This article describes a functional interaction between PP2A and FOXO3a in which PP2A promotes rapid dephosphorylation of FOXO3a at its conserved AKT phosphorylation sites, leading to FOXO3a dissociation from 14-3-3, nuclear translocation, and transcriptional activation in response to inhibition of PI3K signaling.
Abstract
Forkhead box transcription factor FOXO3a, a key regulator of cell survival, is regulated by reversible phosphorylation and subcellular localization. Although the kinases regulating FOXO3a activity have been characterized, the role of protein phosphatases (PP) in the control of FOXO3a subcellular localization and function is unknown. In this study, we detected a robust interaction between FOXO3a and PP2A. We further demonstrate that 14-3-3, while not impeding the interaction between PP2A and FOXO3a, restrains its activity toward AKT phosphorylation sites T32/S253. Disruption of PP2A function revealed that after AKT inhibition, PP2A-mediated dephosphorylation of T32/S253 is required for dissociation of 14-3-3, nuclear translocation, and transcriptional activation of FOXO3a. Our findings reveal that distinct phosphatases dephosphorylate conserved AKT motifs within the FOXO family and that PP2A is entwined in a dynamic interplay with AKT and 14-3-3 to directly regulate FOXO3a subcellular localization and transcriptional activation.
INTRODUCTION
The mammalian forkhead box transcription factors of the O classification (FOXO) are an evolutionarily conserved subgroup of the forkhead family of transcription factors that consist of FOXO1, FOXO3a, FOXO4, and FOXO6 (Anderson et al., 1998). These factors are regulated by an intricate network of posttranslational modifications that allow them to orchestrate the transcriptional control of a wide range of biological processes, such as apoptosis, cell cycle progression, and resistance to oxidative stress (Dansen and Burgering, 2008).
Perhaps the most well understood mechanism to functionally inhibit FOXO transcriptional activity is through Ser/Thr phosphorylation governed largely by prosurvival kinases activated downstream of phosphatidylinositol 3-kinase (PI3K). Phosphorylation of nuclear FOXO3a at three evolutionarily conserved residues (T32, S253, and S315) by AKT (Brunet et al., 1999) and serum- and glucocorticoid-inducible kinase (SGK)-1 (Brunet et al., 2001) promotes its sequestration in the cytoplasmic compartment (Brunet et al., 1999, 2001, 2002). In particular, because S253 overlaps with the nuclear localization signal (NLS), it is thought that phosphorylation of this site interferes with nuclear import by disrupting the integrity of the positively charged NLS. In addition, binding of 14-3-3 to pS253 and pT32 probably contributes to cytoplasmic sequestration of FOXO3a by masking the NLS from nuclear import machinery (Brunet et al., 1999, 2002; Obsilova et al., 2005). Casein kinase (CK) 1 mediates the hierarchical phosphorylation of FOXO3a at S318 and S321, which like FOXO1 (Rena et al., 2002, 2004), is probably to enhance its rate of nuclear export. Adding to the complexity of phosphorylation-dependent regulation of FOXO3a, extracellular signal-regulated kinase (ERK) (Yang et al., 2008) and IκB kinase complex (IKK) (Hu et al., 2004) have recently been shown to converge upon FOXO3a and contribute to its cytoplasmic localization and transcriptional inactivation by directly phosphorylating S294/S344/S425 and S644, respectively. Given the multitude of negative regulatory Ser/Thr phosphoacceptor sites that FOXO3a possesses, an open question that surrounds the control of its nuclear localization and transcriptional activity is the involvement of protein Ser/Thr phosphatases.
Heterotrimeric protein phosphatase 2A (PP2A) accounts for a significant percentage of all Ser/Thr phosphatase activity in most cells and tissues (Virshup, 2000). The PP2A core enzyme, made up of ∼65-kDa scaffolding/A and ∼36-kDa catalytic/C subunits, is regulated by the binding of one of many structurally distinct regulatory B subunits. Assembly of these subunits into the core enzyme can give rise to a large number of PP2A variants differing in substrate specificity and subcellular localization (Janssens and Goris, 2001). Although PP2A has been shown to play a prominent role in activating proapoptotic molecules such as BAD (Chiang et al., 2001, 2003), whether it has a physiological role in directly regulating the nuclear translocation and transcriptional activation of FOXO3a has yet to be elucidated.
We found that PP2A interacts with FOXO3a but 14-3-3 proteins restrain its activity toward the AKT phosphorylation sites T32 and S253, thus revealing a novel role for these proteins in the control of FOXO3a phosphorylation. On inactivation of PI3K/AKT signaling, however, FOXO3a is rapidly dephosphorylated by PP2A at AKT sites, resulting in 14-3-3 dissociation, nuclear translocation, and transcriptional activation. Together, our work provides mechanistic evidence that supports a direct role for PP2A in the rapid nuclear translocation and transcriptional activation of FOXO3a in response to inhibition of PI3K/AKT signaling.
MATERIALS AND METHODS
In Vitro FOXO3a Dephosphorylation Assay
HeLa cells transiently transfected with FLAG-FOXO3a in 100-mm dishes were washed twice with phosphate-buffered saline (PBS) then scraped on ice in phosphatase lysis buffer A (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, and 0.25% [vol/vol] NP-40) supplemented with Complete (Roche Applied Science, Indianapolis, IN) but no phosphatase inhibitors. Aliquots of clarified lysates from one transfected dish containing equal amounts of protein were incubated either with or without 25 μM R18 peptide (BIOMOL Research Laboratories, Plymouth Meeting, PA) for 30 min on ice. Where indicated, fostriecin (FST; Calbiochem, San Diego, CA) was added to the appropriate aliquots on ice for 10 min before initiation of dephosphorylation. Dephosphorylation was performed by incubating lysates for 30 min at 30°C with intermittent mixing before terminating the reaction by boiling in 4× Laemmli reducing sample buffer. For dephosphorylation of FOXO3a by PP2A, three aliquots of radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% [vol/vol] NP-40, 0.5% [wt/vol] sodium deoxycholate, 0.1% [wt/vol] SDS, and 5 mM EDTA) extracts from one 100-mm dish transfected with FLAG-FOXO3a, equalized for protein concentration and volume, were subjected to immunoprecipitation with anti-FLAG (M2) affinity gel (Sigma-Aldrich, St. Louis, MO). Two of the anti-FLAG immunocomplexes containing phospho-FOXO3a were resuspended in phosphatase lysis buffer B [20 mM 3-(N-morpholino)propanesulfonic acid, pH 7.5, 150 mM NaCl, and 14.4 mM β-mercaptoethanol] supplemented with Complete (Roche Applied Science) but no phosphatase inhibitors and subjected to treatment with the indicated units of purified PP2A core enzyme (Millipore, Billerica, MA) for 30 min at 30°C with intermittent mixing. The remaining immunocomplex was incubated in phosphatase lysis buffer B alone. Boiling in 4× Laemmli reducing sample buffer terminated reactions. FOXO3a dephosphorylation was analyzed by immunoblotting with the appropriate phospho-FOXO3a antibodies.
In Vivo FOXO Dephosphorylation Assay
To pharmacologically inhibit phosphatases, cells in 60-mm dishes were pretreated with either 100 nM okadaic acid (OA; Calbiochem) or 5 μM tautomycetin (TC; Tocris Bioscience, Ellisville, MO) for 2 h at 37°C in complete DMEM. Cells were maintained in the presence of phosphatase inhibitors during dephosphorylation. Dephosphorylation of FOXOs was induced by treatment with 20 μM each of LY294002 (LY; Calbiochem) and AKT-I VIII (selective for AKT-1 and AKT-2; Calbiochem) in serum-free DMEM at 37°C for the time points indicated in figure legends. For experiments involving insulin treatment, cells were cultured for 20 h in serum-free DMEM and then treated for 30 min with 100 nM insulin (Invitrogen, Carlsbad, CA) before LY/AKT-I treatment. Where indicated, cells were pretreated with 100 nM rapamycin (Calbiochem) or 20 μM of the Jun-NH2-terminal kinase (JNK) inhibitor SP600125 (Calbiochem) for 30 min at 37°C in DMEM supplemented with 10% fetal bovine serum and l-glutamine. Whole-cell lysates were obtained by lysing cells in either RIPA buffer or NP-40 buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% [vol/vol] NP-40, and 5 mM EDTA) supplemented with Complete and Halt phosphatase inhibitor cocktails. Protein concentrations were normalized before boiling in 4× Laemmli reducing sample buffer. FOXO3a dephosphorylation was analyzed by immunoblotting with the appropriate phospho-FOXO3a antibodies.
RESULTS
OA-sensitive Phosphatases Promote FOXO3a Dephosphorylation at AKT and CK1 Sites after PI3K Inhibition
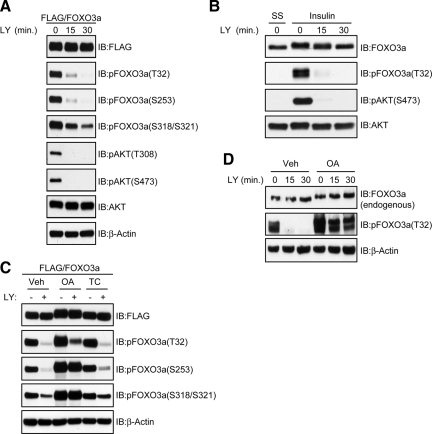
Because FOXO3a is directly phosphorylated by the PI3K-regulated AGC kinases AKT (Brunet et al., 1999) and SGK-1 (Brunet et al., 2001), we sought to establish the kinetics of FOXO3a dephosphorylation at the relevant sites after PI3K inhibition. Therefore, HeLa cells expressing FLAG-FOXO3a were subjected to an in vivo dephosphorylation assay in the presence of the PI3K inhibitor LY. As shown in Figure 1A, dephosphorylation of FOXO3a at T32 and S253 was rapid and paralleled that of AKT at the activation loop (T308) and hydrophobic motif (S473) after LY treatment. The dephosphorylation of CK1 sites (S318/S321), however, was not as rapid. Because no phosphospecific antibodies specific for S315 exist, we were unable to assess the kinetics of S315 dephosphorylation. Similarly, acute stimulation of serum-starved HeLa cells with insulin followed by LY treatment led to the rapid dephosphorylation of endogenous FOXO3a (Figure 1B).
Figure 1.
OA-sensitive phosphatases promote dephosphorylation of FOXO3a at AKT and CK1 sites in vivo after PI3K inhibition. (A) HeLa cells transfected with a FLAG-FOXO3a expression plasmid were grown in the presence of serum and then either lysed (0 min) or treated with 20 μM LY in the absence of serum for the indicated time points before lysis. Whole-cell lysates were immunoblotted (IB) with the indicated antibodies. (B) Serum-starved (SS) HeLa cells were either lysed (0 min) or pretreated for 30 min with 100 nM insulin after which cells were either lysed (0 min) or treated with 20 μM LY in the absence of insulin for the indicated time points before lysis. Whole-cell lysates were IB with the indicated antibodies. (C) Cells transfected with FLAG-FOXO3a were pretreated with 100 nM OA, 5 μM TC, or dimethyl sulfoxide (DMSO; Veh) for 2 h in the presence of serum. Cells were then either lysed (−), treated with 20 μM LY alone, or in combination with OA and TC as indicated for 30 min in the absence of serum (+) before lysis. Whole-cell lysates were IB with the indicated antibodies. (D) Effect of OA on endogenous FOXO3a dephosphorylation was analyzed as described in C.
Because PP1 and PP2A are the most abundant phosphatases in mammalian cells, we investigated their contribution to FOXO3a dephosphorylation by acutely treating HeLa cells expressing FLAG-FOXO3a with either 100 nM of the PP2A-selective inhibitor OA or 5 μM of the PP1-selective inhibitor TC. Previous work has shown that these doses of OA and TC differentiate between the activities of PP2A and PP1 in a variety of cell lines (Favre et al., 1997; Mitsuhashi et al., 2003). As shown in Figure 1C, we observed a significant decrease in the electrophoretic mobility of FOXO3a that was suggestive of hyperphosphorylation in cells treated with OA but not with TC. Indeed, although OA treatment attenuated the LY-induced dephosphorylation of FOXO3a at AKT and CK1 sites, TC had little influence on this process. The dephosphorylation of endogenous FOXO3a was similarly affected by OA (Figure 1D). In summary, these data indicate that PP2A and/or PP2A-like enzymes promote rapid dephosphorylation of FOXO3a after acute inhibition of PI3K.
Identification of PP2A as a FOXO3a Binding Partner
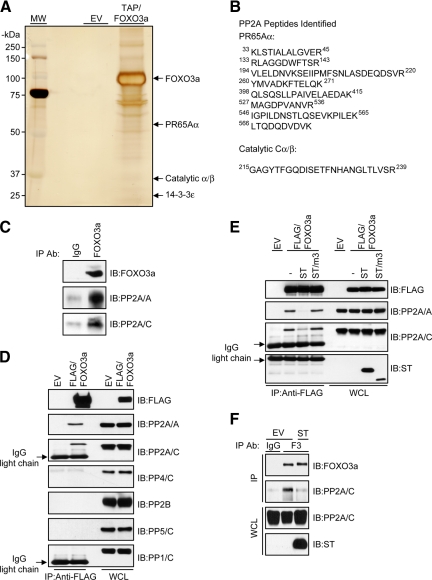
To gain further insight into the phosphatase-dependent regulation of FOXO3a, we used a nonbiased tandem affinity purification (TAP) approach to search for Ser/Thr phosphatases that copurified with FOXO3a under native conditions. Cell extracts from HeLa cells engineered to stably express a FLAG-hemagglutinin (HA)-tagged FOXO3a allele (TAP/FOXO3a) were sequentially immunopurified with FLAG and HA affinity matrices in parallel with cell extracts derived from HeLa cells engineered to express an empty TAP vector (EV). In agreement with a recent study (Rinner et al., 2007), mass spectrometric analysis of TAP/FOXO3a-associated proteins with molecular masses of ∼65 and ∼36 kDa revealed peptides from two subunits of PP2A, namely, the scaffolding/Aα and catalytic/C subunits, respectively. A well-established binding partner of FOXO3a, 14-3-3, was also identified as a constituent of TAP/FOXO3a complexes, thus validating our TAP approach (Figure 2, A and B). Further studies demonstrated an interaction between the endogenous PP2A core enzyme and endogenous FOXO3a, thus providing evidence for a physiological function of this complex (Figure 2C). An interaction between FOXO3a and the catalytic subunit of other OA-sensitive phosphatases, however, could not be detected (Figure 2D).
Figure 2.
PP2A interacts with FOXO3a in vivo. (A) Immune complexes isolated from whole-cell lysates of HeLa cells stably transfected with either an EV or TAP/FOXO3a expression plasmid were resolved by SDS-PAGE and silver stained. Gel slices containing the PP2A subunits found to associate with FOXO3a are indicated. Markers indicating molecular mass (MM) are shown to the left of the gel. (B) Shown are the peptide sequences of the PP2A subunits identified by mass spectrometric analysis. (C) HeLa whole-cell lysates were immunoprecipitated (IP) with either a control immunoglobulin (Ig)G or an anti-FOXO3a IgG. Immunocomplexes were immunoblotted (IB) with the indicated antibodies. (D) FLAG-FOXO3a was IP from HeLa whole-cell lysates, and immunocomplexes were IB with the indicated antibodies. (E) FLAG-FOXO3a was IP from whole-cell lysates derived from HeLa cells transfected with the indicated expression plasmids. − indicates cotransfection with an empty vector. Immunocomplexes were IB with the indicated antibodies. (F) HeLa cells were transfected with either an EV or ST expression plasmid. Whole-cell lysates were IP with either a control IgG or an anti-FOXO3a IgG (F3) as indicated and immunocomplexes were IB with the indicated antibodies (top). An aliquot of the IP input taken from the initial whole-cell lysate (WCL) was resolved on a separate gel and subject to IB with the indicated antibodies (bottom). (G) FLAG-FOXO3a was IP from whole-cell lysates derived from HeLa cells treated with either DMSO (−) or 100 nM OA (+) for 2 h in the presence of serum. Immunocomplexes were IB with the indicated antibodies. IB for 14-3-3 served as a positive control for the co-IP.
To characterize the interaction between FOXO3a and PP2A, we coexpressed FOXO3a and the small t antigen (ST) of simian virus 40 (SV40) in HeLa cells. PP2A is the major cellular target of ST and by directly binding to Aα, ST prevents the incorporation of multiple B subunits into the core enzyme, thus altering the substrate specificity of PP2A (Pallas et al., 1990; Chen et al., 2004). Consistent with these findings, we observed that wild-type ST, but not a PP2A binding-deficient mutant lacking amino acids 111-174 (ST/m3) (Sontag et al., 1993), dramatically reduced the interaction between FOXO3a and the PP2A core enzyme (Figure 2E). Moreover, ST disrupted the endogenous FOXO3a/PP2A complex (Figure 2F). In a separate approach to inhibit PP2A function, we treated cells expressing FLAG-FOXO3a with OA. Similar to ST, OA efficiently disrupted the interaction between FOXO3a and PP2A (Figure 2G). Together, these findings show that PP2A is the predominant Ser/Thr phosphatase that interacts with FOXO3a and this interaction is likely to be mediated by a PP2A heterotrimer.
14-3-3 Proteins Protect AKT Phosphorylation Sites on FOXO3a from PP2A Activity without Impeding Core Enzyme Interaction with FOXO3a
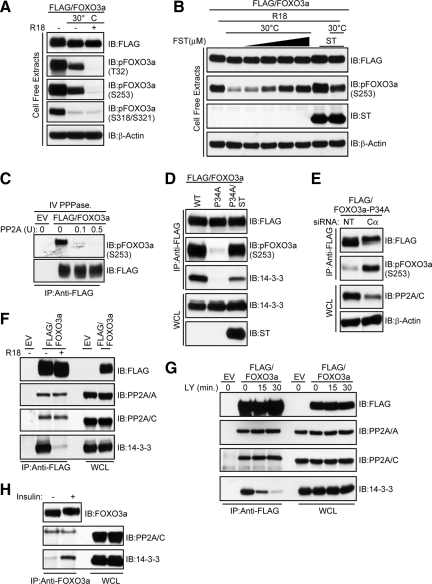
Because maintenance of FOXO3a phosphorylation at T32 and S253 is critical for its cytoplasmic localization (Brunet et al., 1999), we hypothesized that binding of 14-3-3 to these sites protects them from phosphatase activity. To test this hypothesis, we used a nonphosphorylated peptide, R18, which has been shown to displace 14-3-3 from its phosphorylated binding partners (Petosa et al., 1998; Wang et al., 1999). As predicted, the R18 peptide efficiently displaced 14-3-3 from FOXO3a (data not shown). Lysates containing phosphorylated FLAG-FOXO3a were then subjected to an in vitro dephosphorylation assay in a buffer compatible with phosphatase but not kinase activity. As shown in Figure 3A, T32 and S253 were completely dephosphorylated when 14-3-3 was displaced by R18, suggesting the presence of an active phosphatase(s). Dephosphorylation of S318 and S321, sites not implicated in 14-3-3 binding, was unaffected by R18.
Figure 3.
14-3-3 proteins protect AKT phosphorylation sites of FOXO3a from PP2A activity without impeding its binding to FOXO3a. (A) Lysates containing FLAG-FOXO3a were prepared in phosphatase lysis buffer A, and aliquots were either left on ice or dephosphorylated in the presence (+) or absence (−) of 25 μM R18 peptide. Reactions were analyzed by immunoblotting (IB) with the indicated antibodies. (B) Lysates from HeLa cells transfected with a FLAG-FOXO3a expression plasmid alone or in combination with a ST expression plasmid were prepared as in A. Aliquots were either left on ice or dephosphorylated in the presence of 25 μM R18 peptide. Where indicated, FST (1–10 μM) was added on ice for 10 min before initiating dephosphorylation. Reactions were analyzed by IB with the indicated antibodies. (C) Phosphorylated FLAG-FOXO3a was immunoprecipitated (IP) and incubated with the indicated units (U) of purified PP2A. Immunocomplexes were IB with the indicated antibodies. (D) FOXO3a was IP from lysates derived from HeLa cells transfected with FLAG-FOXO3a-WT (WT), FLAG-FOXO3a-P34A (P34A), or FLAG-FOXO3a-P34A and ST (P34A/ST) expression plasmids. Immunocomplexes were IB with the indicated antibodies. (E) HeLa cells were cotransfected with a FLAG-FOXO3a-P34A expression plasmid and either nontargeting siRNAs (NT) or siRNAs targeting the PP2A catalytic subunit (Cα). FOXO3a was IP and immunocomplexes were IB with the indicated antibodies (top). Then, 5% of the IP input taken from the initial whole-cell lysate (WCL) was resolved on a separate gel and subject to IB with the indicated antibodies (bottom). (F) FLAG-FOXO3a was IP from HeLa cell lysates in either the absence (−) or presence (+) of 25 μM R18 peptide. Immunocomplexes were IB with the indicated antibodies. (G) HeLa cells transfected with a FLAG-FOXO3a expression plasmid were grown in the presence of serum and then either lysed (0 min) or treated with 20 μM LY in the absence of serum for the indicated time points before lysis. FOXO3a was IP from lysates and immunocomplexes were IB with the indicated antibodies. (H) HeLa cells were serum-starved and either lysed (−) or treated for 30 min with 100 nM insulin (+) before lysis. Whole-cell lysates were IP with an anti-FOXO3a immunoglobulin (Ig)G and immunocomplexes were (IB with the indicated antibodies.
Because our data suggest that PP2A is the predominant Ser/Thr phosphatase that interacts with FOXO3a, we addressed its contribution to T32 and S253 dephosphorylation in cell-free extracts containing ST and FST, a more selective inhibitor of PP2A than OA (Walsh et al., 1997). In the presence of R18, FST inhibited dephosphorylation of FOXO3a at S253 in a dose-dependent manner at concentrations far lower than the IC50 value for PP1/PP5. Dephosphorylation of S253 was also dramatically impaired in the presence of ST (Figure 3B). To demonstrate that PP2A has the capacity to directly dephosphorylate FOXO3a, we performed an in vitro phosphatase assay. As shown in Figure 3C, increasing amounts of purified PP2A effectively dephosphorylated FOXO3a at S253.
It is thought that 14-3-3 dimers interact with sequences in FOXO3a containing high- (pT32 and P34) and low (p253)-affinity binding sites (Yaffe et al., 1997; Brunet et al., 1999; Obsil et al., 2003). Because P34 is predicted to be a critical determinant of FOXO3a/14-3-3 complex formation (Yaffe et al., 1997), we mutated this residue to address whether 14-3-3 binding protects FOXO3a from PP2A activity in vivo. As shown in Figure 3D, the inability of FOXO3a-P34A to maintain a stable interaction with 14-3-3 significantly compromised S253 phosphorylation, even in the presence of serum. Expression of ST, however, was able to restore S253 phosphorylation to levels comparable to those observed in wild-type FOXO3a despite hampered 14-3-3 binding. Similar results were obtained upon small interfering RNA (siRNA)-mediated silencing of the predominant PP2A catalytic subunit isoform Cα (Figure 3E).
Because our in vitro and in vivo findings demonstrate a phosphoprotective role for 14-3-3 toward sites T32 and S253 of FOXO3a, we hypothesized that the increased susceptibility of these sites to PP2A activity upon disassembly of FOXO3a/14-3-3 complexes resulted from an enhanced association between FOXO3a and PP2A. Contrary to our hypothesis, R18 was able to efficiently dissociate 14-3-3 from FOXO3a in lysates without a concomitant increase in the amount of endogenous core enzyme in immunocomplexes containing FOXO3a (Figure 3F). This finding was corroborated in intact cells by demonstrating that dissociation of 14-3-3 from FOXO3a after PI3K inhibition did not result in an enhanced association between the PP2A core enzyme and FOXO3a (Figure 3G). Furthermore, insulin-induced binding of endogenous FOXO3a to 14-3-3 did not promote disassembly of the endogenous FOXO3a/PP2A complex (Figure 3H). Collectively, our findings strongly suggest that 14-3-3 proteins protect FOXO3a from PP2A without impeding its binding to FOXO3a.
PP2A Inhibition Impairs Dephosphorylation of FOXO3a at AKT and CK1 Sites in Vivo Independently of Sustained SGK and AKT Activity
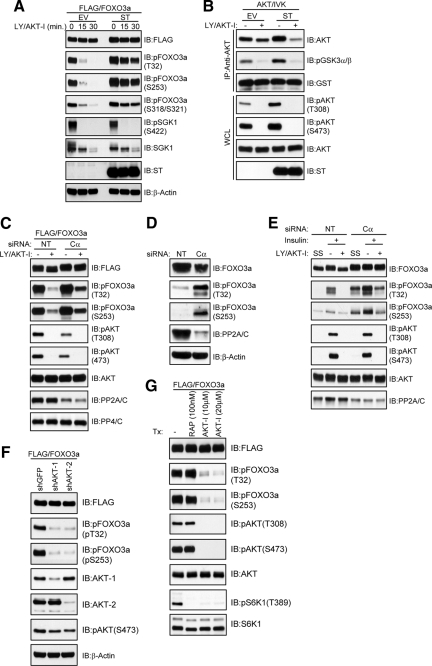
Because it is possible that PP2A inhibition could prevent FOXO3a dephosphorylation in cells by hyperactivating AKT (Brunet et al., 1999) and SGK-1 (Brunet et al., 2001), we performed in vivo dephosphorylation assays in the presence of LY and a pleckstrin homology (PH) domain-dependent inhibitor of AKT-1 and AKT-2 kinase activity (AKT-I; Barnett et al., 2005; Logie et al., 2007). ST expression in HeLa cells dramatically impaired FOXO3a dephosphorylation at AKT/SGK-1 (T32/S253) and CK1 sites (S318/S321) in the absence of sustained hydrophobic motif phosphorylation of SGK-1 (S422) and in HeLa cells with stable SGK-1 knockdown (Supplemental Figure S1A). Furthermore, in vitro kinase assays and phospho-AKT immunoblotting revealed that although ST elevated endogenous AKT activity under steady-state conditions in the presence of serum, this activity was not sustained after LY/AKT-I treatment (Figure 4B). To demonstrate that the impairment in FOXO3a dephosphorylation by ST relied upon its ability to bind PP2A, we expressed ST/m3 and observed that this mutant was unable to either bind to PP2A or prevent FOXO3a dephosphorylation (Supplemental Figure S1, B and C, respectively). Importantly, these findings positively correlate with the observed effects of ST and ST/m3 on the interaction between PP2A and FOXO3a (Figure 2E) and strongly suggest that recruitment of PP2A to FOXO3a is critical for its ability to dephosphorylate FOXO3a. In a separate approach, we inhibited PP2A function through siRNA-mediated silencing of the catalytic subunit. Although we could only achieve partial silencing of the catalytic subunit, probably due to its essential role in cell viability (Gotz et al., 1998), the efficiency of ectopic FOXO3a dephosphorylation at T32 and S253 was markedly reduced compared with cells transfected with nontargeting siRNA, despite complete dephosphorylation of AKT (Figure 4C). Similarly, siRNA-mediated silencing of the catalytic subunit led to a dramatic elevation in endogenous FOXO3a phosphorylation not only under steady-state conditions in the presence of serum but also after LY/AKT-I treatment of insulin-stimulated cells (Figure 4, D and E, respectively).
Figure 4.
PP2A inhibition blocks FOXO3a dephosphorylation at AKT and CK1 sites in vivo after AKT inhibition. (A) A FLAG-FOXO3a expression plasmid was cotransfected into HeLa cells with either an EV or ST expression plasmid. Cells were grown in serum and then either lysed (0 min) or treated with 20 μM each of LY and AKT-I for the indicated time points in the absence of serum before lysis. Whole-cell lysates were immunoblotted (IB) with the indicated antibodies. (B) HeLa cells were transfected with either an EV or ST expression plasmid and then either lysed (−) or treated with 20 μM each of LY and AKT-I in the absence of serum for 30 min (+) before lysis. AKT was immunoprecipitated (IP) and then subjected to an in vitro kinase (IVK) assay using a glutathione transferase (GST)-glycogen synthase kinase (GSK)3α/β-peptide as the substrate. Reaction products (top) and whole-cell lysates (WCL; bottom) were IB with the indicated antibodies. (C) HeLa cells were cotransfected with a FLAG-FOXO3a expression plasmid and either nontargeting siRNAs (NT) or siRNAs targeting the PP2A catalytic subunit (Cα) and treated as described in B. IB for PP4/C served as a positive control for the specificity of PP2A/C knockdown. (D) HeLa cells were transfected with either NT or siRNAs targeting the PP2A catalytic subunit (Cα). Whole cell lysates were immunoblotted with the indicated antibodies. (E) HeLa cells were transfected as described in D and then were either serum starved and lysed (SS) or pretreated for 30 min with 100 nM insulin after which cells were either lysed (−) or treated with 20 μM each of LY and AKT-I for 30 min (+) in the absence of insulin before lysis. Whole-cell lysates were immunoblotted with the indicated antibodies. (F). HeLa cells stably expressing shGFP, shAKT-1, or shAKT-2 were transfected with a FLAG-FOXO3a expression plasmid. Whole cell lysates were IB with the indicated antibodies. (G) HeLa cells transfected with a FLAG-FOXO3a expression plasmid were treated with DMSO (−), 100 nM rapamycin (RAP), or the indicated concentrations of AKT-I for 30 min in the presence of serum before lysis. Whole-cell lysates were IB with the indicated antibodies.
Although ST caused a dramatic impairment in FOXO3a dephosphorylation that did not depend upon either AKT or SGK-1 activity, it is possible that this effect of ST was mediated by the activation of other PI3K-dependent AGC kinases. Therefore, we monitored the phosphorylation status of FOXO3a at T32 and S253 upon short hairpin RNA (shRNA)-mediated silencing of either AKT-1 or AKT-2 in HeLa cells. As shown in Figure 4F, the phosphorylation of FOXO3a at T32 and S253 was almost abolished by knockdown of either isoform. We achieved similar results by treating cells with AKT-I (Figure 4G). Given this result, we performed coimmunoprecipitation assays to exclude the possibility that the impaired dephosphorylation of FOXO3a by ST was due to an enhanced interaction with AKT isoforms. Although a robust interaction occurred between endogenous AKT-1 and its recently identified substrate, S phase kinase-associated protein 2 (Gao et al., 2009), we were unable to detect an interaction between FOXO3a and either endogenous AKT-1 or endogenous AKT-2, even in the presence of ST (Supplemental Figure S2). Together, these data indicate that inhibition of PP2A does not prevent FOXO3a dephosphorylation by activating upstream kinases.
Because our findings suggest that ST disrupts a holoenzyme(s) to prevent FOXO3a interaction with PP2A and hence its dephosphorylation, we asked whether a particular regulatory subunit accompanied the PP2A core enzyme in its interaction with FOXO3a. Immunoblotting of FOXO3a immunocomplexes for regulatory subunits identified B56ε as a prominent FOXO3a binding partner. We were unable, however, to demonstrate an interaction between FOXO3a and B55 family members (Supplemental Figure S3A). Because ST has been shown to destabilize B56ε-containing holoenzymes (Chen et al., 2005), we addressed whether the impairment in FOXO3a dephosphorylation by ST was due largely in part to disruption of B56ε-containing holoenzymes. Compared with ST, knockdown of B56ε was less efficient at disrupting the interaction between the PP2A catalytic subunit and FOXO3a (Supplemental Figure S3B). Furthermore, knockdown of B56ε was unable to prevent dephosphorylation of FOXO3a at either the AKT or CK1 sites (Supplemental Figure S3C).
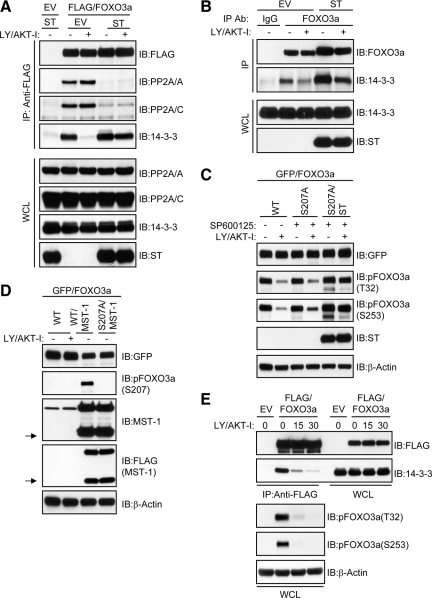
Dephosphorylation of FOXO3a at T32 and S253 by PP2A Facilitates Dissociation of 14-3-3 Independently of Mammalian sterile20-like kinase (MST) and JNK Signaling
Having observed that PP2A and 14-3-3 do not compete for binding to FOXO3a and that ST impaired FOXO3a/PP2A complex formation as well as FOXO3a dephosphorylation after LY/AKT-I treatment, we addressed the direct involvement of PP2A in the dissociation of 14-3-3 from FOXO3a given its phosphoprotective role toward T32/S253. As shown in Figure 5A, ST prevented not only the interaction between ectopic FOXO3a and the endogenous PP2A core enzyme but also its dissociation from 14-3-3 after LY/AKT-I treatment. This finding was corroborated in the context of endogenous proteins (Figure 5B) and strongly suggests that PP2A promotes the dissociation of 14-3-3 from FOXO3a by directly dephosphorylating T32/S253. It is possible, however, that PP2A inhibition could indirectly sustain FOXO3a phosphorylation by preventing activation of MST-1 and JNK signaling cascades, which have been shown to converge upon the FOXO3a/14-3-3 complex to promote 14-3-3 dissociation in response to apoptotic signals (Sunayama et al., 2005; Lehtinen et al., 2006). Therefore, to address whether MST/JNK signaling to FOXO3a facilitates its dephosphorylation after inhibition of PI3K/AKT, we relieved FOXO3a of input from MST and JNK signaling by expressing a FOXO3a mutant resistant to MST-1 phosphorylation, green fluorescent protein (GFP)-FOXO3a-S207A (Lehtinen et al., 2006), in cells treated with a JNK kinase inhibitor. As shown in Figure 5C, both wild-type and mutant GFP-FOXO3a were dephosphorylated at T32 and S253 to similar extents after LY/AKT-I treatment. ST, however, compromised dephosphorylation of the S207A mutant.
Figure 5.
PP2A-mediated dephosphorylation of T32 and S253 facilitates the disassembly of FOXO3a/14-3-3 complexes independent of MST and JNK signaling. (A) A FLAG-FOXO3a expression plasmid was cotransfected into HeLa cells with either an EV or ST expression plasmid. Cells were grown in serum and then either lysed (−) or treated with 20 μM each of LY and AKT-I in the absence of serum for 30 min (+) before lysis. FOXO3a was immunoprecipitated (IP) from whole-cell lysates (WCL) and immunocomplexes were immunoblotted (IB) with the indicated antibodies (top). Then, 5% of the IP input taken from the initial WCL was resolved on a separate gel and subject to IB with the indicated antibodies (bottom). (B) HeLa cells were transfected with either an EV or ST expression plasmid and treated as described in A. Whole-cell lysates were IP with either control immunoglobulin (Ig)G or an anti-FOXO3a IgG as indicated and immunocomplexes were IB with the indicated antibodies (top). An aliquot of the IP input taken from the initial WCL was resolved on a separate gel and subject to IB with the indicated antibodies (bottom). (C) HeLa cells were transfected with wild type GFP-FOXO3a (WT), GFP-FOXO3a-S207A (S207A), or GFP-FOXO3a-S207A and ST (S207A/ST) expression plasmids then pretreated for 30 min with either DMSO (−) or 20 μM SP600125 (+) as indicated. Cells were then lysed or treated with 20 μM each of LY and AKT-I alone or in the continued presence of 20 μM SP600125 for 30 min (+) before lysis. Whole-cell lysates were analyzed by IB with the indicated antibodies. (D) HeLa cells were transfected with wild type GFP-FOXO3a (WT), GFP-FOXO3a-WT, and FLAG-MST-1 (WT/MST-1), or GFP-FOXO3a-S207A and FLAG-MST-1 (S207A/MST-1) expression plasmids and then either lysed (−) or treated with 20 μM each of LY and AKT-I for 30 min in the absence of serum (+) as indicated. Whole-cell lysates were IB with the indicated antibodies. The arrows to the left of the indicated blots designate caspase-cleaved MST-1. (E) HeLa cells were transfected with either an EV or a FLAG-FOXO3a expression plasmid and treated as described in A. FOXO3a was IP from whole-cell lysates and immunocomplexes were IB with the indicated antibodies (top). Then, 5% of the IP input taken from the initial WCL was resolved on a separate gel and subject to IB with the indicated antibodies (bottom).
To further exclude the involvement of MST-1 in the rapid dissociation of 14-3-3 from FOXO3a after inhibition of PI3K/AKT signaling, we monitored the phosphorylation status of S207. In support of a recent study demonstrating that caspase cleavage of MST-1 is required for its ability to efficiently phosphorylate FOXO3a at S207 (Jang et al., 2007), overexpression of MST-1 generated a highly active ∼36-kDa caspase cleavage product that corresponded to phosphorylation of FOXO3a at S207. We were unable, however, to observe either caspase cleavage of endogenous MST-1 or phosphorylation of FOXO3a at S207 after brief treatment with LY/AKT-I (Figure 5D). Further studies demonstrated that LY/AKT-I-induced dissociation of 14-3-3 from FOXO3a did not precede but rather coincided with T32 and S253 dephosphorylation (Figure 5E). Together, these findings clearly show that immediately following inhibition of PI3K/AKT, MST/JNK signaling cascades are not used to promote dissociation of 14-3-3 from FOXO3a and its subsequent dephosphorylation. Rather, PP2A associated with FOXO3a directly dephosphorylates T32 and S253 to facilitate release of 14-3-3.
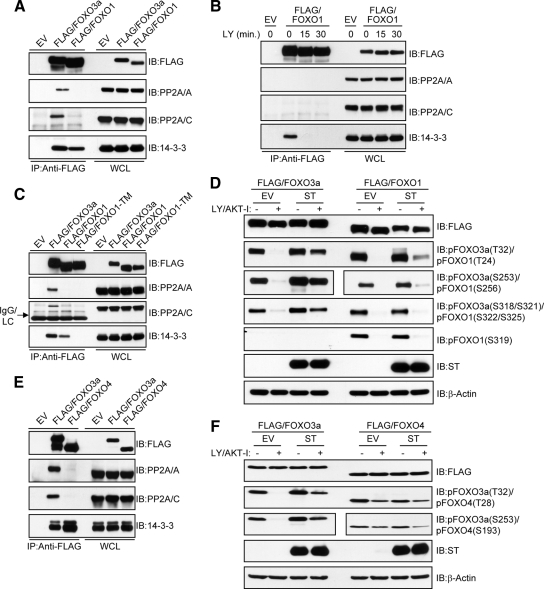
PP2A Differentially Interacts with and Dephosphorylates FOXO Family Members at Conserved AKT Phosphorylation Sites
We next sought to determine whether the evolutionarily conserved AKT phosphorylation sites of other FOXO transcription factors are subject to direct regulation by PP2A. As predicted, both FOXO3a and FOXO1 interacted with 14-3-3 under steady-state conditions in the presence of serum. The interaction between FOXO3a and the PP2A core enzyme, however, was much more prominent compared with FOXO1 (Figure 6A). Although this observation strongly suggests that PP2A is not a FOXO1 phosphatase, it is possible that our inability to detect a robust interaction between PP2A and FOXO1 resulted from competitive binding with 14-3-3. Indeed, this scenario has been observed with other PP2A substrates such as BAD (Chiang et al., 2003). To test this possibility, HeLa cells expressing FLAG-FOXO1 were treated with LY/AKT-I to induce dissociation from 14-3-3. As shown in Figure 6B, even though FOXO1 was rapidly dissociated from 14-3-3, a concomitant increase in PP2A binding was not observed. We further showed that FOXO1-TM, which is resistant to AKT-mediated phosphorylation and thus unable to bind 14-3-3 (Zhao et al., 2004), did not display an increased interaction with PP2A compared with wild-type FOXO1 (Figure 6C). These results clearly demonstrate that the lack of robust PP2A core enzyme binding to FOXO1 is not due to a competitive mechanism involving 14-3-3. Given the significant difference in PP2A binding to FOXO3a and FOXO1, we predicted that PP2A inhibition would have little effect on FOXO1 dephosphorylation at either the conserved AKT (Rena et al., 1999) or CK1 (Rena et al., 2002; Rena et al., 2004) phosphorylation sites after LY/AKT-I treatment. Indeed, ST was unable to prevent FOXO1 dephosphorylation at either the AKT (T24, S256, and S319) or CK1 phosphorylation sites (S322/S325) to the same extent as observed for FOXO3a (Figure 6D). We also addressed whether PP2A could interact with FOXO4 and observed a very weak interaction in HeLa cells (Figure 6E). Furthermore, although the kinetics of FOXO4 dephosphorylation at its conserved AKT phosphorylation sites (T28 and S193) (Kops et al., 1999) were not nearly as rapid as those observed for FOXO3a or FOXO1, ST was without effect (Figure 6F). These findings suggest that PP2A is not involved in directly dephosphorylating the conserved AKT phosphorylation sites of all FOXO family members. More importantly, the finding that ST significantly compromised the dephosphorylation of AKT sites on FOXO3a, but not the corresponding sites on either FOXO1 or FOXO4, further demonstrates that impaired FOXO3a dephosphorylation by PP2A inhibition is not due to sustained AKT activity.
Figure 6.
PP2A selectively dephosphorylates the AKT phosphorylation sites of FOXO family members. (A) HeLa cells were transfected with either an EV or FLAG-FOXO3a and FLAG-FOXO1 expression plasmids. Whole-cell lysates were immunoprecipitated (IP) with anti-FLAG(M2) and immunocomplexes were immunoblotted (IB) with the indicated antibodies. (B) HeLa cells transfected with a FLAG-FOXO1 expression plasmid were grown in serum and then either lysed (0 min) or treated with 20 μM LY in the absence of serum for the indicated time points before lysis. FOXO1 was IP from lysates and immunocomplexes were IB with the indicated antibodies. (C) HeLa cells were transfected with either an EV or FLAG-FOXO3a, FLAG-FOXO1, and FLAG-FOXO1-TM expression plasmids. Whole-cell lysates were IP with anti-FLAG(M2) and immunocomplexes were IB with the indicated antibodies. (D) Effect of ST on the dephosphorylation of FOXO1 was compared with FOXO3a. Cells were grown in serum and then either lysed (−) or treated with 20 μM each of LY and AKT-I in the absence of serum for 30 min (+) before lysis. Where indicated, membranes were cut to IB pFOXO3a at S253 and pFOXO1 at S256 with separate phosphospecific antibodies. The same phospho-specific antibody was used to detect pFOXO3a at T32 and pFOXO1 at T24. Similarly, the same phosphospecific antibody was used to detect pFOXO3a at S318/S321 and pFOXO1 at S322/S325. (E) Co-IP of PP2A with FLAG-FOXO4 was performed as described in A. (F) Effect of ST on the dephosphorylation of FOXO4 was compared with FOXO3a as described in D.
PP2A Is Required for Efficient Nuclear Import and Transcriptional Activation of FOXO3a after Inhibition of PI3K/AKT Signaling
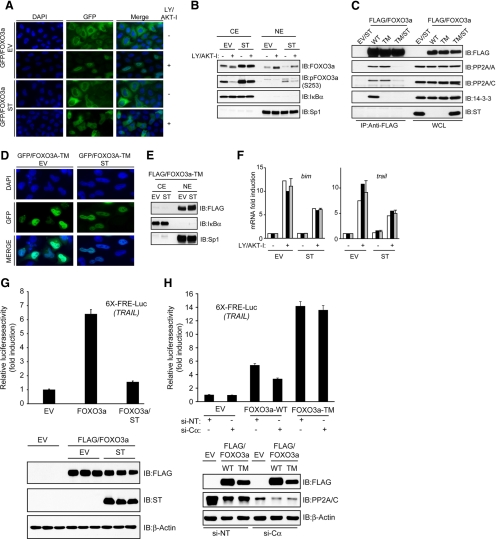
Because ST compromised the dissociation of 14-3-3 from FOXO3a following inhibition of PI3K/AKT signaling, we posited that the nuclear translocation and transcriptional activation of FOXO3a would also be compromised. Indeed, fluorescence microscopy studies demonstrated that translocation of GFP-FOXO3a from the cytoplasmic to nuclear compartment of HeLa cells after LY/AKT-I treatment was compromised by ST (Figure 7A). Cellular fractionation studies also revealed an enhanced accumulation of total and phosphorylated endogenous FOXO3a in the cytoplasmic compartment of cells expressing ST under steady-state conditions in the presence of serum and after LY/AKT-I treatment (Figure 7B). To address whether the effects of ST on FOXO3a nuclear translocation were due largely to impaired T32 and S253 dephosphorylation, we coexpressed ST and FOXO3a-TM, a mutant that is resistant to AKT-mediated phosphorylation and 14-3-3 binding, thus allowing for its constitutive nuclear localization (Brunet et al., 1999). Although ST disrupted the interaction between FOXO3a-TM and PP2A (Figure 7C), fluorescence microscopy (Figure 7D) and cellular fractionation (Figure 7E) studies revealed that FOXO3a-TM was still able to localize to the nuclear compartment, even in the presence of serum. We also sought to examine the effects of PP2A inhibition on the nuclear translocation of other FOXOs. Consistent with a previous study (Zhao et al., 2004), mutation of all three AKT phosphorylation sites within FOXO1 to alanine (FOXO1-TM) promoted its nuclear localization in the presence of serum and this was unaffected by ST (Supplemental Figure S4A). Furthermore, ST was unable to prevent the nuclear translocation of either wild-type FOXO1 or FOXO4 in response to LY/AKT-I treatment (Supplemental Figure S4, B and C, respectively), which is consistent with their weak interaction with PP2A and unrestrained dephosphorylation of AKT phosphorylation sites in the presence of ST (Figure 6D).
Figure 7.
PP2A is required for efficient nuclear translocation and transcriptional activation of FOXO3a following PI3K/AKT inhibition. (A) GFP-FOXO3a expression plasmid was cotransfected into HeLa cells with either an EV or ST expression plasmid. Cells grown in serum were either fixed (−) or treated with 20 μM each of LY and AKT-I for 30 min in the absence of serum (+) before fixation. GFP-FOXO3a and 4,6-diamidino-2-phenylindole (DAPI)-stained nuclei were visualized by fluorescence microscopy. (B) HeLa cells were transfected with either an EV or ST expression plasmid. Cells grown in serum were either fractionated (−) or treated with 20 μM each of LY and AKT-I for 30 min in the absence of serum (+) before fractionation. Cytoplasmic (CE) and nuclear (NE) extracts were immunoblotted (IB) with the anti-IκBα and -Sp1 antibodies to evaluate extract purity. (C) FOXO3a was immunoprecipitated (IP) from whole-cell lysates derived from HeLa cells transfected with wild-type FLAG-FOXO3a (WT), FLAG-FOXO3a-TM (TM), or FLAG-FOXO3a-TM and ST (TM/ST) expression plasmids. Immunocomplexes were IB with the indicated antibodies. (D) A GFP-FOXO3a- TM expression plasmid was cotransfected into HeLa cells with either an EV or ST expression plasmid. GFP-FOXO3a-TM and DAPI-stained nuclei were visualized by fluorescence microscopy. (E) Analysis of FLAG-FOXO3a-TM subcellular localization by fractionation. (F) BaF3/pMIG (EV) and BaF3/pMIG/ST (ST) cells were grown in the presence of interleukin (IL)-3 and either lysed (−) or treated with 20 μM each of LY and AKT-I for 7h in the absence of IL-3 (+) before lysis. Quantitative real-time RT-PCR was used to calculate the mean fold induction in bim (left) and trail (right) transcripts after LY/AKT-I treatment relative to untreated BaF3/pMIG cells, which is shown by gray columns. Results shown are a representative of three independent experiments performed in duplicate (black and white columns) each time. Error bars represent the SD from a representative experiment. (G) HeLa cells with a chromosomally integrated luciferase reporter plasmid containing six-tandem FRE from the human TRAIL promoter were transfected with the indicated expression plasmids (6X-FRE/TRAIL-Luc). The increase in firefly luciferase activity is shown as the mean fold induction over the activity in cells transfected with EV alone. In each experiment, triplicate dishes for each transfection group were analyzed. The result presented in this figure is a representative of two independent experiments. Each column represents the mean of the triplicate values, and the error bars represent the SD of the mean for this particular experiment (top). Cell extracts from the presented reporter experiment were analyzed by immunoblotting with the indicated antibodies (bottom). (H) The HeLa reporter cell line was transfected with an empty vector (EV), wild-type FLAG-FOXO3a (WT), or FLAG-FOXO3a-TM (TM) expression plasmids together with either nontargeting siRNAs (NT) or siRNAs targeting the PP2A catalytic subunit (Cα) as indicated. Results were analyzed as described in G.
Previous studies have shown that Foxo3a is indispensable for the transactivation of bcl-2–interacting mediator of cell death (bim; Essafi et al., 2005) and tumor necrosis factor-related apoptosis-inducing ligand (trail) promoters (Ghaffari et al., 2003) in the BaF3 proB cell line after cytokine withdrawal. Indeed, shRNA-mediated silencing of Foxo3a in BaF3 cells significantly impaired the induction of Bim expression (BimEL and BimL) by LY/AKT-I treatment compared with shGFP control cells (Supplemental Figure S5). Given the dependence of FOXO3a nuclear translocation on PP2A activity, we addressed the consequence of PP2A inhibition on its transcriptional activity by analyzing bim and trail transcript levels after LY/AKT-I treatment in BaF3 cells stably expressing ST. As shown in Figure 7F, real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis revealed that the mRNA levels of both trail and bim were significantly reduced in BaF3 cells expressing ST after LY/AKT-I treatment, compared with vector control BaF3 cells. Given that we were unable to detect either Foxo1 or Foxo4 expression in BaF3 cells (data not shown) and knockdown of Foxo3a compromised Bim induction by LY/AKT-I treatment, the effect of ST on bim and trail transcript levels strongly suggests a mechanism involving inhibition of PP2A-mediated dephosphorylation and activation of endogenous Foxo3a. ST also significantly inhibited FOXO3a transcriptional activity in a HeLa reporter cell line harboring a chromosomally integrated luciferase reporter plasmid driven by FOXO response elements (FRE) of the TRAIL promoter (Figure 7G). Furthermore, siRNA-mediated silencing of the PP2A catalytic subunit in the HeLa reporter cell line significantly inhibited the transcriptional activity of wild-type FOXO3a but not that of FOXO3a-TM, which is impervious to AKT-mediated phosphorylation (Figure 7H).
Collectively, these findings provide strong evidence that PP2A promotes the nuclear translocation and transcriptional activation of FOXO3a in response to PI3K/AKT inhibition through the dephosphorylation of T32 and S253.
DISCUSSION
Stringent regulation of FOXO subcellular localization is critical for the appropriate transcriptional control of cell cycle progression, DNA damage repair, and apoptosis (Dansen and Burgering, 2008). This is underscored by the identification of multiple prosurvival kinases such as AKT, SGK-1, ERK, and IKK that converge upon FOXO3a in a coordinated manner to promote its inactivation, in part, through nuclear exclusion (Brunet et al., 1999, 2001; Hu et al., 2004; Yang et al., 2008). Although FOXO3a can be targeted for proteosomal degradation by deregulation of these kinases (Hu et al., 2004; Yang et al., 2008), it is quite stable in untransformed cells and thus likely to undergo continuous shuttling between nuclear and cytoplasmic compartments due to the dynamic equilibrium between kinases and antagonistic phosphatase(s). The identity and mechanism of action of the phosphatase(s) that participate in the regulation of this equilibrium, however, have remained elusive. In this study, we identified PP2A as a FOXO3a binding partner (Figure 2) and suspected that this interaction represented a link to understanding the phosphatase-dependent control of FOXO3a subcellular localization and transcriptional activity. Indeed, we found FOXO3a dephosphorylation, nuclear localization, and transcriptional activation to be critically dependent upon its interaction with PP2A.
Because PP2A is a phosphatase for numerous kinases (Millward et al., 1999), it is imperative that in designating a candidate phosphoprotein as a novel PP2A substrate, one considers the upstream kinases that converge upon the putative PP2A substrate, their relative contribution to the phosphorylation of site(s) in question, and the effects of PP2A on the activity of these upstream kinases. Previous studies that identified FOXO3a as substrate of AKT and SGK-1 either relied upon overexpression of dominant-interfering forms of these kinases (Brunet et al., 1999, 2001) or genetic disruption of the complex of mTOR, mLST8, and rictor (Sarbassov et al., 2005), which may have generated spurious results due to the simultaneous inactivation of endogenous forms of both kinases as well as other AGC kinases. In thus study, however, we revealed that both AKT-1 and AKT-2 are necessary for FOXO3a phosphorylation at T32 and S253. Based upon the ability of AKT to be negatively regulated by PP2A (Trotman et al., 2006; Padmanabhan et al., 2009), we inhibited AKT activity directly in the context of PP2A inhibition and showed a dramatic impairment in FOXO3a dephosphorylation, unequivocally demonstrating a direct role for PP2A activity in the dephosphorylation of FOXO3a (Figure 4).
Although FOXO1, FOXO3a, and FOXO4 are negatively regulated through a conserved mechanism involving the phosphorylation of three consensus AKT sites (Burgering and Kops, 2002), it is uncertain whether a conserved phosphatase-dependent mechanism exists to antagonize AKT at these sites. Our study addressed this question and provides evidence suggesting that PP2A, although playing a significant role in the direct and rapid dephosphorylation of FOXO3a at AKT sites, is not the prominent phosphatase for the corresponding sites on either FOXO1 or FOXO4 (Figure 6). The slower kinetics of FOXO4 dephosphorylation at T28 and S193 after AKT inhibition suggests that phosphatase-dependent control of these sites is unique, perhaps involving a mechanism dependent upon Ras-Ral-mediated phosphorylation of C-terminal residues T447 and T451 (De Ruiter et al., 2001). Because we observed a weak interaction between FOXO4 and PP2A, however, it is unclear whether PP2A is incorporated into this putative mechanism. Furthermore, although the inability of either wild-type FOXO1 or FOXO1-TM to form a robust interaction with PP2A suggests that FOXO1 subcellular localization is not a critical determinant for PP2A binding, the apparent selectivity of PP2A toward FOXO3a remains uncertain and raises multiple questions for future studies. For example, is the ability of FOXO1 and FOXO4 to interact with PP2A dictated by specific substrate-targeting regulatory B subunits that are expressed in a cell-type specific manner? Alternatively, do FOXO1 and FOXO4 lack certain structural motifs present in FOXO3a that allow for binding and direct regulation by PP2A? Although we found AKT site dephosphorylation and nuclear translocation of FOXO1 and FOXO4 to occur in a PP2A-independent manner, we cannot exclude the possibility that PP2A indirectly regulates their function in the nuclear compartment by modulating the activity of upstream kinases that directly phosphorylate other functionally relevant sites.
PP2A regulatory subunits impart substrate selectivity and subcellular targeting to the phosphatase. For example, it has been reported that PP2A holoenzymes containing B56α, B56β, and B56ε are localized to the cytoplasmic compartment (McCright et al., 1996). Although we found B56ε to interact with FOXO3a, this interaction did not seem to be essential for FOXO3a dephosphorylation at either the AKT or CK1 sites. Perhaps the effectiveness of OA and ST in preventing FOXO3a dephosphorylation is a reflection of holoenzymes with functional redundancy interacting with FOXO3a. Indeed, ST can disrupt the assembly and substrate targeting of multiple holoenzymes (Pallas et al., 1990; Chen et al., 2004) and was more efficient than B56ε knockdown in displacing PP2A from FOXO3a. Similarly, a recent study showed that OA-induced phosphorylation of the PP2A catalytic subunit at Y307 likely disrupts the integrity of holoenzymes containing B56α, B56β, and B56ε (Longin et al., 2007). In light of this, further studies are needed to address the contribution of other B56 family members in the recruitment of PP2A to FOXO3a.
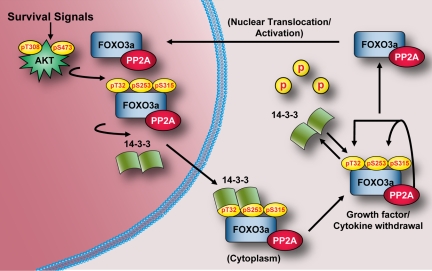
Based upon our findings, we propose a model for PP2A-mediated dephosphorylation of FOXO3a (Figure 8) in which there exists a constant cycling between AKT-dependent phosphorylation/14-3-3 binding and PP2A-dependent dephosphorylation/14-3-3 dissociation. Despite a robust interaction between FOXO3a and PP2A when AKT is active, the phosphorylation of T32/S253 is highly favored due to the protective effect of 14-3-3 binding. When PI3K/AKT signaling is attenuated, however, the equilibrium is rapidly shifted and PP2A is poised to dephosphorylate FOXO3a at T32/S253 in the cytoplasm, which results in destabilized 14-3-binding, nuclear translocation, and transcriptional activation. Although a role for PP2A in the control of FOXO3a phosphorylation at ERK (Yang et al., 2008) and IKK (Hu et al., 2004) sites was not addressed, PP2A inhibition failed to interfere with either the nuclear localization or transcriptional activity of FOXO3a-TM, indicating that the predominant mechanism by which PP2A activates FOXO3a function is through AKT site dephosphorylation (Figure 7). Furthermore, although we did not determine whether the CK1 sites on FOXO3a are a direct target of PP2A, the observed dephosphorylation of these sites probably contributes to FOXO3a nuclear localization by shifting the equilibrium away from enhanced nuclear export, as has been reported previously (Rena et al., 2002, 2004). Our observation that FOXO3a underwent dephosphorylation and nuclear translocation in the absence of its phosphorylation at S207 by MST-1 supports the contention that PP2A-mediated dephosphorylation of T32/S253 is the driving force for rapid dissociation of 14-3-3 and nuclear translocation of FOXO3a after PI3K/AKT inhibition. Indeed, we observed that 14-3-3 and PP2A do not compete for binding to FOXO3a and the kinetics of T32/S253 dephosphorylation paralleled those of FOXO3a/14-3-3 complex disassembly. Furthermore, a recent report demonstrated that caspase cleavage of MST-1 is required for acquisition of catalytic activity toward FOXO3a and this is prevented by direct phosphorylation of MST-1 by AKT (Jang et al., 2007). Only after prolonged periods of dephosphorylation at the AKT site, however, does MST-1 become susceptible to caspase cleavage and competent to phosphorylate FOXO3a at S207, which prohibits 14-3-3 binding to facilitate nuclear retention.
Figure 8.
Model for PP2A as a positive effector of FOXO3a function. In the presence of growth factors and cytokines that promote maximal AKT activation (phosphorylation at T308 and S473), FOXO3a bound to PP2A in the nucleus is phosphorylated at T32, S253, and S315. Phosphorylation of FOXO3a at T32 and S253 creates phosphorylation-dependent docking sites for 14-3-3 proteins, which facilitate the nuclear export and cytoplasmic sequestration of FOXO3a. After inhibition of PI3K/AKT signaling, phosphorylation of FOXO3a is no longer favored, and the equilibrium is switched such that PP2A bound to FOXO3a is poised to rapidly dephosphorylate FOXO3a at T32 and S253 to facilitate its dissociation from 14-3-3, nuclear translocation, and transcriptional activation.
The present report establishes a functional interaction between PP2A and FOXO3a that is critical for the dephosphorylation-dependent nuclear accumulation and transcriptional activation of FOXO3a in response to inhibition of AKT signaling. Furthermore, as FOXO3a is gaining prominence as a tumor suppressor, our findings may provide evidence for disassembly of the FOXO3a/PP2A complex as an important event in SV40 ST-mediated cellular transformation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Joseph Avruch (Massachusetts General Hospital/Harvard Medical School), Azad Bonni (Harvard Medical School), Boudewijn Burgering (University Medical Center Utrecht), James Griffin (Dana Farber Cancer Institute/Harvard Medical School), Kun-Liang Guan (University of California, San Diego Medical Center), Marc Mumby (University of Texas Southwestern Medical Center), and William Sellers (Novartis Institutes for BioMedical Research) for reagents. We are grateful to Jack Lawler and Susan Glueck for critical reading of the manuscript. This work was supported by U.S. Public Health Service grants CA131664 and CA105306 (to R. K.), 2-T32-HL07623-22 and 5-F32-CA132365-02 (to A. S.), and a Lady Tata Memorial Trust Award (to O. B.).
Abbreviations used:
- Bim
Bcl-2–interacting mediator of cell death
- CK
casein kinase
- FOXO
forkhead box, class O
- JNK
Jun-NH2-terminal kinase
- MST
mammalian sterile20-like kinase
- PI3K
phosphatidylinositol-3-kinase
- PP2A
protein phosphatase 2A
- SGK
serum- and glucocorticoid-inducible kinase
- SV40 ST
simian virus 40 small t antigen
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-09-0795) on January 28, 2010.
REFERENCES
- Anderson M. J., Viars C. S., Czekay S., Cavenee W. K., Arden K. C. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics. 1998;47:187–199. doi: 10.1006/geno.1997.5122. [DOI] [PubMed] [Google Scholar]
- Barnett S. F., et al. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem. J. 2005;385:399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Brunet A., Kanai F., Stehn J., Xu J., Sarbassova D., Frangioni J. V., Dalal S. N., DeCaprio J. A., Greenberg M. E., Yaffe M. B. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J. Cell Biol. 2002;156:817–828. doi: 10.1083/jcb.200112059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A., Park J., Tran H., Hu L. S., Hemmings B. A., Greenberg M. E. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol. Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering B. M., Kops G. J. Cell cycle and death control: long live Forkheads. Trends Biochem. Sci. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- Chen W., Arroyo J. D., Timmons J. C., Possemato R., Hahn W. C. Cancer-associated PP2A Aalpha subunits induce functional haploinsufficiency and tumorigenicity. Cancer Res. 2005;65:8183–8192. doi: 10.1158/0008-5472.CAN-05-1103. [DOI] [PubMed] [Google Scholar]
- Chen W., Possemato R., Campbell K. T., Plattner C. A., Pallas D. C., Hahn W. C. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell. 2004;5:127–136. doi: 10.1016/s1535-6108(04)00026-1. [DOI] [PubMed] [Google Scholar]
- Chiang C. W., Harris G., Ellig C., Masters S. C., Subramanian R., Shenolikar S., Wadzinski B. E., Yang E. Protein phosphatase 2A activates the proapoptotic function of BAD in interleukin-3-dependent lymphoid cells by a mechanism requiring 14-3-3 dissociation. Blood. 2001;97:1289–1297. doi: 10.1182/blood.v97.5.1289. [DOI] [PubMed] [Google Scholar]
- Chiang C. W., Kanies C., Kim K. W., Fang W. B., Parkhurst C., Xie M., Henry T., Yang E. Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Mol. Cell. Biol. 2003;23:6350–6362. doi: 10.1128/MCB.23.18.6350-6362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansen T. B., Burgering B. M. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 2008;18:421–429. doi: 10.1016/j.tcb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- De Ruiter N. D., Burgering B. M., Bos J. L. Regulation of the Forkhead transcription factor AFX by Ral-dependent phosphorylation of threonines 447 and 451. Mol. Cell. Biol. 2001;21:8225–8235. doi: 10.1128/MCB.21.23.8225-8235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essafi A., Fernandez de Mattos S., Hassen Y. A., Soeiro I., Mufti G. J., Thomas N. S., Medema R. H., Lam E. W. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–2329. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- Favre B., Turowski P., Hemmings B. A. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J. Biol. Chem. 1997;272:13856–13863. doi: 10.1074/jbc.272.21.13856. [DOI] [PubMed] [Google Scholar]
- Gao D., Inuzuka H., Tseng A., Chin R. Y., Toker A., Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat. Cell Biol. 2009;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari S., Jagani Z., Kitidis C., Lodish H. F., Khosravi-Far R. Cytokines and BCR-ABL mediate suppression of TRAIL-induced apoptosis through inhibition of forkhead FOXO3a transcription factor. Proc. Natl. Acad. Sci. USA. 2003;100:6523–6528. doi: 10.1073/pnas.0731871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J., Probst A., Ehler E., Hemmings B., Kues W. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Calpha. Proc. Natl. Acad. Sci. USA. 1998;95:12370–12375. doi: 10.1073/pnas.95.21.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M. C., et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- Jang S. W., Yang S. J., Srinivasan S., Ye K. Akt phosphorylates MstI and prevents its proteolytic activation, blocking FOXO3 phosphorylation and nuclear translocation. J. Biol. Chem. 2007;282:30836–30844. doi: 10.1074/jbc.M704542200. [DOI] [PubMed] [Google Scholar]
- Janssens V., Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops G. J., de Ruiter N. D., De Vries-Smits A. M., Powell D. R., Bos J. L., Burgering B. M. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- Lehtinen M. K., et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Logie L., Ruiz-Alcaraz A. J., Keane M., Woods Y. L., Bain J., Marquez R., Alessi D. R., Sutherland C. Characterization of a protein kinase B inhibitor in vitro and in insulin-treated liver cells. Diabetes. 2007;56:2218–2227. doi: 10.2337/db07-0343. [DOI] [PubMed] [Google Scholar]
- Longin S., Zwaenepoel K., Louis J. V., Dilworth S., Goris J., Janssens V. Selection of protein phosphatase 2A regulatory subunits is mediated by the C terminus of the catalytic subunit. J. Biol. Chem. 2007;282:26971–26980. doi: 10.1074/jbc.M704059200. [DOI] [PubMed] [Google Scholar]
- McCright B., Rivers A. M., Audlin S., Virshup D. M. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J. Biol. Chem. 1996;271:22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- Millward T. A., Zolnierowicz S., Hemmings B. A. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi S., Shima H., Tanuma N., Matsuura N., Takekawa M., Urano T., Kataoka T., Ubukata M., Kikuchi K. Usage of tautomycetin, a novel inhibitor of protein phosphatase 1 (PP1), reveals that PP1 is a positive regulator of Raf-1 in vivo. J. Biol. Chem. 2003;278:82–88. doi: 10.1074/jbc.M208888200. [DOI] [PubMed] [Google Scholar]
- Obsil T., Ghirlando R., Anderson D. E., Hickman A. B., Dyda F. Two 14-3-3 binding motifs are required for stable association of Forkhead transcription factor FOXO4 with 14-3-3 proteins and inhibition of DNA binding. Biochemistry. 2003;42:15264–15272. doi: 10.1021/bi0352724. [DOI] [PubMed] [Google Scholar]
- Obsilova V., Vecer J., Herman P., Pabianova A., Sulc M., Teisinger J., Boura E., Obsil T. 14-3-3 protein interacts with nuclear localization sequence of forkhead transcription factor FoxO4. Biochemistry. 2005;44:11608–11617. doi: 10.1021/bi050618r. [DOI] [PubMed] [Google Scholar]
- Padmanabhan S., Mukhopadhyay A., Narasimhan S. D., Tesz G., Czech M. P., Tissenbaum H. A. A PP2A regulatory subunit regulates C. elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation. Cell. 2009;136:939–951. doi: 10.1016/j.cell.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas D. C., Shahrik L. K., Martin B. L., Jaspers S., Miller T. B., Brautigan D. L., Roberts T. M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Petosa C., Masters S. C., Bankston L. A., Pohl J., Wang B., Fu H., Liddington R. C. 14-3-3 ζ binds a phosphorylated Raf peptide and an unphosphorylated peptide via its conserved amphipathic groove. J. Biol. Chem. 1998;273:16305–16310. doi: 10.1074/jbc.273.26.16305. [DOI] [PubMed] [Google Scholar]
- Rena G., Bain J., Elliott M., Cohen P. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 2004;5:60–65. doi: 10.1038/sj.embor.7400048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G., Guo S., Cichy S. C., Unterman T. G., Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J. Biol. Chem. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- Rena G., Woods Y. L., Prescott A. R., Peggie M., Unterman T. G., Williams M. R., Cohen P. Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion. EMBO J. 2002;21:2263–2271. doi: 10.1093/emboj/21.9.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinner O., Mueller L. N., Hubalek M., Muller M., Gstaiger M., Aebersold R. An integrated mass spectrometric and computational framework for the analysis of protein interaction networks. Nat. Biotechnol. 2007;25:345–352. doi: 10.1038/nbt1289. [DOI] [PubMed] [Google Scholar]
- Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sontag E., Fedorov S., Kamibayashi C., Robbins D., Cobb M., Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- Sunayama J., Tsuruta F., Masuyama N., Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J. Cell Biol. 2005;170:295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman L. C., Alimonti A., Scaglioni P. P., Koutcher J. A., Cordon-Cardo C., Pandolfi P. P. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup D. M. Protein phosphatase 2A: a panoply of enzymes. Curr. Opin. Cell Biol. 2000;12:180–185. doi: 10.1016/s0955-0674(99)00074-5. [DOI] [PubMed] [Google Scholar]
- Walsh A. H., Cheng A., Honkanen R. E. Fostriecin, an antitumor antibiotic with inhibitory activity against serine/threonine protein phosphatases types 1 (PP1) and 2A (PP2A), is highly selective for PP2A. FEBS Lett. 1997;416:230–234. doi: 10.1016/s0014-5793(97)01210-6. [DOI] [PubMed] [Google Scholar]
- Wang B., Yang H., Liu Y. C., Jelinek T., Zhang L., Ruoslahti E., Fu H. Isolation of high-affinity peptide antagonists of 14-3-3 proteins by phage display. Biochemistry. 1999;38:12499–12504. doi: 10.1021/bi991353h. [DOI] [PubMed] [Google Scholar]
- Yaffe M. B., Rittinger K., Volinia S., Caron P. R., Aitken A., Leffers H., Gamblin S. J., Smerdon S. J., Cantley L. C. The structural basis for 14-3-3, phosphopeptide binding specificity. Cell. 1997;91:961–971. doi: 10.1016/s0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]
- Yang J. Y., et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat. Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Gan L., Pan H., Kan D., Majeski M., Adam S. A., Unterman T. G. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO 1, characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem. J. 2004;378:839–849. doi: 10.1042/BJ20031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.