Abstract
Speech comprehension remains largely preserved in older adults despite significant age-related neurophysiological change. However, older adults’ performance declines more rapidly than that of young adults when listening conditions are challenging. We investigated the cortical network underlying speech comprehension in healthy aging using short sentences differing in syntactic complexity, with processing demands further manipulated through speech rate. Neural activity was monitored using blood oxygen level–dependent functional magnetic resonance imaging. Comprehension of syntactically complex sentences activated components of a core sentence-processing network in both young and older adults, including the left inferior and middle frontal gyri, left inferior parietal cortex, and left middle temporal gyrus. However, older adults showed reduced recruitment of inferior frontal regions relative to young adults; the individual degree of recruitment predicted accuracy at the more difficult fast speech rate. Older adults also showed increased activity in frontal regions outside the core sentence-processing network, which may have played a compensatory role. Finally, a functional connectivity analysis demonstrated reduced coherence between activated regions in older adults. We conclude that decreased activation of specialized processing regions, and limited ability to coordinate activity between regions, contribute to older adults’ difficulty with sentence comprehension under difficult listening conditions.
Keywords: cognitive aging, fMRI, language, sentence processing, speech
Introduction
Successful language comprehension relies on the recruitment of cortical centers to support specific linguistic processes, and the organization of these regions into effective networks. Considering these 2 facets is especially interesting with regard to adult aging, as age-related neuroanatomical changes would be expected to impact the efficacy of both components. However, older adults’ language processing is generally quite good, despite age-related neurophysiological and cognitive changes, with accuracy declines typically becoming apparent only in situations presenting increased perceptual or cognitive challenge (Wingfield and Stine-Morrow 2000). A central question, then, is how older adults are able to maintain such good performance despite significant changes in cortical anatomy and related changes in cognitive ability. We see this as a specific instance of a fundamental issue in neurobiology: namely, how stable behavior can be produced despite wide variability in underlying neural parameters (Prinz et al. 2004).
To address these issues we examined how young and older adult listeners process short, syntactically complex sentences. We chose these stimuli primarily because of their robust cognitive effects: Regardless of the specific manipulation used, sentences with complex syntactic constructions reliably result in more comprehension errors and longer processing times compared with syntactically simpler sentences (Just and Carpenter 1992; Ferreira et al. 1996; Vos et al. 2001; Waters and Caplan 2004). In addition, due to age-related cognitive decline in cognitive abilities such as working memory and information processing speed, older adults are often reported to demonstrate difficulty processing syntactically complex sentence structures on a wide variety of tasks (Kemper 1986, 1987; Obler et al. 1991; Kemper et al. 2001). A syntactic manipulation thus allows us to test a restricted set of cognitive operations that are known to be differentially affected in adult aging.
In a previous behavioral study using 2 levels of syntactic complexity, we have shown that older adults’ difficulty with syntactically complex sentences is exacerbated when processing challenge is increased by presenting sentences at a rapid rate of speech (Wingfield et al. 2003). In the current study we employ this same manipulation, enabling us to examine neural activity supporting sentence comprehension in older adults both when they are generally successful (at slower speech rates) and when the task becomes more difficult (at faster speech rates).
Prior neuroimaging studies of sentence processing, using both written and spoken material, have delineated a core sentence-processing network that appears to be involved in processing many types of syntactically complex sentences (Caplan et al. 1999; Ni et al. 2000; Peelle et al. 2004; Fiebach et al. 2005). These regions reflect a combination of syntactic parsing, verbal working memory, and semantic integration. One region commonly associated with syntactic processing is the left inferior frontal gyrus (IFG), although the precise location appears to depend on particular task requirements. In many of these same studies verbal working memory requirements appear to be supported by dorsolateral prefrontal cortex, including dorsal IFG and portions of middle frontal gyrus (MFG), as well as inferior parietal cortex. Finally, increased lexical–semantic processing is reflected by activity in posterior middle temporal gyrus (MTG). We thus expect listeners, regardless of age, to rely on these components for successful comprehension (Grossman et al. 2002).
Although we anticipate older adults’ sentence processing to be supported largely by these core regions, given that older adults find comprehending syntactically complex sentences more difficult than young adults we also expect some differences in the underlying patterns of neural recruitment. A straightforward prediction is that there would be regions within the core sentence comprehension network that older adults are less able to recruit than young adults, leading to decreased efficiency of sentence processing. In fact, a common finding in the neuroimaging literature is that older adults make less use of specialized brain regions than young adults (Park et al. 2004; Gutchess et al. 2005; Duarte et al. 2008). However, this decrease in use of specialized regions is often accompanied by the recruitment of additional brain regions not observed in young adults; such extra activation is often interpreted as playing a compensatory role in older adults’ performance (Cabeza 2002; Wingfield and Grossman 2006). One possibility is that older adults recruit some areas to a greater degree than young adults to compensate for under-recruitment of more specialized regions. In the context of sentence processing, this may involve the recruitment of more general purpose working memory regions.
As noted above, older adults’ processing of syntactically complex sentences is generally not as effective as young adults’, suggesting that, if present, compensatory activity may not be sufficient to overcome age-related limitations. One possibility is that rather than being truly compensatory, increases in activation may reflect an inappropriate strategy on the part of older adults, or may reflect a loss of specialization of cortical processing regions (Park et al. 2004). A second possibility is that older adults may effectively recruit additional brain areas in service of normal speech comprehension, but that these regions are not able to effectively compensate when processing challenge is increased. This would fit well with previous studies showing exaggerated age effects when stimuli are more challenging.
A third possible explanation relates to a reduction in necessary coordination between brain regions. Although studies of neural connectivity are not common in older adults, several recent investigations have found that older adults may show different patterns of functional connectivity than young adults (Grady et al. 2003; Daselaar et al. 2006). Andrews-Hanna et al. (2007) examined correlations between portions of the “default” network in older adults, and found significant decreases in correlated activity relative to young adults. Furthermore, individual variability in the strength of these correlations between medial prefrontal cortex and posterior cingulate predicted performance on behavioral measures of executive function, memory, and processing speed. These results suggest that differences in neural coherence may render older adults less efficient at tasks that require coordination of multiple brain systems.
In the current experiment, young and older adults heard spoken sentences with 2 degrees of syntactic complexity. We used time compression to present these sentences at 3 different speech rates in order to further manipulate processing challenge. Our primary interest was to determine the potential relationship between comprehension success and neural activity in the older adults relative to the young adults.
Method
Participants
The older adults were 20 community-dwelling volunteers (11 females) who ranged in age from 60 to 77 years (M = 64.8, SD = 4.5). The group had a mean of 15.5 years of formal education (SD = 2.3), and a mean Shipley vocabulary score (Zachary 1986) typical of well-educated adults of 15.3 (SD = 1.9), out of a maximum possible score of 20. Twenty young adult participants (11 female) were recruited from the surrounding community and ranged in age from 19 to 27 years (M = 22.4, SD = 2.6). The young adult participants had a mean of 15.0 years of formal education at time of testing (SD = 1.8) and a mean Shipley vocabulary score of 15.1 (SD = 2.0). (A vocabulary score was not obtained for one young adult participant.) Both groups were thus well educated and well matched for vocabulary score. All participants reported themselves to be right-handed native speakers of American English and in good health, with no history of neurological disorder. None were taking psychoactive medication. The older adults had good hearing for their ages. Sixteen older adults’ hearing was tested using pure tone audiometry; of these, 13 had pure tone averages (average thresholds at 1, 2, and 4 kHz) of ≤25 dB HL in their better ear, with 3 being only slightly higher. The remaining older adult participants, and all young adults, had hearing ≤25 dB HL in both ears, assessed using an automated screening procedure (Reilly et al. 2007). Participants were paid for their participation. Informed consent was obtained from all participants according to a protocol approved by the University of Pennsylvania Institutional Review Board.
All of the 20 older adults whose data we report scored above 60% on the object-relative sentences at the slowest speech rate. Four additional older adult participants performed below 60% accuracy on the object-relative sentences at the slowest speech rate. Postexperiment interviews indicated this was due to a lack of understanding regarding the task; these participants were replaced and not analyzed.
Stimuli
The stimuli were based on 60 meaningful 6-word sentences containing a center-embedded subject-relative clause. We used short sentences in part to minimize working memory demands that might be associated with longer constructions. From each of these sentences we constructed another sentence that had the same words and characters as the original, but in which the meaning was expressed using an object-relative clause structure. Two sets of sentences were constructed for each subject-relative and object-relative clause sentence that had the same words and structures but with one having a male character (e.g., king, brother) and one having a female character (e.g., queen, sister) performing the action. A variety of verbs were used. This procedure resulted in a total of 240 sentences, 60 of each of the following types:
Subject-relative clause, male agent: “Men that assist women are helpful.”
Object-relative clause, male agent: “Women that men assist are helpful.”
Subject-relative clause, female agent: “Women that assist men are helpful.”
Object-relative clause, female agent: “Men that women assist are helpful.”
Using this procedure, 120 sentences with a subject-relative clause structure and 120 with an object-relative clause structure were created, with equal numbers having a male or a female character as the agent of the action. Although both types of sentences feature embedded clauses and are therefore not “simple” sentences for listeners to comprehend, object-relative constructions require greater syntactic processing and are empirically more difficult to understand. Between the 4 versions of each sentence, the only difference was word order; lexical information was therefore equated across conditions.
All sentences were recorded by a female speaker of American English at a fast-normal speech rate of approximately 205 words per minute (wpm). These sentences were then time-compressed to 80%, 65%, and 50% of original speaking time, corresponding to 258, 321, and 410 wpm, respectively. Time compression was performed using the pitch-synchronous overlap and add (PSOLA) technique (Moulines and Charpentier 1990) as implemented in Praat software (Institute of Phonetic Sciences, University of Amsterdam, Amsterdam, The Netherlands, available from www.praat.org). We compressed the speech signal uniformly; that is, silence and sound were shortened by equal amounts, maintaining relative durations of speech information. The pitch remained unchanged. The PSOLA method largely preserves transient information such as formant transitions that are important for speech comprehension.
Stimuli for our control condition were composed of sentences lowpass filtered at 250 Hz. Half of these were spoken by the same speaker as the main study, and half by a male speaker. The sentences were completely unintelligible, but remaining pitch information allowed identification of talker sex. These control stimuli thus controlled for many low level acoustic features while providing no lexical or semantic information. Although this condition was available as an acoustic control condition, we focused our analyses on differences between sentence types, and thus the data in this condition were not required for any analysis.
Procedure
Each participant heard all 240 sentences evenly divided between subject-relative and object-relative sentences. Eighty sentences were presented at each of the 3 speech rates, equally divided between sentence type. Half of each sentence type had a male agent and half had a female agent. For each participant half of the sentences were presented in an ascending order of speech rates: 40 sentences at 258 wpm, 40 sentences at 321 wpm, and 40 sentences at 410 wpm. When this sequence was completed, the remaining sentences were presented in reverse order, going from the fastest speech rate to the slowest speech rate. This was done to minimize task switching demands and perceptual normalization processes that would be caused by randomizing speech rates. Each block of sentences at a particular speech rate corresponded to one continuous block of scanning (imaging run). The particular sentences heard at each speech rate were counterbalanced across participants. Due to technical difficulties data were unavailable for at least one scanning run for 2 young and 2 older participants. However, these participants still had data in all conditions.
Following each sentence, participants were instructed to press 1 of 2 keys to indicate whether the character performing the action was a male or female. For the control condition, participants indicated whether the sex of the speaker was male or female. Accuracy and response time were recorded for each trial. Stimuli were presented binaurally over earphones at a comfortable listening level that was maintained across all conditions of the experiment. E-Prime 1.0 (Psychology Software Tools, Inc., Pittsburgh, PA) was used to present stimuli and record response times.
The main experiment was preceded by a familiarization session to ensure that the instructions were understood, and to familiarize participants with the sound of time-compressed speech so as to minimize any effect of perceptual learning during the main experiment (Peelle and Wingfield 2005). This session, conducted outside the scanner just prior to the main experiment, consisted of 16 sentences that included both subject-relative and object-relative clause sentences presented at the various speech rates used in the main experiment. These sentences were not used in the main experiment.
Image Acquisition and Analysis
Functional magnetic resonance imaging (MRI) data were acquired on a Siemens Trio scanner (Siemens Medical Systems, Erlangen, Germany) at 3 T, beginning with acquisition of a T1-weighted structural volume using a magnetization prepared rapid acquisition gradient echo protocol (repetition time [TR] = 1620 ms, echo time [TE] = 3 ms, flip angle = 15°, 1-mm slice thickness, 192 × 256 matrix, resolution = 0.98 × 0.98 × 1 mm). Blood oxygenation level–dependent functional MRI images were acquired with fat saturation, 3-mm isotropic voxels, flip angle of 15°, TR = 8 s, acquisition time (TA) = 3 s, TEeff = 30 ms, and a 64 × 64 matrix. We used a sparse imaging design in which the TR was longer than the TA to allow presentation of sentences in the absence of echoplanar scanner noise (Edmister et al. 1999; Hall et al. 1999).
Image processing and statistical analyses were performed using SPM5 software (Wellcome Trust Centre for Functional Neuroimaging, London, UK). Analysis of imaging data was conducted in an event-related manner and restricted to descriptions that resulted in a correct response by the participant. Data were initially analyzed separately for each participant. The first 4 volumes of each scanning run were discarded to allow for equilibration effects. Low-frequency drifts were removed with high-pass filtering with a cut-off period of 128 s and autocorrelations were modeled using a first-order autoregressive model. Images for each participant were realigned to the first image in the series (Friston et al. 1995) and coregistered to the structural image (Ashburner and Friston 1997). The transformation required to bring a participant's images into standard Montreal Neurological Institute (MNI) space were calculated using tissue probability maps (Ashburner and Friston 2005), and these warping parameters were then applied to all functional images for that participant. The data were spatially smoothed with a 10-mm full-width half maximum isotropic Gaussian kernel prior to model estimation. Each event onset was convolved with a canonical hemodynamic response function to arrive at a predicted neural response. Additional regressors were included to account for scanning run effects. (Note that because we presented only a single speech rate for each imaging run, it would be inappropriate to analyze the imaging data for main effects of speech rate, as these were confounded by session effects.)
Unless otherwise specified all comparisons were done using a voxel-wise threshold of P < 0.001, corrected for whole-brain set-level significance using random field theory at P < 0.05 (Worsley et al. 1992). After thresholding, only clusters exceeding an extent of 15 voxels were further considered for interpretation. Cluster coordinates are reported in the space of the MNI152 average brain template.
Results
Behavioral Data
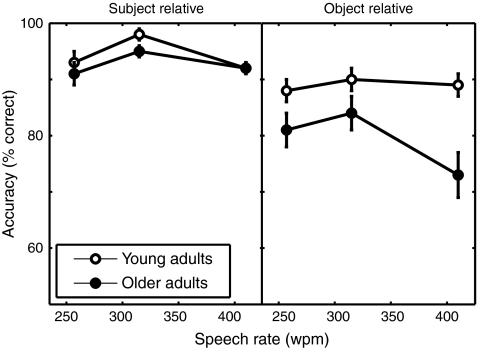
We first examined the accuracy data, plotted in Figure 1, as a function of the experimental conditions. Inspection of these data suggests that older adults performed comparably to the young adults for the easier subject-relative sentences, but were differentially affected by the more difficult object-relative sentences, with this age effect exaggerated at the fastest speech rate. To evaluate these impressions we analyzed the data using a 2 (Syntax) × 3 (Speech Rate) × 2 (Age) mixed design analysis of variance (ANOVA). There was a main effect of Syntax, reflecting the extra difficulty of the object-relative sentences, F1,38 = 45.47, MSE = 0.011, P < 0.001 (subject-relative: M = 0.935 proportion correct [SE = 0.007]; object-relative M = 0.843 proportion correct [SE = 0.016]). A main effect of Speech rate reflected the slight increase at the middle speech rate and overall difficulty of the faster sentences, F2,76 = 12.79, MSE = 0.004, P < 0.001 (slower: M = 0.883 proportion correct [SE = 0.011]; medium: M = 0.918 proportion correct [SE = 0.011]; fast: M = 0.867 proportion correct [SE = 0.014]). Older adults’ worse performance overall was supported by a main effect of Age, F1,28 = 6.60, MSE = 0.027, P < 0.05 (young: M = 0.917 proportion correct [SE = 0.015]; older: M = 0.862 proportion correct [SE = 0.015]). Most importantly, we found a significant Syntax × Speech rate × Age interaction, F2,76 = 6.50, MSE = 0.003, P < 0.01. These results are consistent with our previous study showing that older adults’ performance is differentially affected by complex syntax relative to young adults, and further impacted by the increased processing challenges associated with a faster presentation rate (Wingfield et al. 2003).
Figure 1.
Mean (±SE) accuracy data for the sentence comprehension task in the scanner. The left panel shows accuracy for young and older adults for subject-relative sentences, the right panel for the more syntactically complex object-relative sentences. For each sentence type, accuracy is plotted as a function of speech rate.
Effects of Syntactic Complexity and Age on Neural Activation
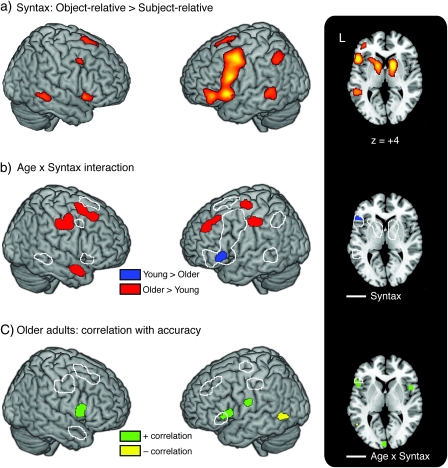
In all imaging analyses, only trials resulting in correct responses were entered into our model. We first identified regions that showed an increased response to syntactically complex sentences—a core sentence-processing network—collapsed across speech rate and age group. Results from this analysis are shown in Figure 2a, with maxima listed in Table 1. Regions showing an increased response for more complex sentences across both age groups are largely consistent with previous studies and included bilateral ventral IFG/anterior insula, bilateral MFG, bilateral MTG, and left inferior parietal lobe. As is evident from the axial slice in Figure 2a, the large frontal clusters also extended subcortically to encompass bilateral caudate and putamen, and extended into right inferior frontal regions. Precuneus, anterior cingulate, medial superior frontal gyrus (SFG), and supplemental motor area (SMA) also demonstrated increased activity for the more complex object-relative sentences. This core sentence-processing network is largely congruent with previous studies.
Figure 2.
Brain areas showing responses related to processing syntactically complex sentences. (a) Increases in neural activity associated with syntactic complexity (object-relative sentences > subject-relative sentences) collapsed across Age and Speech Rate reveals a core sentence-processing network. (b) Age × Syntax interaction. Regions in which young adults show a greater syntax-related response are in blue, and those in which older adults show a greater syntax-related response in red. White outline indicates the regions showing syntax-related increases collapsed across age. (c) Correlation between syntax-related activity at the fast speech rate and accuracy at this rate in the older adults. Green regions indicate a positive correlation, yellow regions a negative correlation. White outline illustrates regions that showed a significant Age × Syntax interaction.
Table 1.
Regions and peak coordinates showing an effect of syntax (object-relative > subject-relative)
| Region | Coordinates |
Z score | ||
| x | y | z | ||
| L anterior insulaa | −30 | 22 | −6 | 5.47 |
| R anterior insulaa | 32 | 18 | −6 | 5.24 |
| L dorsal MFG/precentral gyrusa | −42 | 2 | 46 | 4.91 |
| L dorsal IFGa | −50 | 14 | 24 | 4.85 |
| L ventral IFG | −42 | 40 | −6 | 4.51 |
| R pallidum | 14 | 8 | 0 | 4.91 |
| L pallidum | −14 | 6 | 4 | 4.78 |
| Medial SFG/anterior cingulatea | −4 | 28 | 38 | 4.19 |
| SMA | −4 | 6 | 64 | 3.86 |
| L posterior MTGa | −56 | −40 | 0 | 3.83 |
| Precuneousa | −4 | −64 | 38 | 3.81 |
| R posterior MTGa | 60 | −38 | −4 | 3.77 |
| L inferior parietala | −48 | −54 | 48 | 3.57 |
| R dorsal MFGa | 44 | 10 | 46 | 3.47 |
Indicates maximum was included in network analysis.
In addition to examining the main effect of syntax, we investigated the Syntax × Speech Rate interaction. This analysis failed to reveal any significant voxels, nor was there a significant Syntax × Speech Rate × Age interaction, indicating that syntax-related neural responses were largely consistent across the different speech rates for all participants.
We next turn to regions where young and older adults showed different patterns of syntax-related activation, collapsed across speech rate, by looking for Syntax × Age interactions (i.e., where the object-relative > subject-relative contrast differed as a function of age). The results of this analysis are shown in Figure 2b, with maxima listed in Table 2. For comparison purposes, the regions showing a syntax-related increase (from Fig. 2a) are outlined in white. After selecting significant voxels based on the F test, we classified voxels as showing a young > older response (blue) or an older > young response (red) based on the sign of the parameter estimate. The only region in which young adults showed significantly more syntax-related increases than older adults was in the left IFG/anterior insula, a region appearing in the main effect of syntax that we consider part of a core sentence-processing network. The areas in which older adults showed greater syntax-related activity than young adults fell outside the core sentence-processing network. These additional regions included left MFG and right SFG, bilateral precentral gyrus, and right temporal pole.
Table 2.
Regions and peak coordinates showing an Age × Syntax interaction
| Region | Coordinates |
Z score | ||
| x | y | z | ||
| R temporal pole | 38 | 12 | −28 | 4.26 |
| R precentral gyrus | 50 | −4 | 52 | 4.01 |
| L postcentral gyrus | −54 | −20 | 48 | 3.97 |
| R SFG | 24 | 12 | 60 | 3.80 |
| L ventral IFG | −52 | 28 | −2 | 3.79 |
| L MFG | −26 | 46 | 34 | 3.74 |
| L precentral gyrus | −30 | −14 | 68 | 3.48 |
| L hippocampus | −26 | −10 | −24 | 3.45 |
| R hippocampus | 26 | −6 | −24 | 3.38 |
The observation that older adults recruited different regions in response to a comprehension challenge than young adults raises the related question of whether these increases supported successful performance. To investigate the issue of whether this increased activity in older adults was truly compensatory, we performed a whole-brain regression analysis within the older adults to see if activity associated with the most difficult condition (fast object-relative sentences) was related to performance. (We did not perform a similar analysis with the young adults because their accuracy remained quite high, and it was the individual variability in older adults’ accuracy scores that was of interest.) As with the previous analyses, only data from trials that resulted in a correct behavioral response were analyzed. Regions showing a correlation with accuracy in the older adults are shown in Figure 2c, with maxima listed in Table 3. For this analysis, we used a slightly more lenient voxel-wise threshold of P < 0.005, maintaining set-level whole-brain correction at P < 0.05. For reference, regions showing a Syntax × Age interaction (Fig. 2b) are shown outlined in white. Regions showing a positive correlation between neural activity and accuracy include bilateral IFG and anterior insula, as well as a more dorsal portion of left IFG. Left posterior MTG showed a negative correlation with performance for these sentences. To see if there were any regions in which increased activity led to faster response times, we repeated this correlation analysis using older adults’ response times from the fast object-relative sentences, but no regions reached significance using this same threshold.
Table 3.
Regions and peak coordinates showing a correlation between activity and accuracy for fast object-relative sentences in older adults
| Region | Coordinates |
Z score | ||
| x | y | z | ||
| Calcarine fissure | −2 | −96 | 6 | 3.73 |
| Posterior cingulate | 6 | −44 | 34 | 3.72 |
| L hippocampus | −28 | −26 | −6 | 3.36 |
| R IFG/operculum | 50 | 14 | 8 | 3.33 |
| L precentral gyrus | −48 | −10 | 24 | 3.32 |
| R inferior IFG | 24 | 38 | −6 | 3.27 |
| Orbital SFG | 4 | 48 | −2 | 3.22 |
| L IFG | −50 | 16 | 10 | 3.00 |
| R anterior insula | 40 | 24 | −2 | 2.96 |
| L posterior MTG | −56 | −60 | 2 | 2.93 |
Network Analysis
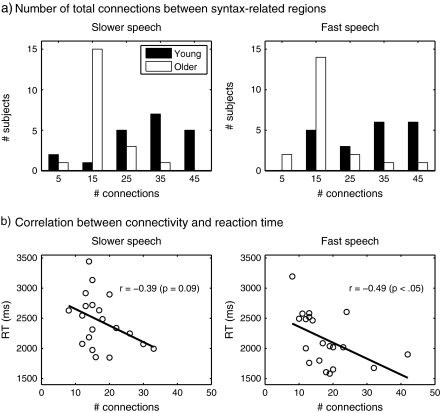
Coordination of activity across different brain regions is a necessary feature of successful cognitive processing, and differences in functional connectivity would likely result in behavioral consequences. To assess the degree to which participants showed coherent activity across brain regions we performed a correlation analysis on data for cluster maxima identified as responding differentially to syntax. For each participant, we extracted the time series from each of 10 peak voxels showing an effect of syntax, identified above (Fig. 2a; Table 1). Linear trends within each scanning run, as well as grand average means of each scanning run, were removed, and then the coordination between all regions was analyzed using bivariate Pearson correlations, producing a measure of effect size (Pearson r) and significance level for each of 45 (10C2) possible connections. For each participant we used a Bonferroni correction to control for false positives; thus, a correlation had to be significant at P < 0.05/45 to be considered significant. We were then able to use the total number of connections reaching significance as a summary measure of overall inter-regional coherence for each participant.
The distributions of the number of significant connections for young and older adults at the slower and fastest rates of speech are shown in Figure 3a. Overall, young listeners showed significantly greater connectivity than older listeners, evidenced by a greater number of connections at both the slower [t(19) = 5.83, P < 0.0001] and fast [t(19) = 5.97, P < 0.0001] speech rates. To ascertain whether this measure of neural connectivity was indeed reflected in older listeners’ behavioral performance, we correlated the number of significant connections seen in older adults with their response times to correct responses for all sentences at the same 2 speech rates, plotted in Figure 3b. Response times were used to provide a more sensitive measure of processing efficiency than accuracy scores, and also because they allowed us to examine older adults’ behavior at the slower speech rate (where accuracy showed little inter-individual variability). At the slow speech rate the correlation of response times and network connectivity was not significant [Pearson r(18) = −0.39, P = 0.09]. However, the same analysis reached significance at the faster rate [Pearson r(18) = −0.49, P < 0.05]. This suggests that, at least at the more difficult faster rate of speech, older adults who demonstrated greater inter-regional coordination were able to perform the task more efficiently.
Figure 3.
(a) Histogram of number of possible connections between brain regions activated in young and older adults at the slower and fast speech rates (out of a maximum of 45 possible, see text for details). (b) For older adults, correlation between the number of connections shown by each participant and their reaction time for correct responses to sentences at the slower and fast rates of speech.
We performed the same correlation analysis on young adults, for whom there was not a significant relationship between number of connections and reaction time at either the slow [Pearson r(18) = −0.19, P = 0.41] or faster [Pearson r(18) = −0.02, P = 0.92] rate (data not shown). Given the overall greater connectivity in young adults, we hypothesize that connectivity may only begin to exert a significant influence beyond some lower bound, which would be consistent with the age differences seen in the current data.
Discussion
Speech comprehension is a complex, time-dependent activity in which previously received and incoming information must be simultaneously processed. In order to accomplish this demanding cognitive task, listeners must coordinate several specialized brain regions. In the current study, we examined the issue of resource availability in healthy older adults, in the context of both relatively successful behavioral performance and under increased processing challenge, in which performance decreased. We found that older adults generally showed less efficient patterns of processing than young adults, both in terms of the focal regional activation and network connectivity.
Below we discuss these different patterns of resource allocation and changes in functional connectivity. First we discuss the core network of regions identified as supporting syntactic processing, followed by an additional focus on areas showing significant age effects. Finally, we assess the functional connectivity between these regions in both young and older adults.
Core Processing Resources Supporting Syntactic Comprehension
The regions we identified as supporting syntactic processing are largely in agreement with prior studies of both written and spoken sentence comprehension. For example, Grossman et al. (2002) presented young and older adults with written sentences that varied in sentence type (object relative or subject relative) and gap distance (short or long antecedent gap). All sentences resulted in posterior MTG activation, a large swathe covering left inferior and middle frontal gyri, and activity in left inferior parietal cortex. Elaborated below, these regions accurately reflect the combination of grammatical, short-term working memory, and semantic resources required to comprehend sentence meaning.
As indicated previously, processing syntactically complex sentences reliably recruits left IFG, with occasional observations of additional right hemisphere activations in homologous regions (Just et al. 1996; Caplan et al. 1998, 1999; Keller et al. 2001). Although left inferior frontal activity related to syntax is often associated with pars triangularis of the left IFG, the precise locus of this activity varies considerably among tasks (Kaan and Swaab 2002). Our finding of more ventral IFG activity, encompassing pars orbitalis and including anterior insula, is not uncommon in sentence-processing studies (Keller et al. 2001; Grossman et al. 2002; Friederici et al. 2003; Peelle et al. 2004). Some studies suggest the nearby frontal operculum plays a specific role in grammatical processing (Friederici et al. 2003, 2006). However, the ventral IFG activation we observe is also near portions of IFG implicated in semantic processing and response competition (Thompson-Schill et al. 1997; Devlin et al. 2003), narrative production (Troiani et al. 2008), and repetition suppression in a lexical priming study (Orfanidou et al. 2006). In addition, left IFG activation can also be seen in during the comprehension of syntactically simple sentences (Davis and Johnsrude 2003; Rodd et al. 2005). Together these studies suggest the possibility of a more general role for ventral IFG and anterior insula in semantic integration. Thus, although left ventral IFG is clearly important for processing syntactically complex sentences, additional work is needed to establish the precise operations carried by this region.
In addition to demands of syntactic processing, comprehending spoken sentences places a burden on verbal working memory resources to store linguistic information for analysis. At least some of this working memory burden may be borne by left IFG (Fiebach et al. 2005), although we argue that working memory is primarily supported by MFG and inferior parietal cortex. Inferior frontal activity associated with syntactic processing often extends dorsolaterally (Grossman et al. 2002; Peelle et al. 2004) to include regions of the MFG that support working memory in both linguistic and nonlinguistic contexts (Rypma et al. 1999; D'Esposito et al. 2000; Buchsbaum et al. 2005). Converging evidence for the role of these frontal regions in supporting working memory operations relevant for language processing comes from voxel-based morphometric studies of patients with neurodegenerative disease. Peelle et al. (2008), for example, reported that comprehension of sentences containing embedded clauses in patients with progressive nonfluent aphasia was related to gray matter density in left inferior frontal and middle frontal gyri, regions that also correlated with verbal working memory. These findings are in agreement with Amici et al. (2007), who also found that both backward digit span and comprehension of multiclausal relative sentences were both significantly related to gray matter density in left inferior and middle frontal gyri in patients with neurodegenerative disease.
We also observed a cluster in inferior parietal cortex that showed significantly greater activity for object-relative than subject-relative sentences. Inferior parietal regions are often proposed to support forms of short-term memory (Awh et al. 1996; Jonides et al. 1997; Jonides et al. 1998; Buchsbaum et al. 2005), therefore may also be recruited to manage working memory demands associated with sentence comprehension. This is supported by observations of these same inferior parietal regions in sentence comprehension tasks, including differential activation for syntactically complex sentences (Keller et al. 2001; Cooke et al. 2002; Grossman et al. 2002). We believe activity in this region reflects phonological working memory necessary for the retention of verbal information for syntactic analysis.
The posterior portion of MTG has been implicated in lexical–semantic processing in a variety of studies (Kotz et al. 2002; Raettig and Kotz 2008; Peelle et al. 2009). This region is consistently activated by lexical and sentence stimuli (Binder et al. 2000; Crinion et al. 2003; Rodd et al. 2005), and damage to posterior MTG is often associated with auditory comprehension deficits (Hickok and Poeppel 2007). In at least one report posterior lateral temporal cortex also responded more to sentences than random word lists (Vandenberghe et al. 2002), suggesting a role in contextual integration (but see Humphries et al. 2006, in which similar comparisons show more anterior activity). We believe that more complex syntactic structures rely on these integrative lexical–semantic processes to a greater degree.
Finally, we observed activity in regions in medial SFG/SMA that we believe is due to participants needing to inhibit prepotent responses. One likely contributor to the difficulty of syntactically complex sentences is that they tend to occur with less frequency than simpler forms; thus, for a given sentence, the simpler subject–verb–object interpretation is likely the meaning the listener presumes, until incoming information proves otherwise. Overriding this default interpretation may require recruitment of resources related to response inhibition, which we believe is supported by the SFG in this study (Liddle et al. 2001; Aron and Poldrack 2006; Taylor et al. 2007; Xue et al. 2008). We note that several other regions responding to syntax are also seen in studies of response inhibition, including STG, MFG, inferior parietal lobule, and even left IFG (Swick et al. 2008). However, unlike the SFG, these regions appear in multiple studies of syntactic complexity; we thus think it likely that the task demands of our current experiment (requiring a speeded response and using short stimuli) accentuated the response inhibition component, driving the increased SFG/SMA activation.
In summary, both young and older adults showed syntax-related increases in activity largely consistent with previous studies, reflecting the allocation of processes related to grammar, working memory, and executive control to sentence processing. We now turn our attention to those regions showing significant age effects, and the possible implication of these differences for older adults’ performance.
Age-Related Differences in Sentence Processing
When processing syntactically complex object-relative sentences, young adults showed significantly greater activity than older adults in left ventral IFG. Particularly interesting is the finding that activity in this same region was associated with better performance in older adults at the fastest speech rate; in other words, the degree of older adults’ success at sentence comprehension can be predicted by their ability to recruit this core resource.
As noted previously, left ventral IFG is consistently implicated in the processing of grammatically complex sentences. Our current data thus suggest an age-related decrease in the ability to make use of specialized language processing regions. Potential causes of such a decline might include decreases in gray matter thickness (Salat et al. 2004), reduced white matter pathway integrity (Madden et al. 2009), or the inability to coordinate activity across regions (Andrews-Hanna et al. 2007). Irrespective of the cause of these differences, the consequences for behavior seem clear: those older participants who showed greater activation in these regions specialized for language processing had significantly better performance.
Given older adults’ decreased activation in left IFG and apparent lack of compensatory activity within the core sentence-processing network, how are they able to process these cognitively demanding stimuli accurately? At least a partial answer seems provided by the frontal regions that older adults recruit more than young adults in response to syntactically complex sentences. We think these areas are recruited to support working memory demands related to sentence comprehension. First, in young adults, working memory related activity (in nonlanguage tasks) can extend dorsally to this point (Cohen et al. 1997; D'Esposito et al. 1998). Second, there is also evidence from nonaphasic patients with frontotemporal dementia that suggests an important role for right frontal regions in sentence comprehension. These patients have frontal atrophy, but its distribution is typically less focal than in progressive nonfluent aphasia, and often shows lateralization differentially affecting the right frontal cortex (reviewed in Peelle and Grossman 2008). This distribution of atrophy in these patients is associated with a limitation in executive resources (Rahman et al. 1999; Libon et al. 2007), which in turn impair sentence comprehension. However, comprehension of all sentences (not just syntactically complex sentences) is impaired (Peelle et al. 2007), suggesting a general—as opposed to “syntax specific”—role for these regions.
Broadly speaking, our results are in good agreement with investigations in other domains of age-related cognitive change. A common expectation in the aging literature is that when older adults are unable to activate specialized cortical regions as well as young adults, they upregulate additional areas to compensate (Cabeza 2002; Cabeza et al. 2002; Wingfield and Grossman 2006). An important point is that this extra activation should be related to some measure of successful task performance. In the current study, the regions that older adults activated to a greater extent than young adults did not predict older adults’ accuracy in the most difficult condition. We hypothesize that the increases in older adults’ activity were sufficient to support their more accurate performance at the slower rate of speech, but were unable to compensate at the faster rate. This would be consistent both with the drop in behavioral accuracy at this faster rate, as well as the lack of correlation between these regions and older adults’ performance in this condition. However, this interpretation is only tentative, because due to older adults’ uniformly high accuracy at the slower speech rate we were unable to directly link this good performance to patterns of increased activation.
Functional Connectivity in Sentence Processing
In addition to areas of focal activation involved in sentence processing, we also investigated the integration of processing across brain regions. As a basic measure of functional connectivity in our task, for each participant we examined correlations between regions showing an effect of syntax, operationalizing coherent network activity for each participant as the number of possible connections between regions. We then used this summary measure to investigate relationships between global neural connectivity and behavior.
We found that for both slower and fast speech rates, the young adults demonstrated significantly greater coherence in their patterns of activity than the older adults, and evidence that this difference had behavioral consequences for the older adults at the fast speech rate. This is consistent with data from Andrews-Hanna et al. (2007) who examined age-related changes in connectivity. They found that not only is aging associated with reductions in functional connectivity between brain networks, but that these disruptions were associated with poor performance across multiple cognitive domains. Our connectivity analysis suggests that some of older adults’ difficulty in sentence comprehension tasks may reflect a lack of coordination between activated brain regions.
Conclusions
The literature on language comprehension in adult aging is uniform in showing 2 features of spoken sentence comprehension in adult aging. The first is that, in spite of significant declines in working memory and processing speed, older adults’ comprehension of spoken language remains at a generally good level (Wingfield and Stine-Morrow 2000). The second is that significant age declines appear when the older adult is confronted by complex sentences, particularly under difficult listening conditions (e.g., Wingfield et al. 2003). Our research task is thus to explain the 2 sides of this coin in normal aging: the spared comprehension of less complex speech heard at normal speech rates, and the comprehension failures that occur in the face of rapid, syntactically complex speech.
Our imaging data suggest that the generally good comprehension of spoken sentences under ordinary circumstances is carried by a network that overlaps the core sentence-processing network seen in young adults. Our current results also reveal patterns of neural regulation that underlie the age-related declines one sees when the processing challenge is especially high. One source of this decline is older adults’ reduced ability to recruit regions of left ventral IFG within the core sentence-processing network, with the degree of this failure predicting performance in the most difficult processing condition. The reason the age-related performance decline is typically one of degree, rather than catastrophic failure, may rest on increasing recruitment of additional frontal regions related to working memory support. Our data suggest that a second source of older adults’ performance decline is a reduction in coordinated activity in the relevant regions of the brain necessary to support sentence comprehension. Together, these findings highlight the importance of both local activity and network connectivity in linguistic processing, and implicate failures at both stages to account for older adults’ performance declines in speech comprehension.
Funding
National Institutes of Health (NS54575, AG04517, AG17586, AG15116, NS53488, and NS44266).
Acknowledgments
We are grateful to Kristina Rehm for assistance in study design and stimuli preparation, and to Jamie Reilly for helpful comments on the manuscript. Portions of this work were presented at the Annual Meeting of the Society for Neuroscience, San Diego, CA, November 2007. Conflict of Interest: None declared.
References
- Amici S, Brambati SM, Wilkins DP, Ogar J, Dronkers NL. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J Neurosci. 2007;27:6282–6290. doi: 10.1523/JNEUROSCI.1331-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Multimodal image coregistration and partitioning— a unified framework. Neuroimage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: evidence from positron emission tomography. Psychol Sci. 1996;7:25–31. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Olsen RK, Koch P, Berman KF. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron. 2005;48:687–697. doi: 10.1016/j.neuron.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G. Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. J Cogn Neurosci. 1998;10:541–552. doi: 10.1162/089892998562843. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G. PET studies of syntactic processing with auditory sentence presentation. Neuroimage. 1999;9:343–351. doi: 10.1006/nimg.1998.0412. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Cooke A, Zurif EB, DeVita C, Alsop D, Koenig P, Detre J, Gee J, Pinango M, Balogh J, Grossman M. Neural basis for sentence comprehension: grammatical and short-term memory components. Hum Brain Mapp. 2002;15:80–94. doi: 10.1002/hbm.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion JT, Lambon Ralph MA, Warburton EA, Howard D, Wise RJS. Temporal lobe regions engaged during normal speech comprehension. Brain. 2003;126:1193–1201. doi: 10.1093/brain/awg104. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Davis MH, Johnsrude IS. Hierarchical processing in spoken language comprehension. J Neurosci. 2003;23:3423–3431. doi: 10.1523/JNEUROSCI.23-08-03423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. The effects of aging on the neural correlates of subjective and objective recollection. Cereb Cortex. 2008;18:2169–2180. doi: 10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmister WB, Talavage TM, Ledden PJ, Weisskoff RM. Improved auditory cortex imaging using clustered volume acquisitions. Hum Brain Mapp. 1999;7:89–97. doi: 10.1002/(SICI)1097-0193(1999)7:2<89::AID-HBM2>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira F, Henderson JM, Anes MD, Weeks PA, McFarlane DK. Effects of lexical frequency and syntactic complexity in spoken-language comprehension: evidence from the auditory moving-window technique. J Exp Psychol Learn Mem Cogn. 1996;22:324–335. [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD. Revisiting the role of Broca's area in sentence processing: syntactic integration versus syntactic working memory. Hum Brain Mapp. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Fiebach CJ, Schlesewsky M, Bornkessel ID, von Cramon DY. Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb Cortex. 2006;16:1709–1717. doi: 10.1093/cercor/bhj106. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- Grady CL, McIntosh AR, Craik FIM. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Chen W, Gee J. Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage. 2002;15:302–317. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangeert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, Gurney EM, Bowtell RW. “Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp. 1999;7:213–223. doi: 10.1002/(SICI)1097-0193(1999)7:3<213::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. J Cogn Neurosci. 2006;18:665–679. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marshuetz C, Willis CR. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal working memory load affects regional brain activation as measured by PET. J Cogn Neurosci. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension: individual differences in working memory. Psychol Rev. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kaan E, Swaab TY. The brain circuitry of syntactic comprehension. Trends Cogn Sci. 2002;6:350–356. doi: 10.1016/s1364-6613(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Keller TA, Carpenter PA, Just MA. The neural bases of sentence comprehension: a fMRI examination of syntactic and lexical processing. Cereb Cortex. 2001;11:223–237. doi: 10.1093/cercor/11.3.223. [DOI] [PubMed] [Google Scholar]
- Kemper S. Imitation of complex syntactic constructions by elderly adults. Appl Psycholinguist. 1986;7:277–287. [Google Scholar]
- Kemper S. Life span changes in syntactic complexity. J Gerontol. 1987;42:323–328. doi: 10.1093/geronj/42.3.323. [DOI] [PubMed] [Google Scholar]
- Kemper S, Marquis J, Thompson M. Longitudinal change in language production: effects of aging and dementia on grammatical complexity and propositional content. Psychol Aging. 2001;16:600–614. doi: 10.1037//0882-7974.16.4.600. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Cappa SF, von Cramon DY, Friederici A. Modulation of the lexical-semantic network by auditory semantic priming: an event-related functional MRI study. Neuroimage. 2002;17:1761–1772. doi: 10.1006/nimg.2002.1316. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Moore P, Farmer J, Antani S, McCawley G, Cross K, Grossman M. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68:369–375. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulines E, Charpentier F. Pitch-synchronous waveform processing techniques for text-to-speech synthesis using diphones. Speech Commun. 1990;9:453–467. [Google Scholar]
- Ni W, Constable RT, Mencl WE, Pugh KR, Fulbright RK, Shaywitz SE, Shaywitz BA, Gore JC, Shankweiler D. An event-related neuroimaging study distinguishing form and content in sentence processing. J Cogn Neurosci. 2000;12:120–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Obler LK, Fein D, Nicholas M, Albert ML. Auditory comprehension and aging: decline in syntactic processing. Appl Psycholinguist. 1991;12:433–452. [Google Scholar]
- Orfanidou E, Marslen-Wilson WD, Davis MH. Neural response suppression predicts repetition priming of spoken words and pseudowords. J Cogn Neurosci. 2006;18:1237–1252. doi: 10.1162/jocn.2006.18.8.1237. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci USA. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Cooke A, Moore P, Vesely L, Grossman M. Syntactic and thematic components of sentence processing in progressive nonfluent aphasia and nonaphasic frontotemporal dementia. J Neurolinguistics. 2007;20:482–494. doi: 10.1016/j.jneuroling.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Grossman M. Language processing in frontotemporal dementia: a brief review. Lang Linguistics Compass. 2008;2:18–35. [Google Scholar]
- Peelle JE, McMillan C, Moore P, Grossman M, Wingfield A. Dissociable patterns of brain activity during comprehension of rapid and syntactically complex speech: evidence from fMRI. Brain Lang. 2004;91:315–325. doi: 10.1016/j.bandl.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Grossman M. Interaction between process and content in semantic memory: an fMRI study of noun feature knowledge. Neuropsychologia. 2009;47:995–1003. doi: 10.1016/j.neuropsychologia.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Gee J, Moore P, McMillan C, Vesely L, Grossman M. Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. J Neurolinguistics. 2008;21:418–432. doi: 10.1016/j.jneuroling.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Wingfield A. Dissociations in perceptual learning revealed by adult age differences in adaptation to time-compressed speech. J Exp Psychol Hum Percept Perform. 2005;31:1315–1330. doi: 10.1037/0096-1523.31.6.1315. [DOI] [PubMed] [Google Scholar]
- Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- Raettig T, Kotz SA. Auditory processing of different types of pseudo-words: an event-related fMRI study. Neuroimage. 2008;39:1420–1428. doi: 10.1016/j.neuroimage.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Rahman S, Sahakian BJ, Hodges JR, Rogers RD, Robbins TW. Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain. 1999;122:1469–1493. doi: 10.1093/brain/122.8.1469. [DOI] [PubMed] [Google Scholar]
- Reilly J, Troiani V, Grossman M, Wingfield A. An introduction to hearing loss and screening procedures for behavioral research. Behav Res Methods. 2007;39:667–672. doi: 10.3758/bf03193038. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cereb Cortex. 2005;15:1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PC, Nobre AC, Rushworth MFS. Subsecond changes in top-down control exerted by human medial frontal cortex during conflict and action selection: a combined transcranial magnetic stimulation-electroencephalography study. J Neurosci. 2007;27:11343–11353. doi: 10.1523/JNEUROSCI.2877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiani V, Fernández-Seara MA, Wang Z, Detre JA, Ash S, Grossman M. Narrative speech production: an fMRI study using continuous arterial spin labeling. Neuroimage. 2008;40:932–939. doi: 10.1016/j.neuroimage.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Nobre AC, Price CJ. The response of left temporal cortex to sentences. J Cogn Neurosci. 2002;14:550–560. doi: 10.1162/08989290260045800. [DOI] [PubMed] [Google Scholar]
- Vos SH, Gunter TC, Schriefers H, Friederici AD. Syntactic parsing and working memory: the effects of syntactic complexity, reading span, and concurrent load. Lang Cogn Process. 2001;16:65–103. [Google Scholar]
- Waters GS, Caplan D. Verbal working memory and on-line syntactic processing: evidence from self-paced listening. Q J Exp Psychol. 2004;57A:129–163. doi: 10.1080/02724980343000170. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Stine-Morrow EAL. Language and speech. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2nd ed. Mahwah (NJ): Lawrence Erlbaum Associates; 2000. pp. 359–416. [Google Scholar]
- Wingfield A, Grossman M. Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. J Neurophysiol. 2006;96:2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Peelle JE, Grossman M. Speech rate and syntactic complexity as multiplicative factors in speech comprehension by young and older adults. Aging Neuropsychol Cogn. 2003;10:310–322. [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex. 2008;18:1923–1932. doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]
- Zachary R. Shipley Institute for Living Scale. Los Angeles: Western Psychological Services; 1986. [Google Scholar]