Abstract
The catalytic activity of γ-glutamylcysteine ligase (γ-GCL; EC 6.3.2.2) was compared between relatively young (4-day-old) and old (19-day-old) houseflies (Musca domestica) in order to understand the mechanism of putative deterioration of glutathione homeostasis during the aging process. Hanes–Woolf analyses ([S]/v vs [S]) indicated that γ-GCL had significantly higher affinities for its substrates in the young than in the old flies. The Km values in the young and old flies were, respectively, for glutamate 0.6 and 5.5 mM; for cysteine 0.3 and 4.6 mM; and for ATP 1.2 and 2.9 mM. Furthermore, young but not old flies exhibited substrate-dependent inhibition of γ-GCL activity at >5 mM cysteine indicating a loss of metabolic regulation during aging. The age-associated differences in the affinity of native γ-GCL towards its substrates suggest that de novo synthesis of glutathione would be relatively less efficient in the old houseflies.
Keywords: γ-Glutamylcysteine ligase, Aging, Oxidative stress, Glutathione, Housefly
The tripeptide thiol, γ-glutamylcysteinylglycine, i.e., glutathione (GSH), is often present in millimolar amounts in biological tissues [1–3]. It is synthesized by the consecutive actions of two enzymes, γ-glutamylcysteine ligase (γ-GCL; EC 6.3.2.2), which catalyzes the first and rate-limiting step in the de novo synthesis of GSH, followed by GSH synthetase (GSS; EC 6.3.2.3), which couples glycine to γ-glutamylcysteine (γ-GC), resulting in the end-product of the pathway (Reactions 1 and 2 [4]).
| (Reaction 1) |
| (Reaction 2) |
In vivo, γ-GC is present in nanomolar concentrations [5]. Comparison of the apparent equilibrium constants and mass action ratios has shown that Reaction 1 is considerably displaced from equilibrium [6], thereby suggesting that γ-GCL activity is the primary determinant of the rate of GSH biosynthesis.
Because of its versatility as a reductant, GSH serves multiple biological functions, acting as a cofactor in the enzymatic reduction of peroxides, or as a conjugant of drugs to enhance their water solubility, or in cellular transport of amino acids and thiolation and dethiolation of proteins, among others. Depletion of GSH has been shown to enhance tissue susceptibility to oxidative damage [3,7–9]. Several studies have shown that GSH concentrations in some mammalian tissues decline with age or that the old organisms are relatively less capable of maintaining GSH levels in response to oxidative stress, which can be hypothesized to be reflective of an impairment in γ-GCL activity [10].
γ-GCL enzymes are often heterodimeric [11], consisting of the products of two distinct genes. Molecular cloning of subunits of γ-GCL enzymes has been accomplished [12–14] in several species, including Drosophila melanogaster, where the holoenzyme consists of a regulatory subunit, which is ~31 kDa in size, and a catalytic subunit of ~80 kDa size [12,15,16]. The γ-GCLC subunit alone can catalyze the formation of γ-GC [14], but the activity of γ-GCLC is substantially increased by covalent interactions with GCLR. Meister and co-workers [12] originally suggested that γ-GCL activity could be increased under conditions that deplete intracellular GSH, and hypothesized that an oxidizing environment within the cell could promote disulfide bond formation between the γ-GCLR and γ-GCLC subunits. Kinetically, it is thought that mechanisms by which the GCLR subunit regulates the catalytic activity of DmGCLC include a decrease in the Km for glutamate and a reduction in the feedback inhibition of the holoenzyme by GSH, the end-product of the glutathione biosynthetic pathway [12,17,18]. Since intracellular levels of glutamate are typically relatively low, it has been inferred that the monomeric GCLC subunit would function very poorly in maintaining high cellular GSH levels as compared to the heterodimeric holoenzyme [12].
The literature describing the biochemical regulation of γ-GCL activities is presently rather perplexing. For example, despite the fact that GCLC subunits from various species have been shown to be only marginally active on their own, upregulation of GCLC has been reported to support high levels of intracellular GSH in many species [19,20]. Furthermore, overexpression of the catalytically inactive GCLR subunit alone has been reported to increase intracellular GSH levels by 2-fold, rendering cells resistant to oxidative stress [21]. However, a decrease in GCLR expression following antisense RNA inhibition does not lead to any changes in GSH levels in human hepatoblastoma HepG2 cell cultures [22]. Such seemingly paradoxical results are interpreted to suggest that the metabolic responses to changes in γ-GCL activity and expression probably involve a complex network of regulatory processes, which have yet to be identified. Accordingly, the purpose of the present report was to understand the biochemical interactions which occur in the regulation of GSH biosynthesis in the aging organisms, using the housefly as a model system.
Materials and methods
Chemicals and reagents
HPLC Calibration standards (γ-GC, Cys-Gly, GSH, GSSG, and L-cysteine were obtained from Sigma Chemical (St. Louis, MO). o-Phosphoric acid was purchased from EMD Science (Gibbstown, NJ). Milli-Q grade water was prepared by reverse-osmosis on a Millipore water-purification system. All chemicals were of either analytical grade or of the highest purity commercially available.
Rearing and tissue preparation
Following emergence from their pupal case, adult flies were immobilized on ice, segregated based on their sex, and placed in 1 ft3 (~0.027 m3) cages in clusters of 200 male flies. The male flies, used in this study, were maintained under constant incandescent lighting, at 25 °C and 50% relative humidity. Free access to water and food was provided throughout the experimental period. The average life span of houseflies under these conditions was about 20–22 days. Representative samples of 4- and 19-day-old houseflies (approximately 10 per sample) were immobilized on ice by tapping into a chilled borosilicate test tube. Homogenization was carried out in Kontes glass homogenizers (Vineland, NJ) using 10 volumes of extraction buffer (320 mM sucrose, 1 mM PMSF, 1 mM ε-amino-n-caproic acid, and 10 mM Tris, pH 7.4). Complete protease inhibitor cocktail tablets (Roche, Indianapolis, IN), at a concentration of 1 tablet per 10 ml of extraction buffer were used in some experiments to inhibit endogenous proteases. Crude homogenates were centrifuged at 3000g for 10 min at 4 °C to pellet debris. Small molecular weight compounds were removed from the supernatants by centrifugation through centrifugal filters (Pall, Ann Arbor, MI) with a 10-kDa membrane cut-off (14,000g for 15 min at 4 °C).
Measurement of enzymatic activity
All procedures were carried out at 4 °C unless stated otherwise. Supernatants resulting from 3000g centrifugation of tissue homogenates were passed though 0.45 μm PTFE Acrodisc syringe filters (Gelman Laboratory, Ann Arbor, MI) directly into Pall centrifugal devices. Following centrifugation (at 4 °C, 14,000g for 15 min), the protein samples in the centrifugal devices were washed with 100 μl wash buffer (200 mM sucrose, 1 mM PMSF, 1 mM ε-amino-n-caproic acid, and 10 mM Tris, pH 7.4) and made up to a known volume with wash buffer. Aliquots of the preparation were immediately used for γ-GCL assays, as described below and elsewhere [23]. Following the γ-GCL assay, samples for HPLC analysis were either directly injected onto the HPLC column or stored at −80 °C for no longer than 24 h before analysis.
HPLC based housefly γ-GCL enzyme assay
The γ-GCL assay mixture, prepared immediately prior to the assay, consisted of 5–20 μl aliquots of protein sample (~30–50 μg), 100 mM Tris–HCl, 20 mM MgCl2, (pH 8.2), 10 mM ATP, 5 mM L-cysteine, 50 mM L-glutamate, and 500 μM acivicin in a total assay volume of 250 μl. Assays were usually carried out for 10 min at 25 °C. Reaction linearity with various substrate concentrations, with time, and with enzyme protein content was rigorously tested in preliminary experiments. The specific inhibitor of γ-GCL, L-buthionine-S,R-sulfoximine (L-BSO), was used to determine the specificity of the assay. Briefly, housefly extracts were incubated at room temperature for 10 min with up to 1 mM L-BSO and 10 mM ATP in incubation buffer (0.1M Tris–HCl, 20 mM MgCl2, pH 8.2) and then subjected to the γ-GCL assay. When kinetic data required, L-glutamate was varied between 0 and 10 mM, L-cysteine between 0 and 5 mM, and ATP between 0 and 10 mM. At the end of the assay, reactions were terminated with an equivalent volume of 15 mM o-phosphoric acid. Precipitated protein was removed by centrifugation at 14,000g for 10 min at 4 °C, the supernatant was refiltered through 0.45 μm PTFE Acrodisc syringe filters and injected onto the HPLC either immediately or invariably within 24 h.
HPLC resolution and coulometric detection of aminothiols
Aminothiols were detected by an HPLC based procedure, described in recently published reports [3,23,24]. In brief, sample injection for HPLC analysis was carried out on a Waters 717 plus autosampler and the mobile phase was delivered via a single Waters 515 solvent pump. Resolution of compounds was detected on a reverse-phase C18 Luna column (particle size 5 μm; 250 × 4.6 mm; Phenomenex, Torrance, CA) using isocratic elution with 15 mM o-phosphoric acid (pH 2.0) as the mobile phase at a flow rate of 1.0 ml min−1 [3,23]. The mobile phase was passed through the electrochemical detector for a period of 8 h to ensure baseline current stabilization.
Concentration series of calibration standards, which included γ-GC, Cys-Gly, GSH, GSSG, and cysteine, were prepared in 15 mM o-phosphoric acid, and were injected onto the HPLC at regular intervals to ensure uniform standardization. Each standard or experimental sample was analyzed by HPLC in duplicate and mean peak areas were calculated. Following HPLC resolution, compound detection was carried out with a model 5011 CoulArray electrochemical detector (ESA, Chelmsford, MA), equipped with a two-channel analytical cell. Potentials of +100 and +600 mV were applied on channels 1 and 2, respectively.
Protein determinations
The protein content of the extracts was determined in duplicate using the sodium bicinchoninate (BCA) protein assay (Pierce, Rockford, IL), according to the manufacturer’s instructions. Calibration curves were constructed using BSA standards made up to between 0.2 and 50 μg ml−1 of protein [25].
Results
Native γ-glutamylcysteine from young and old houseflies is dependent on L-cysteine, L-glutamate, and ATP for activity and is inhibited by L-BSO
In preliminary experiments, a series of concentrations of γ-GC, Cys-Gly, GSH, GSSG, and L-cysteine standards were analyzed by HPLC, using the conditions described below. In all cases, concentration calibration curves for all tested aminothiols were invariably linear (R2 ≥ 0.995; data not presented).
γ-GCL preparations obtained from both young and old houseflies were assayed in the presence or absence of different substrates. γ-GCL preparations from both sources were found to have an absolute requirement for L-cysteine, L-glutamate, ATP, and Mg2+ for activity. Using saturating substrate concentrations, product formation was determined to be linear with respect to time and protein concentration during a 60 min reaction period (data not presented).
In order to confirm the presence of active γ-GCL, end-point assays were performed with a specific γ-GCL inhibitor, L-buthionine-S,R-sulfoximine (L-BSO), or without the addition of protein. When tissue extracts were preincubated with 500 μM L-BSO and 5 mM ATP for 5 min before initiation of the assay, γ-GC formation was abolished. L-BSO was chosen as it forms a transition-state “suicide” analogue that interferes with the recognition of the cysteine substrate and has been found to be a specific and potent inhibitor of numerous classes of γ-GCL enzymes [26]. In some studies, the extent of γ-GCL inhibition with L-BSO has been reported to vary according to the origin of the enzyme. For example, L-BSO is a powerful inhibitor of the rat and human enzymes, and a poor inhibitor of the Escherichia coli γ-GCL enzyme [27]. In this study, L-BSO was found to invariably inhibit both old and young housefly γ-GCL totally and irreversibly, abolishing enzyme activity when included in the assay at concentrations of 500 μM. Although γ-GCL from both young and old houseflies was totally inhibited by the inclusion of 500 μM L-BSO in the assay, the kinetics of inhibition, with varied concentrations of L-BSO, differed between old and young houseflies (Toroser and Sohal, unpublished data).
Kinetic characteristics of γ-glutamylcysteine ligase from flies of different ages
A series of experiments were carried out in young and old houseflies to determine apparent kinetic constants for the three known substrates of γ-GCL in γ-GC synthesis. Native γ-GCL from both young and old house-flies was found to have an absolute requirement for the presence of L-glutamate, L-cysteine, ATP, and Mg2+ for synthetic activity. Notably, although significant differences in γ-GCL activity between young and old flies were not observed with saturating substrate assays, highly significant deviations in the affinity of the enzyme for its substrates were observed depending on whether the source of the γ-GCL enzyme was young or old houseflies. Apparent Km values were determined for the young and old housefly γ-GCL enzymes (Figs. 1–3) for comparison to each other and to kinetic constants, recently reported from studies with recombinant and highly purified γ-GCL enzymes ([16,28] and references therein).
Fig. 1.
Determination of apparent kinetic constants for young and old housefly γ-GCL at various concentrations of L-glutamate using Michaelis–Menten saturation plots and Hanes–Woolf transformations. Initial velocity Michaelis–Menten plots (v vs [glutamate]) and Hanes–Woolf analysis ([glutamate]/v vs [glutamate]; inset) of the Michaelis–Menten data of in vitro assays of γ-GCL in young (A) and old (B) houseflies are presented. Experiments were carried out three times in two independent broods of houseflies. Each sample was analyzed in duplicate and representative data are presented.
Fig. 3.
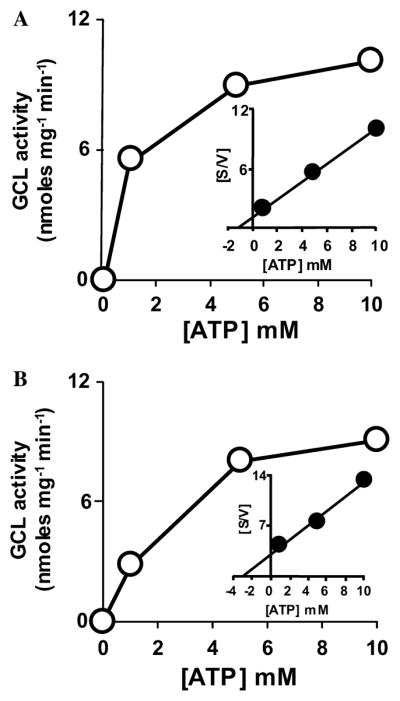
Determination of apparent kinetic constants for young and old housefly γ-GCL at various concentrations of ATP using Michaelis–Menten and Hanes–Woolf kinetic plots. Initial velocity Michaelis–Menten plots (v vs [ATP]) and Hanes–Woolf analysis ([ATP]/v vs [ATP]; inset) of the Michaelis–Menten data of in vitro assays of γ-GCL in young (A) and old (B) houseflies are shown. Experiments were carried out three times in two different broods of houseflies. Each sample was analyzed in duplicate and representative data are presented. All other details are as stated in the legend to Fig. 1.
In brief, Hanes–Woolf analysis of Michaelis–Menten results was employed to study γ-GCL from young and old houseflies. This method is often preferred over other straight-line plot analyses because of its robust and reliable kinetic derivations even in the presence of possible deviations from strict linearity [29]. Hanes–Woolf plots utilize linear regression analysis extended through the y-axis and derive the Km values by extrapolating to the x-axis. The apparent Km values obtained from young and old houseflies for L-glutamate, L-cysteine, and ATP are provided below.
Affinity of γ-GCL for L-glutamate is higher in young than in old houseflies
When the kinetics of L-glutamate utilization was investigated with γ-GCL enzyme preparations from young houseflies, it was found that γ-GC formation vs glutamate concentrations invariably yielded rectangular hyperbolic Michaelis–Menten plots of v vs [S] (Fig. 1A and inset). However, Michaelis–Menten plots and data transformations for γ-GCL from old flies yielded significantly different patterns (Fig. 1B and inset). The apparent Km for L-glutamate for γ-GCL from young flies was 0.60 mM, vs 5.52 mM in old flies (Figs. 1A and B), representing a 9.2-fold decrease in the affinity of γ-GCL for L-glutamate when 4-day-old houseflies were compared to 19-day-old houseflies. In general, the intracellular concentration of glutamate was estimated to range from ~1 to 3 mM. Given these concentrations, the data shown in Fig. 1 suggest that γ-GCL activity in old flies may be limited by L-glutamate availability under conditions of oxidative-stress and GSH depletion. The apparent Km values of L-glutamate for γ-GCL from young and old houseflies, determined in the present study, fall within the broad range that has been reported in other systems in the literature (e.g., see [27] and references therein).
Affinity of γ-GCL for L-cysteine is greater in young than old houseflies
When varied concentrations of cysteine were used as γ-GCL substrate, the apparent Km of γ-GCL, prepared from old houseflies (19-day-old), showed a 15-fold reduction in affinity for this substrate (Fig. 2B) as compared to the young (4-day-old) houseflies (Fig. 2A). For instance, the apparent Km for L-cysteine was 0.30 mM in the young vs 4.60 mM in the old flies (19-day-old; Figs. 2A and B). Interestingly, a reduction in the affinity of rat brain γ-GCL for its L-cysteine substrate was recently reported by Suh et al. [10], where a 40% loss in the apparent catalytic turnover of cerebral γ-GCL was observed. The authors reported that they were able to reverse the loss in γ-GCL activity via an α-lipoic acid induced increase in L-cysteine accumulation.
Fig. 2.
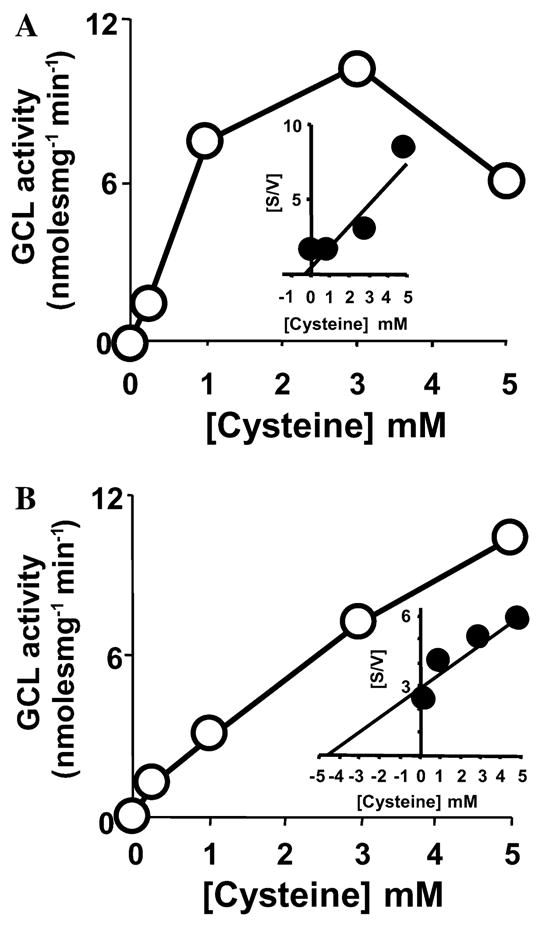
Determination of apparent kinetic constants for young and old housefly γ-GCL at various concentrations of L-cysteine using Michaelis–Menten and Hanes–Woolf kinetic plots. Initial velocity Michaelis–Menten plots (v vs [cysteine]) and Hanes–Woolf analysis ([cysteine]/v vs [cysteine]; inset) of the Michaelis–Menten data of in vitro assays of γ-GCL in young (A) and old (B) houseflies are presented. Experiments were carried out three times in two independent broods of houseflies. Each sample was analyzed in duplicate and representative data are presented. All other details are as stated in the legend to Fig. 1.
γ-GCL activity in young but not old flies shows substrate-dependent feedback inhibition with increasing L-cysteine concentrations
During preliminary experiments with numerous independent broods of houseflies, significant substrate inhibition of γ-GCL activity from young houseflies was evident with increasing levels of L-cysteine, with a threshold of around 5 mM (Fig. 2A). In contrast to the young flies, γ-GCL from old houseflies did not display substrate inhibition with increasing L-cysteine concentrations (Fig. 2B). Inhibition of the young native γ-GCL enzyme at high concentrations of L-cysteine restricted the range of substrate concentrations that could be effectively used for determining the Km of the enzyme for this substrate. Appropriate controls and γ-GCL assays with L-cysteine from a number of independent sources showed that the inhibitory effect seen with L-cysteine concentrations above ~5 mM was due to increasing levels of the substrate and not due to a contaminant in the commercial chemical, an artefactual shift in pH, chelation of necessary metal ions (e.g., Mg2+) or chelation of other necessary assay components such as the ATP substrate. It was inferred that young housefly γ-GCL is feedback regulated in vitro by high concentrations of cysteine, whereas native γ-GCL from old flies does not respond to this potentially regulatory mechanism.
Utilization of ATP by γ-GCL in young and old houseflies
γ-GCL activity from old and young houseflies also differed significantly in their efficiency for ATP utilization. However, proportionately, the decrease in the affinity of the enzyme for this substrate was less severe with advancing age than it was for the L-cysteine and L-glutamate substrates. The apparent Km of γ-GCL obtained from young flies for its ATP substrate was 1.20 mM, whereas it was 2.96 mM in the old flies (Figs. 3A and B). This variation in the Km for ATP suggests that higher in vivo concentrations of ATP may be required for γ-GCL activity in old houseflies to function optimally (Figs. 3A and B).
Discussion
The observed inhibition of housefly γ-GCL activity following addition of L-BSO suggests that the recognition of amino acid substrates at its active site in housefly γ-GCL is analogous to that in mammalian γ-GCL enzymes, as opposed to the E. coli enzyme [30]. It is thought that the reaction catalyzed by γ-GCL proceeds via the formation of a γ-glutamylphosphate intermediate followed by a nucleophilic substitution by Cys. By analogy to the inhibitory mechanism of L-BSO for the mammalian enzyme, a major recognition element for the housefly enzyme is likely to be the side chain of Cys (CH2SH); however, its highly sensitive reaction with L-BSO distinguishes the housefly enzyme from the E. coli, which has an additional requirement for the carboxy group of cysteine, as recently demonstrated by Hiratake et al. [31]. Although additional amino acids were not tested as substrates in the present study, the housefly enzyme may also be able to accommodate additional amino acids, with small hydrophobic side chains in its active site, a property similar to that of the mammalian enzyme, albeit this awaits experimental determination [32]. The recent crystallization of the E. coli enzyme [33,34] has been very informative in terms of the general reaction mechanism of γ-GCL enzymes. Crystallization of the heterodimeric mammalian and Drosophila enzymes would be particularly useful to advance our understanding of the enzyme from metazoan species.
It is noteworthy that the in vivo concentrations of the tested substrates may often be significantly less than the concentrations required for optimal γ-GCL activity of the old housefly enzyme. In particular, it is thought that deficits in L-cysteine availability or transport significantly slow down the ability to restore GSH through de novo biosynthesis following oxidative insults [35,36]. Due to cysteine toxicity, the intracellular levels of this amino acid are relatively low [36]. Elevated levels of cysteine as well as its precursor homocysteine are cytotoxic and associated with several chronic diseases manifested with aging [37–39]. Tissue concentrations of cysteine must be maintained at low levels, while at the same time ensuring an adequate supply of these thiols for utilization by γ-GCL and other essential functions [37,39]. Changes in the in vivo γ-GCL activity as well as mRNA level have been reported in response to increases in cysteine [37,39]. Since, in the present study, the γ-GCL enzyme from old flies was found to have an apparent Km for L-cysteine of 4.6 mM, the enzyme would be capable of only relatively low levels of activity and that under conditions of extreme oxidative challenge, the activity may be inadequate for a synthetic de novo response. The de novo synthetic capacity to replenish depleted GSH levels is of immense interest in many pathologies associated with oxidative stress (e.g., see [40] and references therein). It is noteworthy that some recent papers have reported a relatively high apparent Km for L-cysteine (up to 6.55 mM for recombinant γ-GCL from D. melanogaster [41]. In the present study, native housefly γ-GCL from young and old flies displayed Km values (0.3; 4.6 mM), which fall within the broad spectrum of values that have been reported for other characterized γ-GCL enzymes (see [27,30,42–44] and references therein).
Regulation of mammalian γ-GCL activity is influenced by the formation of transient reversible covalent interactions between the regulatory and catalytic subunits (Fig. 4) [12,26]. The enzyme can be dissociated into its constituent subunits after treatment with reductants such as DTT [30], leading to a much reduced affinity for L-glutamate and a decrease in activity under reducing conditions [16,30]. Based on our results from the kinetic analysis of native housefly GCL, we propose an additional cysteine-dependent regulatory redox sensing mechanism by which the L-cysteine substrate regulates the activity of young γ-GCL. Although the mode of action of the proposed “cysteine-dependent feedback regulation” is not presently known, it is tempting to speculate that cysteine may directly bind to a low-affinity allosteric binding site on the holoenzyme. Similar mechanisms of allostery have been shown to be crucial to living cells and can operate through positive or negative feedback regulation [45]. Interestingly, mammalian γ-GCL is known to catalyze several partial reactions which reflect aspects of the overall catalytic mechanism of the enzyme [31]. When highly purified γ-GCL is incubated with ATP, there is a stoichiometric formation of ADP and Pi [31,32] with rates that can be around 10% of the synthetic activity [32]. It is noteworthy that the ATPase partial enzymatic activity of γ-GCL can be totally inhibited by L-cysteine at a concentration of only 0.5 mM L-cysteine [32].
Fig. 4.
Hypothetical scheme showing aspects of housefly γ-GCL regulation. This regulatory model is based on information obtained here and other studies on mammals and Drosophila. For simplicity, only some of the products in the reaction are included. An increase in in vitro L-cysteine concentrations above 5 mM (this study) and supplementation of rat hepatocyte cell cultures with 2 mM L-cysteine (e.g., see [37,39] and references therein) leads to a decrease in γ-GCL activity and gene expression. γ-GCLc expression is thought to decrease with age presumably due to a reduction in transcriptional activity. Intracellular GSH content can be altered by buthionine-S,R-sulfoximine (L-BSO), a specific inhibitor of γ-GCL. L-BSO decreases GSH levels, inhibits DNA synthesis, and causes depolarization of mitochondria. γ-GCL activity is increased under conditions that deplete intracellular GSH, and in oxidizing conditions within the cell, which promote disulfide bond formation between the γ-GCLR and γ-GCLC subunits. A decrease in the Km for glutamate and a reduction in the feedback inhibition of the holoenzyme by GSH occur when the heterodimer is formed [12,17,18]. It is noteworthy that the enzyme has not been cloned from the housefly, and the catalytic and regulatory subunits are included in the hypothetical scheme only by analogy to classes of γ-GCL enzymes such as the mammalian and Drosophila forms.
The present study found that L-cysteine concentrations in excess of ~5 mM had a significantly inhibitory effect on the synthetic reaction in vitro. It should be noted that, unlike the highly purified preparations used by Meister and co-workers [4,30] and the recombinant protein preparations described in recent studies [16,41], the preparations used for the present study were not exhaustively purified, a factor which may have helped to maintain potentially regulatory allosteric binding site(s) for L-cysteine on regulatory subunits of the enzyme. Interestingly, the Km for L-cysteine of the recombinant Drosophila enzyme has recently been reported to be ~6.55 mM, nearly 22-fold greater than that in the young housefly, but very near to the apparent Km observed in the old flies. The apparent Km value for L-cysteine reported in the present study is consistent with the observed intracellular cysteine concentrations of many systems [46,47]. To our knowledge, no reports presently exist which describe structural variations in γ-GCL that could account for the substrate affinity differences reported here. Although the specific structural mechanisms were not determined, differences in catalytic affinity described here suggest that houseflies of different ages are likely to differ significantly in their physiological response to oxidative stress and GSH depletion.
The inhibitory kinetics of L-BSO, an active-site-directed inhibitor that can be used to probe the recognition of the L-cysteine substrate by γ-GCL, also show significant age-related differences, suggesting that the partial activities of the enzyme, which consist of glutamate binding followed by transient substrate level phosphorylation, are kinetically different in γ-GCL from young and old flies (Toroser and Sohal, unpublished data). Although deduced protein sequence information for the housefly γ-GCL is not yet available, and the substrate binding sites have not been mapped, we suggest that, based on its L-BSO sensitivity, the housefly enzyme may have a thiol at its active site, similar to the human, mouse, and rat enzymes [26]. In other systems, this active-site thiol has to be in a reduced state for full enzymatic activity; in contrast to the other thiols in the enzyme, which have to be in an oxidized state to allow subunit interactions and significant γ-GCL activity.
On the basis of the present observation that an increase in cysteine concentration above a threshold level led to a decrease in in vitro γ-GCL activity in 4-day-old houseflies (Figs. 2A and B), it seems possible that in houseflies L-cysteine directly modulates its own metabolism at the level of γ-GCL enzyme activity. Presumably, catabolism is favored when cysteine is high, and glutathione synthesis occurs when cysteine concentration is low, a mechanism that is also consistent with observations recently made in mammalian systems [39]. Another possible mechanism may involve the separation of the two subunits of the enzyme under reducing conditions represented by high concentrations of cysteine [12,30]. To test our “dissociation” hypothesis we carried out a diagnostic set of experiments involving the determination of the Km for the L-glutamate substrate. In mammalian and Drosophila γ-GCL, the affinity of the γ-GCL heterodimer for its glutamate substrate is up to 16-fold greater than the catalytic subunit alone [16,30]. Estimates of Km values for glutamate at various concentrations of cysteine were obtained from Hanes–Woolf analysis. The apparent Km values for L-glutamate were approximately 0.6 mM for young flies and showed no significant consistent differences at a range of cysteine concentrations (data not presented). In short, Hanes–Woolf plots of glutamate utilization were linear at various cysteine concentrations, suggesting that the mechanism of lowered enzyme function may not be due to a possible subunit dissociation.
Finally, the limiting substrate enzyme assays used in this study show that the rate-limiting synthetic step catalyzed by γ-GCL is apparently impaired in old house-flies. A possible functional consequence would be that the ability for a de novo response to an oxidative insult is attenuated in old houseflies.
Acknowledgments
This study was supported by Grant RO1AG7657 from National Institutes of Health and National Institute on Aging.
Footnotes
Abbreviations: acivicin, (αS,5S)-α-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid; L-BSO, L-buthionine-S,R-sulfoximine; ECD, electrochemical detection; γ-GC, γ-glutamylcysteine; GSH, glutathione (reduced); GSSG, glutathione (oxidized); HPLC, high-performance liquid chromatography.
References
- 1.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 2.Rebrin I, Kamzalov S, Sohal RS. Free Radic Biol Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebrin I, Bayne AC, Mockett RJ, Orr WC, Sohal RS. Biochem J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meister A. Methods Enzymol. 1995;251:3–7. doi: 10.1016/0076-6879(95)51106-7. [DOI] [PubMed] [Google Scholar]
- 5.Risto E, Hebert C, Njalsson R, Norgren S, Rooyackers O, Larsson A. J Inherit Metab Dis. 2002;25:577–584. doi: 10.1023/a:1022095324407. [DOI] [PubMed] [Google Scholar]
- 6.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Proc Natl Acad Sci USA. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohal RS, Sohal BH, Brunk UT. Mech Ageing Dev. 1990;53:217–227. doi: 10.1016/0047-6374(90)90040-m. [DOI] [PubMed] [Google Scholar]
- 8.Sohal RS, Arnold LA, Sohal BH. Free Radic Biol Med. 1990;9:495–500. doi: 10.1016/0891-5849(90)90127-5. [DOI] [PubMed] [Google Scholar]
- 9.Sohal RS, Toy PL, Farmer KJ. Arch Gerontol Geriatr. 1987;6:95–100. doi: 10.1016/0167-4943(87)90001-x. [DOI] [PubMed] [Google Scholar]
- 10.Suh JH, Wang H, Liu RM, Liu J, Hagen TM. Arch Biochem Biophys. 2004;423:126–135. doi: 10.1016/j.abb.2003.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seelig GF, Meister A. Methods Enzymol. 1985;113:390–392. doi: 10.1016/s0076-6879(85)13051-x. [DOI] [PubMed] [Google Scholar]
- 12.Huang CS, Anderson ME, Meister A. J Biol Chem. 1993;268:20578–20583. [PubMed] [Google Scholar]
- 13.Saunders RD, McLellan LI. FEBS Lett. 2000;467:337–340. doi: 10.1016/s0014-5793(00)01148-0. [DOI] [PubMed] [Google Scholar]
- 14.Yan N, Meister A. J Biol Chem. 1990;265:1588–1593. [PubMed] [Google Scholar]
- 15.Galloway DC, Blake DG, Shepherd AG, McLellan LI. Biochem J. 1997;328:99–104. doi: 10.1042/bj3280099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser JA, Kansagra P, Kotecki C, Saunders RD, McLellan LI. J Biol Chem. 2003;278:46369–46377. doi: 10.1074/jbc.M308035200. [DOI] [PubMed] [Google Scholar]
- 17.Misra I, Griffith OW. Protein Expr Purif. 1998;13:268–276. doi: 10.1006/prep.1998.0897. [DOI] [PubMed] [Google Scholar]
- 18.Richman PG, Meister A. J Biol Chem. 1975;250:1422–1426. [PubMed] [Google Scholar]
- 19.Mulcahy RT, Bailey HH, Gipp JJ. Cancer Res. 1995;55:4771–4775. [PubMed] [Google Scholar]
- 20.Yao KS, Godwin AK, Johnson SW, Ozols RF, O’Dwyer PJ, Hamilton TC. Cancer Res. 1995;55:4367–4374. [PubMed] [Google Scholar]
- 21.Tipnis SR, Blake DG, Shepherd AG, McLellan LI. Biochem J. 1999;337:559–566. [PMC free article] [PubMed] [Google Scholar]
- 22.Lu SC. FASEB J. 1999;13:1169–1183. [PubMed] [Google Scholar]
- 23.Gegg ME, Clark JB, Heales SJ. Anal Biochem. 2002;304:26–32. doi: 10.1006/abio.2001.5607. [DOI] [PubMed] [Google Scholar]
- 24.Birago C, Marchei E, Pennino R, Valvo L. J Pharm Biomed Anal. 2001;25:759–765. doi: 10.1016/s0731-7085(01)00379-x. [DOI] [PubMed] [Google Scholar]
- 25.Stoscheck CM. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- 26.Huang CS, Moore WR, Meister A. Proc Natl Acad Sci USA. 1988;85:2464–2468. doi: 10.1073/pnas.85.8.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly BS, Antholine WE, Griffith OW. Epub 2001 Oct 2023. J Biol Chem. 2002;277:50–58. doi: 10.1074/jbc.M107961200. [DOI] [PubMed] [Google Scholar]
- 28.Jez JM, Cahoon RE, Chen S. Epub 32004 Jun 33404. J Biol Chem. 2004;279:33463–33470. doi: 10.1074/jbc.M405127200. [DOI] [PubMed] [Google Scholar]
- 29.Cornish-Bowden A. Methods. 2001;24:181–190. doi: 10.1006/meth.2001.1179. [DOI] [PubMed] [Google Scholar]
- 30.Huang CS, Chang LS, Anderson ME, Meister A. J Biol Chem. 1993;268:19675–19680. [PubMed] [Google Scholar]
- 31.Hiratake J, Irie T, Tokutake N, Oda J. Biosci Biotechnol Biochem. 2002;66:1500–1514. doi: 10.1271/bbb.66.1500. [DOI] [PubMed] [Google Scholar]
- 32.Orlowski M, Meister A. J Biol Chem. 1971;246:7095–7105. [PubMed] [Google Scholar]
- 33.Hibi T, Hisada H, Nakatsu T, Kato H, Oda J. Acta Crystallogr D Biol Crystallogr. 2002;58:316–318. doi: 10.1107/s0907444901019886. [DOI] [PubMed] [Google Scholar]
- 34.Hibi T, Nii H, Nakatsu T, Kimura A, Kato H, Hiratake J, Oda J. Proc Natl Acad Sci USA. 2004;11:11. doi: 10.1073/pnas.0403277101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meister A. Life Sci. 1974;15:177–190. doi: 10.1016/0024-3205(74)90206-9. [DOI] [PubMed] [Google Scholar]
- 36.Puka-Sundvall M, Eriksson P, Nilsson M, Sandberg M, Lehmann A. Brain Res. 1995;705:65–70. doi: 10.1016/0006-8993(95)01139-0. [DOI] [PubMed] [Google Scholar]
- 37.Lee JI, Londono M, Hirschberger LL, Stipanuk MH. J Nutr Biochem. 2004;15:112–122. doi: 10.1016/j.jnutbio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Montine TJ, Picklo MJ, Amarnath V, Whetsell WO, Jr, Graham DG. Exp Neurol. 1997;148:26–33. doi: 10.1006/exnr.1997.6662. [DOI] [PubMed] [Google Scholar]
- 39.Stipanuk MH. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 40.Weijl NI, Elsendoorn TJ, Lentjes EG, Hopman GD, Wipkink-Bakker A, Zwinderman AH, Cleton FJ, Osanto S. Eur J Cancer. 2004;40:1713–1723. doi: 10.1016/j.ejca.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 41.Fraser JA, Saunders RD, McLellan LI. Epub 2001 Nov 1106. J Biol Chem. 2002;277:1158–1165. doi: 10.1074/jbc.M106683200. [DOI] [PubMed] [Google Scholar]
- 42.Lueder DV, Phillips MA. J Biol Chem. 1996;271:17485–17490. doi: 10.1074/jbc.271.29.17485. [DOI] [PubMed] [Google Scholar]
- 43.Board PG, Smith JE, Moore K, Ou D. Biochim Biophys Acta. 1980;613:534–541. doi: 10.1016/0005-2744(80)90109-6. [DOI] [PubMed] [Google Scholar]
- 44.Davis JS, Balinsky JB, Harington JS, Shepherd JB. Biochem J. 1973;133:667–678. doi: 10.1042/bj1330667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunasekaran K, Ma B, Nussinov R. Proteins. 2004;57:433. doi: 10.1002/prot.20232. [DOI] [PubMed] [Google Scholar]
- 46.Himi T, Ikeda M, Yasuhara T, Nishida M, Morita I. J Neural Transm. 2003;110:1337–1348. doi: 10.1007/s00702-003-0049-z. [DOI] [PubMed] [Google Scholar]
- 47.Eck HP, Droge W. Biol Chem Hoppe-Seyler. 1989;370:109–113. doi: 10.1515/bchm3.1989.370.1.109. [DOI] [PubMed] [Google Scholar]




