Abstract
Cell specification and differentiation of cardiomyocytes from mesodermal precursors is orchestrated by epigenetic and transcriptional inputs throughout heart formation. Of the many transcription factor super families that play a role in this process, the basic Helix-loop Helix (bHLH) family of proteins is well represented. The bHLH protein by design allows for dimerization—both as homodimers and heterodimers with other proteins within the family. Although DNA binding is mediated via a short variable cis-element termed an E-box, it is clear that DNAaffinity for these elements as well as the transcriptional input conveyed is dictated largely by the transcriptional partners within the dimer complex. Dimer partner choice has a number of inputs requiring co-expression within a given cell nucleus and dimerization modulation by the level of protein present, and post-translational modifications that can both enhance or reduce protein–protein interactions. Due to these complex interrelationships, it has been difficult to identity bona-fide downstream transcriptional targets and define the molecular pathways regulated of bHLH factors within cardiogenesis, despite the clear roles suggested via loss-of-function animals models. This review focuses on the Hand bHLH proteins—key members of the Twist-family of bHLH factors. Despite over a decade of investigation, questions regarding functional redundancy, downstream targets, and biological role during heart specification and differentiation have still not been fully addressed. Our goal is to review what is currently known and address strategies for gaining further understanding of Hand/Twist gene dosage and functional redundancy relationships within the developing heart that may underlie congenital heart defect pathogenesis.
Keywords: Congenital heart diseases, Basic helix-loop-helix transcription factors, Gene dosage
Hand1 and Hand2 Transcription Factors and Cardiovascular Development
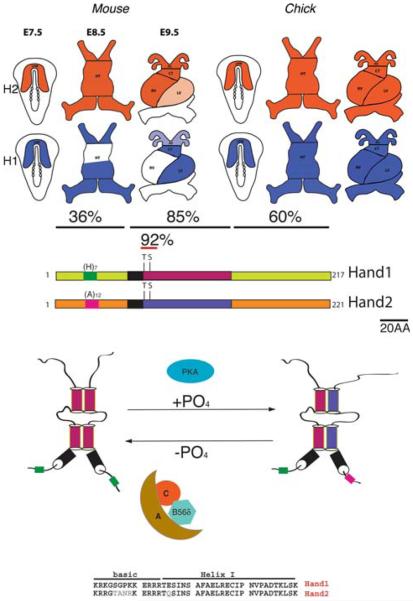
In the mouse, cardiac progenitors can be identified as early as E7.5 (roughly equivalent to day 18 in human embryos) and their presence in the embryo is dependent upon multiple epigenetic and transcriptional inputs from an ever growing number of essential transcription factors [2, 14, 35, 42]. The bHLH factors Hand1 and Hand2 are members of the Twist-family of proteins and are broadly expressed throughout development in a number of tissues including: neural crest that contribute to craniofacial structures, sympathetic and enteric neurons and cardiac outflow tract (OFT); developing limbs; extraembryonic tissues; placenta and most significantly the cardiomyocytes [12, 13]. Hand mRNA is first detected around E7.5 throughout the cardiac crescent [8, 9, 22, 43]. In chick embryos, both Hand genes mark the entire cardiac crescent and expression is completely overlapping through linear heart tube and cardiac chamber septation [43]. However, in mouse Hand1 expression becomes restricted to what will become the left ventricle and OFT, whereas Hand2 expression maintains ubiquitous cardiac expression within the linear heart tube; though restricts to cardiomyocytes that form the right ventricle and OFT (Fig. 1). In antisense knockdown experiments that reduced Hand1 and Hand2 expression in chick embryos, no significant phenotypes were observed; however, simultaneous reduction in Hand mRNA resulted in morphological defects to the heart that resulted in diminished function and death [43]. Targeted mouse knockout models of Hand1 and Hand2 confirm a critical role in cardiogenesis but individual loss-of-function experiments display unique phenotypes [15, 40, 44]. Hand2 systemic knockout embryos die at E9.5 resulting from a hypoplastic right ventricle and OFT vascular defects—corresponding phenotype with expression domains and suggesting a cell autonomous role [44]. Hand1 systemic knockout embryos die at E8.5–9.5 due to extraembryonic mesoderm and placental insufficiencies that involve deregulation of vascular genes [15, 30, 40]. Cardiac-restricted deletion of Hand1 in conditional knockout mice circumvent this superseding extraembryonic phenotype and reveal a hypoplastic left ventricle (again matching phenotype with expression domain), and interestingly the phenotypes observed become more severe on a Hand2 haploinsufficient background [29]. Subtle expression variation within species is not uncommon and given that Hand factors share a high amino acid identity (90%) within the functional bHLH domain, the idea of functional overlap was obvious, especially given the supporting chick data. Conversely, outside the bHLH domain identity is far less conserved (Fig. 1), suggesting that although some functions may be replaceable, it is likely that unique functions for each Hand factor may also exist. Thus, although it is clear that both Hand factors play significant roles in cardiac morphogenesis, given their broad expression profiles in other organ systems and combinatorial nature; the specific molecular networks within the developing heart that utilize Hand factor biological function remain elusive (Table 1).
Fig. 1.
a Schematic of Hand gene expression during early cardiac development. Published findings describe early expression within the anterior later plate mesoderm, which in the chick persists for both Hand genes. In mouse, Hand2 persists until E8.0 and upon rightward looping, restricts to second field lineage cells on the right ventricle and OFT. Hand1 prior to heart tube fusion already displays chamber-specific expression within the primary heart field derived left ventricle and OFT such that by E9.5 coexpression with the myocardial cuff and OFT is observed along with unique expression domains that define the left and right ventricles. b Schematic of Hand1 and Hand2 coding regions showing amino acid identities within the amino terminal, bHLH, and carboxy terminal domains. The positions of the basic DNA-binding domain are shown in black and poly-histidine and polyalanine tracks in the amino terminal portion of each respective protein are indicated. The evolutionarily conserved threonine and serine within Helix I of the bHLH are phosphoregulated via PKA and B56d-containing PP2A. Regulation alters dimer partner choice and effects DNA-binding affinities to various cis-elements. Amino acid alignment of Hand1 and 2 over the basic domain and Helix I showing high level of conservation within the DNA binding and dimerization motifs
Table 1.
Summary of known cardiac-related phenotypes in gain-of-function and loss-of-function analysis for Hand factors
| Gene | Cardiovascular phenotypes | Reference |
|---|---|---|
| Hand1 | Mouse: Systemic Hand1 KO display extraembryonic and placental & vascular defects. Dies at E9-9.5. | [15, 30, 40] |
| Conditional knockout using cardiac-specific Cre drivers show hypoplastic left ventricle and clear gene dosage with Hand2. | [29] | |
| Neural crest inactivation of Hand1 displays no observable phenotypes; however, Hand2 gene dosage effects are observed. | [1] | |
| Hand1 inducible gain-of-function during embryogenesis increases cardiomyocyte proliferation. Expression via knockin to Mlc2v inhibited septum formation expanded ventricular size. | [46] | |
| Hand1 gain-of-function in adult heart predisposes mice to arrhythmias. | [3, 41] | |
| Hand2 | Systemic Hand2 KO mice display right reduction in right ventricle and vascular defects. Dies at E9.5-E10.0 | [44] |
| Cardiac knockout of Hand2 using cTnt-Cre phenocopy a reduction in right ventricle. Hand1 gene dosage effects not evaluated | [31] | |
| Neural crest inactivation of Hand2 display OFT defects. Hand1 gene dosage effects not evaluated | [31] | |
| Chick: Antisense knockdown of either Hand1 or 2 has no observable phenotype; however, knockdown of both genes results in defective heart development | [43] | |
| Fish: The single Hand gene as two mutant alleles termed Hands off these fish display in lower number of myocardial cell precursors and do not maintain Tbx5 expression within the myocardium. | [48] | |
| Fly: Drosophila Hand semi lethal larva—lymph glands missing. Adult flies display abnormal dorsal vessel disorganized musculature and reduced function. | [25] | |
| Twist1 | Mouse: Twist1 loss-of-function exhibits adhesion and emigration defects in cardiac neural crest cells marked by expression of Hand1 and Hand2 | [47] |
Molecular Mechanism of Function: bHLH Partner Choice and Gene Dosage
The bHLH domain is a region of basic amino acids followed directly by an amphipathic α-Helix, which is separated from a second more carboxy α-Helix by a loop domain of varying length [27]. This domain enables protein–protein interactions between two bHLH factors. Dimerization then juxtaposes the two proteins basic domains to form a combined DNA-binding domain that in the majority of bHLH proteins recognizes a canonical sequence termed an E-box (CANNTG) [27]. This super-family of proteins was first identified as important transcriptional and developmental regulatory from study of skeletal muscle—specifically the myogenic regulatory factors (MRFs) MyoD, Myogenin, Myf5, and Mrf4 [27, 33, 34]. Functionally, the MRF proteins dimerize with the ubiquitously expressed E-proteins class of bHLH factors [27], although recent investigations have shown that these factors can interact and bind to non-traditional cis-elements [20]. In contrast to the myogenic bHLH factors, which are expressed exclusively in skeletal muscle and primarily function as heterodimers with E-proteins, Hand1 and Hand2 (and all Twist-family proteins) display a broad range of expression throughout the developing embryo (Fig. 2) and adult, and exhibit dimerization characteristics which include homodimerization, heterodimerization with other members of the Twist-family, dimerization with E-proteins as well as other bHLH factors such as Hey factors [16-18]. Moreover, interactions with other transcription factors and binding to non-canonical cis-elements can also be observed [21, 24, 45].
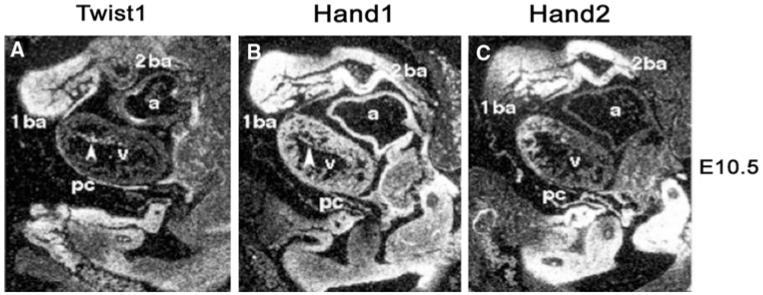
Fig. 2.
In situ hybridization of Twist1, Hand1, and Hand2 in sagitally sectioned E10.5 day mouse embryos. Expression is observed within a broad domain of mesodermally and neural crest-derived cells that make up the craniofacial, cardiac, sympathetic, and enteric nervous system as well as lateral mesoderm derived organs. Given the broad overlap of expression of Twist-family genes during development and the ability of the coded proteins to form transcriptional complexes modulation of where these genes are expressed as well as levels of overall expression are critical to normal development
It is easy to see the convenience of participation in multiple transcriptional complexes, when one considers the board spectrum of tissues where these proteins are expressed. However, to propose this hypothesis there must accompany a mechanism that provides instruction to bHLH dimer choice. First, coexpression and the overall level of bHLH protein present within the nucleus is probably the primary factor governing dimerization. Clearly, two factors cannot interact if they are not coexpressed nor can a factor expressed at 1:100th the levels of its potential partners compete effectively for a rate-limiting dimer partner (provided dimerization affinities are equal). The later supposition is unlikely to hold true and mechanisms that modulate dimerization affinities are in fact in place. One such post-translational mechanism that we have shown plays a role in Hand1 and Hand2 dimer regulation is phosphorylation of evolutionarily conserved residues within the Helix I of the bHLH domain [17]. Via protein kinase A (PKA) and B56d-containing protein phosphatase 2A (PP2A), a charge on Helix I is modulated and these changes can affect not only dimer affinities for various partners but also DNA-binding affinities to specific cis-elements (Fig. 1) [17, 19]. Thus, the level of gene expression in union with post-translational modifications to the bHLH dimer pool within a cell may direct the formation of the specific bHLH complexes required to drive specific developmental transcriptional programs.
In addition, recent work in the Riley lab showed that in addition to dimer affinities, Helix I phosphoregulation of Hand1 may also regulate subcellular localization [26]. Hand1 promotes the differentiation of trophoblast giant cells. Expression Hand1T107;S109A within Rcho1 stem cell trophoblast cells [38] fails to induce differentiation compared to wild type Hand1. Examination of cell localization using GFP-fusion proteins demonstrated that Hand1T107;S109A is sequestered in the nucleoli via interactions with the inhibitor of myogenic factor, I-mfa [26]. Phosphorylation of Hand1 within Helix I is necessary for release from within the nucleoli fitting into the over riding regulation model proposed above—thereby limiting access to the factor where it functions. The idea that Hand1 (and perhaps Hand2) are regulated via sequestration from the transcriptional compartment in cardiogenesis will be interesting to investigate further.
To date, published data currently point toward post-translational modifications and cellular localization as defining the regulatory mechanisms. However, the remaining element to create the bHLH dimer pool; namely gene dosage effects, are also observed between Twist1, Hand1, and Hand2 factors. During conditional ablation of Hand1 within the myocardium haploinsufficiency of Hand2 was able to exacerbate the observed Hand1 phenotype [29]. In these studies, cardiomyocyte deletion of Hand1 via αMHC-Cre results in VSDs and overriding aorta as well as thickened and shorten AV valves. Use of the Nkx2.5-Cre driver, which is expressed earlier and more directly upstream of Hand1 for cardiomyocyte deletion yielded similar results suggesting that Hand1 is being deleted early enough in cardiogenesis as Nkx2.5 null mice fail to express Hand1 in the left ventricle [14]. Moreover, removing a single Hand2 allele on this background results in embryonic lethality at E10.5 with ventricular phenotypes clearly indicating that gene dosage is required to normal embryogenesis. Furthermore, less Hand1 expressing cells (as visualized via a Hand1LacZ allele) are observed in the left ventricle and heart morphology shows a thin and poorly trabeculated myocardium [29]. As expected Hand1 conditional/Hand2 systemic double knockouts display a more grossly hypoplastic heart that is looped but contains only a single ventricle and atria via morphological analysis [29]. However, gene dosage effects seem to require a minimum threshold before having any cardiovascular effects, as no phenotypes are observed within either Hand1 or Hand2 heterozygous or double heterozygous Hand mice. Future insights into the limit of dosage effects may be gained using Hand2 conditional mice to focus upon cardiomyocytes-restricted autonomous defects.
Perhaps, the best example of Twist-Hand gene dosage effects is the autosomal dominant human disease Saethre Chotzen syndrome (SCS) that exemplifies post-translational modifications, dimer choice, and gene dosage most clearly. Craniofacial and limb phenotypes are largely observed in SCS patients, and this congenital defect is largely attributed to mutations in the TWIST1 gene. Significantly, 73 documented null, missense and nonsense mutations have been associated with SCS in humans [23]. Recently, we identified a number of SCS-causing human mutations in TWIST1 that alter phosphoregulation via PKA and B56δ-containing PP2A [18]. Realizing that Twist1 haploinsufficiency partially phenocopied specific Hand2 gain-of-function in the developing limb [11, 28] resembling SCS-like phenotypes within the mouse limb, we reasoned that this was an example of Twist-family gene dosage. To test this, we reduced gene dosage of Hand2 to match that in Twist1 haploinsufficient mice and were able to clearly genetically demonstrate that Hand2 heterozygousity rescues Twist1-mediated SCS phenotypes in vivo. Similarly, overexpression of Twist1 rescues Hand2 gain-of-function limb phenotypes; however, overexpression of a phosphorylation Twist1 mutation that models a human TWIST1 SCS allele does not rescue the Hand2-mediated limb phenotypes [18]. Collectively these experiments define a paradigm for Twist-family bHLH gene regulation where the level of expression and the post-translational state of the proteins themselves set up a bHLH dimer pool that conveys defined biological function. In this example, Twist1 and Hand2 functionally and genetically interact in an antagonistic fashion, and we speculate that in other tissues different, and perhaps more complex relationships may be operating.
Recently, we uncovered a novel role for Twist1 within the cardiac neural crest (cNCC) adding Twist as a new bHLH player within cardiac OFT development [47]. Twist1 null mice die at E11.5 and present hypoplastic limbs along with extensive craniofacial defects associated with NCC cell populations [5]. Closer examination of the cardiac OFT of Twist1 null mice reveals a cell adhesion defect where clumps of cells that are uncharacteristic of the loose mesenchymal phenotype of OFT cushion formation [47]. Lineage analysis identifies these “nodule” cells as NCC origin and perhaps most striking, the cNCC, which contribute to OFT nodules are specifically marked by expression of Hand1 and Hand2. Also evident were cNCC emigration defects; however, proliferation and cell death of NCC cells in Twist1 null mice was indistinguishable from wild-type littermates [47]. This emigration defect could be directly traced back to the neural tube where an anomalous expansion of Wnt1-Cre marked cells are encountered suggesting that the NCC cells become trapped in the neural tube and these trapped cells simply revert to a default CNS developmental program. Given that Twist1 expression is not detectable within the neural tube but is observed in the surrounding mesenchyme, we believe this NCC trapping is a non-cell autonomous effect, which may be independent of the aggregation phenotype observed in the cNCC that do escape, emigrate and are marked by Hand gene expression. Experiments to explore gene dosage effects on the crest nodule phenotype are currently underway.
Roadblocks to Overcome Before We Can Understand bHLH Mode of Action In Vivo
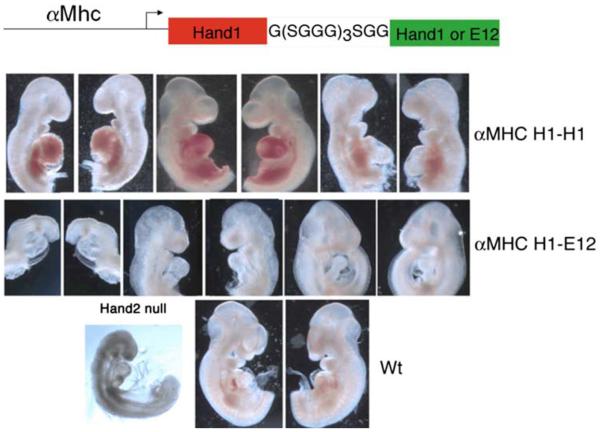
In order to expand our understanding of the biochemical and genetic relationships of Twist-family proteins within the developing heart, the field will be required to continue to dissect the functional and genetic relationships, define true redundancy relationships (can functional heterodimers be formed and/or isolated) and define the unique roles these factors play in building the heart. The rub is that any loss-of-function or gain-of-function approach has ripple effects on unintended bHLH factors coexpressed within the cells, wherever the balance has been altered. Gaining an improved understanding of that balance, the code, is of paramount importance for us to gaining a true understanding of Twist-family proteins and their functional role in heart formation. What is lacking has been the elucidation of direct transcriptional targets, which likely reflects the importance of the aforementioned dimer-regulatory mechanisms. Conflicting data sets showing activation or repression in vitro may reflect the specific dimer complex forming (or being inhibited from forming) and biological and arti-factual interactions within the system studied. Although highly artificial in nature, the idea of using tethered bHLH factors, where translation of two bHLH factors as a single polypeptide that (in theory) could force the two linked coding regions to form a transcriptional complex, could be used to initially probe some of the aforementioned outstanding questions. In the fly for example, tethered Twist homodimers and Twist/E-protein heterodimers can indeed activate/repress mesoderm and somatic muscle formation when expressed [4]. Work from the Spicer lab elegantly shows Twist expression using tethered gain-of-function approaches results in craniofacial development [6, 7]. In our own work using a tethered dimer approach, we can correlate observed limb phenotypes between specific Twist1 and Hand2 tethered dimers with those generated by expression of Twist1 phosphorylation mutants [19]. Expanding upon this approach via the use of the cardiac-specific α-myosin heavy chain (αMHC), promoter to drive Hand1 dimers also yields consistent and provocative data that underscores the importance of genetic balance and dimmer rules (Fig. 3). In E9.5 day F0 transgenic expressing αMHC-Hand1-Hand1 homodimers, only pericardial bleeding is encounter in a high percentage of embryos. In contrast, expression of αMHC-Hand1-E12 results in embryos that have severely hypoplastic right ventricles (Fig. 3). What is striking is that these embryos appear to phenocopy the Hand2 systemic deletion defects. Thus, if there is a critical balance between Hand1 and Hand2 in the developing ventricle and loss of Hand2 promotes the formation of additional Hand1-E12 heterodimers, one can see how analysis of a loss-of function phenotype of a bHLH factor could become overly complex. Similarly, over-expression of a monomeric bHLH factor may effectively titrate out endogenously expressed bHLH proteins, thereby creating a hidden los-of-function requirement for unknown transcription factor(s).
Fig. 3.
Schematic of Hand1 tethered dimers expressed via the αMHC-promoter. Three F0 E9.5 day embryos expressing Hand1-Hand1 (top row) within the heart display pericardial bleeding cardiac morphological defects. Hand1-E12 tethered expression (middle row) results in loss of right ventricular structures phenocopying the Hand2 systemic knockout (lower row). Wild-type mice included for comparison
Further compounding the unquantified nature of Twist-family dimer diversity in steady state is the lack of reliable specific antibodies. In conjunction with the low level of endogenous protein expression, the lack of antibody tools prohibits unbiased screens for downstream targets, although ectopic overexpression can be achieved, the obvious perturbation of the bHLH dimer pool via overexpression could inadvertently pollute the datasets of such screens. Perhaps targeting in a high affinity epitope within the coding region of Twist1, Hand1, and Hand2 will be required for obtaining multiple bona fide downstream targets, as well as the targeting of specific tethered dimer complexes to force dimer choice, and thus avoid overexpression and ectopic domains of function.
In addition to these experimental roadblocks, genetic data linking Twist-family proteins to congenital heart disease has also been lacking. When considering the myriad of transcription factors that contribute to cardiogenesis, numerous factors such as, Gata4 and Nkx2.5 have all been identified as causative within human congenital heat disease via identification of haploinsufficiency coding mutations [32, 36, 37]. Although Hand factors have been shown to interact with all these key factors, specifically Gata4 [10] and Nkx2.5 [45], as yet no CHDs have been directly associated with a human HAND mutation. Excitingly, a study of 31 unrelated patients diagnosed with hypoplastic left heart syndrome (HLHS), found that 24 of these patients presented with mutations within HAND1 [39]. These findings clearly support the importance that Hand factors play in heart development and more widespread analysis of CHD patients is likely to result in further identification of additional HAND1 and HAND2 mutations that are causative of disease.
Summary and Future Directions
To move forward in our understanding of the role of Twist-family bHLH factors during cardiogenesis and specifically how the clear functional mechanisms modulate heart formation, we need to gain additional information on the ultimate destination of Hand and Twist-expressing cells within the mature heart via lineage analysis, gain improved understanding of phosphoregulation of dimerization affinities without the complications of over-expression via point mutant-specific knockin alleles, and further dissect issues of gene dosage and functional relationships by replacement alleles where the coding domains of one bHLH factor are replaced with that of a relative. The results of such experiments are likely to fill in the current gaps of how we view this key family of transcription factors and their significance in cardiogenesis.
Acknowledgments
We would like to thank members of the Firulli and Conway labs for scientific assistance. We would also like to thank the Riley Heart Research Center for helpful input during robust group discussions. Support is provided by the Herman B Wells Center for Pediatric Research, the Riley Children's Foundation, and the Division of Pediatric Cardiology and by NIH P01HL085098 (ABF & SJC).
References
- 1.Barbosa AC, Funato N, Chapman S, McKee MD, Richardson JA, Olson EN, Yanagisawa H. Hand transcription factors cooperatively regulate development of the distal midline mesenchyme. Dev Bio. 2007;310:154–168. doi: 10.1016/j.ydbio.2007.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes RM, Firulli AB. A Twist of insight, the role of Twist-Family bHLH factors in development. Int J Dev Biol. 2009;53(7):909–924. doi: 10.1387/ijdb.082747rb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breckenridge R, Zuberi Z, Gomes J, Orford R, Dupays L, Felkin LE, Clark JE, Magee AI, Ehler E, Birks EJ, et al. Over-expression of the transcription factor Hand1 causes predisposition towards arrhythmia in mice. J Molec Cell Cardiol. 2009;47:133–141. doi: 10.1016/j.yjmcc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Castanon I, Von Stetina S, Kass J, Baylies MK. Dimerization partners determine the activity of the Twist bHLH protein during Drosophila mesoderm development. Development. 2001;128:3145–3159. doi: 10.1242/dev.128.16.3145. [DOI] [PubMed] [Google Scholar]
- 5.Chen ZF, Behringer RR. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- 6.Connerney J, Andreeva V, Leshem Y, Muentener C, Mercado MA, Spicer DB. Twist1 dimer selection regulates cranial suture patterning and fusion. Dev Dyn. 2006;235:1345–1357. doi: 10.1002/dvdy.20717. [DOI] [PubMed] [Google Scholar]
- 7.Connerney J, Andreeva V, Leshem Y, Mercado MA, Dowell K, Yang X, Lindner V, Friesel RE, Spicer DB. Twist1 homodimers enhance FGF responsiveness of the cranial sutures and promote suture closure. Dev Biol. 2008;318:323–334. doi: 10.1016/j.ydbio.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross JC, Flannery ML, Blanar MA, Steingrimsson E, Jenkins NA, Copeland NG, Rutter WJ, Werb Z. Hxt encodes a basic helix-loop-helix transcription factor that regulates trophoblast cell development. Development. 1995;121:2513–2523. doi: 10.1242/dev.121.8.2513. [DOI] [PubMed] [Google Scholar]
- 9.Cserjesi P, Brown D, Lyons GE, Olson EN. Expression of the novel basic helix-loop-helix gene eHAND in neural crest derivatives and extraembryonic membranes during mouse development. Dev Biol. 1995;170:664–678. doi: 10.1006/dbio.1995.1245. [DOI] [PubMed] [Google Scholar]
- 10.Dai YS, Cserjesi P, Markham BE, Molkentin JD. The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J Biol Chem. 2002;277:24390–24398. doi: 10.1074/jbc.M202490200. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Teran M, Piedra ME, Kathiriya IS, Srivastava D, Rodriguez-Rey JC, Ros MA. Role of dHAND in the anterior-posterior polarization of the limb bud: implications for the sonic hedgehog pathway. Development. 2000;127:2133–2142. doi: 10.1242/dev.127.10.2133. [DOI] [PubMed] [Google Scholar]
- 12.Firulli AB. A HANDful of questions: the molecular biology of the HAND-subclass of basic helix-loop-helix transcription factors. Gene. 2003;312C:27–40. doi: 10.1016/s0378-1119(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 13.Firulli AB, Conway SJ. Combinatorial transcriptional interaction within the cardiac neural crest: a pair of HANDs in heart formation. Birth Defects Res C Embryo Today. 2004;72:151–161. doi: 10.1002/bdrc.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Firulli AB, Thattaliyath BD. Transcription factors in cardiogenesis: the combinations that may unlock the mysteries of the heart. Int Rev Cytol. 2002;214:1–62. doi: 10.1016/s0074-7696(02)14002-2. [DOI] [PubMed] [Google Scholar]
- 15.Firulli AB, McFadden DG, Lin Q, Srivastava D, Olson EN. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat Genet. 1998;18:266–270. doi: 10.1038/ng0398-266. [DOI] [PubMed] [Google Scholar]
- 16.Firulli BA, Hadzic DB, McDaid JR, Firulli AB. The basic helix-loop-helix transcription factors dHAND and eHAND exhibit dimerization characteristics that suggest complex regulation of function. J Biol Chem. 2000;275:33567–33573. doi: 10.1074/jbc.M005888200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Firulli B, Howard MJ, McDaid JR, McIlreavey L, Dionne KM, Centonze V, Cserjesi P, Virshup DMa, Firulli AB. PKA, PKC and the protein phosphatase 2A influence HAND factor function: a mechanism for tissue specific transcriptional regulation. Mol Cell. 2003;12:1225–1237. doi: 10.1016/s1097-2765(03)00425-8. [DOI] [PubMed] [Google Scholar]
- 18.Firulli BA, Krawchuk D, Centonze VE, Virshup DE, Conway SJ, Cserjesi P, Laufer E, Firulli AB. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nat Genet. 2005;37:373–381. doi: 10.1038/ng1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Firulli BA, Redick BA, Conway SJ, Firulli AB. Mutations within helix I of Twist1 result in distinct limb defects and variation of DNA binding affinities. J Biol Chem. 2007;282:27536–27546. doi: 10.1074/jbc.M702613200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidt AB, Rojas A, Harris IS, Black BL. Determinants of myogenic specificity within MyoD are required for noncanonical E box binding. Mol Cell Biol. 2007;27:5910–5920. doi: 10.1128/MCB.01700-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill AA, Riley PR. Differential regulation of Hand1 homodimer and Hand1-E12 heterodimer activity by the cofactor FHL2. Mol Cell Biol. 2004;24:9835–9847. doi: 10.1128/MCB.24.22.9835-9847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollenberg SM, Sternglanz R, Cheng PF, Weintraub H. Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol Cell Biol. 1995;15:3813–3822. doi: 10.1128/mcb.15.7.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabs EW. TWIST and Saethre-Chotzen syndrome. In: Epstein CJ, Erickson RP, Wynshaw-Boris A, editors. Inborn errors of development. Oxford University Press; New York: 2004. pp. 401–409. [Google Scholar]
- 24.Knofler M, Meinhardt G, Bauer S, Loregger T, Vasicek R, Bloor DJ, Kimber SJ, Husslein P. Human Hand1 basic helix-loop-helix (bHLH) protein: extra-embryonic expression pattern, interaction partners and identification of its transcriptional repressor domains. Biochem J. 2002;361:641–651. doi: 10.1042/0264-6021:3610641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo PCH, Zaffran S, Senatore S, Frasch M. The drosophila hand gene is required for remodeling of the developing adult heart and midgut during metamorphosis. Dev Bio. 2007;311:287–296. doi: 10.1016/j.ydbio.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martindill DMJ, Risebro CA, Smart N, Franco-Viseras MDM, Rosario CO, Swallow CJ, Dennis JW, Riley PR. Nucleolar release of Hand1 acts as a molecular switch to determine cell fate. Nat Cell Biol. 2007;9:1131–1141. doi: 10.1038/ncb1633. see comment. [DOI] [PubMed] [Google Scholar]
- 27.Massari ME, Murre C. Helix-Loop-Helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McFadden DG, McAnally J, Richardson JA, Charité J, Olson EN. Misexpression of dHAND induces ectopic digits in the developing limb bud in the absence of direct DNA binding. Development. 2002;129:3077–3088. doi: 10.1242/dev.129.13.3077. [DOI] [PubMed] [Google Scholar]
- 29.McFadden DG, Barbosa AC, Richardson JA, Schneider MD, Srivastava D, Olson EN. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201. doi: 10.1242/dev.01562. [DOI] [PubMed] [Google Scholar]
- 30.Morikawa Y, Cserjesi P. Extra-embryonic vasculature development is regulated by the transcription factor HAND1. Development. 2004;131:2195–2204. doi: 10.1242/dev.01091. [DOI] [PubMed] [Google Scholar]
- 31.Morikawa Y, Cserjesi P. Cardiac neural crest expression of Hand2 regulates outflow and second heart field development. Circ Res. 2008;103:1422–1429. doi: 10.1161/CIRCRESAHA.108.180083. [DOI] [PubMed] [Google Scholar]
- 32.Nemer G, Fadlalah F, Usta J, Nemer M, Dbaibo G, Obeid M, Bitar F. A novel mutation in the GATA4 gene in patients with Tetralogy of Fallot. Hum Mutat. 2006;27:293–294. doi: 10.1002/humu.9410. [DOI] [PubMed] [Google Scholar]
- 33.Olson EN. Regulation of muscle transcription by the MyoD family. The heart of the matter. Circ Res. 1993;72:1–6. doi: 10.1161/01.res.72.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Olson EN. Signal transduction pathways that regulate skeletal muscle gene expression. Mol Endocrinol. 1993;7:1369–1378. doi: 10.1210/mend.7.11.8114752. [DOI] [PubMed] [Google Scholar]
- 35.Olson EN. A genetic blueprint for growth and development of the heart. Harvey Lect. 2002;98:41–64. [PubMed] [Google Scholar]
- 36.Pashmforoush M, Lu JT, Chen H, Amand TS, Kondo R, Pradervand S, Evans SM, Clark B, Feramisco JR, Giles W, Ho SY, Benson DW, Silberbach M, Shou W, Chien KR. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell. 2004;117:373–386. doi: 10.1016/s0092-8674(04)00405-2. [DOI] [PubMed] [Google Scholar]
- 37.Pehlivan T, Pober BR, Brueckner M, Garrett S, Slaugh R, Van Rheeden R, Wilson DB, Watson MS, Hing AV. GATA4 haploinsufficiency in patients with interstitial deletion of chromosome region 8p23.1 and congenital heart disease. Am J Med Genet. 1999;83:201–206. [PubMed] [Google Scholar]
- 38.Peters TJ, Chapman BM, Soares MJ. Trophoblast differentiation: an in vitro model for trophoblast giant cell development. In: Tuan RS, Lo CW, editors. Developmental biology protocols. Humana Press Inc.; Totowa: 2000. pp. 301–311. [DOI] [PubMed] [Google Scholar]
- 39.Reamon-Buettner SM, Ciribilli Y, Inga A, Borlak J. A loss-of-function mutation in the binding domain of HAND1 predicts hypoplasia of the human hearts. Hum Mol Genet. 2008;17:1397–1405. doi: 10.1093/hmg/ddn027. [DOI] [PubMed] [Google Scholar]
- 40.Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18:271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- 41.Risebro CA, Smart N, Dupays L, Breckenridge R, Mohun TJ, Riley PR. Hand1 regulates cardiomyocyte proliferation versus differentiation in the developing heart. Development. 2006;133:4595–4606. doi: 10.1242/dev.02625. [DOI] [PubMed] [Google Scholar]
- 42.Sakabe M, Matsui H, Sakata H, Ando K, Yamagishi T, Nakajima Y. Understanding heart development and congenital heart defects through developmental biology: a segmental approach. Congenit Anom. 2005;45:107–118. doi: 10.1111/j.1741-4520.2005.00079.x. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava D, Cserjesi P, Olson EN. A subclass of bHLH proteins required for cardiac morphogenesis. Science. 1995;270:1995–1999. doi: 10.1126/science.270.5244.1995. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16:154–160. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- 45.Thattaliyath BD, Firulli BA, Firulli AB. The Basic-Helix-Loop-Helix transcription factor HAND2 directly regulates transcription of the atrial naturetic peptide gene. J Mol Cell Cardiol. 2002;34:1325–1344. doi: 10.1006/jmcc.2002.2085. [DOI] [PubMed] [Google Scholar]
- 46.Togi K, Kawamoto T, Yamauchi R, Yoshida Y, Kita T, Tanaka M. Role of Hand1/eHAND in the dorso-ventral patterning and interventricular septum formation in the embryonic heart. Mol Cell Biol. 2004;24:4627–4635. doi: 10.1128/MCB.24.11.4627-4635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincentz JW, Barnes RM, Rodgers R, Firulli BA, Conway SJ, Firulli AB. An Absence of Twist1 results in aberrant cardiac neural crest morphogenesis. Dev Biol. 2008;320:131–139. doi: 10.1016/j.ydbio.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yelon D, Ticho B, Halpern ME, Ruvinsky I, Ho RK, Silver LM, Stainier DYR. The bHLH transcription factor HAND2 plays parallel roles in zebrafish heart and pectoral fin development. Development. 2000;127:2573–2582. doi: 10.1242/dev.127.12.2573. [DOI] [PubMed] [Google Scholar]