Mutations in mitochondrial genes and inhibitors of OX-Phos make Caenorhabditis elegans resistant to multiple drugs. The anti-oxidant NAC prevents this drug-resistance, indicating that a mechanism responsive to ROS is required. The resistance generated by inhibitors of respiration is reduced in mitochondrial mutants that lack the C. elegans ortholog of PKCε.
Abstract
In a previous genetic screen for Caenorhabditis elegans mutants that survive in the presence of an antimitotic drug, hemiasterlin, we identified eight strong mutants. Two of these were found to be resistant to multiple toxins, and in one of these we identified a missense mutation in phb-2, which encodes the mitochondrial protein prohibitin 2. Here we identify two additional mutations that confer drug resistance, spg-7 and har-1, also in genes encoding mitochondrial proteins. Other mitochondrial mutants, isp-1, eat-3, and clk-1, were also found to be drug-resistant. Respiratory complex inhibitors, FCCP and oligomycin, and a producer of reactive oxygen species (ROS), paraquat, all rescued wild-type worms from hemiasterlin toxicity. Worms lacking mitochondrial superoxide dismutase (MnSOD) were modestly drug-resistant, and elimination of MnSOD in the phb-2, har-1, and spg-7 mutants enhanced resistance. The antioxidant N-acetyl-l-cysteine prevented mitochondrial inhibitors from rescuing wild-type worms from hemiasterlin and sensitized mutants to the toxin, suggesting that a mechanism sensitive to ROS is necessary to trigger drug resistance in C. elegans. Using genetics, we show that this drug resistance requires pkc-1, the C. elegans ortholog of human PKCε.
INTRODUCTION
The appearance of drug-resistant cancers after chemotherapy is a common, serious contributor to morbidity, and combating this requires developing new therapeutics. Circumventing drug resistance ultimately entails understanding both the molecular mechanisms of drug action and the responses to the drug that lead to resistance. We have been exploring the use of Caenorhabditis elegans as a model for understanding these issues, as it is a fast growing multicellular organism with well-developed genetics, a sequenced genome, and known mutants with defects in many important cell signaling pathways. To avoid the problem that the C. elegans genome encodes for effective drug effluent pumps, we chose hemiasterlin as the toxic compound to begin these studies. Hemiasterlins are sponge-derived tripeptides that bind to tubulin and inhibit microtubule assembly. A hemiasterlin analog, HTI-286, is poorly transported by the P-glycoprotein efflux pump and inhibits the growth of human tumor xenografts expressing P-glycoprotein, where paclitaxel and vincristine are ineffective (Loganzo et al., 2003). Also, HTI-286 exhibited strong antitumor effects against docetaxel-refractory prostate cancer (Hadaschik et al., 2008b) and has shown minimal toxicity and significantly delayed human bladder cancer growth in a mouse model (Hadaschik et al., 2008a). Tumor cells resistant to HTI-286 have been isolated that bear mutations in α or β tubulin and show increased microtubule stability (Poruchynsky et al., 2004). Currently, HTI-286 is in clinical trials as a promising chemotherapeutic drug that circumvents P-glycoprotein–mediated resistance (Loganzo et al., 2003; Ayral-Kaloustian and Zask, 2005).
We have chosen to use a hemiasterlin analog almost identical to HTI-286, having a β-3 bromophenyl instead of β-phenyl group. This compound was synthesized as a precursor of a biotinylated probe we used to show that the derivative binds tubulin and is toxic for animal cells at subnanomolar range, like natural hemiasterlin (Zubovych et al., 2006). Previously, we described a genetic screen on C. elegans where we isolated drug-resistant mutants and identified the genetic lesion responsible for drug resistance in one of them as a missense mutation in C. elegans prohibitin-2 (PHB-2), a protein localized to the inner mitochondrial membrane. Now we report the identity of mutations that confer drug resistance in two additional mutant worms from our screen. Both are in proteins known or predicted to locate to mitochondria.
We have shown previously that worms phb-2(ad2154) and (ad2155dm) are resistant to a number of poisons, including other tubulin binders and the DNA topoisomerase I inhibitor camptothecin, while retaining wild-type sensitivity to phalloidin (Zubovych et al., 2006). Because our screen produced resistant animals with mitochondrial protein alterations, in this current work we examined other existing mitochondrial mutants for drug resistance. Among five mutants tested, three of them were resistant (isp-1, clk-1, eat-3), and two sensitive (gas-1 and mev-1) to the hemiasterlin analog. We tested the hypothesis that impaired mitochondrial respiration leads to drug resistance by a combination of pharmacology and genetics. Our work leads to the conclusion that a mechanism sensitive to reactive oxygen species (ROS) is necessary to produce drug resistance involving mitochondria in C. elegans.
MATERIALS AND METHODS
Drugs and Toxins Used
The hemiasterlin analog was synthesized and described previously (Zubovych et al., 2006). Rotenone, antimycin, stigmatellin, myxothiazol, oligomycin, 2-thenoyltrifluoroacetone (TTFA), FCCP, 2-deoxy-d-glucose, taxol, etomoxir sodium, wortmannin, and N-acetyl-l-cysteine (NAC) were purchased from Sigma (St. Louis, MO); peloruside A was from Dr. Jef De Brabander (University of Texas Southwestern Medical Center), cryptophycin-1 was provided by Dr. Gunda Georg (University of Minnesota) and Dr. Richard Himes (University of Kansas), and methyl viologen and 5-thio-d-glucose were from MP Biomedicals (Aurora, OH).
Worm Strains and Culture
Worms were handled at 20°C as described (Brenner, 1974) with slight modifications. All worms were fed on Escherichia coli HB101 bacteria (Boyer and Roulland-Dussoix, 1969). The wild-type N2 Bristol was the parental strain for all mutant strains and was used as the wild type for all comparisons. The wild-type Hawaiian CB4856 was interbred with mutants for experiments that mapped mutations. Other strains used were DA2154 phb-2 (ad2154) II; DA2155 har-1 III, DA2249 spg-7 (ad2249) I; ZG31 hif-1(ia4) V; DA1496 eat-3(ad426) II; DA1116 eat-2(ad1116) II; MQ887 isp-1(qm150) IV; CB4876 clk-1(e2519) III; MQ130 clk-1(qm30) III; CW152 gas-1(fc21) X; TK22 mev-1(kn1) III; GR1310 akt-1 (mg144) V; RB712 daf-18 (ok480) IV; TM173 sod-2(sj173) I; TM134 sod-3(sj134) X; TM259 sod-2(sj173);sod-3(sj134) IK105 pkc-1(nj1)V; IK130 pkc-1(nj3)V; MJ500 tpa-1(k501)IV; MJ563 tpa-1(k530)IV; VC127 pkc-2(ok328)X, JJ1271 glo-1(zu391)X.
Identifying the Mutation in har-1(ad2155dm) and Complementation Testing
Using a standard single-nucleotide polymorphism method (Wicks et al., 2001), we mapped the drug-resistant dominant mutation in ad2155dm to the left arm of chromosome III, between cosmids C32A3 and W03A5. Further analysis of recombinants placed the mutation among cosmids C44F1 and R10E4. This interval was flanked by lin-48 (left border) and tag-310 (right border) and contained 107 genes. Because phb-2 and ad2155dm displayed similar behavior in our assays, we hypothesized that both mutations in these worms might share the same pathway and the genes might show similar expression patterns. We compared the expression of the 107 genes in the interval containing the drug-resistance mutation with PHB-2 (GeneOrienteer 1.40; www.geneorienteer.org/; Zhong and Sternberg, 2006). C16C10.11 had the highest feature score and was the only mitochondrial protein in the region. We amplified the C16C10.11 DNA from the ad2155dm mutant and sequenced PCR products. Sequence analysis revealed a G-to-A transition at nucleotide 218 resulting in a Gly-to-Glu change at 73 aa (G73E). To test if a mutation in C16C10.11 was responsible for the ad2155dm drug-resistant phenotype, we amplified by PCR 1943 base pairs of genomic DNA from the mutant that contained the 850-base pair coding region of C16C10.11 with a 533-base pair upstream and 560-base pair downstream sequence. The primers used for the amplification were GCTAGTAAATCGAATGGCAT and AAGCTTCGAAGCTACCGTA. We injected gonads of wild-type worms with this PCR product (0.15 ng/μl) mixed with DNA encoding a rol-6 (pRF4) mutation as a transformation marker (50 ng/μl). Twenty-seven independent stable transgenic lines were examined for drug resistance, defined as the ability of worms to grow to healthy gravid adults that can move in the presence of hemiasterlin analog. In 19 lines 30–100% of transformed worms were resistant to the hemiasterlin analog.
Mapping the Mutation in the ad2249 Recessive Mutant and Complementation Testing
A recessive mutant, ad2249, was isolated in our genetic screen for hemiasterlin-resistant mutants together with phb-2 and har-1. We crossed ad2249 males with CB4856 hermaphrodites and in the F2 generation selected for drug-resistant progeny, placing 435 drug-resistant animals individually on plates and allowing them to reproduce. Subsequent SNP (single nucleotide polymorphism) analysis of DNA isolated from progeny of these resistant worms assigned the mutation to chromosome I and analysis of worms with recombinant chromosome I mapped the drug-resistant mutation into the region between cosmids W05F2 and T28F2. This region contains 46 genes in total, and only one, spg-7, encoded a mitochondrial protein. We amplified and sequenced all 10840 base pairs of DNA encoding SPG-7 from the ad2249 mutant and found a single G-to-A transition changing E-to-K at amino acid 414. The primers for PCR amplification of the region containing this mutation were GTGAATTTCCTGAAGAACCC and ATCTCGTGATTCGCATCTCT. The resulting 649-base pair PCR product was sequenced and the mutation was confirmed on both DNA strands. E414 is a highly conserved amino acid from yeast to humans (see Figure 1B). To confirm that this mutation was responsible for drug resistance, we obtained strain FX 2312(tm2312/+) from the Mitani laboratory. We amplified by PCR the region that contained the deletion in FX 2312(tm2312/+) using as primers, AATCGCAGTTAGGCTGTGT and CATAGATCTGTCTATCAAAGCG. The resulting DNA fragment was 978 base pairs in wild-type worms but in FX 2312(tm2312/+) heterozygous worms both the 978-base pair fragment and a 584-base pair fragment were present. Sequence analysis of the 584 fragment indicated that the spg-7 gene in FX 2312 (tm2312/+) worms contains a 394-base pair deletion that causes a frame shift in the protein coding sequence that would produce a protein containing the N-terminal 245 SPG-7 amino acids and 40 nonnative residues before terminating. We mated spg-7(ad2249) males with spg-7(tm2312/+) hermaphrodites and examined the F1 progeny for resistance to 1 μM hemiasterlin. This drug concentration converts wild-type worms into rigidly paralyzed, dumpy worms. However, after the mating we observed in the F1 generation healthy and actively moving, nondumpy worms. The spg-7(ad2249) mutant is recessive, and the appearance of hemiasterlin resistance in F1 progeny indicates that ad2249 and tm2312 alleles do not complement each other. We conclude that the ad2249 mutation in the spg-7 gene is responsible for the hemiasterlin resistance phenotype.
Figure 1.
Amino acid sequence alignment of the region mutated in (A) HAR-1 and (B) SPG-7. The amino acid sequence near the mutation site is shown for Saccharomyces cerevisiae, C. elegans, Drosophila melanogaster, Mus musculus, and Homo sapiens. The mutated amino acid in each protein is indicated with an asterisk (*). A complete alignment assembled by the ClustalX program is presented in Supplemental Figures 1 and 2.
Longevity Experiments
To evaluate life span, worms were cultured at 19°C and ∼600 synchronized L1 larvae were grown to young adults and counted (day 1). Adult worms were counted by transferring them individually each day to fresh nematode growth medium (NGM) plates, which separated the initial population from progeny and avoided starvation. Plates were flooded with M9 buffer, and worms were transferred with a P20 plastic pipette tip that had been soaked in M9 buffer supplemented with 1% BSA to prevent worms from adhering to the plastic. This prevented damage to the worms and resulted in complete transfer of the viable adult population. A worm was considered dead if it failed to respond to gentle touch with the pipette tip.
Drug Experiments
For all drug testing, gravid adults were bleached (Emmons et al., 1979), and the remaining intact eggs were allowed to hatch by rocking them gently overnight in M9 buffer in the absence of food (Brenner, 1974). Synchronized L1s were used for all drug tests. Assays were performed in 48-well plates as described previously (Zubovych et al., 2006). N2 wild-type worms were used for all rescue experiments with mitochondrial inhibitors. Briefly, N2 L1 larvae were exposed to increasing amounts of mitochondrial inhibitor in the presence of 1.3 μM the hemiasterlin analog, a concentration that causes N2 larvae to die or infrequently develop into paralyzed and dumpy worms. In experiments testing the effect of NAC to counteract drug resistance, eggs were hatched overnight and cultured thereafter in the presence of 20 mM NAC.
Amplex Red Assay for H2O2 Measurements
The Amplex Red hydrogen peroxide/peroxidase assay kit (Invitrogen, Carlsbad, CA) was used to measure H2O2 production by worms according to a published protocol for C. elegans (Chavez et al., 2007). To measure ROS production in the presence of mitochondrial inhibitors, synchronized L1 larvae were treated with the indicated amount of the inhibitor alone or with addition of 20 mM NAC. DMSO was used as a vehicle control. At the concentrations used, the inhibitors (FCCP and myxothiazol) had no effect on growth, and within 2 d animals developed into L4 larvae. At that stage worms were washed four times with M9 in 15-ml tubes, transferred into microfuge tubes, washed once with 50 mM sodium phosphate buffer, pH 7.4 (reaction buffer), and centrifuged for 1.5 min at 800 × g. Each sample contained 50 μl of tightly packed L4 worms, and 450 μl of 1× reaction buffer was added to each tube. According to the manufacturer's instructions, 500 μl of working solution (100 μM Amplex Red and 0.2 U/ml HRP diluted in reaction buffer) was added to every sample. Absorbance was measured at 560 nm twice, 30 and 90 min from the beginning of the reaction. Tubes were placed on a rotator in the dark, and within 30 min the liquid changed color from transparent to pink, indicating the presence of H2O2. Samples were centrifuged as before, 600 μl of supernatant was transferred to a separate cuvette, and absorbance was measured. After initial measurements, the supernatant was returned; the reaction was allowed to proceed for 1 h, and absorbance was measured again. The amount of H2O2 produced by each worm sample was calculated by comparison to a H2O2 standard curve. Each experiment was reproduced three times with similar results, and data are presented as fold difference of H2O2 produced in 1 h compared with a DMSO control.
Western Blot Analysis
To detect the mitochondrial superoxide dismutase (MnSOD) protein, 50 μl of tightly packed N2, sod-2;sod-3, phb-2;sod-2;sod-3, har-1;sod-2;sod-3, or ad2249;sod-2;sod-3 worms were resuspended in 150 μl of M9 buffer and 50 μl of 4× SDS sample buffer (EMD Chemicals, Gibbstown, NJ) and boiled immediately for 20 min, centrifuged at 14,000 × g for 15 min to remove debris, and then samples were resolved by SDS/PAGE. After electrophoresis, proteins were electrotransferred to nitrocellulose membrane (Trans-Blot, Bio-Rad, Hercules, CA) and immunoblotted with a 1:2000 dilution of rabbit polyclonal anti-MnSOD antibody (Assay Designs, Ann Arbor, MI), followed by a 1:3000 dilution of HRP-conjugated goat anti-rabbit antibody (Bio-Rad). Mouse monoclonal anti-actin antibody (MP Biomedicals) were used as a protein loading control at 1:8000 dilution, followed by a 1:3000 dilution of HRP-conjugated goat anti-mouse antibody (Bio-Rad). For the phospho AMP kinase (p-AMPK) detection, wild-type and mutants lysates were prepared as described above, and then after electrophoresis and electrotransfer the membrane was probed with phospho-Thr172 antibody (Cell Signaling Technology, Danvers, MA) as 1:1000 in 5% BSA, followed by a 1:3000 dilution of HRP-conjugated goat anti-rabbit antibody (Bio-Rad). To visualize the protein bands ECL reagent (Perkin Elmer Life Sciences, Boston, MA) was used according to the manufacturer's instructions.
Imaging Worms Stained with MitoTracker Red
The JJ1271 glo-1(zu391) mutant strain which lacks autofluorescence and birefringent gut granules was used for imaging mitochondria. We crossed the glo-1 animals with our mitochondrial mutant worms and generated the three double mutants: glo-1;phb-2, glo-1;har-1, and glo-1;spg-7. We also made the glo-1;phb-2;har-1 triple mutant. Eggs were collected from gravid adults of each strain and allowed to hatch overnight. The L1 larvae were plated in liquid culture of E. coli HB101 bacteria +2 μg/ml MitoTracker Red CMXRos (Invitrogen) dye in 48-well plates. For hemiasterlin treatment we used 0.6 μM hemiasterlin, the dosage at which wild-type worms survive, but become unhealthy and paralyzed. Mutant worms look normal in 0.6 μM hemiasterlin. After 3 d of incubation, worms were washed and allowed to crawl for 1 h on a bacteria-free agar plate and washed again with M9 and immobilized for 7 min with 0.1% tricaine +0.01% tetramizole (Sigma) in M9 buffer. The immobile worms were applied on freshly made 3% agarose in dH2O on a microscope slide and immediately covered with a coverglass. Worms were viewed with a Deltavision RT deconvolution microscope (Applied Precision, Seattle, WA) provided by the UT Southwestern Live Cell Imaging Core Facility (Dallas, TX). Images were captured and deconvoluted using SoftWoRx software (Applied Precision).
RNA Interference
Bacteria expressing double-stranded RNA (dsRNA) were purchased from Open Biosystems (Huntsville, AL) as individual clones for phb-2 T24H7.1 (cat. no. RCE1182-9359536), har-1 C16C10.11 (cat. no. RCE1182-9359420), and spg-7 Y47G6A.10 (cat. no. RCE1182-9363938). Bacteria were grown overnight at 37°C in liquid LB +100 μg/ml carbenicillin and then induced with 1 mM IPTG for 4 h and seeded onto NGM agar plates supplemented with 100 μg/ml carbenicillin +1 mM IPTG. Wild-type N2 L4 larvae were placed on these plates and allowed to grow for 1 d. Worms were then plated as one animal per well in 48-well plates into corresponding dsRNA-expressing bacteria or HB101 bacteria resuspended in M9 buffer +1 mM IPTG, with or without the 1.5 μM hemiasterlin.
Construction of myc-His C-terminal–tagged Expression Vectors
Vectors used in transient transfections were constructed by PCR amplification of full-length human cDNAs encoding PHB2 and CHCHD2 from their corresponding clones (PHB2, NM_007273.3; CHCHD2, NM_016139.2, OriGene Technologies, Rockville, MD) using the following primer sequences: XbaI-C1qBP. fwd: 5′-GGCTCTAGAATGCTGCCTCTGCTGCGC-3′ and XbaI-AA-C1qBP. rev: 5′-GGCTCTAGAAACTGGCTCTTGACAAAACTCTTG-3′; and XbaI-CHCHD2.fwd: 5′-GGCTCTAGAATGCCGCGTGGAAGCCGAAG-3′ and XbaI-AA-CHCHD2.rev: 5′-GGCTCTAGAATGCCGCGTGGAAGCCGAAG-3′. The resulting PCR products were digested with XbaI and cloned into the pcDNA3.1/myc-His(−) B vector (Invitrogen). All constructs were sequenced from T7 and BGH priming sites to ensure that wild-type sequences were obtained. The QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used to generate the PHB2 E130K and CHCHD2 G65E mutations according to manufacturer's instructions. The following primer sequences were used: qcPHB2-E130K.fwd: 5′-GTCCATTGTCAACAAGGTGCTCAAGAGTG-3′and qcPHB2-E130K.rev: 5′-CACTCTTGAGCACCTTGTTGACAATGGAC-3′; and qcCHCHD2-G65E.fwd: 5′-ACCACTGCAGCTGAAGTGGCTGTGGGC-3′ and qcCHCHD2-G65E.rev: 5′-GCCCACAGCCACTTCAGCTGCAGTGGT-3′. Constructs were sequenced from T7 and BGH priming sites to ensure that the correct mutations were generated. All primers were obtained from Integrated DNA Technologies (Coralville, IA). Transfection-ready DNAs were isolated using the Qiagen EndoFree Plasmid Maxi kit (Chatsworth, CA).
Imaging HeLa Cells Expressing PHB2 and CHCHD2 and Their Mutants
HeLa cells were grown in DMEM (Invitrogen) containing 10% FBS, 1 mM sodium pyruvate, and 10 mM HEPES without antibiotics. Before transfection, cells were plated in four-well dishes fitted with coverslips and allowed to attach overnight. DNA transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Eighteen hours later, the cells were washed twice in prewarmed medium and incubated with 200 nM MitoTracker Red CMXRos (M7512, Molecular Probes/Invitrogen) in normal growth media for 45 min in a 5% CO2 incubator at 37°C. Cells were fixed with 3.7% formaldehyde in PBS for 15 min at 37°C and permeabilized with 0.2% (vol/vol) Triton X-100 in PBS for 8 min at room temperature. The cells were blocked for 30 min in PBS containing 1% (wt/vol) BSA, incubated with mouse monoclonal c-Myc (9E10) primary antibody (M4439, Sigma) diluted 1:250 in PBS containing 1% BSA for 1 h at room temperature, washed three times in NET/Gel, incubated with goat anti-mouse AlexaFluor 488 (Molecular Probes) secondary antibody diluted 1:1000 in PBS containing 1% BSA, washed three times in NET/Gel (50 mM Tris-HCl, pH 8.0, 1 mM EDTA, 150 mM NaCl, 0.25% gelatin, 0.05% NP-40, 0.01% NaN3), and mounted onto slides using Aqua-Poly/Mount (Polysciences, Warrington, PA). Cells were viewed using the Deltavision RT deconvolution microscope, and images were captured and deconvolved using SoftWoRx software (Applied Precision, Seattle, WA). Overlays were generated using ImageJ software (http://rsb.info.nih.gov/ij/).
RESULTS
Identifying Mutations in ad2155dm and ad2249
Drug-resistant mutant worm strains DA2155 (ad2155dm) and DA2249 (ad2249) were two of eight strains isolated in a screen for resistance to a hemiasterlin analog that we described previously (Zubovych et al., 2006). Using standard techniques, we mapped the dominant drug-resistant mutation in ad2155dm to a region on the left arm of chromosome III containing 107 genes (see Materials and Methods). Because the phb-2 (ad2154) mutant identified previously and ad2155dm displayed similar behavior in our drug-resistance assays, we hypothesized both mutations might act in the same drug-resistance pathway. Thus, we analyzed these 107 genes with GeneOrienteer program (Zhong and Sternberg, 2006) to identify any with an expression pattern correlated with PHB-2. C16C10.11 appeared with the highest feature score and was the only mitochondrial protein in the region. We amplified C16C10.11 DNA from the ad2155dm mutant, and sequence analysis revealed a single G-to-A transition at nucleotide 218, resulting in a Gly-to-Glu change (G73E). This amino acid is conserved across species (Figure 1A). To confirm that the mutation in C16C10.11 was responsible for the ad2155dm drug-resistant phenotype, C16C10.11 DNA from ad2155dm was injected into wild-type N2 worms, and the transgenic progeny were found to be drug-resistant (see Materials and Methods). We concluded that the G73E substitution in C16C10.11 is responsible for ad2155dm drug resistance and named the mutant har-1(ad2155dm) for hemiasterlin resistant-1 (referred to as har-1 in this article). HAR-1 contains a mitochondrial localization sequence on the N-terminus and a strongly conserved feature called the CHCH domain: (coiled coil 1)–(helix 1)—(coiled coil 2)–(helix 2), which contains two C-X9-C motifs that might serve as ligands for metal binding. Based on the secondary structure analysis, proteins with the CHCH domain have been divided into three groups (Westerman et al., 2004). HAR-1 belongs to the N-group proteins, which have a CHCH domain located toward the C-terminus and a GXXXGH motif positioned to the N-terminus of the CHCH domain. The human ortholog of HAR-1 is CHCHD2, a protein with an unknown function. short hairpin RNA targeting CHCHD2 in mammalian cells decreases respiration (Baughman et al., 2009).
The recessive mutant ad2249 was isolated in our genetic screen together with phb-2 and har-1. As described in Materials and Methods, we mapped the ad2249 mutation to a region on chromosome I containing 46 genes of which only spg-7, encoding the worm ortholog of the human paraplegin gene, was likely to encode a mitochondrial protein (Atorino et al., 2003; Ishihara et al., 2006). In yeast, prohibitin 2 forms a complex with prohibitin 1 and the ortholog of spg-7 (Steglich et al., 1999). Therefore we amplified DNA encoding spg-7 from the ad2249 mutant and sequenced both strands. We found a G-to-A transition resulting in an E-to-K change at amino acid 414 (Figure 1B). Because spg-7 is a very large gene and the mutation was recessive, to confirm that this mutation was responsible for drug resistance, we used a complementation test with a C. elegans strain, FX 2312(tm2312/+), which contains a small deletion within one copy of spg-7 that results in premature termination of translation (see Materials and Methods for details). Drug-resistant worms were observed in the F1 progeny from a cross between ad2249 males and FX 2312(tm2312/+) hermaphrodites, indicating that the ad2249 and tm2312 alleles do not complement each other and the mutation in spg-7 is responsible for drug resistance in spg-7(ad2249) worms.
Loss of Function of phb-2 and spg-7, But Not har-1, Produces Drug Resistance
To determine if loss of function of phb-2, spg-7, and har-1 conferred drug resistance, we used RNA interference (RNAi). We found that in the absence of drug worms fed on bacteria expressing dsRNAi for har-1 were as healthy as worms fed on HB101. Thus, RNAi for har-1 produced no phenotype. In contrast, feeding worms with bacteria expressing dsRNAi for phb-2 and spg-7 resulted in dramatically reduced brood size. Only 10–25 F1 progeny survived from each phb-2 or spg-7 parent, and many dead eggs were present, indicating that RNAi for phb-2 and spg-7 results in embryonic lethality. To examine the effect on drug resistance, for each of the three genes 27 individual young adult parents were fed bacteria expressing RNAi in the presence of 1.5 μM hemiasterlin. After 3 d of incubation, we observed no progeny surviving from parents fed on HB101 bacteria or har-1 dsRNAi-expressing bacteria in the presence of hemiasterlin. However, under these conditions some progeny (∼5–10% of normal brood size) of worms fed with phb-2 and spg-7 dsRNAi survived and grew into healthy adults. Although phb-2 and spg-7 RNAi resulted in embryonic lethality, the small percentage of animals that did survive, possibly because inhibition of gene expression was incomplete, were hemiasterlin resistant. In conclusion, loss of function of both phb-2 and spg-7 genes results in hemiasterlin resistance. The RNAi experiment was reproduced twice, with 27 parental worms for each gene knockdown.
A Human Ortholog of har-1 Localizes to Mitochondria
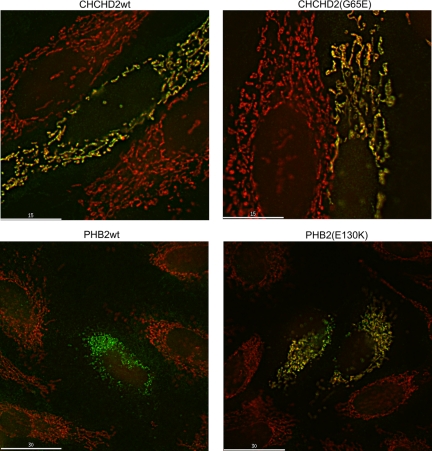
PHB-2 and SPG-7 proteins are known to localize to mitochondria in several species, but the subcellular location of HAR-1 had not been reported. CHCHD2 is the human ortholog of HAR-1. We obtained cDNA for CHCHD2 and introduced into it the mutation found in C. elegans har-1. Wild-type and mutant myc-tagged CHCHD2, with wild-type and mutant PHB-2 for comparison, were expressed transiently in HeLa cells, and the staining pattern with c-myc antibody was compared with MitoTracker Red staining (Figure 2). Both wild-type and mutant CHCHD2 localized exclusively to mitochondria as indicated by the colocalization with MitoTracker Red. For both CHCHD2 and PHB-2, some cells stained only with the c-myc antibody and had no MitoTracker staining (wild-type PHB-2, Figure 2). In these cells the reticular c-myc staining became more punctate, a result that would occur if mitochondria lost membrane potential and fragmented in those cells. We also observed that transient transfection appeared cytotoxic after longer periods, suggesting that overexpressing these proteins was toxic.
Figure 2.
PHB-2 and CHCHD2 (Har-1) mutants localize to mitochondria. Hela cells were transfected with plasmid expression vectors expressing myc-tagged human PHB-2, human PHB-2(E130K), CHCHD2 (the human ortholog of Har-1), and CHCHD2(G65E). After 18 h cells were stained with MitoTracker Red, fixed, and stained with anti-myc antibody (green). PHB2 is known to localize to and CHCHD2 suspected of localizing to mitochondria. Each of the mutants also colocalized with the MitoTracker Red. In some cells such as the one shown for PHB-2 wild type, the myc-staining pattern resembled MitoTracker staining, but no MitoTracker was visible in the cells.
Hemiasterlin Analog Disrupts Mitochondria Networks in Wild-Type C. elegans
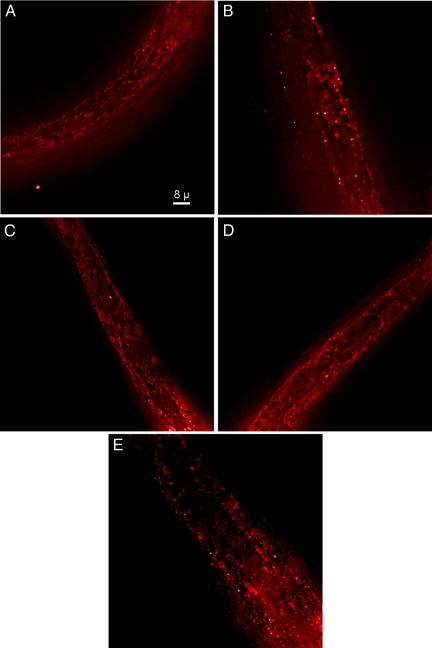
Because mutations that produced drug resistance were in mitochondrial proteins and because microtubules are known to help organize mitochondrial networks, we investigated the effect of the hemiasterlin analog on wild-type and mutant worm mitochondria (Figure 3). Worms were cultured in the presence or absence of a concentration of hemiasterlin that immobilizes wild-type animals but does not kill them. Worms were stained with MitoTracker Red, and images of the same region of the head were taken for each worm strain. Untreated wild-type worms exhibited a reticular pattern of MitoTracker staining typical of mitochondria (Figure 3A); however, this pattern was completely disrupted in worms treated with the drug (Figure 3B). Untreated phb-2, har-1, or spg-7 mutants stained with MitoTracker Red were indistinguishable from wild-type worms (data not shown). Untreated phb-2;har-1 worms, which are more resistant to hemiasterlin than either phb-2 or har-1 single mutants, presented two phenotypes. Half of the worms showed a reticular pattern of MitoTracker staining similar to the wild-type (Figure 3C), and half were very abnormal with mitochondrial staining. (Figure 3E). Because mitochondrial networks were disrupted in at least half the viable drug-resistant phb-2;har-1 worms, it is unlikely that drug resistance is linked to the maintenance of mitochondrial networks.
Figure 3.
The hemiasterlin analog causes mitochondrial networks to fragment in wild-type worms. Images of worms stained with MitoTracker Red were taken with the focal plane in marginal cells in the region of the metacorpus and isthmus in the head of wild-type or phb-2;har-1 double mutant worms in the presence or absence of the hemiasterlin analog. (A) Wild-type worm. (B) Fragmented mitochondria in a wild-type worm treated with 0.6 μM hemiasterlin analog. (C) One of the more normal appearing phb-2;har-1 worms without drug treatment. (D) phb-2;har-1 treated with 0.6 μM drug. (E) An untreated phb-2;har-1 worm with fragmented mitochondria. All images were taken at the same magnification and with the same camera settings.
AMPK Is Activated in phb-2, har-1, and spg-7, Suggesting Impaired ATP Production
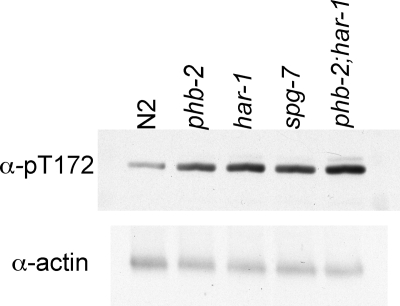
AMPK is activated by an elevated AMP/ATP ratio, and phosphorylation on Thr172 is an important response to cellular metabolic stress (Hardie, 2004). To determine if the mutations in phb-2, har-1, and spg-7 activated AMPK, lysates from each of the worm strains were probed by immunoblotting with an antibody to phosphorylated Thr172 (Figure 4). We observed higher activity of AMPK in the mutant animals compared with wild-type worms, suggesting that the mutants likely produce less ATP than wild-type worms. Lower ATP production suggests that the mitochondria in the mutant worms might work less efficiently.
Figure 4.
AMPK is activated in phb-2, har-1, and spg-7, suggesting impaired ATP production. Wild-type and mutant adult worms were lysed and analyzed by immunoblotting with an antibody that recognizes phosphorylated T172 on AMPK-α. The blot was reprobed with antibody to actin.
Other Mutations in Mitochondrial Proteins Can Alter Drug Sensitivity in C. elegans
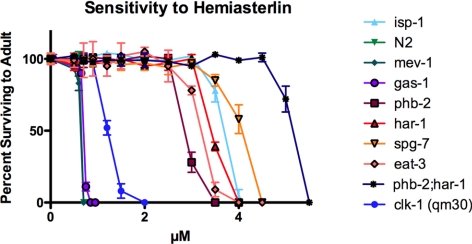
Because all three of the mutations we had identified so far as conferring drug resistance were found in mitochondrial proteins, we asked if other known mitochondrial mutants would show a similar phenotype. We tested four well-studied mutants: gas-1(fc21), mev-1(kn1), isp-1(qm150), and two alleles of clk-1, (e2519) and (qm30), for resistance to the hemiasterlin analog. The first three of these mutants contain missense mutations in complexes I (NADH-ubiquinone oxidoreductase 49-kDa subunit), II (succinate dehydrogenase cytochrome b subunit), and III (Rieske iron sulfur protein), respectively. The two clk-1 mutants possess either a missense mutation (e2519) or deletion (qm30) in the gene encoding the demethoxyubiquinone hydroxylase, an enzyme required for the biosynthesis of ubiquinone. Ubiquinone accepts electrons from both complex I and II in the electron transport chain. All four mutants are defective in oxidative phosphorylation (Feng et al., 2001; Kayser et al., 2001, 2004; Senoo-Matsuda et al., 2001). isp-1 and clk-1 were resistant to hemiasterlin, and gas-1 and mev-1 had wild-type sensitivity (Figure 5). Both isp-1 and clk-1 worms grow slowly and are long lived; in contrast, gas-1 and mev-1 have a slight growth delay and are short lived. Thus, we tested if drug resistance correlates with growth rates and the life span. We found that har-1 and spg-7 mutants grow slightly slower than N2 worms, but phb-2 has wild-type growth, indicating that resistance to the hemiasterlin analog is not correlated with slow growth.
Figure 5.
Not all mutations in mitochondrial proteins confer increased resistance to the hemiasterlin analog. Synchronized L1 larvae of each strain were incubated at the indicated hemiasterlin concentrations for 4–5 d, and healthy mobile adults with eggs were counted. For each concentration, two wells were scored in an experiment. Average values are graphed ± SEM. Note that data for N2 and mev-1 superimpose. Similar results were reproduced in at least three independent experiments.
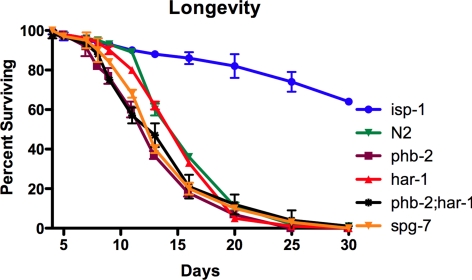
Moreover, our mutants were not long lived; har-1 has a wild-type life span, and phb-2 and spg-7 have slightly shorter life spans than wild-type worms (Figure 6). Because we observed drug resistance in worms with short, normal and prolonged life, we concluded that response to drugs and changes in longevity are independent consequences of mitochondrial alterations.
Figure 6.
The mutants isolated in our screen are not long-lived. phb-2, phb-2;har-1, and spg-7 have slightly shortened life spans, and har-1 is similar to wild type. isp-1worms were used as a long-lived control. Data represent average values of two independent experiments ± SEM.
As our mutants likely have reduced ATP levels (Figure 4), it was possible that drug-resistance was a result of slowed metabolism. To investigate this, we tested two food-deprived mutants, eat-2 (acetylcholine receptor) and eat-3 (mitochondrial dynamin, homologous to Opa1 in humans), for resistance to hemiasterlin. Both eat-2 and eat-3 mutants cannot consume food efficiently because of pharyngeal pumping defects and are starved worms that grow very slowly, but only eat-2 is a long-lived mutant. In agreement with our observations that mitochondrial dysfunction may result in drug resistance that is unrelated to longevity, we found that eat-2 was fully sensitive, and eat-3 was resistant to the hemiasterlin analog. Importantly, phb-2, har-1, spg-7, isp-1, and eat-3 were resistant not only to hemiasterlin but also to dolastatin-10, indicating that resistance for all these mutants was not specific to hemiasterlin (data not shown). The mitochondrial dynamin EAT-3 is orthologous to human OPA-1, and the mutation in eat-3 worms results in fragmented mitochondria with reduced numbers of cristae (Kanazawa et al., 2008). Furthermore, the m-AAA protease paraplegin, in complex with prohibitins, has been reported to process OPA1 and its yeast ortholog, Mgm-1 (Ishihara et al., 2006; Merkwirth and Langer, 2009). This suggests that phb-2, spg-7, and eat-3 might function in the same pathway.
Mitochondrial Inhibitors, But Not Inhibitors of Glycolysis, Protect Wild-Type Worms from the Hemiasterlin Analog
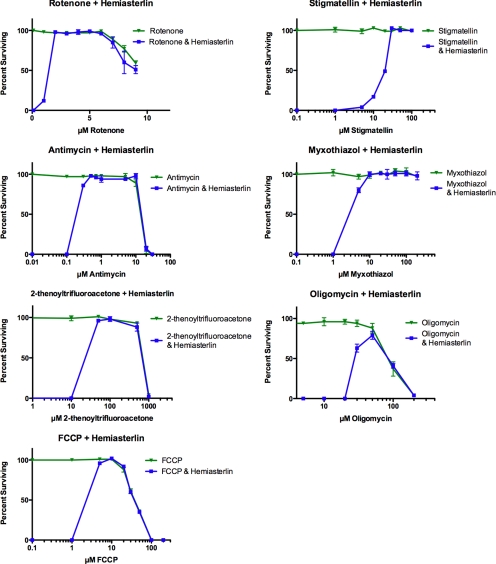
We hypothesized that if certain mitochondrial defects cause drug resistance in our mutants, then partially inhibiting mitochondrial respiration in wild-type N2 worms might confer the same phenotype. If true, growing wild-type worms in the presence of inhibitors that disrupt individual mitochondrial respiration complexes would show the contribution of each complex to hemiasterlin resistance. Inhibitors of respiration complexes I and II represented our special interest, because gas-1 and mev-1 mutants with mutations in those complexes were sensitive to the hemiasterlin analog. However, we found that rotenone, which inhibits complex I, TTFA (complex II inhibitor), antimycin, myxothiazol, and stigmatellin (complex III inhibitors) all rescue wild-type worms from the hemiasterlin analog (Figure 7). FCCP, a potent uncoupler of oxidative phosphorylation, conferred strong resistance to the drug. Oligomycin, an inhibitor of mitochondrial ATP synthase, also protected N2 worms from hemiasterlin, although less efficiently than respiration inhibitors.
Figure 7.
Mitochondrial inhibitors rescue wild-type N2 worms from the hemiasterlin analog. About 400 synchronized N2 L1 larvae were grown in liquid culture in the presence of increasing concentrations of the mitochondrial inhibitor alone or mitochondrial inhibitor plus 1.3 μM hemiasterlin analog. After 3 d of exposure healthy, morphologically normal and mobile L4 and adult worms were counted. Worms exposed to 1.3 μM hemiasterlin analog alone die or develop as paralyzed and dumpy animals. In contrast, when exposed to 1.3 μM hemiasterlin analog in the presence of an inhibitor of mitochondrial respiration, N2 worms grow as healthy, mobile, and morphologically normal animals. TTFA, rotenone, FCCP, oligomycin, and antimycin eventually killed worms at higher concentrations, but were protective at lower dosages. Rotenone, oligomycin, and antimycin also slightly delayed worm growth comparable to untreated animals. Stigmatellin, myxothiazol, and FCCP protected worms from hemiasterlin analog and did not affect animal growth. Experiments with each drug were independently reproduced three times, and average values are graphed ± SEM. For most concentrations tested, the error bars are smaller than the symbol that indicates the data point.
To distinguish between general effects on metabolism or energy levels and changes in mitochondrial respiration as causes of drug resistance, we tested the competitive hexokinase inhibitors, 2-deoxy-glucose and 5-thio-glucose, for their ability to protect worms from the hemiasterlin analog. Hexokinase initiates the first critical step in glycolysis by phosphorylating glucose. The glucose analog 5-thio-glucose cannot be phosphorylated by hexokinase. 2-Deoxyglucose can be phosphorylated but not further metabolized. In contrast to oxidative phosphorylation inhibitors, inhibition of glycolysis with nonmetabolizable glucose analogs could not rescue N2 worms from the hemiasterlin analog (Supplemental Figure S3). Therefore, hemiasterlin resistance appears specific to changes in mitochondrial activity. Importantly, inhibitors of mitochondrial respiration rescue worms not only from the hemiasterlin analog, but from other drugs. For example, cryptophycin-1 at 300 nm concentration killed 100% of N2 larvae, but in the presence of 1.5 μM antimycin more than 70% of animals survived even in 1 μM cryptophycin-1.
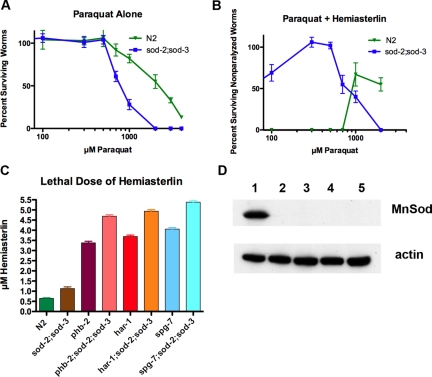
Paraquat Protects Wild-Type Worms from the Hemiasterlin Analog
ROS are byproducts of oxidative phosphorylation, formed by incomplete reduction of molecular oxygen. As a product of normal metabolism, ROS are important cell signaling molecules but can oxidize proteins, damage lipids, and cause DNA mutations. Paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride; PQ2+) is widely used to induce superoxide production. Paraquat enters the mitochondrial matrix and is reduced by complex I to •PQ1+ and then reacts with oxygen to form superoxide •O2−, resulting in mitochondrial oxidative stress (Cocheme and Murphy, 2008). As expected, TM259 sod-2(sj173);sod-3(sj134) worms, which lack MnSOD enzymes, are hypersensitive to paraquat (Figure 8A). Similar to mitochondrial inhibitors, paraquat protected N2 worms from the hemiasterlin analog (Figure 8B), and this protection occurred at much lower paraquat concentrations in TM259 sod-2(sj173);sod-3(sj134) worms, indicating that the protection conferred by paraquat is most likely due to the generation of ROS.
Figure 8.
Inducing ROS with paraquat, or loss of MnSODs, increases resistance to hemiasterlin. (A) The sod-2;sod-3 double mutants are more sensitive to paraquat than N2 worms. (B) Like mitochondrial inhibitors, paraquat protects worms from hemiasterlin toxicity, and sod-2;sod-3 double mutants were protected at a lower paraquat concentration than wild-type worms. Worms were grown in 2 μM hemiasterlin analog, a concentration that kills most larvae, with the few survivors remaining as paralyzed dumpy worms. However, in the presence of paraquat, animals survived and were able to move. (C) Loss of MnSODs increases tolerance to hemiasterlin analog. Worms with the double sod-2;sod-3 mutations are modestly resistant to the toxin, and phb-2;sod-2;sod-3, har-1;sod-2;sod-3, and spg-7;sod-2;sod-3 animals are more resistant than phb-2, har-1, or spg-7, indicating that triple mutants acquired their resistance capacity from both parental strains. Experiments shown in A–C were repeated three times and averages are plotted ± SEM. (D) Immunoblot analysis with anti-MnSOD antibody (top panel) detects a 25-kDa MnSOD band present only in N2 animals (1), but not in sod-2;sod-3 (2), phb-2;sod-2;sod-3 (3), har-1;sod-2;sod-3 (4), or spg-7;sod-2;sod-3 (5). The same blot was reprobed with actin antibody, as a loading control (bottom panel).
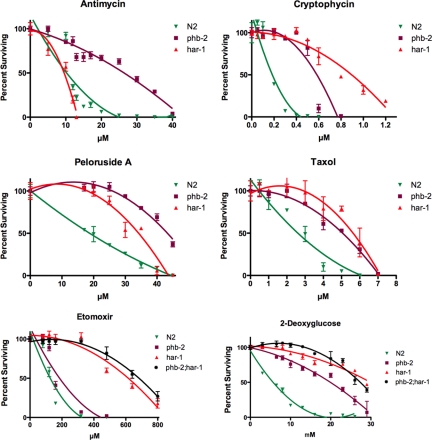
phb-2 and har-1 Show Differential Drug Response
We previously showed that phb-2 is resistant to nine chemically diverse compounds (Zubovych et al., 2006). The compounds tested represented a number of tubulin binders, the DNA replication inhibitor camptothecin and the actin stabilizer phalloidin. Among 11 compounds tested, phb-2 retained wild-type sensitivity to nocodazole (microtubule destabilizer) and phalloidin (stabilizes actin), although nocodazole differs from the other drugs tested in causing abnormal body morphology (dumpy phenotype), but not death, in wild-type worms (Zubovych et al., 2006). The response of the har-1 mutant on those 11 drugs was similar to the response of phb-2 (taxol, cryptophycin, and peloruside data shown; Figure 9). Moreover, the phb-2;har-1 double mutant was more resistant to the hemiasterlin analog than phb-2 or har-1 alone (Figure 5), indicating the synergy of the two mutations.
Figure 9.
phb-2 and har-1 respond differentially to some drugs. har-1 is hypersensitive to antimycin but more tolerant than phb-2 to the inhibitor of fatty acid oxidation, etomoxir sodium. Approximately 400 wild-type (N2) or mutant L1 larvae were treated with indicated drug concentrations for 4 d, and healthy adult worms with eggs were counted. Three wells were scored for each concentration, and average values were graphed ± SEM. Curves were fit to the data using the polynomial option in GraphPad Prism 5.0 (San Diego, CA). Each experiment was reproduced independently three times.
Both phb-2 and har-1 exhibited similar resistance to 2-deoxyglucose (Figure 9). These mutants were also resistant to etomoxir sodium (an inhibitor of fatty acid beta oxidation); however, har-1 was more resistant to this drug than phb-2 (Figure 9). When exposed to paraquat, which induces ROS at complex I, or to antimycin, the complex III inhibitor, our mutants also show differential responses. har-1 is hypersensitive to antimycin, but phb-2 is antimycin-resistant. phb-2 fails to survive in paraquat, and har-1 survives, identical to wild-type (Figure S4). These results suggest that mutations in phb-2 and har-1 affect respiration in different ways.
Mitochondrial Inhibitors Induce ROS Formation in C. elegans
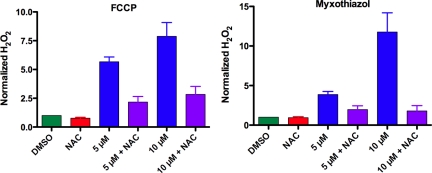
The production of ROS in C. elegans in response to mitochondrial inhibitors has not been previously measured because cell-permeable dyes that work well in mammalian cells do not cross the C. elegans firm exterior cuticle (Balaban et al., 2005). However, recently Chavez et al. (2007) used an Amplex Red assay, which detects H2O2 released into the culture medium, and reported that when fed pathogenic bacteria C. elegans generates more H2O2 than when fed nonpathogenic food. We adapted their method to our experimental system to measure the effect of mitochondrial inhibitors on production of H2O2. Antimycin, TTFA, myxothiazol, stigmatellin, and FCCP all increased H2O2 production in C. elegans (data for myxothiazol and FCCP shown; Figure 10). Although mitochondrial inhibitors induced dose-dependent increases in H2O2 in wild type, in resting conditions (without inhibitor) the rates of H2O2 formation of all mutant strains were similar to the wild type (Figure S5). Thus, if the mutations that conferred drug resistance increased ROS, either the active species is not H2O2 or the difference in H2O2 production between wild-type and mutant worms is less than the Amplex Red assay can detect.
Figure 10.
NAC decreased production of H2O2 by N2 worms treated with myxothiazol or FCCP. Worms were grown for 2 d in the presence of DMSO and 20 mM NAC and in each mitochondrial inhibitor at indicated concentrations with or without 20 mM NAC. H2O2 production was measured as described in Materials and Methods. Results are graphed as the fold difference in H2O2 produced by each sample compared with controls treated with DMSO. Average values of three independent experiments performed on different days were graphed ± SEM.
Worms Lacking MnSOD Are Resistant to the Hemiasterlin Analog
C. elegans has five SOD genes, two of them Fe/Mn-containing mitochondrial enzymes (sod-2 and sod-3) and the other three Cu2+/Zn2+ containing cytosolic and extracellular enzymes (sod-1, sod-4 and sod-5; Doonan et al., 2008). SOD converts superoxide into oxygen and H2O2, an important cellular antioxidant defense. Mitochondrial mutants isp-1, eat-3, and gas-1 overexpress MnSOD (Feng et al., 2001; Kondo et al., 2005; Kanazawa et al., 2008). Sod-2;sod-3 double knockouts were described previously as hypersensitive to oxidative stress (Honda et al., 2008). Not surprisingly, the elimination of MnSODs should raise ROS levels, making sod-2;sod-3 mutants more susceptible to oxidative damage. Western blot analysis with an antibody to MnSOD confirmed the absence of SOD-2 and SOD-3 proteins in the TM259 sod-2(sj173);sod-3(sj134) strain (Figure 8D). We reasoned that if an increase in mitochondrial ROS participates in the resistance of our mutants to drugs, then TM259 sod-2(sj173);sod-3(sj134) worms should be resistant to the hemiasterlin analog compared with wild-type worms. Indeed, sod-2;sod-3 double mutants were resistant to the drug (Figure 8C), although the resistance was modest compared with any mitochondrial mutant alone. We constructed phb-2;sod-2;sod-3, har-1;sod-2;sod-3, and spg-7;sod-2;sod-3 worms and found the triple mutants to be more resistant to the hemiasterlin analog than the mitochondrial mutants alone, confirming that absence of MnSOD makes the drug sensitivity threshold greater even in a mitochondrial mutant background (Figure 8C).
Antioxidant NAC Pharmacologically Sensitizes phb-2, har-1, and spg-7 to the Hemiasterlin Analog
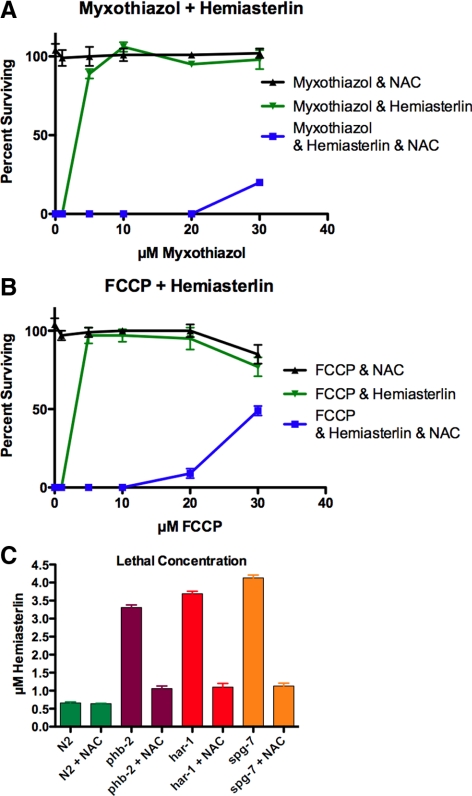
NAC is a thiol compound that acts as a free radical scavenger itself and also provides cells with cysteine, enhancing glutathione synthesis (Atkuri et al., 2007). If generating ROS plays a role in drug resistance, then lowering the ROS levels with antioxidant NAC should enhance sensitivity. Indeed, we observed that NAC reduced the H2O2 production stimulated by mitochondrial inhibitors, as measured by the Amplex Red assay (Figure 10). Pretreating mitochondrial mutants with NAC sensitized them to the hemiasterlin analog, indicating that a ROS-sensitive mechanism contributes to drug resistance in phb-2, har-1, and spg-7 worms (Figure 11C). Along with sensitizing mutants, NAC completely abolished the ability of FCCP and myxothiazol to rescue wild-type worms from hemiasterlin toxicity (Figure 11, A and B). This suggests that either mitochondrial mutations or mitochondrial inhibitors protect worms from hemiasterlin analog by increasing ROS.
Figure 11.
NAC counteracts the effects of mitochondrial inhibitors or mutations on drug resistance. NAC restored sensitivity of mutants to the hemiasterlin analog and reduced the protection provided by mitochondrial inhibitors to N2 worms. (A and B) In the presence of 20 mM NAC, much higher concentrations of myxothiazol (A) or FCCP (B) were required to protect N2 worms from the hemiasterlin analog. (C) NAC sensitized phb-2, har-1, and spg-7 mutants to the hemiasterlin analog, but did not enhance the sensitivity of N2 worms. Concentrations which killed most worms and left rare dumpy, paralyzed survivors were graphed ± SEM; n = 4.
Resistance to Hemiasterlin Analog Does Not Require HIF-1 or AKT Survival Pathways But Does Require Protein Kinase C
It has been proposed previously that respiration injury in mammalian cells leads to inactivation of PTEN and activation of the AKT survival pathway, with active AKT contributing to increased drug resistance (Pelicano et al., 2006). This study used respiration-deficient mammalian cells with damaged mitochondrial DNA (mtDNA) and observed consistent activation of AKT kinase. Wortmannin, a phosphatidylinositol 3′-kinase (PI3K)-Akt pathway inhibitor, enhanced drug sensitivity in these respiration-deficient cells (Pelicano et al., 2006). We could not check the AKT activation level in our mutants because antibody developed against the human AKT phosphorylation site did not recognize the C. elegans phosphorylated protein. However, a mutant with an activating mutation in AKT-1 was isolated as a dauer arrest suppressor of a PI3K knockout strain (Paradis and Ruvkun, 1998), and a PTEN null C. elegans mutant, daf-18, was also available (Rouault et al., 1999). Both akt-1(mg144) and daf-18(ok480) were as sensitive to the hemiasterlin analog as wild-type worms. Additionally, we constructed four double mutant strains: phb-2;akt-1, phb-2;daf-18, har-1;akt-1, and har-1;daf-18 to determine if those double mutants with a potentially activated PI3K-AKT pathway were more drug resistant than mitochondrial mutants alone. However, none of these double mutants showed an increase in drug resistance (data for har-1 shown; Figure 12A). Wortmannin failed to sensitize mutants to the hemiasterlin analog, although the drug was active because it killed larvae at 350 μM and resulted in 50% of larvae converting to dauer at 200 μM, which is evidence that the PI3K pathway was inhibited. Thus it is likely that activation of AKT kinase is not responsible for the drug resistance that we observed in our mitochondrial mutants.
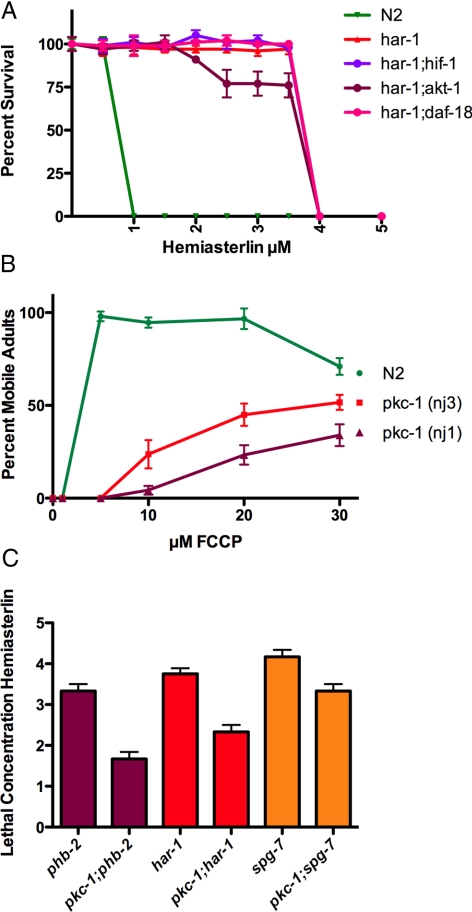
Figure 12.
PKC-1, but not HIF-1 or AKT-1 plays a role in drug resistance caused by mitochondrial dysfunction. (A) Wild-type N2, har-1, and double mutants combining har-1 with hif-1, akt-1, or daf-18 were compared for resistance to hemiasterlin. None of the double mutants had increased resistance compared with the har-1 single mutant. (B) About 250 synchronized N2 or pkc-1 L1 larvae were grown in liquid culture in the concentrations of FCCP shown plus 1.3 μM hemiasterlin analog. After 3 d, healthy, morphologically normal and mobile L4 and adult worms were counted. FCCP protects both the wild-type worms and pkc-1 mutants from hemiasterlin, but pkc-1 mutants were less protected and protection required higher concentrations of FCCP. For each FCCP concentration, three wells were scored in an experiment, and average values ± SEM were graphed. This experiment was reproduced four times. (C) pkc-1(nj3) was crossed into either the phb-2, har-1, or spg-7 mutants, and the sensitivity of single or double mutants to hemiasterlin was compared. For each concentration of hemiasterlin, triplicate wells containing 300 of L1 larvae of each strain were grown for 3 d, and healthy L4 and adult worms were counted. The average concentrations of three independent experiments at which <10% of worms are healthy adults are graphed ± SEM. By t test, p = 0.005 comparing phb-2 and pkc-1;phb-2, p = 0.02 for har-1 and pkc-1;har-1; and p = 0.02 for spg-7 and pkc-1;spg-7.
In mammalian cells, ROS produced by mitochondria may stabilize HIF-1α and turn on cell survival mechanisms depending on that transcription factor (Galanis et al., 2008). We tested the possibility that mitochondrial dysfunction in worms may activate this pathway, providing survival in the presence of the hemiasterlin analog. In C. elegans, hif-1 is a functional homolog of mammalian HIF-1α, and hif-1(ia04) mutants cannot adapt to low oxygen (Jiang et al., 2001). We mated har-1 with hif-1(ia04) to see if double mutant har-1;hif-1 would be more sensitive to hemiasterlin because of the deficiency in HIF-1. However, the double mutant was as resistant to the hemiasterlin analog as was har-1 alone, indicating that har-1 drug resistance does not require HIF-1 (Figure 12A).
ROS have been reported to serve as signals for ischemic preconditioning, in which brief periods of hypoxia are found to protect against ischemia/reperfusion injury to cardiomyocytes (Oldenburg et al., 2003). ROS scavengers prevent preconditioning (Hanley and Daut, 2005; Brennan et al., 2006) and, although the exact mechanism is unknown, the activation of protein kinase C (PKC) ε by ROS was suggested to reduce the opening of the mitochondria permeability transition pore (MPTP) and to activate the mitochondrial K+ ATP channels, protecting tissue from death (Baines et al., 2003; Oldenburg et al., 2003; Otani, 2004; Hanley and Daut, 2005; Brennan et al., 2006; Inagaki et al., 2006; Barnett et al., 2007). C. elegans also exhibit preconditioning, and mitoK+ATP channel activity was detected in purified worm mitochondria (Wojtovich et al., 2008). Four pkc genes, pkc-1, pkc-2, pkc-3, and tpa-1, have been identified in C. elegans, and if ROS act through PKC to confer protection from hemiasterlin, protection should be weaker in pkc mutants. We tested the capability of FCCP to prevent hemiasterlin toxicity in pkc-1(nj1), pkc-1(nj3), pkc-2(ok328), tpa-1(k501), and tpa-1(k530) mutant worm strains. The pkc-2 and tpa-1 mutants were protected from hemiasterlin similarly to the wild-type worms (not shown), but interestingly, we found that in pkc-1 mutants the protection afforded by FCCP was greatly reduced (Figure 12B). The pkc-1 gene is orthologous to the mammalian PKCε involved in preconditioning. The nj3 mutation truncates the PKC-1 protein, and kinase activity is expected to be completely lost in nj3 mutants (Okochi et al., 2005). When pkc-1(nj3) was crossed with phb-2, har-1, and spg-7, the resulting phb-2;pkc-1, har-1;pkc-1, and spg-7;pkc-1 double mutants were found to be more sensitive to hemiasterlin than phb-2, har-1, and spg-7 single mutants (Figure 12C). These data suggest that the mitochondrial mutations or pharmacological inhibitors that confer resistance to antimitotic toxins require PKC-1.
DISCUSSION
In a genetic screen for C. elegans mutants resistant to the antimitotic toxin, hemiasterlin, we obtained eight mutants (Zubovych et al., 2006). We have now identified the genetic lesions in three of these, and all reside in mitochondrial proteins. Two of these, PHB-2 and SPG-7, the worm ortholog of a mitochondrial AAA-metalloprotease, paraplegin, are known to interact physically (Merkwirth and Langer, 2009). In addition, we show that other mitochondrial mutations can produce drug resistance in worms. eat-3, encoding a mitochondrial dynamin; isp-1, with a mutation in the Rieske iron sulfur protein; and clk-1, with a mutation in the ubiquinone biosynthesis protein, were all resistant to the hemiasterlin analog. However, not all mutations in mitochondria result in drug resistance. gas-1 and mev-1, with point mutations in complex I and II proteins, respectively, were as sensitive to the hemiasterlin analog as wild-type worms. It is possible that only certain, fairly specific mutations can provide worms with drug resistance. Alternatively, it is the degree of impairment that may be important. Genome-wide microarray profiling of gas-1, mev-1, and isp-1 have shown that mitochondrial respiratory chain mutants differentially up-regulate basic cellular metabolic pathways, implying numerous possibilities for compensatory adaptation (Falk et al., 2008). For example, drug-sensitive gas-1 mutants have compensated decreased complex I capacity by increasing complex II–dependent oxidative phosphorylation and drug-sensitive mev-1 mutants balance complex II deficiency by increasing activity of complex I, but isp-1, which is drug-resistant, has deficits in both complex I and II (Falk et al., 2008). Likewise, each mitochondrial mutant strain is unique, with distinct metabolic rates, life span and drug sensitivity. In agreement with this, phb-2, har-1, and spg-7 also show different sensitivities to some drugs.
Consistent with the interpretation that impairment of respiration is the key to drug resistance in our mutant worms, we found that exogenous inhibitors of mitochondrial respiration rescue wild-type worms from the hemiasterlin analog. In fact, the rescue was striking, with nontoxic concentrations of the respiration inhibitors completely protecting wild-type worms from a normally lethal dose of the hemiasterlin analog. Addition of the antioxidant NAC fully blocked the protection by mitochondrial inhibitors and by the mutations in phb-2, har-1, and spg-7, indicating that hemiasterlin resistance in mutants, or resistance caused by respiration inhibitors in the wild type, requires a mechanism sensitive to ROS. Furthermore, sod-2;sod-3, mutant worms lacking both MnSODs, which should result in increased mitochondrial ROS, tolerated the hemiasterlin analog better than wild-type worms. When crossed with our phb-2, har-1, or spg-7 mutants, the resulting triple mutants lacking MnSODs were more resistant to the hemiasterlin analog than were single phb-2, har-1, or spg-7 mutants.
Although treating wild-type worms with mitochondrial inhibitors reproducibly stimulated H2O2 production, in resting conditions without inhibitors the Amplex Red assay could not detect differences between the mutants and the wild-type worms. This could simply mean that the level of ROS production required for a drug-resistant phenotype is below the sensitivity range of the assay, which requires that H2O2 be secreted from the animal. Because Amplex Red measures only H2O2, it is also possible that some other ROS species might be necessary for drug resistance. If so, sensing of ROS is likely to be confined to the mitochondria, because the more reactive oxygen species have short half-lives and do not diffuse far. Our data suggest that an increase in superoxide is enough to promote resistance, as paraquat produces superoxide and the absence of MnSOD enzymes would enhance superoxide rather than H2O2 levels. Alternatively, drug resistance might be due to a mechanism that is sensitive to ROS, but the mutants activate that mechanism through a different process independent of ROS.
Interestingly, in mammalian cell culture mtDNA mutations resulted in respiration-deficient ρ0 cells with altered drug responses (Singh et al., 1999; Pelicano et al., 2006). Despite mitochondrial metabolic defects, in one study ρ0 cells demonstrated resistance to common anticancer agents (Pelicano et al., 2006). In another study ρ0 were resistant to cell death induced by adriamycin and photodynamic therapy, but were equally sensitive to alkylating agents and gamma-radiation compared with their respiration-competent parental cells (Singh et al., 1999). Obviously, in mammalian cells mitochondrial defects also influence drug sensitivity in complex ways.
Although some mitochondrial mutants are multidrug-resistant, it is unlikely that resistance is due to overexpression of P-glycoprotein or other drug efflux pumps. In a microarray experiment comparing har-1 to N2 wild-type worms, none of the genes encoding potential efflux pumps was overexpressed in the mutant (Wu and Roth, data not shown). Typically P-glycoprotein results in a very high resistance, such as a thousand fold. In our mutants we observed small resistance, only 2.5–5-fold, depending on the compound. Also, taxol and vinblastine are excellent substrates for P-glycoprotein and hemiasterlin is not, yet resistance to those drugs was equivalent. The fact that our mitochondrial mutants were also resistant to 2-deoxyglucose makes the drug efflux possibility extremely unlikely, because sugars are not transported by P-glycoprotein and any unknown pump exporting 2-deoxyglucose would likely export glucose.
It is possible that either chronic (mutants) or acute (N2 worms grown in mitochondrial inhibitors) mitochondrial dysfunction may serve to “precondition” worms to hemiasterlin analog by activating survival pathways. If so, our results showing that mutants with an activated AKT pathway are not protected from hemiasterlin analog and that loss of HIF-1 does not block resistance in our mitochondrial mutants suggests that these pathways are not necessary for hemiasterlin resistance. In contrast, a pkc-1 mutation suppressed the drug resistance of our mitochondrial mutants and showed reduced protection by FCCP from hemiasterlin, consistent with functioning downstream from the defects or inhibitors. PKC-1 is the kinase most closely related by sequence to the mammalian PKC isoform reported to respond to ROS by preconditioning cells to hypoxic shock. In our “acute” assay, N2 L1 larvae are exposed to hemiasterlin and the mitochondrial inhibitor simultaneously, so the protective effect of the mitochondrial inhibitor must act faster than the toxic effects of the drug. One possibility is that the protective effect occurs at the mitochondria itself, making the mitochondria less able to participate in events leading to death.
Supplementary Material
ACKNOWLEDGMENTS
We thank Shuji Honda (Tokyo Metropolitan Institute of Gerontology) for TM173, TM134, and TM259 C. elegans strains; Leon Avery (University of Texas Southwestern Medical Center) for DA1496 and DA1116 strains; Tokyo Women's Medical University School of Medicine and Shohei Mitani for TM2312, TM537, and TM1752. We also thank Thomas Doundoulakis and Patrick Harran for synthesizing the hemiasterlin analog and Gavin McAlister for help with mapping the mutation in ad2249. Some nematode strains used in this work were provided by Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources (NCRR). This work was supported by grant CA095571 and the Diane and Hal Brierley Distinguished Chair in Biomedical Research and was conducted in a facility constructed with support from Research Facilities Improvement Program Grant C06-RR15437 from NCRR.
Abbreviations used:
- ROS
reactive oxygen species.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-08-0673) on January 20, 2010.
REFERENCES
- Atkuri K. R., Mantovani J. J., Herzenberg L. A. N-acetylcysteine—a safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007;7:355–359. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atorino L., Silvestri L., Koppen M., Cassina L., Ballabio A., Marconi R., Langer T., Casari G. Loss of m-AAA protease in mitochondria causes complex I deficiency and increased sensitivity to oxidative stress in hereditary spastic paraplegia. J. Cell Biol. 2003;163:777–787. doi: 10.1083/jcb.200304112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayral-Kaloustian S., Zask A. Taltobulin—oncolytic drug tubulin polymerization inhibitor antimitotic drug. Drugs Future. 2005;30:254–260. [Google Scholar]
- Baines C. P., Song C. X., Zheng Y. T., Wang G. W., Zhang J., Wang O. L., Guo Y., Bolli R., Cardwell E. M., Ping P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ. Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban R. S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Barnett M. E., Madgwick D. K., Takemoto D. J. Protein kinase C as a stress sensor. Cell Signal. 2007;19:1820–1829. doi: 10.1016/j.cellsig.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman J. M., Nilsson R., Gohil V. M., Arlow D. H., Gauhar Z., Mootha V. K. A computational screen for regulators of oxidative phosphorylation implicates SLIRP in mitochondrial RNA homeostasis. PLoS Genet. 2009;5:e1000590. doi: 10.1371/journal.pgen.1000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brennan J. P., Southworth R., Medina R. A., Davidson S. M., Duchen M. R., Shattock M. J. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc. Res. 2006;72:313–321. doi: 10.1016/j.cardiores.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez V., Mohri-Shiomi A., Maadani A., Vega L. A., Garsin D. A. Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics. 2007;176:1567–1577. doi: 10.1534/genetics.107.072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocheme H. M., Murphy M. P. Complex I is the major site of mitochondrial superoxide production by paraquat. J. Biol. Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- Doonan R., McElwee J. J., Matthijssens F., Walker G. A., Houthoofd K., Back P., Matscheski A., Vanfleteren J. R., Gems D. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008;22:3236–3241. doi: 10.1101/gad.504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W., Klass M. R., Hirsh D. Analysis of the constancy of DNA sequences during development and evolution of the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1979;76:1333–1337. doi: 10.1073/pnas.76.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk M. J., Zhang Z., Rosenjack J. R., Nissim I., Daikhin E., Sedensky M. M., Yudkoff M., Morgan P. G. Metabolic pathway profiling of mitochondrial respiratory chain mutants in C. elegans. Mol. Genet. Metab. 2008;93:388–397. doi: 10.1016/j.ymgme.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Bussiere F., Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev. Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- Galanis A., Pappa A., Giannakakis A., Lanitis E., Dangaj D., Sandaltzopoulos R. Reactive oxygen species and HIF-1 signalling in cancer. Cancer Lett. 2008;266:12–20. doi: 10.1016/j.canlet.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Hadaschik B. A., Adomat H., Fazli L., Fradet Y., Andersen R. J., Gleave M. E., So A. I. Intravesical chemotherapy of high-grade bladder cancer with HTI-286, a synthetic analogue of the marine sponge product hemiasterlin. Clin. Cancer Res. 2008a;14:1510–1518. doi: 10.1158/1078-0432.CCR-07-4475. [DOI] [PubMed] [Google Scholar]
- Hadaschik B. A., Ettinger S., Sowery R. D., Zoubeidi A., Andersen R. J., Roberge M., Gleave M. E. Targeting prostate cancer with HTI-286, a synthetic analog of the marine sponge product hemiasterlin. Int. J. Cancer. 2008b;122:2368–2376. doi: 10.1002/ijc.23406. [DOI] [PubMed] [Google Scholar]
- Hanley P. J., Daut J. K(ATP) channels and preconditioning: a re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms. J. Mol. Cell Cardiol. 2005;39:17–50. doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Hardie D. G. The AMP-activated protein kinase pathway—new players upstream and downstream. J. Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Honda Y., Tanaka M., Honda S. Modulation of longevity and diapause by redox regulation mechanisms under the insulin-like signaling control in Caenorhabditis elegans. Exp. Gerontol. 2008;43:520–529. doi: 10.1016/j.exger.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Inagaki K., Churchill E., Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc. Res. 2006;70:222–230. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Ishihara N., Fujita Y., Oka T., Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. Q., Guo R., Powell-Coffman J. A. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc. Natl. Acad. Sci. USA. 2001;98:7916–7921. doi: 10.1073/pnas.141234698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T., Zappaterra M. D., Hasegawa A., Wright A. P., Newman-Smith E. D., Buttle K. F., McDonald K., Mannella C. A., van der Bliek A. M. The C. elegans Opa1 homologue EAT-3 is essential for resistance to free radicals. PLoS Genet. 2008;4:e1000022. doi: 10.1371/journal.pgen.1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser E. B., Morgan P. G., Hoppel C. L., Sedensky M. M. Mitochondrial expression and function of GAS-1 in Caenorhabditis elegans. J. Biol. Chem. 2001;276:20551–20558. doi: 10.1074/jbc.M011066200. [DOI] [PubMed] [Google Scholar]
- Kayser E. B., Sedensky M. M., Morgan P. G., Hoppel C. L. Mitochondrial oxidative phosphorylation is defective in the long-lived mutant clk-1. J. Biol. Chem. 2004;279:54479–54486. doi: 10.1074/jbc.M403066200. [DOI] [PubMed] [Google Scholar]
- Kondo M., Senoo-Matsuda N., Yanase S., Ishii T., Hartman P. S., Ishii N. Effect of oxidative stress on translocation of DAF-16 in oxygen-sensitive mutants, mev-1 and gas-1 of Caenorhabditis elegans. Mech. Ageing Dev. 2005;126:637–641. doi: 10.1016/j.mad.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Loganzo F., et al. HTI-286, a synthetic analogue of the tripeptide hemiasterlin, is a potent antimicrotubule agent that circumvents P-glycoprotein-mediated resistance in vitro and in vivo. Cancer Res. 2003;63:1838–1845. [PubMed] [Google Scholar]
- Merkwirth C., Langer T. Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim. Biophys. Acta. 2009;1793:27–32. doi: 10.1016/j.bbamcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Okochi Y., Kimura K. D., Ohta A., Mori I. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J. 2005;24:2127–2137. doi: 10.1038/sj.emboj.7600697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg O., Cohen M. V., Downey J. M. Mitochondrial K(ATP) channels in preconditioning. J. Mol. Cell Cardiol. 2003;35:569–575. doi: 10.1016/s0022-2828(03)00115-9. [DOI] [PubMed] [Google Scholar]
- Otani H. Reactive oxygen species as mediators of signal transduction in ischemic preconditioning. Antiox. Redox. Signal. 2004;6:449–469. doi: 10.1089/152308604322899521. [DOI] [PubMed] [Google Scholar]
- Paradis S., Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicano H., et al. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J. Cell Biol. 2006;175:913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poruchynsky M. S., Kim J. H., Nogales E., Annable T., Loganzo F., Greenberger L. M., Sackett D. L., Fojo T. Tumor cells resistant to a microtubule-depolymerizing hemiasterlin analogue, HTI-286, have mutations in alpha- or beta-tubulin and increased microtubule stability. Biochemistry. 2004;43:13944–13954. doi: 10.1021/bi049300+. [DOI] [PubMed] [Google Scholar]
- Rouault J. P., Kuwabara P. E., Sinilnikova O. M., Duret L., Thierry-Mieg D., Billaud M. Regulation of dauer larva development in Caenorhabditis elegans by daf-18, a homologue of the tumour suppressor PTEN. Curr. Biol. 1999;9:329–332. doi: 10.1016/s0960-9822(99)80143-2. [DOI] [PubMed] [Google Scholar]
- Senoo-Matsuda N., Yasuda K., Tsuda M., Ohkubo T., Yoshimura S., Nakazawa H., Hartman P. S., Ishii N. A defect in the cytochrome b large subunit in complex II causes both superoxide anion overproduction and abnormal energy metabolism in Caenorhabditis elegans. J. Biol. Chem. 2001;276:41553–41558. doi: 10.1074/jbc.M104718200. [DOI] [PubMed] [Google Scholar]
- Singh K. K., Russell J., Sigala B., Zhang Y., Williams J., Keshav K. F. Mitochondrial DNA determines the cellular response to cancer therapeutic agents. Oncogene. 1999;18:6641–6646. doi: 10.1038/sj.onc.1203056. [DOI] [PubMed] [Google Scholar]
- Steglich G., Neupert W., Langer T. Prohibitins regulate membrane protein degradation by the m-AAA protease in mitochondria. Mol. Cell. Biol. 1999;19:3435–3442. doi: 10.1128/mcb.19.5.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman B. A., Poutsma A., Steegers E. A., Oudejans C. B. C2360, a nuclear protein expressed in human proliferative cytotrophoblasts, is a representative member of a novel protein family with a conserved coiled coil-helix-coiled coil-helix domain. Genomics. 2004;83:1094–1104. doi: 10.1016/j.ygeno.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Wicks S. R., Yeh R. T., Gish W. R., Waterston R. H., Plasterk R. H. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 2001;28:160–164. doi: 10.1038/88878. [DOI] [PubMed] [Google Scholar]
- Wojtovich A. P., Burwell L. S., Sherman T. A., Nehrke K. W., Brookes P. S. The C. elegans mitochondrial K+(ATP) channel: a potential target for preconditioning. Biochem. Biophys. Res. Commun. 2008;376:625–628. doi: 10.1016/j.bbrc.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W., Sternberg P. W. Genome-wide prediction of C. elegans genetic interactions. Science. 2006;311:1481–1484. doi: 10.1126/science.1123287. [DOI] [PubMed] [Google Scholar]
- Zubovych I., Doundoulakis T., Harran P. G., Roth M. G. A missense mutation in Caenorhabditis elegans prohibitin 2 confers an atypical multidrug resistance. Proc. Natl. Acad. Sci. USA. 2006;103:15523–15528. doi: 10.1073/pnas.0607338103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.