002/1439-7633(20020902)3:9<815::AID-CBIC815>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 82.Klein S, Reuveni H, Levitzki A. Signal transduction by a nondissociable heterotrimeric yeast G protein. Proc Natl Acad Sci USA. 2000;97:3219–3223. doi: 10.1073/pnas.050015797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Janetopoulos C, Jin T, Devreotes P. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science. 2001;291:2408–2411. doi: 10.1126/science.1055835. [DOI] [PubMed] [Google Scholar]
- 84.Bunemann M, Frank M, Lohse MJ. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc Natl Acad Sci USA. 2003;100:16077–16082. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frank M, Thumer L, Lohse MJ, Bunemann M. G Protein activation without subunit dissociation depends on a G-alpha-i-specific region. J Biol Chem. 2005;280:24584–24590. doi: 10.1074/jbc.M414630200. [DOI] [PubMed] [Google Scholar]
- 86.Digby GJ, Lober RM, Sethi PR, Lambert NA. Some G protein heterotrimers physically dissociate in living cells. Proc Natl Acad Sci USA. 2006;103:17789–17794. doi: 10.1073/pnas.0607116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rebois RV, Robitaille M, Gales C, Dupre DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hebert TE. Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci. 2006;119:2807–2818. doi: 10.1242/jcs.03021. [DOI] [PubMed] [Google Scholar]
- 88.Evanko DS, Thiyagarajan MM, Takida S, Wedegaertner PB. Loss of association between activated Galpha q and Gbetagamma disrupts receptor-dependent and receptor-independent signaling. Cell Signal. 2005;17:1218–1228. doi: 10.1016/j.cellsig.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 89.Linder ME, Pang IH, Duronio RJ, Gordon JI, Sternweis PC, Gilman AG. Lipid modifications of G protein subunits. Myristoylation of Go alpha increases its affinity for beta gamma. J Biol Chem. 1991;266:4654–4659. [PubMed] [Google Scholar]
- 90.Azpiazu I, Gautam N. A fluorescence resonance energy transfer-based sensor indicates that receptor access to a G protein is unrestricted in a living mammalian cell. J Biol Chem. 2004;279:27709–27718. doi: 10.1074/jbc.M403712200. [DOI] [PubMed] [Google Scholar]
- 91.Yan K, Kalyanaraman V, Gautam N. Differential ability to form the G protein betagamma complex among members of the beta and gamma subunit families. J Biol Chem. 1996;271:7141–7146. doi: 10.1074/jbc.271.12.7141. [DOI] [PubMed] [Google Scholar]
- 92.Schmidt CJ, Thomas TC, Levine MA, Neer EJ. Specificity of G protein beta and gamma subunit interactions. J Biol Chem. 1992;267:13807–13810. [PubMed] [Google Scholar]
- 93.Ray K, Kunsch C, Bonner LM, Robishaw JD. Isolation of cDNA clones encoding eight different human G protein gamma subunits, including three novel forms designated the gamma 4, gamma 10, and gamma 11 subunits. J Biol Chem. 1995;270:21765–21771. doi: 10.1074/jbc.270.37.21765. [DOI] [PubMed] [Google Scholar]
- 94.Dingus J, Wells CA, Campbell L, Cleator JH, Robinson K, Hildebrandt JD. G Protein betagamma dimer formation: Gbeta and Ggamma differentially determine efficiency of in vitro dimer formation. Biochemistry. 2005;44:11882–11890. doi: 10.1021/bi0504254. [DOI] [PubMed] [Google Scholar]
- 95.Pronin AN, Gautam N. Interaction between G-protein beta and gamma subunit types is selective. Proc Natl Acad Sci USA. 1992;89:6220–6224. doi: 10.1073/pnas.89.13.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee C, Murakami T, Simonds WF. Identification of a discrete region of the G protein gamma subunit conferring selectivity in beta gamma complex formation. J Biol Chem. 1995;270:8779–8784. doi: 10.1074/jbc.270.15.8779. [DOI] [PubMed] [Google Scholar]
- 97.Mervine SM, Yost EA, Sabo JL, Hynes TR, Berlot CH. Analysis of G protein betagamma dimer formation in live cells using multicolor bimolecular fluorescence complementation demonstrates preferences of beta1 for particular gamma subunits. Mol Pharmacol. 2006;70:194–205. doi: 10.1124/mol.106.022616. [DOI] [PubMed] [Google Scholar]
- 98.McIntire WE, MacCleery G, Garrison JC. The G protein beta subunit is a determinant in the coupling of Gs to the beta-1-adrenergic and A2a adenosine receptors. J Biol Chem. 2001;276:15801–15809. doi: 10.1074/jbc.M011233200. [DOI] [PubMed] [Google Scholar]
- 99.Siffert W. G protein polymorphisms in hypertension, atherosclerosis, and diabetes. Annu Rev Med. 2005;56:17–28. doi: 10.1146/annurev.med.56.082103.104625. [DOI] [PubMed] [Google Scholar]
- 100.Rosskopf D, Nikula C, Manthey I, Joisten M, Frey U, Kohnen S, Siffert W. The human G protein beta4 subunit: gene structure, expression, Ggamma and effector interaction. FEBS Lett. 2003;544:27–32. doi: 10.1016/s0014-5793(03)00441-1. [DOI] [PubMed] [Google Scholar]
- 101.Asano T, Morishita R, Ueda H, Kato K. Selective association of G protein beta 4 with gamma 5 and gamma 12 subunits in bovine tissues. J Biol Chem. 1999;274:21425–21429. doi: 10.1074/jbc.274.30.21425. [DOI] [PubMed] [Google Scholar]
- 102.Wells CA, Dingus J, Hildebrandt JD. Role of the chaperonin CCT/TRiC complex in G protein betagamma-dimer assembly. J Biol Chem. 2006;281:20221–20232. doi: 10.1074/jbc.M602409200. [DOI] [PubMed] [Google Scholar]
- 103.Dupre DJ, Robitaille M, Richer M, Ethier N, Mamarbachi AM, Hebert TE. Dopamine receptor-interacting protein 78 acts as a molecular chaperone for Ggamma subunits before assembly with Gbeta. J Biol Chem. 2007;282:13703–13715. doi: 10.1074/jbc.M608846200. [DOI] [PubMed] [Google Scholar]
- 104.Willardson BM, Howlett AC. Function of phosducin-like proteins in G protein signaling and chaperone-assisted protein folding. Cell Signal. 2007;19:2417–2427. doi: 10.1016/j.cellsig.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones MB, Garrison JC. Instability of the G-protein beta5 subunit in detergent. Anal Biochem. 1999;268:126–133. doi: 10.1006/abio.1998.3064. [DOI] [PubMed] [Google Scholar]
- 106.De Vries L, Zheng B, Fischer T, Elenko E, Farquhar MG. The regulator of G protein signaling family. Annu Rev Pharmacol Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- 107.Yost EA, Mervine SM, Sabo JL, Hynes TR, Berlot CH. Live cell analysis of G protein beta5 complex formation, function, and targeting. Mol Pharmacol. 2007;72:812–825. doi: 10.1124/mol.107.038075. [DOI] [PubMed] [Google Scholar]
- 108.Gilman AG. Nobel Lecture. G proteins and regulation of adenylyl cyclase. Biosci Rep. 1995;15:65–97. doi: 10.1007/BF01200143. [DOI] [PubMed] [Google Scholar]
- 109.Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- 110.Ruiz-Velasco V, Ikeda SR, Puhl HL. Cloning, tissue distribution, and functional expression of the human G protein beta4 subunit. Physiol Genomics. 2002;8:41–50. doi: 10.1152/physiolgenomics.00085.2001. [DOI] [PubMed] [Google Scholar]
- 111.Wilcox MD, Dingus J, Balcueva EA, McIntire WE, Mehta ND, Schey KL, Robishaw JD, Hildebrandt JD. Bovine brain Go isoforms have distinct gamma subunit compositions. J Biol Chem. 1995;270:4189–4192. doi: 10.1074/jbc.270.9.4189. [DOI] [PubMed] [Google Scholar]
- 112.McIntire WE, Schey KL, Knapp DR, Hildebrandt JD. A major G protein alpha O isoform in bovine brain is deamidated at Asn346 and Asn347, residues involved in receptor coupling. Biochemistry. 1998;37:14651–14658. doi: 10.1021/bi981642q. [DOI] [PubMed] [Google Scholar]
- 113.McIntire WE, Dingus J, Wilcox MD, Hildebrandt JD. The relationship of G(o)alpha subunit deamidation to the tissue distribution, nucleotide binding properties, and betagamma dimer interactions of G(o)alpha subunit isoforms. J Neurochem. 1999;73:633–640. doi: 10.1046/j.1471-4159.1999.0730633.x. [DOI] [PubMed] [Google Scholar]
- 114.Krumins AM, Gilman AG. Targeted knockdown of G protein subunits selectively prevents receptor-mediated modulation of effectors and reveals complex changes in non-targeted signaling proteins. J Biol Chem. 2006;281:10250–10262. doi: 10.1074/jbc.M511551200. [DOI] [PubMed] [Google Scholar]
- 115.Schwindinger WF, Betz KS, Giger KE, Sabol A, Bronson SK, Robishaw JD. Loss of G protein gamma 7 alters behavior and reduces striatal alpha(olf) level and cAMP production. J Biol Chem. 2003;278:6575–6579. doi: 10.1074/jbc.M211132200. [DOI] [PubMed] [Google Scholar]
- 116.Lobanova ES, Finkelstein S, Herrmann R, Chen YM, Kessler C, Michaud NA, Trieu LH, Strissel KJ, Burns ME, Arshavsky VY. Transducin gamma-subunit sets expression levels of alpha- and beta-subunits and is crucial for rod viability. J Neurosci. 2008;28:3510–3520. doi: 10.1523/JNEUROSCI.0338-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Evanko DS, Thiyagarajan MM, Siderovski DP, Wedegaertner PB. Gbeta gamma isoforms selectively rescue plasma membrane localization and palmitoylation of mutant Galphas and Galphaq. J Biol Chem. 2001;276:23945–23953. doi: 10.1074/jbc.M101154200. [DOI] [PubMed] [Google Scholar]
- 118.Fletcher JE, Lindorfer MA, DeFilippo JM, Yasuda H, Guilmard M, Garrison JC. The G protein beta5 subunit interacts selectively with the Gq alpha subunit. J Biol Chem. 1998;273:636–644. doi: 10.1074/jbc.273.1.636. [DOI] [PubMed] [Google Scholar]
- 119.McIntire WE, MacCleery G, Murphree LJ, Kerchner KR, Linden J, Garrison JC. Influence of differential stability of G protein betagamma dimers containing the gamma11 subunit on functional activity at the M1 muscarinic receptor, A1 adenosine receptor, and phospholipase C-beta. Biochemistry. 2006;45:11616–11631. doi: 10.1021/bi0604882. [DOI] [PubMed] [Google Scholar]
- 120.Lindorfer MA, Myung CS, Savino Y, Yasuda H, Khazan R, Garrison JC. Differential activity of the G protein beta5 gamma2 subunit at receptors and effectors. J Biol Chem. 1998;273:34429–34436. doi: 10.1074/jbc.273.51.34429. [DOI] [PubMed] [Google Scholar]
- 121.Kageyama K, Murakami T, Iizuka K, Sato K, Ichihara K, Tokumitsu Y, Kitabatake A, Kawaguchi H. Translocation of G-protein beta3 subunit from the cytosol pool to the membrane pool by beta1-adrenergic receptor stimulation in perfused rat hearts. Biochem Pharmacol. 1999;58:1497–1500. doi: 10.1016/s0006-2952(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 122.Akgoz M, Kalyanaraman V, Gautam N. Receptor-mediated reversible translocation of the G protein betagamma complex from the plasma membrane to the Golgi complex. J Biol Chem. 2004;279:51541–51544. doi: 10.1074/jbc.M410639200. [DOI] [PubMed] [Google Scholar]
- 123.Saini DK, Kalyanaraman V, Chisari M, Gautam N. A family of G protein betagamma subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J Biol Chem. 2007;282:24099–24108. doi: 10.1074/jbc.M701191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Azpiazu I, Akgoz M, Kalyanaraman V, Gautam N. G protein betagamma11 complex translocation is induced by Gi, Gq and Gs coupling receptors and is regulated by the alpha subunit type. Cell Signal. 2006;18:1190–1200. doi: 10.1016/j.cellsig.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chisari M, Saini DK, Kalyanaraman V, Gautam N. Shuttling of G protein subunits between the plasma membrane and intracellular membranes. J Biol Chem. 2007;282:24092–24098. doi: 10.1074/jbc.M704246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sato M, Cismowski MJ, Toyota E, Smrcka AV, Lucchesi PA, Chilian WM, Lanier SM. Identification of a receptor-independent activator of G protein signaling (AGS8) in ischemic heart and its interaction with Gbetagamma. Proc Natl Acad Sci USA. 2006;103:797–802. doi: 10.1073/pnas.0507467103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuan C, Sato M, Lanier SM, Smrcka AV. Signaling by a non-dissociated complex of G protein betagamma and alpha subunits stimulated by a receptor-independent activator of G protein signaling, AGS8. J Biol Chem. 2007;282:19938–19947. doi: 10.1074/jbc.M700396200. [DOI] [PubMed] [Google Scholar]
- 128.Zeng W, Mak DO, Li Q, Shin DM, Foskett JK, Muallem S. A new mode of Ca2+ signaling by G protein-coupled receptors: gating of IP3 receptor Ca2+ release channels by Gbetagamma. Curr Biol. 2003;13:872–876. doi: 10.1016/s0960-9822(03)00330-0. [DOI] [PubMed] [Google Scholar]
- 129.Wang S, Narendra S, Fedoroff N. Heterotrimeric G protein signaling in the Arabidopsis unfolded protein response. Proc Natl Acad Sci USA. 2007;104:3817–3822. doi: 10.1073/pnas.0611735104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dell EJ, Connor J, Chen S, Stebbins EG, Skiba NP, Mochly-Rosen D, Hamm HE. The betagamma subunit of heterotrimeric G proteins interacts with RACK1 and two other WD repeat proteins. J Biol Chem. 2002;277:49888–49895. doi: 10.1074/jbc.M202755200. [DOI] [PubMed] [Google Scholar]
- 131.Patterson RL, van Rossum DB, Barrow RK, Snyder SH. RACK1 binds to inositol 1,4,5-trisphosphate receptors and mediates Ca2+ release. Proc Natl Acad Sci USA. 2004;101:2328–2332. doi: 10.1073/pnas.0308567100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen S, Lin F, Hamm HE. RACK1 binds to a signal transfer region of G betagamma and inhibits phospholipase C beta2 activation. J Biol Chem. 2005;280:33445–33452. doi: 10.1074/jbc.M505422200. [DOI] [PubMed] [Google Scholar]
- 133.Schmidt CJ, Neer EJ. In vitro synthesis of G protein beta gamma dimers. J Biol Chem. 1991;266:4538–4544. [PubMed] [Google Scholar]
- 134.Yoshikawa DM, Hatwar M, Smrcka AV. G protein beta 5 subunit interactions with alpha subunits and effectors. Biochemistry. 2000;39:11340–11347. doi: 10.1021/bi0005557. [DOI] [PubMed] [Google Scholar]
- 135.Blake BL, Wing MR, Zhou JY, Lei Q, Hillmann JR, Behe CI, Morris RA, Harden TK, Bayliss DA, Miller RJ, Siderovski DP. G beta association and effector interaction selectivities of the divergent G gamma subunit G gamma 13. J Biol Chem. 2001;276:49267–49274. doi: 10.1074/jbc.M106565200. [DOI] [PubMed] [Google Scholar]
- 136.Rahmatullah M, Robishaw JD. Direct interaction of the alpha and gamma subunits of the G proteins. Purification and analysis by limited proteolysis. J Biol Chem. 1994;269:3574–3580. [PubMed] [Google Scholar]
- 137.Azpiazu I, Cruzblanca H, Li P, Linder M, Zhuo M, Gautam N. A G protein gamma subunit-specific peptide inhibits muscarinic receptor signaling. J Biol Chem. 1999;274:35305–35308. doi: 10.1074/jbc.274.50.35305. [DOI] [PubMed] [Google Scholar]
- 138.Azpiazu I, Gautam N. A G protein gamma subunit peptide stabilizes a novel muscarinic receptor state. Biochem Biophys Res Commun. 2006;341:904–910. doi: 10.1016/j.bbrc.2006.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hagiwara K, Wada A, Katadae M, Ito M, Ohya Y, Casey PJ, Fukada Y. Analysis of the molecular interaction of the farnesyl moiety of transducin through the use of a photoreactive farnesyl analogue. Biochemistry. 2004;43:300–309. doi: 10.1021/bi0351514. [DOI] [PubMed] [Google Scholar]
- 140.Hou Y, Azpiazu I, Smrcka A, Gautam N. Selective role of G protein gamma subunits in receptor interaction. J Biol Chem. 2000;275:38961–38964. doi: 10.1074/jbc.C000604200. [DOI] [PubMed] [Google Scholar]
- 141.Mende U, Schmidt CJ, Yi F, Spring DJ, Neer EJ. The G protein gamma subunit. Requirements for dimerization with beta subunits. J Biol Chem. 1995;270:15892–15898. doi: 10.1074/jbc.270.26.15892. [DOI] [PubMed] [Google Scholar]
- 142.Landry S, Hoffman CS. The git5 Gbeta and git11 Ggamma form an atypical Gbetagamma dimer acting in the fission yeast glucose/cAMP pathway. Genetics. 2001;157:1159–1168. doi: 10.1093/genetics/157.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chinault SL, Blumer KJ. The C-terminal tail preceding the CAAX box of a yeast G protein gamma subunit is dispensable for receptor-mediated G protein activation in vivo. J Biol Chem. 2003;278:20638–20644. doi: 10.1074/jbc.M212701200. [DOI] [PubMed] [Google Scholar]
- 144.Zeller CE, Parnell SC, Dohlman HG. The RACK1 ortholog Asc1 functions as a G-protein beta subunit coupled to glucose responsiveness in yeast. J Biol Chem. 2007;282:25168–25176. doi: 10.1074/jbc.M702569200. [DOI] [PubMed] [Google Scholar]
- 145.Yamazaki A, Tatsumi M, Torney DC, Bitensky MW. The GTP-binding protein of rod outer segments. I. Role of each subunit in the GTP hydrolytic cycle. J Biol Chem. 1987;262:9316–9323. [PubMed] [Google Scholar]
- 146.Kleuss C, Hescheler J, Ewel C, Rosenthal W, Schultz G, Wittig B. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991;353:43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- 147.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Different beta-subunits determine G-protein interaction with transmembrane receptors. Nature. 1992;358:424–426. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- 148.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993;259:832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- 149.Macrez-Lepretre N, Kalkbrenner F, Morel JL, Schultz G, Mironneau J. G protein heterotrimer Galpha13beta1gamma3 couples the angiotensin AT1A receptor to increases in cytoplasmic Ca2+ in rat portal vein myocytes. J Biol Chem. 1997;272:10095–10102. doi: 10.1074/jbc.272.15.10095. [DOI] [PubMed] [Google Scholar]
- 150.Macrez N, Morel JL, Mironneau J. Specific galpha11beta3gamma5 protein involvement in endothelin receptor-induced phosphatidylinositol hydrolysis and Ca2+ release in rat portal vein myocytes. Mol Pharmacol. 1999;55:684–692. [PubMed] [Google Scholar]
- 151.Kisselev O, Gautam N. Specific interaction with rhodopsin is dependent on the gamma subunit type in a G protein. J Biol Chem. 1993;268:24519–24522. [PubMed] [Google Scholar]
- 152.Kisselev O, Ermolaeva M, Gautam N. Efficient interaction with a receptor requires a specific type of prenyl group on the G protein gamma subunit. J Biol Chem. 1995;270:25356–25358. doi: 10.1074/jbc.270.43.25356. [DOI] [PubMed] [Google Scholar]
- 153.Jian X, Clark WA, Kowalak J, Markey SP, Simonds WF, Northup JK. Gbeta gamma affinity for bovine rhodopsin is determined by the carboxyl-terminal sequences of the gamma subunit. J Biol Chem. 2001;276:48518–48525. doi: 10.1074/jbc.M107129200. [DOI] [PubMed] [Google Scholar]
- 154.Yasuda H, Lindorfer MA, Woodfork KA, Fletcher JE, Garrison JC. Role of the prenyl group on the G protein gamma subunit in coupling trimeric G proteins to A1 adenosine receptors. J Biol Chem. 1996;271:18588–18595. doi: 10.1074/jbc.271.31.18588. [DOI] [PubMed] [Google Scholar]
- 155.Richardson M, Robishaw JD. The alpha-2A-adrenergic receptor discriminates between Gi heterotrimers of different betagamma subunit composition in Sf9 insect cell membranes. J Biol Chem. 1999;274:13525–13533. doi: 10.1074/jbc.274.19.13525. [DOI] [PubMed] [Google Scholar]
- 156.Lim WK, Myung CS, Garrison JC, Neubig RR. Receptor-G protein gamma specificity: gamma11 shows unique potency for A1 adenosine and 5-HT1A receptors. Biochemistry. 2001;40:10532–10541. doi: 10.1021/bi010950c. [DOI] [PubMed] [Google Scholar]
- 157.Wang Q, Mullah B, Hansen C, Asundi J, Robishaw JD. Ribozyme-mediated suppression of the G protein gamma7 subunit suggests a role in hormone regulation of adenylyl cyclase activity. J Biol Chem. 1997;272:26040–26048. doi: 10.1074/jbc.272.41.26040. [DOI] [PubMed] [Google Scholar]
- 158.Wang Q, Mullah BK, Robishaw JD. Ribozyme approach identifies a functional association between the G protein beta1gamma7 subunits in the beta-adrenergic receptor signaling pathway. J Biol Chem. 1999;274:17365–17371. doi: 10.1074/jbc.274.24.17365. [DOI] [PubMed] [Google Scholar]
- 159.Wang Q, Jolly JP, Surmeier JD, Mullah BM, Lidow MS, Bergson CM, Robishaw JD. Differential dependence of the D1 and D5 dopamine receptors on the G protein gamma 7 subunit for activation of adenylylcyclase. J Biol Chem. 2001;276:39386–39393. doi: 10.1074/jbc.M104981200. [DOI] [PubMed] [Google Scholar]
- 160.Hwang JI, Fraser ID, Choi S, Qin XF, Simon MI. Analysis of C5a-mediated chemotaxis by lentiviral delivery of small interfering RNA. Proc Natl Acad Sci USA. 2004;101:488–493. doi: 10.1073/pnas.0307549100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hou Y, Chang V, Capper AB, Taussig R, Gautam N. G Protein beta subunit types differentially interact with a muscarinic receptor but not adenylyl cyclase type II or phospholipase C-beta 2/3. J Biol Chem. 2001;276:19982–19988. doi: 10.1074/jbc.M010424200. [DOI] [PubMed] [Google Scholar]
- 162.Murphree LJ, Marshall MA, Rieger JM, MacDonald TL, Linden J. Human A2A adenosine receptors: high-affinity agonist binding to receptor-G protein complexes containing Gbeta4. Mol Pharmacol. 2002;61:455–462. doi: 10.1124/mol.61.2.455. [DOI] [PubMed] [Google Scholar]
- 163.Nguyen DK, Montesinos MC, Williams AJ, Kelly M, Cronstein BN. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. J Immunol. 2003;171:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]