Abstract
One influential account asserts that the anterior temporal lobe (ATL) is a domain-general hub for semantic memory. Other evidence indicates it is part of a domain-specific social cognition system. Arbitrating these accounts using functional magnetic resonance imaging has previously been difficult because of magnetic susceptibility artifacts in the region. The present study used parameters optimized for imaging the ATL, and had subjects encode facts about unfamiliar people, buildings, and hammers. Using both conjunction and region of interest analyses, person-selective responses were observed in both the left and right ATL. Neither building-selective, hammer-selective nor domain-general responses were observed in the ATLs, although they were observed in other brain regions. These findings were supported by “resting-state” functional connectivity analyses using independent datasets from the same subjects. Person-selective ATL clusters were functionally connected with the brain's wider social cognition network. Rather than serving as a domain-general semantic hub, the ATLs work in unison with the social cognition system to support learning facts about others.
Keywords: anterior temporal lobe, domain-general, functional connectivity, person knowledge, semantic hub
Introduction
It is now generally accepted that the representation of knowledge in the human brain depends on broadly distributed neural circuits that are differentiated by conceptual categories and their associated perceptual, motor, and affective properties (Martin 2007; Patterson et al. 2007; Barsalou 2008). At least 2 important questions remain unresolved, however. The first is whether a property-based model of the conceptual system is sufficient to support all conceptual phenomena (see Barsalou 1999 for a discussion of these issues). The second pertains to the systemic architecture linking these property regions.
Recently, semantic hub models have grown in influence by offering answers to both of these questions (Rogers et al. 2004; Patterson et al. 2007). With regard to the first, these models assert that property circuits are necessary, but not sufficient to support conceptual knowledge; that in addition to property regions one must posit the presence of an amodal, domain-general representational hub. With regard to the second question, these models assert that the anterior temporal lobe is the domain-general hub through which property regions are connected.
The anterior temporal lobes are regarded as the likely location of the semantic hub, largely on the basis of evidence from semantic dementia patients. Semantic dementia, a variant of frontotemporal dementia, is a progressive degenerative disorder characterized by damage to the anterior temporal lobes in its earliest stages, followed by widespread deterioration in more posterior temporal and frontal cortices (Hodges and Patterson 2007). Semantic dementia patients typically exhibit impaired performance on a variety of semantic memory tests across multiple categories of knowledge, whereas other cognitive abilities remain relatively intact (Hodges et al. 1995; Bozeat et al. 2000; Rogers et al. 2004). Recent studies have shown that deficits in semantic dementia are more highly correlated with pathology along the lateral surface of the anterior temporal lobes, as compared with more medial temporal cortex (Mummery et al. 2000; Levy et al. 2004; Moss et al. 2005).
Upon closer review, however, the neuropsychological evidence for an anterior temporal hub is not so clear as it might first appear. First, the pathology in semantic dementia is not restricted to the anterior temporal lobes. The pathology often extends up into frontal cortex (Hodges and Patterson 2007; Brambati et al. 2009). In addition, voxel-based morphometry demonstrates that semantic memory impairments in semantic dementia patients are as strongly correlated with pathology in the posterior fusiform as to pathology in the anterior temporal lobe (Williams et al. 2005). Second, resection of the temporal lobes to treat intractable epilepsy does not lead to the catastrophic, domain-general semantic memory deficits one might predict if this region is the seat of conceptual knowledge (Drane et al. 2008). Proponents of an anterior temporal hub argue that this simply reflects the fact that the surgery removes abnormal tissue that no longer serves its normal function due to pathology-related reorganization. Although this is undoubtedly true (Yucus and Tranel 2007), it is not, however, as if the surgery or damage to this region is without cognitive consequences. Anterior temporal resection, or damage due to conditions such as herpes encephalitis, is often associated with significant episodic memory deficits, as well as notable domain-specific semantic impairments typically including recognizing and naming famous and familiar people (Damasio et al. 1990; McCarthy and Warrington 1992; Sergent and Signoret 1992; Fukatsu et al. 1999; Tippett et al. 2000; Barton et al. 2001, 2004; Glosser et al. 2003; Tsukiura et al. 2003). These findings suggest that the anterior temporal lobes support person-specific knowledge, with the left hemisphere being relatively more important for person naming.
Given its prominent role in semantic hub models, one would expect a veritable mountain of neuroimaging evidence that the anterior temporal lobes are involved in conceptual processing. Significantly, the majority of imaging studies, whether using positron emission tomography (PET) or functional magnetic resonance imaging (fMRI), have not observed anterior temporal lobe activation during conceptual processing. Instead most find posterior temporal or frontal cortex activations (see Thompson-Schill 2003; Martin 2007). To the extent that anterior temporal activation is observed during conceptual processing, it is usually in the context of social conceptual processing tasks (Zahn et al. 2007, forthcoming; for review see Olson et al. 2007) along with the medial prefrontal cortex (PFC), the posterior superior temporal sulcus (pSTS), the amygdala, and the precuneus; regions that are widely regarded as the brain's social cognition network (Frith 2007). For example, the anterior temporal lobe is frequently activated by theory of mind tasks (Olson et al. 2007), as well as to famous and familiar faces (Sergent and Signoret 1992; Gorno-Tempini et al. 1998; Leveroni et al. 2000; Nakamura et al. 2000; Grabowski et al. 2001; Sugiura et al. 2001; Damasio et al. 2004; Pourtois et al. 2005; Rotshtein et al. 2005). The findings of Tsukiura et al. (2008) are of particular interest to the present study as they used a verbal fact recall task and observed that activity in the left anterior temporal lobe reflects recall of associations between names and faces, whereas right anterior temporal activity reflects recall of faces and person-related semantic information.
Aside from the processing of social concepts, functional neuroimaging evidence for anterior temporal lobe involvement in conceptual processing has been inconsistent. Although this would seem to be a major challenge to the model, proponents of anterior temporal hub accounts cite 2 reasons for this dearth of evidence. First is the claim that fMRI is blind to the anterior temporal lobes (Devlin et al. 2000, 2002). Relative to other brain regions, image quality in the anterior temporal lobes is degraded due to distortions of the magnetic field caused by air–tissue interfaces. Hub proponents have often addressed this problem by using PET imaging, which does not suffer from the same signal deficits, but with spatial resolution that is 2 to 3 times lower than that of most fMRI studies. Indeed, some PET studies have provided support for anterior temporal hub accounts by demonstrating anterior temporal activations during conceptual processing (Vandenberghe et al. 1996; Devlin et al. 2000, 2002; Vandenberghe et al. 2002; Crinion et al. 2003; Davis and Johnsrude 2003; Rogers et al. 2006). Additionally, a parallel literature has developed showing activation of the ATLs during sentence-level processing using both reading and auditory–verbal stimuli (Mazoyer et al. 1993; Dronkers et al. 1994, 2004; Stowe et al. 1999; Friederici and Von Cramon 2000; Friederici et al. 2000; Humphries et al. 2001, 2005; Meyer et al. 2003). These studies often report that the ATL is activated for syntactically correct versus incorrect sentences that control for semantic content, thus indicating a potential role for the ATLs in the representation of syntax.
A second argument put forth for why imaging studies of conceptual processing often do not find anterior temporal activation is that they employ tasks that require subjects to process concepts at a level that is too general to engage the region, or because they compare categories at different levels of specificity. By this account, the aforementioned person-knowledge effects in the anterior temporal lobes do not reflect social information processing per se, but rather the comparison of specific classification (e.g., famous faces) with more general classification (e.g., nonfamous faces, animals, tools) (Tyler et al. 2004; Rogers et al. 2006).
Hub accounts claim that the anterior temporal lobes are the seat of human conceptual knowledge, storing amodal conceptual representations, irrespective of category. On the other hand, a different account asserts that the anterior temporal lobes are domain-specific and involved in the representation of person knowledge. Based on the issues and controversies described so far, directly testing these 2 accounts requires: 1) an fMRI study with adequate signal quality in the anterior temporal lobes; 2) processing of multiple object categories, at least one of which is people; 3) each processed at the same level of specificity; 4) with the same type of information across categories; and 5) a nonconceptual control condition.
To meet these requirements, we used fMRI scan parameters optimized for imaging the anterior temporal lobes to study subjects while they learned facts about 4 different unfamiliar and unique people, places, and hammers, or performed a nonconceptual control task, in this case a Riser Letter Detection task. In the scanner, subjects were presented only written sentences describing the age, location, and occupation/usage of the people, places, and hammers, ensuring that all categories were processed at the same level of specificity and with the same types of information (e.g., see Table 1). If the anterior temporal lobes serve as a hub for domain-general conceptual processing, then we should expect to find anterior temporal lobe regions that respond equally to all 3 categories over and above the nonconceptual Riser Letter Detection Task control condition. If on the other hand the anterior temporal lobes are part of a domain-specific social information processing network, then we should expect to find anterior temporal regions that exhibit reliably greater activation for person information as compared with either building or hammer information. Additionally, if the social information processing account of the anterior temporal lobes is correct, then we should also expect that any person-selective regions in the anterior temporal lobes will exhibit reliable functional connectivity with the previously well-described social-processing circuit distributed throughout the brain. To test this last prediction, subjects also underwent a low-level Vigilance Task scan before performing the Fact Encoding Task scans. During this scan, subjects simply pressed a button whenever they saw a fixation mark change color, which occurred approximately once a minute. With this independent data set we were able to evaluate the entrained “resting-state” functional connectivity of the anterior temporal lobes.
Table 1.
Examples of stimuli
| Stimulus presented in scanner | |
| Hammer | |
| Age | the brooks hammer is eight years old |
| Location | the sperry hammer was built in kansas city |
| Occupation/usage | the carson hammer is used to shatter window glass |
| Person | |
| Age | william is sixty three years old |
| Location | patrick was born in little rock |
| Occupation/usage | alex works as an insurance agent |
| Building | |
| Age | the monroe building is 73 years old |
| Location | the gilbert building is located in baton rouge |
| Occupation/usage | the newport building is used for voter registration |
| Riser detection | |
| acran bcr triol ske tpiin kwlto | |
Note: The stimuli used only lower-case letters so that they would be identical to those used in the Riser Detection Task.
Materials and Methods
Participants
Twelve right-handed, native English-speaking volunteers were paid for their participation (7 females; age range, 20–32 years). All subjects completed health questionnaires and none reported a history of head injury or other neurological problems. In accordance with the National Institutes of Health Institutional Review Board protocols, all subjects read and signed informed consent documents.
Experimental Design
Subjects performed 3 tasks while undergoing fMRI. During the first functional scanning run, subjects performed a simple Vigilance Task. In the subsequent 3 scanning runs, participants performed alternating blocks of the Fact-Learning Task and the Riser Detection Task.
Person-Building-Hammer Fact-Learning Task
Subjects were instructed to remember facts described by short sentences presented in black font against a white background. Each sentence described a fact about 4 unique but novel persons, buildings, or hammers, each labeled with a different proper name (see Table 1 for example stimuli). For each unique exemplar, subjects learned an age, location, and usage/occupation fact (e.g., “the gilbert building is forty-five years old”; “the gilbert building is located in baton rouge”; “the gilbert building is used for community meetings”). Our decision to have subjects learn the same attributes about the 3 different categories’ exemplars was motivated by our desire to have subjects process the 3 categories at the same level of specificity and using similar types of information. We believe this is important because hub proponents have claimed that greater item specificity leads to greater anterior temporal lobe activation (Tyler et al. 2004; Rogers et al. 2006). In addition, the stimulus sentences were balanced across categories for average number of words and letters per sentence.
During the task instruction period prior to entering the scanner, subjects were presented with photographs of each unique entity and given its name but no other information. At the conclusion of the instruction period, subjects were again shown the photographs and asked to recall each exemplar's name. Subjects who were unable to recall the correct name upon seeing its photograph were given extra time to study the photo and learn the corresponding name.
In the scanner, subjects only saw sentences; no pictures were presented. In each 18-s Fact-Learning Task block, subjects read sentences describing the 3 facts for a particular exemplar, each presented for 6-seconds. The presentation orders of sentences describing the individual exemplars were varied within and between categories, and presentation orders of the age, location, and usage/occupation facts were randomized within each block. Subjects were shown the 3 facts about an exemplar once during each run and 3 times over the course of the experiment.
After being removed from the scanner, subjects were asked to first recall the critical information for each fact learned while in the scanner. They were presented with the same sentences they read in the scanner, but with the critical fact replaced with a blank space (e.g., “the gilbert building is located in _________”). After completing the recall trials, subjects were given a forced-choice recognition test for all facts.
Entrained “Resting-State”/Vigilance Task
To evaluate functional connectivity, we chose to use a simple vigilance task because it provides images of the brain's functional connectivity in a more constrained context than the typical “resting-state” scan, whereas keeping the subjects’ information processing load to a minimum. In the vigilance task subjects fixated a cross in the center of a grey background and pressed a button anytime the fixation mark changed colors (mean interchange duration = 60 s, range 30–90 s). These data provided an independent data set for exploring the functional connectivity of brain regions activated in the subsequent Fact-Learning Task scanning runs.
Riser Detection Task
Riser Detection letter strings were constructed by scrambling the letters used in the Fact-Learning Task, and contained the same number of spaces as the text strings in the Fact-Learning Task. By doing so, we controlled for the amounts of visual stimulation and visual scanning between the 2 tasks. There were 13 Riser Detection blocks in each scanning run. In each 18-s Riser Detection Task block subjects saw 3 letter strings, presented individually for 6 s in black font against a white background. The subjects’ task was to count the number of “riser letters” in nonword letter strings and press a button on a response box held in the right hand if the total was an odd number. Subjects were instructed that the letters b, d, f, h, k, l, and t are riser letters because they each have some portion that rises up above the tops of most other lower-case letters. This task is a modified version of the “feature detection task” used by Price et al. (1996).
Imaging Details
Stimuli were back-projected onto a screen at the head of the scanner and viewed by subjects via a mirror mounted on the head coil. Stimulus presentation and response collection both during scanning and the recall and recognition tests were controlled using Eprime (www.pstnet.com).
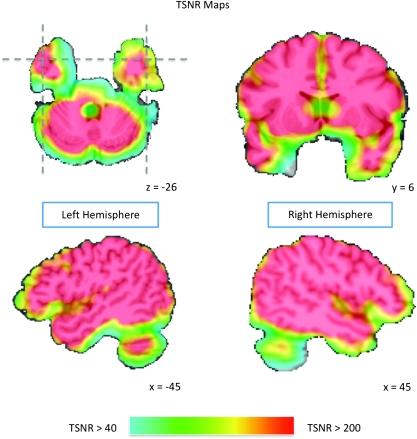
During the Vigilance Task scanning run, 140 echoplanar MR volumes depicting blood oxygenation level dependant (BOLD) contrast were collected with a 3T General Electric scanner. In each echoplanar image (EPI) volume 42 contiguous 3-mm thick slices were collected in the axial plane, ensuring whole-brain coverage (echo time [TE] = 27 ms, repetition time [TR] = 3500 ms, flip angle = 90°, voxel size = 2.3 mm × 2.3 mm × 3 mm). The 3 Fact-Learning Task runs used the same volume parameters, although 143 volumes were collected per run. High-resolution structural images were collected as the first and last scans in each session (TE = 6 ms, TR = 25 ms, flip angle = 15°, voxel size = 0.9 mm × 0.9 mm × 1.2 mm). A General Electric 8-channel send-receive head coil was used for all functional and structural scanning runs, with a SENSE factor of 2 used to minimize EPI distortions in anterior temporal regions while also reducing gradient coil heating over the course of the scan session. As demonstrated by measurements of temporal signal-to-noise (the ratio of the average signal intensity to the signal standard deviation), signal quality in the anterior temporal lobes was very good (see Fig. 1).
Figure 1.
Temporal signal-to-noise ratio (TSNR) maps showing EPI image quality over the anterior temporal lobes. The color gradient indicates the TSNR of the smoothed EPI time course data overlaid on the AFNI Talairach N27 atlas brain. TSNR was calculated by dividing the mean signal intensity at a voxel by the standard deviation of its signal time course. The color map is thresholded at a TSNR of 40, with all areas in red indicating a TSNR of at least 200. Simulations indicate that a TSNR of 40 (indicated in the map by light blue) is the minimum to reliably detect effects between conditions in fMRI data (Murphy et al. 2007). Note that virtually all of the anterior temporal lobes far exceed this threshold, with many anterior temporal regions exceeding a TSNR of 200.
Prior to statistical analyses, image preprocessing was conducted using the AFNI software package (Cox 1996). The first MP–RAGE anatomical scan was coregistered to the second MP–RAGE, and the 2 were then averaged to produce a single high-quality anatomical image of the subject's brain. Next, each subject's EPI volumes were coregistered to the 130th volume of the final EPI scanning run, and smoothed in the axial plane with an isotropic 6-mm full width half max Gaussian kernel. Following application of slice time correction, and removal of the first 3 volumes from each run, EPI signal intensity measurements at each time point were normalized to reflect the percent signal change from the voxel's signal time course mean.
fMRI Statistical Analyses
Multiple regression was used to analyze the Fact-Learning Task data. The regression model included one regressor for each of the 3 fact categories (people, buildings, and hammers) with the Riser Detection Task periods composing the signal baseline. The 3 task regressors were constructed by convolving a box-car function with a width of 18-s beginning at the onset of a condition's blocks with a gamma-variate function to adjust the predictor variable for the delay and shape of the BOLD response. In addition, regressors of no interest were included to account for each run's signal mean, linear, quadratic, and cubic signal trends, as well as 6 motion parameters (3 translations and 3 rotations).
Subjects’ beta maps for each condition were then transformed to Talairach space, and resampled to a 2-mm isotropic resolution. Finally, a repeated measures random effects ANOVA was used on the aggregated group data to evaluate differences between conditions at the population-level.
We used the conjunction analysis methods described by Nichols et al. (2005) to identify regions where the activity patterns across conditions conformed to domain-specific and domain-general response patterns. A domain-specific response was defined as a cluster of activity where a particular condition exhibited reliably greater activity than each of the other Fact-Learning Task conditions. For example, to qualify as a person-selective region, each voxel in a cluster of activity had to satisfy 2 separate statistical tests: person > building AND person > hammer. Because this conjunction assumes a particular directionality, each of the individual tests were thresholded at P < 0.05 one-tailed within the 3 regions of interest (ROIs) described below, and at P < 0.005 one-tailed outside the ROIs. As described by Nichols et al. (2005), the conservative estimate of the probability of a conjunction is the P-value associated with the minimum statistic among the conjoined tests, which in this case is P < 0.05 one-tailed in the ROIs and P < 0.005 one-tailed outside the ROIs. To implement corrections for multiple comparisons at the P < 0.05 level, we used Monte Carlo simulations implemented in AFNI's AlphaSim to identify the required cluster-size threshold, given the voxel-wise probability and the volume in the statistical map (see below) separately for each of the tests in a conjunction. Because the clusters of activity for each test in a conjunction were corrected for multiple comparisons, and should thus be regarded as reliable, so too can the intersections between the clusters. Nevertheless, because it is possible that small areas of intersection between clusters from different statistical tests could be induced by spatial smoothing and resampling, we applied a small cluster-size threshold of at least 10 voxels (defined in the original scanning resolution) on all areas of conjunction.
In contrast to the domain-specific clusters, domain-general clusters were defined as regions where responses for all 3 categories were reliably greater than the Riser Detection Task, but where activity did not differ between categories in the Fact-Learning Task. To this end, we used conjunction analyses similar to those used to identify domain-specific clusters. First we identified regions where each of the categories in the Fact-Learning Task responded reliably above the Riser Detection Task with a P-value of 0.05 one-tailed in the ROIs and P-value of 0.005 one-tailed outside the ROIs, again with each test corrected separately for multiple comparisons at the P < 0.05 level using cluster-size correction (see below). Importantly, the conjunction probability for domain-general clusters was equal to the conjunction probability of the domain-specific clusters. Finally, to remove regions showing a bias toward a particular category, a mask was applied to the data to remove all regions exhibiting a difference with P < 0.25 between any 2 categories in the Fact-Learning Task. Again, as with the domain-specific regions, a cluster-size threshold of at least 10 voxels was applied to all areas of conjunction to ameliorate concerns that smoothing or resampling induced the observed domain-general clusters.
There were 3 region of interest volumes used in the cluster-size threshold calculations: the anterior temporal lobes, the posterior middle temporal gyrus, and the parahippocampal gyrus. The anterior temporal lobes were defined as all areas in the temporal lobes anterior to the limen insula (Insausti et al. 1998; located at approximately y = 3 in the left hemisphere and y = 5 in the right hemisphere of the AFNI Talairach N27 atlas brain). This ROI included only temporal cortex, and did not include any portion of the amygdala. Within the volume of this region, defined bilaterally, a cluster-size threshold for individual tests among the conditions was determined to be at least 1056 mm3 (132 resampled voxels sharing at least one edge). The posterior middle temporal gyrus between y = −40 and y = −69 was selected as a ROI given its association with tool processing (for review see Beauchamp and Martin 2007). Within this region, the cluster-size threshold was determined to be at least 1216 mm3 (152 voxels sharing at least one edge). The parahippocampal gyrus was also selected as a ROI given its association with location representation (Epstein and Kanwisher 1998; Epstein et al. 1999). Within this region, the cluster-size threshold was determined to be at least 992 mm3 (124 voxels sharing at least one edge). Finally, outside these 3 ROIs, clusters of activity had to exceed a size threshold defined by the volume of the brain minus the volumes of the 3 ROIs, rendering a cluster-size threshold of at least 848 mm3 (106 voxels sharing at least one edge). (The cluster size threshold for the regions outside the ROIs is smaller than the cluster size threshold within the ROIs because the P-value threshold outside the 3 ROIs is more stringent by an order of magnitude; P < 0.05 vs. P < 0.005.)
Functional connectivity analyses were implemented on the subjects’ Vigilance Task scanning run, with seed voxels determined by the highest average t-values across the statistical contrasts used in the group conjunction analyses. The connectivity analyses proceeded in the following manner. First, at the subject-level, multiple regression was used to model the run's signal mean, linear, quadratic, and cubic signal trends, as well as 6 motion parameter regressors. In addition, the average signal time course from the subject's ventricles was included to further account for global signal changes. The residual time course for each voxel was then used in the subsequent analyses. Time course residuals for the anterior temporal lobe seed voxels were then used as predictors in separate regression analyses, to produce a map of the correlations between each voxel in the brain and a given seed voxel. These r-values were then converted to Z-values using Fisher's r-to-Z transformation. Next, the subjects’ Z-maps were included in a random effects, one-sample t-test to identify voxels whose means differed from zero with P < 0.0005. Finally, these statistical maps were corrected for multiple comparisons at the P < 0.05 level by applying a cluster-size threshold of at least 296 mm3 (37 voxels sharing at least one edge). The resulting maps show brain regions where activity across subjects was reliably correlated with a seed-voxel's time course while subjects performed the Vigilance Task scanning run, a dataset that was independent of the Fact Encoding Task scanning runs.
Results
Behavioral Results
Responses to color change events in the Vigilance Task were quick and accurate (RT: M = 614 ms, SD = 159 ms; detection accuracy: M = 70%, SD = 22%). In contrast, subjects found it difficult to provide responses on the Riser Detection Task within the allotted time for each trial (RT: M = 4768 ms, SD = 130 ms; detection accuracy: M = 26%, SD = 13%, responses occurring earlier than 2 standard deviations from the response mean were filtered out, as were responses occurring later than the 6-second trial duration). The riser detection accuracy scores reflect the fact that subjects had to perform the task under significant time constraints, rather than indicating that they were performing the task poorly. The letter strings presented to subjects were rather long because they were constructed by scrambling the fact-learning sentences, and as a result it was difficult for subjects to provide responses before the stimuli disappeared from the screen.
After scanning, subjects demonstrated good recall of the information presented during the Fact-Learning Task (Person fact recall: M = 72%, SD = 17%; Building: M = 63%, SD = 17%; Hammer: M = 65%, SD = 21%). Although subjects recalled more person facts than building facts, t(11) = 4.31, P < 0.005, person and hammer fact recall were equivalent, t(11) = 1.13, P = 0.28, as was recall of building and hammer facts, t(11) = 0.38, P = 0.71. As with recall, recognition performance was good for all categories (Person fact recognition: M = 87%, SD = 17%; Building: M = 77%, SD = 17%; Hammer: M = 74%, SD = 24%). Although subjects recognized more person facts than hammer facts, t(11) = 2.55, P < 0.05, person and building fact recognition were not reliably different, t(11) = 1.88, P = 0.09, nor were recognition of building and hammer facts, t(11) = 0.49, P = 0.63.
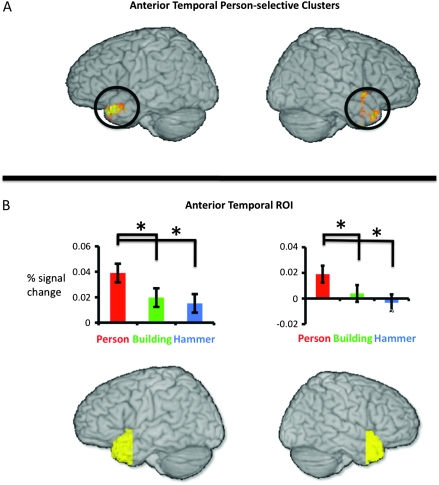
The Anterior Temporal Lobes are Engaged while Acquiring Person Knowledge
Two lateral anterior temporal regions exhibited person-selective responses (Fig. 2A, Table 2). The 2 clusters, located bilaterally in homologous locations in the temporal pole and superior temporal gyri, responded more during person-fact encoding than during building-fact or hammer-fact encoding. Aside from these 2 regions, there were no other category-selective responses in the anterior temporal lobes. To demonstrate the robustness of the person-selective effects to a different voxel selection strategy (Kreigeskorte et al. 2009), and to assess whether statistical mapping was even necessary to observe person-selective responses in this region, we used an anatomical region of interest approach to examine the average response across all voxels in the anterior temporal lobe ROIs for each of the 3 conditions. As can be seen in Figure 2B, person-fact encoding produced greater activation than either building- or hammer-fact encoding across the entirety of the left and right anterior temporal lobes, but no differences were observed between buildings and hammers (left anterior temporal: person > building, t(11) = 1.95, one-tailed P = 0.04; person > hammer, t(11) = 2.27, one-tailed P = 0.02; building versus hammer, t(11) = 0.65, 2-tailed P = 0.53; right anterior temporal: person > building, t(11) = 2.10, one-tailed P = 0.03; person > hammer, t(11) = 2.75, one-tailed P = 0.01; building versus hammer, t(11) = 1.14, 2-tailed P = 0.29).
Figure 2.
Person-selective responses in the anterior temporal lobes. (A) person-selective clusters in the anterior temporal lobes identified using conjunction analyses. The rendered surfaces show the person-selective clusters in the left and right hemispheres where person > building AND person > hammer with P < 0.05 and cluster-size corrected for the volume of the anterior temporal lobes. (B) Activity in the anterior temporal lobe ROIs. The rendered surfaces show the extent of the anterior temporal ROIs in the left and right hemispheres. The bar graphs demonstrate the average percent signal change across subjects in the left and right anterior temporal ROIs relative to the nonconceptual (riser detection) control task. In both ROIs, the responses to person-fact encoding were reliably greater than the responses to building- or hammer-fact encoding. Responses during building- and hammer-fact encoding were not different from each other. Error bars on bar charts in both panels indicate ±1 standard error of the subject means.
Table 2.
Anterior temporal lobe activations
| Contrast | Side/location | Coordinates |
Volume (mm3) | ||
| x | Y | z | |||
| Person-selective clusters | |||||
| L superior temporal gyrus | −47 | 17 | −22 | 848 | |
| R superior temporal gyrus | 47 | 17 | −26 | 800 | |
| Domain-general clusters | |||||
| None | |||||
Note: Coordinates are listed in Talairach space. We do not report peak t-scores in the table because the reported clusters reflect conjunctions of statistical contrast maps for which the constituent peak t-values might be located in different voxels. The reported coordinates are the locations of each cluster's peak average t-value for person > building and person > hammer. Reporting the t-statistic associated with this average t score would be misleading, as this value would underestimate the true differences between the 2 conditions, even though it would itself clear the threshold for statistical significance. See Supplemental Table 3 for the locations and peak t-scores for the individual contrasts contributing to this conjunction analysis.
No domain-general responses were observed anywhere in the anterior temporal lobes. In other words, there were no regions in the anterior temporal lobes where activity was equivalent for person, building, and hammer fact learning and where these 3 conditions produced reliably greater activation than the Riser Detection Task, our nonsemantic control condition.
Fact Encoding Effects Outside the Anterior Temporal Lobes
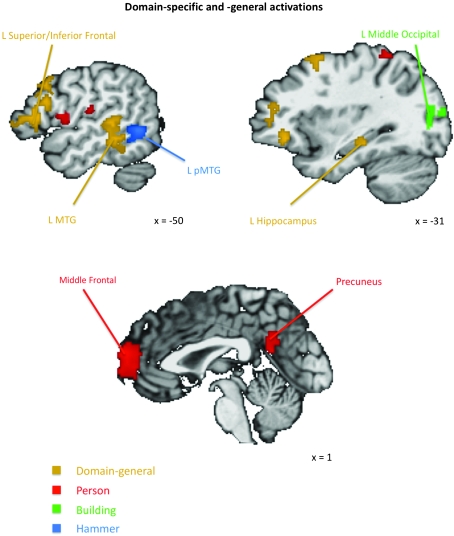
Although the current experiment's focus is the function of the anterior temporal lobes, domain-specific and domain-general responses were observed in other brain regions (Fig. 3).
Figure 3.
Domain-specific and domain-general responses outside the anterior temporal lobes indentified using conjunction analyses. Domain-general responses (shown in gold) were observed in various regions outside the anterior temporal lobes, including the left inferior and superior frontal gyri, the left middle temporal gyrus, and the hippocampus. A hammer-selective cluster (shown in blue) was observed in the left middle temporal gyrus (L pMTG) immediately posterior to a domain-general cluster. More medially, building-selective clusters (shown in green) were observed in left and right middle occipital gyri. Person-selective clusters (shown in red) were observed along the midline in the medial PFC and the precuneus, among other regions. All clusters are corrected for multiple comparisons.
Domain-Specific Encoding Effects
Outside the anterior temporal lobes, person-selective encoding effects were observed in regions commonly implicated in social processing, including the medial PFC, precuneus and posterior cingulate, and the right pSTS. In addition, the superior parietal lobule was also activated bilaterally, as was the left insula (see Table 3). Contrary to our prediction, place-selective effects were not observed in the parahippocampal gyrus. Instead, large areas of place-selective activity were observed bilaterally in the lingual, cuneus, and middle occipital gyri, as well as the right cerebellum. Finally, as predicted, hammer-selective activity was observed in the left posterior middle temporal gyrus.
Table 3.
Domain-specific and domain-general activations outside the anterior temporal lobes
| Contrast (mm3) | Side/location | Coordinates |
Volume | ||
| x | y | z | |||
| Person | |||||
| Midline medial PFC | −1 | 59 | 16 | 4896 | |
| Midline post. cingulate/precuneus | −5 | −51 | 26 | 3224 | |
| L superior parietal lobule | −19 | −51 | 58 | 952 | |
| R posterior STS | 47 | −59 | 12 | 872 | |
| L inferior frontal operculum | −51 | 5 | 12 | 552 | |
| L superior frontal gyrus | −7 | 49 | 32 | 336 | |
| L insula | -43 | −19 | 16 | 224 | |
| R superior parietal lobule | 25 | −41 | 60 | 200 | |
| Building | |||||
| L lingual/middle occipital gyrus | −9 | −81 | −2 | 4112 | |
| R lingual/middle occipital gyrus | 11 | −81 | −4 | 4032 | |
| R cerebellum | 11 | −71 | −16 | 552 | |
| Hammer | |||||
| L middle temporal gyrus | −51 | −55 | 0 | 576 | |
| Domain-general | |||||
| L superior/inferior frontal gyrus | −23 | 17 | 52 | 10488 | |
| L middle temporal gyrus | −53 | −31 | −2 | 3648 | |
| R cerebellum | 35 | −65 | −40 | 1456 | |
| L parahipp. gyrus/hippocampus | −35 | −29 | −10 | 1400 | |
| L angular gyrus | −41 | −57 | 28 | 968 | |
Note: Coordinates are listed in Talairach space. We do not report peak t-scores in the table because the reported clusters reflect conjunctions of statistical contrast maps for which the constituent peak t-values might be located in different voxels. The reported coordinates are the locations of each cluster's peak average t-value for the contrast maps contributing to the conjunction analysis. Reporting the t-statistic associated with this average t-score would be misleading, as this value would underestimate the true differences between the 2 conditions, even though it would itself clear the threshold for statistical significance. See Supplemental Tables 4–7 for the locations and peak t-scores for the individual contrasts contributing to these conjunction analyses.
Domain-General Encoding Effects
Although domain-general encoding effects were not observed in the anterior temporal lobes, other brain regions did exhibit these effects (see Table 3). For example, a large area of domain-general activation was observed to stretch from the left inferior frontal gyrus into the middle frontal gyrus. Additionally, domain-general activation was observed in the left hippocampus, the left middle temporal gyrus, left angular gyrus, and the right cerebellum.
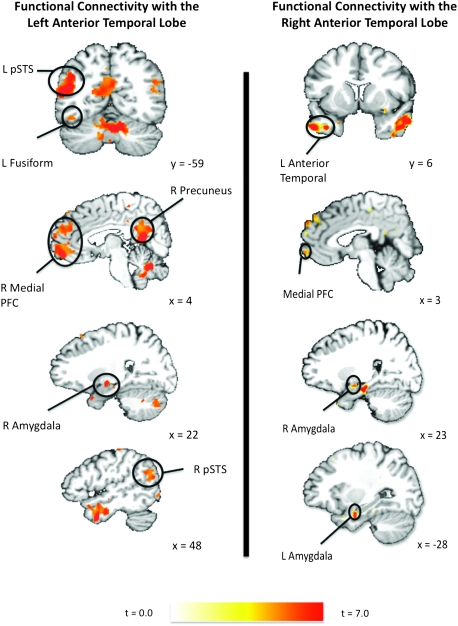
Functional Connectivity: Anterior Temporal Person-Selective Regions are Part of the Wider Social Cognitive Network
Further support for the person-selective nature of the anterior temporal lobes comes from functional connectivity analyses using independent data sets. We used the Vigilance Task scanning runs to examine the functional connectivity with the peak activations in the left and right anterior temporal person-selective clusters identified in the Fact-Learning Task. The left anterior temporal person-selective cluster was functionally connected with brain regions frequently implicated in social cognition, including the medial PFC, the pSTS, the amygdala, and the precuneus/posterior cingulate bilaterally, and in the left lateral portion of the fusiform gyrus (Fig. 4). In addition to the other social-processing regions, activity in the left anterior temporal person-selective cluster was tightly coupled with activity in the corresponding region in the right anterior temporal lobe. Finally, activity in the left anterior temporal person-selective region was correlated with activity in regions known to support more general information processing, including the left inferior frontal gyrus and the left hippocampus (see Supplemental Table 1 for a complete list of regions functionally connected with the left anterior temporal seed voxel). As with the left hemisphere, activity in the right anterior temporal person-selective cluster was correlated with activity in regions previously implicated in social processing, including the medial PFC bilaterally, the amygdala bilaterally, the left posterior cingulate/precuneus, the left fusiform gyrus, and the left anterior temporal lobe (Fig. 4). In addition, this region was functionally connected with a host of more general information processing areas, including the left inferior frontal gyrus, the left perirhinal cortex, and the superior frontal gyrus bilaterally (see Supplemental Table 2 for complete list).
Figure 4.
The person-selective clusters in the anterior temporal lobes are functionally connected with the wider social cognition network. Color overlays indicate clusters of functional connectivity with the anterior temporal seed voxels measured in the independent Vigilance Task scanning run. The left and right anterior temporal seed voxels were identified as those voxels in each hemisphere with the highest average t-value for the person > building and person > hammer t-maps in the Fact-Learning Task scanning runs. The depicted functional connectivity t-maps were obtained as follows. First, for each subject a Pearson correlation map was constructed showing correlation between each voxel and an anterior temporal seed voxel. Second, these r-maps were converted to Z score maps. Finally, these Z-maps were included in a random effects, one-sample t-test to identify voxels whose means differed from zero with P < 0.0005 and cluster-size corrected for multiple comparisons across the whole brain at P < 0.05.
Discussion
Person-Selectivity in the Anterior Temporal Lobes
In the present study the anterior temporal lobes exhibited strong category-selectivity while subjects learned facts about people, relative to building- and hammer facts. The person-selective responses in the conjunction analyses were observed in nearly identical anterolateral regions of the superior temporal gyri and temporal poles in the 2 hemispheres. Domain-general effects were not observed in the anterior temporal lobes, although they were found in other brain regions, including the hippocampus and left inferior frontal gyrus. The absence of domain-general anterior temporal effects in our data cannot be due to poor signal quality because we observed statistically reliable clusters of activity in the lateral anterior temporal cortex, the anterior temporal region with the highest temporal signal-to-noise ratios in the present data (Fig. 1), and the area predicted to be the domain-general semantic hub based on pathology in semantic dementia (Mummery et al. 2000; Levy et al. 2004; Moss et al. 2005).
Eschewing cluster mapping altogether, we evaluated separately for each hemisphere the average response of the entire temporal lobes anterior to the limen insula. Even when using this gross anatomical-ROI approach, the anterior temporal lobes responded selectively when encoding information about people. In both hemispheres, the response profile was highly person-specific, with little difference in the responses to buildings and hammers.
The conjunction analysis was an extremely conservative measure requiring significantly greater activity for the person-fact learning than building-fact learning and greater activity for the person-fact learning than hammer-fact learning. Additionally, each of these tests independently had to reach significance after correction for multiple comparisons. The fact that we replicated the person-fact selectivity in the ROI analysis, which aggregated activity across the entire anterior temporal lobe, demonstrates the robustness of this effect. Including all the voxels in the anterior temporal lobe did not wash out the statistically reliable categorical effects observed in the conjunction analysis.
The findings of the cluster-mapping and anatomical-ROI analyses were further strengthened by the functional connectivity profiles of the anterior temporal lobes, with the present study being the first to describe the functional connectivity of this region. The anterior temporal person-selective clusters, identified in the Fact-Learning Task scans, were found to be functionally connected with virtually the entire social cognition network, as measured during the independent Vigilance-Task scan. The functional connectivity findings reported here agree with tracer studies in the macaque, where strong anatomical connectivity is observed between the temporal pole and the amygdala, superior temporal gyrus, area TE (potential monkey homologue of human fusiform gyrus), and the medial frontal cortex (Moran et al. 1987; Kondo et al. 2003).
Given the results of the conjunction analyses, the anterior temporal ROI analyses, and the functional connectivity analyses on independent data, we can be confident that the person-selectivity observed in the anterior temporal lobes was not a product of the particular statistical-mapping procedure, or the particular task, or the particular stimuli presented to subjects, or even the particular seed voxel within the anterior temporal lobe. Rather the results appear to reflect this region's underlying function and connectivity within a network supporting social cognition.
Social Conceptual Processing in the Anterior Temporal Lobes
Recently, Zahn et al. (2007) reported activation of the anterior superior temporal gyrus when subjects made meaning-relatedness judgments for social concepts. In the present study, the person-specific effects in the anterior temporal lobe stretched from the middle temporal gyrus up into the superior temporal gyrus. Given the differences in the paradigms and stimuli, it is remarkable how much agreement exists between our findings, and those reported by Zahn et al. (2007).
Zahn and colleagues observed a reliable difference between social and animal concepts in the superior temporal gyrus, with much weaker effects of each condition versus fixation in the middle temporal gyrus. They speculate that there may exist an inferior–superior gradient for multisensory versus abstract person-specific knowledge, with the former located in middle temporal gyrus, and the latter located in the superior temporal gyrus. Although this is one explanation for these findings, it is not the only explanation. Alternatively, it could be that the anterior temporal lobes are relatively more responsive to animate than inanimate entities, with the superior temporal gyrus being particularly responsive for human-animate attributes (such as the social abstract concepts used by Zahn et al.). By this account, we observed more inferior middle temporal activity, in addition to the superior temporal activity, because we compared animate to inanimate entities (e.g., people vs. buildings and hammers). This account also finds support in the both our ROI analyses using the entirety of the anterior temporal lobes, and in the functional connectivity findings, which showed correlated spontaneous fluctuations between our anterior temporal lobe person-selective regions and the wider social/animacy network.
Yet another possibility is that in Zahn and colleagues’ data the signal quality might be poorer in middle temporal gyrus than in superior temporal gyrus. Zahn and colleagues only observed middle temporal activity in statistical comparisons that presumably have much higher contrast-to-noise ratios, namely the social and animal concepts versus a simple fixation baseline. Note, however, that this contrast does not control for many nonconceptual differences between the task performed by subjects (e.g., reading words and making meaning-relatedness judgments) and the fixation baseline condition. By this account, we may have observed more inferior effects, in addition to the superior temporal effects, because of better signal quality over this region (e.g., see Fig. 1 and refer to Imaging Details section).
Although we are not certain which of the above-described explanations account for the differences between our findings and those reported by Zahn and colleagues, we strongly believe that the overall findings of the 2 studies exhibit significant agreement and are mutually supportive.
Person-Selectivity in the Anterior Temporal Lobes Does Not Simply Reflect Encoding Effort
Given that subjects generally remembered more person facts than building or hammer facts, one might argue that the person selectivity in the anterior temporal lobes simply reflects encoding effort. There are at least 5 arguments against this account. First, not all brain regions responded selectively for person-fact encoding. Indeed, as just described, many regions responded selectively to other categories. This suggests that there was not a general encoding effort effect for the person facts. Second, regions such as the left inferior frontal gyrus and the hippocampus that would be expected to show a task difficulty or encoding effort effect do not exhibit selectivity for person-fact encoding, but rather responded in a domain-general fashion (e.g., responded equally to all categories). Third, given that we have much more experience learning new information about people, relative to buildings and hammers, it seems unlikely that one would find more activation for learning person facts relative to the other categories if the activity in this region is driven by encoding effort. Fourth, better person fact recall (vs. building fact recall) or recognition (vs. hammer fact recognition) does not guarantee differences between conditions at encoding. The recall and recognition differences could be entirely mediated by storage or retrieval processes. Finally, and perhaps most convincingly, using independent, non-task-related data we observed functional connectivity between the person-selective clusters in the anterior temporal lobes and the wider social cognition network, a finding that strongly supports our interpretation that the activation observed in this area reflects its role in social cognition, not encoding effort.
Domain-Specificity Outside the Anterior Temporal Lobes
Outside the anterior temporal lobes, we observed other domain-specific effects. Encoding hammer facts selectively engaged a posterior region of the left middle temporal gyrus. This finding was predicted a priori, given that the region is consistently activated during conceptual processing of tool categories and tool-related verbs using both pictorial and linguistic stimuli (e.g., Beauchamp et al. 2002; Kemmerer et al. 2008; for recent reviews see Thompson-Schill 2003; Beauchamp and Martin 2007; Martin and Simmons 2008). Large place-selective responses occurred bilaterally in the cuneus and up into middle occipital gyrus. This region, near the transverse occipital sulcus, has been previously implicated in scene perception, navigation, and the representation of large-scale features (such as buildings) in the visual environment (Levy, Hasson et al. 2004; Epstein et al. 2007; MacEvoy and Epstein 2007). Finally, in addition to the anterior temporal lobes, learning facts about people elicited category-selective responses in other social cognition regions. Person-selective responses were observed in the medial PFC, a region that supports mentalizing about others’ mental states (Amodio and Frith 2006; Frith 2007); the right pSTS, a region commonly implicated in the perception and conceptualization of biological motion (Beauchamp et al. 2002, 2003); and the precuneus, a region implicated in social perspective-taking and representation of the self (Cavanna and Trimble 2006).
Domain-General Responses
We found no evidence for a domain-general hub in the anterior temporal lobes. This does not mean however that hub theories in general are incorrect. Rather, it only means that if a domain-general representational hub exists in the brain, it is not in the anterior temporal lobes. In fact, we did find domain-general areas. One region was a large area in left frontal cortex stretching from the inferior frontal gyrus up to the middle frontal gyrus. Based on findings from earlier research, this region serves as a control-center for conceptual processing, guiding retrieval and postretrieval selection of property information stored in posterior cortex, irrespective of category (Bookheimer 2002; Thompson-Schill 2003; Badre and Wagner 2007). Similarly, domain-general responses were observed in the hippocampus, a region long known to support the acquisition of new knowledge (Squire and Zola 1998). It is unlikely that either of these regions serve as representational hubs in the sense previously attributed to the anterior temporal lobes. For example, although damage to the left inferior frontal gyrus results in word-finding deficits, it does not disrupt conceptual knowledge per se (Baldo and Shimamura 1998; Thompson-Schill et al. 1998; Price et al. 1999). Similarly, although damage to the hippocampus greatly affects new learning, it does not result in conceptual deficits for previously acquired knowledge (Levy et al. 2004).
We also observed domain-general responses in the left middle temporal gyrus (immediately anterior to the domain-specific “hammer” cluster), the left angular gyrus, and the right cerebellum, all regions shown previously to be engaged when subjects learn new facts and associations (Maguire and Frith 2004; Addis and McAndews 2006). Of these regions, the left middle temporal gyrus may be of particular interest in future studies, as it is often implicated in domain-general conceptual processing (Hickok and Poeppel 2007; Lau et al., 2008).
Conceptual Processing during the Fact-Learning Task
Semantic memory/conceptual processing involves retrieving information about objects and words that is not immediately available in a stimulus itself. This is perhaps most easily recognized in the case of conceptual processing for words, where the word itself is merely an arbitrary symbol, and so understanding its meaning necessarily requires attributions and inferences about the word's referent. A bedrock principle in cognitive psychology is that reading words automatically activates word meaning (e.g., consider the ubiquity of Stroop effects). Thus, reading the sentence stimuli in our task engaged our subjects’ conceptual systems. Given this, we simply needed to ensure that they actually read the sentence stimuli. To accomplish this we told subjects to remember the information they learned because their memory would be tested at the end of the study.
Our task allowed us to directly compare 3 familiar categories for which subjects had a great deal of previously acquired conceptual knowledge, while being reasonably certain that subjects processed the categories at the same level of specificity and with the same amount of knowledge about the specific exemplars presented in the scanner. Although the specific exemplars were unfamiliar, subjects’ comprehension of the sentence stimuli meant that the task engaged retrieval of pre-existing category-knowledge. Good evidence for this comes from the neuroanatomical distribution of the activations we observed. Consider the person-fact learning condition. Learning facts about specific peoples’ occupations, ages, and places of birth activated regions previously demonstrated to represent biological motion (posterior STS; Beauchamp et al. 2002, 2003), mentalizing about other's mental states (medial PFC; Amodio and Frith 2006; Frith 2007), and social perspective-taking and representation of the self (precuneus; Cavanna and Trimble 2006). The facts learned by subjects about a particular person did not contain references to that person's physical motions, their mental states, or social interactions, and thus these activations are neural signatures of conceptual inferences about the exemplars. Similarly, the hammer facts never described the hammers in motion, yet we can deduce that subjects were engaged in conceptual inference about the hammer exemplars because we observed activation in a region of the middle temporal gyrus known to represent nonbiological (tool) motion (Beauchamp et al. 2002). These activations further strengthen our confidence that the fact-learning task was successful at engendering conceptual processing, and warrants our claims about the anterior temporal lobe's role in conceptual processing.
Because all 3 conditions required fact learning, comparisons among the categories should cancel-out domain-general conceptual processes, leaving only domain-specific conceptual processes. Now, in light of this, consider the claims of the domain-general semantic hub account, which asserts that the anterior temporal lobes are the domain-general hub of the human conceptual system irrespective of the task context through which conceptual information is accessed. In fact, hub models explicitly claim that the anterior temporal semantic hub is engaged in any and all varieties of conceptual processing tasks (e.g., see Patterson et al. 2007). If this is correct, then we should have observed greater anterior temporal lobe activation for all categories compared to the nonsemantic control task, and equivalent activations for all categories in our task. We did not. This leaves us with only 2 options. The first option is that the anterior temporal lobes are domain-specific for person knowledge during sentence comprehension, but the same tissue is domain-general in other task contexts (perhaps after consolidation from the hippocampus to the neocortex), and also exhibiting strong functional connectivity with the wider social cognition network. The second option is that the anterior temporal lobes are domain-specific for person knowledge regardless of the conceptual processing context, and also strongly functionally connected to the wider social cognition circuit. Option one assumes a remarkable switch in domain selectivity from one task context to another, and we can think of no evidence for such a switch either in the anterior temporal lobe or indeed anywhere else in the brain. Option 2 is also a more parsimonious account.
Conclusion
Rather than serving as a domain-general conceptual hub, the anterior temporal lobes appear to support person knowledge. Using both typical statistical-mapping approaches, as well as gross anatomical-ROI analyses, we observed person-selectivity in both the left and right anterior temporal lobes. Further, in independent data sets these regions were functionally connected with the social cognition network. Future studies should seek to better understand the information content within the anterior temporal lobes. In this regard, it is important to note that there exists both neuropsychological and neuroimaging evidence that this regions plays a critical role in the representation of unique entities (Nakamura et al. 2000; Grabowski et al. 2001; Damasio et al. 2004; Tranel 2006). The present study compared responses among different categories of unique entities, rather than between unique and nonunique entities. As such, it will be important for future studies to clarify the relationship between the unique entity findings, and the results reported here.
More generally, the findings reported here help to clarify the architecture of the human conceptual system. As demonstrated in many earlier studies, conceptual knowledge is supported by a widely distributed network of property regions that represent in part the content of conceptual representations, as well as auxiliary regions such as the hippocampus and left inferior frontal gyrus that support memory acquisition and retrieval processes generally. The precise architecture of this system, namely the nodes through which regions are functionally connected, remains an important and controversial question. In the present study, no evidence was obtained in support of the claim that the anterior temporal lobe is a domain-general representational hub. Rather, the findings strongly suggest that the anterior temporal lobe is a component in a network supporting an important class of knowledge: social concepts (Zahn et al. 2007). Describing how the components of this and other conceptual processing networks connect and communicate is a major challenge for all neural theories of the human conceptual system. Developing a better understanding of both the functional and structural connectivity among these regions, and how these connections develop over the lifespan and change with experience, remains a critical and unfinished task.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Institute of Mental Health Intramural Research Program.
Supplementary Material
Acknowledgments
We would like to thank Ziad Saad, Gang Chen, and Steve Gotts for helpful discussions. Conflict of Interest: None decleard.
References
- Addis DR, McAndews MP. Prefrontal and hippocampal contributions to the generation and binding of semantic associations during successful encoding. Neuroimage. 2006;33:1194–1206. doi: 10.1016/j.neuroimage.2006.07.039. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and frontal cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP. Letter and category fluency in patients with frontal lobe lesions. Neuropsychology. 1998;12:259–267. doi: 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Perceptual symbol systems. Behav Brain Sci. 1999;22:577–609. doi: 10.1017/s0140525x99002149. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annu Rev Psychol. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Barton JJS, Cherkasova MV, Hefter R. Covert priming effect of faces in prosopagnosia. Neurology. 2004;63:2062–2068. doi: 10.1212/01.wnl.0000145772.77040.24. [DOI] [PubMed] [Google Scholar]
- Barton JJS, Cherkasova MV, O'Connor M. Covert recognition in acquired and developmental prosopagnosia. Neurology. 2001;57:1161–1168. doi: 10.1212/wnl.57.7.1161. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;34:149–159. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. FMRI responses to video and point-light displays of moving humans and manipulable objects. J Cogn Neurosci. 2003;15:991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Martin A. Grounding object concepts in perception and action: evidence from fMRI studies of tools. Cortex. 2007;43:461–468. doi: 10.1016/s0010-9452(08)70470-2. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Rankin KP, Narvid J, Seeley WW, Dean D, Rosen HJ, Miller BL, Ashburner J, Gorno-Tempini ML. Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiol Aging. 2009;30:103–111. doi: 10.1016/j.neurobiolaging.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioral correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Lambon Ralph MA, Warburton EA, Howard D, Wise RJS. Temporal lobe regions engaged during normal speech comprehension. Brain. 2003;126:1193–1201. doi: 10.1093/brain/awg104. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Face agnosia and the neural substrates of memory. Annu Rev Neurosci. 1990;13:89–109. doi: 10.1146/annurev.ne.13.030190.000513. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Davis MH, Johnsrude IS. Hierarchical processing in spoken language comprehension. J Neurosci. 2003;23:3423–31. doi: 10.1523/JNEUROSCI.23-08-03423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, Matthews PM, Tyler LK. Susceptibility-induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Moss HE, Fadili MJ, Tyler LK. Is there an anatomical basis for category-specificity? Semantic memory studies in PET and fMRI. Neuropsychologia. 2002;40:54–75. doi: 10.1016/s0028-3932(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Aylward E, Ojemann JG, Johnson LC, Silbergeld DL, Miller JW, Tranel D. Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia. 2008;46:1242–1255. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ. A reconsideration of the brain areas involved in the disruption of morphosyntactic comprehension. Brain Lang. 1994;47(3):461–463. [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. In: Hickok G, Poeppel D, editors. The new functional neuroanatomy of language: a special issue of cognition. Vol. 92. New York: Elsevier; 2004. pp. 145–177. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS, Jablonski K, Feiler AM. Visual scene processing in familiar and unfamiliar environments. J Neurophysiol. 2007;97:3670–3683. doi: 10.1152/jn.00003.2007. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Meyer M, Von Cramon DY. Auditory language comprehension: an event related fMRI study on the processing of syntactic and lexical information. Brain Lang. 2000;74(2):289–300. doi: 10.1006/brln.2000.2313. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Von Cramon DY. Syntax in the brain: linguistic versus neuroanatomical specificity. Behav Brain Sci. 2000;23(1):32–33. [Google Scholar]
- Frith CD. The social brain? Philos Trans R Soc Lond B Biol Sci. 2007;362:671–678. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukatsu R, Fujii T, Tsukiura T, Yamadori A, Otsuki T. Proper name anomia after left temporal lobectomy: a patient study. Neurology. 1999;52:1096–1099. doi: 10.1212/wnl.52.5.1096. [DOI] [PubMed] [Google Scholar]
- Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61:81–86. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RSJ. The neural systems sustaining face and proper-name processing. Brain. 1998;121:2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Ponto LL, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham N, Patterson K. Charting the progression in semantic dementia: implications for the organization of semantic memory. Memory. 1995;3:463–495. doi: 10.1080/09658219508253161. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 2007;6:1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- Humphries C, Buchsbaum B, Hickok G. Role of anterior temporal cortex in auditory sentence comprehension: an fMRI study. Neuroreport. 2001;12:1749–1752. doi: 10.1097/00001756-200106130-00046. [DOI] [PubMed] [Google Scholar]
- Humphries C, Love T, Swinney D, Hickok G. Response of anterior temporal cortex to syntactic and prosodic manipulations during sentence processing. Hum Brain Mapp. 2005;26:128–138. doi: 10.1002/hbm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laskso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perhirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Kemmerer D, Gonzalez Castillo J, Talavage T, Patterson S, Wiley C. Neuroanatomical distribution of five semantic components of verbs: evidence from fMRI. Brain Lang. 2008;107:16–43. doi: 10.1016/j.bandl.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in Macaque monkeys. J Comp Neurol. 2003;465:499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- Kreigeskorte N, Simmons WK, Bellgowan PSF, Baker C. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau EF, Phillips C, Poeppel D. A cortical network for semantics: (de)constructing the N400. Nat Rev Neurosci. 2008;9:920–933. doi: 10.1038/nrn2532. [DOI] [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci. 2000;20:878–886. doi: 10.1523/JNEUROSCI.20-02-00878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DA, Bayley PJ, Squire LR. The anatomy of semantic knowledge: medial vs. lateral temporal lobe. Proc Natl Acad Sci USA. 2004;101:6710–6715. doi: 10.1073/pnas.0401679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Hasson U, Harel M, Malach R. Functional analysis of the periphery effect in human building related areas. Hum Brain Mapp. 2004;22:15–26. doi: 10.1002/hbm.20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEvoy SP, Epstein RA. Position selectivity in scene- and object-responsive occipitotemporal regions. J Neurophysiol. 2007;98:2089–2098. doi: 10.1152/jn.00438.2007. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. The brain network associated with acquiring semantic knowledge. Neuroimage. 2004;22:171–178. doi: 10.1016/j.neuroimage.2003.12.036. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Simmons WK. Structural Basis of semantic memory. In: Eichenbaum H, Bryne J, editors. Memory systems. Vol. 3. Learning and memory: a comprehensive reference. 4 Vols. London, Oxford: Elsevier; 2008. pp. 113–130. [Google Scholar]
- Mazoyer BM, Tzourio N, Frak V, Syrota A, Murayama N, Levrier O, Salamon G, Dehaene S, Cohen L, Mehler J. The cortical representation of speech. J Cogn Neurosci. 1993;5:467–479. doi: 10.1162/jocn.1993.5.4.467. [DOI] [PubMed] [Google Scholar]
- McCarthy RA, Warrington EK. Actors but not scripts: the dissociation of people and events in retrograde amnesia. Neuropsychologia. 1992;30:633–644. doi: 10.1016/0028-3932(92)90068-w. [DOI] [PubMed] [Google Scholar]
- Meyer M, Alter K, Friederici A. Functional MR imaging exposes differential brain responses to syntax and prosody during auditory sentence comprehension. J Neuroling. 2003;16:277–300. [Google Scholar]
- Moran MA, Mufson EJ, Mesulam MM. Neural inputs into the temporopolar cortex of the rhesus monkey. J Comp Neurol. 1987;256:88–103. doi: 10.1002/cne.902560108. [DOI] [PubMed] [Google Scholar]
- Moss HE, Rodd JM, Stamatakis EA, Bright P, Tyler LK. Anteromedial temporal cortex supports fine grained differentiation among objects. Cereb Cortex. 2005;15:616–627. doi: 10.1093/cercor/bhh163. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges J. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Murphy K, Bodurka J, Bandettini PA. How long to scan? The relationship between fMRI temporal signal to noise ratio and necessary scan duration. NeuroImage. 2007;34:565–574. doi: 10.1016/j.neuroimage.2006.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Sato N, Nakamura A, Sugiura M, Kato T, Hatano K, Ito K, Fukuda H, Schormann T, et al. Functional delineation of the human occipito-temporal areas related to f ace and scene processing. A PET study. Brain. 2000;123:1903–1912. doi: 10.1093/brain/123.9.1903. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, Segheir ML, Lazeyras F, Vuilluemier P. View-independent coding of face identity in frontal and temporal cortices is modulated by familiarity: an event-related fMRI study. Neuroimage. 2005;24:1214–1224. doi: 10.1016/j.neuroimage.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Price CJ, Mummery CJ, Moore CJ, Frackowiak RS, Friston KJ. Delineating necessary and sufficient neural systems with functional imaging studies of neuropsychological patients. J Cogn Neurosci. 1999;11:371–382. doi: 10.1162/089892999563481. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Frackowiak RSJ. Demonstrating the implicit processing of visually presented words and pseudowords. Cereb Cortex. 1996;6:62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Hocking J, Noppeney U, Mechelli A, Gorno-Tempini ML, Patterson K, Price C. Anterior temporal cortex and semantic memory: Reconciling findings from neuropsychology and functional imaging. Cogn Affect Behav Neurosci. 2006;6:201–213. doi: 10.3758/cabn.6.3.201. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Henson RN, Treves A, Driver J, Dolan RJ. Morphing marilyn into maggie dissociates physical and identity face representations in the brain. Nat Neurosci. 2005;8:107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- Sergent J, Signoret JL. Functional and anatomical decomposition of face processing: evidence from prosopagnosia and PET study of normal subjects. Philos Trans R Soc Lond B Biol Sci. 1992;335:55–62. doi: 10.1098/rstb.1992.0007. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Episodic memory, semantic memory, and amnesia. Hippocampus. 1998;8:205–11. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Stowe LA, Paans AMJ, Wijers AA, Zwarts F, Mulder G, Vaalburg W. Sentence comprehension and word repetition: a positron emission tomography investigation. Psychophysiology. 1999;36:786–801. [PubMed] [Google Scholar]
- Sugiura M, Kawashima R, Nakamura K, Sato N, Nakamura A, Kato T, Hatao K, Schormann T, Zilles K, Sato K, et al. Activation reduction in anterior temporal cortices during repeated recognition of faces and personal acquaintances. Neuroimage. 2001;13:877–890. doi: 10.1006/nimg.2001.0747. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D'Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci USA. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring “how” from “where.”. Neuropsychologia. 2003;41:280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Tippett LJ, Miller LA, Farah MJ. Prosopamnesia: a selective impairment in face learning. Cogn Neuropsychol. 2000;17:241–255. doi: 10.1080/026432900380599. [DOI] [PubMed] [Google Scholar]
- Tranel D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology. 2006;20:1–10. doi: 10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Suzuki C, Shigemune Y, Mochizuki-Kawai H. Differential contributions of the anterior temporal and medial temporal lobe to the retrieval of memory for person identity information. Hum Brain Mapp. 2008;29:1343–1354. doi: 10.1002/hbm.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Toshikatsu F, Fukatsu R, Otsuki T, Okuda J, Umetsu A, Suzuki K, Tabuchi M, Yanagawa I, Nagasaka T, et al. Neural basis of the retrieval of people's names: evidence from brain-damaged patients and fMRI. J Cogn Neurosci. 2003;14:922–937. doi: 10.1162/089892902760191144. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Bright P, Acres K, Abdallah S, Rodd JM, Moss HE. Processing objects at different levels of specificity. J Cogn Neurosci. 2004;16:351–362. doi: 10.1162/089892904322926692. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RSJ. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Nobre AC, Price CJ. The response of left temporal cortex to sentences. J Cogn Neurosci. 2002;14:550–60. doi: 10.1162/08989290260045800. [DOI] [PubMed] [Google Scholar]
- Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. Neuroimage. 2005;24:1042–1051. doi: 10.1016/j.neuroimage.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Yucus CJ, Tranel D. Preserved proper naming following left anterior temporal lobectomy is associated with early age of seizure onset. Epilepsia. 2007;48:2241–2252. doi: 10.1111/j.1528-1167.2007.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.