Abstract
Members of the Wnt family of signaling molecules are expressed differentially along the dorsal–ventral axis of the developing neural tube. Thus we asked whether Wnt factors are involved in patterning of the nervous system along this axis. We show that Wnt-1 and Wnt-3a, both of which are expressed in the dorsal portion of the neural tube, could synergize with the neural inducers noggin and chordin in Xenopus animal explants to generate the most dorsal neural structure, the neural crest, as determined by the expression of Krox-20, AP-2, and slug. Overexpression of Wnt-1 or Wnt-3a in the neuroectoderm of whole embryos led to a dramatic increase of slug and Krox-20-expressing cells, but the hindbrain expression of Krox-20 remained unaffected. Enlargement in the neural crest population could occur even when cell proliferation was inhibited. Wnt-5A and Wnt-8, neither of which is expressed in the dorsal neuroectoderm, failed to induce neural crest markers. Overexpression of glycogen synthase kinase 3, known to antagonize Wnt signaling, blocked the neural-crest-inducing activity of Wnt-3a in animal explants and inhibited neural crest formation in whole embryos. We suggest that Wnt-1 and Wnt-3a have a role in patterning the neural tube along its dorsoventral axis and function in the differentiation of the neural crest.
The Wnt gene family encodes a group of cysteine-rich secreted glycoproteins involved in a wide range of activities during embryogenesis (1, 2). For example, Drosophila Wingless (Wg); mouse Wnt-1; and Xenopus wnt-2b (Xwnt-2b), Xwnt-3a, Xwnt-8, and Xwnt-8b have been implicated in dorsoventral axis formation in that they can fully restore dorsal axis formation in UV-ventralized embryos and/or can cause axis duplication when their RNAs are expressed on the ventral side of an early frog embryo (3–8). Mice lacking Wnt-4, which is normally expressed in the developing mesenchyme of the kidney, present kidney defects suggesting that Wnt-4 is playing a key role in the mesenchyme-to-epithelium transition, which occurs during nephron formation (9). A Wnt-7a dorsalizing signal has been shown to be critical in setting the normal polarity of the mouse limb, because mice with a disrupted Wnt-7a activity display a dorsal-to-ventral transformation of the limb mesoderm and lack posterior digits, further implicating this Wnt factor in anterior–posterior patterning of the limb (10). The molecular mechanisms involved in the signaling cascade of Wnt factors have been clarified largely through genetics studies on Wingless in Drosophila, and more recently, evidence has emerged for the striking conservation of the Wnt/wg signaling pathway between invertebrates and vertebrates (11).
We have attempted to determine whether members of the Wnt family can exert a dorsoventral patterning function within the nervous system. Two lines of evidence have led us to investigate this possibility. (i) Some Wnt molecules, among them Wnt-1 and Wnt-3a, are selectively expressed in the dorsal but not in the ventral region of the developing nervous system (6, 12). (ii) Recent results in frog embryos have shown that Xwnt-3a is capable of inducing Krox-20 when coexpressed with the neural inducer noggin (13). Krox-20, a zinc-finger transcription factor expressed in rhombomeres 3 and 5 of the hindbrain, also defines a population of cephalic neural crest cells adjacent to rhombomere 5 that will contribute to the third branchial arch (14). Although induction of Krox-20 by Xwnt-3a has been proposed to reflect posteriorization of the neural tissue induced by noggin (13), we reasoned that the dorsally expressed Wnt molecules could also have an important role in the regulation of the formation of the most dorsal neural structure, the neural crest (15).
MATERIALS AND METHODS
RNA and DNA Injections.
Capped RNAs were synthesized by using the Megascript in vitro transcription kit (Ambion, Austin, TX) with mGpppG (Boehringer Mannheim) and linearized template DNAs: mouse Wnt-1 (4), Xwnt-3a (6), Xwnt-5A (16), Xwnt-8 (7), noggin (17), chordin (18), and human glycogen synthase kinase 3β (GSK-3β) (19). In explant experiments, RNAs were injected into both blastomeres at the two-cell stage in the animal-pole region, and animal caps were isolated at the blastula stage (stage 8/9) and cultured to equivalent stage 23/26 as described (20). Unless differently specified, 100 pg of Wnt RNAs, 150 pg of noggin RNA, 3 ng of GSK-3β RNA, 700 pg of chordin RNA, and 350 pg of CS2+Wnt plasmids were injected per embryo. Xwnt-3a was cloned into the ClaI and XbaI sites of CS2+ vector (21), and Wnt-1 was cloned into the NcoI and EcoRI sites of a modified version of pCS2+.
Northern Blot Hybridization.
RNA extraction, electrophoresis with 5–7 μg of RNA per lane, and blotting for 48 hr were performed as described (20). Hybridization probes, Xenopus Krox-20 (14), nrp-1 (22), otx-2 (17), AP-2 (23), slug (24), wnt-1 (6), FKH-1 (25), pax-3 (26), and shh (27) cDNAs were synthesized by using a DNA labeling kit (α-dCTP) from Pharmacia (Ready-To-Go). Filters were washed and analyzed by PhosphorImager (Molecular Dynamics).
Lineage Tracing and in Situ Hybridization.
Embryos were coinjected with RNA encoding nuclear localized β-galactosidase (β-gal; 0.5 ng) and Wnt (10–40 pg) or GSK-3β (1 ng) RNAs, or Wnt (100 pg) DNA into one blastomere at the two-cell stage in the animal-pole region. By stage 20–25, embryos were processed successively for 5-bromo-4-chloro-3-indolyl β-d-galactoside staining (19) and in situ hybridization. Antisense RNA probes were synthesized in vitro by using a digoxigenin labeling kit (Boehringer Mannheim). Whole-mount in situ hybridization was performed as described by Harland (28).
Inhibition of Cell Division by Hydroxyurea and Aphidicolin (HUA) Treatment.
Embryos were incubated at the early gastrula stage in a mixture of hydroxyurea (Sigma, 20 mM) and aphidicolin (Sigma, 150 mM) until equivalent stage 25, as described (29). Embryos were then fixed for hematoxylin/eosin staining (19) or nuclear staining using 4,6-diamidino-2-phenylindole (Molecular Probes) or processed for in situ hybridization.
Rescue of UV-Ventralized Embryos.
UV-ventralized embryos were generated and injected as described (19) and scored by using the dorsoanterior index of Kao and Elinson (30). One picogram of Xwnt-8, 5 pg of Xwnt-3a, and 1 ng of GSK-3β RNAs were used in these experiments.
RESULTS
Wnt-1 or Wnt-3a Induce Neural Crest Fates in Neuralized Animal Explants.
We tested the effect of expressing mouse Wnt-1, Xwnt-3a, Xwnt-5A, and Xwnt-8 in animal explants that were neuralized by the influence of noggin. The appropriate synthetic RNAs were injected into the animal region of two-cell embryos; animal caps were isolated at the blastula stage, cultured, and tested for the expression of several markers: the pan-neural marker nrp-1 (22) and the forebrain marker otx-2 (17), both of which are excluded from the neural crest; the hindbrain/cephalic neural crest marker Krox-20 (14); the cephalic neural crest cell marker AP-2, which is also expressed at a low level in the epidermis (23); and slug, a marker of all premigratory neural crest cells (24). As previously reported (17, 22) and illustrated in Fig. 1a, noggin induced expression of the forebrain (otx-2) and pan-neural (nrp-1) markers and reduced the basal expression of AP-2 attributable to an epidermal contribution. Coexpression of Wnt-1 or Xwnt-3a with noggin led to a dramatic reduction of nrp-1 and otx-2 RNAs and to a strong induction of Krox-20, AP-2, and slug RNAs whereas Xwnt-3a alone induced some of the neural crest markers at a lower level (Fig. 1 a–c). The mesoderm marker α-cardiac actin was never induced in the explants expressing the crest markers (data not shown). Xwnt-5A and Xwnt-8, alone or in combination with noggin (Fig. 1b), failed to induce any of the neural crest markers; further, both reduced the overall neuralization mediated by noggin, as reported for Xwnt-8 (31). Like noggin, the neural inducer chordin (18) also synergized with Xwnt-3a to induce crest markers (Fig. 1c).
Figure 1.
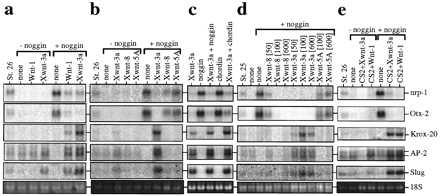
Expression of neural crest markers in animal cap explants injected with Wnt RNAs or DNAs, as seen by RNA blotting. RNAs were extracted from animal explants injected with Wnt RNAs or DNAs (CS2+Wnt), alone (−noggin), or with noggin RNA (+noggin), from whole embryos at the indicated stage and from uninjected explants (none). The blots were sequentially hybridized with Krox-20, AP-2, slug, nrp-1, and otx-2 probes. Ethidium bromide-stained 18S ribosomal RNA is shown as a loading control. In some experiments (two of six), coexpression of Xwnt-8 and noggin induced low levels of slug RNA but consistently failed to induce Krox-20 or AP-2 (b and d). See Materials and Methods for levels of RNA injected, except for d, where the amount of injected RNA in pg is indicated. a–e represent five experiments.
To test for possible effects of RNA concentration on the differential activity of Wnt1/Xwnt-3a vs. Xwnt-5A/Xwnt-8, increasing amounts of Wnt RNAs were injected in combination with a constant amount of noggin RNA (Fig. 1d). Xwnt-5A and Xwnt-8 failed to induce Krox-20, AP-2, or slug expression even at the highest level of injected RNA (600 pg per embryo), whereas Xwnt-3a could induce these markers at 50 pg of RNA. Moreover, the same RNA preparations of Xwnt-8 and Xwnt-3a are highly effective in the axis duplication assay (data not shown) or in rescue of UV-ventralized embryos (see below, Fig. 4a) in a similar concentration range of 1–10 pg. These results illustrate that Wnt-1 and Xwnt-3a are capable of modifying the character of the neural tissue induced by noggin by activating a set of neural-crest-specific genes at the expense of more general neural markers. In contrast, Xwnt-5A and Xwnt-8, which are excluded from the dorsal aspect of the nervous system (16, 32), failed to exert this function. Previously, Wnt-1, Xwnt-3a, and Xwnt-8 have been classified in the same group of biological activities, based on their ability to restore dorsal axis formation in UV-ventralized embryos and to cause axis duplication upon expression on the ventral side of the embryo (33), yet the assay described herein shows that these Wnt proteins are not equivalent in every biological context.
Figure 4.
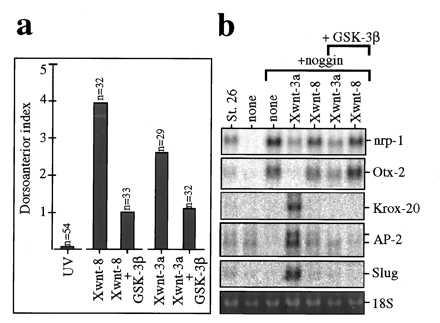
Both the axis inducing and neural crest inducing activities of Xwnt-3a are antagonized by GSK-3β. (a) GSK-3β partially inhibits axis rescue by Xwnt-3a. Partial rescue is observed in UV-treated embryos (DAI 0.1) injected with Xwnt-8 RNA (DAI 3.9) or Xwnt-3a RNA (DAI 2.6); rescue is inhibited when Xwnt-8 plus GSK-3β RNAs (DAI 1) or Xwnt-3a plus GSK-3β RNAs (DAI 1.2) are coinjected. (b) GSK-3β inhibits neural crest induction by Xwnt-3a. Northern blot from animal explants injected with Xwnt-3a or Xwnt-8 RNAs plus noggin RNA, with (+GSK-3β) or without GSK-3β RNA. Coinjection of GSK-3β and noggin RNAs leads to the same repertoire of gene expression as noggin RNA alone (data not shown). DAI, dorsoanterior index (30).
To evaluate whether Wnt-1 and Xwnt-3a can activate neural crest markers if expressed after the midblastula transition, Wnt-1 or Xwnt-3a expression plasmids (in pCS2+, ref. 21) were injected alone or in combination with noggin mRNA into the early embryo, and animal caps from these embryos were tested for the induction of neural crest markers. Injection of Wnt-1 or Xwnt-3a plasmid synergized with noggin to induce Krox-20, AP-2, and slug RNAs (Fig. 1e) in a similar way as injection of Wnt-1 or Xwnt-3a RNA. This result suggests that a Wnt-1/Xwnt-3a signal is required at or after gastrulation to generate a neural crest phenotype, consistent with the timing of crest development in vivo (15).
Targeted Overexpression of Wnt-1 or Xwnt-3a in the Neuroectoderm Expands the Domain of the slug- and Krox-20-Expressing Neural Crest Population.
Because Wnt-1 and Xwnt-3a elicit expression of neural crest markers in neuralized animal explants, we examined whether they could do so in the whole embryo in vivo. Wnt RNAs were injected with RNA encoding β-gal, as lineage tracer, into the animal region of one of the blastomeres at the two-cell stage. At the neurula stage, embryos were examined by in situ hybridization with Krox-20 and slug probes. A dramatic increase of the population of cells expressing Krox-20 (Fig. 2b) and slug (Fig. 2e) was observed in a large proportion of embryos injected with Wnt-1 or Xwnt-3a (Table 1). Supernumerary Krox-20/slug-expressing cells were always located in the injected half, as determined by colocalization with β-gal staining. Importantly, Krox-20 expression in rhombomeres 3 and 5 remained unaffected in these embryos (Fig. 2 b and g), consistent with the observation that the hindbrain and neural crest expression of Krox-20 can be independently activated (34). Neither Xwnt-5A nor Xwnt-8 were able to induce Krox-20 or slug upon RNA injection (Fig. 2 c and f and Table 1). Wnt-1 or Xwnt-3a plasmid injections likewise generated an increase in the population of neural crest cells expressing Krox-20, although the extent of the population increase was less than in the case of RNA injection (Fig. 2h and Table 1).
Figure 2.
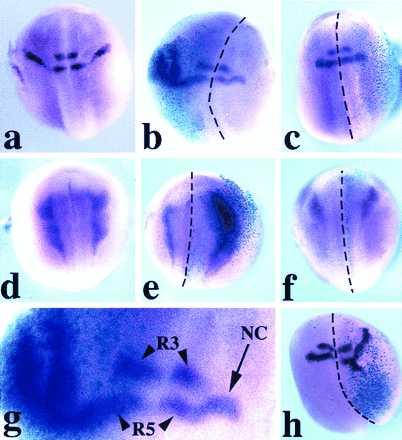
Expression of Krox-20 (a–c, g, and h) and slug (d–f) in Xenopus embryos (stage 20–25), as seen by whole-mount in situ hybridization. The embryos are viewed from the dorsal side, and anterior is to the top. Krox-20 expression in a control embryo (a) is in two stripes (rhombomeres 3 and 5) in the hindbrain and a stream of neural crest cells adjacent to rhombomere 5. Xwnt-3a-injected embryo (b); in the injected half (note the turquoise β-gal staining in the left half) the stream of Krox-20-expressing cells is dramatically expanded while still arising from a zone adjacent to rhombomere 5. In an Xwnt-8-injected embryo (c), the pattern of expression of Krox-20 is unaffected. Slug expression in a control embryo (d). Xwnt-3a-injected embryo (e) shows an enlargement of the domain of expression of slug within the injected right half (see β-gal staining). An Xwnt-8-injected embryo (f) presents a pattern of slug expression in the injected half (right side, β-gal staining) comparable to the uninjected half. (g) Higher magnification of the hindbrain region of the embryo presented in b. Rhombomere 3 (R3) and rhombomere 5 (R5) expression of Krox-20 is not affected; NC indicates stream of neural crest. CS2+Xwnt-3a-injected embryo (h) presents an expanded domain of expression of Krox-20 in the injected half (see β-gal staining). The dotted lines indicate the dorsal midline. Among the embryos injected in the animal pole region, 30% showed some level of axis duplication when injected with Wnt-1, Xwnt-3a, or Xwnt-8 RNAs.
Table 1.
Number of embryos presenting ectopic expression of Krox-20 or slug upon Wnt RNA or DNA injections
| Embryos with ectopic expression of Krox-20 or slug, n/total, n
|
||||||
|---|---|---|---|---|---|---|
| RNA injection
|
DNA injection
|
|||||
| Wnt-1 | Xwnt-3a | Xwnt-5A | Xwnt-8 | Wnt-1 | Xwnt-3a | |
| Krox-20 | 13/49 | 49/80 | 1/37 | 0/25 | 8/13 | 27/60 |
| Slug | 25/36 | 12/30 | 1/30 | 0/24 | ND | ND |
ND, not determined.
Induction of Neural Crest by Xwnt-3a Can Occur After Inhibition of Cell Proliferation.
In mouse embryos, ectopic expression of Wnt-1 in the entire spinal cord under the control of the Hox-B4 enhancer has been reported to increase the number of dorsal cells undergoing mitosis without affecting the overall polarity of the spinal cord (35). We therefore tested whether the increase in the neural crest after Wnt-1/Xwnt-3a injection was due primarily to an increase in cell proliferation. Embryos were injected with Wnt-1 or Xwnt-3a RNA and β-gal RNA in one blastomere at the two-cell stage and transferred at the early gastrula stage into a mixture of DNA synthesis inhibitors, HUA, that has been shown to block cell division without affecting cell differentiation (29). The embryos were cultured in the presence of the drugs to equivalent stage 25 and were then hybridized with a Krox-20 probe. As reported (29), the development of embryos treated with HUA was delayed with an overall normal pattern of differentiation (Fig. 3 a–c and e) but with great reduction in cell number (Fig. 3 d and f). In situ hybridization revealed the expected expression pattern of Krox-20 within the neural crest with reduced expression in rhombomere 3 (Fig. 3 g and h). Upon Xwnt-3a injection, embryos cultured in control medium (Fig. 3i) or embryos treated with HUA (Fig. 3j) showed a similar enlargement of the domain of expression of Krox-20 within the neural crest and at a similar frequency (Table 2). This result suggests that the increase in the number of Krox-20-expressing cells observed after injection of Wnt-1 or Xwnt-3a is not contingent upon cell proliferation and, thus, is likely to reflect a change in cell fate within the neuroectoderm. Consistent with this observation, induction of neural-crest-specific genes in animal explants occurs at the expense of more general neural markers (Fig. 1). These results do not exclude the possibility that Wnt-1 and Xwnt-3a may also stimulate some level of neural crest cell proliferation in embryos not exposed to HUA. The inability of Wnt-1 to change the overall polarity of the neuraxis in transgenic mice as reported (35) may be due to the fact that at the time of the ectopic expression of Wnt-1, cell fate decisions had already been made in the spinal cord.
Figure 3.
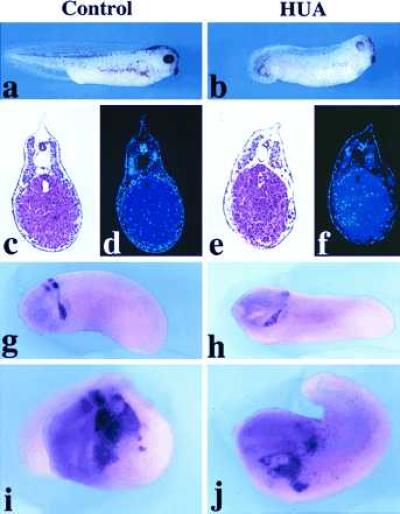
Expression of Krox-20 after inhibition of cell proliferation by HUA. General appearance of a control embryo (a) and an HUA-treated embryo (b) at equivalent stage 35. Hematoxylin-eosin staining (c and e) and 4,6-diamidino-2-phenylindole (nuclear) staining (d and f) of sections of a control embryo (c and d) and an HUA-treated embryo (e and f). The neural crest expression of Krox-20 is unaffected in HUA-treated embryos although rhombomere expression is reduced (h) as compared with control untreated embryo (g). The increase in neural crest cells expressing Krox-20 generated upon Xwnt-3a RNA injection appears similar in untreated (i) and in HUA-treated (j) embryos.
Table 2.
HUA treatment does not affect enlargement of the Krox-20 expression domain generated by Xwnt-3a RNA injection
| Embryos with ectopic expression of Krox-20, n/total, n
| |||
|---|---|---|---|
| Control
|
HUA treatment
|
||
| Uninjected | Xwnt-3a | Uninjected | Xwnt-3a |
| 0/17 | 16/24 | 0/23 | 24/34 |
The Neural Crest-Inducing Activity of Xwnt-3a Requires Inhibition of GSK-3.
In axis duplication or UV-rescue assays, the dorsalizing function of Xwnt-8 is antagonized by overexpression of GSK-3 (19). UV rescue by Xwnt-3a can also be inhibited by overexpression of GSK-3 (Fig. 4a), suggesting that the dorsalizing function of Xwnt-3a is also mediated by an inhibition of GSK-3. We therefore asked whether neural crest induction by Xwnt-3a requires inhibition of GSK-3. As shown in Fig. 4b, coinjection of GSK-3 RNA completely blocked the induction of Krox-20, AP-2, and slug by the combination of noggin and Xwnt-3a RNAs. These observations suggest that Xwnt-3a axis specification and neural-crest-inducing activities both require inhibition of GSK-3 activity.
On the basis of the above results, we tested whether GSK-3 could inhibit neural crest formation in the whole embryo. GSK-3 RNA was coinjected with β-gal RNA into the animal region of one of the blastomeres at the two-cell stage, and embryos were examined by in situ hybridization at stage 20–25. In these embryos (n = 80), the Krox-20-expressing neural crest stream arising from rhombomere 5 was either absent (24%) or greatly reduced (45%) in the injected half (Fig. 5 a–c) whereas the rhombomere expression of Krox-20 was unmodified (Fig. 5 b and c). The remaining embryos (31%), in which β-gal staining was absent from the dorsal region (Fig. 5d), were unaffected; we suggest that in these embryos the GSK-3 RNA did not become localized into the cells that are normally involved in neural crest formation. These results indicate that inhibition of GSK-3 is required for neural crest formation in vivo. Thus, with the ability of Wnt-1 and Xwnt-3a to induce crest markers in explants and in vivo, these results strongly implicate a Wnt-like signal in neural crest differentiation in the embryo.
Figure 5.
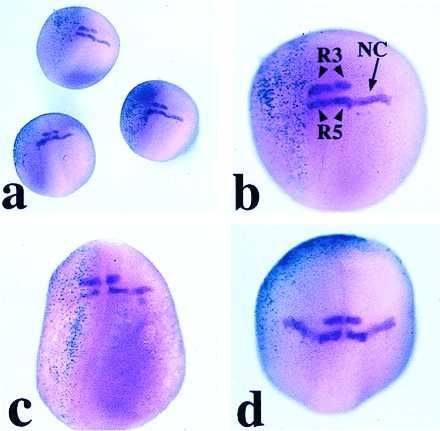
Expression of Krox-20 in embryos injected with 1 ng of GSK-3β RNA, as seen by whole-mount in situ hybridization. The embryos are viewed from the dorsal side, and anterior is to the top. (a) Group of three embryos coinjected on the left side with GSK-3β and β-gal RNAs (turquoise staining). (b and c) GSK-3β-injected embryos at a higher magnification. The stream of Krox-20-positive neural crest cells (NC) arising from rhombomere 5 (R5) is absent from the injected half (left side), whereas the rhombomere expression is unaffected. When β-gal staining is not detected in the dorsal region of the injected embryo the neural crest expression of Krox-20 is unaffected, as shown in d, where β-gal staining is ventral-anterior.
Xwnt-3a Has a General Dorsalizing Activity in the Neuraxis.
We further tested whether the neural-crest-inducing activity of Xwnt-3a was part of a more general process of dorsalization of neural tissue by looking at additional markers expressed along the dorsoventral axis of the central nervous system. We found that two dorsally restricted genes pax-3 (36) and wnt-1 (37) were strongly activated by the combination of Xwnt-3a and noggin RNA, but two ventrally restricted genes, shh (38) and FKH-1/HNF3β (25), were not induced (Fig. 6). This result suggests that in addition to its ability to generate neural crest fates, Xwnt-3a activates a range of dorsal markers in the neuroectoderm.
Figure 6.

Expression of dorsal and ventral neural genes in animal explants injected with noggin RNA, Xwnt-3a RNA, or a combination of both. Animal explants coexpressing noggin and Xwnt-3a activate a broad range of dorsally expressed genes (Krox-20, slug, wnt-1, and pax-3) but failed to activate ventrally expressed genes (shh and FKH-1).
DISCUSSION
In this article we report an analysis of the influence of Wnt signaling factors on the patterning of the neural tube along its dorsoventral axis. Such a role might be inferred from the highly differentiated expression patterns of different Wnt family members along this axis. We find that Wnt-1 and Xwnt-3a, the dorsally expressed Wnt molecules, induce neural crest markers in neuralized ectoderm, in explants and in the whole embryo (Figs. 1 and 2). Xwnt-3a has some ability to activate neural crest markers in animal explants, without initial neuralization of the tissue (Fig. 1). Although not fully understood, this result suggests that neural crest formation can be achieved in the ectoderm independently of the induction of neural plate tissue or that Xwnt-3a favors the innate tendancy of the ectoderm to become neuralized (39, 40).
A role for Xwnt-3a in posteriorization of the neural tube has been proposed, based on the activation of Krox-20 in noggin-expressing animal explants (13). Although patterning effects that would lead to changes of both posteriorizing and dorsalizing nature are certainly compatible, we wish to stress the evidence for a dorsalizing effect that is supported by the induction of the crest markers slug and AP-2, and of wnt-1, which is expressed at the dorsal midline as far rostrally as the diencephalon (37). The induction of Krox-20 and pax-3 is compatible with both posteriorizing and dorsalizing effects. Nevertheless, Xwnt-3a does not change the anteroposterior pattern of Krox-20 in the region of rhombomeres 3 and 5 but rather affects formation of neural crest fates in this region of the central nervous system. Moreover, the caudal neural marker HoxB9 (41) failed to be induced by Xwnt-3a (ref. 13 and unpublished results).
The specificity of neural crest marker induction by Wnt-1 and Xwnt-3a is emphasized by the fact that Xwnt-8 and Xwnt-5A fail to induce such markers; this is especially notable in the case of Xwnt-8, which is known to be equally or more effective than Wnt-1 and Xwnt-3a in the axis duplication and rescue assays (7). Induction by Xwnt-3a requires inhibition of GSK-3 (Fig. 4) as does Wnt signaling in other biological systems (11). This fact allowed us to test whether neural crest formation in vivo has a requirement for Wnt signaling. Overexpression of GSK-3, which counteracts the down-regulation of this kinase during Wnt signaling, inhibited the formation of neural crest in otherwise unmanipulated embryos (Fig. 5). This result indicates that crest induction by overexpression of Wnt-1 or Xwnt-3a reflects a pathway that is active during normal neural crest formation in vivo. Because the detection of slug transcripts has been reported (24) at a time prior to the earliest documented expression of Xwnt-1 and Xwnt-3a (6), we suggest that the Wnt factors are involved in the maintenance of the neural crest differentiation program rather than in its initial specification, reminiscent of the role proposed for Dorsalin-1 (42). Numerous studies, especially compelling in Drosophila, have shown that the maintenance of differential gene expression in specific cells and tissues is controlled separately from its initiation, both processes being essential during embryogenesis (43).
Previous studies indicate that neural crest development can be mediated through a bone morphogenetic protein (BMP)-like signal produced by nonneural ectoderm and by the dorsal neural tube (44). The neural inducers noggin and chordin act by neutralizing a BMP signal in the ectoderm (39, 40); on the basis of this fact, it has been proposed that neural crest forms in a region where BMP and anti-BMP signals reach an appropriate balance (45). We found that the level of BMP-4 transcripts was unchanged in animal explants expressing noggin and Wnt-1 or Xwnt-3a (data not shown), indicating that the Wnt effect we describe is probably not mediated by up-regulation of BMP-4; however, it remains possible that other BMPs or nontranscriptional regulation could be involved. On the other hand, a recent report showing that BMPs up-regulate Wnt-1 and Wnt-3a in the chicken dorsal neural tube (46) suggests the possibility that neural crest induction by BMPs may be mediated, in part, by Wnt factors. In addition, the RNA helicase eIF4AII, a component of the translation initiation complex (eIF4F), and members of the fibroblast growth factor family have been shown to initiate expression of neural crest markers in animal explants (45, 47). The way in which the different neural-crest-inducing factors interact remains to be determined.
Mice with targeted disruption of the wnt-1 gene present severe truncation of the midbrain and the cerebellum whereas the rest of the neural tube and other tissues appeared to be normal (48, 49). Gene targeting of wnt-3a gave rise to embryos that lack caudal somites and have a disrupted notochord (50), illustrating the role of this gene in the regulation of dorsal mesodermal fate. Our findings imply possible functional compensation of the disrupted wnt-1 and wnt-3a genes by each other, in a manner commonly observed in mouse null mutants. Indeed, wnt-1/wnt-3a double mutant mice show defects in three major neural crest derivatives, including components of the head skeleton, cranial and dorsal root ganglia, and melanocyte precursors (51), implying that a broad loss of dorsal Wnt signaling in the neural tube interferes with neural crest formation. These results and our observations strongly argue for an important role of Wnt-1 and Wnt-3a in the patterning of the neuroectoderm along its dorsoventral axis.
Acknowledgments
We are grateful to Drs. Shinji Takada and Andrew McMahon for communication of results prior to publication. We thank Drs. Anne Bang, Milan Jamrich, Roberto Mayor, Randall Moon, Thomas Sargent, and Yun-Bo Shi for plasmids; and Thomas Sargent, Michael Rebagliati, and Joseph Breen for comments on the manuscript.
ABBREVIATIONS
- β-gal
β-galactosidase
- BMP
bone morphogenetic protein
- HUA
hydroxyurea plus aphidicolin
- GSK-3
glycogen synthase kinase 3
References
- 1.Nusse R, Varmus H E. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 2.Moon R T, Brown J D, Torres M. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- 3.Chakrabarti A G, Matthews G, Colman A, Dale L. Development (Cambridge, UK) 1992;115:355–369. doi: 10.1242/dev.115.1.355. [DOI] [PubMed] [Google Scholar]
- 4.McMahon A P, Moon R T. Cell. 1989;58:1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- 5.Landesman Y, Sokol S Y. Mech Dev. 1997;63:199–209. doi: 10.1016/s0925-4773(97)00041-5. [DOI] [PubMed] [Google Scholar]
- 6.Wolda S L, Moody C J, Moon R T. Dev Biol. 1993;155:46–57. doi: 10.1006/dbio.1993.1005. [DOI] [PubMed] [Google Scholar]
- 7.Christian J L, McMahon J A, McMahon A P, Moon R T. Development (Cambridge, UK) 1991;111:1045–1056. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- 8.Cui Y, Brown J D, Moon R T, Christian J L. Development (Cambridge, UK) 1995;121:2177–2186. doi: 10.1242/dev.121.7.2177. [DOI] [PubMed] [Google Scholar]
- 9.Stark K, Vainio S, Vassileva G, McMahon A P. Nature (London) 1995;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 10.Parr B A, McMahon A P. Nature (London) 1995;374:350–353. doi: 10.1038/374350a0. [DOI] [PubMed] [Google Scholar]
- 11.Miller J R, Moon R T. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- 12.Parr B A, Shea M J, Vassileva G, McMahon A P. Development (Cambridge, UK) 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- 13.McGrew L L, Lai C-J, Moon R T. Dev Biol. 1995;172:337–342. doi: 10.1006/dbio.1995.0027. [DOI] [PubMed] [Google Scholar]
- 14.Bradley L C, Snape A, Bhatt S, Wilkinson D G. Mech Dev. 1992;40:73–84. doi: 10.1016/0925-4773(93)90089-g. [DOI] [PubMed] [Google Scholar]
- 15.Le Douarin N M. The Neural Crest. Cambridge, U.K.: Cambridge Univ. Press; 1982. [Google Scholar]
- 16.Moon R T, Campbell R M, Christian J L, McGrew L L, Shih J, Fraser S. Development (Cambridge, UK) 1993;119:97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- 17.Lamb T L, Knecht A K, Smith W C, Stachel S E, Economides A N, Stahl N, Yancopolous G D, Harland R M. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- 18.Sasai Y, Lu B, Steinbeisser H, De Robertis E M. Nature (London) 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- 19.He X, Saint-Jeannet J-P, Woodgett J, Varmus H E, Dawid I B. Nature (London) 1995;374:617–622. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- 20.Taira M, Otani H, Saint-Jeannet J-P, Dawid I B. Nature (London) 1994;372:677–679. doi: 10.1038/372677a0. [DOI] [PubMed] [Google Scholar]
- 21.Turner D L, Weintraub H. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- 22.Knecht A K, Good P J, Dawid I B, Harland R M. Development (Cambridge, UK) 1995;121:1927–1936. doi: 10.1242/dev.121.6.1927. [DOI] [PubMed] [Google Scholar]
- 23.Winning R S, Shea L J, Marcus S J, Sargent T D. Nucleic Acids Res. 1991;19:3709–3714. doi: 10.1093/nar/19.13.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayor R, Morgan R, Sargent M. Development (Cambridge, UK) 1995;121:767–777. doi: 10.1242/dev.121.3.767. [DOI] [PubMed] [Google Scholar]
- 25.Dirksen M L, Jamrich M. Genes Dev. 1992;6:599–608. doi: 10.1101/gad.6.4.599. [DOI] [PubMed] [Google Scholar]
- 26.Bang A, Papalopulu N, Kintner C, Goulding M D. Development (Cambridge, UK) 1997;124:2075–2085. doi: 10.1242/dev.124.10.2075. [DOI] [PubMed] [Google Scholar]
- 27.Stolow M A, Shi Y B. Nucleic Acids Res. 1995;23:2555–2562. doi: 10.1093/nar/23.13.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harland R M. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- 29.Harris W A, Hartenstein V. Neuron. 1991;6:499–515. doi: 10.1016/0896-6273(91)90053-3. [DOI] [PubMed] [Google Scholar]
- 30.Kao K R, Elinson R P. Dev Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- 31.Cunliffe V, Smith J C. EMBO J. 1994;13:349–359. doi: 10.1002/j.1460-2075.1994.tb06268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christian J L, Moon R T. Genes Dev. 1993;7:13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- 33.Du S J, Purcell S M, Christian J L, McGrew L L, Moon R T. Mol Cell Biol. 1995;15:2625–2634. doi: 10.1128/mcb.15.5.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieto M A, Sechrist J, Wilkinson D G, Bronner-Fraser M. EMBO J. 1995;14:1697–1710. doi: 10.1002/j.1460-2075.1995.tb07159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickinson M E, Krumlauf R, McMahon A P. Development (Cambridge, UK) 1994;120:1453–1471. doi: 10.1242/dev.120.6.1453. [DOI] [PubMed] [Google Scholar]
- 36.Goulding M D, Chalepakis G, Deutsch U, Erselius J R, Gruss P. EMBO J. 1991;10:1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkinson D G, Bailes J A, McMahon A P. Cell. 1987;50:79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- 38.Echelard Y, Epstein D J, St-Jacques B, Shen L, Mohler J, McMahon J A, McMahon A P. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman L B, de Jesus-Escobar J M, Harland R. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 40.Piccolo S, Sasai Y, Lu B, de Robertis E M. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharpe C R, Fritz A, De Robertis E M, Gurdon J B. Cell. 1987;50:749–758. doi: 10.1016/0092-8674(87)90333-3. [DOI] [PubMed] [Google Scholar]
- 42.Basler K, Edlund T, Jessell T M, Yamada T. Cell. 1993;73:687–702. doi: 10.1016/0092-8674(93)90249-p. [DOI] [PubMed] [Google Scholar]
- 43.Kennison J A. Annu Rev Genetics. 1995;29:289–303. doi: 10.1146/annurev.ge.29.120195.001445. [DOI] [PubMed] [Google Scholar]
- 44.Liem K F, Tremml G, Roelink H, Jessell T M. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- 45.Morgan R, Sargent M G. Development (Cambridge, UK) 1997;124:2751–2760. doi: 10.1242/dev.124.14.2751. [DOI] [PubMed] [Google Scholar]
- 46.Marcelle C, Stark M R, Bronner-Fraser M. Development (Cambridge, UK) 1997;124:3955–3963. doi: 10.1242/dev.124.20.3955. [DOI] [PubMed] [Google Scholar]
- 47.Mayor R, Guerrero N, Martinez C. Dev Biol. 1997;189:1–12. doi: 10.1006/dbio.1997.8634. [DOI] [PubMed] [Google Scholar]
- 48.McMahon A P, Bradley A. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 49.Thomas K R, Capecchi M R. Nature (London) 1990;346:847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- 50.Takada S, Stark K L, Shea M J, Vassileva G, McMahon J A, McMahon A P. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- 51.Ikeya M, Lee S M K, Johnson J E, McMahon A P, Takada S. Nature (London) 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]


