This article shows that globular adiponectin regulates vital cues of mesoangioblast, such as proliferation, survival, and migration toward myotubes and the myogenic properties. In vivo experiments confirm that globular adiponectin increases the survival, engraftment, and localization to muscle of mesoangioblasts in α-sarcoglycan-null mice.
Abstract
Mesoangioblasts are progenitor endowed with multipotent mesoderm differentiation ability. Despite the promising results obtained with mesoangioblast transplantation in muscle dystrophy, an improvement of their efficient engrafting and survival within damaged muscles, as well as their ex vivo activation/expansion and commitment toward myogenic lineage, is highly needed and should greatly increase their therapeutic potential. We show that globular adiponectin, an adipokine endowed with metabolic and differentiating functions for muscles, regulates vital cues of mesoangioblast cell biology. The adipokine drives mesoangioblasts to entry cell cycle and strongly counteracts the apoptotic process triggered by growth factor withdrawal, thereby serving as an activating and prosurvival stem cell factor. In addition, adiponectin provides a specific protection against anoikis, the apoptotic death due to lack of anchorage to extracellular matrix, suggesting a key protective role for these nonresident stem cells after systemic injection. Finally, adiponectin behaves as a chemoattractive factor toward mature myotubes and stimulates their differentiation toward the skeletal muscle lineage, serving as a positive regulator in mesoangioblast homing to injured or diseased muscles. We conclude that adiponectin exerts several advantageous effects on mesoangioblasts, potentially valuable to improve their efficacy in cell based therapies of diseased muscles.
INTRODUCTION
Adiponectin is an adipocyte-derived hormone endowed with antidiabetic, anti-inflammatory, and antiatherogenic properties and exerting insulin-sensitizing metabolic effects (Kadowaki et al., 2006; Guerre-Millo, 2008). The hormone, via ligation to its receptors, is known to act in skeletal muscle, where it regulates glucose and lipid metabolism (Berg et al., 2002). All biological activities are mediated by two atypical G-protein–coupled receptors, named AdipoR1 and AdipoR2 (Yamauchi et al., 2003). The adipokine exhibits insulin-sensitizing, fat-burning, and anti-inflammatory properties (Yamauchi et al., 2001; Lihn et al., 2005; Kadowaki et al., 2006; Ouchi and Walsh, 2007). It increases glucose uptake in cultured myocytes or isolated mouse muscle and alters lipid metabolism through the stimulation of muscle fatty acid oxidation (Yamauchi et al., 2002, 2003). Adiponectin has recently been indicated as a potent prodifferentiative factor for myoblasts, acting on both their differentiation and fusion into mature myofibers (Fiaschi et al., 2009). Notably, adiponectin is directly produced in muscle after inflammatory cytokine treatment, suggesting a role for proinflammatory cues in adiponectin autocrine production from muscle (Delaigle et al., 2004).
Regeneration of adult skeletal muscle involves the activation, proliferation, and differentiation of quiescent satellite cells residing in a dedicated niche close to the basal lamina of myofibers (Buckingham, 2006; Le Grand and Rudnicki, 2007). The progeny of satellite cells associated with isolated myofibers are commonly considered as a model of quiescence, activation, and commitment to skeletal muscle lineage (Sherwood et al., 2004; Dhawan and Rando, 2005). Several mesodermal cells, including adipose-derived stem cells and mesoangioblasts, have been indicated as potent and active contributors for muscle regeneration (Minasi et al., 2002; Cossu and Bianco, 2003; Oreffo et al., 2005; Rodriguez et al., 2005). These cells may be rooted in a skeletal lineage within the muscle niche and are currently considered as possible candidates for cell therapy of muscle degenerative diseases (Leri et al., 2005; Cossu and Biressi, 2005; Chamberlain et al., 2007). Mesoangioblasts are attractive candidates for future stem cell therapy as their intraarterial delivery corrects morphology and function of muscles in α-sarcoglycan-null mice (Sgca-null) or dystrophic dogs, where it induces extensive recovery of normal dystrophin expression (Sampaolesi et al., 2003, 2006). In addition to adipocytes-derived stem cells and mesoangioblasts, a multiplicity of different cells with myogenic properties have been isolated in the few last years. These include pericytes (Dellavalle et al., 2007), muscle-derived stem cells (Qu-Petersen et al., 2002), side population cells (Asakura et al., 2002; LaBarge and Blau, 2002; Bachrach et al., 2004), Ac133+ cells (Torrente et al., 2004), and stem and/or precursor cells from muscle endothelium (Tamaki et al., 2002) and sinovium (De Bari et al., 2003).
Notably, adiponectin recently has been reported to control hematopoietic stem cell proliferation through a p38 mitogen-activating protein kinase (MAPK)-mediated pathway (DiMascio et al., 2007), as well as to promote endothelial progenitor cell number, inducing formation of tubular structures and serving as a chemoattractant factor during vasculogenesis (Shibata et al., 2008). In addition, during regeneration of injured lesions, muscle nonresident stem cells derived from adipose tissue have been shown to produce both adiponectin and hepatocyte growth factor (HGF; Ando et al., 2008).
Having in mind its role as a stem cell regulatory factor and a muscle-active agent, we used mesoangioblasts as a model of progenitors to investigate the role of adiponectin in their activation and commitment toward a muscle lineage. Our data show that the adipokine strongly activate mesoangioblasts to repress apoptotic pathways and enter the cell cycle. Furthermore, adiponectin serves as a chemoattractant factor toward mature muscle fibers and promotes fusion of mesoangioblasts with myoblasts, likely helping in the regeneration process.
MATERIALS AND METHODS
Materials
Unless specified all reagents were obtained from Sigma (St. Louis, MO) except PVDF membrane (Millipore, Bedford, MA); anti-AMP kinase (AMPK), anti-muscle myosin heavy chain (MHC), anti-AdipoR1, anti-AdipoR2, anti-Bim, anti-myogenin and anti-actin antibodies (Santa Cruz Biotechnology, Santa Cruz, CA); anti-phospho-p38 (Thr180/Tyr182), anti-p38, anti-phospho-MAPK (Thr202/Tyr204), and anti-phospho-AMPK (Thr172) antibodies (Cell Signaling Technology, Beverly, MA); anti-caveolin-3 (Transduction Laboratories, Lexington, KY). Globular adiponectin was from Alexis (San Diego, CA), and Alexa 488 fluorescent secondary antibodies was from Molecular Probes (Eugene, OR). Diff-Quik staining kit was from Medion Diagnostics (Miami, FL). The carboxyfluorescein FLICA (fluorescent-labeled inhibitor of caspases) apoptosis detection kit was from ImmunoChemistry Technologies (Bloomington, MN).
Animals
Sgca-null C57BL/6 mice were a kind gift of K. Campbell (Iowa University, Iowa City, IA). Animals were housed in the pathogen-free facility at San Raffaele Scientific Institute and treated in accordance with the European Community guidelines and with the approval of the Institutional Ethical Committee. Animals were used when 4 mo old, because at this stage Sgca-null mice are characterized by the development of histopathological features of muscle dystrophy with ongoing fiber degeneration and modest spontaneous regeneration (Duclos et al., 1998). All the mice used in the experiments were treated with tacrolimus, 2.5 mg/kg, to prevent a possible rejection of the green fluorescent protein (GFP)- or nLacZ-expressing mesoangioblasts.
Real-Time PCR
Total RNA (400 ng) was reverse-transcribed using the TaqMan Reverse Transcription Reagents Kit (Applied Biosystems, Foster City, CA). Reverse transcription was performed in a final volume of 80 μl containing 500 mM KCl, 0.1 mM EDTA, 100 mM Tris-HCl (pH 8.3), 5.5 mM MgCl2, 500 μM of each dNTP, 2.5 μM random examers, 0.4 U/μl RNase inhibitor, and 1.25 U/μl Multiscribe Reverse Transcriptase. The reverse transcription reaction was performed at 25°C for 10 min, 48°C for 30 min, and 95°C for 3 min. Measurement of gene expression was performed by quantitative real-time PCR (7500 Fast Real-Time PCR System, Applied Biosystems). The amount of target, normalized to an endogenous reference (eukaryotic 18S RNA, endogenous control, Applied Biosystems) was given by 2−ΔΔCT calculation (Livak and Schmittgen, 2001). For each sample, 12.5 ng of cDNA was added to 10 μl of PCR mix containing each primer/probe mix and 1× Universal Master Mix. The samples were then subjected to 40 cycles of amplification at 95°C for 15 s and 60°C for 60 s. TaqMan Reverse Transcription Reagents kit, all primer/probe mixes. TaqMan Gene Expression Assays: Adipo R1(ID number Mm01291331_m1), Adipo R2 (ID number Mm01184032_m1), and 1× Universal Master Mix were from Applied Biosystems.
Cell Cultures
Murine D16 mesoangioblasts were cultured in DMEM supplemented with 20% fetal bovine serum (FBS) in 5% CO2 humidified atmosphere. L6 rat myoblasts were maintained in DMEM containing 10% FBS in 5% CO2 humidified atmosphere. GFP- or nLacZ-expressing D16 mesoangioblasts were generated by standard lentiviral transduction as previously described (Minasi et al., 2002; Sampaolesi et al., 2003). For muscle differentiation of D16/L6 cocultures, cells were treated for 4 d with differentiating medium composed of DMEM containing 2% horse serum as previously reported (Galvez et al., 2006; Sciorati et al., 2006).
Cell Proliferation Assay
Mesoangioblasts, 5 × 104, were seeded in a six-well plates in growing medium. After 24 h cells were serum-deprived overnight, and then serum-free medium was added to the cells with or without adiponectin (1 μg/ml). After 72 h cells were trypsinized and counted using an hemocytometer. For thymidine incorporation assay, [3H]thymidine (1 μCi/well) was added to the cells during the last 2 h of treatment. Cells were washed twice with ice-cold phosphate buffered saline (PBS), and then 500 μl of 10% trichloroacetic acid was added to the cells for 5 min at 4°C. Samples were then lysed in 0.2 N NaOH for 10 min at 37°C, and finally, incorporation of [3H]thymidine was measured by scintillation counting. Protein content has been used for normalization of data.
Western Blot Analysis
Seventy percent confluent D16 mesoangioblasts were stimulated, at different times, with adiponectin (1 μg/ml) and then lysed for 10 min on ice in complete RIPA lysis buffer (0.1% SDS, 0.5% deoxycholate, 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 2 mM EGTA, 1 mM sodium orthovanadate, 1 mM phenyl-methanesulphonyl-fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin). Lysates were clarified by centrifugation. An equal amount of each sample was run on SDS/PAGE and transferred onto PVDF membrane. Immunoblots were performed as already described (Fiaschi et al., 2007) and analyzed by a Kodak Gel Logic 2200 for dedicated chemiluminescent image acquisition (Eastman Kodak, Rochester, NY).
Flow Cytometric Analysis of Apoptotic Cell Death
D16 cells were serum-deprived for 12 h and then maintained for 48 h in serum-free medium with or without adiponectin (1 μg/ml). The percentage of cells undergoing apoptosis was assayed by the Guava Nexin Kit, according to the manufacturer's instructions (Guava Technologies, Hayward, CA). Briefly, cells were stained with phycoerythrin-conjugated annexin V (annexin-V-PE) and nexin 7–aminoactinomycin D (7-AAD) in cold 1× nexin buffer in a 50-μl reaction volume. After incubation for 20 min on ice, samples were diluted with cold nexin buffer and analyzed by a Guava Personal Cytometer (Guava Technologies). The analyzer threshold was adjusted on the flow cytometer channel to exclude most of the subcellular debris to reduce the background noise. To quantify cells displaying phosphatidylserine translocation on the cell plasma membrane, we determined those annexin-V-PE–positive cells that were 7-AAD negative. As 7-AAD only binds DNA to those cells having a nonintact plasma membrane (due both to disruption during detaching and necrosis by the treatments), Annexin-V-PE–stained cells that were 7-AAD negative were considered apoptotic.
Tetramethyl-Rhodamine Methyl Ester Staining
D16 mesoangioblasts, 1 × 105, were plated on glass coverslips in growing medium, serum-deprived for 12 h, and then maintained for 48 h in serum-free medium with or without adiponectin (1 μg/ml). For mitochondrion visualization D16 cells were stained for 15 min at 37°C with 1 μM tetramethyl-rhodamine methyl ester (TMRM). Loading of TMRM in metabolically active mitochondria is driven by mitochondrial membrane potential that is maintained in healthy living cells. The disruption of this membrane potential causes an abrupt decrease in mitochondrial fluorescence that is a distinctive feature of programmed cell death. This potentiometric dye (excitation 543 nm, emission 590 nm) was evaluated by confocal microscopy (Leica TCS SP5; Deerfield, IL). TMRM fluorescence intensity was measured for each treatment using the proper tool relative to the software LAS-AF (Leica TCS SP5).
Analysis of Caspase Activation
D16 mesoangioblasts, 1 × 105, were plated on glass coverslips in growing medium, serum-deprived for 12 h, and then maintained for 48 h in serum-free medium with or without adiponectin (1 μg/ml). Analysis of caspases activation was performed using the carboxyfluorescein FLICA apoptosis detection kit according to manufacturer's protocol (ImmunoChemistry Technologies). Briefly, nonpermeabilized D16 cells were washed once with phosphate buffer and incubated with specific FLICA peptide for 15 min at 37°C. Propidium iodide was added to the cells during the final 5 min of incubation. Cells were then washed once with phosphate buffer, mounted with glycerol plastine, and observed with a confocal fluorescence microscope (Leica TCS SP5).
Immunofluorescence
D16 mesoangioblasts were cultured on glass coverslips in growing medium. For immunofluorescence cells were washed with PBS and fixed in 3% paraformaldehyde for 20 min at 4°C. Fixed cells were permeabilized with three washes with TBST (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Triton X-100) and then blocked with 5.5% horse serum in TBST for 1 h at room temperature. Cells were then incubated with specific primary antibodies, diluted 1:100 in TBS (50 mM Tris-HCl, pH 7.4, 150 mM NaCl), overnight at 4°C. Cells were then washed once with TBST and once with TBST with 0.1% bovine serum albumin (BSA) and incubated with secondary antibodies (diluted 1:100) for 1 h at room temperature in TBST with 3% BSA. After extensive washes in TBST cells were mounted with glycerol plastine and observed with a confocal fluorescence microscope (Leica TCS SP5).
Migration Assay
Murine mesoangioblasts (5 × 104, serum-deprived overnight) were seeded in the upper chamber of a transwell (membrane diameter: 6.5 mm; pore size: 8 μm) in serum-free medium with or without adiponectin (1 μg/ml). Undifferentiated or 4-d differentiated C2C12 cells were cultured in the lower chamber of the transwell in the presence of serum-free medium during the migration experiment. Mesoangioblasts in the upper chamber were suspended in serum-free medium, and adiponectin was added to 4-d differentiated myotubes in the lower chamber in serum-depleted medium. The number of mesoangioblasts that crossed the membrane pores was evaluated after 16 h. Nonmigrated cells were mechanically removed from the upper side of the transwell system, whereas the cells in the lower side of the filter membrane was colored using Diff-Quik staining kit (Biomap, Milan, Italy) according the manufacturer's instruction. The number of migrated cells was obtained by counting three to five random fields of the lower face of the transwell membrane at 10× magnification.
Differentiation Experiments
D16 mesoangioblasts were cultured for 48 h in serum-free medium with or without adiponectin (1 μg/ml). The same number of untreated or adiponectin-treated cells was added to a culture of growing L6 rat myoblasts (1:2 ratio), and muscle differentiation of the coculture was carried on for 4 d in differentiating medium. The cells were then fixed, and myotubes and nuclei were revealed by immunofluorescence using anti-MHC antibodies and propidium iodide, respectively. We calculated the differentiation index as the percentage of muscle MHC-positive cells above total nuclei and the fusion index as the average number of nuclei in muscle MHC-positive cells containing at least three nuclei above the total nuclei, respectively (Filigheddu et al., 2007). The percentage of mesoangioblasts fused to rat myotubes was obtained by counting the number of mouse nuclei in MHC-positive rat fibers on the basis of different staining with propidium iodide because of their different heterochromatin distribution (Brunelli et al., 2004).
In Vivo Transplantation
D16 mesoangioblasts (expressing nuclear LacZ or GFP) were starved 12 h, and then adiponectin (1 μg/ml) was added for 16 h. The day after, 5 × 105 cells were harvested, suspended in 30 μl of PBS without calcium and magnesium, and injected with a 30-gauge syringe in the TA (tibialis anterior) muscle of dystrophic Sgca-null mice (Duclos et al., 1998). Three mice per cell type were transplanted. Control mice were injected with vehicle. After 3 d mice were killed, and muscle samples from control or cell-transplanted mice were analyzed for GFP fluorescence under a Nikon stereomicroscope (Melville, NY) or were frozen in liquid nitrogen–cooled isopentane. For D16 nLacZ-transplanted muscles, serial 8-μm-thick sections were cut with a Leica cryostat, and fixed with 4% paraformaldehyde for 10 min, and standard X-Gal staining followed by quick Eosin staining was performed. The number of nuclear LacZ-positive nuclei per TA section was counted double in blind, and the numbers were analyzed with GraphPad Prism (GraphPad Software, San Diego, CA).
Bead Implantation
Affi-Gel Blue beads (100–200 mesh, Bio-Rad, Richmond, CA) were incubated with adiponectin (1 μg/ml) for 30 min and then with GFP-expressing mesoangioblasts for an additional 30 min. After a wash with PBS, a final volume of 20 μl, containing adiponectin-conjugated beads and 2.5 × 105 mesoangioblasts, was injected in the TA of dystrophic Sgca-null mice. Control mice received beads treated with 0.1% BSA and the same number of mesoangioblasts. After 24 h mice were killed, muscles were explanted, and GFP fluorescence was visualized using a Nikon stereomicroscope. Fluorescence intensity was measured using ImageJ software (http://rsb.info.nih.gov/ij/).
Statistical Analysis
Data are presented as means ± SD from at least three experiments. Analysis of densitometry was performed using Quantity One Software (Bio-Rad). Results were normalized versus control expression levels. Statistical analysis of the data were performed by Student's t test. p ≤ 0.05 was considered statistically significant.
RESULTS
Adiponectin Induces Proliferation of Murine Mesoangioblasts
We initially examined whether mesoangioblasts express receptors for adiponectin. mRNA analysis by real-time PCR revealed that mesoangioblasts express both AdipoR1 and AdipoR2 messenger and protein as confirmed by immunoblot analysis (Figure 1, A and B).
Figure 1.
Expression of adiponectin receptors AdipoR1 and AdipoR2 in mesoangioblasts. (A) Amount of AdipoR1 and AdipoR2 mRNA by real-time PCR. Total RNA was used for the amplification of mRNA of adiponectin receptors using as housekeeping gene 18S rRNA. The amount of target, normalized to the endogenous reference (18S RNA), was given by the 2−ΔΔCT calculation and was reported as arbitrary units (a.u.). (B) Analysis of AdipoR1 and AdipoR2 expression by immunoblot. An equal amount of total proteins were run in each lane after protein assay with Bradford method, as shown by actin immunoblot. Real-time PCR is the mean of three independent assays, whereas the blot is representative of three different experiments. n.r., nonrelated band.
To test whether the hormone can act as a growth factor (GF) in mesoangioblasts, serum-deprived cells were cultured for 72 h with 1 μg/ml adiponectin and then counted with a hemocytometer. We observed that the treatment of mesoangioblasts with adiponectin leads to a 100% increase in cell number (Figure 2A). The effect of adiponectin on mesoangioblast proliferation was then evaluated by [3H]thymidine incorporation. Our results showed that adiponectin treatment induces ∼50% of increase in thymidine incorporation (Figure 2B), thereby demonstrating that adiponectin acts as GF in mesoangioblasts. In keeping with these observations, adiponectin is able to elicit a sustained activation of the p42/p44 MAPK two master regulators of mitogenic signaling (Figure 2C).
Figure 2.
Adiponectin induces the growth of mesoangioblasts. (A) Mesoangioblasts were serum-deprived for 24 h, and where indicated, adiponectin (1 μg/ml) was added to serum-free medium for 72 h. Cells were then counted using an hemocytometer. (B) Analysis of [3H]thymidine incorporation by mesoangioblasts after the treatment with adiponectin (1 μg/ml). Cells were treated as in A, and [3H]thymidine was added during the last 2 h of incubation. These results correspond to the mean of four different experiments. *p < 0.001 and **p < 0.005 versus control. (C–E) Analysis of the signaling pathways activated by adiponectin stimulation. Mesoangioblasts were serum-deprived overnight and then stimulated with adiponectin (1 μg/ml) for the indicated period. Immunoblot analysis for the detection of the phosphorylation level of p42/p44 MAPK (Thr202/Tyr204), Akt (Ser473), p38 MAPK (Thr180/Tyr182), and AMPK (Thr182) was performed using specific phospho-antibodies. p42/p44 MAPK (C), p38 (D), and AMPK (E) immunoblots were used for normalization. Bar graph represents the phosphorylation level of the signaling proteins calculated by the ratio between the phosphorylated and total protein obtained in four different experiments. *p < 0.001 and **p < 0.005 versus time 0.
One of the key features for the activation of stem cells is the activation of p38 MAPK. This protein in vivo is required for satellite cell activation regulating the quiescent state of stem cells (Jones et al., 2005). We observed that adiponectin is able to induce a transient but strong increase of p38 MAPK phosphorylation, which reaches a maximum of activation 10 min after stimulation (Figure 2D). To further complete the analysis of adiponectin signaling in mesoangioblasts, we assayed the activation of AMPK, an important signaling molecule that exerts many of the metabolic effects of adiponectin in vivo in muscle metabolism, including regulation of glucose uptake and lactate production (Yamauchi et al., 2002, 2003). We observed that in mesoangioblasts adiponectin leads only to slight activation of AMPK (Figure 2E), suggesting only a partial role of AMPK activation in mesoangioblasts.
Adiponectin Protects Murine Mesoangioblasts from Apoptosis Induced by GF Withdrawal
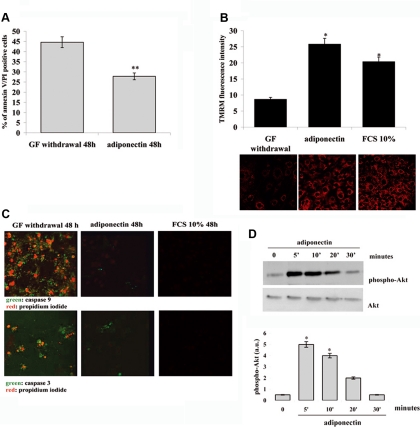
After the observation that adiponectin induces proliferation in mesoangioblasts, we investigated the possibility that adiponectin protects from apoptosis as well. Apoptotic death in mesoangioblasts was induced by GF withdrawal and, after 24 h of serum deprivation, 1 μg/ml adiponectin was added for additional 48 h. Cells were then stained with annexin V and propidium iodide and the evaluation of apoptotic cells were carried on by cytofluorimetric analysis. We observed that ∼45% of cells are driven to apoptosis due to the continuous serum deprivation. Conversely, the treatment with adiponectin, 1 μg/ml, leads to a decrease in apoptotic cells (26%) with a protection from apoptosis of ∼40% (Figure 3A), thereby suggesting that adiponectin protects mesoangioblasts from apoptosis induced by GF withdrawal.
Figure 3.
Adiponectin protects mesoangioblasts from apoptosis induced by GF withdrawal. Cells were serum-deprived overnight and then treated with serum-free medium with or without adiponectin (1 μg/ml) for 48 h. (A) Analysis of annexin V– and propidium iodide–positive cells by flow cytofluorimetry. (B) Analysis of mitochondria membrane potential through the staining of the cells with TMRM fluorescent probe. *p < 0.001 and **p < 0.005 versus GF withdrawal. (C) Detection of caspase-9 and -3 activation by confocal miscroscope. Mesoangioblasts were stained with FLICA probe (green) for analysis of activated caspases and with propidium iodide (red) to analyze plasma membrane modifications. (D) Phosphorylation of serine/treonine kinase Akt by adiponectin. Mesoangioblasts were serum-deprived overnight and then stimulated with adiponectin (1 μg/ml) for the indicated period. Activation of Akt was detected by immunoblot using anti-phospho-serine 473 antibodies, whereas anti-Akt antibodies were used for normalization. The Western blot is representative of three different experiments with similar results. Bar graphs in B and D are the mean of four independent experiments, whereas images are representative of three different experiments with similar results. *p < 0.001 versus time 0.
To further confirm the antiapoptotic function of adiponectin in mesoangioblasts, we analyzed mitochondrial membrane depolarization, a common event occurring during early apoptosis. Mesoangioblasts, treated as above, were stained with TMRM fluorescent probe. Confocal microscope analysis reveals that treatment for 48 h with adiponectin leads to a strong protection from mitochondrial depolarization induced by GF withdrawal. Of note, the protective effect exerted by adiponectin has a similar extent compared with 10% FBS, suggesting adiponectin as a powerful antiapoptotic factor (Figure 3B).
A typical feature of apoptosis is the activation of caspases, a family of cysteine proteases that plays an essential roles in this process. To further confirm the involvement of adiponectin in the survival of mesoangioblasts after GF withdrawal, we analyzed the activation of caspase-3 and -9. Caspase-9 becomes activated due to the formation of apoptosome after the exit of cytochrome c from mitochondria, whereas caspases-3 is the final executor in the apoptotic event (Li and Yuan, 2008). Analysis of activation of caspase-3 and -9 was carried out by confocal microscope, using two specific probes emitting green fluorescence after their binding to the corresponding activated caspases. In addition, propidium iodide labeling of DNA was used as an additional marker of apoptotic cells. Confocal microscope analysis reveals that treatment for 48 h with adiponectin leads to a strong protection from caspase-3 and -9 activation induced by GF withdrawal, as well as DNA labeling with propidium iodide (Figure 3C). Again, the protective effect exerted by adiponectin has a comparable extent compared with 10% FCS. In keeping with its role of a prosurvival agent, adiponectin is able to elicit a sustained Akt phosphorylation/activation, a well-known kinase that plays a key role in prosurvival processes (Figure 3D).
Adiponectin Protects Mesoangioblasts from Anoikis
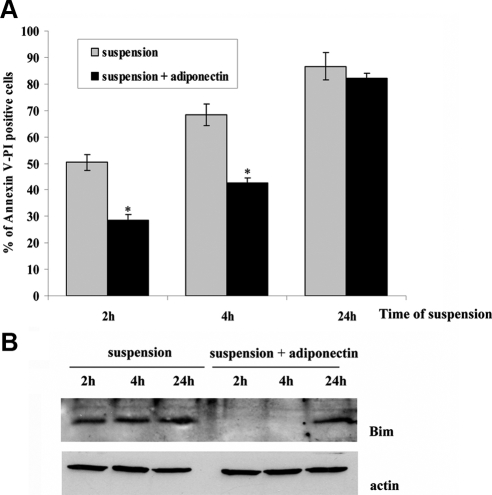
Anoikis, the apoptosis death induced by loss of matrix attachment, is a common event in untransformed adherent cells, including epithelial, endothelial, muscle, and some fibroblastoid cells (Meredith et al., 1993; Gilmore, 2005). Hence, the successful homing of nonresident stem cells to injured muscle is likely correlated with a transient but efficient protection from anoikis, thus permitting to stem cells to survive into the bloodstream. We therefore analyzed if adiponectin, which is able to protect mesoangioblasts from apoptosis induced by GF withdrawal, might counteract anoikis as well. Mesoangioblasts were pretreated with 1 μg/ml adiponectin for 12 h, detached, and maintained in suspension in serum-deprived medium with or without 1 μg/ml adiponectin for 2, 4, and 24 h, respectively. Cells were then stained with annexin V and propidium iodide, and the evaluation of apoptotic cells was performed by cytofluorimetric analysis. We observed that in mesoangioblasts adiponectin induces a clear protection from anoikis, reaching 50% of protection after 2 h of suspension and then decreasing to 38% after 4 h of matrix detachment. Conversely, adiponectin is almost ineffective for longer suspension times (Figure 4A).
Figure 4.
Adiponectin protects mesoangioblasts from anoikis. Mesoangioblasts were serum-deprived overnight and then pretreated with serum-free medium with or without adiponectin (1 μg/ml) for 12 h. Cells were then detached and maintained in suspension in serum-free medium with or without adiponectin (1 μg/ml) for 2, 4, and 24 h. (A) Apoptotic mesoangioblasts treated as described above were analyzed by annexin V and propidium iodide labeling. Bar graph is the mean of four different experiments. *p < 0.001 versus suspension. (B) Detection of Bim expression in mesoangioblasts treated as described above. The Western blot is representative of three different experiments with the same result.
Among proteins belonging to the BH3-only family, Bim has been reported to play a selective role in the induction of anoikis, acting as a specific sensor of matrix detachment (Reginato et al., 2003). In survival conditions Bim is continuously destroyed by an ubiquitin degradation pathway. We therefore analyzed Bim accumulation in response to adiponectin in the same conditions described above. Figure 4B shows that Bim accumulates 2, 4, and 24 h after matrix detachment, suggesting that the apoptotic pathway is activated. Conversely, the presence of adiponectin leads to a strong degradation of Bim 2 and 4 h after suspension, suggesting that the apoptotic pathway is repressed. On the contrary, Bim is accumulated in the presence of adiponectin 24 h after matrix detachment, in agreement with annexin V and propidium iodide labeling. These findings suggest that adiponectin acts as a prosurvival agent both in GF withdrawal and matrix detachment conditions.
Adiponectin Enhances Migration of Mesoangioblasts In Vitro
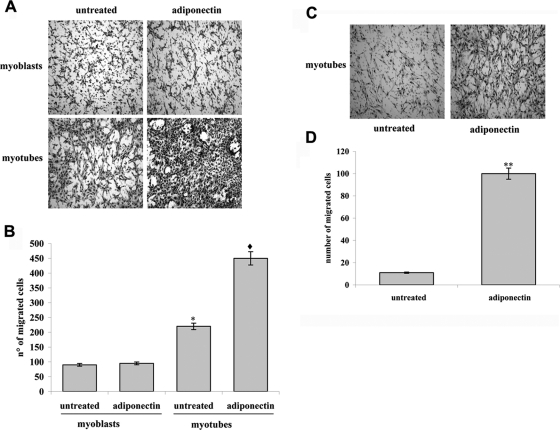
A key characteristic of nonresident stem cells is their ability to migrate toward a damaged muscle thereby improving efficient delivery of cells to target tissues during cell therapy. We therefore tested whether the treatment of mesoangioblasts with adiponectin affects the promigratory capacity of the cells toward both undifferentiated and 4-d differentiated C2C12 cells, seeded in the lower chamber of the Boyden transwell. Mesoangioblasts were serum-starved for 12 h and then seeded in the upper chamber of the transwell in the presence or absence of adiponectin (1 μg/ml). We found that adiponectin greatly increases the migration of mesoangioblasts toward myotubes (4–5-fold), whereas it failed to improve the movement of the cells toward undifferentiated C2C12 cells (Figure 5, A and B). These findings suggest that adiponectin acts as a promigratory factor for mesoangioblasts, enhancing their ability to move toward myotubes.
Figure 5.
Adiponectin enhances mesoangioblasts migration in vitro. Mesoangioblasts were serum-deprived overnight and then seeded in the upper Boyden chamber for assay. Adiponectin (1 μg/ml) was added in the upper (A and B) or lower (C and D) Boyden chamber. C2C12 myoblasts (C2C12, 0 d) or 4-d differentiated myotubes (C2C12, 4 d) were plated in the lower chamber. (A and C) Representative images of migrated mesoangioblasts after staining with hematoxylin-eosin. (B and D) Bar graph represents the mean of migrated cells counted in three different fields for each experiment. *p < 0.005 versus untreated 0 d, ♦p < 0.001 versus adiponectin 0 d, and **p < 0.001 versus untreated.
In addition, because of the recent observation that adiponectin is directly expressed by differentiated C2C12 cells (Fiaschi et al., 2009), we tested the hypothesis of a chemoattractant role of this adipokine for mesoangioblasts. We repeat the experiment described above in which mesoangioblasts move toward differentiated C2C12 myotubes, except that we added adiponectin (1 μg/ml) in the lower chamber of the Boyden chamber assay (Figure 5C). The results indicate that adiponectin is a strong chemoattractant for mesoangioblasts, because it increases their attraction compared with that of differentiated C2C12 myotubes, which is ∼10-fold (Figure 5D).
Differentiation of Mesoangioblasts into Skeletal Muscle Cells Is Enhanced by Adiponectin
Mesoangioblasts can differentiate into skeletal muscle lineage only when cocultured with myogenic cells. In this condition mesoangioblasts fuse with muscle cells to form myotubes (Minasi et al., 2002). Recently, we demonstrated that adiponectin acts as a myogenic factor in murine myoblasts C2C12. In this cell line, the chronic treatment with adiponectin (1 μg/ml) induces muscle differentiation with the expression of skeletal muscle markers and the increase in differentiation and fusion indexes (Fiaschi et al., 2009). We therefore tested whether adiponectin affects the ability of mesoangioblasts to fuse with muscle cells and to express skeletal muscle markers. To this end, we used cocultures of D16 mouse mesoangioblasts and L6 rat myoblasts. This method allows evaluating the heterotypic fusion of mouse mesoangioblasts with rat myotubes, by labeling nuclei with propidium iodide and analyzing the chromatin distribution. Indeed, mouse nuclei have been reported to have a more condensed chromatin distribution/staining compared with rat nuclei (Sciorati et al., 2006). Mesoangioblasts were cultivated for 48 h in medium without serum, with or without adiponectin (1 μg/ml) and, after normalization of their cell number, viable mesoangioblasts were added to a culture of proliferating rat L6 myoblasts. Muscle differentiation of coculture was carried on for 4 d. Cells were then fixed and stained with anti-MHC antibodies to visualize myotubes and with propidium iodide. As shown in Figure 6A, the treatment with adiponectin leads to an increase of differentiation index in D16/L6 coculture. In addition, in coculture containing adiponectin-treated mesoangioblasts we detected an increase in the fusion between murine and rat nuclei (Figure 6B). These findings were further supported by the analysis of skeletal muscle markers expressing in two cocultures. Indeed, we observed an increase of both early and late skeletal markers (MHC, myogenin, and caveolin-3) in adiponectin-treated-D16/L6 cocultures compared with L6 cocultures with untreated D16 mesoangioblasts (Figure 6C). The same increase of specific skeletal muscle markers was observed when the experiment was performed using D16/murine C2C12 myoblasts coculture (data not shown). A representative image of differentiated L6/mesangioblast coculture was shown in Figure 6D. We observed longer and abundant myotubes formed in cocultures containing adiponectin-treated mesoangioblasts compared with cocultures containing untreated mesoangioblasts. These findings demonstrate that the treatment of mesoangioblasts with adiponectin increases their ability to fuse with rat myoblasts and elevates the differentiation properties of the cocultures.
Figure 6.
Adiponectin increases skeletal muscle differentiation in vitro. Mesoangioblasts were serum-deprived overnight and then treated for 48 h with serum-free medium with or without adiponectin (1 μg/ml). The same number of mesoangioblasts was then added to a culture of rat L6 myoblasts (ratio 1:2). Myogenic differentiation was carried on for 4 d. Cells were then fixed and stained with anti-muscle myosin heavy chain (mMHC, green) and with propidium iodide (red) to label the nuclei. (A) Differentiation index of D16 mesoangioblasts per L6 coculture. (B) The graph shows the fusion index between nuclei of the murine mesoangioblasts and rat L6 myoblasts in mMHC-expressing cells with more than two nuclei compared with the total number of rat and murine nuclei. (C) Representative Western blot showing the expression of skeletal muscle markers in rat L6/murine D16 coculture after 4 d of differentiation. (D) Representative image of L6-D16 coculture after 4 d of differentiation obtained by confocal microscopy showing higher amount of myotubes in coculture containing adiponectin-treated mesoangioblasts compared with untreated. Inset shows the magnification of the myotube in the rectangle, revealing the presence of two nuclei of mesoangioblasts. The different chromatin distribution of the nuclei between rat L6 and murine D16 (arrow) is shown. *p < 0.005 versus untreated; **p < 0.001 versus untreated.
Adiponectin Increases In Vivo the Survival, Engraftment, and Localization of Mesoangioblasts to Skeletal Muscle of Dystrophic Mice
Mesoangioblasts isolated from dorsal aorta of C57BL/6 mouse embryos and injected into muscle of Sgca-null mice are able to engraft and repair, both morphological and functional, the diseased muscle of these mice (Sampaolesi et al., 2003). We investigated whether adiponectin can improve the survival and the engraftment of these cells in skeletal muscle of Sgca-null mice. To this end, nuclear nLacZ (nLacZ)- or GFP-expressing mesoangioblasts were treated for 16 h with adiponectin (1 μg/ml) and then injected in the TA muscle of Sgca-null mice. Three days after injection animals were killed, and TA muscles were explanted for detecting GFP-expressing mesoangioblasts. We found an increased green fluorescence (corresponding to transplanted mesoangioblasts) in TA of mice transplanted with adiponectin-treated cells compared with controls, suggesting that adiponectin enhances in vivo the survival of mesoangioblasts (Figure 7A). A great increase of mesoangioblasts survival due to adiponectin was clearly observed 24 h after injection (Figure 2B). Seventy-two hours after injection, the section of the muscles of mice transplanted with adiponectin-treated mesoangioblasts show a great and statistical significant improvement of the engraftment of ∼79% (calculated as percentage of the mean of nLacZ-positive cells between untreated and adiponectin-treated cells) compared with muscle transplanted with untreated cells (Figure 7B). Finally, we tested whether in vivo adiponectin induces the localization of mesoangioblasts in skeletal muscle of dystrophic mice. Beads coated with adiponectin and control beads are injected in the TA muscles of Sgca-null mice together with GFP-expressing mesoangioblasts. Figure 7C shows that adiponectin is able to attract mesoangioblasts in a localized site of muscle, and it does not allow transplanted cells to disperse. The chemoattractive effect of adiponectin on mesoangioblasts is indicated by the small area (GFP-positive area) in which mesoangioblasts are localized (3.28% compared with total area) compared with the larger area taken by untreated beads and mesoangioblasts (30.62% compared with total area). Overall these findings demonstrated that in vivo adiponectin has a pleiotropic effect on mesoangioblasts, increasing their survival, engraftment, and localization to skeletal muscle of dystrophic mice.
Figure 7.
Adiponectin stimulates survival, chemoattraction and engrafting of mesoangioblasts in skeletal muscle of dystrophic mice. (A) Adiponectin increases mesoangioblasts survival in vivo. D16 mesoangioblasts expressing GFP were treated for 16 h with adiponectin (1 μg/ml) and then injected in the TA of Sgca-null mice. The level of expression of GFP was visualized under a stereomicroscope 3 d after injection. Bar graph shows the fluorescence intensity of GFP in lateral and medial surface of the TA muscles measured using ImageJ. Bar, 2 mm. A representative experiment is shown (n = 3). (B) Adiponectin increases the engraftment of mesoangioblasts. Top, nuclear LacZ-expressing mesoangioblasts were treated for 16 h with adiponectin and then injected in the TA of Sgca-null mice. After 24 h muscles were recovered, and a X-gal staining was performed for 4 h. Blue staining revealed an increased survival and engraftment in muscles injected with adiponectin-treated mesoangioblasts compared with control. Bar, 2 mm. A representative experiment is shown (n = 3). Bottom, 3 d after injection the muscles were recovered, and nLacZ-positive nuclei were detected by X-Gal staining. Bar, 100 μm. p = 0.028 (calculated with GraphPad). (C) Adiponectin enhances the localization of mesoangioblasts to TA of Sgca-null mice. Beads bounded with adiponectin or incubated with 0.1% BSA were injected in the TA of Sgca-null mice. The chemoattractive effect due to adiponectin was calculated as the percentage of positive GFP area near the site of injection and are reported in the plot below (arrowhead). The fluorescence intensity of GFP was measured using ImageJ software. Bar, 1 mm. A representative experiment is shown (n = 3).
DISCUSSION
Here we report that globular adiponectin, beside its ability to control several skeletal muscle metabolic functions, behaves on nonresident muscle progenitors as a stem cell factor. Our data show that the adipokine is able to 1) drive mesoangioblasts into the cell cycle, promoting their proliferation; 2) protect them by both GF- and anchorage-withdrawal by repressing the apoptotic and anoikis pathways and promoting their engraftment to dystrophic muscles; 3) behave as a chemoattractant factor toward mature and differentiated fibers, promoting homing of transplanted cells to diseased muscles; and 4) push them into a skeletal muscle lineage, promoting their fusion with myoblasts and the differentiation index.
Despite the promising results for the use of stem cells in muscle regeneration, several problems still hamper their therapeutic development, including 1) the efficient engrafting and survival of nonresident cells in the toxic environment of the damaged muscle, 2) the induction of in vivo and in vitro proliferation/expansion of progenitor cells, and 3) the commitment/activation steps of stem cells toward myogenic lineage. In these years several alternative cell therapies, relying on non-muscle-resident stem cells endowed with myogenic potential have been proposed, thus opening new avenues for cell therapy of skeletal myopathies (Cossu and Sampaolesi, 2004; Leri et al., 2005; Oreffo et al., 2005; Wagers and Conboy, 2005; Porada et al., 2006). These include mesoangioblasts, multipotent mesodermal progenitors, isolated from arterial vessels, which have been shown to restore muscle structure and function in dystrophic mice (Sciorati et al., 2006; Galvez et al., 2006; Guttinger et al., 2006) and dogs (Sampaolesi et al., 2006), as well as bone marrow-derived cells and mesenchymal stem cells isolated from subcutaneous adipose tissue (Long et al., 2005; Oreffo et al., 2005; Porada et al., 2006). The current understanding of the extrinsic physiologically relevant agonists regulating the above mentioned key biological processes in muscle progenitors is still very limited and is highly warranted to enhance their therapeutic efficacy toward degenerative diseases. Consequently the identification of new modulators of stem cells endowed with myogenic properties can individuate new pharmacological approaches to enhance the therapeutic efficacy of stem cell delivery. To date HGF, fibroblast GF (FGF; Sheehan and Allen, 1999), and tumor necrosis factor-α (TNF-α) have been shown to recruit resident satellite cells to division (Li, 2003). In addition, it has been reported that the entry of muscle satellite cells into the cell cycle requires sphingolipid signaling (Nagata et al., 2006) and that HGF promotes muscle repair by stimulating the proliferation and migration of myogenic precursors toward the wounded area (Volonte et al., 2005). In addition, sphingosine-1 phosphate has been indicated as a modulators of mesoangioblasts, thereby affecting their proliferation and protecting them from apoptosis induced by GF removal (Donati et al., 2007). Furthermore, the treatment of mesoangioblasts with nitric oxide donors increases their migration, their ability to fuse with myotubes and acts as survival agent (Sciorati et al., 2006). We now include adiponectin among stem cell growth and survival factors as indicated by its ability to improve both survival and engraftment to dystrophic muscles of ex vivo–treated mesoangioblasts. In keeping with common signal transduction pathways activated by other stem cell factors, adiponectin activates p38 and p42/44 MAPKs. The peculiarity of adiponectin compared with sphingosine 1-phosphate is that the adipokine is produced by adipose tissue in an inverse relationship with fat mass, and its level is strongly decreased in diabetic patients (Weyer et al., 2001; Rasouli and Kern, 2008). To date, a decrease of plasma adiponectin content correlates with obesity, diabetes, and insulin resistance, conditions that can be reversed in mice by the treatment with exogenous adiponectin (Yamauchi et al., 2001). This may have severe consequences on the regenerative potential in obese and diabetic, muscle-diseased patients. In keeping with this speculation there is an acknowledged correlation between progression of diabetes and decreased skeletal muscle contractility, induction of atrophy, and impairment of regeneration (Vignaud et al., 2007). In addition high glucose and diabetes have been correlated with adipogenic differentiation of muscle stem cells (Aguiari et al., 2008), suggesting that hyperglycemia may represent a feed-forward cycle, driving uncommitted muscle precursors to form adipose depots and thereby to decrease muscle function and regeneration.
One of the main limit concerning nonresident satellite cells is their inability to reach the damaged tissue upon systemic injection, although they have been shown to restore dystrophin expression in mdx mice when injected directly in the damaged tissue (Partridge et al., 1989). Conversely, the main disadvantages of nonresident cells, such as mesoangioblasts and mesenchymal stem cells, are their limited survival upon systemic injection without proper matrix attachment and survival GFs as well their effective differentiation into myofibers (Cossu and Bianco, 2003; Sherwood et al., 2004; Oreffo et al., 2005). We now report that adiponectin protects mesoangioblasts from the withdrawal of survival factors, implying a general down-regulation of proapoptotic signaling. We observed activation of the prosurvival protein Akt, down-regulation of caspase-3 and 9, maintenance of mitochondrial integrity, and inhibition of phosphatidyl-serine exposure, an early apoptotic marker. Sphingosine-1-phosphate has been reported to exert the same effect in the protection of mesoangioblasts from apoptotic commitment (Donati et al., 2007). In keeping with the common activity of the two factors it has been reported that, at least in endothelial cells, adiponectin elicits its biological response via activation of sphingosine kinase and the formation of sphingosine-1-phosphate (Kase et al., 2007). In this respect, it will be very useful to know whether the effects of adiponectin in mesoangioblasts occurs, at least in part, through a cross-talk with sphingosine kinase. In contrast, up to now the activity of adiponectin in protecting mesoangioblasts from anoikis, the apoptotic death due to lack of proper attachment to extracellular matrix, is a unique feature of the adipokine. Protection from anoikis, confirmed by delayed annexin V staining and accumulation of Bim, the main BH3-only proapoptotic protein specifically associated with anoikis, may be extremely useful for these stem cells after systemic injection, prolonging their survival while circulating and allowing them to reach the injured site in muscle.
A second peculiar characteristic of adiponectin in the regulation of mesoangioblasts is its ability to behave as a promigratory factor and chemoattractant. Mesoangioblasts have already been reported to move toward mature C2C12 myotubes. The factors involved in this chemoattraction are TNF-α, stromal cell derived factor-1 (SDF-1), FGF, high mobility group box (HMBG-1; Galvez et al., 2006). The exogenous addition of adiponectin is able to enhance the motility of mesoangioblasts toward mature fibers. It is therefore conceivable that adiponectin, which is produced by myotubes (Delaigle et al., 2004; Fiaschi et al., 2009), may cooperate with other regulators and act in loco during muscle homing and establishment of the regeneration niche. Inflammation may concur to help the regeneration process through secretion of proinflammatory cytokines as TNF-α and interferon-γ, which in turn stimulate the autocrine production of adiponectin by mature fibers (Delaigle et al., 2004). Second, inflammatory cells may enhance the local concentration of globular adiponectin by elastase proteolytic activity on the full-length muscle-inactive hormone (Waki et al., 2005). The role of locally produced adiponectin in the injured site is further strengthened by the observation that during regeneration of injured lesions, muscle nonresident stem cells derived from adipose tissue have been shown to produce both adiponectin and HGF (Ando et al., 2008). Although the role of the apparent synergy between adiponectin and HGF in differentiation or distribution to muscle injured sites of these nonresident stem cells is still unknown, the possibility that these two factors cooperate and synergize in stem cell activation is attracting.
The final contribution to muscle regeneration given by cell-based therapy is the engrafting of nonresident stem cells into damaged muscle. We report herein in vivo evidence that adiponectin enhances the ability of mesoangioblasts to resist the apoptogenic environment of Sgca-null dystrophic muscles, as well as the attraction and engrafting of ex vivo–treated cells to diseased muscles. In addition, our data using an in vitro fusion assay with differentiating myoblasts confirm that adiponectin increases mesoangioblast ability to fuse with preexisting fibers and to enhance their differentiation and maturation. This evidence supports the fact that adiponectin may contribute to the fusogenic ability of mesoangioblasts, an uncommon but mandatory feature among stem cell–regulating factors. Indeed, only nitric oxide has been proved so far to enhance the fusion of mesoangioblasts with myofibers, with a clear correlation with the therapeutic efficacy of mesoangioblasts for dystrophic muscle regeneration (Sciorati et al., 2006).
The effect of adiponectin on muscle cells is greatly pleiotropic and multifaceted. The hormone is active in the metabolism of muscle cells, shifting them compared with catabolic routes, is produced directly by injured fibers (Delaigle et al., 2004), regulates proliferation and survival of stem cells, acts as a chemoattractant factor for stem cells compared with that of mature fibers, promotes fusion of stem cells with resident muscle cells, and finally promotes differentiation into mature fibers. Hence our data indicate adiponectin as a stem cell GF in mesoangioblasts, confirming that the role of the adipokine in muscle goes beyond its metabolic activity. These findings provide a plausible molecular mechanism linking muscle regeneration to diabetes and other obesity-related diseases that are associated with hypoadiponectinemia. Thus, therapeutic approaches designed at increasing adiponectin production could be useful for treating muscle congenital or acquired myopathies, for which stem cell therapies have been proposed.
ACKNOWLEDGMENTS
We thank Anna Innocenzi and Diego Covarello (San Raffaele Scientific Institute) for technical assistance and Gonzalo Ugarte for the helpful suggestions about Affi-Gel beads. This work was supported by the Tuscany Region Studies on Rosiglitazone (TRESOR), the Italian Association for Cancer Research (AIRC), The Tuscany Tumor Institute (ITT), Consorzio Interuniversitario Biotecnologie, and Cassa di Risparmio di Firenze.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-04-0310) on January 20, 2010.
REFERENCES
- Aguiari P., et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc. Natl. Acad. Sci. USA. 2008;105:1226–1231. doi: 10.1073/pnas.0711402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando Y., Inaba M., Sakaguchi Y., Tsuda M., Quan G. K., Omae M., Okazaki K., Ikehara S. Subcutaneous adipose tissue-derived stem cells facilitate colonic mucosal recovery from 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats. Inflamm. Bowel Dis. 2008;14:826–838. doi: 10.1002/ibd.20382. [DOI] [PubMed] [Google Scholar]
- Asakura A., Seale P., Girgis-Gabardo A., Rudnicki M. A. Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach E., Li S., Perez A. L., Schienda J., Liadaki K., Volinski J., Flint A., Chamberlain J., Kunkel L. M. Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc. Natl. Acad. Sci. USA. 2004;101:3581–3586. doi: 10.1073/pnas.0400373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A. H., Combs T. P., Scherer P. E. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- Brunelli S., et al. Msx2 and necdin combined activities are required for smooth muscle differentiation in mesoangioblast stem cells. Circ. Res. 2004;94:1571–1578. doi: 10.1161/01.RES.0000132747.12860.10. [DOI] [PubMed] [Google Scholar]
- Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr. Opin. Genet. Dev. 2006;16:525–532. doi: 10.1016/j.gde.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Cossu G., Bianco P. Mesoangioblasts–vascular progenitors for extravascular mesodermal tissues. Curr. Opin. Genet. Dev. 2003;13:537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Cossu G., Biressi S. Satellite cells, myoblasts and other occasional myogenic progenitors: possible origin, phenotypic features and role in muscle regeneration. Semin. Cell Dev. Biol. 2005;16:623–631. doi: 10.1016/j.semcdb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Cossu G., Sampaolesi M. New therapies for muscular dystrophy: cautious optimism. Trends Mol. Med. 2004;10:516–520. doi: 10.1016/j.molmed.2004.08.007. [DOI] [PubMed] [Google Scholar]
- De Bari C., Dell'Accio F., Vandenabeele F., Vermeesch J. R., Raymackers J. M., Luyten F. P. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J. Cell Biol. 2003;160:909–918. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaigle A. M., Jonas J. C., Bauche I. B., Cornu O., Brichard S. M. Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology. 2004;145:5589–5597. doi: 10.1210/en.2004-0503. [DOI] [PubMed] [Google Scholar]
- Dellavalle A., et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Dhawan J., Rando T. A. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- DiMascio L., Voermans C., Uqoezwa M., Duncan A., Lu D., Wu J., Sankar U., Reya T. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J. Immunol. 2007;178:3511–3520. doi: 10.4049/jimmunol.178.6.3511. [DOI] [PubMed] [Google Scholar]
- Donati C., Cencetti F., Nincheri P., Bernacchioni C., Brunelli S., Clementi E., Cossu G., Bruni P. Sphingosine 1-phosphate mediates proliferation and survival of mesoangioblasts. Stem Cells. 2007;25:1713–1719. doi: 10.1634/stemcells.2006-0725. [DOI] [PubMed] [Google Scholar]
- Duclos F., et al. Progressive muscular dystrophy in alpha-sarcoglycan-deficient mice. J. Cell Biol. 1998;142:1461–1471. doi: 10.1083/jcb.142.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaschi T., Buricchi F., Cozzi G., Matthias S., Parri M., Raugei G., Ramponi G., Chiarugi P. Redox-dependent and ligand-independent trans-activation of insulin receptor by globular adiponectin. Hepatology. 2007;46:130–139. doi: 10.1002/hep.21643. [DOI] [PubMed] [Google Scholar]
- Fiaschi T., Cirelli D., Comito G., Gelmini S., Ramponi G., Serio M., Chiarugi P. Globular adiponectin induces differentiation and fusion of skeletal muscle cells. Cell Res. 2009;19:584–597. doi: 10.1038/cr.2009.39. [DOI] [PubMed] [Google Scholar]
- Filigheddu N., et al. Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol. Biol. Cell. 2007;18:986–994. doi: 10.1091/mbc.E06-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez B. G., Sampaolesi M., Brunelli S., Covarello D., Gavina M., Rossi B., Constantin G., Torrente Y., Cossu G. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J. Cell Biol. 2006;174:231–243. doi: 10.1083/jcb.200512085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore A. P. Anoikis. Cell Death. Differ. 2005;12(Suppl 2):1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M. Adiponectin: an update. Diabetes Metab. 2008;34:12–18. doi: 10.1016/j.diabet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Guttinger M., Tafi E., Battaglia M., Coletta M., Cossu G. Allogeneic mesoangioblasts give rise to alpha-sarcoglycan expressing fibers when transplanted into dystrophic mice. Exp. Cell Res. 2006;312:3872–3879. doi: 10.1016/j.yexcr.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Tyner K. J., Nibarger L., Stanley H. M., Cornelison D. D., Fedorov Y. V., Olwin B. B. The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J. Cell Biol. 2005;169:105–116. doi: 10.1083/jcb.200408066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase H., et al. Globular adiponectin induces adhesion molecule expression through the sphingosine kinase pathway in vascular endothelial cells. Life Sci. 2007;81:939–943. doi: 10.1016/j.lfs.2007.08.002. [DOI] [PubMed] [Google Scholar]
- LaBarge M. A., Blau H. M. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- Le Grand F., Rudnicki M. A. Skeletal muscle satellite cells and adult myogenesis. Curr. Opin. Cell Biol. 2007;19:628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri A., Kajstura J., Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol. Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- Li J., Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- Li Y. P. TNF-alpha is a mitogen in skeletal muscle. Am. J. Physiol. Cell Physiol. 2003;285:C370–C376. doi: 10.1152/ajpcell.00453.2002. [DOI] [PubMed] [Google Scholar]
- Lihn A. S., Pedersen S. B., Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes. Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long M. A., Corbel S. Y., Rossi F. M. Circulating myogenic progenitors and muscle repair. Semin. Cell Dev. Biol. 2005;16:632–640. doi: 10.1016/j.semcdb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Meredith J. E., Jr, Fazeli B., Schwartz M. A. The extracellular matrix as a cell survival factor. Mol. Biol. Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minasi M. G., et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Partridge T. A., Matsuda R., Zammit P. S. Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J. Cell Biol. 2006;174:245–253. doi: 10.1083/jcb.200605028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreffo R. O., Cooper C., Mason C., Clements M. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1:169–178. doi: 10.1385/SCR:1:2:169. [DOI] [PubMed] [Google Scholar]
- Ouchi N., Walsh K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Porada C. D., Zanjani E. D., Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr. Stem Cell Res. Ther. 2006;1:365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- Qu-Petersen Z., et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J. Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli N., Kern P. A. Adipocytokines and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 2008;93:S64–S73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginato M. J., Mills K. R., Paulus J. K., Lynch D. K., Sgroi D. C., Debnath J., Muthuswamy S. K., Brugge J. S. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat. Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- Rodriguez A. M., et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J. Exp. Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaolesi M., et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M., et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- Sciorati C., Galvez B. G., Brunelli S., Tagliafico E., Ferrari S., Cossu G., Clementi E. Ex vivo treatment with nitric oxide increases mesoangioblast therapeutic efficacy in muscular dystrophy. J. Cell Sci. 2006;119:5114–5123. doi: 10.1242/jcs.03300. [DOI] [PubMed] [Google Scholar]
- Sheehan S. M., Allen R. E. Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor. J. Cell. Physiol. 1999;181:499–506. doi: 10.1002/(SICI)1097-4652(199912)181:3<499::AID-JCP14>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Sherwood R. I., Christensen J. L., Conboy I. M., Conboy M. J., Rando T. A., Weissman I. L., Wagers A. J. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Shibata R., Skurk C., Ouchi N., Galasso G., Kondo K., Ohashi T., Shimano M., Kihara S., Murohara T., Walsh K. Adiponectin promotes endothelial progenitor cell number and function. FEBS Lett. 2008;582:1607–1612. doi: 10.1016/j.febslet.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki T., Akatsuka A., Ando K., Nakamura Y., Matsuzawa H., Hotta T., Roy R. R., Edgerton V. R. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J. Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrente Y., et al. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J. Clin. Invest. 2004;114:182–195. doi: 10.1172/JCI20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignaud A., Ramond F., Hourde C., Keller A., Butler-Browne G., Ferry A. Diabetes provides an unfavorable environment for muscle mass and function after muscle injury in mice. Pathobiology. 2007;74:291–300. doi: 10.1159/000105812. [DOI] [PubMed] [Google Scholar]
- Volonte D., Liu Y., Galbiati F. The modulation of caveolin-1 expression controls satellite cell activation during muscle repair. FASEB J. 2005;19:237–239. doi: 10.1096/fj.04-2215fje. [DOI] [PubMed] [Google Scholar]
- Wagers A. J., Conboy I. M. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122:659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Waki H., Yamauchi T., Kamon J., Kita S., Ito Y., Hada Y., Uchida S., Tsuchida A., Takekawa S., Kadowaki T. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790–796. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- Weyer C., Funahashi T., Tanaka S., Hotta K., Matsuzawa Y., Pratley R. E., Tataranni P. A. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]