Abstract
The negative impact of external interference on working memory (WM) performance is well documented; yet, the mechanisms underlying this disruption are not sufficiently understood. In this study, electroencephalogram and functional magnetic resonance imaging (fMRI) data were recorded in separate experiments that each introduced different types of visual interference during a period of WM maintenance: distraction (irrelevant stimuli) and interruption (stimuli that required attention). The data converged to reveal that regardless of the type of interference, the magnitude of processing interfering stimuli in the visual cortex (as rapidly as 100 ms) predicted subsequent WM recognition accuracy for stored items. fMRI connectivity analyses suggested that in the presence of distraction, encoded items were maintained throughout the delay period via connectivity between the middle frontal gyrus and visual association cortex, whereas memoranda were not maintained when subjects were interrupted but rather reactivated in the postinterruption period. These results elucidate the mechanisms of external interference on WM performance and highlight similarities and differences of distraction and multitasking.
Keywords: distraction, EEG, fMRI, human, interference, working memory
Introduction
Our ability to maintain relevant sensory information in mind in the presence of external interference is critical for successfully interacting with an environment that often overloads our limited cognitive resources. Working memory (WM), the theoretical construct that underlies the temporary storage and manipulation of information, is compromised by external interference (Baddeley 1986; Sakai 2003; Sakai and Passingham 2004; Sreenivasan and Jha 2007; Yoon et al. 2006). However, the underlying neural mechanisms by which this disruption occurs are not fully understood, notably in terms of the influence of different types of interference. External interference can be divided into 2 general categories. One involves encountered stimuli that are entirely irrelevant and should be ignored (i.e., distractions), whereas the other involves interfering stimuli that necessitate attention as a secondary task (i.e., interruptions). It is unclear if WM performance is differentially impacted by these 2 types of interference and if there are overlapping or distinct neural mechanisms of WM disruption.
One strategy to investigate the mechanisms underlying the influence of interference on WM is to explore neural measures of stimulus representation in areas of sensory cortex that process interfering stimuli. Several recent studies have investigated the impact distraction has on WM performance by recording activity modulation in visual association cortex (VAC) while distracting stimuli (DSs) were presented during a delayed recognition task. Gazzaley, Cooney, Rissman, and D'Esposito (2005); Gazzaley et al. (2008) demonstrated with electroencephalography (EEG) and functional magnetic resonance imaging (fMRI) that older adults who allocated the most attention to distracting information, as reflected by modulation of early event-related potentials (ERPs) and blood oxygen level–dependent (BOLD) signal modulation in stimulus-selective VAC, exhibited the poorest performance on a WM task. Similarly, an EEG study in healthy young adults demonstrated that a failure to ignore distracting information, also identified by modulation of early ERPs, was associated with neural markers of increased WM load during the maintenance period and diminished WM performance (Zanto and Gazzaley 2009). Furthermore, the impact of interference by distracting information on subsequent WM performance occurs in the VAC within 100 ms of the onset of complex visual stimuli (Rutman et al. 2009). These findings emphasize the impact that processing-irrelevant distractors have on the maintenance of relevant information. To our knowledge, previous research has not yet addressed the spatiotemporal dynamics of the impact interruption has on WM.
Another strategy for investigating the impact that interference has on WM is to explore the role of cortical control areas. The ability to maintain information over a delay period and to allocate attention toward or away from interference involves top–down control from the prefrontal cortex (PFC) via communication with sensory cortices. Previous neuroimaging and electrophysiological studies have analyzed the time period when a distractor was present and reported activity in several PFC regions, including the middle frontal gyrus (MFG) and inferior frontal gyrus (IFG) (Dolcos et al. 2008; Jha et al. 2004). IFG activity has been implicated in both inhibition (Aron et al. 2004; D'Esposito, Postle, Jonides, and Smith 1999; Jha et al. 2004) and selection of attention (Jha et al. 2004), whereas the MFG has been primarily associated with WM maintenance and manipulation (D'Esposito, Postle, Ballard, and Lease 1999). Less research has been performed to evaluate the neural mechanisms involved when an interfering stimulus interrupts ongoing WM maintenance by requiring attention as a secondary task (i.e., multitasking). Sakai et al. (2002a) found that higher degrees of sustained activity within the MFG (Brodmann's area [BA] 46) before interruption was associated with correct trials. In another study, Sakai et al. showed sustained activity within this area before interruption but noted using data from a no-memory + interruption condition that this area also became engaged in processing the interruptor (an arithmetic calculation) (Sakai et al. 2002b). Postle et al. (2003) concluded from a study investigating the impact of interruption on WM maintenance that the PFC does not store mnemonic representations when interruptions are introduced in a WM task. The specific role of the PFC in mediating the impact of distractions and interruptions on WM processes is still unclear.
It should be noted that these studies all report univariate fMRI data, which offer isolated measures of activity within selected brain regions independent of activity in other regions. Functional connectivity analysis, by exploring interactions between brain regions, or neural networks, may allow us to better assess the role of the PFC during concurrent maintenance of relevant information and interference processing. Studies have reported significant functional connectivity between the MFG and VAC during the maintenance of visual information in WM tasks (Gazzaley et al. 2004). It has also been demonstrated that the strength of MFG–VAC connectivity is associated with the amount of attentional allocation to relevant and irrelevant stimuli, such that the degree of connectivity predicts the modulation of VAC activity (Gazzaley et al. 2007). Thus, we hypothesized that MFG–VAC connectivity plays a role in resisting the impact of distraction on WM via maintenance of representations of relevant stimuli. Interruption demands the execution of concurrent goals, and we hypothesized that due to resource limitations the PFC may be incapable of both actively maintaining stored memoranda via PFC–VAC connectivity while simultaneously supporting another goal. Evidence for this comes from a study by Yoon et al. (2006) that showed MFG–VAC connectivity was disrupted when participants were presented with an interfering stimulus to which they were instructed to attend. Furthermore, Miller et al. (1996) implanted electrodes in both the PFC and the inferior temporal cortex of monkeys to investigate maintenance activity before and during interruptors and reported that activity in the PFC was maintained throughout the delay, whereas the interruption disrupted responses in visual areas.
A collective view of these findings suggests that WM disruption by external interference involves distinct mechanisms dependent upon the type of interference. However, distraction and interruption have not been studied within the context of a single experiment. In the current study, we utilized a novel experimental design and both EEG and fMRI to characterize how distraction and interruption disrupt WM. The paradigm employed was a delayed recognition WM task in which an interfering stimulus presented during the delay period was either a distractor or interruptor. In the first experiment, we capitalized on the temporal resolution of EEG to study the precise timing of processing stimuli at each stage of the task (encode, interference and probe) and evaluated the relationship of neural indices of modulation (e.g., enhancement of interruptors and suppression of distractors relative to passively viewed stimuli) to subsequent WM performance. In the second experiment, we exploited the spatial resolution of fMRI to explore the relationship between VAC activity modulation within stimulus-specific cortical nodes and WM performance and then assessed the role of top–down modulation in interference using functional connectivity analyses focused on PFC–VAC connections. This analytical approach enabled us to explore the role of the PFC during simultaneous events of interacting with interfering stimuli and maintaining stored memoranda. Specifically, we performed the following analyses. 1) We tested whether maintenance of stored memoranda was associated with PFC–VAC networks and if this differed between types of interference. If maintenance connectivity was disrupted by interference, we searched for PFC networks associated with reactivating stored memoranda and if this connectivity correlated with WM performance. 2) We examined whether maintenance of stored items (via PFC–VAC connectivity) correlated with the amount of suppression of DS. 3) We evaluated if PFC–VAC connectivity correlated with allocation of attention toward the interrupting stimulus (IS). In summary, convergent approaches in EEG and fMRI experiments are directed at characterizing temporal and spatial characteristics of top–down control processes engaged as healthy young adults perform a WM task in the presence of different types of interference.
Experiment 1—EEG
Materials and Methods
Participants
EEG was recorded from 21 young, healthy participants (ages 18–30 years, mean = 23.3, 14 males) as they performed the experimental task. Participants volunteered, gave consent, and were monetarily compensated to participate in the study. They were prescreened to have normal or corrected-to-normal vision and no use of medication known to affect cognitive state. One participant's neural and behavioral data were removed from analysis due to their failure to perform the task (i.e., no responses to interfering stimuli or probes).
Stimuli
The stimuli consisted of grayscale images of faces and were novel across all tasks, across all runs, and across all trials of the experiment. The stimuli consisted of a variety of neutral expression male and female faces across a large age range. Hair and ears were removed digitally, and a blur was applied along the contours of the face as to remove any potential nonface-specific cues. All images were 225 pixels wide and 300 pixels tall (14 × 18 cm) and were presented foveally, subtending 3° of visual angle from fixation.
Paradigm
A delayed recognition paradigm was used and consisted of 4 distinct tasks presented in blocks, no interference (NI), DS (participants were informed that the distractor was irrelevant), IS (participants made a judgment about the interfering stimulus), and passive view (PV—no memory requirement). Each run was preceded by an instruction slide informing the participant which one of the 4 tasks they would be performing for the duration of the run (see Fig. 1A). Each trial began with the presentation of a face (encode) displayed for 800 ms, followed by a delay period (D1—3 s), the presentation of a face stimulus as a distractor only in the DS and IS tasks (distractor—800 ms), a second delay period (D2—3 s), and the presentation of a face (probe, duration—1 s). The participants were instructed to make a match/nonmatch button press response at the probe as quickly as possible, without sacrificing accuracy. This was followed by a self-paced intertrial interval (ITI).
Figure 1.
Experimental paradigm. All participants performed 4 tasks, which were blocked and counterbalanced. (A) Experiment 1, EEG. (B) Experiment 2, fMRI.
In the NI task, participants were instructed to keep the encoded image of the face in mind and respond to the probe. In the DS task, participants were instructed to actively ignore the distracting face stimulus while maintaining the representation of the encoded stimulus. In the IS task, participants were instructed to respond with a button press to the interfering stimulus only if they determined that the interrupting face image was of a male older than 40 years and to not respond if the face image was of a female or a male younger than 40 years. Ten percent of the trials in IS were catch trials, where the interrupting face stimulus was a male older than 40 years, and these trials were removed from further analysis because the neural data were confounded by a button response. An additional 9 trials (10%) were included in this task to account for these discarded trials. In the PV control task, participants were instructed not to memorize either of the face stimuli. At the probe, participants made a button press to indicate the direction of an arrow (balanced the demands for a decision-driven motor response in the other tasks). Each task was counterbalanced and repeated twice, with 40 trials in each run. These parameters were chosen in order to collect approximately 80 trials within each task and keep the recording time under 1.5 h. Incidental long-term memory was assessed with a surprise postexperiment recognition test after the main experiment. The data from this test will not be discussed in this paper.
Electrophysiological Recordings
Electrophysiological signals were recorded at 1024 Hz through a 24-bit BioSemi ActiveTwo 64-channel Ag-AgCl–active electrode EEG acquisition system (Cortech Solutions, LLC, Wilmington, NC). Electrode offsets were maintained between ± 20 mV. Raw EEG data were referenced to the average off-line. All preprocessing and further analyses were completed using BrainVision Analyzer (BrainVision, LLC, Richardson, TX). Eye artifacts were removed through independent component analysis by excluding components consistent with topographies for blinks and eye movements and the electrooculogram time series. One-second epochs were extracted from the data beginning 200 ms before stimulus onset and ending 800 ms after stimulus onset. The 200-ms period before stimulus onset was used to baseline correct the ERP. Epochs for cue, probe, and interfering stimuli were then cleaned of trials with excessive peak-to-peak deflections (±50 μV), amplifier clipping, or other artifacts. Epochs from all trials were then split by task, filtered (1–30 Hz), and averaged. ERP peak latencies were obtained from lateral occipitotemporal scalp sites over preselected latency ranges. In the analysis, dependent variables were peaks and latencies of stimulus-locked ERPs. Peak amplitudes/latencies were selected as the largest positive/negative deflection within the following time windows for each component (P100—positive deflection between 80–140 ms, N170—negative deflection between 140–240 ms). Peak amplitude was calculated as an 8-ms area centered around the peak amplitude deflection (±4 ms) for each individual. Across-participant statistics were calculated using amplitudes and latencies obtained from each participant. Analyses utilized paired t-tests with a false-discovery rate (FDR) correction for multiple comparisons (Benjamini and Hochberg 1995).
Electrode of Interest
A within-experiment localization to detect an electrode of interest (EOI) was performed by averaging responses to all face stimuli within the experiment (including all tasks, cue, interference, and probe stimuli). P100 and N170 EOIs were selected for each participant from a selection group of the lateral occipitotemporal electrodes (electrodes P10, PO8, P8, O2, P9, PO7, P7, and O1) as the maximal evoked response. P100 and N170 peaks were defined as the largest positive/negative (respectively) peak at the occipitotemporal electrodes within the following time windows: P100, 80–120 ms; N170, 140–200 ms. These time windows and the search for the EOI within the lateral occipitotemporal electrodes were guided by past studies investigating evoked responses to face stimuli (Goffaux et al. 2003; Herrmann et al. 2005).
Indices of Attentional Modulation
The following attentional indices were used in the analyses: enhancement—defined as the difference between activity measures associated with interruptors and passively viewed intervening stimuli—and suppression—defined as the difference between activity measures associated with passively viewed intervening stimuli and distractors. These measures were calculated such that a positive value always indicated greater enhancement above baseline or greater suppression below baseline. Thus, for P100 amplitude: enhancement = IS − PV, suppression = PV − DS, and for N170 latency indices: enhancement = PV − IS, suppression = DS − PV. The calculations were reversed to maintain the convention because an earlier peak latency (lower number) is associated with enhancement and a later peak latency is associated with suppression (Gazzaley, Cooney, McEvoy, et al. 2005).
Results
Behavioral Data
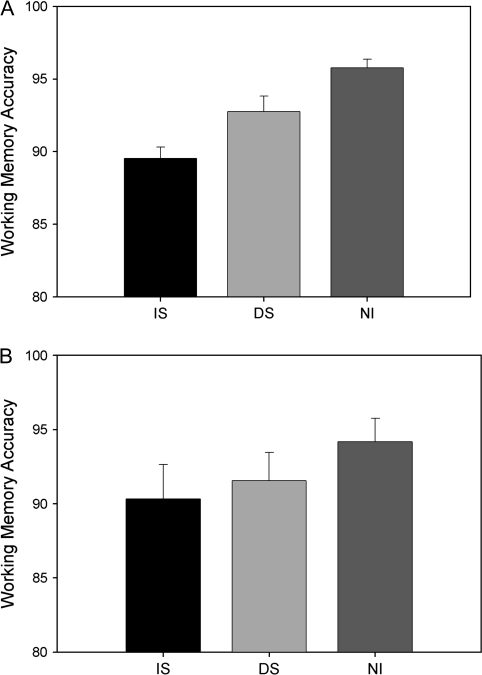
Behavioral analyses revealed a disruptive effect of interference on WM performance. Participants performed with highest accuracy when NI was present (NI = 96%, standard error [SE] = 1%). When a distractor stimulus was introduced, accuracy dropped significantly (DS = 93%, SE = 1%, P < 0.05). When the participants were instructed to attend to an IS to make a judgment decision, their performance declined further relative to both NI and DS (IS = 89%, SE = 1%; P < 0.0001 and P < .01, respectively) (see Fig. 2A). In regard to misses and false alarms, in NI the miss rate was 3% (SE = 1%), and the false alarm rate was 5% (SE = 1%); in DS, the miss rate was 5% (SE = 1%), and false alarm rate was 9% (SE = 2%), and in IS, the miss rate was 10% (SE = 3%), and the false alarm rate was 11% (SE = 2%). Miss rate was significantly different across all conditions (i.e., ND < ID < AD, all P < 0.05), and the false alarm rate in the setting of interference (IS and DS) was greater than without interference (NI) (IS = DS > NI, P < 0.05); there was a trend toward IS > DS (P = 0.07). Results from an analysis of reaction times (correct trials only) were similar to the accuracy findings. Specifically, responses in the interruptor condition were fastest in the NI condition (656 ms, SE = 23 ms), followed by the distractor condition (675 ms, SE = 23 ms) and then interruption (768 ms, SE = 27 ms). Statistically, all conditions were different from one another, except for the NI and distractor condition comparison (P = 0.12).
Figure 2.
Behavioral performance. (A) Experiment 1: WM accuracy. Participants performed best in the NI task, followed by DS, and then IS (all comparisons are significantly different, P < 0.05). (B) Experiment 2: WM accuracy. Accuracy was not significantly different in any of the tasks, but NI trended toward higher accuracy compared with IS (P < 0.1) and DS (P < 0.1) tasks.
EEG Data
EEG analysis focused on the P100 and N170 ERP components, as these have previously been shown to be selective for face stimuli and modulated by attention (Bentin et al. 1996; Gazzaley, Cooney, McEvoy, et al. 2005; Herrmann et al. 2005; Hillyard and Anllo-Vento 1998; Liu et al. 2002). ERPs were time locked to the onset of the cue, interfering, and probe stimuli to evaluate the differential response within each stage across tasks.
Cue
The P100 amplitude and latencies to the cues did not differ between any of the conditions (all P < 0.05). For the N170, the amplitude of the cue stimuli in IS was more negative than in PV (P < 0.05). N170 latencies were earliest for the cue stimuli from the WM conditions (i.e., NI, IS, DS) compared with the passively viewed stimuli (all P < 0.05), and there were no differences between the WM conditions (all P > 0.05). In summary, for N170 amplitude IS = DS = NI = PV (but IS < PV), and for N170 latency IS = DS = NI < PV. Thus, there were no differences in early ERPs for the cue stimuli of the WM tasks, supporting an interpretation of no global change in attention between the tasks.
Interference
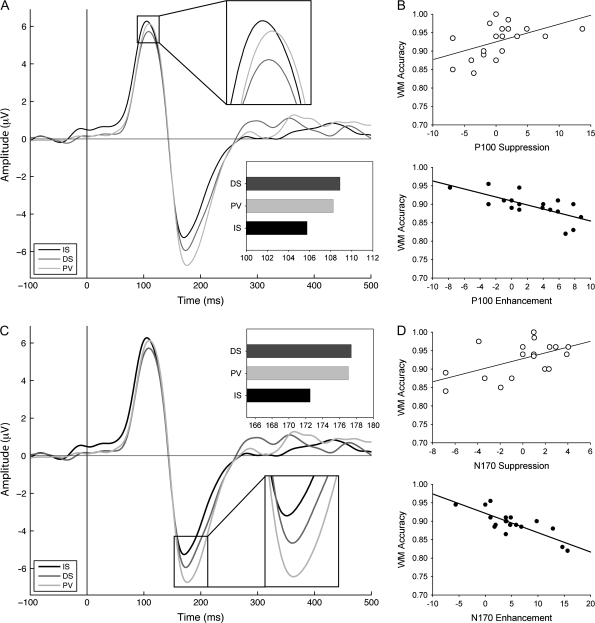
The P100 amplitude was significantly lower for the distractors compared with the interruptors (P < 0.01). It was also significantly lower than the PV intervening stimulus (P = 0.01). There was no significant difference in the P100 amplitude between interruptors and PV intervening stimuli. P100 latencies for the interruptors were significantly earlier than the PV intervening stimuli (P < 0.05) and the DSs (P < 0.05). There was no significant difference in P100 latency between distractors and PV (see Fig. 3A). In summary, for P100 amplitude IS = PV > DS, and for P100 latency IS < PV = DS.
Figure 3.
Modulation of occipitotemporal EOI ERPs: (A and C) ERPs to interruptors (IS), passively viewed stimuli (PV), and distractors (DS). (A) P100 latency reveals significant enhancement. (B) The amount that participants allocate attention toward an interruptor (IS, enhancement) negatively correlates with their WM performance (R = −0.7, P < 0.001). Likewise, the amount of attention allocated away from a distractor (DS, suppression) positively correlates with WM (R = 0.5, P < 0.05). (C) N170 results showing significant enhancement of the N170 latency. (D) The same significant correlations were obtained as for the P100, such that the amount of attention allocated toward the interruptor and away from distractors predicts WM performance (R = −0.76, P < 0.0001; R = 0.64, P < 0.005, respectively). These results are replicated in the fMRI findings (Fig. 4).
For the N170, the amplitude was significantly more negative for the PV stimulus than for both the IS and the DS interfering stimuli (P < 0.05), and IS and DS did not differ (P > 0.05). N170 latencies were earliest to the IS stimuli and were significantly earlier than the PV stimuli (P = 0.001) and the DS stimuli (P < 0.001). There was no significant difference in N170 latency between distractors and PV stimuli (see Fig. 3C). In summary, for N170 amplitude PV > IS = DS, and for N170 latency IS < PV = DS.
Probe
P100 and N170 amplitude and latency comparisons for the probe stimuli across WM conditions did not show significant differences. Thus, task-dependent changes were not seen at this stage of the response selection.
Neural–Behavioral Correlations
In order to evaluate if ERP measures during the 3 stimulus stages of the task predicted subsequent WM performance, linear regression analysis was used to explore correlations between the P100 and N170 amplitude and latency data and WM recognition accuracy. Specifically, we utilized the attentional indices enhancement and suppression (described in the Materials and Methods).
For the cue and probe stimuli, no significant correlations existed between attentional indices and WM accuracy. For the interfering stimuli, P100 and N170 amplitude modulation indices showed no significant correlations with WM performance. However, analysis of P100 latency indices revealed that enhancement of the interruptor was negatively correlated with WM accuracy (R = −0.7, P < 0.001) (see Fig. 3B,D), such that those individuals who enhanced the representation of the IS the most exhibit the poorest WM performance. Analysis also revealed that suppression of the distractor was significantly correlated with WM accuracy across participants (R = 0.5, P < 0.05) (see Fig. 3B,D). In addition, there was a negative correlation between enhancement and suppression indices (R = −0.76, P < 0.01) across participants. As a control, we tested whether the P100 and N170 latency to PV intervening stimuli (the baseline condition) correlated with IS or DS performance and found neither correlation reached significance.
Notably, the same significant correlations were found for N170 latency attentional indices of enhancement of ISs and suppression of DSs. Specifically, analyses of N170 latencies revealed that enhancement to the interruptor was correlated with WM accuracy (R = −0.76, P < 0.0001), and suppression of distractors was correlated with WM accuracy (R = 0.64, P < 0.005). In addition, comparable to the P100 latency data, there was a negative correlation between these enhancement and suppression indices (R = −0.53, P < 0.05) across participants.
Although there was no significant suppression of distractors using either the N170 or P100 latency for the population, there was a strong correlation with WM performance, and so we explored the hypothesis that the high-performing participants exhibited significant suppression of the irrelevant information. We split the participants into 3 subgroups based on their WM accuracy on the DS task (i.e., high performing, low performing, and a middle group: 6 participants in each group). Splitting the participants into 3 groups ensured that the high-performing participants all performed better than the low performers (i.e., a median split resulted in participants that performed with the same accuracy in both high- and low-performing groups). Unpaired t-tests revealed that the high-performing participants significantly suppressed the distractors (P = 0.05) using both P100 and N170 latencies, whereas low-performing participants did not significantly suppress distractors. Moreover, high- and low-performing groups showed a significant difference in suppression index (P < 0.01). Similarly, when the group was split by performance in the interruptor condition, the low-performing group showed significant enhancement of the P100 and N170 latencies (P < 0.05), whereas the high-performing group did not (P > 0.05). Again, the 2 groups differed significantly in their enhancement indices (P < .05).
Discussion
Impact of Interference on WM
It is well established that interference impairs WM processes and performance (Chao and Knight 1998; Sakai et al. 2002a; Jha et al. 2004; Postle et al. 2005). This was replicated in the current study, as both delayed recognition tasks that included interfering face stimuli during the WM maintenance period significantly diminished WM recognition accuracy. Interestingly, there was a differential impact of interrupting vs distracting interference in terms of the magnitude of the effect. If the intervening stimulus was to be attended (i.e., interruptor), it had a more detrimental impact on WM performance than if it was a distraction that could be ignored.
Neural Responses to Interference and Impact on Performance
ERP analyses time locked to the onset of the cue, interference, and probe stimuli were used to identify markers of attentional allocation. Specifically, enhancement of the interruptor was manifest for both the P100 and N170 latencies, such that the peak latency was earlier for the IS than the passively viewed intervening stimulus. This is a replication of previous findings that revealed that P100 and N170 latencies are markers of selective attention for faces (Gazzaley, Cooney, McEvoy et al. 2005; Gazzaley et al. 2008). Both the P100 and N170 have been localized to visual areas in lateral VAC (Gomez Gonzalez et al. 1994). Modulation of P100 and N170 latency most likely reflects the time for these cortical regions to reach maximal synchronized activity. Earlier latencies have been shown to occur for faces that are attended to (i.e., enhancement relative to passive), whereas later latencies (i.e., slowing of processing) occur for faces that are ignored (i.e., suppression relative to passive) (Gazzaley, Cooney, McEvoy, et al. 2005). In the current data set, there was no significant suppression of the N170 and P100 latencies for the distractor as has been observed previously, perhaps because it was more difficult to anticipate the time of distractor onset in this experiment. These same indices of attentional modulation revealed that there was no difference in attentional allocation to the cue or probe stimulus for the 3 WM conditions. This suggests that the impact of interference on WM performance is occurring at the time of interference and not the result of changes in processing the cue or probe.
To further evaluate the impact of interference on WM performance, we capitalized on individual differences that exist in the ability to allocate attention toward and away from both types of interfering stimuli. Regression analysis revealed that individual variability in attentional modulation indices only to the interfering stimuli predicted subsequent WM performance. Specifically, the amount of attention directed toward the interruptor negatively correlated with WM recognition performance. Likewise, the degree of suppression of the distractor correlated positively with WM performance. Furthermore, the same participants who did not “excessively” enhance the interruptor were those who were best able to ignore the distractor. These findings reveal that although interruptors have an overall more disruptive influence on WM performance, increased processing of either type of interference will have a detrimental impact on WM. This serves to highlight a commonality in the impact attentional allocation to different types of interfering information has on WM.
Lastly, the results reveal that the influence of interference processing on WM performance is very rapid, occurring within 100 ms of stimulus presentation. This information was only obtainable with the higher resolution of EEG and informs the basic mechanism of WM interference by supporting an early processing stage model. Two views exist in terms of which processing stage attentional allocation occurs, early sensory processing (Jonides 1983) or later stages of informational processing (Duncan 1980) (also see Luck and Vecera 2002). The present findings extend previous research that demonstrates that the mechanism of attentional allocation occurs during the early stages of sensory processing and that it is at this time point that interference impacts WM (Rutman et al. 2009; Zanto and Gazzaley 2009).
Experiment 2—fMRI
Materials and Methods
Participants
Twenty-two young healthy adults (ages 18–32 years, mean = 24.57, 13 males) with normal or corrected-to-normal vision volunteered, gave consent, and were monetarily compensated to participate in the study. Participants were prescreened to exclude individuals using medication known to affect cognitive state. Two participants’ data were not included in the final analysis due to a failure to follow task instructions (i.e., did not respond to ISs).
Stimuli
The stimuli consisted of grayscale images of faces and natural scenes. The same face images were used as in experiment 1, and images of scenes were not digitally modified beyond resizing to match the face images and gray scaling.
Paradigm
This experiment utilized a delayed recognition paradigm, where interference was introduced in the middle of the delay period for 3 of the 4 tasks. The task was similar to that used in experiment 1, in terms of the tasks (IS, DS, NI, PV) but differed in several important aspects: 1) Due to the lower temporal resolution of fMRI, we opted to use incongruent interference (i.e., scene WM task, with faces as interfering stimuli). This allowed us to pursue our aim of investigating the neural response to interfering face stimuli, while minimizing the influence on the BOLD response of maintaining a scene in WM. 2) The delay periods were extended from 3 to 7.2 s to allow the BOLD response to decay after stimulus offset. 3) A static ITI of 9 s was used in favor of the self-paced ITI present in experiment 1 (see Fig. 1B).
Each WM trial began with the presentation of a natural scene (encode) displayed for 800 ms, followed by a delay period (D1 = 7.2 s), the presentation of a face stimulus as interference (distractor = 800 ms) in the IS and DS tasks, a second delay period (D2 = 7.2 s), and the presentation of a scene (probe, duration = 1 s). The participants were instructed to make a match/nonmatch button press response as quickly as possible without sacrificing accuracy. Each task was counterbalanced and repeated twice, with 16 trials in each run. These parameters were chosen in order to collect 32 trials for each task.
fMRI Acquisition and Processing
All images were acquired on a Siemens 3T Trio Magnetom. Images were collected with a 2-s repetition time (TR) and 1.78 × 1.78 × 3.5 voxel size. For functional data, thirty-three 3.0-mm oblique axial T2*-weighted gradient-echo slices (TR = 2000 ms, echo time = 25 ms, 90° flip angle, and 250 mm2 field of view in a 128 × 128 matrix) were collected. Images were corrected for slice timing, motion artifacts, and Gaussian smoothed to 5-mm full width at half maximum. Data were modeled using a general linear model (GLM) in SPM5. Group whole-brain maps were calculated from Montreal Neurological Institute-normalized data. In addition, high-resolution anatomical (T1-MPRAGE) data sets were collected.
Data Analysis
Region of interest localization.
A separate localizer task was used to identify face-selective areas in the VAC, the fusiform face area (FFA) (Kanwisher et al. 1997), and scene-selective areas, the parahippocampal place area (PPA) (Epstein and Kanwisher 1998). In this task, participants performed a 1-back task during 10 blocks of 16-s blocks of face stimuli, scene stimuli, and rest. Participants were instructed to indicate when a match (1-back) occurred within a block with a simple button press. Blocked face and scene stimuli regressors were contrasted to generate SPM[T] images, and from these contrasts, regions of interest (ROIs) were identified. A face-selective ROI (FFA) was then identified as the cluster of 35 contiguous voxels with the highest t value within the right fusiform gyrus of each participant; the right FFA has been shown to be most strongly activated by faces, and thus, it was used as a seed in beta-series correlations (Bentin et al. 1996; Kanwisher et al. 1997). A scene-selective ROI (PPA) was also identified as the cluster of 35 contiguous voxels with the highest t value within the left parahippocampal gyrus of each participant. The left PPA has been shown to be the most selective for scenes (Epstein and Kanwisher 1998) and was used in beta-series correlations. The decision of the ROI voxel extent was based on the methodology of similar studies (Gazzaley, Cooney, Rissman, and D'Esposito 2005; Gazzaley et al. 2007; Gazzaley et al. 2004; Rissman et al. 2004) and was used in order to achieve a reasonable balance between regional specificity (diminished by the use of a larger cluster) and susceptibility to noise (a problem with smaller seeds).
fMRI univariate analysis.
BOLD responses were modeled as events convolved with the canonical hemodynamic response function (HRF). The onsets of temporally adjacent covariates were spaced at least 3.6 s apart to minimize the contamination of residual activity and autocorrelation (Zarahn et al. 1997). All responses were analyzed, though trials when participants failed to respond to the probe were modeled separately and not included in the final analysis.
To generate BOLD time courses, signal and baseline (intercept) were each averaged across the ROI for each time point (TR). The baseline was extracted within the ROI from the session-specific intercept term of the GLM at every time point. Then, the data were multiplied by a scaling factor (100/baseline) to generate the percent change in signal. Finally, the percent signal change (PSΔ) was averaged across trials to form an average time course. The following formula was used to compute PSΔ: (signal − baseline) × 100/baseline. Analysis of the BOLD time course signal involved t-tests at each time point to investigate where DS and IS differed. Analyses were focused on the time points between encode and probe peaks, including interference period and both delay periods (corrected for multiple comparisons with FDR).
fMRI functional connectivity analysis.
Whole-brain maps of functional connectivity were generated by extracting beta values for each stage of every trial from each participant's ROI and correlating these values across trials with each voxel in a whole-brain analysis (Gazzaley et al. 2004; Rissman et al. 2004). A new GLM design matrix was constructed in which each trial stage (cue, delay1, distractor, delay2, and probe) from each trial was coded with a unique covariate. This resulted in a total of 640 covariates of interest being entered into the GLM (5 task stages per trial × 32 trials per condition × 4 task conditions). As a secondary analysis, to reduce autocorrelation and detracting variance from parameter estimation of stimulus locked events, a separate GLM design matrix was constructed for the beta-series correlation analysis of the cue, interference, and probe trial stages alone, which resulted in a total of 384 covariates of interest being entered into the GLM.
In the IS task, participants always responded correctly to the interrupting face when it was in fact a male older than 40 years, but occasionally they responded to nontarget faces as well (i.e., false alarms). Throughout both experimental blocks of IS, participants responded on average with 4.15 (σ = 1.565) false alarms to ISs, thus resulting in fewer useable trials for this task. To avoid a potential confound of statistical power differences between tasks, an iterative resampling method was employed (100 repetitions) to equilibrate the samples contributed by each task.
To correct for discrepancies in the overall magnitude of beta correlations between participants, z scores across the voxels of each participant's correlation map were calculated to move each participant into the same range and thus facilitate group comparisons. It was necessary to exclude the ventral–posterior quadrant from this analysis because this area contained an excessive number of suprathreshold voxels due to local autocorrelations. This was reasonable as this region was not within the focus of the connectivity analysis.
Correction for multiple comparisons.
Where applicable, we performed a Monte Carlo simulation similar to AlphaSim in the AFNI toolbox (Cox 1996) except that actual data were utilized to calculate cluster sizes with corrected P values. Statistics utilizing this correction are explicitly stated. Throughout all analyses, clusters were defined within an 18-connected voxel neighborhood, consistent with previous fMRI research investigating the reliability of functional responses across participants (Seghier et al. 2008). Connected voxels are defined as those that surpass a magnitude threshold and are connected to adjacent suprathreshold voxels by a face or an edge.
Indices of attentional modulation.
The following attentional indices were used in the analyses: enhancement—defined as the difference between activity measures associated with interruptors and passively viewed intervening stimuli (IS–PV)—and suppression—defined as the difference between activity measures associated with passively viewed intervening stimuli and distractors (PV–DS). These measures were calculated such that a positive value always indicated greater enhancement above baseline or greater suppression below baseline.
Results
Behavioral Data
Participants performed the WM task with high accuracy when NI was present (94.2%, SE = 3%). When a distraction was present (DS), accuracies dropped (91.6%, SE = 2%) with a trend toward significance (P = 0.09). When participants were interrupted (IS), their performance again diminished (90.3%, SE = 3%), with a trend toward a significant decline from NI (P = 0.08) but not relative to DS (P = 0.4), see Figure 2B. Further analyses revealed that in NI the miss rate was 8% (SE = 3%), and the false alarm rate was 5% (SE = 3%); in DS the miss rate was 9% (SE = 3%), and false alarm rate was 6% (SE = 2%), and in IS the miss rate was 10% (SE = 3%), and the false alarm rate was 7% (SE = 2%). Miss rates and false alarm rates were not significantly different across conditions (i.e., NI = DS = IS). RT did not show significant differences across the WM tasks (NI = 1089 ms, SE = 86 ms; DS = 1072 ms, SE = 87 ms; IS = 1063 ms, SE = 75 ms).
Univariate Activity
Cue.
BOLD activity within the PPA during the cue period did not differ between WM tasks. The BOLD response to cue stimuli was higher for IS, DS, and NI compared with passively viewed cue stimuli (P < 0.05).
Interference.
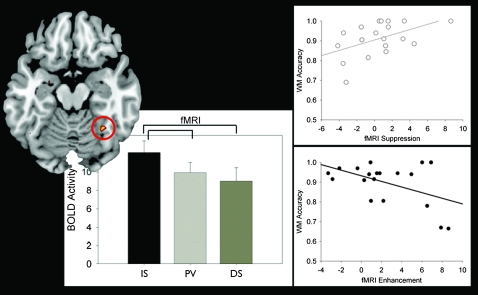
Analysis revealed that BOLD activity within the FFA for interfering face stimuli differed depending on task. The BOLD response to ISs was higher than distractor stimuli (DS), (P < 0.01). Furthermore, the BOLD response to IS was higher than passively viewed intervening stimuli (PV) (P < 0.01), which was not significantly different from the DSs (see Fig. 4). In summary, IS > PV = DS.
Figure 4.
FFA modulation and correlations with WM accuracy: The BOLD response in the FFA to interruptors (IS), passively viewed stimuli (PV), and distractors (DS) are presented in the bar graphs. The BOLD response was highest in response to the interruptors and lowest to the distractors (enhancement [IS > PV, P < 0.01]). Right panels: The amount that participants allocate attention toward an interruptor or away from a distractor (vs. passively viewed intervening stimuli) correlates with their WM performance (R = −0.54, P < 0.05; R = 0.53, P < 0.05, respectively). These results replicate the EEG findings (Fig. 3).
PFC activity was evaluated to identify potential control regions involved in modulation of VAC activity. A comparison of responses to interruptors vs distractors revealed that several areas within the PFC, including MFG, IFG, and BA 10 were more active when participants were interrupted than when distracted (see Table 1).
Table 1.
Univariate activity contrast
| Brain region | BA | x | y | z | Peak t value |
| IS interruptor > DS distractor | |||||
| L inferior temporal cortex | 20 | −41 | −13 | −30 | 5.2 |
| R fusiform gyrus | 37 | 37 | 42 | 20 | 4.08 |
| R fusiform gyrus | 37 | 28 | −55 | 13 | 6.13 |
| L lingual gyrus | 19 | −26 | −56 | −8 | 4.36 |
| R inferior temporal gyrus | 20/37 | 43 | −47 | −11 | 5.5 |
| L middle occipital gyrus | 18 | −23 | −91 | 2 | 4.38 |
| L caudate | 25 | −11 | 12 | 6 | 4.92 |
| R insula | 47 | 31 | 28 | 3 | 5.31 |
| R superior occipital gyrus | 17 | 18 | −100 | 10 | 5.82 |
| R caudate | — | 6 | 6 | 11 | 5.26 |
| RMFG | 10 | 25 | 56 | 9 | 4.99 |
| L middle occipital gyrus | 19 | −35 | −73 | 9 | 4.08 |
| L thalamus | — | −12 | −8 | 8 | 4.2 |
| R anterior cingulate cortex | 32 | 4 | 46 | 15 | 5.6 |
| L calcarine | 17 | −12 | −79 | 9 | 4.9 |
| R middle temporal gyrus | 37 | 40 | −63 | 10 | 5.16 |
| R putamen | 48 | 26 | 5 | 16 | 4.57 |
| Medial anterior cingulate cortex | 24 | −1 | 26 | 19 | 4.8 |
| RMFG | 45 | 47 | 34 | 27 | 5.34 |
| L inferior frontal gyrus | 45/48 | −42 | 24 | 26 | 4.46 |
| R anterior cingulate cortex | 32 | 6 | 34 | 27 | 4.34 |
| R precentral gyrus | 44 | 40 | 10 | 38 | 5.36 |
| R cuneus | 18/31 | 13 | −69 | 36 | 4.76 |
| R superior temporal gyrus | 39 | 38 | −55 | 34 | 4.54 |
| L precentral gyrus | 6 | −39 | 4 | 38 | 4.43 |
| L superior medial frontal gyrus | 6 | 1 | 32 | 45 | 6.36 |
| R inferior parietal lobule | 40 | 36 | −51 | 46 | 4.5 |
| RSFG | 9 | 6 | 51 | 51 | 4.87 |
| R inferior parietal lobule | 7/39 | 44 | −59 | 53 | 4.27 |
| L supplementary motor area | 6 | −2 | 17 | 57 | 4.25 |
| R superior medial frontal gyrus | 6/8 | 4 | 34 | 58 | 4.53 |
| LSFG | 6 | −27 | −2 | 71 | 4.16 |
| DS distractor> IS Interruptor | |||||
| L middle temporal gyrus | 39 | −41 | −66 | 23 | 4.33 |
| L precuneus | 19 | −33 | −81 | 42 | 4.89 |
Maintenance period.
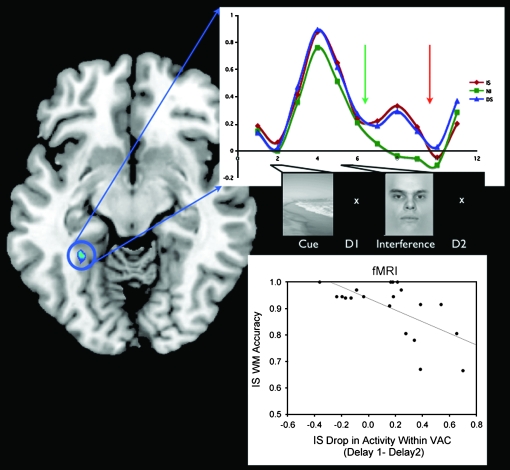
The delay periods before and after the interfering stimuli were analyzed to evaluate the impact of different types of interference on neural signatures of maintenance of the encoded stimulus (i.e., a scene). Analysis revealed that the PPA time course followed a similar pattern through delay 1, such that there was no difference between DS and IS (see Fig. 5), whereas activity was significantly higher in delay 2 in DS than IS (P < 0.05). Further analysis of the reduction in PPA activity from delay 1 to delay 2 (i.e., after interference) revealed that in IS those participants who experienced the largest drop in PPA activity performed worst on the WM task (R = −0.64, P < 0.005).
Figure 5.
Time course of BOLD activity within the PPA and correlation with WM accuracy. The time series of the percent signal change in the PPA in IS, DS, and NI are plotted. The amount of decrement in the PPA signal in IS compared with DS is significant during delay2 (orange arrow, P < 0.05). Bottom right: the amount that PPA activity drops in IS between delay1 (green arrow) and delay2 negatively correlates with WM accuracy on the IS task.
Probe.
Activity within the PPA during the probe period did not differ between the 3 WM conditions (IS, DS, NI). The BOLD response to probe stimuli was higher for IS, DS, and NI compared with PV probe stimuli (P < 0.05).
BOLD Activity–Behavioral Correlations
To evaluate the influence of VAC activity associated with interfering stimuli on WM performance (i.e., face stimuli as reflected in the FFA), the data were subjected to a linear regression analysis (comparable to the analyses in experiment 1). This revealed that the amount participants' suppressed the distractors (PV–DS) correlated with their WM accuracy (R = 0.53, P < 0.05), and the degree to which they enhanced the interruptors (FFA: IS–PV) negatively correlated with their WM accuracy (R = −0.54, P < 0.05, see Fig. 4). Further analysis revealed a negative correlation between the enhancement and suppression indices from each participant (R = −0.47, P < 0.05), revealing that individuals who direct more attention to the interruptors also do so to the distractors (i.e., less suppression).
Splitting the participants into 3 subgroups based on their WM performance, as was done in experiment 1, revealed differences in attentional indices between subgroups, with the high-performing group significantly suppressing distracting faces (P < 0.05) and the low-performing group not significantly suppressing the distracting faces. Furthermore, there was a significant difference in the amount of suppression between high- and low-performing groups (P < 0.05). Similarly, the 2 groups differed in their neural enhancement indices (P < 0.05), such that the low-performing group showed significant enhancement of the interruptors (P < 0.05) whereas the high-performing group did not (P > 0.05).
Connectivity Analysis
PFC–VAC connectivity and encoded stimuli.
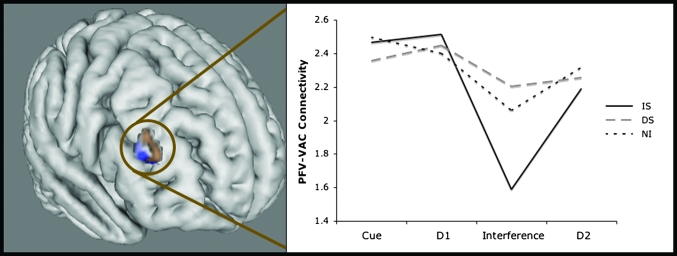
Previous studies have shown that the PFC and VAC are functionally connected during both WM encoding and maintenance of relevant stimuli (Gazzaley et al. 2004, 2007). In this multivariate analysis, we first searched for PFC regions whose across-trial activity pattern was significantly correlated with PPA activity (because the cue stimuli were scenes) during the encoding period for the 3 WM tasks combined (IS, DS, and NI, corrected by PV). Three ROIs within the PFC were identified (P < 0.05, corrected for multiple comparisons): right MFG, right superior frontal gyrus (SFG), and dorsal medial frontal gyrus (dmFG) regions. Given that the MFG connectivity was most significant (MFG: P = 0.007, SFG: P = 0.05, dmFG: P = 0.05) and previous literature has revealed its role in resistance to interference (Sakai et al. 2002) and WM maintenance (Leung et al. 2002), we focused further analyses on the MFG ROI. Peak and average z scores were extracted from each participant's correlation maps from the right MFG ROI during 4 stages of the task (encode, delay1, distractor, and delay2). The right MFG ROI exhibited differential connectivity during the interference period, such that connectivity to the PPA was significantly reduced for the interruptor (IS) compared with the distractor (DS) (P < 0.05), as well as compared with the same time period when no interfering stimuli were presented (NI) (P < 0.05) (FDR corrected for conditions and intervals) (see Fig. 6). MFG–PPA connectivity in DS and NI was not statistically different from one another during the interference period (P = 0.56). Furthermore, significant MFG–PPA connectivity existed at the time of interference only in the DS and NI conditions (IS: P = 0.1; DS: P = .01; NI: P = 0.01). Across the other stages of the task (encode, delay1, and delay2), IS, DS, and NI connectivity did not differ significantly. These results suggest that MFG–PPA connectivity drops in IS during the interference period but is maintained in NI and DS.
Figure 6.
MFG connectivity with PPA during WM maintenance. An area in the right MFG (brown) was identified with connectivity analysis using a PPA seed during the encoding period of the 3 WM tasks contrasted against PV (IS + DS + NI − 3PV). Connectivity between the PPA and the MFG area is maintained throughout the trial in both NI and DS whereas in IS connectivity declines during the interruption. A whole-brain correlation analysis using suppression indices as a regressor shows that in DS, stronger connectivity between the PPA and the right MFG (blue) is associated with greater suppression of the distractor. Right MFG is an ROI, not a statistical map, and the correlation analysis regressed with suppression has the cortex masked to highlight the area of interest.
In light of this finding, we investigated if there was evidence that scene memoranda may be reactivated in delay period 2 after the interruption (IS). Functional connectivity analyses revealed significant PPA–left MFG connectivity in delay 2 (t = 5.785, P < 0.00001). Further analysis, via a whole-brain regression analysis between PPA connectivity in delay 2 and WM accuracy, revealed connectivity between PPA-left MFG-predicted WM performance (P < 0.001).
PFC–VAC connectivity and suppression of distractors.
To gain insight into PFC contribution to suppression of VAC activity in the presence of face distractors (DS), and its impact on WM maintenance of the encoded scene stimuli, we performed an across-participant, whole-brain linear regression analysis: connectivity maps associated with maintenance of scene memoranda in the distractor period (PPA seed connectivity) vs suppression indices associated with the distracting face stimuli (FFA: PV–DS activity). Positive correlations in this analysis revealed brain regions that have greater connectivity with the PPA when activity in the FFA is suppressed. This analysis revealed a robust positive correlation within the right MFG (P < 0.001), which overlaps with the MFG region identified previously as being involved in maintenance of the stored memoranda (see Fig. 6). This suggests that connectivity between the right MFG and PPA is also related to the degree that participants suppress the face distractor.
PFC–VAC connectivity and enhancement of interruptors.
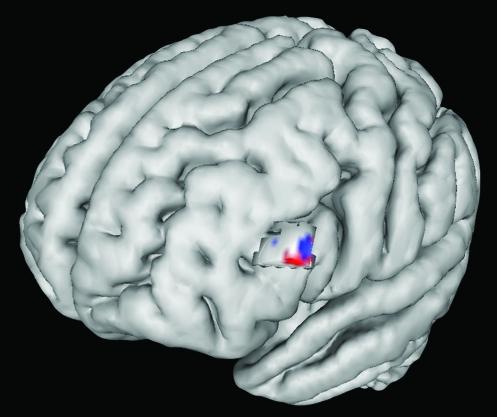
An across-participant, whole-brain regression analysis was performed to identify PFC control regions that may mediate the enhancement of activity in the VAC to the face interruptors (IS): connectivity maps associated with modulation of face interruptors (FFA seed connectivity) vs enhancement indices associated with the interrupting face stimuli (FFA: IS–PV activity). This analysis revealed a strong correlation within the left IFG (P < 0.0005), suggesting that functional connectivity between the IFG and FFA is associated with the degree that participants enhance the IS representation (see Fig. 7). This region overlaps with the PFC region that exhibits the greatest activity in IS vs DS during the interference period. These results suggest that IFG is involved in attentional allocation toward the interruption.
Figure 7.
Left IFG connectivity with FFA during interruption. An area in the left IFG (blue) was more active in IS than DS during the interference period with univariate analysis. Connectivity between this region (red) and the FFA was also found to correlate with enhancement of the BOLD signal in the FFA during interruption in the IS task. Cortical activity masked to highlight area of interest.
Discussion
The Impact of Interference on WM Performance
Incongruent stimuli (i.e., different category for cue and interfering stimuli) were utilized in the fMRI experiment in order to facilitate the investigation of both WM maintenance of stored memoranda (scenes) and mechanisms associated with interference (faces). This would not have been possible with congruent cue and interference stimuli, as carried out in the EEG experiment, due to the temporal lag of the hemodynamic response. It has previously been shown that congruent interference has a greater impact on WM performance than incongruent interference (Jha et al. 2004; Sreenivasan and Jha 2007; Yoon et al. 2006). Our results support this, as participants show a significant decline in performance when congruent interference was present in experiment 1, and only a strong trend toward significant WM disruption in experiment 2. The lack of significance in experiment 2 may also be due to a fewer number of trials used in the fMRI experiment. Despite a reduced behavioral impact by interference in this experiment, in accordance with the results of the EEG experiment, analysis of activity modulation in the FFA at the time of the interfering face stimulus replicated the finding that participants who were best able to suppress distractors exhibited the highest WM accuracy, and participants who allocated the least amount of attention to interruptors performed best on that WM task. Taken together, this suggests that a key component of successful WM in the setting of both types of interference is to allocate the least amount of attention to the interfering stimulus.
The Impact of Interference on WM Maintenance
The visual cortex has been implicated as a site where stored visual memoranda are likely represented (Serences et al. 2009). Maintained activity in the VAC has previously been shown to exist over the delay period (Ranganath et al. 2004), and a loss of delay period activity that is attributable to an interfering stimulus is assumed to reflect disruption of the memory trace (Miller and Desimone 1994). Investigation of PPA activity across the trial stages revealed that in the presence of interruption, activity associated with the memoranda is significantly more diminished in the delay period after the interruptor, compared with the same period after a distractor. We interpret these results to suggest that a representation of the encoded scene is maintained in the setting of distraction, whereas it is “released” in the context of an IS that requires attention.
Top–Down Control Networks in the Setting of Different Types of Interference
Previous evidence suggests that the PFC is involved in distinct and concurrent processes during the delay period in the setting of interference: 1) allocating attention toward or away from interference, 2) maintaining relevant memoranda in mind, and/or 3) reactivating representations if maintenance is disrupted. PFC univariate activity was evident during the period of distraction/interruption in both interference tasks. We used functional connectivity analyses to parse out which areas of the PFC are communicating with stimulus-specific VAC regions in the context of these different ongoing processes and how this varies across types of interference. fMRI connectivity analyses demonstrated that in the NI and DS tasks the MFG maintains connectivity with the scene-selective PPA across all task stages, whereas in IS, connectivity drops significantly at the time the interruptor is presented. We interpret this finding, in the context of the decrease in univariate activity in the PPA after interruptors (described above), to suggest that in the setting of either no external interference or an irrelevant distractor stimulus, a representation of the encoded stimulus is maintained via top–down control from the MFG to the VAC, whereas maintenance via network connectivity is interrupted when a participant engages in a secondary task. It is important to note that this correlational data does not reveal directionality of PFC control; this interpretation is based on an extension of previous findings in the literature regarding the PFC role in top–down modulation (review; Gazzaley and D'Esposito 2007).
These findings are consistent with previous fMRI studies that have reported univariate data in support of a role of the MFG in resisting interference. As mentioned in the introduction, Sakai et al. (2002a) showed that in a spatial WM task, sustained MFG activity in the delay period preceding interruption was associated with successful WM. Likewise, Olesen et al. (2006) suggested that stronger activity within the MFG in adults compared with children may reflect the maintenance of stored memoranda in the setting of distraction. More support for this theory comes from Dolcos et al. (2007) who demonstrated that activation within the MFG may reflect executive control mechanisms used to maintain WM content and lower the influence of distracting information. There also has been data reported regarding the role of the PFC and VAC in distraction resolution. Jha and colleagues (Jha et al. 2004; Sreenivasan and Jha 2007) showed that both the PFC and VAC showed greater activation during congruent distraction. They hypothesized that during congruent distraction, the PFC and VAC interact to support delay-spanning interference resolution and suggested that the processing of distractors may be attenuated due to attentional biasing toward the to-be-remembered information. This assertion is supported by the current data, which reveals that participants with the highest MFG–PPA connectivity exhibited the greatest suppression of the irrelevant face distractors. Thus, maintaining the stored memoranda over the delay period via PFC–VAC networks may serve to suppress encountered irrelevant information. Alternatively, because directionality cannot be directly interpreted from these data, the reverse could be true, such that the degree to which the distractors are represented in the VAC disrupts WM maintenance as reflected by reduced MFG–PPA activity.
Evidence from the univariate VAC data in the current experiment demonstrates that the amount of attention allocated to the interfering stimulus negatively correlates with WM performance. Thus, we probed whether a PFC region also mediates enhancement of the interrupting faces. Our results demonstrate that left IFG–FFA connectivity was positively correlated with the degree of enhancement in the FFA. These data suggest that poorer performing participants allocate too much attention toward the secondary task via top–down control from the IFG. The IFG has been previously been proposed to be involved in selection of information among competing alternatives (Thompson-Schill et al. 1997).
Univariate and connectivity data from the current study, as well as a previous fMRI investigation (Yoon et al. 2006), suggest that stored memoranda are released from WM maintenance when attention is directed toward an interruptor. However, the question arises as to when the representations of the memoranda are reactivated to perform the WM recognition task. Previous studies have proposed a role of the MFG in refreshing information that has been previously encoded during the delay period (Johnson et al. 2003; Miller et al. 2008). The current connectivity analysis adds a new level of detail by revealing that this area is functionally connected to the PPA during the delay period after the interruptor, while subjects are refreshing information as instructed. Furthermore, subjects that have the highest MFG–PPA connectivity following interruption perform best on the WM task. Additionally, the PPA time course revealed that participants who showed the least decline of PPA activation levels in delay2 compared with delay1 also performed best on the WM task. These findings demonstrate that reactivation of the encoded stimulus representation occurs during the postinterference delay period. It should be noted that reactivation activity during the probe has also been demonstrated, as revealed by Sakai and colleagues who reported a reactivation signal in the parahippocampal area after interruption of rehearsal (Sakai et al. 2002b). They did not, however, have a second delay period in this experiment, and thus, the reactivation potentially could have occurred during this period had they included a second delay.
Conclusions
In this study, EEG and fMRI were used to identify mechanisms that underlie WM disruption by different types of external interference. As predicted, interfering stimuli that demanded attention due to task goals (interruptors) had a more detrimental impact on WM performance than interfering stimuli that were irrelevant (distractors). We demonstrated that measures of attentional allocation, as reflected by the representation of interfering stimuli in the visual cortex using both modalities, predicted WM performance for both types of interference (distraction and interruption). The significance of replication between the fMRI and EEG results should not be understated, as these 2 techniques measure different aspects of the underlying neural activity and therefore provide converging information about the cortical mechanisms underlying these effects.
In the EEG experiment, we determined that WM disruption occurs at the presentation time of the interfering stimuli, and this impact occurs within the first 100 ms of stimulus onset. In the fMRI experiment, we evaluated top–down neural networks underlying the allocation of attention in the context of interference and how WM maintenance was impacted. Functional connectivity analysis allowed us to assess which PFC subregions, via their connectivity with the stimulus-selective VAC regions, were involved in maintenance vs interference processing. Analyses suggested that modulation of VAC activity at the time of interference, the maintenance of the stored memoranda across the delay period in the distractor task, and the refreshing of memoranda during the postinterference delay period in the interruptor task are all associated with top–down control via distinct PFC–VAC networks. These results further reveal that participants recruit different prefrontal networks depending upon the nature of interference.
Our findings demonstrate that higher performing young individuals suppress distracting information and direct less attention to ISs, which require an interaction (i.e., multitasking), whereas lower performing individuals allocate excessive attention to interfering stimuli of both types. Normal aging has been shown to be associated with a selective deficit in suppressing distraction information (Gazzaley et al. 2008; Gazzaley, Cooney, Rissman, and D'Esposito 2005). Future studies will investigate top–down control mechanisms of interference resolution in older adults and explore whether alterations in the mechanisms that underlie them lead to differential WM impairment in the setting of different types of interference.
Funding
National Institutes of Health (K08 AG025221, R01-AG030395); American Federation of Aging Research; University of California, Office of the President (UCOP) Presidential Postdoctoral Fellowship (to W.C.C.).
Acknowledgments
We thank Dr Peter Wais, Chips McSteeley, and Dr Ezequiel Morsella for critically reading the manuscript and for their helpful comments. Conflict of Interest: None declared.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Contribution of human prefrontal cortex to delay performance. J Cogn Neurosci. 1998;10:167–177. doi: 10.1162/089892998562636. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proc Natl Acad Sci U S A. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, Diaz-Granados P, Wang L, Mccarthy G. Opposing influences of emotional and non-emotional distracters upon sustained prefrontal cortex activity during a delayed-response working memory task. Neuropsychologia. 2008;46:326–335. doi: 10.1016/j.neuropsychologia.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Miller B, Kragel P, Jha A, Mccarthy G. Regional brain differences in the effect of distraction during the delay interval of a working memory task. Brain Res. 2007;1152:171–181. doi: 10.1016/j.brainres.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The locus of interference in the perception of simultaneous stimuli. Psychol Rev. 1980;87:272–300. [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D'Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci U S A. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D'Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, D'Esposito M. Unifying prefrontal cortex function: executive control, neural networks and top-down modulation. In: Cummings J, Miller B, editors. The human frontal lobes. New York: The Guildford Press; 2007. [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D'Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;(17 suppl 1):i125–135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Desposito M. Functional connectivity during working memory maintenance. Cogn Affect Behav Neurosci. 2004;4:580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- Goffaux V, Jemel B, Jacques C, Rossion B, Schyns P. ERP evidence for task modulations on face perceptual processing at different spatial scales. Cogn Sci. 2003;27:313–325. [Google Scholar]
- Gomez Gonzalez CM, Clark VP, Fan S, Luck SJ, Hillyard SA. Sources of attention-sensitive visual event-related potentials. Brain Topogr. 1994;7:41–51. doi: 10.1007/BF01184836. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Ehlis A, Ellgring H, Fallgatter A. Early stages (P100) of face perception in humans as measured with event-related potentials (ERPs) J Neural Transm. 2005;112:1073–1081. doi: 10.1007/s00702-004-0250-8. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci U S A. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AP, Fabian SA, Aguirre GK. The role of prefrontal cortex in resolving distractor interference. Cogn Affect Behav Neurosci. 2004;4:517–527. doi: 10.3758/cabn.4.4.517. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Anderson AW. fMRI evidence for an organization of prefrontal cortex by both type of process and type of information. Cereb Cortex. 2003;13:265–273. doi: 10.1093/cercor/13.3.265. [DOI] [PubMed] [Google Scholar]
- Jonides J. Further toward a model of the mind's eye's movement. Bull Psychon Soc. 1983;21:247–250. [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J Cogn Neurosci. 2002;14:659–671. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher N. Stages of processing in face perception: an MEG study. Nat Neurosci. 2002;5:910–916. doi: 10.1038/nn909. [DOI] [PubMed] [Google Scholar]
- Luck S, Vecera S. Attention. In: Yantis S, editor. Stevens’ handbook of experimental psychology. New York: Wiley; 2002. pp. 235–286. [Google Scholar]
- Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BT, Verstynen T, Johnson MK, D'Esposito M. Prefrontal and parietal contributions to refreshing: an rTMS study. NeuroImage. 2008;39:436–440. doi: 10.1016/j.neuroimage.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Olesen P, Macoveanu J, Tegner J, Klingberg T. Brain activity related to working memory and distraction in children and adults. Cereb Cortex. 2006;17:1047–1054. doi: 10.1093/cercor/bhl014. [DOI] [PubMed] [Google Scholar]
- Postle BR, Desposito M, Corkin S. Effects of verbal and nonverbal interference on spatial and object visual working memory. Mem Cogn. 2005;33:203–212. doi: 10.3758/bf03195309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Druzgal TJ, D'Esposito M. Seeking the neural substrates of visual working memory storage. Cortex. 2003;39:927–946. doi: 10.1016/s0010-9452(08)70871-2. [DOI] [PubMed] [Google Scholar]
- Ranganath C, DeGutis J, D'Esposito M. Category-specific modulation of inferior temporal activity during working memory encoding and maintenance. Brain Res Cogn Brain Res. 2004;20:37–45. doi: 10.1016/j.cogbrainres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rutman AM, Clapp WC, Chadick JZ, Gazzaley A. Early top-down control of visual processing predicts working memory performance. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21257. Epub ahead of print, May 4, doi:10.1162/jocn.2009.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K. Reactivation of memory: role of medial temporal lobe and prefrontal cortex. Rev Neurosci. 2003;14:241–252. doi: 10.1515/revneuro.2003.14.3.241. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal selection and medial temporal lobe reactivation in retrieval of short-term verbal information. Cereb Cortex. 2004;14:914–921. doi: 10.1093/cercor/bhh050. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002a;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Parahippocampal reactivation signal at retrieval after interruption of rehearsal. J Neurosci. 2002b;22:6315–6320. doi: 10.1523/JNEUROSCI.22-15-06315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Khateb A. Group analysis and the subject factor in functional magnetic resonance imaging: analysis of fifty right-handed healthy subjects in a semantic language task. Hum Brain Mapp. 2008;29:461–477. doi: 10.1002/hbm.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Ester EF, Vogel EK, Awh E. Stimulus-specific delay activity in human primary visual cortex. Psychol Sci. 2009;20:207–214. doi: 10.1111/j.1467-9280.2009.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan KK, Jha AP. Selective attention supports working memory maintenance by modulating perceptual processing of distractors. J Cogn Neurosci. 2007;19:32–41. doi: 10.1162/jocn.2007.19.1.32. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Curtis CE, D'Esposito M. Differential effects of distraction during working memory on delay-period activity in the prefrontal cortex and the visual association cortex. NeuroImage. 2006;29:1117–1126. doi: 10.1016/j.neuroimage.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. J Neurosci. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D'Esposito M. A trial-based experimental design for fMRI. NeuroImage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]