Abstract
A retroviral insertion into the c-myb gene, which resulted in a 3' truncation, was found in an in vitro-derived myeloid cell line. The retroviral insertion occurred at precisely the same nucleotide at which another murine leukemia virus insertion occurred in an in vivo-induced myeloid leukemia. These findings suggest that comparable events may be required for the derivation of myeloid cell lines in vitro and for induction of myeloid leukemia in vivo.
Full text
PDF

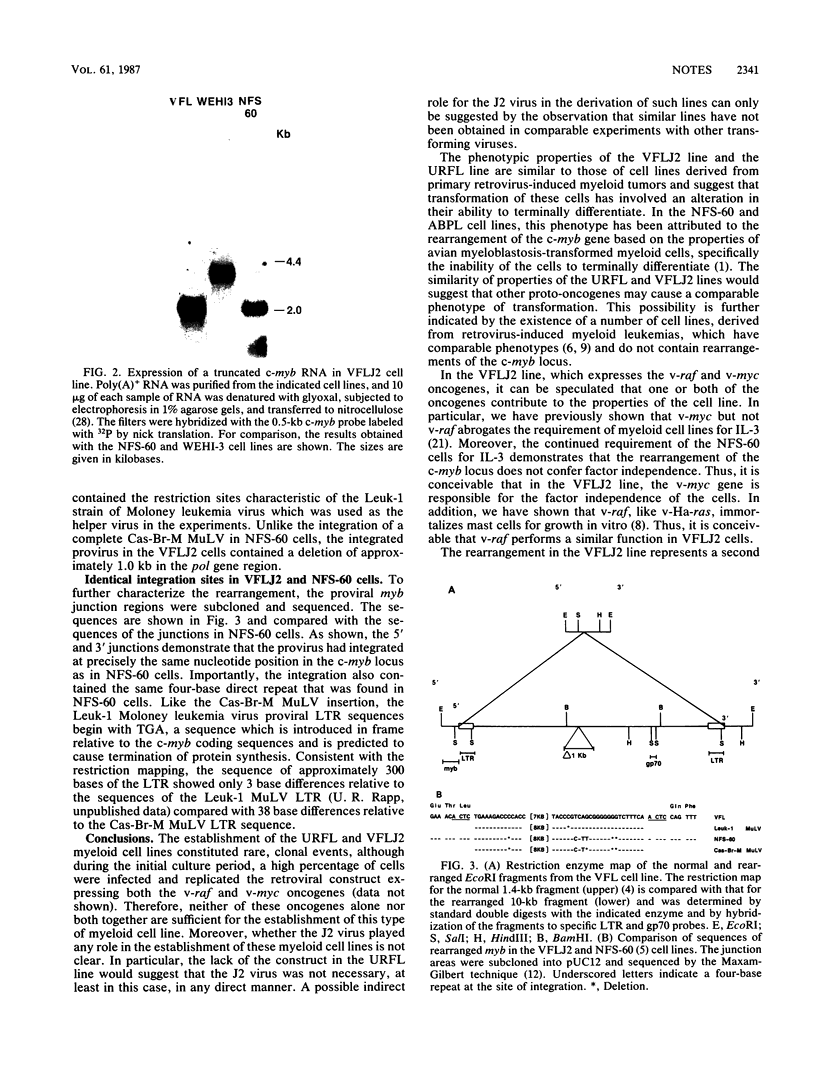


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., Hayman M. J., Graf T. Myeloblasts transformed by the avian acute leukemia virus E26 are hormone-dependent for growth and for the expression of a putative myb-containing protein, p135 E26. EMBO J. 1982;1(9):1069–1073. doi: 10.1002/j.1460-2075.1982.tb01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Scott D., Teich N. M. Isolation and characterisation of a bipotential haematopoietic cell line. Nature. 1979 Feb 8;277(5696):471–474. doi: 10.1038/277471a0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Moscovici C. Genetic structure of avian myeloblastosis virus, released from transformed myeloblasts as a defective virus particle. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5120–5124. doi: 10.1073/pnas.77.9.5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D. L., Glick B., Trusko S., Freeman N. Enhanced c-Ki-ras expression associated with Friend virus integration in a bone marrow-derived mouse cell line. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1651–1655. doi: 10.1073/pnas.83.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Fanshier L., Bishop J. M., Moscovici C., Moscovici M. G. The genome and the intracellular RNAs of avian myeloblastosis virus. Cell. 1981 Jan;23(1):279–290. doi: 10.1016/0092-8674(81)90292-0. [DOI] [PubMed] [Google Scholar]
- Holmes K. L., Palaszynski E., Fredrickson T. N., Morse H. C., 3rd, Ihle J. N. Correlation of cell-surface phenotype with the establishment of interleukin 3-dependent cell lines from wild-mouse murine leukemia virus-induced neoplasms. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6687–6691. doi: 10.1073/pnas.82.19.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Greenberger J. S., Henderson L., Yetter R. A., Morse H. C., 3rd Phenotypic characteristics of cell lines requiring interleukin 3 for growth. J Immunol. 1982 Oct;129(4):1377–1383. [PubMed] [Google Scholar]
- Ihle J. N., Morse H. C., 3rd, Keller J., Holmes K. L. Interleukin 3 dependent retrovirus induced lymphomas: loss of the ability to terminally differentiate in response to differentiation factors. Curr Top Microbiol Immunol. 1984;113:86–91. doi: 10.1007/978-3-642-69860-6_16. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Weinstein Y. Immunological regulation of hematopoietic/lymphoid stem cell differentiation by interleukin 3. Adv Immunol. 1986;39:1–50. doi: 10.1016/s0065-2776(08)60347-8. [DOI] [PubMed] [Google Scholar]
- Keller J. R., Weinstein Y., Hursey M., Ihle J. N. Interleukins 2 and 3 regulate the in vitro proliferation of two distinguishable populations of 20-alpha-hydroxysteroid dehydrogenase-positive cells. J Immunol. 1985 Sep;135(3):1864–1871. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- May W. S., Ihle J. N. Affinity isolation of the interleukin-3 surface receptor. Biochem Biophys Res Commun. 1986 Mar 28;135(3):870–879. doi: 10.1016/0006-291x(86)91009-0. [DOI] [PubMed] [Google Scholar]
- Moscovici C. Leukemic transformation with avian myeloblastosis virus: present status. Curr Top Microbiol Immunol. 1975;71:79–101. doi: 10.1007/978-3-642-66193-8_2. [DOI] [PubMed] [Google Scholar]
- Moscovici M. G., Jurdic P., Samarut J., Gazzolo L., Mura C. V., Moscovici C. Characterization of the hemopoietic target cells for the avian leukemia virus E26. Virology. 1983 Aug;129(1):65–78. doi: 10.1016/0042-6822(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Mushinski J. F., Potter M., Bauer S. R., Reddy E. P. DNA rearrangement and altered RNA expression of the c-myb oncogene in mouse plasmacytoid lymphosarcomas. Science. 1983 May 20;220(4599):795–798. doi: 10.1126/science.6687762. [DOI] [PubMed] [Google Scholar]
- Palaszynski E. W., Ihle J. N. Evidence for specific receptors for interleukin 3 on lymphokine-dependent cell lines established from long-term bone marrow cultures. J Immunol. 1984 Apr;132(4):1872–1878. [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Radke K., Beug H., Kornfeld S., Graf T. Transformation of both erythroid and myeloid cells by E26, an avian leukemia virus that contains the myb gene. Cell. 1982 Dec;31(3 Pt 2):643–653. doi: 10.1016/0092-8674(82)90320-8. [DOI] [PubMed] [Google Scholar]
- Ralph P., Ho M. K., Litcofsky P. B., Springer T. A. Expression and induction in vitro of macrophage differentiation antigens on murine cell lines. J Immunol. 1983 Jan;130(1):108–114. [PubMed] [Google Scholar]
- Rapp U. R., Cleveland J. L., Brightman K., Scott A., Ihle J. N. Abrogation of IL-3 and IL-2 dependence by recombinant murine retroviruses expressing v-myc oncogenes. Nature. 1985 Oct 3;317(6036):434–438. doi: 10.1038/317434a0. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Cleveland J. L., Fredrickson T. N., Holmes K. L., Morse H. C., 3rd, Jansen H. W., Patschinsky T., Bister K. Rapid induction of hemopoietic neoplasms in newborn mice by a raf(mil)/myc recombinant murine retrovirus. J Virol. 1985 Jul;55(1):23–33. doi: 10.1128/jvi.55.1.23-33.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Keller J., Schultz A. M., Holmes K. L., Medicus R., Ihle J. N. Infection of immune mast cells by Harvey sarcoma virus: immortalization without loss of requirement for interleukin-3. Mol Cell Biol. 1985 Sep;5(9):2257–2264. doi: 10.1128/mcb.5.9.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Weeks M. O., Shih T. Y., Ruscetti S. K., Dexter T. M. Markedly elevated levels of an endogenous sarc protein in a hemopoietic precursor cell line. Mol Cell Biol. 1981 Jan;1(1):66–74. doi: 10.1128/mcb.1.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten G., Cuypers H. T., Boelens W., Robanus-Maandag E., Verbeek J., Domen J., van Beveren C., Berns A. The primary structure of the putative oncogene pim-1 shows extensive homology with protein kinases. Cell. 1986 Aug 15;46(4):603–611. doi: 10.1016/0092-8674(86)90886-x. [DOI] [PubMed] [Google Scholar]
- Shen-Ong G. L., Morse H. C., 3rd, Potter M., Mushinski J. F. Two modes of c-myb activation in virus-induced mouse myeloid tumors. Mol Cell Biol. 1986 Feb;6(2):380–392. doi: 10.1128/mcb.6.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Potter M., Mushinski J. F., Lavu S., Reddy E. P. Activation of the c-myb locus by viral insertional mutagenesis in plasmacytoid lymphosarcomas. Science. 1984 Nov 30;226(4678):1077–1080. doi: 10.1126/science.6093260. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Strommer J. N., Hillyard R. L., Komaromy M. C., Baluda M. A. Cellular sequences are present in the presumptive avian myeloblastosis virus genome. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5177–5181. doi: 10.1073/pnas.77.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Ihle J. N., Lavu S., Reddy E. P. Truncation of the c-myb gene by a retroviral integration in an interleukin 3-dependent myeloid leukemia cell line. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5010–5014. doi: 10.1073/pnas.83.14.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]




