Abstract
Uncertainty about potential negative future outcomes can cause stress and is a central feature of anxiety disorders. The stress and anxiety associated with uncertain situations may lead individuals to overestimate the frequency with which uncertain cues are followed by negative outcomes, an example of covariation bias. Using functional magnetic resonance imaging, we found that uncertainty-related expectations modulated neural responses to aversion. Insula and amygdala responses to aversive pictures were larger after an uncertain cue (that preceded aversive or neutral pictures) than a certain cue (that always preceded aversive pictures). Anticipatory anterior cingulate cortex (ACC) activity elicited by the cues was inversely associated with the insula and amygdala responses to aversive pictures following the cues. Nearly 75% of subjects overestimated the frequency of aversive pictures following uncertain cues, and ACC and insula activity predicted this uncertainty-related covariation bias. Findings provide the first evidence of the brain mechanisms of covariation bias and highlight the temporal dynamics of ACC, insula, and amygdala recruitment for processing aversion in the context of uncertainty.
Keywords: anterior cingulate cortex, covariation bias, emotion, expectancy, fMRI
Introduction
Knowledge about upcoming adverse circumstances can be helpful in terms of preparing for and potentially avoiding such events. However, there is often uncertainty about whether an upcoming aversive event will actually occur and how dangerous or negative it will be. Such uncertainty is central to worry and negative expectations about future events that can be debilitating in individuals suffering from anxiety disorders (Dugas et al. 1998; Barlow 2002; Lohr et al. 2007; Krain et al. 2008; Simmons et al. 2008). The resolution of uncertainty can be achieved through the detection of contingencies between environmental cues and subsequent aversive events. Such contingency detection allows individuals to explain past events and more appropriately prepare for the future (Alloy and Tabachnik 1984). This process, however, is subject to errors involved in the perception and interpretation of contingencies, and can result in “illusory correlations,” or the identification of relationships between cues and subsequent outcomes that in reality are not related (Chapman and Chapman 1967, 1969).
Illusory correlation paradigms have been used to identify overestimates of the covariance of fear-relevant cues and subsequent aversive outcomes, known as covariation biases (Tomarken et al. 1989). The covariation bias has primarily been investigated as it relates to phobias and other anxiety disorders (Tomarken et al. 1989; de Jong et al. 1992; Pauli et al. 1996, 1998; Amin and Lovibond 1997; Kennedy et al. 1997; Hermann et al. 2004). Biased estimates of covariance have also been identified in individuals without anxiety symptoms, given an adequately salient or fear-relevant cue (Tomarken et al. 1989; Pury and Mineka 1997; de Jong et al. 1998). Uncertainty about potential aversive stimuli is a fear-relevant cue (Freeston et al. 1994; Barlow 2002; Buhr and Dugas 2002) that would be expected to result in overestimates of the relationship between uncertain cues and aversive outcomes.
Previous research has demonstrated that the anterior cingulate cortex (ACC), insula, and amygdala are recruited under conditions of uncertainty (Critchley et al. 2001; Davis and Whalen 2001; Volz et al. 2003; Hsu et al. 2005; Paulus 2005; Grinband et al. 2006; Krain et al. 2006; Rosen and Donley 2006; Belova et al. 2007; Dunsmoor et al. 2007; Hasler et al. 2007; Herry et al. 2007; Platt and Huettel 2008; Preuschoff et al. 2008). Importantly, these same broad regions have also been shown to be activated by a wide range of aversive stimuli and conditioning paradigms (Büchel et al. 1998, 1999; Ploghaus et al. 1999; Davis and Whalen 2001; Craig 2002, 2003; LeDoux 2002; Han et al. 2003; Phillips et al. 2003; Mackiewicz et al. 2006; Nitschke, Dixon, et al. 2006; Nitschke, Sarinopoulos, et al. 2006; Bissière et al. 2008). Uncertainty about the likelihood of an aversive event may serve to enhance responses to aversion when such events do occur, as illustrated by studies showing that aversive events are more stressful when associated with or preceded by uncertainty than certainty (Peeke and Grings 1968; Grings and Schell 1971; Lykken et al. 1972; Craske et al. 1995; Nader and Balleine 2007). Although further attention is needed to identify the specific regions within the ACC, insula, and amygdala showing overlap in the literatures on uncertainty and on aversion, these 3 brain areas are promising candidates for a modulatory neural network that can account for such uncertainty-enhanced responses to aversion. Indeed, a recent study found greater amygdala responses to an aversive noise (unconditioned stimulus) that was paired with a conditioned stimulus on 50% of trials than to the same noise paired with a conditioned stimulus on all trials (Dunsmoor et al. 2008).
The current study investigated the temporal unfolding of activity in the ACC, insula, and amygdala that transpires between the introduction of uncertainty and subsequent aversive stimuli. Modifying a paradigm previously shown to activate the ACC, insula, and amygdala in anticipation of and response to aversive pictures (Mackiewicz et al. 2006; Nitschke, Sarinopoulos, et al. 2006), we added an anticipatory cue signaling uncertain outcomes. Building on insula and amygdala findings for aversion and uncertainty (Ploghaus et al. 1999; Hsu et al. 2005; Mackiewicz et al. 2006; Nitschke, Sarinopoulos, et al. 2006; Dunsmoor et al. 2007, 2008; Herry et al. 2007), we predicted larger insula and amygdala responses to aversive pictures following an uncertain cue that subjects were told could be followed by aversive or neutral pictures than a certain cue that was paired with aversive pictures on all trials. Based on research implicating the ACC in the modulation of aversion expectancy (Ploghaus et al. 2003; Petrovic et al. 2005; Sarinopoulos et al. 2006) and top-down emotion regulation (Phan et al. 2005; Etkin et al. 2006; Urry et al. 2006; Johnstone et al. 2007), we hypothesized that individual differences in cue-elicited anticipatory ACC activation would be inversely associated with insula and amygdala responses to pictures following the cues. The specific subregions expected to show these effects were the anterior insula and mid-insula (Craig 2009) as well as the pregenual and subgenual ACC (Etkin et al. 2006; Urry et al. 2006; Johnstone et al. 2007). In addition, this study investigated an uncertainty-related covariation bias. After the scan, subjects estimated the frequency of uncertain cues followed by aversive pictures, and we tested whether these covariation estimates were associated with activity in the hypothesized modulatory network comprising the ACC, insula, and amygdala.
Materials and Methods
Subjects
Forty right-handed healthy undergraduate students (18 women and 22 men; age M = 20.65, SD = 1.53) who responded to flyers posted in University of Wisconsin-Madison buildings participated in the study. Subjects reported no medical, neurological, or psychiatric problems and took no medications. Four subjects were dropped from analyses—2 women due to technical difficulties with functional magnetic resonance imaging (fMRI) data acquisition, one man due to excessive movement during fMRI data acquisition, and one man who did not follow task instructions for the fMRI paradigm—resulting in a final sample of 36 subjects (age M = 20.4, SD = 1.4). All subjects gave informed consent in accord with study approval by the Human Subjects Committee of the University of Wisconsin Medical School and were paid for their participation.
Experimental Design and Stimuli
As shown in supplementary Figure S1, each trial consisted of an anticipatory cue presented for 1 s, followed by a black screen presented for 6, 7, 8, 9, or 10 s. This was followed by the presentation of an aversive or neutral picture for 1 s and another black screen presented for 6, 7, 8, 9, or 10 s. For aversive trials, the cue was an “X,” which was always followed by an aversive picture. For neutral trials, the cue was an “O,” which was always followed by a neutral picture. For uncertain trials, the cue was a “?,” which was followed by either an aversive or neutral picture at exactly a 50/50 ratio. Subjects were instructed about all cue-picture pairings prior to scanning, but were not informed of the proportion of aversive versus neutral pictures that followed the uncertain cue. All cues were white and presented on a black background and were of similar size. Trial order was pseudorandomized, with the stipulation that no trial type (aversive, neutral, or uncertain) was presented more than twice in a row. Trial length varied from 14 to 22 s, with an average trial length of 18 s. The duration of the interstimulus interval (6–10 s) following the anticipatory cue and picture presentation was randomized across trials. In designing the experimental paradigm, simulations using the RSGgen program provided by Analysis of Functional NeuroImages (AFNI) (Cox 1996) indicated that this trial structure employing variable interstimulus intervals optimized the separation and estimation of the hemodynamic response for the anticipation and response periods, while minimizing the number of trials and subject time in the scanner.
There were a total of 3 functional scan runs, each consisting of 8 aversive trials, 8 neutral trials, and 8 uncertain trials. The 3 scan run lengths were 8:52, 9:00, and 8:46, which included a 30-s black screen at the start of each run. Using a response box during the fMRI experiment, subjects were instructed to press one button after each cue and after each picture, and to press a second button if they saw a square in place of the cue or picture. Because subjects were not instructed to press these buttons immediately after the cue or picture, reaction time data for button presses were abnormally long (typically greater than 1 s) and therefore were not analyzed. There were 2 trials with a square in the first functional run, 3 in the second run, and 2 in the third run. These trials were employed to help maintain subjects’ attention to the cue and picture stimuli and due to their infrequency were not modeled or analyzed.
During the fMRI experiment, subjects viewed 75 pictures from the International Affective Picture Set (Lang et al. 1999), with no picture shown more than once. The aversive pictures were carefully selected from the most unpleasant and arousing in the picture set (e.g., mutilated bodies, attack scenes), based on published norms (Lang et al. 1999). Pictures with neutral valence and low arousal ratings comprised the neutral pictures (e.g., household items). Of the 72 pictures on trials included in analyses (the remaining 3 were on trials with a square in place of the cue), 36 were aversive and 36 were neutral. Of the 36 aversive pictures, 24 were presented on aversive trials and the remaining 12 on uncertain trials. Similarly, 24 of the neutral pictures were presented on neutral trials and 12 on uncertain trials. Males and females viewed slightly different sets of neutral and aversive pictures in order to yield equivalent valence and arousal ratings (Lang et al. 1999) across gender. Within each gender, the 36 aversive pictures were divided into 3 sets of 12, such that the aversive pictures preceded by “?” cues in 1/3 of the participants were preceded by “X” cues in the other 2/3 of the participants (similar counterbalancing also took place for neutral pictures).
fMRI Data Acquisition
Anatomical and functional data were collected on a General Electric 3.0 Tesla system (Waukesha, WI) equipped with a quadrature head coil. Whole-brain anatomical images were acquired using an axial T1-weighted 3D spoiled gradient-recalled echo scan (SPGR; repetition time/echo time [TR/TE] = 35/8 ms, flip angle [α] = 30°, number of excitations [NEX] = 1, field of view [FOV] = 24 × 24 cm, matrix = 256 × 192, slice thickness/gap = 1.2 mm, 124 slices). Whole-brain functional images were acquired using sagittal T2*-weighted echo-planar scans (EPI; TR/TE = 2000/30 ms, α = 90°, NEX = 1, FOV = 24 × 24 cm, matrix = 64 × 64, in-plane resolution = 3.75 × 3.75 mm, slice thickness/gap = 4/1 mm, 30 interleaved slices [a total of 263–270 whole-brain slices collected per functional run]). Four EPI images with identical acquisition parameters but with TEs of 30, 31, 33, and 36 ms were acquired to create field maps to correct for image distortion.
A Silent Vision system (Avotec, Inc., Jensen Beach, FL) displayed the stimuli via a pair of stereoscopic goggles. Head movement was restricted using a customized bite bar, which consisted of dental impression compound affixed to an acrylic plate. Both the goggles and the bite bar were mounted directly to the head coil. Approximately one week before the fMRI experimental session, all subjects were positioned in a mock scanner, including head coil, goggles, bite bar, response box, and digitized scanner sounds. After being instructed about all cue-picture pairings, subjects viewed an abbreviated version of the experimental paradigm, using pictures not shown during the experimental session.
fMRI Data Analysis
All fMRI data processing was done with AFNI version 2.41 software (Cox 1996). Data processing included reconstruction with smoothing in Fourier space using a Fermi filter, 6-parameter rigid-body motion correction, volume registration, and removal of skull and gross artifacts. A high-pass temporal Fourier filter (cutoff = 0.017 Hz) was applied to each of the 3 functional runs and then the data were concatenated. In-house software was used to correct for image distortion at each time point based on the field maps. Single-subject time series were analyzed using a general linear model (GLM) with separate regressors for the anticipation and picture periods, formed by convolving stimulus functions with an ideal hemodynamic response function. Resultant beta weights were converted to percentage signal change. The resultant percentage signal change maps from the GLM were Gaussian-blurred with full width at half maximum = 4 mm and transformed into the standardized Talairach space via identification of anatomical landmarks on the high-resolution SPGR anatomical scan (Talairach and Tournoux 1988; Lancaster et al. 2000). This was done by interactively placing the Talairach landmarks within the anatomical image, notably the anterior commissure and posterior commissure as well as the left, right, anterior, posterior, cranial, and caudal outer edges of the brain. We then ran an automated data-to-atlas warping based on the Talairach proportional grid transformation.
To assess the extent of signal loss resulting from differential magnetic susceptibility coefficients at bone/air/tissue boundaries, we calculated signal-to-noise ratios (SNR) resulting from the above fMRI data acquisition parameters in insula, amygdala, and ACC clusters identified in this report. For comparison purposes, we calculated SNR from Talairach-defined clusters in regions with minimal signal loss such as the superior frontal gyrus and precuneus (Talairach and Tournoux 1988; Lancaster et al. 2000). SNR was determined independently for each voxel by dividing the mean time series signal by the standard deviation of that time series signal. Regional SNR estimates were obtained by averaging across voxels in each region of interest (ROI) mask. As expected, there was some signal loss in ventral aspects of the amygdala, which compromised our ability to detect effects there (Mackiewicz et al. 2006). Adequate signal was observed for all functional activations reported here. SNR values ranged from 60 to 81 for clusters in the superior frontal gyrus and precuneus. For the areas circled in Figure 1, SNR values for the insula activations ranged from 68 to 82, and SNR values for the amygdala activations ranged from 44 to 53. SNR values for the ACC areas in Figures 2 and 3 ranged from 32 to 81.
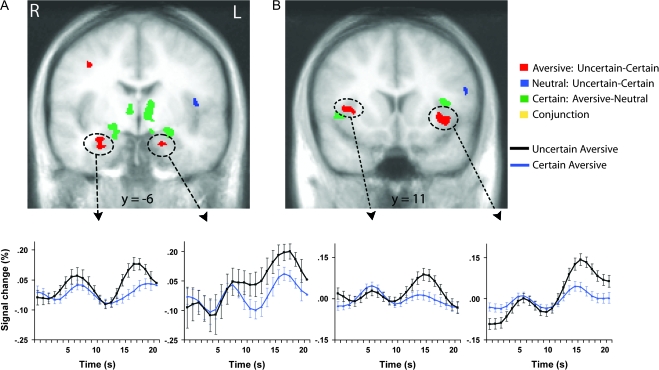
Figure 1.
Amygdala (A) and mid-insula (B) regions responding to uncertainty and aversion. Red areas showed greater activation to aversive pictures preceded by the uncertain cue than to aversive pictures preceded by the certain aversive cue. Blue areas showed greater activation to neutral pictures preceded by the uncertain cue than to neutral pictures preceded by the certain neutral cue. Green areas showed greater activation to aversive pictures preceded by the certain aversive cue than to neutral pictures preceded by the certain neutral cue. Each contrast corresponds to a voxelwise t-test (P < 0.05, corrected). Time series plots of the circled clusters illustrate average percentage signal change across all time points of trials with aversive pictures preceded by the uncertain cue (black) and trials with aversive pictures preceded by the certain aversive cue (blue). Error bars are for the SEM after adjusting for between-subject variance (Loftus and Masson 1994). R = right; L = left.
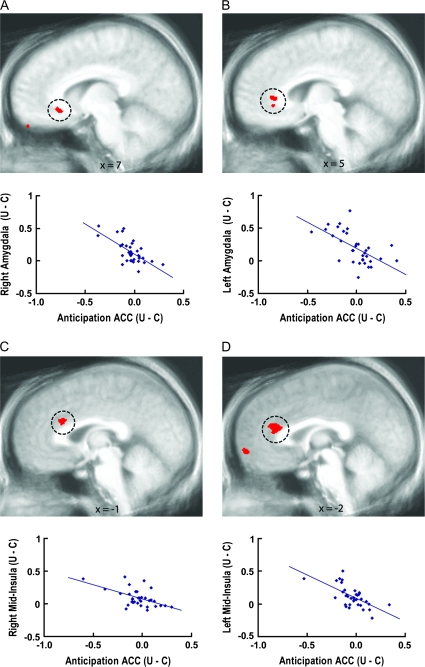
Figure 2.
Anticipatory ACC activation associated with amygdala and mid-insula responses modulated by uncertainty. Voxelwise regressions (P < 0.05, corrected) indicated relationships between anticipatory activation in circled ACC regions and activation in the right amygdala (A), left amygdala (B), right mid-insula (C), and left mid-insula (D) areas circled in Figure 1 that were modulated by uncertainty. Plots illustrate that individual differences in anticipatory ACC activity were inversely associated with mid-insula and amygdala responses, such that individuals with greater ACC activation to the uncertain cue than the certain aversive cue showed the smallest uncertainty-related enhancement of mid-insula and amygdala activation to aversive pictures. U = uncertain condition; C = certain condition.
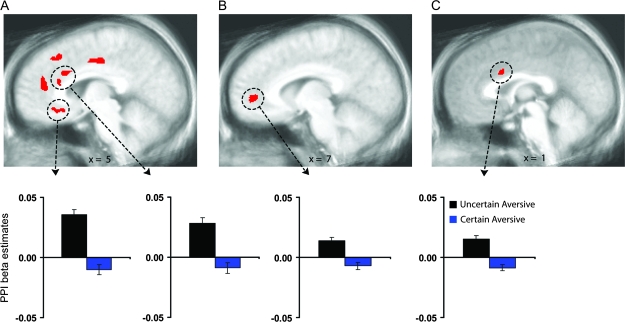
Figure 3.
Greater coupling of ACC with the amygdala and mid-insula following uncertainty. Circled ACC regions showed greater coupling with the right amygdala (A), left amygdala (B), and left mid-insula (C) clusters circled in Figure 1 (P < 0.05, corrected) during aversive pictures preceded by the uncertain cue than during aversive pictures preceded by the certain cue. Bar graphs of the circled clusters illustrate average psychophysical interaction (PPI) beta coefficients of the aversive pictures preceded by the uncertain cue (black) and aversive pictures preceded by the certain aversive cue (blue). Error bars are for the SEM after adjusting for between-subject variance (Loftus and Masson 1994).
To statistically evaluate the main hypothesis that insula and amygdala regions would activate more to aversive pictures preceded by the uncertain cue (“?”) than to aversive pictures preceded by the certain aversive cue (“X”), a voxelwise whole-brain Student's paired t-test compared these 2 conditions. Using the Talairach-defined anatomical boundaries of the 2 structures (Talairach and Tournoux 1988; Lancaster et al. 2000), we applied a small volume correction for multiple comparisons using Monte Carlo simulations (AlphaSim in AFNI). The spatial correlation of the input data and an uncorrected P value threshold of 0.005 resulted in a minimum cluster size of 155 mm3 for this voxelwise t-test to achieve a corrected P < 0.05. The same voxelwise t-test and correction procedure were applied for 2 additional relevant contrasts: aversive pictures preceded by the certain aversive cue (“X”) versus neutral pictures preceded by the certain neutral cue (“O”), and certain aversive cues (“X”) versus uncertain cues (“?”).
To test the hypothesis that the observed difference between aversive pictures following uncertain and certain cues in the insula and amygdala would be associated with activation in ACC regions during the anticipation period, we first extracted percent signal change values for the 2 aversive picture conditions from the insula and amygdala using the functional ROIs circled in Figure 1. Difference scores (aversive pictures following uncertain cue-aversive pictures following certain cue) for each subject were calculated for each of those insula and amygdala ROIs. We then performed voxelwise regressions, regressing these percent signal change difference scores (uncertain–certain) for the picture period in the insula and amygdala seed regions on the uncertain versus certain contrast brain map for the anticipation period. Based on research implicating the ACC in modulating expectancies of aversion (Ploghaus et al. 2003; Petrovic et al. 2005; Sarinopoulos et al. 2006), as well as emotion regulation and fear extinction (Phelps et al. 2004; Phan et al. 2005; Etkin et al. 2006; Urry et al. 2006; Johnstone et al. 2007; Egner et al. 2008), the search volume for these analyses was restricted to the ACC (Talairach and Tournoux 1988; Lancaster et al. 2000), using the same method described above for the voxelwise t-test. A small volume correction for multiple comparisons for an uncorrected P value threshold of 0.005 corresponded to a minimum cluster size of 167 mm3 in order to achieve a corrected map-wise P < 0.05. The analogous analytic strategy was also implemented for regressions examining associations with ACC activations during the picture period, in order to assess relations within the same temporal epoch as the insula and amygdala responses to the aversive pictures. Such within-period regressions are far more common than the aforementioned cross-period regressions (Phelps et al. 2004; Etkin et al. 2006; Urry et al. 2006; Johnstone et al. 2007).
In addition to the above cross-subject regression analyses, psychophysiological interactions (PPI) methods (Friston et al. 1997) were used to calculate within-subject covariation of activation (coupling) during the picture period. Separate voxelwise multiple regression analyses were conducted for aversive pictures following certain cues and for aversive pictures following uncertain cues. Each regression analysis included 2 first-order main effect terms and their second-order interaction. The first-order terms were 1) the modeled hemodynamic response for aversive pictures following certain or uncertain cues and 2) the extracted time series from the amygdala and insula using the functional ROIs circled in Figure 1. The key term for determining condition-specific coupling is the second-order interaction term, derived here by multiplying those 2 first-order terms. Resultant interaction beta weights from each regression analysis were compared with one another using voxelwise Student's paired t-tests for each of the identified insula and amygdala ROIs. The small volume correction threshold of 167 mm3 was again applied to achieve a corrected map-wise P < 0.05 across the ACC.
To obtain a measure of uncertainty-related covariation bias, the experimental manipulation of uncertainty was implemented using an illusory correlation paradigm (Chapman and Chapman 1967; Tomarken et al. 1989), with the uncertain cue uncorrelated with the aversive and neutral pictures (i.e., uncertain cue followed by aversive and neutral pictures at 50/50 ratio). Immediately following the scan, subjects were asked to provide their estimation of the percentage of uncertain cues that were followed by aversive pictures during the scan. To assess a general negativity bias not related to uncertainty, subjects were also asked to estimate the percentage of certain aversive cues relative to certain neutral cues (which were presented at a 50/50 ratio). Two subjects did not follow instructions to provide percentages and were excluded from analyses, resulting in a sample of 34 for these analyses. Covariation bias was operationalized as each subject's reported estimate of the percentage of uncertain cues followed by aversive pictures. To assess the association of individual differences in covariation bias and uncertainty-related brain activation patterns, a voxelwise regression was conducted that regressed the subjects’ covariation estimates on the brain contrast map comparing activation to aversive pictures following uncertain cues versus aversive pictures following certain cues. Similar voxelwise regressions were also conducted for the anticipation period (uncertain cue–certain aversive cue) and for each condition separately.
To determine whether observed associations with covariation bias could be explained by a general negativity bias or negative affect, correlations with all areas identified for the above voxelwise regressions for the covariation estimates were assessed after partialing out general negativity bias scores (subjects’ reported estimates of the percentage of certain aversive cues relative to certain neutral cues) as well as negative affect scores on the Positive and Negative Affective Schedule (PANAS; Watson et al. 1988). Comprised of a 10-item Negative Affect scale and a 10-item Positive Affect scale, the PANAS asks subjects to rate the degree to which each emotional adjective descriptor applies to them in general. All subjects completed the PANAS after providing the covariation estimates at the end of the scan. In addition to the aforementioned partial correlations, voxelwise regressions were conducted, regressing scores for the Negative Affect scale on the contrast map for aversive pictures following uncertain cues versus aversive pictures following certain cues, and on brain maps of those uncertain and certain conditions separately. Similar regressions were conducted for the anticipation period, and for the Positive Affect scale. For all regression analyses involving covariation bias and self-reported affect, the search volume was restricted to insula, amygdala and ACC regions (Talairach and Tournoux 1988; Lancaster et al. 2000), using the thresholds determined above.
To assess asymmetry, significant insula and amygdala clusters identified in the voxelwise t-tests above were dilated 250% and used to identify the homologous cluster in the opposite hemisphere (Nitschke, Sarinopoulos, et al. 2006). Average percentage signal change values from the dilated clusters on the same and homologous side were extracted, and the values entered into appropriate repeated-measures analyses of variance (ANOVAs) with Hemisphere (Left, Right) as a within-subjects factor. Clusters identified in the voxelwise regression analyses above were also dilated and used to identify the homologous cluster in the opposite hemisphere. Correlation coefficients associated with each cluster on the same and homologous side were then extracted and the values were statistically compared by means of Fisher's Z score tests. Results of these procedures without dilation and with a larger dilation of 500% resulted in similar conclusions about asymmetry. With respect to concerns about the use of selection statistics and test statistics that are not inherently independent (Kriegeskorte et al. 2009; Vul et al. 2009), this assessment of laterality may overestimate the degree of asymmetry.
Results
Behavioral Rating Data
Although the uncertain cue was followed by aversive or neutral pictures at exactly a 50/50 ratio for all subjects, 25 of the 34 subjects estimated that greater than 50% of the uncertain cues were followed by aversive pictures, and the mean estimate was significantly higher than the true 50/50 ratio (M = 63.68, SD = 10.47; t(33) = 7.62, P < 0.001). Only 9 subjects accurately estimated 50%, and none estimated that there were more neutral than aversive pictures following the uncertain cues. Half of the subjects overestimated the percentage of uncertain cues followed by aversive pictures at rates of 65% or higher, up to 85%. In addition, subjects demonstrated a general negativity bias in their estimations of the proportion of certain aversive to certain neutral cues, which were also presented at exactly a 50/50 ratio for all subjects (M = 62.88, SD = 8.03; t(33) = 9.36, P < 0.001). There was a significant correlation between covariation bias scores and general negativity bias scores (r = 0.527, P < 0.002). Scores on the Positive and Negative Affect Schedule (PANAS) were similar to normative data (Watson et al. 1988; Crawford and Henry 2004) for both the Negative Affect scale (M = 12.78, SD = 3.30) and the Positive Affect scale (M = 31.25, SD = 6.08). Scores for the 2 PANAS scales were not correlated with covariation bias scores or general negativity bias scores (all ps > 0.20).
Brain Activation to Aversion and Uncertainty
Bilateral mid-insula and amygdala responses to aversive pictures were larger following the uncertain cue (“?”) than the certain aversive cue (“X”). Figure 1 illustrates that these areas were distinct from bilateral insula and amygdala areas that showed greater activation to aversive pictures preceded by the certain aversive cue (“X”) than neutral pictures preceded by the certain neutral cue (“O”) (supplementary Table S1). The uncertainty effects for the mid-insula and amygdala did not change over the course of the experiment, as indicated by nonsignificant interactions for Uncertainty (aversive pictures preceded by the uncertain cue, aversive pictures preceded by the certain cue) x Run (1, 2, 3) ANOVAs conducted on a voxelwise basis as well as on the mid-insula and amygdala ROIs circled in Figure 1. supplementary Figure S2 depicts a subgenual ACC area that showed the same uncertainty-enhanced responses to aversive pictures.
Asymmetry was assessed for each uncertainty- and aversion-related cluster shown in Figure 1 (see Methods). The left insula clusters showing uncertainty and aversion effects were asymmetric, as indicated by an Uncertainty × Hemisphere interaction (F1,35 = 6.07, P < 0.02) and a Valence × Hemisphere interaction (F1,35 = 6.12, P < 0.02), respectively. All other insula and amygdala activations in Figure 1 were bilateral, as indicated by nonsignificant interactions with Hemisphere.
The only area showing differential activation between the certain aversive cue and the uncertain cue was in the left anterior insula (1674 mm3, x = −35, y = 18, z = 6), with greater activation observed for the certain aversive cue. This left anterior insula finding for anticipatory activity was asymmetric, as indicated by an Uncertainty × Hemisphere interaction (F1,35 = 7.43, P < 0.01). For the sake of comparison, no region of the anterior insula, mid-insula, amygdala, or ACC showed differential activation between the certain neutral cue and the uncertain cue, or between neutral pictures following the uncertain cue and the neutral pictures following the certain neutral cue (blue in Fig. 1 and supplementary Fig. S2), although there was an operculum/posterior insula cluster for the latter (317 mm3, x = −43, y = −4, z = 10; Fig. 1A).
Anticipatory ACC activity (uncertain cue–certain aversive cue) was inversely associated with mid-insula and amygdala responses circled in Figure 1 for the corresponding contrast comparing aversive pictures following uncertain and certain cues (Fig. 2, supplementary Fig. S3, supplementary Table S1), as revealed by cross-period voxelwise regressions. Regarding the specific ACC regions (Pezawas et al. 2005; Palomero-Gallagher et al. 2008; Johansen-Berg et al. 2008; Beckmann et al. 2009; Nitschke et al. 2009) showing these effects, supragenual ACC areas were associated with the mid-insula responses (Fig. 2C,D), whereas subgenual and pregenual ACC areas were associated with the respective right and left amygdala responses (Fig. 2A,B). Correlations of anticipatory ACC activity with each of the bilateral mid-insula and amygdala regions remained significant after partialing out ACC activation to the pictures in the same areas (all ps < 0.005).
Subgenual ACC responses to the pictures (aversive pictures following uncertain cues-aversive pictures following certain cues) were inversely associated with the left mid-insula and bilateral amygdala responses circled in Figure 1 for the same contrast, as indicated by within period voxelwise regressions (supplementary Fig. S4, supplementary Table S1). These correlations remained significant after partialing out anticipatory activation in these subgenual ACC areas (all ps < 0.001). The anticipatory ACC areas above did not overlap with these subgenual ACC areas for picture viewing, and both sets of areas remained significant when entered into simultaneous regressions predicting the mid-insula and amygdala modulation (all ps < 0.05).
In addition to these cross-subject connectivity analyses, within-subject connectivity was assessed using PPI methods to test the coupling of ACC activation with concurrent mid-insula and amygdala activation in response to the aversive pictures. The ACC showed greater functional connectivity with left mid-insula and bilateral amygdala responses to aversive pictures following uncertain cues relative to aversive pictures following certain cues (Fig. 3, supplementary Fig. S5, supplementary Table S1). Consistent with the locations found for the anticipatory ACC associations above, a supragenual ACC area showed coupling with the mid-insula responses (Fig. 3C), whereas subgenual and pregenual ACC areas were coupled with the respective right and left amygdala responses (Fig. 3A,B). An additional supragenual ACC locus was also present for the right amygdala (Fig. 3A). No ACC areas exhibited greater functional connectivity for the certain condition.
Relations among Brain Activation, Covariation Bias, and Self-Reported Affect
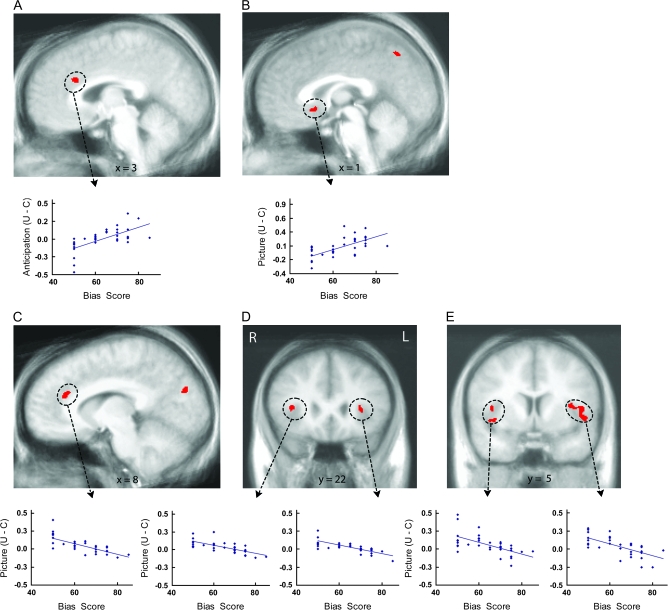
A voxelwise regression indicated that greater anticipatory supragenual ACC activation for uncertain cues relative to certain aversive cues was associated with higher covariation estimates (i.e., overestimations of the percentage of uncertain cues followed by aversive pictures) provided by the subjects directly after the scan (Fig. 4A, supplementary Table S2). Similarly, greater subgenual ACC activation in response to aversive pictures following uncertain cues (relative to aversive pictures following certain cues) was associated with higher covariation estimates (Fig. 4B, supplementary Table S2). The opposite pattern was observed for supragenual ACC and insula responses to the pictures (Fig. 4C–E, supplementary Table S2), with greater activation to aversive pictures following uncertain cues (relative to aversive pictures following certain cues) associated with lower covariation estimates. The anterior and mid-insula effects in Figure 4D,E were bilateral, as indicated by formal tests of asymmetry. The mid-insula effects overlapped with the bilateral insula areas showing the uncertainty effect circled in Figure 1B. Correlations for all the ACC and insula regions in Figure 4 remained significant after partialing out general negativity bias scores and PANAS Negative Affect scores (all ps < 0.005). Voxelwise regressions conducted for uncertain and certain conditions separately resulted in no ACC, insula, or amygdala effects.
Figure 4.
Individual differences in covariation bias associated with uncertainty-related activation. Voxelwise regressions (P < 0.05, corrected) indicated relationships between covariation bias scores and uncertainty-related activation in the ACC and insula. Top plots illustrate positive associations between covariation estimates and greater anticipatory activation to the uncertain cue than the certain aversive cue in the supragenual ACC (A) and greater activation to aversive pictures preceded by the uncertain cue than aversive pictures preceded by the certain aversive cue in the subgenual ACC (B). Bottom plots illustrate negative associations between covariation estimates and activation to aversive pictures preceded by the uncertain cue than aversive pictures preceded by the certain aversive cue in the supragenual ACC (C), anterior insula (D) and mid-insula (E) regions. U = uncertain condition; C = certain condition; R = right; L = left.
In contrast to the findings for covariation bias, there were no ACC, insula, or amygdala effects for voxelwise regressions that regressed PANAS Negative Affect scores on the brain contrast map comparing activation to uncertain versus certain conditions for the anticipation and picture periods. Instead, voxelwise regressions conducted for uncertain and certain conditions separately revealed that higher Negative Affect scores were associated with greater supragenual ACC and insula activation to the uncertain cue and certain aversive cue, as well as the aversive pictures preceded by both uncertain and certain cues (supplementary Figs S6, S7; supplementary Table S2). All insula associations were right anterior (supplementary Fig. S7A–C) except the association for aversive pictures preceded by certain cues, which was left mid-insula (supplementary Fig. S7D). Unlike the bilateral insula effects for covariation bias, all insula regions in supplementary Figure S7 were asymmetric (all ps < 0.04), as indicated by formal tests of asymmetry. These correlations remained significant after partialing out covariation estimates (all ps < 0.001). No findings were observed for regressions using PANAS Positive Affect scores, and no association with the amygdala was observed for covariation bias or PANAS.
Discussion
This experiment investigated the impact of expectations on subsequent neural responses to aversive pictures in 2 contexts: uncertainty signaled by a cue that could be followed by either an aversive or neutral picture, and certainty signaled by a cue that was always followed by an aversive picture. Expectations produced by these cues modulated mid-insula and amygdala responses to aversion, with greater activation observed in response to aversive pictures preceded by an uncertain cue than aversive pictures preceded by a certain cue. Individual differences in anticipatory ACC activity were inversely related to these bilateral mid-insula and amygdala responses, such that individuals with greater ACC activation to the uncertain than certain cue showed the smallest uncertainty-related enhancement of mid-insula and amygdala activation to aversive pictures. In addition, ACC coupling with the mid-insula and amygdala was greater for aversive pictures preceded by an uncertain cue than aversive pictures preceded by a certain cue. Finally, the ACC and insula were correlated with overestimations of the frequency of aversive pictures following the uncertain cue, providing the first evidence of brain mechanisms contributing to covariation bias.
The impact of uncertainty on insula and amygdala responses to aversion likely affects emotion-related functions of these brain areas. Current conceptualizations of insula function emphasize interoception and the detailed representation of the body's internal state, including peripheral signals relevant for emotion (Craig 2002, 2009; Critchley 2004, 2005; Critchley et al. 2004). In a recent review, Craig (2009) argued that the insula is centrally involved in self-awareness more broadly, generating a stable representation of the self over time and allowing for the representation and awareness of current feelings as well as predictions about future feelings. Findings here indicate that uncertainty results in modulation of the mid-insula, which in Craig's model integrates emotionally salient environment stimuli such as uncertainty with the representation of bodily signals previously mapped in the posterior insula. His model posits that this integrative process culminates in a final representation of a given moment in time in the anterior insula, which is the predominant location of the aversion effects here and in other reports (Wicker et al. 2003; Kong et al. 2006; Nitschke, Sarinopoulos, et al. 2006; Craig 2009). Moreover, the left insula effects for uncertainty and aversion, but not the right insula effects, were asymmetric, as was the greater left anterior insula anticipatory activity to the certain aversive than uncertain cue. The proposed asymmetry of parasympathetic influences to the left insula (Craig 2005) suggests that certainty about an upcoming aversive stimulus may activate the parasympathetic system, as might the presentation of aversive pictures after uncertain cues have been shown. Associations of the left insula with positive affect predicted by Craig's model were not found, although the expected associations for the right insula with negative affect were observed (supplementary Fig. S7A–C). Formal tests of asymmetry, such as those used in the present report, have rarely been applied to the insula and are needed to further address Craig's proposed roles for each insula in autonomic and emotional behavior.
Present findings add to a growing number of reports showing that uncertainty results in larger responses in the amygdala (Rosen and Donley 2006; Herry et al. 2007; Whalen 2007; Belova et al. 2007; Dunsmoor et al. 2008), an area critically involved in vigilance for motivationally salient events across a broad range of emotional contexts (Davis and Whalen 2001; Baxter and Murray 2002; LeDoux 2002; Schwartz et al. 2003; Phelps et al. 2004; Mackiewicz et al. 2006; Nitschke, Sarinopoulos, et al. 2006; Sarinopoulos et al. 2006; Murray 2007). Uncertainty about the occurrence of aversive outcomes may increase the motivational salience of a situation. In support of the increased amygdala activation observed for aversive events following uncertain cues in humans, Belova et al. (2007) identified specific amygdala neurons in macaques that responded more to unexpected than expected aversive stimuli.
To investigate neural mechanisms predicting the observed insula and amygdala modulation, we examined activity during the anticipatory phase that defined the context on any given trial as either certain or uncertain. The findings of an inverse association between anticipatory ACC activity on uncertain versus certain trials and bilateral mid-insula and amygdala responses to subsequent aversive pictures are consistent with prior reports implicating the ACC in anticipation-driven modulatory functions (Ploghaus et al. 2003; Petrovic et al. 2005; Phan et al. 2005; Sarinopoulos et al. 2006) and top-down modulation of insula and amygdala responsivity (Etkin et al. 2006; Sarinopoulos et al. 2006; Egner et al. 2008). Anticipatory ACC activity may play a causal role in modulating subsequent neural firing in the insula and amygdala during the perception of aversive pictures. Extensive monosynaptic white matter tracts connect the ACC to both the insula and the amygdala (Augustine 1996; Roberts et al. 2007; Bissière et al. 2008; Johansen-Berg et al. 2008; Beckmann et al. 2009). However, the correlational nature of fMRI data precludes definitive conclusions about causation. Alternatively, the insula and amygdala responses to aversive pictures may influence anticipatory ACC activity on subsequent trials. Causality could be further assessed using the same paradigm in cingulotomy patients or people with ACC damage. Absent insula and amygdala modulation in such individuals, in conjunction with the findings here, would provide support for causative effects of the ACC on mid-insula and amygdala responses to aversion.
An important question following from the findings of anticipatory ACC activity is whether the ACC continues to show evidence of regulatory functions after picture presentation. Indeed, individual differences in ACC responses to the aversive pictures showed the same inverse association with the left mid-insula and bilateral amygdala, replicating recent findings from an emotion regulation paradigm (Urry et al. 2006; Johnstone et al. 2007) and an emotional Stroop task (Etkin et al. 2006; Egner et al. 2008). Complementing these cross-subject connectivity analyses that average ACC activity over all trials of each condition, we also conducted within-subject connectivity analyses examining intraindividual variation on a trial-by-trial basis. These functional connectivity analyses indicated that ACC coupling with the mid-insula and amygdala was consistently stronger in response to aversive pictures following the uncertain cue than aversive pictures following the certain cue. These data suggest that uncertainty results in tighter coupling of the ACC to insula and amygdala responses to negative outcome.
The precise anatomical location of ACC findings is of considerable import for discussions about function and clinical implications (Pezawas et al. 2005; Johansen-Berg et al. 2008; Palomero-Gallagher et al. 2008; Beckmann et al. 2009; Nitschke et al. 2009). The functions of the different sectors of the ACC identified in this report continue to be an active area of investigation (Devinsky et al. 1995; Vogt et al. 1995, 2003, 2005; Bush et al. 2000; Critchley 2004, 2005; Nitschke and Mackiewicz 2005; Etkin et al. 2006; Egner et al. 2008). Findings for associations with anticipatory brain activity (Fig. 2) and for PPI-based coupling (Fig. 3) converge to suggest that the supragenual ACC is particularly important in the top-down modulation of the insula, whereas subgenual and pregenual sectors of the ACC may preferentially modulate the right and left amygdala, respectively. The latter findings are consistent with other studies highlighting subgenual and pregenual ACC in regulation and top-down modulation of the amygdala (Phan et al. 2005; Etkin et al. 2006; Urry et al. 2006; Johnstone et al. 2007; Egner et al. 2008) and are consistent with known anatomical connections between these areas and the relative absence of monosynaptic projections between the supragenual ACC and the amygdala (van Hoesen et al. 1993; Carmichael and Price 1995; Johansen-Berg et al. 2008; Beckmann et al. 2009).
Recruitment of the ACC and insula nodes of this modulatory network was reliably associated with uncertainty-related covariation bias. ACC and insula responses to uncertainty during the scan predicted individual differences in covariation estimates provided after the scan about the percentage of uncertain cues followed by aversive pictures. Individuals with greater anticipatory activity in the supragenual ACC following the uncertain than certain cue showed biased covariation estimates. Subgenual ACC responses to the aversive pictures showed the same pattern. Both of these areas overlapped with the respective ACC areas found to be associated with insula responses to the aversive pictures (Fig. 2, supplementary Fig. S4), suggesting that although ACC activation may be adaptive in downregulating insula and amygdala responses to aversion, this comes at the cost of distorting perceptions about aversive experiences in these individuals. This downregulation of the insula would be expected to compromise its function in updating bodily representations in these individuals (Craig 2002, 2003, 2009; Damasio 2003; Critchley 2004, 2005), thereby resulting in less accurate perceptions about their exposure to aversion when faced with uncertainty. Supporting this interpretation, bilateral anterior insula and mid-insula responses to the pictures were inversely associated with covariation bias. Supragenual ACC responses to the aversive pictures showed the same pattern as these insula areas, consistent with the tight supragenual ACC coupling with mid-insula responses to aversive pictures following the uncertain cue.
The findings for covariation bias were not explained by negative affect. Instead of showing uncertainty-related effects (e.g., uncertain cue–certain aversive cue), negative affect was positively associated with greater supragenual ACC, right anterior insula, and left mid-insula activity for certain and uncertain cues as well as for the aversive pictures following each cue. The indiscriminate recruitment of the supragenual ACC and insula across these different contexts in individuals endorsing higher levels of negative affect may be pathognomic in patients with anxiety and mood disorders (Nitschke and Heller 2005; Paulus and Stein 2006). Unlike the insula findings for covariation bias, these right anterior insula and left mid-insula findings for negative affect were asymmetric. These effects are consistent with findings for the right anterior insula in low trait resilience (Waugh et al. 2008) and for the left mid-insula in state negative affect (Mériau et al. 2009), although asymmetry was not statistically evaluated in those reports. Because negative affect and a general negativity bias (estimated proportion of certain aversive versus neutral cues) did not account for the associations of uncertainty-related covariation bias to ACC and insula activity, research is needed to address other potential contributing factors, such as expectancy bias (Davey 1992), memory bias for emotional events (Christianson 1992; Phelps 2006; Mackiewicz et al. 2006), or cardiorespiratory activity (Rainville et al. 2005).
The current study can be grounded in the context of reinforcement learning models, which posit that learning is a result of discrepancies between an organism's predictions about future events and the occurrence of such events (Rescorla and Wagner 1972; Schultz et al. 1997; Niv and Schoenbaum 2008). Research into the neural basis of such discrepancies, termed prediction errors, has predominantly focused on reward prediction errors generated by dopaminergic neurons (Schultz et al. 1997; Niv and Schoenbaum 2008). There have been recent proposals that the anterior insula plays an analogous role in signaling probabilities of risk and risk prediction errors, thus promoting learning in the context of risky or unpredictable circumstances (Paulus and Stein 2006; Preuschoff et al. 2008). In the current study, it might be hypothesized that increased mid-insula activity to aversive pictures following uncertain cues reflects risk prediction errors. The inverse relationship between covariation estimates and insula activation to aversive pictures following uncertain cues would support this hypothesis if higher covariation estimates after the scan reflected greater risk predictions following uncertain cues during the scan. If this were the case, a stronger covariation bias would indicate greater risk predictions and smaller risk prediction errors for aversive pictures. Of note, differences in brain activation to expected versus unexpected outcomes may occur for reasons other than prediction error signaling, such as attention modulation or the encoding of stimulus value (Belova et al. 2007; Niv and Schoenbaum 2008). Activity associated with prediction errors could be assessed in future work with our anticipation paradigm by monitoring expectancies/predictions of aversion on a trial-by-trial basis. Indeed, a limitation of the present study is that this version of our paradigm did not include on-line ratings of expectancies that could be used to assess expectancy bias and prediction errors, or on-line affective ratings that could be used to assess subjective responses to the aversive pictures.
In sum, the impact of aversion on the brain is contingent upon expectations about whether the aversive outcome is a definite certainty or only a possibility. The neural mechanisms of this phenomenon include the ACC, insula, and amygdala. Extending prior work demonstrating recruitment of these regions in response to a wide range of aversive stimuli, mid-insula and amygdala activation to aversive pictures was greater after an uncertain cue that was sometimes followed by an aversive picture as compared with a certain cue that was always followed by an aversive picture. Anticipatory activity in the supragenual ACC predicted this impact of uncertainty on mid-insula responses to aversion, whereas anticipatory activity in the subgenual and pregenual ACC predicted this impact of uncertainty on amygdala responses to aversion. Findings for covariation bias suggest that multiple ACC and insula areas influence perceptions of cue–outcome contingencies, resulting in the observed overestimations of aversive pictures following the uncertain cue. This study highlights the importance of chronometry in the context of uncertainty and aversion by dissociating anticipatory ACC effects from neural responses to aversive stimuli that follow. Individual differences in ACC and insula activation have behavioral significance of particular relevance to anxiety disorders, both in terms of disposition toward negative affect and a covariation bias that accompanies uncertainty about aversive outcomes.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institute of Mental Health (R01-MH74847, K02-MH082130, and K08-MH63984) to J.B.N.; and core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30-HD03352).
Supplementary Material
Acknowledgments
We gratefully acknowledge Andrew Alexander, Michael Anderle, Krystal Cleven, Danielle Green, Ron Fisher, Daniel McFarlin, Terrence Oakes, Desmond Oathes, Adrian Pederson, and Hillary Schaefer for their contributions to this project, and Ned Kalin and Richie Davidson for their comments on an earlier version of this manuscript. Conflict of Interest: None declared.
References
- Alloy LB, Tabachnik N. Assessment of covariation by humans and animals: the joint influence of prior expectations and current situational information. Psychol Rev. 1984;91:112–149. [PubMed] [Google Scholar]
- Amin JM, Lovibond PF. Dissociations between covariation bias and expectancy bias for fear-relevant stimuli. Cogn Emot. 1997;11:273–289. [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic. 2nd ed. New York: Guilford Press; 2002. [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;7:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissière S, Plachta N, Hoyer D, McAllister KH, Olpe HR, Grace AA, Cryan JF. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biol Psychiatry. 2008;63:821–831. doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J Neurosci. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Buhr K, Dugas MJ. The intolerance of uncertainty scale: psychometric properties of the English version. Behav Res Ther. 2002;40:931–945. doi: 10.1016/s0005-7967(01)00092-4. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;262:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Chapman LL, Chapman JP. Genesis of popular but erroneous psychodiagnostic observations. J Abnorm Psychol. 1967;72:193–204. doi: 10.1037/h0024670. [DOI] [PubMed] [Google Scholar]
- Chapman LL, Chapman JP. Illusory correlation as an obstacle to the use of valid psychodiagnostic signs. J Abnorm Psychol. 1969;74:271–280. doi: 10.1037/h0027592. [DOI] [PubMed] [Google Scholar]
- Christianson SA. The handbook of emotion and memory: research and theory. Hillsdale (NJ): Lawrence Earlbaum Associates; 1992. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craske MG, Glover D, DeCola J. Predicted versus unpredicted panic attacks: acute versus general distress. J Abnorm Psychol. 1995;104:214–223. doi: 10.1037//0021-843x.104.1.214. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA. 2004;101:6333–6334. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Looking for Spinoza: joy, sorrow, and the feeling brain. Orlando: Harcourt; 2003. [Google Scholar]
- Davey GCL. An expectancy model of laboratory preparedness effects. J Exp Psychol. 1992;121:24–40. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- de Jong PJ, Merckelbach H, Arntz A, Nijman H. Covariation detection in treated and untreated spider phobics. J Abnorm Psychol. 1992;101:724–727. doi: 10.1037//0021-843x.101.4.724. [DOI] [PubMed] [Google Scholar]
- de Jong PJ, Merckelbach H, Bögels S, Kindt M. Illusory correlation and social anxiety. Behav Res Ther. 1998;36:1063–1073. doi: 10.1016/s0005-7967(98)00099-0. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dugas MJ, Gagnon F, Ladouceur R, Freeston MH. Generalized anxiety disorder: a preliminary test of a conceptual model. Behav Res Ther. 1998;36:215–226. doi: 10.1016/s0005-7967(97)00070-3. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behav Neurosci. 2007;121:635–642. doi: 10.1037/0735-7044.121.4.635. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage. 2008;40:811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza D, Kandel E, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Freeston MH, Rheaume J, Letarte H, Dugas MJ, Ladouceur R. Why do people worry? Pers Individ Dif. 1994;17:791–802. [Google Scholar]
- Friston KJ, Büchel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Grinband J, Hirsch J, Ferrera VP. A neural representation of categorization uncertainty in the human brain. Neuron. 2006;49:757–763. doi: 10.1016/j.neuron.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Grings WW, Schell AM. Effects of trace versus delay conditioning, interstimulus interval variability, and instructions on UCR diminution. J Exp Psychol. 1971;90:136–140. doi: 10.1037/h0031343. [DOI] [PubMed] [Google Scholar]
- Han CJ, O'Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, Koch C, Anderson DJ. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc Natl Acad Sci USA. 2003;100:13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007;27:6313–6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann C, Ofer J, Flor H. Covariation bias for ambiguous social stimuli in generalized social phobia. J Abnorm Psychol. 2004;113:646–653. doi: 10.1037/0021-843X.113.4.646. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Lüthi A, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SJ, Rapee RM, Mazurski EJ. Covariation bias for phylogenetic versus ontogenetic fear-relevant stimuli. Behav Res Ther. 1997;35:415–422. doi: 10.1016/s0005-7967(96)00128-3. [DOI] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel M, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27:715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Gotimer K, Hefton S, Ernst M, Castellanos FX, Pine DS, Milham MP. A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biol Psychiatry. 2008;63:563–568. doi: 10.1016/j.biopsych.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage. 2006;32:477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system: technical manual and affective ratings. Gainesville: University of Florida; 1999. [Google Scholar]
- LeDoux JE. The synaptic self. New York: Viking Penguin; 2002. [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within subjects designs. Psychol Bull Rev. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Lohr JM, Olatunji BO, Sawchuk CN. A functional analysis of danger and safety signals in anxiety disorders. Clin Psychol Rev. 2007;27:114–126. doi: 10.1016/j.cpr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Macindoe I, Tellegen A. Perception: autonomic response to shock as a function of predictability in time and locus. Psychophysiology. 1972;9:318–333. doi: 10.1111/j.1469-8986.1972.tb03215.x. [DOI] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proc Natl Acad Sci USA. 2006;103:14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mériau K, Wartenburger I, Kazzer P, Prehn K, Villringer A, van der Meer E, Heekeren HR. Insular activity during passive viewing of aversive stimuli reflects individual differences in state negative affect. Brain Cogn. 2009;69:73–80. doi: 10.1016/j.bandc.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Nader K, Balleine B. Ambiguity and anxiety: when a glass half full is empty. Nat Neurosci. 2007;10:807–808. doi: 10.1038/nn0707-807. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Dixon GE, Sarinopoulos I, Short SJ, Cohen JD, Smith EE, Kosslyn SM, Rose RM, Davidson RJ. Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nat Neurosci. 2006;9:435–442. doi: 10.1038/nn1645. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W. Distinguishing neural substrates of heterogeneity among anxiety disorders. Int Rev Neurobiol. 2005;67:1–42. doi: 10.1016/S0074-7742(05)67001-8. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Mackiewicz KL. Prefrontal and anterior cingulate contributions to volition in depression. Int Rev Neurobiol. 2005;67:73–94. doi: 10.1016/S0074-7742(05)67003-1. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, Kalin NH. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y, Schoenbaum G. Dialogues on prediction errors. Trends Cogn Sci. 2008;12:265–272. doi: 10.1016/j.tics.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol. 2008;508:906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli P, Montoya P, Martz GE. Covariation bias in panic-prone individuals. J Abnorm Psychol. 1996;105:658–662. doi: 10.1037//0021-843x.105.4.658. [DOI] [PubMed] [Google Scholar]
- Pauli P, Wiedemann G, Montoya P. Covariation bias in flight phobics. J Anxiety Disord. 1998;12:555–565. doi: 10.1016/s0887-6185(98)00033-4. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Neurobiology of decision-making: quo vadis? Cogn Brain Res. 2005;23:2–10. doi: 10.1016/j.cogbrainres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Peeke SS, Grings WW. Magnitude of UCR as a function of variability in the CS-UCS relationship. J Exp Psychol. 1968;77:64–69. doi: 10.1037/h0025769. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing–induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DJ, Nathan PA, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Platt ML, Huettel SA. Risky business: the neuroeconomics of decision making under uncertainty. Nat Neurosci. 2008;11:398–403. doi: 10.1038/nn2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus A, Becerra L, Borras C, Borsook D. Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends Cogn Sci. 2003;7:197–200. doi: 10.1016/s1364-6613(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pury CLS, Mineka S. Covariation bias for blood-injury stimuli and aversive outcomes. Behav Res Ther. 1997;35:35–47. doi: 10.1016/s0005-7967(96)00075-7. [DOI] [PubMed] [Google Scholar]
- Rainville P, Bao QV, Chrétien P. Pain-related emotions modulate experimental pain perception and autonomic responses. Pain. 2005;118:306–318. doi: 10.1016/j.pain.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Roberts AC, Tomic DL, Parkinson CH, Roeling TA, Cutter DJ, Robbins TW, Everitt BJ. Forebrain connectivity of the prefrontal cortex in the marmoset monkey. Callithrix jacchus.: an anterograde and retrograde tract-tracing study. J Comp Neurol. 2007;502:86–112. doi: 10.1002/cne.21300. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Donley MP. Animal studies of amygdala function in fear and uncertainty: relevance to human research. Biol Psychol. 2006;73:49–60. doi: 10.1016/j.biopsycho.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sarinopoulos I, Dixon GE, Short SJ, Davidson RJ, Nitschke JB. Brain mechanisms of expectation associated with insula and amygdala response to aversive taste: implications for placebo. Brain Behav Immun. 2006;20:120–132. doi: 10.1016/j.bbi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neurosci Lett. 2008;430:92–97. doi: 10.1016/j.neulet.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system—an approach to cerebral imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Tomarken AJ, Mineka S, Cook M. Fear-relevant selective associations and covariation bias. J Abnorm Psychol. 1989;98:381–394. doi: 10.1037//0021-843x.98.4.381. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoesen GW, Morecraft RJ, Vogt BA. Connections of the monkey cingulate cortex. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus: a comprehensive handbook. Boston (MA): Birkhauser; 1993. pp. 249–284. [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky AE, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt LJ, Farber NB, Bush G. Architecture and neurocytology of monkey cingulate gyrus. J Comp Neurol. 2005;485:218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY. Predicting events of varying probability: uncertainty investigated by fMRI. Neuroimage. 2003;19:271–280. doi: 10.1016/s1053-8119(03)00122-8. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scale. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Wager TD, Fredrickson BL, Noll DC, Taylor SF. The neural correlates of trait resilience when anticipating and recovering from threat. Soc Cogn Affect Neurosci. 2008;3:322–332. doi: 10.1093/scan/nsn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends Cogn Sci. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.