SUMMARY
Many pathogenic bacteria utilize the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway for the biosynthesis of isopentenyl diphosphate and dimethylallyl diphosphate, two major building blocks of isoprenoid compounds. The fifth enzyme in the MEP pathway, 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (ME-CPP) synthase (IspF), catalyzes the conversion of 4-diphosphocytidyl-2-C-methyl-D-erythritol 2-phosphate (CDP-ME2P) to ME-CPP with a corresponding release of cytidine 5-monophosphate (CMP). Since there is no ortholog of IspF in human cells IspF is of interest as a potential drug target. However, study of IspF has been hindered by a lack of enantiopure CDP-ME2P. Herein, we report the first synthesis of enantiomerically pure CDP-ME2P from commercially available D-arabinose. Cloned, expressed, and purified M. tuberculosis IspF was able to utilize the synthetic CDP-ME2P as a substrate, a result confirmed by mass spectrometry. A convenient, sensitive, in vitro IspF assay was developed by coupling the CMP released during production of ME-CPP to mononucleotide kinase, which can be used for high throughput screening.
INTRODUCTION
Mycobacterium tuberculosis is the etiological agent of tuberculosis (TB) and, as of 2007, roughly 1/3 of the world’s population was infected with tubercle bacilli (World Health Organization, WHO Report 2007). This, and the fact that both multi-drug resistant TB (MDR-TB) and extensively drug resistant TB (XDR-TB) are rapidly spreading(Zhao et al., 2009) has renewed the urgency for the development of new treatments for this disease. Here we report continued efforts to characterize and identify inhibitors of isoprenoid biosynthesis in pathogenic bacteria.
Isoprenoid compounds, such as polyprenols, polyprenyl phosphates, carotenoids, sterols, monoterpenes, sesquiterpenes, lipoquinones, etc., are found in all living organisms(Edwards and Ericsson, 1999), and a number of isoprenoid compounds are found in mycobacteria that are essential for bacterial survival(Brennan and Crick, 2007). To date, two different biosynthetic pathways are known to lead to the synthesis of isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP), universal precursors of isoprenoids(Rohmer, 1999; Brennan and Crick, 2007). The mevalonate pathway(Buhaescu and Izzedine, 2007), first identified in mammals, and the non-mevalonate or methylerythritol phosphate (MEP) pathway found in plants (MEP pathway in plants is constricted to the chloroplasts), apicomplexan protozoa, and many eubacteria, including human pathogens such as M. tuberculosis, Escherichia coli, Streptococcus pneumoniae, Pseudomonas aeruginosa, Campylobacter jejuni, Salmonella enterica Serovar Typhi, Mycobacterium leprae, Staphylococcus aureus and Plasmodium falciparum (Rohmer, 1999; Skorupinska-Tudek et al., 2008; Rohmer, 2007; Eoh et al., 2008).
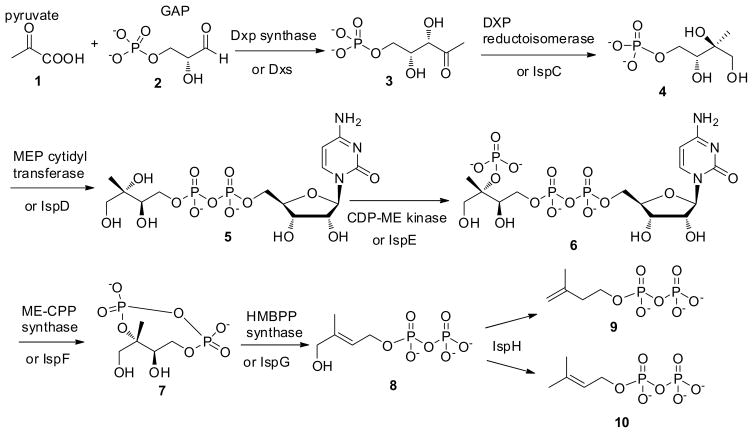
In the MEP biosynthetic pathway (Fig. 1), 1-deoxy-D-xylulose 5-phosphate 3 (Dxp) is made by condensing pyruvate 1 and glyceraldehyde 3-phosphate 2 catalysed by Dxp synthase (Dxs). Subsequently 3 undergoes intramolecular rearrangement and reduction by Dxp reductoisomerase (IspC) enzyme to synthesize 4. 4 is coupled with cytidine triphosphate (CTP) using MEP cytidyltransferase (IspD) to synthesize 5 as the major product. 5 is subsequently phosphorylated by CDP-ME kinase (IspE) at the 2-hydroxyl position to form 4-diphosphocytidyl-2-C-methyl-D-erythritol-2-phosphate 6 (CDP-ME2P) which is cyclized by ME-CPP synthase (IspF)(Buetow et al., 2007; Campbell and Brown, 2002; Fellermeier et al., 2001; Rohdich et al., 2001; Steinbacher et al., 2002; Ramsden et al., 2009; Richard et al., 2002; Lehmann et al., 2002) to form 2-C-methyl-D-erythritol 2, 4-cyclodiphosphate 7 (ME-CPP). The cyclic diphosphate undergoes reductive elimination to form 1-hydroxy-2-methyl-2-E-butenyl 4-diphosphate 8 (HMBPP), a reaction catalysed by IspG and IspH (LytB) is utilized subsequently to generate IPP 9 and DMAPP 10.
Figure 1.
MEP biosynthetic pathway.
Since the MEP pathway is not found in human cells it is generally considered to be a source of good targets for the development of antimicrobials(Illarionova et al., 2006), antimalarials(Giessmann et al., 2008), and herbicidal agents(Ershov, 2007), a hypothesis being explored by many researchers. However, a major difficulty faced by researchers in this area is the lack of availability of pure substrates. Access to MEP pathway intermediates and their analogues is essential to ongoing biochemical investigations and development of new antibiotics targeting the respective enzymes. Recently we reported the synthesis of CDP-ME(Narayanasamy et al., 2008), ME-CPP(Narayanasamy and Crick, 2008) and kinetic studies of mycobacterial Dxs(Bailey et al., 2002), IspC(Dhiman et al., 2003), IspD(Eoh et al., 2007), and IspE(Narayanasamy et al., 2008). In order to extend our research to include IspF, we were in need of compound 6; however the previously reported enzymatic synthesis of 6 is tedious, expensive and leads to low yields(Illarionova et al., 2006; Herz et al., 2000; Luttgen et al., 2000). Herein, we report the first chemical synthesis of enantiomerically pure 6 and its use as a substrate to initiate studies of IspF.
RESULTS AND DISCUSSION
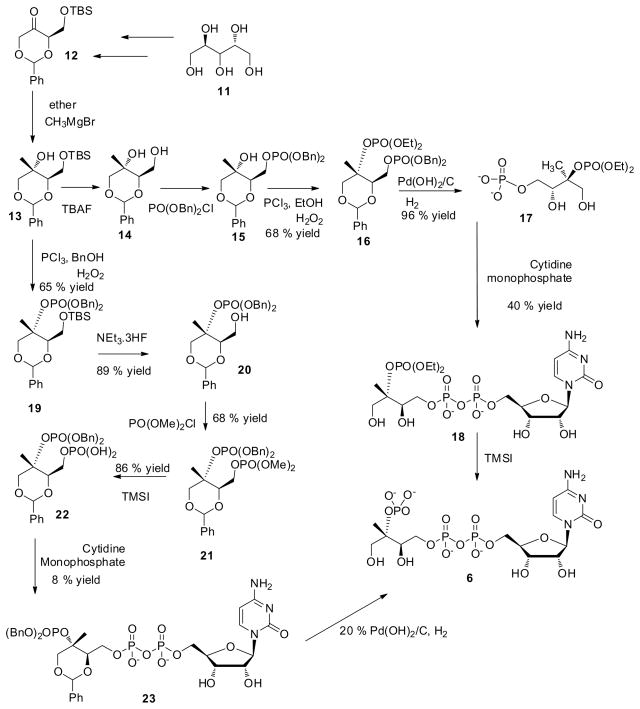
Interestingly, although reported for the synthesis of 4 and 5, there is no procedure for synthesis of enantiomerically pure 16, 21 or 6 from commercially available D-arabinose. To initiate the reactions leading to 6, we extended the studies on the synthesis of methylerythritol described in the literature(Urbansky et al., 2004). Thus, the tertiary hydroxyl group of 13 is phosphorylated by PCl3 followed by benzylation using benzyl alcohol to yield dibenzyl phosphite and subsequent oxidation to dibenzyl phosphate, 19 using a hydrogen peroxide solution. The TBS group in 19 is deprotected by triethylamine hydrofluoride in good yield and the primary hydroxyl group of 20 is phosphorylated using dimethyl phosphochloridate in a n-BuLi solution. The methyl in the dimethyl phosphate 21 is deprotected using TMSI as previously reported(Zygmunt et al., 1978) before coupling to give 22 in stable form. We assumed that the benzyl protected methylerythritol 22 could be efficiently coupled with cytidine monophosphate (CMP) to give the final product 23; unfortunately, this reaction only afforded an 8 % yield of 23, presumably due to high stearic hindrance. Hydrogenolysis of 23 using 20 % Pd(OH)2/C yielded pure 6, but the overall yield for this route to 6 is unacceptably low. Therefore an alternate route was developed. Initially, an attempt was made to use dibenzyl phosphochloridate in pyridine to selectively protect the primary hydroxyl of 14, but that reaction was unsuccessful, so 14 was activated with n-BuLi at low temperature and then reacted with freshly prepared dibenzyl phosphochloridate to yield 15. The free tertiary hydroxyl group was phosphorylated using PCl3 at low temperature, followed with ethanol to give diethyl phosphite, which was subsequently oxidized to diethyl phosphate, 16, in good yield (Fig. 2). Subsequently the benzyl deprotection was carried out by hydrogenolysis in one step using 20 % Pd(OH)2/C in the presence of hydrogen to yield 17.
Figure 2.
Enantiomeric synthesis of 6.
Cytidine monophosphate was titrated with triethylamine leading to the formation of the corresponding triethylammonium cytidine 5′-monophosphate. The phosphoester moiety was activated by triflouroacetyl anhydride followed by conversion to phosphoramide by treatment with methylimidazole and then coupling with the tributylammonium salt of 17; this reaction was completed in 4 h and quenched with 1 M aq. NH4HCO3. The crude material was purified by passing through a benzyl-DEAE cellulose column using a gradient of 10–500 mM aq. NH4HCO3 and fractions containing the product, 18, were collected and lyophilized giving 40 % yield. Examination of 18 was done by 1H-NMR, 13C-NMR, 31P-NMR and MS (see supplemental information, SI). The phosphate moiety of 18 was deprotected using TMSI as reported in literature and purification by column chromatography on Whatman fibrous cellulose using 2-propanol/methanol/water (4:2:4) solution(Illarionova et al., 2006) gave an 84 % yield of 6. Since the deprotected 6 is not stable it was subjected to analysis by MS and used immediately for the kinetic study of IspF. The nature and structure of product 6 was confirmed by MS, 1H-NMR, 31P-NMR (see SI) and utilization by pure recombinant enzyme.
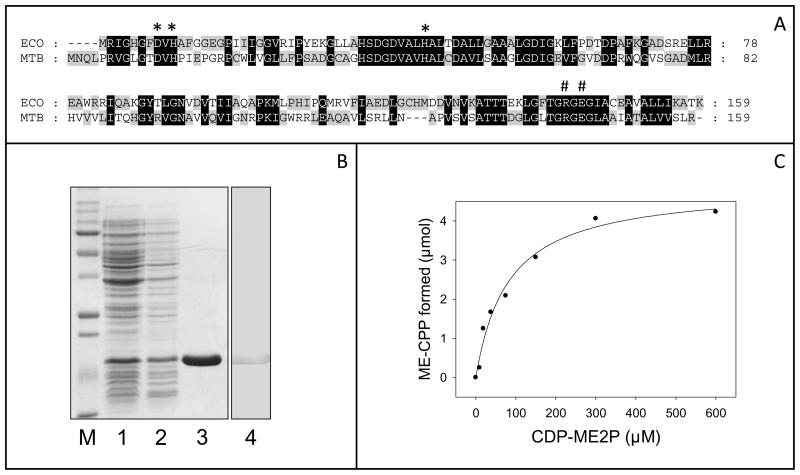
An amino acid sequence alignment of Rv3581c with E. coli IspF(Herz et al., 2000) showed 40% overall similarity and suggested that Rv3581c is the M. tuberculosis ortholog of IspF (Fig. 3A). Rv3581c is 480 bp in length, encoding a polypeptide of 159 amino acids with a molecular weight of 17.7 kDa, which is predicted to be cytosolic(Sgraja et al., 2008). M. tuberculosis IspF expression in the heterologous host, E. coli, was confirmed by Western blot analysis using an anti–His antibody (Fig. 3B) and purified by immobilized metal affinity chromatography. Interestingly, all purification steps require the inclusion of 1 mM Zn2+ in order to maintain activity (see SI).
Figure 3.
Partial alignment, purification and characterization of IspF. Panel A: Partial alignment of putative M. tuberculosis (MTB) IspF and E. coli (ECO) IspF. Identities are indicated in black boxes and similarities in gray. Conserved amino acids reported to be involved in substrate specificity (#) and the Zn2+ binding (*) are indicated. Panel B: Expression and purification of His tagged M. tuberculosis IspF. SDS-PAGE and Western blot analysis of protein fractions from E. coli transfomed with pET28a(+)::Rv3581c. Lane 1, cell lysate prior to IPTG induction. Lane 2, cell lysate after IPTG treatment. Lane 3, purified His-tagged IspF visualized by Coomassie Brilliant Blue 250R. Lane 4, Western blot analysis of purified IspF using an anti-His antibody. Panel C: The effect of CDP-ME2P concentration on M. tuberculosis IspF activity. Reaction mixtures are described in the Experimental Procedures. ADP generated from this reaction was detected using ADP Quest HS Kinase kit and a Synergy™ HT Multi-Detection Microplate Reader with an excitation wavelength of 520 nm and emission wavelength of 590 nm.
Initially, a radioisotope based assay was utilized to confirm the activity of the recombinant protein. When synthesized CDP-ME and [γ-32P]ATP were incubated in presence of IspE, TLC analysis of the reaction mixture clearly showed the formation of a product with the reported chromatographic properties of CDP-ME2P (Fig. 4, lane 2). Addition of M. tuberculosis IspF to the mixture resulted in the formation of a new product that had chromatographic properties on TLC and mass spectral data similar to those previously reported for ME-CPP generated by an assay in which IspE and IspF were coupled (Testa et al., 2006) (Fig. 4, lane 3). In addition, crude reaction mixtures containing enantiomerically pure CDP-ME2P and M. tuberculosis IspF were analyzed by mass spectrometry in the absence of CDP-ME and IspE. In these assays, a decrease in the CDP-ME2P molecular ion and the appearance of a molecular ion corresponding to ME-CPP (Narayanasamy and Crick, 2008; Urbansky et al., 2004) was observed (data not shown). Although this protocol confirmed the formation of product, it is cumbersome for the analysis of multiple samples.
Figure 4.
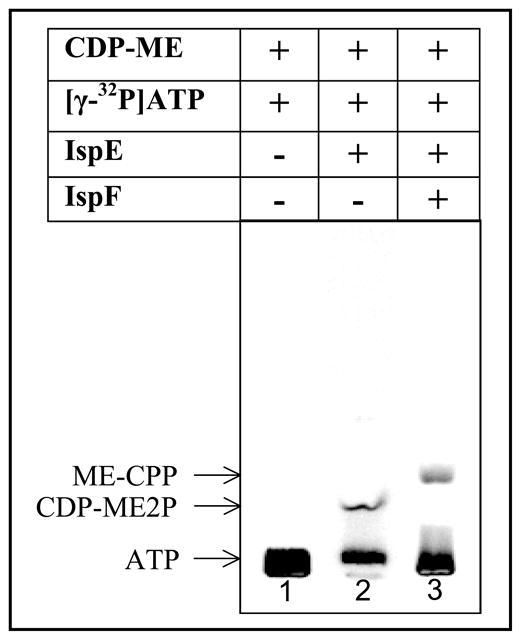
Determination of activity of recombinant M. tuberculosis IspF. Three different reaction mixtures containing the indicated compounds were analyzed. The arrows indicate the migration of authentic compounds.
In general, IspF catalyzes an unusual cyclization reaction producing ME-CPP and releasing CMP (Herz et al., 2000); in previous reports analysis of IspF assay products were based on TLC or HPLC (Herz et al., 2000; Rohdich et al., 2001), which is not suitable for high throughput screening. To develop a more convenient assay, which is applicable to high throughput screening and to determine kinetic properties of M. tuberculosis IspF, CMP generation was coupled to nucleotide monophosphate kinase (NMK) and the ADP Quest HS Kinase Assay Kit (GE Healthcare, UK) to monitor ADP formation (Figure S1 in SI). The activity of pure recombinant IspF was linear with increasing protein concentration up to 281.5 pmol and reaction time up to 30 minutes. The coupled assay showed classic saturation kinetics with increasing concentration of CDP-ME2P (Fig. 3C). From this data the KmCDP-ME2P and Vmax values of 81.1 μM and 81.6 nmolS−1 respectively were calculated. The constants, kcat and kcat/Km for M. tuberculosis IspF were calculated to be 7.3×10−3 S−1 and 5.4×10−4 μM−1min−1 respectively.
SIGNIFICANCE
We successfully synthesized enantiopure 6 by two routes. Although this compound was unstable under usual storage conditions, the derivative 18 can be stored at −20° C and easily deprotected to form 6. In the synthetic scheme of 6 reported here, radiolabeling also can be easily introduced during the methylation, reduction and/or coupling steps if required. To determine the kinetic properties of M. tuberculosis IspF using 6, the enzyme was identified bioinformatically, overexpressed, purified in the presence of 1 mM Zn2+ and utilized to develop a spectrophotometry based in vitro assay. This assay was used to generate the first kinetic characterization of an IspF enzyme and, importantly, it can be used for high throughput screening to identify IspF inhibitors. We also observed that the kcat/Km value 5.4×10−4 μM−1min−1 of M. tuberculosis IspF was much lower than that reported for M. tuberculosis IspD and IspE(Eoh et al., 2007) suggesting that the catalytic efficiency of IspF is lower than the preceding two steps on the biosynthetic pathway.
EXPERIMENTAL PROCEDURES
M. tuberculosis H37Rv strain genomic DNA was provided by Dr. John T. Belisle of Colorado State University (NIH/NIAID Contract N01-AI-75320, “Tuberculosis Research Material and Vaccine Testing”). All PCR reagents and cloning materials were purchased from Qiagen (Valencia, CA). His-select nickel affinity resin, ATP and reagents were obtained from Sigma-Aldrich (St. Louis, MO). Nucleotide monophosphate kinase (NMK) and phosphorylase inhibitor were purchased from Roche. The ADP Quest HS Kinase Assay Kit was purchased from GE healthcare Bio–Sciences Corp. UK. All other reagents and solvents were of at least analytical grade. [γ-32P]ATP (6000 Ci/mmol) was purchased from Amersham Biosciences (Pittsburgh, PA).
Procedure for synthesis of 16
To alcohol 15 (0.275 mmol) in dcm (6 mL), pyridine (0.91 mmol) was added at −13 °C, followed by addition of phosphorus trichloride (0.28 mmol). After 2 h of stirring at 13 °C, completion of reaction was checked by TLC and then dry ethanol (0.605 mmol) was added at 13 °C and stirred at 16 °C for 2 h. After the completion of the reaction it was cooled to 0 °C and hydrogen peroxide (0.99 mmol) solution was added. The reaction mixture was stirred at room temperature for 4 h and then diluted with dichloromethane. Subsequently the organic phase was washed with 10 % sodium metabisulfite solution, dilute HCl and brine solution. The organic phase was dried with MgSO4, solvents were evaporated at reduced pressure and the crude mixture was purified by flash column chromatography using 80:20 (EtOAc: hexane) as eluent and gave a 68 % yield of 16.
Procedure for synthesis of 18
To CMP (0.05 mmol) in acetonitrile (0.5 mL), N, N, dimethyl aniline (0.21 mmol) and triethylamine (0.05 mmol) were added consequently at 0 °C. Trifluoroacetic anhydride (0.26 mmol) in acetonitrile was added slowly to the above mixture and stirred for 15 minutes. Excess TFA and anhydride was removed under reduced pressure. Then 1-methyl imidazole (0.15 mmol) and triethylamine (0.26 mmol) in acetonitrile was slowly added and the mixture was stirred for 30 more minutes. The activated CMP obtained was added to 0.04 mmol of 16 and activated 4 A° molecular sieves in acetonitrile at 0 °C and stirred for 4h. The mixture was then extracted with chloroform and the aqueous layer was lyophilized. The dried compound was dissolved in 100 mM aq. ammonium bicarbonate and purified through Bio-Gel® P-2 gel fine column using 100 mM aq. ammonium bicarbonate followed by further purification on a benzyl DEAE cellulose anion exchange column, eluted by a gradient of 0–0.5 M aq. ammonium bicarbonate leading to a 40 % yield of 18 as the major product.
In vitro radiochemical IspF assay
IspF assays were performed in reaction mixtures containing 50 mM Tris-Cl (pH 7.0), 100 μM [γ-32P]ATP (10 dpm/nmol), 2 mM DTT, 100 μM CDP-ME, 5 mM MgCl2, 97.2 pmol M. tuberculosis IspE, and 112.6 pmol M. tuberculosis IspF in a 50 μl final reaction volume. Reactions were initiated by addition of purified M. tuberculosis IspF, incubated at 37 °C for 30 min, and terminated by the addition of 10 mM of EDTA (pH 8.0). TLC analysis was performed by transferring 10 μl of the reaction mixture to a TLC plate (Polygam Sil N-HR, Macherey and Nagel) and developing with n-propanol/ethyl acetate/H2O (6:1:3, v/v/v). Distribution of the radioactivity on the TLC plates was analyzed using a Molecular Dynamics Typhoon 8600 Phosphoimager.
MS based in vitro IspF assay
The IspF activity was also assessed by monitoring ME-CPP formation by MS. Reaction mixture contained 50 mM MOPS (pH 8.0), 5 mM of MgCl2, 100 μM CDP-ME2P, 1 mM phosphatase inhibitor, in a final volume of 50 μl. In all cases, the reactions were started by the addition of 112.6 pmol purified IspF, incubated at 37 °C for 30 minutes and terminated by the addition of 10 mM EDTA. The reaction mixture was completely dried, dissolved in methanol/H2O (1:1) and analysed on an Agilent 6210 mass spectrometer.
Spectrophotometry based in vitro IspF assay
The IspF activity was assessed by monitoring CMP release, which was coupled to NMK to generate ADP (Fig. 2 in SI). The ADP Quest HS Kinase Assay Kit was used to detect ADP formation. Reactions were performed in 96-well black microplates with clear bottoms (Costar, Bethesda, MD); each reaction mixture contained 50 mM MOPS (pH 8.0), 5 mM of MgCl2, 100 μM CDP-ME2P, 1 mM phosphatase inhibitor, 0.05 U of NMK, and 200 μM ATP in a final volume of 50 μl. In all cases, the reactions were started by the addition of 112.6 pmol of purified IspF, incubated at 37 °C for 30 minutes and terminated by the addition of 10 mM EDTA. Subsequently, 25 μl of reagent A and 50 μl of reagent B of the ADP Quest HS Kinase Assay Kit were added and incubated at room temperature for 15 min. Fluorescence was measured by Synergy™ HT Multi-Detection Microplate Reader (BioTek Instruments, Inc. Winooski, VT) with an excitation wavelength of 530 nm and emission wavelength of 590 nm.
Kinetic characterization of Rv3581c
The effect of CDP-ME2P concentrations on reaction rates was determined by adding various concentrations of the compound to the reactions. Results presented are the average of duplicate experiments. The Km and Vmax values of substrates for the enzyme were calculated by non-linear regression analysis using SigmaPlot V.8.02A.
Supplementary Material
Acknowledgments
This research was supported by NIH/NIAID grant AI65357-040010.
Footnotes
This material is free via the internet
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- Bailey AM, Mahapatra S, Brennan PJ, Crick DC. Identification, cloning, purification, and enzymatic characterization of Mycobacterium tuberculosis 1-deoxy-D-xylulose 5-phosphate synthase. Glycobiology. 2002;12:813–820. doi: 10.1093/glycob/cwf100. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Crick DC. The cell-wall core of Mycobacterium tuberculosis in the context of drug discovery. Current Topics in Medicinal Chemistry. 2007;7:475–488. doi: 10.2174/156802607780059763. [DOI] [PubMed] [Google Scholar]
- Buetow L, Brown AC, Parish T, Hunter WN. The structure of mycobacteria 2C-methyl-D-erythritol-2,4-cyclodiphosphate synthase, an essential enzyme, provides a platform for drug discovery. Bmc Structural Biology . 2007;7 doi: 10.1186/1472-6807-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhaescu I, Izzedine H. Mevalonate pathway: A review of clinical and therapeutical implications. Clinical Biochemistry. 2007;40:575–584. doi: 10.1016/j.clinbiochem.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Campbell TL, Brown ED. Characterization of the depletion of 2-C-methyl-D-erythritol-2,4-cyclodiphosphate synthase in Escherichia coli and Bacillus subtilis. Journal of Bacteriology. 2002;184:5609–5618. doi: 10.1128/JB.184.20.5609-5618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman RK, Schaeffer ML, Scherman H, Bailey AM, Brennan PJ, Crick DC. Characterization of IspC, 1-deoxy-D-xylulose-5-phosphate reductoisomerase from Mycobacterium tuberculosis. Glycobiology. 2003;13:877. [Google Scholar]
- Edwards PA, Ericsson J. Sterols and isoprenoids: Signaling molecules derived from the cholesterol biosynthetic pathway. Annual Review of Biochemistry. 1999;68:157–185. doi: 10.1146/annurev.biochem.68.1.157. [DOI] [PubMed] [Google Scholar]
- Eoh H, Brennan PJ, Crick DC. The Mycobacterium tuberculosis MEP (2C-methyl-D-erythritol 4-phosphate) pathway as a new drug target. Tuberculosis (Edinb) 2008 doi: 10.1016/j.tube.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eoh H, Brown AC, Buetow L, Hunter WN, Parish T, Kaur D, Brennan PJ, Crick DC. Characterization of the Mycobacterium tuberculosis 4-diphosphocytidyl-2-C-methyl-D-erythritol synthase: Potential for drug development. Journal of Bacteriology. 2007;189:8922–8927. doi: 10.1128/JB.00925-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershov YV. 2-C-methylerythritol phosphate pathway of isoprenoid biosynthesis as a target in identifying new antibiotics, herbicides, and immunomodulators: A review. Applied Biochemistry and Microbiology. 2007;43:115–138. [PubMed] [Google Scholar]
- Fellermeier M, Raschke M, Sagner S, Wungsintaweekul J, Schuhr CA, Hecht S, Kis K, Radykewicz T, Adam P, Rohdich F, Eisenreich W, Bacher A, Arigoni D, Zenk MH. Studies on the nonmevalonate pathway of terpene biosynthesis - The role of 2C-methyl-D-erythritol 2,4-cyclodiphosphate in plants. European Journal of Biochemistry. 2001;268:6302–6310. doi: 10.1046/j.0014-2956.2001.02585.x. [DOI] [PubMed] [Google Scholar]
- Giessmann D, Heidler P, Haemers T, Van Calenbergh S, Reichenberg A, Jomaa H, Weidemeyerd C, Sanderbrand S, Wiesner J, Link A. Towards new antimalarial drugs: Synthesis of non-hydrolyzable phosphate mimics as feed for a predictive QSAR study on 1-deoxy-D-xylulose-5-phosphate reductoisomerase inhibitors. Chemistry & Biodiversity. 2008;5:643–656. doi: 10.1002/cbdv.200890060. [DOI] [PubMed] [Google Scholar]
- Herz S, Wungsintaweekul J, Schuhr CA, Hecht S, Luttgen H, Sagner S, Fellermeier M, Eisenreich W, Zenk MH, Bacher A, Rohdich F. Biosynthesis of terpenoids: YgbB protein converts 4-diphosphocytidyl-2C-methyl-D-erythritol 2-phosphate to 2C-methyl-D-erythritol 2,4-cyclodiphosphate. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2486–2490. doi: 10.1073/pnas.040554697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illarionova V, Kaiser J, Ostrozhenkova E, Bacher A, Fischer M, Eisenreich W, Rohdich F. Nonmevalonate terpene biosynthesis enzymes as antiinfective drug targets: Substrate synthesis and high-throughput screening methods. Journal of Organic Chemistry. 2006;71:8824–8834. doi: 10.1021/jo061466o. [DOI] [PubMed] [Google Scholar]
- Lehmann C, Lim K, Toedt J, Krajewski W, Howard A, Eisenstein E, Herzberg O. Structure of 2C-methyl-D-erythrol-2,4-cyclodiphosphate synthase from haemophilus influenzae: Activation by conformational transition. Proteins-Structure Function and Bioinformatics. 2002;49:135–138. doi: 10.1002/prot.10182. [DOI] [PubMed] [Google Scholar]
- Luttgen H, Rohdich F, Herz S, Wungsintaweekul J, Hecht S, Schuhr CA, Fellermeier M, Sagner S, Zenk MH, Bacher A, Eisenreich W. Biosynthesis of terpenoids: YchB protein of Escherichia coli phosphorylates the 2-hydroxy group of 4-diphosphocytidyl-2C-methyl-D-erythritol. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1062–1067. doi: 10.1073/pnas.97.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanasamy P, Crick DC. Enantiomeric Synthesis of 2-C-Methyl-D-Erythritol 2,4-Cyclodiphosphate. Heterocycles. 2008;76:243–247. doi: 10.3987/com-08-s(n)72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanasamy P, Eoh H, Crick DC. Chemoenzymatic synthesis of 4-diphosphocytidyl-2-C-methyl-D-erythritol: a substrate for IspE. Tetrahedron Lett. 2008;49:4461–4463. doi: 10.1016/j.tetlet.2008.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden NL, Buetow L, Dawson A, Kemp LA, Ulaganathan V, Brenk R, Klebe G, Hunter WN. A Structure-Based Approach to Ligand Discovery for 2C-Methyl-d-erythritol-2,4-cyclodiphosphate Synthase: A Target for Antimicrobial Therapy (dagger) J Med Chem. 2009 doi: 10.1021/jm801475n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard SB, Ferrer JL, Bowman ME, Lillo AM, Tetzlaff CN, Cane DE, Noel JP. Structure and mechanism of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase - An enzyme in the mevalonate-independent isoprenoid biosynthetic pathway. Journal of Biological Chemistry. 2002;277:8667–8672. doi: 10.1074/jbc.C100739200. [DOI] [PubMed] [Google Scholar]
- Rohdich F, Eisenreich W, Wungsintaweekul J, Hecht S, Schuhr CA, Bacher A. Biosynthesis of terpenoids - 2C-methyl-D-erythritol 2,4-cyclodiphosphate synthase (IspF) from Plasmodium falciparum. European Journal of Biochemistry. 2001;268:3190–3197. doi: 10.1046/j.1432-1327.2001.02204.x. [DOI] [PubMed] [Google Scholar]
- Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Natural Product Reports. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- Rohmer M. Diversity in isoprene unit biosynthesis: The methylerythritol phosphate pathway in bacteria and plastids. Pure and Applied Chemistry. 2007;79:739–751. [Google Scholar]
- Sgraja T, Alphey MS, Ghilagaber S, Marquez R, Robertson MN, Hemmings JL, Lauw S, Rohdich F, Bacher A, Eisenreich W, Illarionova V, Hunter WN. Characterization of Aquifex aeolicus 4-diphosphocytidyl-2C-methyl-D-erythritol kinase - ligand recognition in a template for antimicrobial drug discovery. Febs Journal. 2008;275:2779–2794. doi: 10.1111/j.1742-4658.2008.06418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupinska-Tudek K, Poznanski J, Wojcik J, Bienkowski T, Szostkiewicz I, Zelman-Femiak M, Bajda A, Chojnacki T, Olszowska O, Grunler J, Meyer O, Rohmer M, Danikiewicz W, Swiezewska E. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of dolichols in plants. Journal of Biological Chemistry. 2008;283:21024–21035. doi: 10.1074/jbc.M706069200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbacher S, Kaiser J, Wungsintaweekul J, Hecht S, Eisenreich W, Gerhardt S, Bacher A, Rohdich F. Structure of 2C-methyl-D-erythritol-2,4-cyclodiphosphate synthase involved in mevalonate-independent biosynthesis of isoprenoids. Journal of Molecular Biology. 2002;316:79–88. doi: 10.1006/jmbi.2001.5341. [DOI] [PubMed] [Google Scholar]
- Testa CA, Lherbet C, Pojer F, Noel JP, Poulter CD. Cloning and expression of IspDF from Mesorhizobium loti. Characterization of a bifunctional protein that catalyzes non-consecutive steps in the methylerythritol phosphate pathway. Biochimica et Biophysica Acta-Proteins and Proteomics. 2006;1764:85–96. doi: 10.1016/j.bbapap.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Urbansky M, Davis CE, Surjan JD, Coates RM. Synthesis of enantiopure 2-C-methyl-D-erythritol 4-phosphate and 2,4-cyclodiphosphate from D-arabitol. Organic Letters. 2004;6:135–138. doi: 10.1021/ol0362562. [DOI] [PubMed] [Google Scholar]
- Zhao M, Li X, Xu P, Shen X, Gui XH, Wang LL, DeRiemer K, Mei J, Gao Q. Transmission of MDR and XDR tuberculosis in Shanghai, China. Plos One . 2009;4 doi: 10.1371/journal.pone.0004370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt J, Kafarski P, Mastalerz P. Preparation of oxoalkanephosphonic acids. Synthesis-Stuttgart. 1978:609–612. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.