After skin wound, released growth factors and extracellular nucleotides regulate the different phases of healing, including re-epithelialization. Here, we show that, in keratinocytes, purinergic P2Y2 receptors inhibit the motogenic IGF-I/PI3K pathway. Therefore, extracellular nucleotides may play key roles during skin remodelling after wound.
Abstract
Insulin-like growth factor-I (IGF-I) activation of phosphoinositol 3-kinase (PI3K) is an essential pathway for keratinocyte migration that is required for epidermis wound healing. We have previously reported that activation of Gα(q/11)-coupled-P2Y2 purinergic receptors by extracellular nucleotides delays keratinocyte wound closure. Here, we report that activation of P2Y2 receptors by extracellular UTP inhibits the IGF-I–induced p110α-PI3K activation. Using siRNA and pharmacological inhibitors, we demonstrate that the UTP antagonistic effects on PI3K pathway are mediated by Gα(q/11)—and not G(i/o)—independently of phospholipase Cβ. Purinergic signaling does not affect the formation of the IGF-I receptor/insulin receptor substrate-I/p85 complex, but blocks the activity of a membrane-targeted active p110α mutant, indicating that UTP acts downstream of PI3K membrane recruitment. UTP was also found to efficiently attenuate, within few minutes, the IGF-I–induced PI3K-controlled translocation of the actin-nucleating protein cortactin to the plasma membrane. This supports the UTP ability to alter later migratory events. Indeed, UTP inhibits keratinocyte spreading and migration promoted by either IGF-I or a membrane-targeted active p110α mutant, in a Gα(q/11)-dependent manner both. These findings provide new insight into the signaling cross-talk between receptor tyrosine kinase and Gα(q/11)-coupled receptors, which mediate opposite effects on p110α-PI3K activity and keratinocyte migration.
INTRODUCTION
Skin lesions initiate the release of various growth factors in the wound bed, cytokines, and low-molecular-weight soluble factors such extracellular nucleotides (Werner and Grose, 2003). Together, these factors orchestrate a cascade of events leading to a proper wound healing, which includes reepithelialization. During this step, basal keratinocytes, i.e., the epidermis epithelial cells, undergo morphological changes required for their migration from the wound margin over the denuded area (Patel et al., 2006; Hosoya et al., 2008). Insulin-like growth factor-I (IGF-I), acting through paracrine/autocrine regulatory loops, is a peptide growth factor mainly secreted by dermal cells (macrophages and fibroblasts) but also by keratinocytes (although controversy remains regarding keratinocyte IGF-I production; Edmondson et al., 2003). Its biological function is mediated by its cognate tyrosine kinase receptor (IGF-IR), which is expressed by basal keratinocytes (Hodak et al., 1996; Sadagurski et al., 2006). The involvement of IGF-I in wound healing is supported by its up-regulation after wounding and the lack of expression in nonhealing wound (Edmondson et al., 2003). Moreover, gene-mediated IGF-I expression by basal keratinocytes in the wound bed increases the rate of reepithelialization (Jeschke et al., 2005; Semenova et al., 2008; Todorovic et al., 2008). IGF-I is also a strong promoter of in vitro keratinocyte migration (Ando and Jensen, 1993; Haase et al., 2003; Sadowski et al., 2003; Semenova et al., 2008). Thus, an increase of keratinocyte migration may partly explain the acceleration of wound reepithelialization mediated by IGF-I (Semenova et al., 2008).
The first step in the signal transduction by IGF-IR is its autophosphorylation followed by the subsequent recruitment and the extensive tyrosine phosphorylation of insulin-receptor substrate-1 (IRS-1), which then initiates several distinct signaling pathways including phosphatidylinositol 3-kinase (PI3K; Baserga, 2000). Once recruited to the plasma membrane, PI3K phosphorylates phosphatidylinositol (4,5)-bisphosphate (PIP2) to produce phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which serves as a docking signal for the pleckstrin homology (PH) domain–carrying proteins, such as the protein kinase B/Akt (Akt). Class IA PI3Ks are heterodimers composed of a p110 catalytic subunit (three isoforms: α, β, and δ) and a smaller regulatory subunit (five different isoforms: p85α, p85β, p55α, p50α, and p55γ; Hawkins et al., 2006) and are among of the most important proteins that control cell migration (Merlot and Firtel, 2003; Cain and Ridley, 2009). In the epidermis, keratinocytes strongly express p110α and p110β (Pankow et al., 2006), and the expression of an active p110α mutant is sufficient to promote lamellipodium formation, cell spreading, and cell migration in vitro (Haase et al., 2003; Pankow et al., 2006). Furthermore, in murine skin biopsies, pharmacological inhibition of PI3K suppresses keratinocyte migration (Pankow et al., 2006).
As underlined above, extracellular nucleotides (ATP, UTP) are released in the wound bed by many different cell types including platelets and keratinocytes (Lazarowski et al., 2003; Holzer and Granstein, 2004). It has been reported that damaged cultured keratinocytes can release ATP at micromolar concentration, which is sufficiently high to activate their cognate receptors (Burrell et al., 2005; Yin et al., 2007). ATP and UTP as other extracellular nucleotides exert their function by activating two classes of purinergic receptors, the G-protein–coupled receptors (P2YRs) and the ATP-gated ion channels (P2XRs; Burnstock, 2006). In the epidermis, basal keratinocytes mainly express P2Y1R and P2Y2R (Dixon et al., 1999; Greig et al., 2003a). After wound, the expression and distribution of P2Y1R and P2Y2R is modulated, indicating that these receptors may participate to the healing process (Greig et al., 2003b). In keratinocytes, the ATP/UTP-sensitive Gαq-coupled P2Y2R was found to be the main functional receptor activated by UTP (Lee et al., 2001; Koizumi et al., 2004; Yoshida et al., 2006; Inoue et al., 2007). Moreover, a growing body of evidence supports that P2Y2R is an important mediator of cell migration in several cell types (Chaulet et al., 2001; Pillois et al., 2002; Bagchi et al., 2005; Kaczmarek et al., 2005; Wang et al., 2005; Chen et al., 2006; Yu et al., 2008). However, in keratinocytes, the function of P2Y2R has been proven to be antimotogenic. Indeed, we previously showed that activation of P2Y2R by UTP in keratinocytes alters lamellipodia dynamics, disorganizes actin cytoskeleton in a Gαq-dependent manner, and suppresses serum-induced keratinocyte migration in a wound-healing in vitro assay (Taboubi et al., 2007).
In the present study, we used IGF-I as a highly potent activator of the PI3K-dependent keratinocyte migration, in order to gain insight into the molecular mechanism governing the negative cross-talk between P2Y2R and IGF-I/PI3K signaling and its role in the regulation of keratinocyte motility. Our results reveal novel roles of purinergic signals as negative regulators of the IGF-I/PI3K–induced keratinocyte migration. Our work suggests that extracellular nucleotides released in the wound bed may play an important role in the modulation of keratinocyte migration during epidermis reepithelialization after injury.
MATERIALS AND METHODS
Reagents and Antibodies
Purinergic agonists and 4-hydroxytamoxifen (4-OHT) were from Sigma Aldrich (Lyon, France). IGF-I was from Amersham Pharmacia Biotech (Les Ulis, France). LY294002, U73122, and pertussis toxin (PTX) were from Calbiochem (Darmstadt, Germany). YM-254890 was a generous gift from J. Takasaki (Astellas Pharma, Ikibara, Japan; Takasaki et al., 2004). Measurement of [Ca2+]i rise was performed as previously described (Lee et al., 2001). PIK-75 (N-((1E)-(6-bromoimidazo[1,2-a]pyridin-3-yl)methylene)-N′-methyl-N″-(2-methyl-5 nitrobenzene)-sulfonohydrazide, HCl), a selective inhibitor of p110α and TGX-221 ((±)-7-methyl-2-(morpholin-4-yl)-9-(1-phenylaminoethyl)-pyrido[1,2-a]-pyrimidin-4-one), a selective inhibitor of p110β were from Calbiochem. Rabbit polyclonal antibodies against (phospho)-Akt (Ser-473), Akt, and (phospho)-GSK-3α/β were purchased from Cell Signaling (Danvers, MA). Rabbit polyclonal anti-Gα(q/11), -IRS-1, -IGF-IR β chain and mouse monoclonal anti-phospho-Tyr (PY) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti- p85-PI3K antibody was purchased from Upstate Biotechnology (Lake Placid, NY). Rabbit antiserum against cortactin was a generous gift of Ellen Van Obberghen-Shilling (Centre Antoine-Lacassagne, Nice, France).
Cell Lines, Treatments, and Transfections
The human keratinocyte cell line HaCaT (Boukamp et al., 1988), was cultured in Dulbecco's modified Eagle's medium (DMEM; Lonza, Basel, Switzerland) supplemented with 10% fetal calf serum (FCS; Lonza) in a humidified atmosphere of 5% CO2 at 37°C. Stable HaCaT cell lines were generated by retroviral transfection of HaCaT cells with the vector pLXIN-Myr-p110α*-mER (Myr-p110α*-mER clone) or with the empty vector (vector-transfected clone; Leenders et al., 2004; Pankow et al., 2006). Activation of Myr-p110α*-mER was achieved by addition of 4-OHT (1 μM, overnight) to the medium. For inhibition of PI3K, Gα(q/11), PLCβ, and G(i/0) proteins, cells were preincubated with either LY294002 (LY; 20 μM, 60 min), YM-254890 (YM; 3 μM, 5 min), U-73122 (5 μM, 30 min), or PTX (100 ng/ml, overnight), respectively. Gα(q/11) small interfering RNA (siRNA) nucleofection experiments were performed as previously described (Taboubi et al., 2007). Human endothelial cells (HUVECs) were cultured as previously described (Taboubi et al., 2007).
Cell Spreading Assays
Cells were preincubated with LY294002 or YM-254890 as indicated above. Wells were precoated with a laminin-5 (LM-5)-enriched matrix as previously described (Taboubi et al., 2007), and then extracellular nucleotides and/or IGF-I were spotted onto the wells just before cell seeding. Cells (1.5 × 104 cells/cm2) were seeded and incubated for the indicated times at 37°C and then fixed with 3.7% formaldehyde. Three random fields per well from duplicate wells were pictured under a 10× objective, and the cell surface of 100 cells per experiment was measured using the ImageJ software (NIH; http://rsb.info.nih.gov/ij).
Migration Assays
Cells were serum-starved overnight, trypsinized, and then seeded on a LM-5–enriched matrix (104 cells/cm2). Cells were then allowed to migrate at 37°C, 5% CO2 for 2 h. Migration was analyzed using an inverted Nikon microscope at 20× magnification (Melville, NY). Time-lapse recording started after cell adhesion and spreading (20 min). Three fields per well were imaged and followed at 10-min intervals over 2 h with a Coolsnap HQ camera (Photometrics, Tucson, AZ) operated by NIS-elements AR 2.30 software (Nikon). Manual single-cell tracking was performed using Metamorph software (Roper Scientific, Evry, France) as described previously (Sadok et al., 2008). Migration parameters calculated from each individual cell were determined from time-lapse movies. They include total migration distance, distance to origin, velocity, and directional persistence of cell migration. Total migration distance represents the sum of distances between each measurement over a period of 2 h. The distance to origin was determined as the net translocation between the initial and the final position. Velocity was calculated as the total migration distance divided by 2 h. Directional persistence was calculated as the ratio of the distance to origin to the total migration distance. For each parameter, results are expressed as the mean ± SD from at least 60 individual cells.
Immunofluorescence
For cortactin labeling, cells were treated as indicated in the figure legends and then fixed in 3.7% formaldehyde and blocked with 3% bovine serum albumin (BSA). Cells were labeled overnight at 4°C with anti-cortactin serum (1/3000 dilution) and then with the appropriate fluorescent secondary antibody and rhodamine-coupled phalloidin. Cells were observed under immersion oil 63×/1.4 Pan Apochromat objectives on a confocal Leica SP5 microscope (Leica Microsystems, Nanterre, France).
Assays for PI3K Activity
HaCaT cells were cultured to 80% confluence in 10-cm2 dishes, and then quiescence was induced by incubating cells overnight in serum-free medium containing 0.5% BSA. Cells were then stimulated with IGF-I (50 ng/ml) either alone or with UTP (100 μM) for 1, 4, or 10 min at 37°C. Cells were then lysed in 1 ml of Nonidet P-40-containing lysis buffer with protease and phosphatase inhibitors. Equal amounts of protein lysates were incubated with 5 μl of anti-p85-PI3K antibody for 1 h at 4°C and then bound with protein A-agarose beads for 1 h at 4°C. Immunoprecipitates were assayed for their content in p110α-lipid kinase activity by using an inverted competitive ELISA according to the manufacturer's recommendations (Echelon Biosciences, Salt Lake City, UT).
Immunoblotting and Immunoprecipitation
Keratinocytes were lysed on ice in a buffer containing 25 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1% Triton X-100, 0.1% sodium deoxycholate, 4 mM EDTA, 50 mM NaF, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 1 mM PMSF, 1 μg/ml leupeptin, and 1 μg/ml aprotinin for 15 min at 4°C. Cell lysates were centrifuged for 10 min at 10,000 × g to eliminate cell debris. Equal amounts of protein (Protein Assay Kit, Bio-Rad, Hercules, CA) were separated by SDS-PAGE and then transferred to Hybond-C nitrocellulose membranes (Amersham Pharmacia Biotech). Membranes were probed with the appropriate primary antibody (2 μg/ml) and then with a peroxidase-conjugated secondary antibody. Bound immunocomplexes were detected using the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech). For immunoprecipitation, cell lysates were precleared by incubation with 25 μl of normal rabbit or mouse IgG serum, followed by incubation with the appropriate primary antibody (5 μg/ml) overnight at 4°C. Cell lysates were then incubated with protein A-agarose beads for 1 h at 4°C. The beads were washed three times with the lysis buffer and used for immunoblotting as described above.
RESULTS
UTP Inhibits IGF-I—induced Early Phase Activation of p110α-PI3K/Akt Pathway
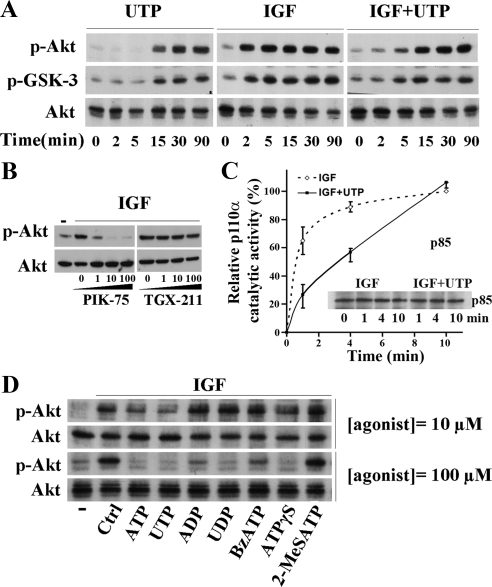
Like other GPCRs, P2Y receptors can activate PI3K pathway (Irino et al., 2008). Here, we stimulated HaCat cells with 100 μM of extracellular UTP, a noncytotoxic concentration widely used to activate P2YRs in keratinocytes (Dixon et al., 1999; Lee et al., 2001; Koizumi et al., 2004; Yoshida et al., 2006; Inoue et al., 2007; Taboubi et al., 2007). Similarly, we found that UTP stimulated the phosphorylation of the ser/thr protein kinase Akt and the glycogene synthase kinase-3 (GSK-3), two PI3K effectors (Figure 1A, left). However, phosphorylation of both proteins was detectable only 15 min after UTP treatment. On the other hand, IGF-I (50 ng/ml) activated the PI3K pathway earlier; within 2 min of treatment (Figure 1A, middle). Noteworthy, costimulation of keratinocytes by IGF-I and UTP resulted in a notable delay in the onset of both Akt and GSK-3 phosphorylation (15 vs. 2 min, Figure 1A, right). Similar results were obtained using ATP instead of UTP (data not shown). Thus, ATP and UTP elicited opposite dual regulatory signals toward PI3K/Akt pathway, i.e., an already reported activating signal and an unusual inhibitory signal revealed when the PI3K pathway was activated by growth factors such as IGF-I.
Figure 1.
UTP inhibits the IGF-I–induced PI3K signaling pathway through P2Y2/P2Y4 receptors. (A) HaCaT keratinocytes were stimulated for the indicated times with UTP (100 μM), IGF-I (50 ng/ml), or both. (B) Cells were pretreated with increasing doses of p110α inhibitor (PIK-75) or p110β inhibitor (TGX-211) for 5 min and then stimulated with IGF-I (50 ng/ml, 5 min). (C) Cells were stimulated with IGF-I (IGF; 50 ng/ml) either alone or supplemented with UTP (100 μM; IGF+UTP) for 1, 4, and 10 min. Cells were lysed and PI3K was immunoprecipitated using an anti-p85 antibody. p110α catalytic activity, i.e., production of PIP3, was measured in the immunoprecipitated material by inverted ELISA assay. p85 immunoblot shows that equivalent amount of PI3K were analyzed. For more details, see Materials and Methods. Data are expressed as a mean ± SD from two independent experiments made in triplicates. (D) Cells were stimulated for 5 min with IGF-I (50 ng/ml) either alone (Ctrl) or in the presence of various purinergic receptor agonists at 10 μM and 100 μM as indicated; (−), untreated cells. Cell lysates were analyzed by Western blot using anti-phospho-Akt (p-Akt), anti-phospho-GSK3 (p-GSK-3), and anti-Akt (Akt) antibodies as indicated. Data shown are representative of three independent experiments.
We previously published that UTP treatment induces the release of a GFP protein fused with the pleckstrin-homology domain of Akt from the plasma membrane indicating a decrease in the amount of plasma membrane PIP3 (Taboubi et al., 2007). Moreover, we observed that in contrast to wild type Akt, a membrane-targeted mutant of Akt (myr-Akt) was still phosphorylated after UTP cell stimulation (unpublished data). Thus, it is likely that UTP inhibited Akt phosphorylation by disturbing its membrane targeting through the control of PIP3 amount. As keratinocytes express p110α and p110β catalytic PI3K subunits (Pankow et al., 2006), we first sought to identify which p110 isoform is responsible for the Akt phosphorylation induced by IGF-I. For this purpose, before IGF-I stimulation, HaCat cells were pretreated with increasing doses of either PIK-75 or TGX-221, two selective pharmacological inhibitors of p110α and p110β subunit, respectively. Immunoblot analyses shown on Figure 1B revealed that IGF-I failed to stimulate Akt phosphorylation when p110α was inhibited, whereas no alteration in Akt phosphorylation was observed when p110β was blocked. As a control and in agreement with previous reports that documented activation of p110β by GPCRs (Kurosu et al., 1997; Maier et al., 1999), we found that TGX-221 inhibited the late induction of Akt phosphorylation by UTP (Figure S1A).
Using in vitro enzymatic assays of immunopurified PI3K, we showed that IGF-I induced a rise in p110α-PI3K lipid kinase activity that reached a plateau 2 min after treatment. By contrast, at this time, the assayed lipid kinase function was twofold lower when p110α-PI3K was purified from cells costimulated with both IGF-I and UTP compared with the one obtained from cells stimulated by IGF-I alone. In the presence of UTP, the amount of produced PIP3 reached the plateau only 10 min after stimulation (Figure 1C). From these experiments, we concluded that, the loss of Akt phosphorylation observed in previous experiments was primarily due to the transient inhibition of IGF-I–induced p110α-PI3K activation.
Finally, in order to better characterize the purinergic receptor involved in the PI3K pathway inhibition, we carried out pharmacological analyses using a wide range of P2YR agonists. ATP is a broad agonist of P2Y and P2X receptors, whereas UTP is selective for P2Y2R and P2Y4R. ATPγS can activate P2Y2R, but is a more potent agonist of P2Y11R; ADP activates P2Y1R; 2-MeSATP is selective for P2Y1R and P2X3R; UDP activates P2Y6R, and BzATP is a P2Y11R and P2X7R agonist (Burnstock, 2006). As shown on Figure 1D, at a concentration of 100 μM all agonists (except 2-MeSATP) decreased IGF-I–induced Akt phosphorylation. In contrast, at 10 μM, only ATP and UTP (and to a lesser extent ATPγS) were still able to exert this inhibition. As cultured keratinocytes overexpress P2Y2R compared with P2Y4R (Yoshida et al., 2006) and data not shown), our results strongly suggest that P2Y2R is presumably responsible for the early and transient inhibition of IGF-I–activated p110α-PI3K signaling pathway.
Extracellular UTP Inhibits IGF-I-induced PI3K Signaling via Gα(q/11) in a PLCβ-independent Manner
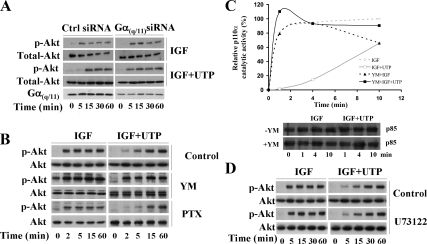
GPCRs can trigger different signals depending on the coupled G protein. P2Y2R was initially reported to be mainly coupled to Gα(q/11) (Erb et al., 2006). However, previous studies from the Erb's group have shown that, in addition to Gα(q/11), P2Y2R can also be coupled to and signal through Gα0 or Gα12 (Erb et al., 2001; Bagchi et al., 2005; Wang et al., 2005; Liao et al., 2007). To identify the G protein involved in the inhibition of PI3K signaling, we used a validated sequence of siRNA targeting Gα(q/11) (Barnes et al., 2005; Atkinson et al., 2006; Taboubi et al., 2007). In Gα(q/11) siRNA-transfected cells, UTP lost its ability to restrain IGF-I–induced PI3K pathway as evaluated by Akt phosphorylation (Figure 2A). This result was confirmed using YM-254890, a pharmacological cyclic depsipeptide that selectively inhibits Gα(q/11) (Figure 2B; Takasaki et al., 2004). Moreover, YM-254890 blocked the capacity of UTP to inhibit p110α-PI3K lipid kinase activity (Figure 2C). To test for a possible role of G(i/0), we used PTX, a G(i/0)specific inhibitor (Albert and Robillard, 2002). PTX treatment of HaCaT cells did not alter the UTP inhibitory effect toward the IGF-I–induced PI3K pathway (Figure 2B, bottom). PTX efficiency was controlled by its ability to inhibit ATP-induced Erk1,2 phosphorylation in human endothelial cells (Figure S1B; Montiel et al., 2006). We further examined the putative role of phospholipase Cβ (PLCβ), the main reported downstream effector of Gα(q/11) (Hubbard and Hepler, 2006). For this purpose, we used U73122 (5 μM), a potent inhibitor of PLCβ (Stam et al., 1998). We verified that, in HaCat cells, at this concentration, U73122 was able to inhibit UTP-induced Erk1,2 phosphorylation (Yang et al., 2004) and intracellular Ca2+ flux (Lee et al., 2001; Figure S1C) without any cytotoxic effect (not shown). As shown on Figure 2D, addition of U73122 did not alter the ability of UTP to inhibit IGF-I–induced Akt phosphorylation. Taken together, these data indicate that UTP required Gα(q/11), but not G(i/0), to inhibit the p110α-PI3K pathway. In addition, PLCβ, although activated by Gα(q/11), was not involved in this inhibitory pathway.
Figure 2.
UTP inhibits IGF-I–induced PI3K signaling in a Gα(q/11) but not a G(i/0)-dependent manner. (A) HaCaT keratinocytes were transiently nucleofected with Gαq siRNA or nontargeting siRNA as a control (Ctrl siRNA) as described in Materials and Methods. Forty-eight hours after transfection, cells were stimulated with IGF-I, either alone or in presence of UTP for the indicated times. (B) HaCaT keratinocytes were pretreated with YM-254890 (YM, 3 μM, 5 min) or pertussis toxin (PTX; 100 ng/ml, 18 h) to selectively inhibit Gα(q/11) and G(i/0), respectively. Cells were then stimulated with IGF-I (50 ng/ml), alone or supplemented with UTP (100 μM) for the indicated times. (C) Cells were treated with YM-254890 as in B, and p110α-PI3K activity was measured as described in Figure 1C. Data are representative of two independent experiments. (D) To inhibit the PLCβ, cells were pretreated with U73122 (5 μM, 30 min) and then treated as in A. Cell lysates were analyzed by Western blot using anti-phospho-Akt (p-Akt) and anti-Akt (Akt) antibodies as indicated. Data shown are representative of three independent experiments.
UTP Does Not Interfere with p110α-PI3K Plasma Membrane Recruitment
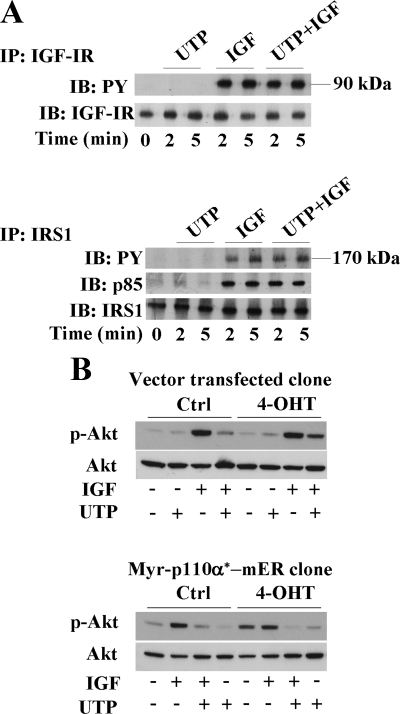
We next attempted to specify the molecular mechanism whereby purinergic signaling interfered with PI3K pathway. Neither IGF-IR surface expression (measured by flow cytometry; data not shown), nor IGF-IR autophosphorylation on tyrosine residues (Figure 3A, top) were modified by UTP treatment. Furthermore, UTP did not reduce tyrosine phosphorylation of IRS-1, the immediate downstream substrate of IGF-IR, and did not change the capacity of IRS-1 to recruit the p85-PI3K regulatory subunit (Figure 3A, bottom). Collectively, these results indicate that UTP did not alter assembly of the IGF-IR/IRS-1/PI3K complex. To investigate further, we examined the impact of UTP on the signaling function of an inducible active form of the p110α catalytic subunit that is targeted to plasma membrane by myristoylation (Myr-p110α*-mER; Leenders et al., 2004; Pankow et al., 2006). Activation of Myr-p110α*-mER protein was induced by addition of 4-OHT. Figure 3B (top) shows that, in empty vector-transfected cells, 4-OHT had no effect on the basal level of Akt phosphorylation. As expected in these cells, UTP still inhibited IGF-I–induced Akt phosphorylation. In agreement with a previous work (Pankow et al., 2006), we observed that Myr-p110α*-mER activation by 4-OHT–induced Akt phosphorylation. Interestingly, this agonist-independent Akt phosphorylation was strongly inhibited by UTP (Figure 3B, bottom). Together, all these results indicate that inhibition of PI3K signaling pathway by UTP occurred downstream of the PI3K recruitment to the plasma membrane.
Figure 3.
UTP inhibits the PI3K signaling pathway downstream of p110α-PI3K membrane recruitment. (A) HaCaT keratinocytes were stimulated for 2- and 5-min with UTP (100 μM), IGF-I (50 ng/ml), or both. Immunoprecipitations were performed on cell lysates with an anti-IGF-I receptor (IGF-IR) antibody (top) or anti-IRS1 antibody (bottom). Immunoprecipitates were analyzed by Western blot using anti-phospho-tyrosine antibody (PY) and anti-p85-PI3K antibody (p85), as indicated. Anti-IRS1 (IRS1) and anti-IGF-IR (IGF-IR) antibodies were used as controls. (B) Myr-p110α*-mER–expressing HaCaT clone and vector-transfected clone were treated with either 4-hydroxytamoxifen (4-OHT) or solvent (Ctrl) and then stimulated with IGF-I (50 ng/ml) (IGF) and/or UTP (100 μM; UTP) for 5 min. Cell lysates were analyzed by Western blot using anti-phospho-Akt (p-Akt) and anti-Akt antibody (Akt) as loading control. Data shown are representative of three independent experiments.
Extracellular UTP Blocks PI3K-dependent Cortactin Recruitment to the Lamellipodium
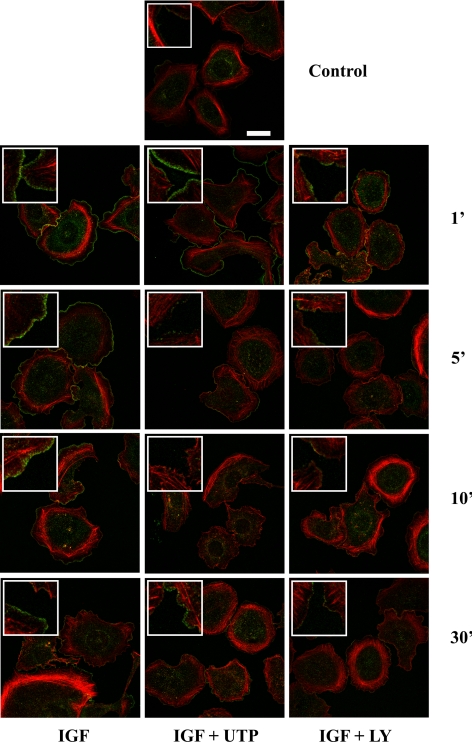
In keratinocytes, lamellipodium extension is driven by p110α-PI3K activation (Haase et al., 2003; Pankow et al., 2006). In our previous work, we showed that within few minutes UTP treatment induces actin cytoskeleton disorganization and a transient lamellipodium withdrawal (Taboubi et al., 2007). Among the large array of proteins involved in cortical actin regulation, cortactin plays a key role as a promoter of actin filament branching and subsequent lamellipodium extension (Bryce et al., 2005; Kowalski et al., 2005; Ammer and Weed, 2008). Growth factors lead to cortactin relocalization from internal cytoplasmic region to the cortical actin network. Although the precise mechanism controlling cortactin translocation is not clearly established, it has been reported that, in keratinocytes, PI3K is involved in this process (Ceccarelli et al., 2007). In HaCat cells, we observed a clear cortactin translocation from the cytosol to the rim of membrane ruffles, with a maximal recruitment at early time points of IGF-I stimulation (1 and 5 min) (Figure 4). In LY294002-treated cells, cortactin redistribution was potently blocked, confirming the involvement of PI3K in this process. As shown in Figure 4, UTP (100 μM) transiently perturbed the IGF-I–induced cortactin relocalization. Although we observed a short delay, UTP-induced inhibition of both cortactin recruitment and p110α-PI3K function were timely correlated (see also Figures 1 and 2). Moreover, the kinetics of the cortactin translocation inhibition induced by UTP is very similar to that of lamellipodium withdrawal described elsewhere (Taboubi et al., 2007), suggesting that cortactin is oppositely regulated by UTP and IGF-I to control lamellipodium formation. Thus, we identified cortactin recruitment to the plasma membrane as a critical early event downstream of the PI3K activation by IGF-I that is inhibited by extracellular UTP and may affect later processes such as cell spreading and motility (see below).
Figure 4.
UTP blocks PI3K-dependent cortactin membrane translocation. HaCaT keratinocytes were treated or not with LY294002 (LY; 30 μM, 60 min) and then stimulated with IGF-I (IGF; 50 ng/ml), either alone or in the presence of UTP (100 μM) for the indicated period of time. Untreated cells were used as a control (Ctrl). Before confocal analysis, filamentous actin organization was revealed by phalloidin staining (red), and cortactin was labeled using an anti-cortactin antibody (green). Scale bar, 20 μm.
Extracellular UTP Inhibits PI3K-induced Keratinocyte Spreading and Motility in a Ga(q/11)-dependent Manner
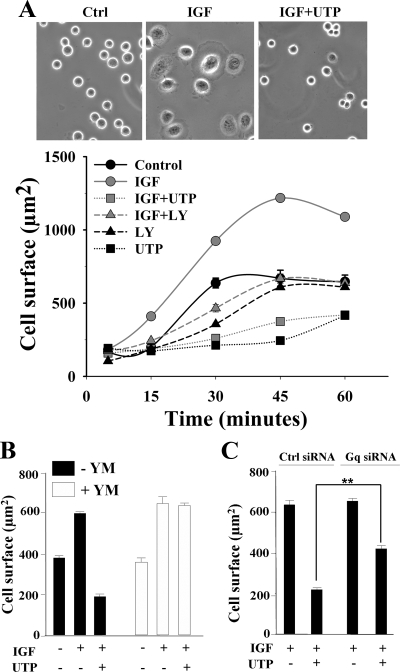
In accordance with data from Watt's group (Haase et al., 2003), 20 min after seeding, IGF-I (50 ng/ml) stimulated lamellipodium formation and increased cell spreading in keratinocytes (Figure 5A, top). In the presence of UTP (100 μM), cells remained round and failed to extend any lamellipodium (Figure 5A, top). Quantification of time course cell spreading revealed that after 45 min IGF-I increased by twofold the mean cell size (Figure 5A, bottom). Cells pretreated with LY294002 behave like control cells, indicating that IGF-I–induced cell spreading is largely due to the activation of the PI3K pathway. In presence of UTP (100 μM), HaCat cells spread poorly, even 60 min after seeding. Notably, UTP inhibited IGF-I–induced and basal cell spreading, suggesting that P2Y2R was not only able to block PI3K-dependent but also PI3K-independent cell spreading. We obtained similar results with normal human keratinocytes (data not shown). As reported above, UTP inhibited PI3K pathway in a Gα(q/11)-dependent manner (see Figure 2). Therefore, we next examined whether Gα(q/11) activation was also required for UTP to exert its inhibitory effect on PI3K-dependent cell spreading. UTP failed to inhibit IGF-I–induced cell spreading in cells where Gα(q/11) function was repressed either by pharmacological inhibition (YM-254890, 3 μM; Figure 5B) or by siRNA-mediated knockdown of its expression (Figure 5C).
Figure 5.
UTP inhibits IGF-I–induced PI3K-dependent cell spreading through Gαq activation. (A) HaCaT keratinocytes were allowed to spread on LM-5–enriched matrix in the presence of IGF-I 50 ng/ml (IGF), either alone or supplemented with UTP 100 μM (IGF+UTP). Top, phase-contrast microphotographs show cell-spreading inhibition by UTP 20 min after seeding. Bottom, a time-course spreading of HaCat cells on LM-5. When indicated, cells were preincubated with LY294002 (LY; 30 μM, 60 min). Cell surface was quantified as described in Materials and Methods. Data are expressed as the mean ± SEM (n = 100) and are representative of three independent experiments. (B) Cells were pretreated with YM-254890 (YM; 3 μM, 5 min), and then spreading assays were performed as described in A. (C) Cells were nucleofected with a nontargeting siRNA (Ctrl siRNA) or a Gq siRNA. Forty-eight hours after transfection, spreading assays were performed as described in A. Data were statistically analyzed using the Student's t test. **p < 0.001.
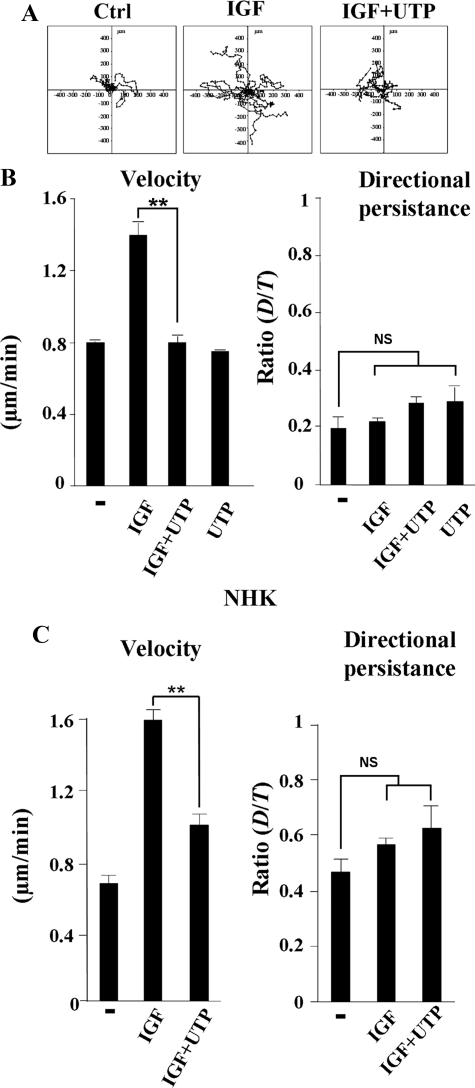
Using an in vitro wound healing assay, we previously reported that purinergic signaling delays keratinocyte migration (Taboubi et al., 2007). Here, we performed a random two-dimensional motility assay using time-lapse videomicroscopy (Sadok et al., 2008). This technique allows a more precise identification of the migratory parameters that might be affected by purinergic signaling, i.e., cell velocity or directional persistence. Representative migration paths (where the origin of each cell track has been set to the coordinate (x = 0, y = 0) for 10 cells are shown on Figure 6A. Analysis of the trajectory obtained from time-lapse recordings of each individual cell during 2 h, indicated that control untreated and UTP-treated cells migrated slowly (0.77 ± 0.06 μm/min) whereas IGF-I–stimulated cells exhibited an enhanced migration velocity (1.5 ± 0.1 μm/min; Figure 6B). Neither IGF-I nor UTP significantly affected cell directionality. Interestingly, in the presence of UTP, IGF-I failed to increase cell velocity (0.8 ± 0.05 μm/min; Figure 6B). This inhibition was sustained and persisted even 10 h after the beginning of the recording (data not shown). Similar results were obtained when normal human keratinocytes were used instead of HaCaT cells (Figure 6C). Thus, IGF-I did not control cell directionality but promoted keratinocyte migration by increasing cell speed, an essential function which is totally abolished by UTP.
Figure 6.
Effect of UTP on IGF-I–induced keratinocyte random migration. (A) Presentation of 10 HaCaT keratinocyte migration paths during 120 min: untreated cells (Ctrl), cells stimulated by IGF-I (50 ng/ml), either alone (IGF) or in combination with UTP (100 μM) (IGF+UTP). (B and C) Motility assays were performed with HaCat cells (B) or normal human keratinocytes (C; NHK) as described in A. In each experimental condition, trajectory of at least 40 individual cells was analyzed. Cell velocity and directional persistence were calculated from time-lapse movies as described in Materials and Methods. Data are expressed as the mean ± SD and are representative of three independent experiments. Data are statistically analyzed using the Student's t test. **p < 0.001; NS, p > 0.5.
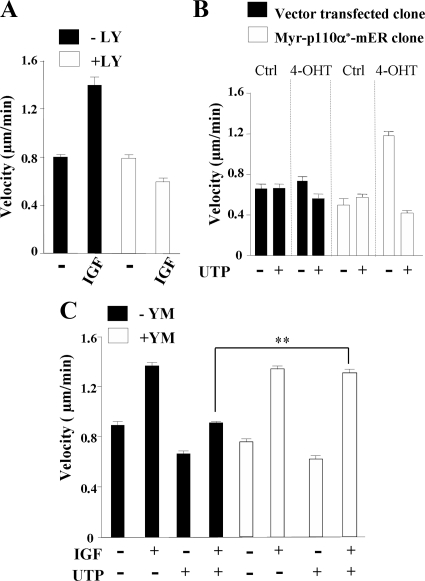
As shown on Figure 7A, PI3K inhibition by LY294002 abrogated the IGF-I–induced increase of cell velocity. To confirm the role of p110α-PI3K, we conducted a second series of motility assays with vector-transfected or Myr-p110α*-mER HaCat clones. We first verified that these cells behave like untransfected HaCat cells upon stimulation by IGF-I and UTP (data not shown). As already reported, activation of Myr-p110α*-mER by 4-OHT promotes HaCat migration in wound healing assays (Pankow et al., 2006). Figure 7B shows that, in random motility assays, activation of the p110α-PI3K mutant by 4-OHT–induced an increase in cell velocity (1.2 ± 0.09 μm/min, □) compared with empty vector–transfected cells (0.47 ± 0.02 μm/min, ■). As reported above for IGF-I (see Figure 6), no increase in cell migration persistence was noted (not shown). Importantly, UTP was able to inhibit cell velocity of cells expressing activated Myr-p110α*-mER (Figure 7B). Using YM-254890 (3 μM), we showed that Gα(q/11) also plays a crucial role in the signaling pathway driven by extracellular UTP to slow down keratinocyte migration (Figure 7C). Taken together, these results clearly show that activation of Gα(q/11)by extracellular nucleotides inhibited p110α-dependent keratinocyte velocity.
Figure 7.
Gα(q/11) activation inhibits p110α-PI3K–dependent keratinocyte motility. (A) HaCaT keratinocytes were pretreated with or without LY294002 (LY; 10 μM) and then with IGF-I (50 ng/ml) and assayed for 2D random migration. (B) Random motility assays were performed with Myr-p110α*-mER HaCaT clone (□) and vector-transfected clone (■). PI3K activation was induced by 4-OHT treatment (4-OHT); solvent-treated cells were used as control (Ctrl). Cells were treated with UTP (100 μM) as indicated. (C) HaCaT cells were pretreated with YM-254890 (YM; 3 μM, 5 min) and then stimulated with IGF-I (50 ng/ml; IGF) with or without UTP (100 μM; UTP) as indicated, and motility assays were performed as described in A. In each experimental condition, trajectories of 40 cells were analyzed, and cell velocity was calculated as described in Materials and Methods. Data are expressed as the mean ± SD and are representative of three independent experiments. Data were statistically analyzed using the Student's t test. **p < 0.001.
DISCUSSION
We previously reported that purinergic receptor activation by extracellular nucleotides reduces keratinocyte wound healing by inhibiting growth factor motogenic effects (Taboubi et al., 2007). An important issue coming from this study was to decipher the molecular mechanism governing this opposite cross-talk between Gq-coupled P2Y receptors and RTKs. Here, we show that P2Y2R signaling inhibits IGF-I–induced p110α-PI3K lipid kinase activity in a Gα(q/11)-dependent manner. Moreover, our data show that extracellular UTP impeded IGF-I–induced PI3K-mediated keratinocyte migration by inhibiting both cell spreading and cell velocity.
As other GPCRs, P2Y2R has been reported to mostly transactivate RTKs or to synergistically cross-talk with their downstream effectors (reviewed in Erb et al., 2006). However, this does not constitute an absolute rule. Indeed, several reports clearly show that GPCRs can also dampen the PI3K/Akt pathway, thus inducing physiopathological repercussions, as illustrated in insulin resistance-associated hypertension and several cardiovascular diseases (Velloso et al., 1996; Folli et al., 1997; Howes et al., 2003; Motley et al., 2003). GPCRs can antagonize RTK-induced PI3K/Akt signal transduction using different molecular mechanisms. These include RTK dephosphorylation (Lin et al., 2003), dephosphorylation of the adaptator protein IRS (Ueda et al., 2004), or alteration of IRS-1/p85-PI3K interaction (Folli et al., 1997; Motley et al., 2002, 2003), all events that ultimately can lead to the inhibition of PI3K activation (Velloso et al., 1996; Folli et al., 1997; Ballou et al., 2000; Bousquet et al., 2006). Conversely, GPCRs have also been shown to stimulate phosphatase and tensin homologue on chromosome 10 (PTEN) activation thus impeding PI3K pathway (Sanchez et al., 2005). In HaCat cells, this possibility with regards to P2Y2R remains to be investigated in future studies. It has also been shown that GPCR-induced PIP2 depletion due to PLCβ activation can compromise PIP3 production (Howes et al., 2003). However, we report here that in HaCat cells, the U73122-insensitive inhibition of Akt phosphorylation by UTP (Figure 2) indicating that alteration of IGF-I signaling by UTP does not require PLCβ activation. In parallel with our own observations, other groups have also described PLCβ-independent inhibition of Akt phosphorylation by GPCR (Ballou et al., 2003; Ueda et al., 2004; Golebiewska and Scarlata, 2008).
Importantly, we show that purinergic signaling reduced IGF-I promoted p110α-PI3K lipid kinase activity in HaCat cells by more than 60% (Figure 1C). Extracellular nucleotides may therefore trigger intracellular signaling, leading to the inhibition of p110α-PI3K activation by IGF-I. However, our data supports a model in which UTP does not inhibit the p110α-PI3K membrane recruitment, the initial step of its activation by growth factors. Two lines of evidence confirm this. First, UTP-inhibited Akt phosphorylation induced by a wide variety of signals, including cell stimulation by IGF-I (Figures 1–3), epidermal growth factor (EGF; unpublished observation), fetal calf serum (Taboubi et al., 2007). Second, the signaling function of a membrane-targeted active mutant of p110α subunit (myr-p110α*-mER) was also inhibited by UTP (Figure 3). Finally, we did not find any evidence for a dissociation of the IGF-R/IRS-1/p85 complex by UTP (Figure 3).
Using siRNA knockdown approach and YM-254890, a specific pharmacological inhibitor (Uemura et al., 2006), we showed that Gα(q/11) is essential to convey the inhibitory signal from P2Y2R to PI3K (Figure 2). It has already been reported that Gq-coupled receptors can inhibit PI3K/Akt pathway (Ballou et al., 2000; Motley et al., 2003; Ueda et al., 2004). In particular, in vitro and in vivo studies from the Lin's group have shown that active Gαq can selectively bind to the p110α subunit but not to p110β or p110γ and can inhibit its lipid kinase activity (Ballou et al., 2000, 2003, 2006; Bommakanti et al., 2000). However, in HaCat cells, we were unable to coimmunoprecipitate endogenous p110α-PI3K and Gαq, which does not imply a resilient interaction between these two proteins. Gα(q/11) can trigger multiple signals by directly linking various effectors such as PLCβ, p63RhoGEF, or p110α-PI3K (Ballou et al., 2003; Rojas et al., 2007). In a recent work, Golebieska and Scarlata (2008) have demonstrated the presence of stable and separate pools of Gαq-PLCβ and Gαq-PI3K complexes by using Foster resonance energy transfer. Thus, in a given cell type, there may be a different subset of Gαq preassociated with one of these effectors, and these complexes would then selectively direct the signal in either direction, i.e., PLCβ activation or PI3K inhibition. Finally, because P2Y2R are also coupled to other G proteins (Erb et al., 2001; Liao et al., 2007), we cannot completely rule out their possible involvement in PI3K inhibition. However, using PTX we showed that PI3K inhibition did not require the coupling of P2Y2R with G(i/0) (Figure 2). The exact mechanism whereby activation of Gαq by UTP can inhibit p100α-PI3K function remains unclear and is still under investigation in our laboratory.
In the current study, we consistently observed that UTP inhibited the early phase of the p110α-PI3K signaling function (1–10 min after cell stimulation by IGF-I). It is now well documented that cell stimulation by RTKs induces two waves of PI3K product accumulation (Jones et al., 1999; Yip et al., 2007). The late peak of PIP3 (rising between 3 and 7 h) is required for cell cycle progression (Jones et al., 1999), whereas the early and transient PIP3 accumulation (between 0.5 and 5 min) controls actin remodeling and protrusion formation at the leading edge of migrating cells (Merlot and Firtel, 2003; Mouneimne et al., 2004; Yip et al., 2007; Kolsch et al., 2008). In agreement with these reports, we observed a fast translocation of the actin-nucleating protein cortactin to the cell edges in IGF-I stimulated HaCat cells (Figure 4). Moreover, this early PI3K-dependent cortactin relocalization was inhibited by UTP in a time correlated manner with the inhibition of PI3K by Gq-coupled P2Y2R (Figures 1, 2 and 4). It has also been also proposed that the early PI3K activation can alter the conformation of other signaling proteins, e.g., the protein kinase C and therefore can trigger signals over a relatively long period of time (Balciunaite and Kazlauskas, 2001). Thus, it is actually conceivable that UTP, by altering the first wave of PI3K activation, may have an immediate profound impact on actin dynamics and may later reduce cell spreading and cell motility (Figures 5 and 7).
Finally, it should be noted that in sharp contrast to the data presented here and our own previously published results, several other publications have reported that P2Y2R stimulates cell migration in several other cell types (Chaulet et al., 2001; Pillois et al., 2002; Bagchi et al., 2005; Kaczmarek et al., 2005; Wang et al., 2005; Chen et al., 2006; Yu et al., 2008). Studies from the Erb's group have partially deciphered the signaling pathway downstream of P2Y2R that promotes astrocyte migration. The authors reported that P2Y2R interacts with αv integrin to access and activate G0 or G12 and in turn induce cell migration through the activation of downstream pathways (Erb et al., 2001; Bagchi et al., 2005; Liao et al., 2007). Interestingly, they provide several data indicating that a mutant of P2Y2R that is unable to bind αv integrin and to promote cell migration still activates Gq/PLCβ pathway. Thus, in a given cell type, it appears that P2Y2R may be coupled to different G proteins and may therefore be able to induce distinct signaling pathways.
Acute wounds normally heal by a well-orchestrated, tightly regulated series of processes including hemostasis, inflammation, and tissues formation and remodeling. In aging individuals or in a pathophysiological context such as diabetes mellitus or venus insufficiency, skin lesions most often result in chronic nonhealing ulcers (Menke et al., 2007). Exogenous growth factors have been reported as important mediators that enhance tissues remodeling and induce faster wound closure (Werner and Grose, 2003). During the inflammatory phase of skin wound healing, extracellular nucleotides are abundant and most likely participate to the regulation of numerous events such as platelet activation (Gachet, 2008), pain transmission (Dussor et al., 2009), or neutrophil and phagocyte recruitment (Chen et al., 2006; Elliott et al., 2009). Our current finding that P2Y2R inhibits signaling of motogenic growth factors on cultured keratinocytes suggests that extracellular nucleotides may have additional functions during wound healing by delaying the reepithelialization phase. Thus, an excess of extracellular ATP in chronic wound might be deleterious. Expanding our understanding of this pathway in wound healing, maybe then translated to clinical benefit in the form of preventive or curative treatments of wound healing disorders.
Supplementary Material
ACKNOWLEDGMENTS
We greatly appreciate the gift of myr-p110α*-mER HaCaT clones from Dr. Sabine Werner and Sandra Pankow (Institute of Cell Biology, Department of Biology, ETH Zurich, CH-8093 Zurich, Switzerland). We thank Anne Sophie Sabatier (DIPTA, Aix en Provence, France) for technical assistance for normal human keratinocytes culture. We are grateful to Dr. Jun Takazaki (Astellas Pharma, Drug Discovery Research and Molecular Medicine Research Labs, Ibaraki, Japan) for the gift of Gα(q/11) pharmacological inhibitor, YM-254890. We thank Dr Hervé Ansanay (CISBIO, Bagnols-sur-Cèze Cedex, France) and Dr. Jacques Nunes (INSERM U599, Marseille, France) for helpful discussions. The work was financed by Cancéropole Provence-Alpes-Côte d'Azur and DIPTA. The work was supported by Région Provence Alpes Côte d'Azur/DIPTA fellowships to S.T.
Abbreviations used:
- GPCR
G protein–coupled receptor
- IGF-I
insulin-like growth factor-I
- PI3K
phosphoinositide 3-kinase
- PIP2
phosphatidylinositol (4,5)bisposphate
- PIP3
phosphatidylinositol (3,4,5) trisphosphate
- PLCβ
phospholipase C β
- P2Y2R
P2Y2 receptor
- RTK
receptor tyrosine kinase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-06-0497) on January 20, 2010.
REFERENCES
- Albert P. R., Robillard L. G protein specificity: traffic direction required. Cell Signal. 2002;14:407–418. doi: 10.1016/s0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- Ammer A. G., Weed S. A. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil. Cytoskelet. 2008;65:687–707. doi: 10.1002/cm.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando Y., Jensen P. J. Epidermal growth factor and insulin-like growth factor I enhance keratinocyte migration. J. Invest. Dermatol. 1993;100:633–639. doi: 10.1111/1523-1747.ep12472297. [DOI] [PubMed] [Google Scholar]
- Atkinson P. J., Young K. W., Ennion S. J., Kew J. N., Nahorski S. R., Challiss R. A. Altered expression of G(q/11alpha) protein shapes mGlu1 and mGlu5 receptor-mediated single cell inositol 1,4,5-trisphosphate and Ca(2+) signaling. Mol. Pharmacol. 2006;69:174–184. doi: 10.1124/mol.105.014258. [DOI] [PubMed] [Google Scholar]
- Bagchi S., Liao Z., Gonzalez F. A., Chorna N. E., Seye C. I., Weisman G. A., Erb L. The P2Y2 nucleotide receptor interacts with alphav integrins to activate Go and induce cell migration. J. Biol. Chem. 2005;280:39050–39057. doi: 10.1074/jbc.M504819200. [DOI] [PubMed] [Google Scholar]
- Balciunaite E., Kazlauskas A. Early phosphoinositide 3-kinase activity is required for late activation of protein kinase Cepsilon in platelet-derived-growth-factor-stimulated cells: evidence for signalling across a large temporal gap. Biochem. J. 2001;358:281–285. doi: 10.1042/0264-6021:3580281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou L. M., Chattopadhyay M., Li Y., Scarlata S., Lin R. Z. Galphaq binds to p110alpha/p85alpha phosphoinositide 3-kinase and displaces Ras. Biochem. J. 2006;394:557–562. doi: 10.1042/BJ20051493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou L. M., Cross M. E., Huang S., McReynolds E. M., Zhang B. X., Lin R. Z. Differential regulation of the phosphatidylinositol 3-kinase/Akt and p70 S6 kinase pathways by the alpha(1A)-adrenergic receptor in rat-1 fibroblasts. J. Biol. Chem. 2000;275:4803–4809. doi: 10.1074/jbc.275.7.4803. [DOI] [PubMed] [Google Scholar]
- Ballou L. M., Lin H. Y., Fan G., Jiang Y. P., Lin R. Z. Activated G alpha q inhibits p110 alpha phosphatidylinositol 3-kinase and Akt. J. Biol. Chem. 2003;278:23472–23479. doi: 10.1074/jbc.M212232200. [DOI] [PubMed] [Google Scholar]
- Barnes W. G., Reiter E., Violin J. D., Ren X. R., Milligan G., Lefkowitz R. J. beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J. Biol. Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- Baserga R. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 2000;19:5574–5581. doi: 10.1038/sj.onc.1203854. [DOI] [PubMed] [Google Scholar]
- Bommakanti R. K., Vinayak S., Simonds W. F. Dual regulation of Akt/protein kinase B by heterotrimeric G protein subunits. J. Biol. Chem. 2000;275:38870–38876. doi: 10.1074/jbc.M007403200. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet C., et al. Direct binding of p85 to sst2 somatostatin receptor reveals a novel mechanism for inhibiting PI3K pathway. EMBO J. 2006;25:3943–3954. doi: 10.1038/sj.emboj.7601279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce N. S., Clark E. S., Leysath J. L., Currie J. D., Webb D. J., Weaver A. M. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr. Biol. 2005;15:1276–1285. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling. Br. J. Pharmacol. 2006;147(Suppl. 1):S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell H. E., Wlodarski B., Foster B. J., Buckley K. A., Sharpe G. R., Quayle J. M., Simpson A. W., Gallagher J. A. Human keratinocytes release ATP and utilize three mechanisms for nucleotide interconversion at the cell surface. J. Biol. Chem. 2005;280:29667–29676. doi: 10.1074/jbc.M505381200. [DOI] [PubMed] [Google Scholar]
- Cain R. J., Ridley A. J. Phosphoinositide 3-kinases in cell migration. Biol. Cell. 2009;101:13–29. doi: 10.1042/BC20080079. [DOI] [PubMed] [Google Scholar]
- Ceccarelli S., Cardinali G., Aspite N., Picardo M., Marchese C., Torrisi M. R., Mancini P. Cortactin involvement in the keratinocyte growth factor and fibroblast growth factor 10 promotion of migration and cortical actin assembly in human keratinocytes. Exp. Cell Res. 2007;313:1758–1777. doi: 10.1016/j.yexcr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Chaulet H., Desgranges C., Renault M. A., Dupuch F., Ezan G., Peiretti F., Loirand G., Pacaud P., Gadeau A. P. Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circ. Res. 2001;89:772–778. doi: 10.1161/hh2101.098617. [DOI] [PubMed] [Google Scholar]
- Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., Nizet V., Insel P. A., Junger W. G. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Dixon C. J., Bowler W. B., Littlewood-Evans A., Dillon J. P., Bilbe G., Sharpe G. R., Gallagher J. A. Regulation of epidermal homeostasis through P2Y2 receptors. Br J Pharmacol. 1999;127:1680–1686. doi: 10.1038/sj.bjp.0702653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussor G., Koerber H. R., Oaklander A. L., Rice F. L., Molliver D. C. Nucleotide signaling and cutaneous mechanisms of pain transduction. Brain Res. Rev. 2009;60:24–35. doi: 10.1016/j.brainresrev.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson S. R., Thumiger S. P., Werther G. A., Wraight C. J. Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr. Rev. 2003;24:737–764. doi: 10.1210/er.2002-0021. [DOI] [PubMed] [Google Scholar]
- Elliott M. R., et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb L., Liao Z., Seye C. I., Weisman G. A. P2 receptors: intracellular signaling. Pfluegers Arch. 2006;452:552–562. doi: 10.1007/s00424-006-0069-2. [DOI] [PubMed] [Google Scholar]
- Erb L., et al. An RGD sequence in the P2Y(2) receptor interacts with alpha(V)beta(3) integrins and is required for G(o)-mediated signal transduction. J. Cell Biol. 2001;153:491–501. doi: 10.1083/jcb.153.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folli F., Kahn C. R., Hansen H., Bouchie J. L., Feener E. P. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J. Clin. Invest. 1997;100:2158–2169. doi: 10.1172/JCI119752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet C. P2 receptors, platelet function and pharmacological implications. Thromb. Haemost. 2008;99:466–472. doi: 10.1160/TH07-11-0673. [DOI] [PubMed] [Google Scholar]
- Golebiewska U., Scarlata S. Galphaq binds two effectors separately in cells: evidence for predetermined signaling pathways. Biophys. J. 2008;95:2575–2582. doi: 10.1529/biophysj.108.129353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig A. V., James S. E., McGrouther D. A., Terenghi G., Burnstock G. Purinergic receptor expression in the regeneration epidermis in a rat model of normal and delayed wound healing. Exp. Dermatol. 2003b;12:860–871. doi: 10.1111/j.0906-6705.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- Greig A. V., Linge C., Terenghi G., McGrouther D. A., Burnstock G. Purinergic receptors are part of a functional signaling system for proliferation and differentiation of human epidermal keratinocytes. J. Invest. Dermatol. 2003a;120:1007–1015. doi: 10.1046/j.1523-1747.2003.12261.x. [DOI] [PubMed] [Google Scholar]
- Haase I., Evans R., Pofahl R., Watt F. M. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J. Cell Sci. 2003;116:3227–3238. doi: 10.1242/jcs.00610. [DOI] [PubMed] [Google Scholar]
- Hawkins P. T., Anderson K. E., Davidson K., Stephens L. R. Signalling through Class I PI3Ks in mammalian cells. Biochem. Soc. Trans. 2006;34:647–662. doi: 10.1042/BST0340647. [DOI] [PubMed] [Google Scholar]
- Hodak E., Gottlieb A. B., Anzilotti M., Krueger J. G. The insulin-like growth factor 1 receptor is expressed by epithelial cells with proliferative potential in human epidermis and skin appendages: correlation of increased expression with epidermal hyperplasia. J. Invest. Dermatol. 1996;106:564–570. doi: 10.1111/1523-1747.ep12344044. [DOI] [PubMed] [Google Scholar]
- Holzer A. M., Granstein R. D. Role of extracellular adenosine triphosphate in human skin. J. Cutan. Med. Surg. 2004;8:90–96. doi: 10.1007/s10227-004-0125-5. [DOI] [PubMed] [Google Scholar]
- Hosoya A., Lee J. M., Cho S. W., Kim J. Y., Shinozaki N., Shibahara T., Shimono M., Jung H. S. Morphological evidence of basal keratinocyte migration during the re-epithelialization process. Histochem. Cell Biol. 2008;130:1165–1175. doi: 10.1007/s00418-008-0499-3. [DOI] [PubMed] [Google Scholar]
- Howes A. L., Arthur J. F., Zhang T., Miyamoto S., Adams J. W., Dorn I. G., Woodcock E. A., Brown J. H. Akt-mediated cardiomyocyte survival pathways are compromised by G alpha q-induced phosphoinositide 4,5-bisphosphate depletion. J. Biol. Chem. 2003;278:40343–40351. doi: 10.1074/jbc.M305964200. [DOI] [PubMed] [Google Scholar]
- Hubbard K. B., Hepler J. R. Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal. 2006;18:135–150. doi: 10.1016/j.cellsig.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Inoue K., Hosoi J., Denda M. Extracellular ATP has stimulatory effects on the expression and release of IL-6 via purinergic receptors in normal human epidermal keratinocytes. J. Invest. Dermatol. 2007;127:362–371. doi: 10.1038/sj.jid.5700526. [DOI] [PubMed] [Google Scholar]
- Irino Y., Nakamura Y., Inoue K., Kohsaka S., Ohsawa K. Akt activation is involved in P2Y12 receptor-mediated chemotaxis of microglia. J. Neurosci. Res. 2008;86:1511–1519. doi: 10.1002/jnr.21610. [DOI] [PubMed] [Google Scholar]
- Jeschke M. G., Schubert T., Krickhahn M., Polykandriotis E., Klein D., Perez-Polo J. R., Przkora R., Herndon D. N. Interaction of exogenous liposomal insulin-like growth factor-I cDNA gene transfer with growth factors on collagen expression in acute wounds. Wound Repair Regen. 2005;13:269–277. doi: 10.1111/j.1067-1927.2005.130309.x. [DOI] [PubMed] [Google Scholar]
- Jones S. M., Klinghoffer R., Prestwich G. D., Toker A., Kazlauskas A. PDGF induces an early and a late wave of PI 3-kinase activity, and only the late wave is required for progression through G1. Curr. Biol. 1999;9:512–521. doi: 10.1016/s0960-9822(99)80235-8. [DOI] [PubMed] [Google Scholar]
- Kaczmarek E., Erb L., Koziak K., Jarzyna R., Wink M. R., Guckelberger O., Blusztajn J. K., Trinkaus-Randall V., Weisman G. A., Robson S. C. Modulation of endothelial cell migration by extracellular nucleotides: involvement of focal adhesion kinase and phosphatidylinositol 3-kinase-mediated pathways. Thromb. Haemost. 2005;93:735–742. doi: 10.1267/THRO05040735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S., Fujishita K., Inoue K., Shigemoto-Mogami Y., Tsuda M., Inoue K. Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem. J. 2004;380:329–338. doi: 10.1042/BJ20031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsch V., Charest P. G., Firtel R. A. The regulation of cell motility and chemotaxis by phospholipid signaling. J. Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski J. R., Egile C., Gil S., Snapper S. B., Li R., Thomas S. M. Cortactin regulates cell migration through activation of N-WASP. J. Cell Sci. 2005;118:79–87. doi: 10.1242/jcs.01586. [DOI] [PubMed] [Google Scholar]
- Kurosu H., Maehama T., Okada T., Yamamoto T., Hoshino S., Fukui Y., Ui M., Hazeki O., Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. J. Biol. Chem. 1997;272:24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- Lazarowski E. R., Boucher R. C., Harden T. K. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Lee W. K., Choi S. W., Lee H. R., Lee E. J., Lee K. H., Kim H. O. Purinoceptor-mediated calcium mobilization and proliferation in HaCaT keratinocytes. J. Dermatol. Sci. 2001;25:97–105. doi: 10.1016/s0923-1811(00)00117-1. [DOI] [PubMed] [Google Scholar]
- Leenders F., et al. PKN3 is required for malignant prostate cell growth downstream of activated PI 3-kinase. EMBO J. 2004;23:3303–3313. doi: 10.1038/sj.emboj.7600345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z., Seye C. I., Weisman G. A., Erb L. The P2Y2 nucleotide receptor requires interaction with alpha v integrins to access and activate G12. J. Cell Sci. 2007;120:1654–1662. doi: 10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. Y., Ballou L. M., Lin R. Z. Stimulation of the alpha1A adrenergic receptor inhibits PDGF-induced PDGF beta receptor Tyr751 phosphorylation and PI 3-kinase activation. FEBS Lett. 2003;540:106–110. doi: 10.1016/s0014-5793(03)00233-3. [DOI] [PubMed] [Google Scholar]
- Maier U., Babich A., Nurnberg B. Roles of non-catalytic subunits in gbetagamma-induced activation of class I phosphoinositide 3-kinase isoforms beta and gamma. J. Biol. Chem. 1999;274:29311–29317. doi: 10.1074/jbc.274.41.29311. [DOI] [PubMed] [Google Scholar]
- Menke N. B., Ward K. R., Witten T. M., Bonchev D. G., Diegelmann R. F. Impaired wound healing. Clin. Dermatol. 2007;25:19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Merlot S., Firtel R. A. Leading the way: Directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J. Cell Sci. 2003;116:3471–3478. doi: 10.1242/jcs.00703. [DOI] [PubMed] [Google Scholar]
- Montiel M., de la Blanca E. P., Jimenez E. P2Y receptors activate MAPK/ERK through a pathway involving PI3K/PDK1/PKC-zeta in human vein endothelial cells. Cell Physiol. Biochem. 2006;18:123–134. doi: 10.1159/000095180. [DOI] [PubMed] [Google Scholar]
- Motley E. D., Eguchi K., Gardner C., Hicks A. L., Reynolds C. M., Frank G. D., Mifune M., Ohba M., Eguchi S. Insulin-induced Akt activation is inhibited by angiotensin II in the vasculature through protein kinase C-alpha. Hypertension. 2003;41:775–780. doi: 10.1161/01.HYP.0000051891.90321.12. [DOI] [PubMed] [Google Scholar]
- Motley E. D., Kabir S. M., Gardner C. D., Eguchi K., Frank G. D., Kuroki T., Ohba M., Yamakawa T., Eguchi S. Lysophosphatidylcholine inhibits insulin-induced Akt activation through protein kinase C-alpha in vascular smooth muscle cells. Hypertension. 2002;39:508–512. doi: 10.1161/hy02t2.102907. [DOI] [PubMed] [Google Scholar]
- Mouneimne G., Soon L., DesMarais V., Sidani M., Song X., Yip S. C., Ghosh M., Eddy R., Backer J. M., Condeelis J. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J. Cell Biol. 2004;166:697–708. doi: 10.1083/jcb.200405156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow S., Bamberger C., Klippel A., Werner S. Regulation of epidermal homeostasis and repair by phosphoinositide 3-kinase. J. Cell Sci. 2006;119:4033–4046. doi: 10.1242/jcs.03175. [DOI] [PubMed] [Google Scholar]
- Patel G. K., Wilson C. H., Harding K. G., Finlay A. Y., Bowden P. E. Numerous keratinocyte subtypes involved in wound re-epithelialization. J. Invest. Dermatol. 2006;126:497–502. doi: 10.1038/sj.jid.5700101. [DOI] [PubMed] [Google Scholar]
- Pillois X., Chaulet H., Belloc I., Dupuch F., Desgranges C., Gadeau A. P. Nucleotide receptors involved in UTP-induced rat arterial smooth muscle cell migration. Circ. Res. 2002;90:678–681. doi: 10.1161/01.res.0000013700.98464.8e. [DOI] [PubMed] [Google Scholar]
- Rojas R. J., Yohe M. E., Gershburg S., Kawano T., Kozasa T., Sondek J. Galphaq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. J. Biol. Chem. 2007;282:29201–29210. doi: 10.1074/jbc.M703458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M., Yakar S., Weingarten G., Holzenberger M., Rhodes C. J., Breitkreutz D., Leroith D., Wertheimer E. Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation. Mol. Cell. Biol. 2006;26:2675–2687. doi: 10.1128/MCB.26.7.2675-2687.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadok A., Bourgarel-Rey V., Gattacceca F., Penel C., Lehmann M., Kovacic H. Nox1-dependent superoxide production controls colon adenocarcinoma cell migration. Biochim. Biophys. Acta. 2008;1783:23–33. doi: 10.1016/j.bbamcr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Sadowski T., Dietrich S., Koschinsky F., Sedlacek R. Matrix metalloproteinase 19 regulates insulin-like growth factor-mediated proliferation, migration, and adhesion in human keratinocytes through proteolysis of insulin-like growth factor binding protein-3. Mol. Biol. Cell. 2003;14:4569–4580. doi: 10.1091/mbc.E03-01-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T., Thangada S., Wu M. T., Kontos C. D., Wu D., Wu H., Hla T. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc. Natl. Acad. Sci. USA. 2005;102:4312–4317. doi: 10.1073/pnas.0409784102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova E., Koegel H., Hasse S., Klatte J. E., Slonimsky E., Bilbao D., Paus R., Werner S., Rosenthal N. Overexpression of mIGF-1 in keratinocytes improves wound healing and accelerates hair follicle formation and cycling in mice. Am. J. Pathol. 2008;173:1295–1310. doi: 10.2353/ajpath.2008.071177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam J. C., Michiels F., van der Kammen R. A., Moolenaar W. H., Collard J. G. Invasion of T-lymphoma cells: cooperation between Rho family GTPases and lysophospholipid receptor signaling. EMBO J. 1998;17:4066–4074. doi: 10.1093/emboj/17.14.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboubi S., Milanini J., Delamarre E., Parat F., Garrouste F., Pommier G., Takasaki J., Hubaud J. C., Kovacic H., Lehmann M. G alpha(q/11)-coupled P2Y2 nucleotide receptor inhibits human keratinocyte spreading and migration. FASEB J. 2007;21:4047–4058. doi: 10.1096/fj.06-7476com. [DOI] [PubMed] [Google Scholar]
- Takasaki J., Saito T., Taniguchi M., Kawasaki T., Moritani Y., Hayashi K., Kobori M. A novel Galphaq/11-selective inhibitor. J. Biol. Chem. 2004;279:47438–47445. doi: 10.1074/jbc.M408846200. [DOI] [PubMed] [Google Scholar]
- Todorovic V., Pesko P., Micev M., Bjelovic M., Budec M., Micic M., Brasanac D., Ilic-Stojanovic O. Insulin-like growth factor-I in wound healing of rat skin. Regul. Pept. 2008;150:7–13. doi: 10.1016/j.regpep.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Ueda H., Morishita R., Narumiya S., Kato K., Asano T. Galphaq/11 signaling induces apoptosis through two pathways involving reduction of Akt phosphorylation and activation of RhoA in HeLa cells. Exp. Cell Res. 2004;298:207–217. doi: 10.1016/j.yexcr.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Uemura T., Takamatsu H., Kawasaki T., Taniguchi M., Yamamoto E., Tomura Y., Uchida W., Miyata K. Effect of YM-254890, a specific Galphaq/11 inhibitor, on experimental peripheral arterial disease in rats. Eur. J. Pharmacol. 2006;536:154–161. doi: 10.1016/j.ejphar.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Velloso L. A., Folli F., Sun X. J., White M. F., Saad M. J., Kahn C. R. Cross-talk between the insulin and angiotensin signaling systems. Proc. Natl. Acad. Sci. USA. 1996;93:12490–12495. doi: 10.1073/pnas.93.22.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Kong Q., Gonzalez F. A., Sun G., Erb L., Seye C., Weisman G. A. P2Y nucleotide receptor interaction with alpha integrin mediates astrocyte migration. J. Neurochem. 2005;95:630–640. doi: 10.1111/j.1471-4159.2005.03408.x. [DOI] [PubMed] [Google Scholar]
- Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Yang L., Cranson D., Trinkaus-Randall V. Cellular injury induces activation of MAPK via P2Y receptors. J. Cell. Biochem. 2004;91:938–950. doi: 10.1002/jcb.10774. [DOI] [PubMed] [Google Scholar]
- Yin J., Xu K., Zhang J., Kumar A., Yu F. S. Wound-induced ATP release and EGF receptor activation in epithelial cells. J. Cell Sci. 2007;120:815–825. doi: 10.1242/jcs.03389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S. C., El-Sibai M., Coniglio S. J., Mouneimne G., Eddy R. J., Drees B. E., Neilsen P. O., Goswami S., Symons M., Condeelis J. S., Backer J. M. The distinct roles of Ras and Rac in PI 3-kinase-dependent protrusion during EGF-stimulated cell migration. J. Cell Sci. 2007;120:3138–3146. doi: 10.1242/jcs.005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Kobayashi D., Ohkubo S., Nakahata N. ATP stimulates interleukin-6 production via P2Y receptors in human HaCaT keratinocytes. Eur. J. Pharmacol. 2006;540:1–9. doi: 10.1016/j.ejphar.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Yu N., Erb L., Shivaji R., Weisman G. A., Seye C. I. Binding of the P2Y2 nucleotide receptor to filamin A regulates migration of vascular smooth muscle cells. Circ. Res. 2008;102:581–588. doi: 10.1161/CIRCRESAHA.107.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.