Abstract
Differentiation of pluripotent low-energy requiring stem cells into the high-energy expenditure cardiac lineage requires coordination of genomic programming and energetic system maturation. Here, in amurine embryonic stem cell cardiac differentiation model, emergence of electrical and beating activity in cardiomyocytes developing within embryoid bodies was coupled with the establishment of the mitochondrial network and expansion of the creatine kinase (CK) phosphotransfer system. Stem cell cardiogenesis was characterized by increased total CK activity, an isoform shift manifested by amplified muscle CK-M mRNA levels and protein content, and the appearance of cardiac-specific CK-MB dimers. Treatment of differentiating stem cells with BMP2, a cardiogenic growth factor, promoted CK activity. CK-M clustered around developing myofibrils, sarcolemma, and the perinuclear compartment, whereas CK-B was tightly associated with myofibrillar α-actinin, forming wire-like structures extending from the nuclear compartment to the sarcolemma. Developmentally enhanced phosphotransfer enzyme-anchoring protein FHL2 coalesced the myofibrillar CK metabolic signaling circuit, providing an energetic continuum between mitochondria and the nascent contractile machinery. Thus, the evolving CK-catalyzed phosphotransfer network integrates mitochondrial energetics with cardiogenic programming, securing the emergence of energy-consuming cardiac functions in differentiating embryonic stem cells.
Keywords: differentiation, embryonic stem cells, mitochondria, phosphotransfer, energetic communication
Introduction
Differentiation of embryonic stem (ES) cells is a fundamental process that secures lineage specification and ultimately organogenesis.1 Specialization into cardiac myocytes is an early event in embryogenesis, as the forming heart is necessary to sustain developing hemodynamics.2,3 Cardiac differentiation requires the establishment of an energetic infrastructure capable of supporting the energy needs of emerging electromechanical activity.4,5 Although organized intracellular energetic communication is critical for optimal heart function,6 little is known regarding the development of the bioenergetic infrastructure that underlies early steps in stem cell cardiogenesis. Such knowledge is required to advance the understanding of molecular principles defining developmental energetics and to foster the application of energy-competent lineage-specified stem cell progeny.7–9
Cardiac differentiation of ES cells is characterized by expansion and maturation of the mitochondrial network and by a switch in energy metabolism from glycolysis to the more efficient oxidative phosphorylation.10–12 Induction of cardiogenic programming has been linked to restructuring of the metabolic transcriptome and integration of mitochondria with nascent force-producing sarcomeres.10 Disruption of respiratory chain function prevents mitochondrial organization and development of the energetic system, causing deficient sarcomerogenesis and contractile malfunction.10,13 Mitochondrial communication with adenosine 5′-triphosphatases (ATPases) in the highly structured cell environment is catalyzed by phosphotransfer pathways, as demonstrated in the adult heart.14–16 In development, significant changes occur in the creatine kinase (CK) system, a principal pathway in the cellular phosphotransfer network critical to the supply of ATP for myofibrillar contraction and signaling to metabolic sensors.17–21 Specifically, during embryonic heart development, the prominence of the brain CK-BB isoform decreases, whereas that of the muscle CK-MM and cardiac-specific CK-MB increase.18,20,22 The most important changes occur during perinatal development with the appearance of the mitochondrial CKmit isoform.20,22,23 Also important in differentiation is the adaptor protein FHL2, which anchors phosphotransfer enzymes to sites of high-energy consumption in the cardiac sarcomere.24 In this regard, metabolic feedback loops, which sense the cellular energy state and rely on organized phosphotransfer pathways structurally supported by anchoring proteins, have been implicated in directing nucleocytoplasmic communication, facilitating information exchange and genetic programming.25–28 Despite advances in defining the process of metabolic maturation during differentiation,10,29,30 the evolution of metabolic circuits in stem cell–based cardiogenesis remains poorly understood.
Here, we demonstrate developmental restructuring of the CK system that integrates mitochondrial energetics with stem cell cardiogenesis. Specifically, we found that cardiac differentiation of ES cells was linked to an isoform shift and intracellular rearrangement of the CK phosphotransfer system, expanding the metabolic signaling network in support of augmented energy needs. Such energetic “rewiring” was paralleled by developmental enhancement of the anchoring protein FHL2, which integrated CK circuits to provide an energetic and signaling continuum between the nucleus, mitochondria, sarcolemma, and myofibrils necessary for information exchange and electro-contractile activity of the newly formed cardiomyocytes.
Material and Methods
Stem Cell Differentiation
Murine ES cells, maintained in Glasgow Minimum Essential Medium (BioWhittaker–Cambrex), were differentiated in media containing 20% fetal bovine serum using the hanging-drop method.7,31,32 Forming embryoid bodies were resuspended in differentiation medium and allowed to grow before plating. Beating embryoid bodies were dissociated using 96 U/mL collagenase type 4 (Worthington Biochemical Corporation) and 0.1 mg/mL pancreatin (Sigma–Aldrich). Isolated cardiomyocytes were separated by Percoll (Sigma–Aldrich) gradient and were cultured on gelatin-coated polystyrene dishes or poly-L-lysine-coated glass slides.10,31,32
Enzyme Assays and Western Blot
Whole-cell extracts were made in 150 mM NaCl, 5 mM EDTA, 60 mM TRIZMA base, 0.2% Triton X-100, and Complete Mini EDTA-Free Protease Inhibitor Cocktail (Roche Applied Science).25 Protein concentration was determined using a detergent-compatible protein assay kit (Bio-Rad). CK activity was measured using a CK kit (Diagnostic Chemicals Limited) and a Beckman DU 7400 spectrophotometer. A portion of extracts was separated through SDS-PAGE, transferred to a PVDF membrane, and probed with antibodies against CK-B, CK-M, GAPDH (Santa Cruz Biotechnologies), FHL2, and α-tubulin (Abcam).
Confocal Microscopy
To monitor plasmalemmal and mitochondrial membrane potential, cells were incubated with 1.3 µM RH237 (Invitrogen)33 and/or 0.5 µM JC-1 (Invitrogen)34 and washed with phosphate-buffered saline solution. Alternatively, cells were incubated with 3 µM MitoTracker Red CMH2XRos (Invitrogen) and fixed in 3% paraformaldehyde. Cells were stained with one or more of the following antibodies: anticardiac α-actinin, anti-CK-B, anti-CK-M (Santa Cruz Biotechnologies), anti-FHL2 (Abcam), and the nuclear probe 4′6-diamidino-2-phenylindole-2HCl (DAPI, Invitrogen). Alexa Fluor secondary antibodies (Invitrogen) allowed fluorescent images to be obtained on an LSM 510 Laser Scanning System (Carl Zeiss) and analyzed using the Zeiss LSM Image Browser.35,36
Zymograms
Activities of CK isoforms were assayed using a 0.8% agarose gel to separate individual isoforms, followed by the Hydragel 7 ISO-CK kit (Sebia) to detect activity. Gel scans were analyzed with the NIH ImageJ 1.36b software.
Metabolic Gene Profiling
Total RNA isolated from ES cells or derived cardiomyocytes were screened using the mouse genome 430 2.0 array (Affymetrix).35 Expression profiles were analyzed with the bioinformatics suite Genespring GX 7.3 (Agilent Technologies). Gene lists were quality filtered to remove genes with expression levels below background and limited to report genes that changed by 1.5-fold or greater during cardiac differentiation.37
Statistics
Comparisons between groups were performed by two-tailed Student’s t tests. Data are presented as mean ± SEM; n refers to sample size. P < 0.05 was considered significant.
Results
Coordinated Development of Mitochondria, Myofibrils, and Electrical Activity in Stem Cell Cardiogenesis
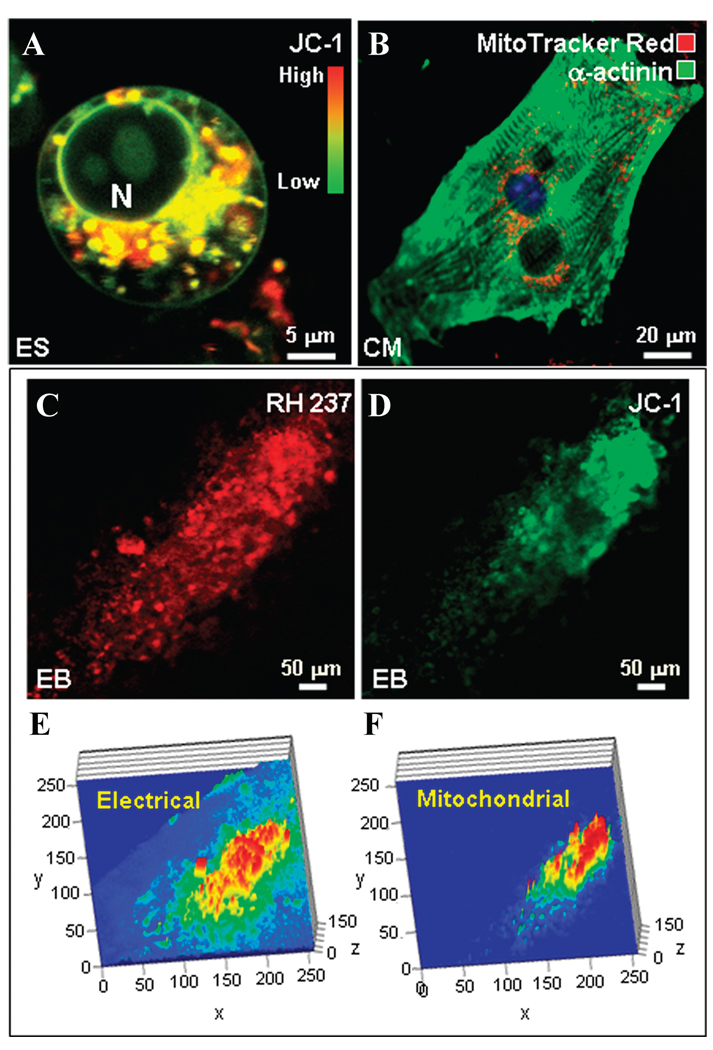
Quiescent ES cells self-maintain primarily through glycolytic metabolism, rather than oxidative phosphorylation.10 ES cells visualized with the inner mitochondrial membrane potential–sensitive probe JC-1 featured sparse mitochondria with variable degrees of polarization within a primitive cell architecture (Fig. 1A).Mitochondria displayed a round morphology and clustered in a perinuclear fashion (Fig. 1A). A distinct feature of ES cells, lost in developing cardiomyocytes, was labeling of the plasma membrane and nuclear envelope, indicating the presence of JC-1-detectable electrical charges and/or an environment favorable for forming fluorescent dye aggregates.34 ES cell–derived cardiomyocytes displayed a structured myofibrillar mesh to secure contractile function, with mitochondria strategically located not only around the nucleus, but also intercalated between developing sarcomeres (Fig. 1B). The organization of emerging cardiomyocytes into syncytia within embryoid bodies was delineated by a tight correlation between electrical activity staining with RH237, a probe for plasma membrane potential, and mitochondrial electrical charge probed with JC-1 (Fig. 1C and D). Intensity maps of co-localized electrical and mitochondrial activities revealed beating areas that organized around a core represented by the highest electrical and mitochondrial activities (Fig. 1E and F). Thus, cardiogenesis involves maturation of mitochondria along with a buildup of the electrical/contractile machinery, processes requiring coordinated energetic signaling and communication with transcriptional and translational events underlying cardiac programming.
Figure 1.
Cardiac differentiation requires coordination of emerging functional, electrical, and mitochondrial activities. (A) An embryonic stem (ES) cell visualized by the mitochondrial membrane potential–sensitive probe JC-1 has sparse mitochondria (red indicates hyperpolarized mitochondria; green indicates those with normal to low membrane potential) clustered around the nucleus (N) and a rather primitive cell architecture. (B) Stem cell–derived cardiomyocytes (CM) have a developed myofibrillar network (α-actinin immunostaining, green) and abundant mitochondria (MitoTracker Red, red) that localize around the nucleus and intercalate with myofibrils. (C) Developing cardiomyocytes within the beating area of an embryoid body demonstrate a higher cellular membrane potential (RH237, red) and (D) greater numbers of mitochondria, constituting a stronger JC-1 signal. (E) Intensity maps indicate that the electrical activity of the beating area is strongest at the core and (F) correlates with mitochondrial signals. Width and length (in µm) of the area of interest are represented on the×and y axes, respectively; intensity (arbitrary units) is plotted on the z axis.
Restructuring of the CK System during Stem Cell Cardiac Differentiation
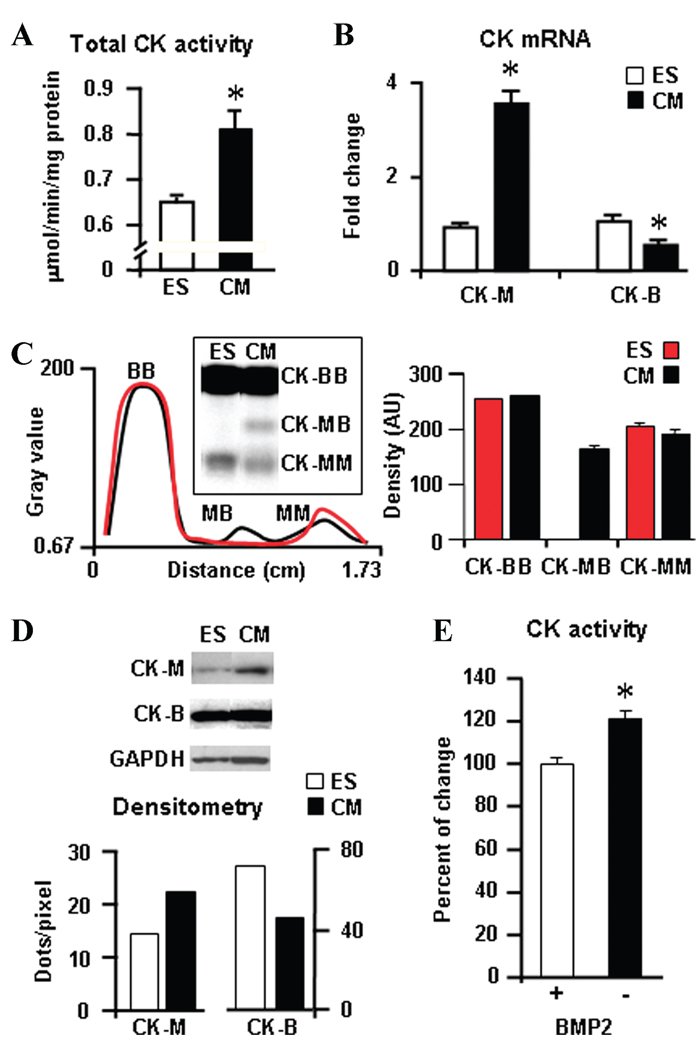
Cardiogenesis was associated with a significant increase in total CK activity, with an average change of 33.6% ± 0.1% in derived cardiomyocytes versus undifferentiated stem cells (n = 4 different sets of experiments, P < 0.01; Fig. 2A). Compared to the ES cell source, cardiac progeny demonstrated an increase in muscle CK-M isoform mRNA levels, from a 0.93-fold ± 0.09 to 3.58-fold ± 0.3 change above background (Fig. 2B), linked with the appearance of the cardiac-specific CK-MB isoform detected in zymograms (Fig. 2C) and an augmentation of protein levels of CK-M in Western blots (Fig. 2D). Concomitantly, the brain-type CK-B isoform was halved both in transcript number and protein levels in cardiomyocytes (n = 3) compared to ES cells (n = 3); in other words, we found a 1.07-fold ± 0.15 to 0.54-fold ± 0.02 change and from 72.3 to 45.5 dots/pixel, respectively (Fig. 2B and D). Mitochondrial Mi-CK isoform transcripts were below detection limits in the mouse genome gene array or zymograms. Treatment of ES cells with 5 ng/mL of the bone morphogenetic protein 2 (BMP2), which facilitates stem cell cardiac differentiation,31 significantly increased CK activity by 23%, on average (n = 3, P < 0.03; Fig. 2E).Thus, cardiogenic programming is associated with restructuring of the CK system at transcriptional, protein, and catalytic levels.
Figure 2.
Creatine kinase (CK) isoform pattern restructures during cardiogenesis. ES cell cardiogenesis is associated with (A) increased total CK activity, (B) an increase in muscle CK-M isoform, and a decrease in CK-B mRNA levels by gene array analysis. (C) Zymogram detected the appearance of cardiac-specific CK-MB dimers in ES cell–derived cardiomyocytes. (D) Western blots show augmented protein levels of CK-M and a decrease of CK-B in ES cell–derived cardiomyocytes. Quantification was based on the ratio of CK isoforms to the GAPDH protein used as a loading control. (E) The cardiogenic factor BMP2 (5 ng/mL) promoted the activity of the CK energy transfer pathway in differentiating ES cells.
Formation of CK Isoform Network
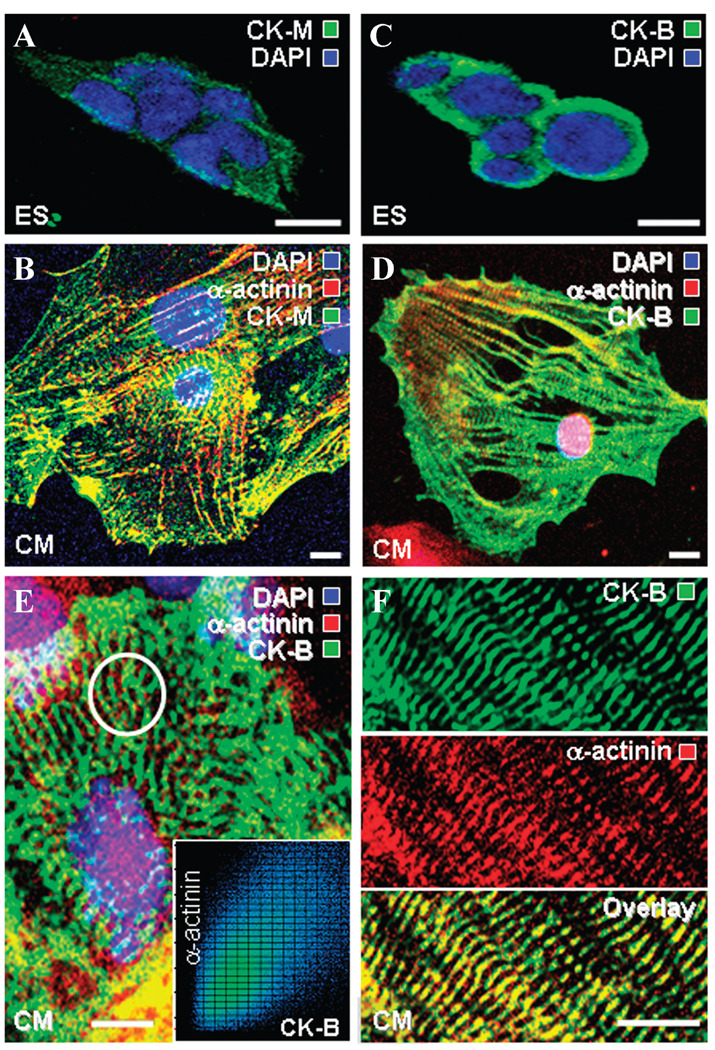
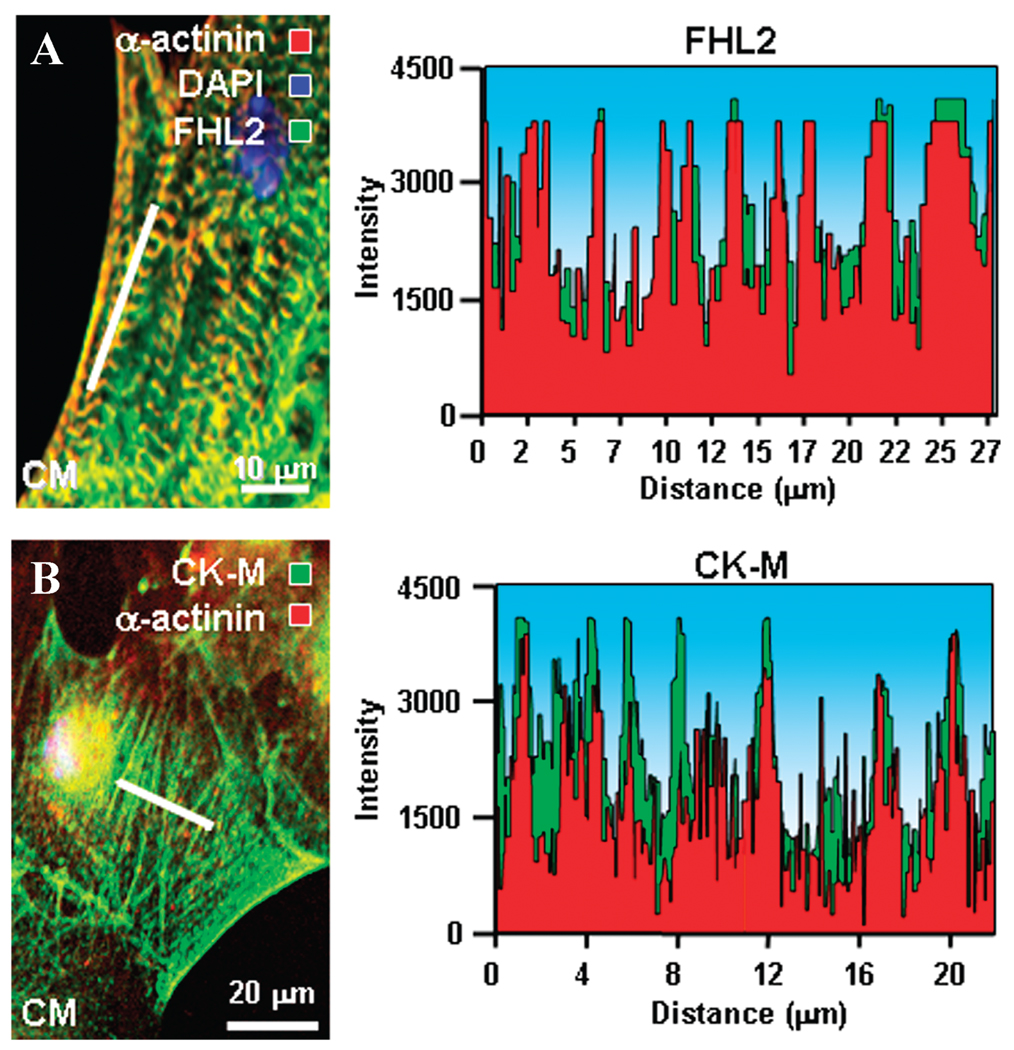
In contrast to ES cells with CK isoforms distributed rather indiscriminately through the cytosol (Fig. 3A and C), stem cell–derived cardiomyocytes displayed an elaborate distribution of CK-M and CK-B intercalating and coalescing with developing α-actinin-containing myofibrils (Fig. 3B and D). In addition, the muscle isoform CK-M showed distribution within the perinuclear compartment and sarcolemma (Fig. 3B). The juxtaposition of CK-B with α-actinin created wire-like structures extending from the nuclear compartment to the sarcolemma in derived cardiomyocytes (Fig. 3E). Quantitative co-localization analysis showed that CK-B and α-actinin immunostaining were tightly correlated (Fig. 3E, inset), indicating an association between these proteins. CK-B “energetic wires” (Fig. 3F, upper) and myofibrillar α-actinin (Fig. 3F, middle) overlapped (Fig. 3F, lower), further suggesting positioning of phosphotransfer enzymes in the microenvironment of ATP consumption sites within contractile proteins. Thus, developmental restructuring targets CK isoforms to specific intracellular locales, providing necessary infrastructure for efficient ATP delivery to cellular ATPases and metabolic feedback signaling to mitochondria.
Figure 3.
Development of the CK isoform phosphotransfer network during cardiogenesis. Immunocytochemistry indicates that compared to (A) ES cells, (B) cardiomyocytes have higher CK-M (green) levels associated with the developing myofibrils (α-actinin, red), perinuclear compartment, and sarcolemma (the nucleus identified using DAPI, blue). CK-B (green), also detected by immunofluorescence, localized (C) rather indiscriminately throughout the cytosol of ES cells, but (D) associated with developing myofibrils (α-actinin, red) in ES cell–derived cardiomyocytes. (E) A “wired” cardiomyocyte: CK-B (green) forms wire-like structures extending from the nuclear compartment to the sarcolemma. Inset: quantitative colocalization analysis of circled area (E) indicates that CK-B distribution tightly correlates with α-actinin immunostaining. (F) In ES cell–derived cardiomyocytes, CK-B “energetic wires” (upper) and myofibrillar α-actinin “wires” (middle) overlap (lower panel) indicating association. Scale bars: 10 µm.
Upregulated Anchoring Protein FHL2 Integrates with Myofibrillar/CK Network
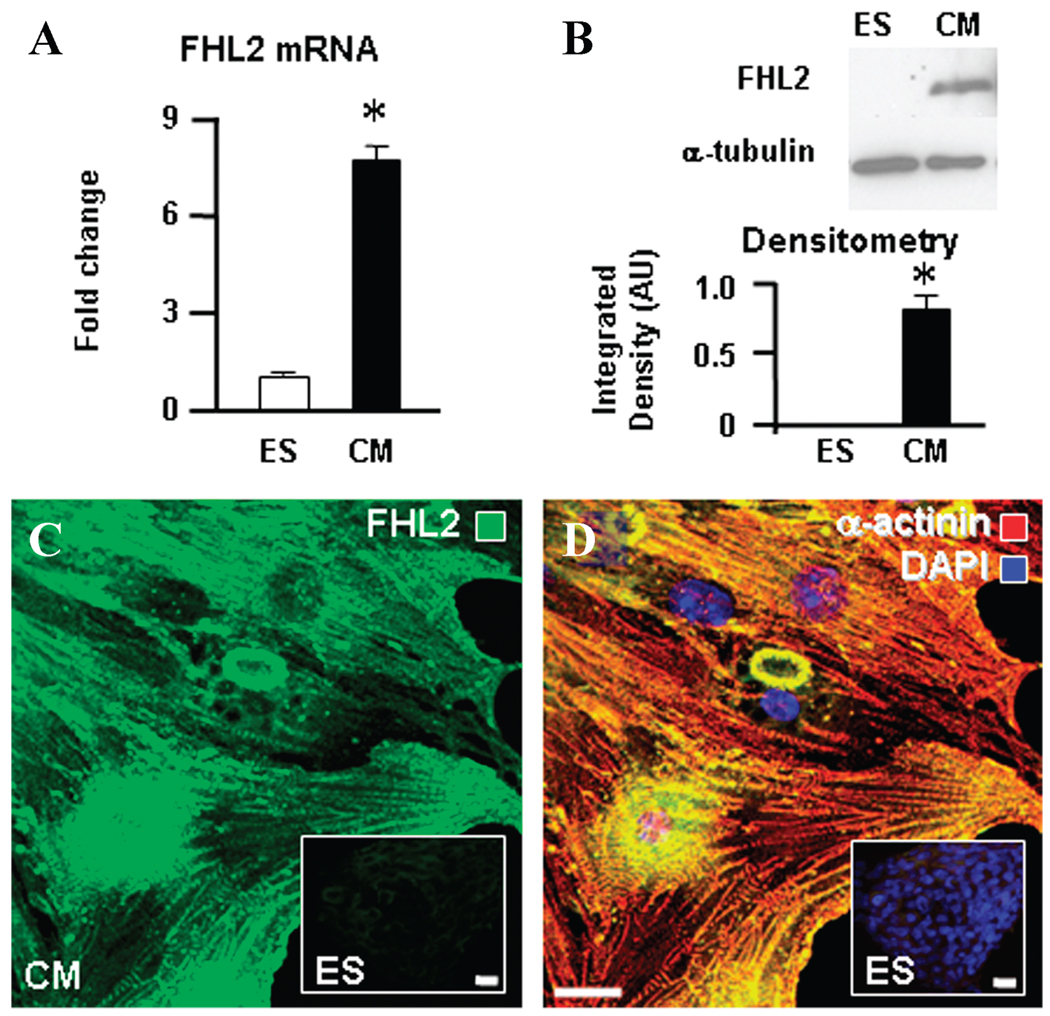
ES cell–derived cardiomyocytes demonstrated marked upregulation of the phosphotransfer enzyme anchor and 4.5 LIM domains containing protein 2 (FHL2). The mRNA copy number of FHL2, indicated by the corresponding gene array signal, increased more than 7-fold, from 1.01-fold ± 0.11 to 7.65-fold ± 0.55 (n = 3; Fig. 4A). FHL2 protein could not be detected by immunoblot in ES cells but became evident following cardiomyocyte derivation—from below detection to 0.83 AU normalized to α-tubulin (Fig. 4B). Confocal immunocytochemistry verified the distinct abundance of FHL2 within cardiac progeny (Fig. 4C) compared with the embryonic source (Fig. 4C, inset). Image overlay indicated co-localization of FHL2 with myofibrillar α-actinin in derived cardiac cells (Fig. 4D) in contrast to ES cells essentially lacking either protein (Fig. 4D, inset). On confocal close-up (Fig. 5A, left) and quantitative image scan (Fig. 5A, right), the overlapping pattern suggested that FHL2 is integral to the myofibrillar structure. Supporting an anchoring role for FHL2, the muscle-specific CK-M isoform co-localized and intercalated with myofibrillar α-actinin, securing a continuum between mitochondria and myofibrils (Fig. 5B). Thus, enhanced FHL2 expression in cardiac development facilitates phosphotransfer enzyme positioning within myofibrils, providing structural support for a functional energetic network.
Figure 4.
Developmental enhancement of the phosphotransfer enzyme anchoring protein FHL2. (A) ES cell–derived cardiomyocytes have a markedly increased FHL2 mRNA level and (B) protein content by Western blot. (C) Immunocytochemistry of FHL2 intracellular localization (green) indicates high abundance in cardiomyocyte progeny compared with the ES cell source (inset). (D) Image overlay indicates FHL2 (green) co-localization with myofibrillar α-actinin (red), which is absent in ES cells (inset), with the nucleus identified using DAPI (blue). Scale bars: 20 µm.
Figure 5.
Integration of FHL2 and CK-M with myofibrillar α-actinin. (A) Confocal image (left) and image scan (right) indicate an overlapping pattern of FHL2 and α-actinin intracellular localization. (B) Confocal image (left) and image scan (right) indicate that the phosphotransfer enzyme CK-M (green) colocalizes and intercalates with α-actinin (red).
Discussion
Stem cell differentiation is an energy-dependent process that requires constant metabolic signaling between the cytosol and nucleus to adjust developmental gene expression in accordance with the energy needs of the emerging cell phenotype.10,12 In contrast to the ES cell source with limited energetic demands, the nascent cell type typically exhibits elaborate functions that necessitate the development of a competent energetic system.11,13 In particular, stem cell cardiogenesis involves robust electrical and functional activities that force additional energy requirements and coordinated signal communication.10
Here, we demonstrate that in murine ES cell cardiac differentiation, development of the mitochondrial network and contractile apparatus is paralleled by isoform shifts and intracellular rearrangements of the CK phosphotransfer system involved in energetic and metabolic signaling. Developmental enhancement of the phosphotransfer enzyme anchoring protein FHL2 integrated myofibrillar/CK circuits to provide an energetic continuum between mitochondrial, myofibrillar, and nuclear compartments. Upregulation of phosphotransfer pathways was accelerated by cardiogenic growth factor treatment. Collectively, these data suggest that maturation of cardiogenic growth factor–responsive energetic and metabolic signaling circuits is an initial event leading to ES cell cardiac differentiation. In this way, developmental enhancement and intracellular positioning of CK phosphotransfer enzymes would facilitate integration of energetic, genetic, and metabolic signaling processes necessary for stem cell cardiogenesis.
Evidence is accumulating that energetic signal communication is catalyzed through circuits composed of phosphotransfer enzymes capable of bidirectional relay of metabolic information between intracellular compartments.5,15,16,20,28 ES cells have a primitive architecture, high nuclear-to-cytosolic volume ratio, and sparse mitochondria,35–37 and they rely on glycolytic metabolism to meet energy needs.10 During cardiac differentiation, emerging electrical and functional activities require adequate energy supply and coordination between myofibrillar and mitochondrial network formation as well as with transcriptional and translational events.10,12,35 To maximize energetic efficiency, mitochondria are aligned with myofibrils to minimize diffusion distances for high-energy phosphoryl-carrying molecules.10,15 Additionally, phosphotransfer systems must be recruited to ensure energetic communication to distant energy-consuming processes in the nucleus and cell membrane and inside myofibrils.15,17,18,21,22,25
The transcriptome of an ES cell undergoing cardiogenesis demonstrated upregulation of the gene encoding the creatine kinase CK-M isoform, leading to the appearance of the cardiac-specific CK-MB isoform. CK isoforms constitute the major phosphotransfer circuit in adult cardiomyocytes, connecting mitochondrial energetics with ATP-consuming processes in myofibrils, sarcolemma, the sarcoplasmic reticulum, and the nucleus.6,14,15,28,38 As demonstrated here in ES cell–derived cardiomyocytes, the CK-M subunit–containing isoforms associate and intercalate with nascent myofibrils, which would maintain a high local ATP:ADP ratio and the free energy of ATP hydrolysis necessary for continuous contractile activity.6,15,21 This observation is in accordance with reports showing ordered molecular clusters of CK-M multimers inside myofibrils39 and a developmental shift in isoform composition toward CK-MM and CK-MB in muscle and cardiac cell types and toward CK-BB in the brain.17,22,40,41 In adult cardiomyocytes, typically the major CK isoforms are CK-MM (50%–60%) and CKmit (30%–40%) with a lower presence of CK-MB (5%–10%) and CK-BB (2%–4%), collectively constituting the mature CK-catalyzed phosphotransfer network.20,41,42 In skeletal muscle fibers, CK-M is bound to the sarcomeric M-band, whereas CK-B is confined to the I-band region.43 Here, we provide the first demonstration of CK-B forming molecular wires connecting nuclear, sarcolemmal, and cytosolic compartments in stem cell–derived cardiomyocytes. CK-B tightly associated with α-actinin staining, indicating an interaction between these proteins to position phosphotransfer enzymes close to ATP consumption sites. These data also demonstrate that CK-B is integral to the cellular energetic infrastructure designed for efficient ATP delivery, ATP hydrolysis product removal from cellular ATPases, and metabolic feedback signaling to mitochondria.40 Thus, cardiomyocyte differentiation is associated with a timely enhancement and targeted restructuring of CK circuits to meet increased demands for energetic communication and processing of cellular information.
Formation of multienzyme complexes, along with metabolic coupling of sequential and non-sequential enzymatic reactions, plays a fundamental role in metabolic regulation.15,16,27,28 Stem cell cardiac differentiation was associated with significant developmental enhancement of the phosphotransfer enzyme anchoring protein FHL2 that interacts with titin and tethers CK, as well as parallel phosphotransfer systems catalyzed by adenylate kinase and phosphofructokinase at sites of high energy consumption in the cardiac sarcomere.24 Also, FHL2 interacts with integrins and transcription factors and has been more recently implicated in cell differentiation.24,44 The intracellular localization of FHL2 here indicated that this protein is integral to the developing myofibrillar network. Concomitantly, CK-M appeared in intermyofibrillar spaces, intercalated with myofibrils and forming a bundled signaling system. In this regard, present data reveal previously unrecognized features underlying the developmental maturation of the intracellular energetic signaling matrix in support of cell differentiation and energy provision for emerging specialized functions.
In summary, the development of the CK system is an integral mechanism for establishing the cellular energetic infrastructure in cardiogenesis linking the expanded mitochondrial network with cellular ATP consumption sites. As shown here, a number of mechanisms during stem cell cardiac differentiation support the emerging energetic and metabolic signaling network. This includes a CK isoform shift, total phosphotransfer activity change, and intracellular positioning close to ATP consumption sites through association with the anchoring protein FHL2 or directly with myofibrils. In this way, developmental enhancement and restructuring of the CK phosphotransfer network provide an energetic continuum between mitochondria and distinct cellular compartments integrating metabolic signaling processes necessary for stem cell cardiogenesis. With increased understanding of molecular processes underlying stem cell–based cardiopoiesis,7,10,35,36,45 the present findings establish metabolic circuits in cardiac programming and reveal targets for directed cardiogenesis and regeneration.
Acknowledgments
S. C. is a Bonner Scholar in Biochemistry and Molecular Biology, and recipient of Mayo Clinic M.D./Ph.D. Training Program funding. This work was supported by grants from the National Institutes of Health, Marriott Heart Disease Research Program, Marriott Foundation, Ted Nash Long Life Foundation, Ralph Wilson Medical Research Foundation, and Asper Foundation.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Solter D. From teratocarcinomas to embryonic stem cells and beyond: A history of embryonic stem cell research. Nat. Rev. Genet. 2006;7:319–327. doi: 10.1038/nrg1827. [DOI] [PubMed] [Google Scholar]
- 2.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava D. Making or breaking the heart: From lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taegtmeyer H, Wilson CR, Razeghi P, Sharma S. Metabolic energetics and genetics in the heart. Ann. N.Y. Acad. Sci. 2005;1047:208–218. doi: 10.1196/annals.1341.019. [DOI] [PubMed] [Google Scholar]
- 6.Saks V, Dzeja PP, Schlattner U, et al. Cardiac system bioenergetics: Metabolic basis of the Frank-Starling law. J. Physiol. 2006;571:253–273. doi: 10.1113/jphysiol.2005.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behfar A, Perez-Terzic C, Faustino RS, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J. Exp. Med. 2007;204:405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrell DK, Niederländer NJ, Perez-Terzic C, et al. Pharmacoproteomics: Advancing the efficacy and safety of regenerative therapeutics. Clin. Pharmacol. Ther. 2007;82:316–319. doi: 10.1038/sj.clpt.6100310. [DOI] [PubMed] [Google Scholar]
- 9.Mimeault M, Hauke R, Batra SK. Stem cells: A revolution in therapeutics—recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies Clin. Pharmacol. Ther. 2007;82:252–264. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- 10.Chung S, Dzeja PP, Faustino RS, et al. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovasc. Med. 2007;4 Suppl 1:S60–S67. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St John JC, Ramalho-Santos J, Gray HL, et al. The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning Stem Cells. 2005;7:141–153. doi: 10.1089/clo.2005.7.141. [DOI] [PubMed] [Google Scholar]
- 12.Facucho-Oliveira JM, Alderson J, Spikings EC, et al. Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J. Cell Sci. 2007;120:4025–4034. doi: 10.1242/jcs.016972. [DOI] [PubMed] [Google Scholar]
- 13.Spitkovsky D, Sasse P, Kolossov E, et al. Activity of complex III of the mitochondrial electron transport chain is essential for early heart muscle cell differentiation. FASEB J. 2004;18:1300–1302. doi: 10.1096/fj.03-0520fje. [DOI] [PubMed] [Google Scholar]
- 14.Ingwall JS. Transgenesis and cardiac energetics: New insights into cardiac metabolism. J. Mol. Cell. Cardiol. 2004;37:613–623. doi: 10.1016/j.yjmcc.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Saks V, Kuznetsov AV, Vendelin M, et al. Functional coupling as a basic mechanism of feed-back regulation of cardiac energy metabolism. Mol. Cell. Biochem. 2004;256–257:185–199. doi: 10.1023/b:mcbi.0000009868.92189.fb. [DOI] [PubMed] [Google Scholar]
- 16.Dzeja PP, Chung S, Terzic A. Integration of adenylate kinase and glycolytic and glycogenolytic circuits in cellular energetics. In: Saks V, editor. Molecular System Bioenergetics. Weinheim, Germany: Wiley-VCH; 2007. pp. 265–301. [Google Scholar]
- 17.Wegmann G, Huber R, Zanolla E, et al. Differential expression and localization of brain-type and mitochondrial creatine kinase isoenzymes during development of the chicken retina: Mi-CK as a marker for differentiation of photoreceptor cells. Differentiation. 1991;46:77–87. doi: 10.1111/j.1432-0436.1991.tb00868.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoerter JA, Ventura-Clapier R, Kuznetsov A. Compartmentation of creatine kinases during perinatal development of mammalian heart. Mol. Cell. Biochem. 1994;133–134:277–286. doi: 10.1007/BF01267960. [DOI] [PubMed] [Google Scholar]
- 19.van Deursen J, Wieringa B. Approaching the multifaceted nature of energy metabolism: Inactivation of the cytosolic creatine kinases via homologous recombination in mouse embryonic stem cells. Mol. Cell. Biochem. 1994;133–134:263–274. doi: 10.1007/BF01267959. [DOI] [PubMed] [Google Scholar]
- 20.Saupe KW, Spindler M, Hopkins JC, et al. Kinetic, thermodynamic, and developmental consequences of deleting creatine kinase isoenzymes from the heart. Reaction kinetics of the creatine kinase isoenzymes in the intact heart. J. Biol. Chem. 2000;275:19742–19746. doi: 10.1074/jbc.M001932200. [DOI] [PubMed] [Google Scholar]
- 21.Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J. Exp. Biol. 2003;206:2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- 22.Ingwall JS, Kramer MF, Woodman D, Friedman WF. Maturation of energy metabolism in the lamb: Changes in myosin ATPase and creatine kinase activities. Pediatr. Res. 1981;15:1128–1133. doi: 10.1203/00006450-198108000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Dowell RT. Mitochondrial component of the phosphorylcreatine shuttle is enhanced during rat heart perinatal development. Biochem. Biophys. Res. Commun. 1986;141:319–325. doi: 10.1016/s0006-291x(86)80371-0. [DOI] [PubMed] [Google Scholar]
- 24.Lange S, Auerbach D, McLoughlin P, et al. Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J. Cell. Sci. 2002;115:4925–4936. doi: 10.1242/jcs.00181. [DOI] [PubMed] [Google Scholar]
- 25.Dzeja PP, Bortolon R, Perez-Terzic C, et al. Energetic communication between mitochondria and nucleus directed by catalyzed phosphotransfer. Proc. Natl. Acad. Sci. USA. 2002;99:10156–10161. doi: 10.1073/pnas.152259999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spirin V, Gelfand MS, Mironov AA, Mirny LA. A metabolic network in the evolutionary context: Multiscale structure and modularity. Proc. Natl. Acad. Sci. USA. 2006;103:8774–8779. doi: 10.1073/pnas.0510258103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kholodenko BN. Untangling the signalling wires. Nat. Cell Biol. 2007;9:247–249. doi: 10.1038/ncb0307-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallimann T, Tokarska-Schlattner M, Neumann D, et al. The phosphocreatine circuit: Molecular and cellular physiology of creatine kinases, sensitivity to free radicals, and enhancement by creatine supplementation. In: Saks V, editor. Molecular System Bioenergetics. Weinheim, Germany: Wiley-VCH; 2007. pp. 195–264. [Google Scholar]
- 29.Portman MA. The adenine nucleotide translocator: Regulation and function during myocardial development and hypertrophy. Clin. Exp. Pharmacol. Physiol. 2002;29:334–338. doi: 10.1046/j.1440-1681.2002.03654.x. [DOI] [PubMed] [Google Scholar]
- 30.Funes JM, Quintero M, Henderson S, et al. Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc. Natl. Acad. Sci. USA. 2007;104:6223–6228. doi: 10.1073/pnas.0700690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behfar A, Zingman LV, Hodgson DM, et al. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J. 2002;16:1558–1566. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Terzic C, Behfar A, Mery A, et al. Structural adaptation of the nuclear pore complex in stem cell-derived cardiomyocytes. Circ. Res. 2003;92:444–452. doi: 10.1161/01.RES.0000059415.25070.54. [DOI] [PubMed] [Google Scholar]
- 33.Muller W, Windisch H, Tritthart HA. Fluorescent styryl dyes applied as fast optical probes of cardiac action potential. Eur. Biophys. J. 1986;14:103–111. doi: 10.1007/BF00263067. [DOI] [PubMed] [Google Scholar]
- 34.Reers M, Smiley ST, Mottola-Hartshorn C, et al. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–417. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Terzic C, Faustino RS, Boorsma BJ, et al. Stem cells transform into a cardiac phenotype with remodeling of the nuclear transport machinery. Nat. Clin. Pract. Cardiovasc. Med. 2007;4 Suppl 1:S68–S76. doi: 10.1038/ncpcardio0763. [DOI] [PubMed] [Google Scholar]
- 36.Faustino RS, Behfar A, Perez-Terzic C, Terzic A. Genomic chart guiding embryonic stem cell cardiopoiesis. Genome Biol. 2008;9:R6. doi: 10.1186/gb-2008-9-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodgson DM, Behfar A, Zingman LV, et al. Stable benefit of embryonic stem cell therapy in myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H471–H479. doi: 10.1152/ajpheart.01247.2003. [DOI] [PubMed] [Google Scholar]
- 38.Abraham MR, Selivanov VA, Hodgson DM, et al. Coupling of cell energetics with membrane metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J. Biol. Chem. 2002;277:24427–24434. doi: 10.1074/jbc.M201777200. [DOI] [PubMed] [Google Scholar]
- 39.Wegmann G, Zanolla E, Eppenberger HM, Wallimann T. In situ compartmentation of creatine kinase in intact sarcomeric muscle: The actomyosin overlap zone as a molecular sieve. J. Muscle Res. Cell. Motil. 1992;13:420–435. doi: 10.1007/BF01738037. [DOI] [PubMed] [Google Scholar]
- 40.Jost CR, Van Der Zee CE, In’t Zandt HJ, et al. Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur. J. Neurosci. 2002;15:1692–1706. doi: 10.1046/j.1460-9568.2002.02001.x. [DOI] [PubMed] [Google Scholar]
- 41.Hall N, DeLuca M. Developmental changes in creatine phosphokinase isoenzymes in neonatal mouse hearts. Biochem. Biophys. Res. Commun. 1975;66:988–994. doi: 10.1016/0006-291x(75)90737-8. [DOI] [PubMed] [Google Scholar]
- 42.Saks VA, Chernousova GB, Voronkov II, et al. Study of energy transport mechanism in myocardial cells. Circ. Res. 1974;35 Suppl 3:138–149. [PubMed] [Google Scholar]
- 43.Stolz M, Wallimann T. Myofibrillar interaction of cytosolic creatine kinase (CK) isoenzymes. J. Cell Sci. 1998;111:1207–1216. doi: 10.1242/jcs.111.9.1207. [DOI] [PubMed] [Google Scholar]
- 44.Lai CF, Bai S, Uthgenannt BA, et al. Four and half lim protein 2 (FHL2) stimulates osteoblast differentiation. J. Bone Miner. Res. 2006;21:17–28. doi: 10.1359/JBMR.050915. [DOI] [PubMed] [Google Scholar]
- 45.Arrell DK, Niederländer NJ, Faustino RS, et al. Cardioinductive network guiding stem cell differentiation revealed by proteomic cartography of TNFα-primed endodermal secretome. Stem Cells. 2008;26:387–400. doi: 10.1634/stemcells.2007-0599. [DOI] [PubMed] [Google Scholar]