Abstract
Viewing objects with the intention to act upon them may activate task-irrelevant motor responses. Many manufactured objects are associated with two action classes: grasping in accordance with object structure and skillful use consistent with object function. We studied the potential for within-object competition during action selection by comparing initiation latencies for “conflict” objects (with competing structure and function responses) to “non-conflict” objects (with a single response). We demonstrated a novel pattern of within-object interference wherein actions involving conflict objects were slowed when participants skillfully used those objects (grasp-on-use interference) as well as a second pattern of interference when conflict objects were grasped after skillfully using the same objects in previous blocks (long-term use-on-grasp interference). These data suggest that actions to common objects are influenced by competition between rapid but briefly maintained grasp responses and slower but longer-lasting use responses, and advance our understanding of the process and neural substrates of selection for action.
Object and action processing are tightly coupled. Object recognition and selection often occur concomitant with the preparation of a motor plan to be executed once the object is found. For example, while searching through a kitchen drawer, one may have in mind both the visual appearance of a large wooden spoon and the action of grasping it. It is increasingly recognized that action preparation has a facilitative effect upon object selection (Botvinick, Buxbaum, Bylsma, & Jax, 2009; Craighero, Fadiga, Umiltá, & Rizzolatti, 1996; Pavese & Buxbaum, 2002). Conversely, viewing objects may prime their associated actions (Castiello, 1996; Humphreys & Riddoch, 2001; Tucker & Ellis, 1998).
Many familiar manufactured objects are associated with multiple actions depending on the actor’s goal (Ansuini et al., 2006; 2008). The vast majority of prior action priming studies have examined prehensile actions such as precision (pinch) and power (clench) grips, which are used to grasp and move objects based on currently available visual information about their structural properties (e.g., shape, size, and orientation; Prabhu, Lemon, & Haggard, 2007). Prehensile actions differ in several respects from a second class of actions, functional use actions, which are strongly linked to object identity (Buxbaum, Veramonti, & Schwartz, 2000) and are associated with activation of conceptual information (Buxbaum & Saffran, 1998). Thus, an object such as a calculator may be associated with at least two responses: a structural “clench” response for grasping and a conceptual “poking” response to skillfully use the calculator and achieve a functional goal. As will be explained below, we conceptualize a calculator as a “conflict” object because it is associated with two conflicting responses.
Given that objects may activate their associated actions, and vice versa, there are a number of unanswered questions concerning interactions with manipulable objects. What factors govern the activation of responses to/by manufactured objects associated with multiple responses? Are all actions associated with an object facilitated during response planning? How long can actions interfere with (or facilitate) object processing? Though clearly relevant to real-life object interactions, these questions have not been studied empirically.
Previous work has shown that “grasp to move” and “skilled use” hand actions (hereafter “grasp” and “use”, respectively) are associated with different activation patterns in neuroimaging paradigms (Buxbaum, Kyle, Tang, & Detre, 2006; Culham & Valyear, 2006; Creem-Regehr & Lee, 2005; Johnson-Frey, 2004) and are disrupted by lesions in different neuroanatomic loci (Buxbaum, Kyle, Grossman, & Coslett, 2007; Buxbaum, Sirigu, Schwartz, & Klatzky, 2003; Sirigu et al., 1996). In response to such data, we (Buxbaum, 2001) and others (Johnson-Frey, 2004; Pisella, Binkofski, Lasek, Toni, & Rossetti, 2006) have proposed two major routes to action which appear to be rooted in dorso-dorsal and ventro-dorsal visual pathways (Rizzolatti & Mattelli, 2003). The first is a bilateral system, localized in part to the superior parietal lobules and intraparietal sulci, that is specialized for object acquisition (grasping and moving). This “Grasp” system encodes current constraints on action imposed by the body and environment, maintains information for milliseconds to seconds, and may operate independent of long-term conceptual information (Cant, Westwood, Valyear, & Goodale, 2005; Garofeanu, Kroliczak, Goodale, & Humphrey, 2004). The second is a left-lateralized system centered on the inferior parietal lobule that is specialized for storage of familiar object-linked actions. This “Use” system subserves conceptual knowledge about functional actions (Buxbaum & Saffran, 1998) and maintains information over longer periods of time.
There is little research on whether activated representations in the Use and Grasp systems may be competitive versus facilitative. Recent work suggests Stroop-like interference occurs between object-related structural or functional actions, on the one hand, and trained actions arbitrarily associated with objects on the other (Bub, Masson, & Cree, 2008). However, direct competition between function- and structure-based responses within the same familiar object has not been explored.
In this study we sought to test several predicted patterns of interference on initiating actions to manipulable objects. We capitalized on the fact that “conflict” objects, such as calculators, are associated with different actions for structural and functional responses (in this case, clench to grasp, poke to use), whereas many other “non-conflict” objects, such as drinking glasses, are associated with one dominant action based on both structure and function (clench to grasp and use). We predicted, first, that as use but not grasp responses require activation of conceptual representations, initiating use movements would take longer than grasp movements. Second, because use activations are slowed by the activation of conceptual representations, we predicted the nature of the within-object interference for conflict objects would depend on the required task. When producing use responses, we predicted participants’ initiation latencies would be greater for conflict than for non-conflict objects because task-irrelevant grasp activations would temporally-precede use activations, allowing them to interfere with use responses. Conversely, when producing grasp responses, we predicted there would be no initiation time differences between conflict and non-conflict objects because task-irrelevant use activations would not be sufficiently activated before grasp responses could be produced. Finally, we predicted different longevities of activated information for grasp versus use. Because priming in the conceptual system can last weeks (Cave, 1997) whereas priming for grasping actions and other dorso-dorsal stream functions lasts only seconds (Jax & Rosenbaum, 2007, 2009), use activations should interfere with actions occurring many minutes later, whereas grasp activations should decay rapidly and have little effect on subsequent actions.
Confirmation of these predictions would suggest that multiple actions associated with single familiar objects may compete for the control of behavior, determined by task goals and by the characteristics of the underlying processing systems.
Method
Participants were fourteen (8 females) right-handed college students. Before the experiment participants were familiarized with the objects (Table 1) by viewing them individually for 2 seconds each. Participants performed two tasks. In the grasp task, they were instructed to reach for and “position your hand on the object as you would to hand it to another person”. In the use task, instructions were to reach for and “position your hand on the object as you would to use it”. Instructions were chosen so that a single movement was made in both tasks without any required subsequent movement (actually moving/using the object). Half of the participants performed four blocks of 22 use trials/block followed by a brief instruction break and then four blocks of 22 grasp trials/block (use-then-grasp order). The remaining participants completed the opposite task order (grasp-then-use order). Within each block, participants interacted with “conflict” objects on 10 trials, “non-conflict” objects on 10 trials, and a red cylinder on 2 no-go trials (included to encourage object identification before movement initiation). Trial types were randomly intermixed.
Table 1.
| Conflict Objects | Non-conflict Objects | |||
|---|---|---|---|---|
| Object | Grasp posture | Use posture | Object | Grasp/Use posture |
| Calculator | clench | poke | Floss | Clench |
| Blender | clench | poke | ice cream scoop | Clench |
| shaving cream | clench | poke | salt shaker | Clench |
| light switch | clench | poke | Baseball | Clench |
| Toaster | clench | poke | dish detergent | Clench |
| pump soap | clench | palm | Flashlight | Clench |
| 3-hole punch | clench | palm | Sponge | Clench |
| Stapler | clench | palm | Glue | Clench |
| Padlock | clench | pinch | Cup | Clench |
| Timer | clench | pinch | Screwdriver | Clench |
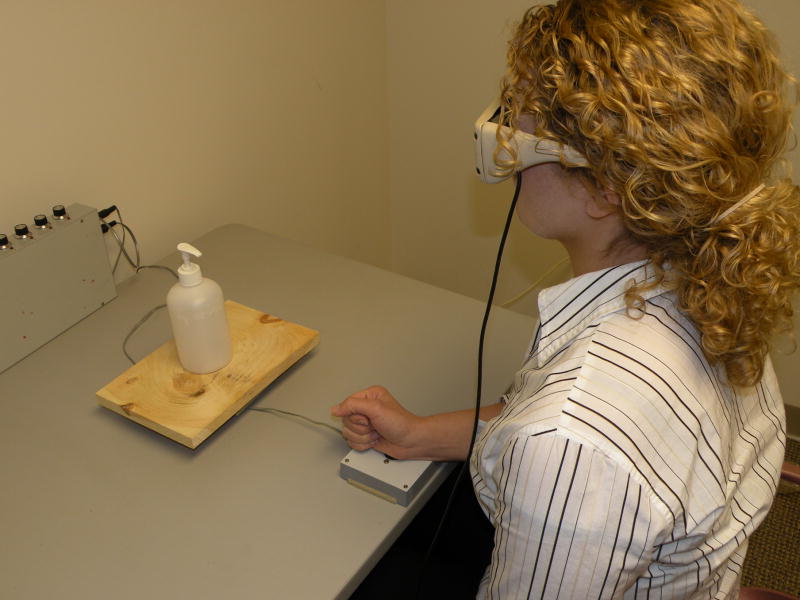
Trials began with the participant holding down a start button with their right hand in a fist with the thumb up (Figure 1), causing LCD goggles (Plato Technologies) to occlude the participant’s vision. The experimenter then placed an object on a platform 20 cm in front of the start button. After a warning tone and random delay (1000–1500 ms) the goggles cleared. The participant then made a movement to grasp or use the object, depending on the block’s instructions, or withheld a response if the no-go object was presented. Because the study employed everyday objects as stimuli, with different movements required to complete the grasp and use movements across objects, analyses focused on initiation latencies (time between goggles opening until hand lift-off), which would not be affected by these variables. Similar use of initiation times can be found in other studies of dorsal stream functioning (Garofeanu et al., 2004; Cant et al., 2005), and is supported by research indicating that neurons in anterior intraparietal cortex (AIP; a dorsal stream structure) are active before movement initiation (Baumann, Fluet, & Scherberger, 2009).
Figure 1.
Experimental setup.
Because different hand postures were often required for the use of conflict (poke, palm) and non-conflict objects (pinch, clench; see Table 1), a control study was completed to insure there were no systematic differences in initiation times for the four hand postures (poke, palm, pinch, clench). Ten participants not in the main study completed the control task using the procedure described above except that objects were replaced by photographs of a right hand in one of the four hand postures. Movements were made to a neutral object affording all four hand postures (3 cm radius ball).
Results
Responses not corresponding to the typical hand postures produced for those objects in pilot testing (2.1% of trials) and trials with initiation times 3 standard deviations or more outside of that participant’s mean for a given block, task, and object type (2.9% of trials) were removed from analysis.
Initiation times in the control task were analyzed using a single-factor (Posture: clench, pinch, palm, poke) ANOVA. After confirming sphericity (Mauchly’s W = .58, p = .523), no effect of Posture was found (F(3,27) = .29, p = .83). Mean initiation times (and s.e.) for the clench, pinch, palm, and poke hand postures were 475.61 (22.18), 479.50 (24.12), 481.61 (24.50), and 478.45 (19.81), respectively.
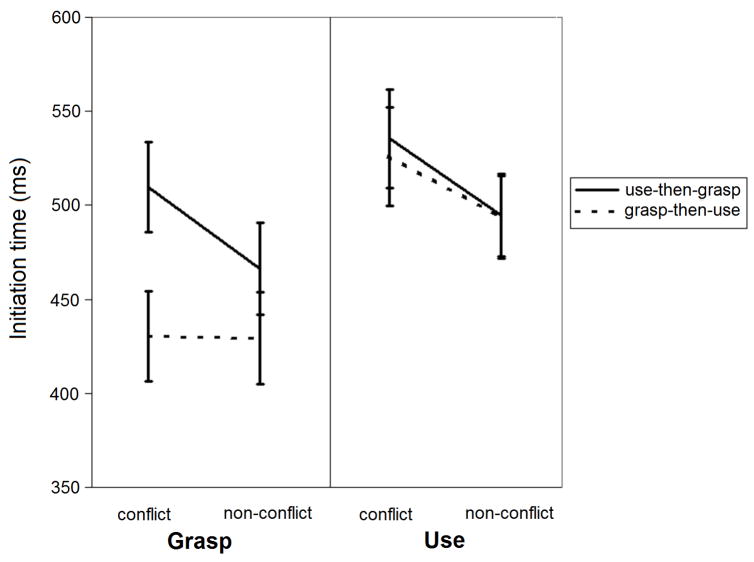
Mean initiation time results for the grasp and use tasks (Figure 2) were analyzed with a 2 (Task: grasp, use) × 2 (Object: conflict, non-conflict) × 2 (Order: use-then-grasp, grasp-then-use) ANOVA. The results indicated main effects of Task (Use > Grasp; F(1,12) = 16.51, p = .002) and Object1 (Conflict > Non-conflict; F(1,12) = 54.58, p < .001) as well as interactions between Task and Object, F(1,12) = 6.44, p = .026, between Object and Order, F(1,12) = 10.29, p = .008, and, most importantly, a three-way interaction between Task, Object, and Order, F(1,12) = 8.98, p = .011. The three-way interaction was explored by examining the interaction between Object and Order for each Task (i.e., separately for the two sides of Figure 2). In the use task (right side of Figure 2), only the main effect of Object was significant (p < .001). In the grasp task (left side of Figure 2), there were main effects of Object and Order (p < .001) and an interaction between Object and Order (p < .001), with the effect of Object being significant in the use-then-grasp order (p < .001) but not in the grasp-then-use order (p = .88). Thus, interference in the grasp task was significantly greater if the same object had been used earlier in the experiment, a pattern we termed “long-term use-on-grasp interference”.
Figure 2.
Mean initiation times (±1 SE) for the grasp and use tasks.
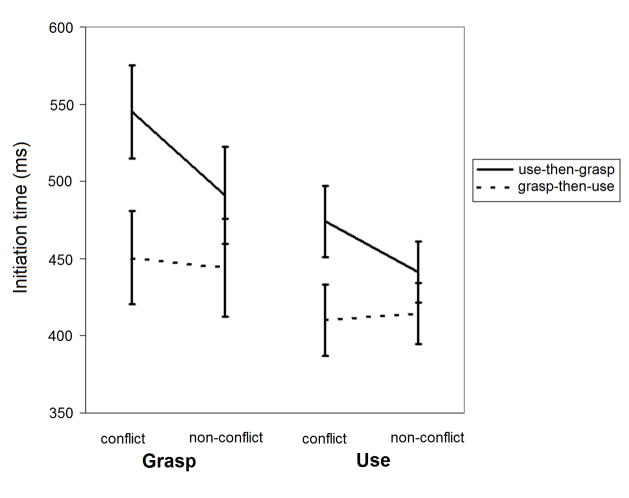
To explore the duration of use-on-grasp interference (Figure 3), we used separate 2 (Object: conflict, non-conflict) × 2 (Order: use-then-grasp, grasp-then-use) ANOVAs for grasp trials in the early (blocks 1–2) and late (blocks 3–4) phases. Interactions between Object and Order were observed during the early (p = .001; left side of Figure 3) and late (p = .012; right side of Figure 3) phases. In these interactions the effect of Object was significant for the use-then-grasp order of both phases (solid lines in Figure 3; p < .001 and p = .004 for early and late, respectively), a finding not observed in the grasp-then-use order (dashed lines in Figure 3; p = .456 and p = .686 for early and late, respectively). These analyses confirm that use-on-grasp interference remained well after the change in tasks occurred.
Figure 3.
Mean initiation times (±1 SE) comparing early and late phases of the grasp task.
Discussion
This study extends prior findings that intending to act upon objects affects object processing by demonstrating that both task-relevant and task-irrelevant attributes of a single familiar object may compete for the control of action. Two forms of interference were observed as a function of the type of action prepared, which we will discuss in turn.
First, latencies to initiate use movements to objects associated with different grasp actions were longer than to objects associated with the same grasp actions. For example, initiating movement to use a calculator with a “poking” action was slowed by the task-irrelevant activation of the “clench” action required to grasp the calculator. This within-object grasp-on-use interference occurred regardless of whether or not the to-be-used object was recently grasped. The absence of task order effects indicates that while an interfering grasp response is activated in a task-irrelevant manner, it remains active only briefly. Although evidence for grasp-on-use interference required comparing different sets of objects, data from the grasp task can rule out confounds emanating from visual and semantic processing-stage differences (e.g., perception; recognition). Any differences at these stages would be predicted to influence both the grasp and use tasks, although no effect of Object was observed in the grasp task of the grasp-then-use order. Similarly, the control task ruled out the possibility that differences in hand postures required to use conflict and non-conflict objects could explain the difference in initiation times between the two object types in the use task.
The second form of interference, long-term use-on-grasp interference, occurred when participants produced grasp responses, and resulted from experience gained at least several minutes earlier. Differences in initiation of grasp responses for conflict and non-conflict objects were only observed when those objects had recently been used. Thus, for the identical task with the same exact objects, previous use experience clearly interfered with participant’s ability to grasp conflict objects. This use-on-grasp interference lasted approximately 20 minutes, the time it took to complete all grasp blocks. The long-lasting nature of this interference effect, along with the observation that no order effect was observed when switching from grasp to use, suggests that it cannot be explained by general task switching difficulties (Monsell, 2003).
We propose that the intention to act on an object triggers a race-like competition between functional and structural responses during action selection. Only functional responses require activation of long-term conceptual representations; thus, structural responses can be activated more quickly than functional responses. This difference in activation timing explains why grasp responses requires less time to initiate than use responses. In addition, the proposal that grasp responses “win the race” with use responses, and can thus interfere with the production of use movements, explains why conflict effects are observed asymmetrically when grasp is performed before use.
On the race model, there are at least two possible explanations for use-on-grasp interference. First, prior object-specific information may remain active after object use. For example, lingering activation from making a “poke” response to use a calculator may cause interference later when a “clench” response is required to grasp that same calculator. Second, use-on-grasp interference could be attributable to task-level, rather than object-specific, interference. That is, repeatedly using objects may bias the motor system to activate use responses when viewing objects, even if those particular objects were not recently used. Similar biasing effects leading to privileged processing in one stream over another as a function of recent experience has been reported in other motor (Tessari & Rumiati, 2004) and cognitive domains (Cohen, Dunbar, & McClelland, 1990). Because participants always interacted with the same objects during use and grasp blocks, additional studies will be required to adjudicate between the two explanations.
In keeping with both neuroscientific (Desimone and Duncan, 1995; Duncan, Humphreys, and Ward, 1997) and behavioral (Tipper, Howard, and Jackson, 1997) theories of action selection, the present account accords a central role to feature-based competition. Unlike other models of selection for action focusing on multi-object arrays (Ward, 1999), competing features influencing grasp-on-use interference are located within single objects. Others have proposed that multiple features of the same object may be processed in parallel without interference (Duncan, 1984), suggesting that attention can operate in object-based frames. However, when one feature has greater behavioral relevance than another, feature-based selection may accelerate reaction times associated with the relevant features at the cost of processing less-relevant features more slowly (Wegener et al., 2008). Such response time disparities may result from active inhibition of irrelevant object features (Fanini, Nobre, & Chelazzi 2006; Nobre, Rao, & Chelazzi 2006).
A widely distributed and integrated network of brain regions in frontal, temporal, and parietal cortex are likely involved in resolving attentional competition, whether in object-based or feature-based frames (see Duncan, 2006). Posterior brain regions such as the lateral occipital area have been implicated in the spread of attention in object-based reference frames (He et al., 2008; de-Wit, Kentridge, & Milner, 2009), and the dorsolateral prefrontal cortex may play a role in the top-down modulation of object-based attention driven by behavioral relevance (Sinnett, Snyder, & Kingstone, 2009).
In conclusion, these data support prior claims that preparing object-direction actions may activate task-irrelevant motor responses (Castiello, 1996; Humphreys & Riddoch, 2001; Tucker & Ellis, 1998; Rafal, Ward, & Danziger, 2006). To our knowledge, we are the first to examine action selection of multiple responses linked to the same familiar object, a situation with clear relevance to everyday life. Similarly, because participants interacted with actual 3-dimensional objects and initiated responses based on non-arbitrary mappings, our methods were more naturalistic than other studies (Tucker & Ellis, 1998; Bub, Masson, & Cree, 2008). Finally, elucidation of the processing characteristics of structural and functional activations associated with objects creates a bridge to the substantial literature on priming of conceptual representations (e.g., Grill-Spector, Henson, & Martin, 2006) and encourages synergy between the study of action selection and object representations.
Acknowledgments
The work was supported by NIH grants R01-NS036387 and T32-HD007425.
Footnotes
Initiation time differences between conflict and non-conflict objects could not be explained by differences in required movement distance (distance between the start button and typical object contact point), as these two variables were not correlated (r = .088, p = .60).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ansuini C, Giosa L, Turella L, Altoè G, Castiello U. An object for an action, the same object for other actions: effects on hand shaping. Experimental Brain Research. 2008;185(1):111–119. doi: 10.1007/s00221-007-1136-4. [DOI] [PubMed] [Google Scholar]
- Ansuini C, Santello M, Massaccesi S, Castiello U. Effects of end-goal on hand shaping. Journal of Neurophysiology. 2006;95(4):2456–2465. doi: 10.1152/jn.01107.2005. [DOI] [PubMed] [Google Scholar]
- Baumann MA, Fluet M, Scherberger H. Context-specific grasp movement representation in the macaque anterior intraparietal area. Journal of Neuroscience. 2009;29(20):6436–6448. doi: 10.1523/JNEUROSCI.5479-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Buxbaum LJ, Bylsma L, Jax SA. Toward an integrated account of object and action selection: A computational analysis and empirical findings from reaching-to-grasp and tool use. Neuropsychologia. 2009;47(2):671–683. doi: 10.1016/j.neuropsychologia.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bub DN, Masson ME, Cree GS. Evocation of functional and volumetric gestural knowledge by objects and words. Cognition. 2008;106(1):27–58. doi: 10.1016/j.cognition.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ. Ideomotor apraxia: A call to action. Neurocase. 2001;7:445–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle K, Grossman M, Coslett HB. Left inferior parietal representations for skilled hand-object interactions: Evidence from stroke and corticobasal degeneration. Cortex. 2007;43(3):411–423. doi: 10.1016/s0010-9452(08)70466-0. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Tang K, Detre JM. Neural substrates of knowledge of hand postures for object grasping and functional object use: Evidence from fMRI. Brain Research. 2006;1117(1):175–185. doi: 10.1016/j.brainres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Saffran EM. Knowing “how” vs. “what for”: A new dissociation. Brain and Language. 1998;65:73–86. [Google Scholar]
- Buxbaum LJ, Sirigu A, Schwartz MF, Klatzky RL. Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia. 2003;41(8):1091–1113. doi: 10.1016/s0028-3932(02)00314-7. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Veramonti T, Schwartz MF. Function and manipulation tool knowledge in apraxia: Knowing ‘What for’ but not ‘how’. Neurocase. 2000;6:83–97. [Google Scholar]
- Cave BC. Very long-lasting priming in picture naming. Psychological Science. 1997;8:322–325. [Google Scholar]
- Cant JS, Westwood DA, Valyear KF, Goodale MA. No evidence for visuomotor priming in a visually guided action task. Neuropsychologia. 2005;43:216–226. doi: 10.1016/j.neuropsychologia.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychological Review. 1990;97(3):332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Current Opinion in Neurobiology. 2006;16(2):205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Castiello U. Grasping a fruit: selection for action. Journal of Experimental Psychology: Human Perception and Performance. 1996;22(3):582–603. doi: 10.1037//0096-1523.22.3.582. [DOI] [PubMed] [Google Scholar]
- Craighero L, Fadiga L, Umiltá CA, Rizzolatti G. Evidence for visuomotor priming effect. Neuroreport. 1996;8:347–349. doi: 10.1097/00001756-199612200-00068. [DOI] [PubMed] [Google Scholar]
- Creem-Regehr S, Lee J. Neural representations of graspable objects: are tools special. Cognitive Brain Research. 2005;22(3):457–469. doi: 10.1016/j.cogbrainres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- de-Wit LH, Kentridge RW, Milner AD. Object-based attention and visual area LO. Neuropsychologia. 2009;47(6):1483–1490. doi: 10.1016/j.neuropsychologia.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Duncan J. Selective attention and the organization of visual information. Journal of Experimental Psychology: General. 1984;113(4):501–517. doi: 10.1037//0096-3445.113.4.501. [DOI] [PubMed] [Google Scholar]
- Duncan J. EPS Mid-Career Award 2004: brain mechanisms of attention. Quarterly Journal of Experimental Psychology (Colchester) 2006;59(1):2–27. doi: 10.1080/17470210500260674. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys G, Ward R. Competitive brain activity in visual attention. Current Opinion in Neurobiology. 1997;7(2):255–261. doi: 10.1016/s0959-4388(97)80014-1. [DOI] [PubMed] [Google Scholar]
- Fanini A, Nobre AC, Chelazzi L. Selecting and ignoring the component features of a visual object. A negative priming paradigm Visual Cognition. 2006;14:584–618. [Google Scholar]
- Garofeanu C, Kroliczak G, Goodale MA, Humphrey GK. Naming and grasping common objects: A priming study. Experimental Brain Research. 2004;159:55–64. doi: 10.1007/s00221-004-1932-z. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Science. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- He X, Humphreys G, Fan S, Chen L, Han S. Differentiating spatial and object-based effects on attention: an event-related brain potential study with peripheral cueing. Brain Research. 2008;1245:116–125. doi: 10.1016/j.brainres.2008.09.092. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Riddoch MJ. Detection by action: Neuropsychological evidence for action-defined templates in search. Nature. 2001;4:84–88. doi: 10.1038/82940. [DOI] [PubMed] [Google Scholar]
- Jax SA, Rosenbaum DA. Hand path priming in manual obstacle avoidance: Evidence that the dorsal stream does not only control visually guided actions in real time. Journal of Experimental Psychology: Human Perception and Performance. 2007;33:425–441. doi: 10.1037/0096-1523.33.2.425. [DOI] [PubMed] [Google Scholar]
- Jax SA, Rosenbaum DA. Hand path priming in manual obstacle avoidance: Rapid decay of dorsal stream information. Neuropsychologia. 2009;47:1573–1577. doi: 10.1016/j.neuropsychologia.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH. The neural bases of complex tool use in humans. Trends in Cognitive Sciences. 2004;8(2):71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Sciences. 2003;7(3):134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Rao A, Chelazzi L. Selective attention to specific features within objects: behavioral and electrophysiological evidence. Journal of Cognitive Neuroscience. 2006;18(4):539–61. doi: 10.1162/jocn.2006.18.4.539. [DOI] [PubMed] [Google Scholar]
- Pavese A, Buxbaum L. Action matters: The role of action plans and object affordances in selection for action. Visual Cognition. 2002;9(45):559–590. [Google Scholar]
- Pisella L, Binkofski F, Lasek K, Toni I, Rossetti Y. No double-dissociation between optic ataxia and visual agnosia: multiple sub-streams for multiple visuo-manual integrations. Neuropsychologia. 2006;44(13):2734–2748. doi: 10.1016/j.neuropsychologia.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Prabhu G, Lemon R, Haggard P. On-line control of grasping actions: object-specific motor facilitation requires sustained visual input. Journal of Neuroscience. 2007;27(46):12651–12654. doi: 10.1523/JNEUROSCI.4308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafal RD, Ward R, Danziger S. Selection for action and selection for awareness: Evidence from hemispatial neglect. Brain Research. 2006;1080(1):2–8. doi: 10.1016/j.brainres.2005.01.108. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Experimental Brain Research. 2003;153(2):146–157. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- Sinnett S, Snyder JJ, Kinstone A. Role of the lateral prefrontal cortex in visual object-based selective attention. Experimental Brain Research. 2009;194(2):191–196. doi: 10.1007/s00221-008-1687-z. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273:1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- Tessari A, Rumiati RI. The strategic control of multiple routes in imitation of actions. Journal of Experimental Psychology: Human Perception and Performance. 2004;30(6):1107–1116. doi: 10.1037/0096-1523.30.6.1107. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Howard LA, Jackson SR. Selective reaching to grasp: Evidence for distractor interference effects. Visual Cognition. 1997;4:1–38. [Google Scholar]
- Tucker M, Ellis R. On the relations between seen objects and components of potential actions. Journal of Experimental Psychology: Human Perception and Performance. 1998;24(3):830–846. doi: 10.1037//0096-1523.24.3.830. [DOI] [PubMed] [Google Scholar]
- Ward R. Interactions between perception and action systems: A model for selective attention. In: Humphreys GW, Duncan J, Treisman A, editors. Attention, space, and action: Studies in cognitive neuroscience. Oxford: Oxford University Press; 1999. pp. 311–332. [Google Scholar]
- Wegener D, Ehn F, Aurich MK, Galashan FO, Kreiter AK. Feature-based attention and the suppression of non-relevant object features. Vision Research. 2008;48(27):2696–2707. doi: 10.1016/j.visres.2008.08.021. [DOI] [PubMed] [Google Scholar]