Abstract
This study compared rates of regional atrophy in Alzheimer's disease (AD), frontotemporal dementia (FTD), and semantic dementia (SD). Cross-sectional studies have shown that different dementia syndromes are associated with different patterns of regional brain tissue loss. Rates of atrophy over time may be useful for differential diagnosis, and could be used to monitor disease progression, serving as an outcome measure for clinical trials. We studied patients with AD (n=12), FTD (n=13), SD (n=20), and normal controls (n=23) longitudinally with structural MRI, using BRAINS2 software to measure frontal, temporal and parietal lobe volumes. In FTD the rate of frontal lobe atrophy over one year was greater than in any other group, while SD showed the highest rate in the temporal lobes. Atrophy in these regions progressed twice as quickly in FTD and SD compared with AD. Atrophy was not significantly faster for AD in any brain region compared with the other groups. Regional atrophy over time was significantly faster in FTD and SD compared with AD, and the regions of greatest atrophy were specific for each syndrome. Measuring specific regions of cerebral volume changes by serial neuroimaging may serve as a useful biomarker outcome measure for clinical trials in neurodegenerative diseases.
Keywords: longitudinal, MRI, alzheimer's disease, frontotemporal dementia
INTRODUCTION
Many studies have shown that specific dementia syndromes are associated with particular patterns of volume loss in the brain, usually reflecting the clinical features in that syndrome. In the case of frontotemporal dementia (FTD), the region of greatest abnormality is found in the frontal lobes, consistent with the behavioral and frontal/executive deficits that characterize this disease, while in semantic dementia (SD) the regions of greatest abnormality are in the anterior and inferior temporal lobes which correlates with the profound semantic deficits seen in these patients.1-8 These patterns are very different compared with the regions of tissue loss indicated in AD, which affects the medial temporal, posterior temporal and parietal regions most severely.9-10 The prominent involvement in these regions is consistent with the memory, language and visuospatial dysfunction characteristic of AD2,4,11 as well at the pathological evidence suggesting that the hippocampus is one of the earliest sites of involvement in AD.12
In contrast to the well-established data on comparing dementias cross-sectionally, relatively little is known about how tissue loss in FTD and SD over time differs from the patterns seen with other dementias.13 This is important because rates of change specific to a given pathology may have utility for differential diagnosis, and may be used to monitor disease progression, potentially providing an outcome measure for clinical trials. One study compared rates of whole brain atrophy and ventricular expansion with serial MRI in autopsied patients with AD, FTLD, other neurodegenerative diseases, and controls, but did not look at specific regions of tissue loss.14 The FTLD group showed the highest whole brain atrophy rates, whereas AD atrophy rates were higher than controls, but similar to or lower than other diagnostic groups.14 Several MRI studies have shown that hippocampal atrophy progresses over time in AD, providing a potential measure of disease progression.15-17
A few serial MR studies have examined longitudinal brain tissue loss in FTD and/or SD using techniques such as voxel-based mophemetry7,18-20 and manual outlining.21 As would be expected, these studies reveal that longitudinal change in FTD is most prominent in the frontal and temporal regions,7,21-22 and some studies have identified changes more specifically in the medial and orbital frontal regions.19 In SD, atrophy over time involves the temporal regions with some involvement of orbital and medial frontal regions and the insula.20 All of these studies used morphometric procedures where patients' brains are reshaped to fit into a common atlas space. While valid, such techniques are known to yield different results compared with more straightforward region-of-interest (ROI) techniques where a relevant region is identified directly on each patient's brain and measured.23 In addition, because they examine changes over the entire brain at a resolution of about 1 cm, the statistical power of these techniques is lessened by the number of comparisons being conducted in each analysis. Lastly, nearly all these studies compared volume changes in FTD and SD with that in controls, rather than other dementias.
The goal of the current study was to compare rates of brain atrophy in FTD and SD with those in AD, as well as healthy controls. In contrast to the majority of prior studies, we used ROI based techniques that do not involve reshaping the brain and we examined a limited number of regions based on a-priori knowledge of relevant regions for these dementias, including the frontal and temporal lobes for FTD and SD, and the parietal lobes for AD.
METHODS
Subjects
FTD (n=13), SD (n=20), AD (n=12) patients, and normal controls (n=23) were studied longitudinally with structural MRI for research purposes (see Table 1 for demographic information). All subjects were evaluated at the University of California, San Francisco, (UCSF) Memory and Aging Center (MAC) by a team of experienced clinicians, including a behavioral neurologist or geriatric psychiatrist, a neuropsychologist, and a nurse. Research diagnoses of AD, FTD, and SD were made using established research criteria.24-25 Every attempt was made to obtain MRI scans one year apart. To be considered for the study, participants had to have at least two serial MRI scans at an interval of approximately one year. Mean MMSE of dementia subjects was 23.1 ± 7.0. Mean interval between scans was 14.0 months ± 6.9. There were no significant differences between the groups for education, gender, MMSE score (of dementia subjects), duration of symptoms (of dementia subjects, which was estimated from the caregiver's report upon initial evaluation), and interval between MRI scans. Controls were significantly older than AD and FTD patients.
Table 1.
General demographic and functional information of all subjects
| Controls | AD | FTD | SD | |
|---|---|---|---|---|
| Age | 67.1 (6.9)* | 60.0 (8.3) | 62.0 (6.2) | 62.7 (6.3) |
| Education | 17.2 (2.3) | 15.3 (3.5) | 14.7 (3.8) | 16.6 (3.4) |
| Sex (M/F) | 10/13 | 8/4 | 9/4 | 14/6 |
| MMSE | 29.6 (0.5)** | 22.2 (5.5) | 22.6 (9.5) | 24.1 (6.0) |
| Symptom Duration | N/A | 5.6 (2.4) | 7.4 (5.5) | 4.6 (2.5) |
| Months b/w scans | 15.9 (9.8) | 14.6 (4.0) | 12.6 (7.2) | 12.5 (2.5) |
Note:
Differs from AD and FTD patients at p<.05.
Differs from the patient groups at p<.01.
Image Acquisition
MRI scans were obtained on a 1.5-T Magnetom VISION system (Siemens Inc., Iselin, NJ) equipped with a standard quadrature head coil. Structural MRI sequences included: (i) 2D FLASH MRI along three orthogonal directions, 3 mm thick slices, ∼ 15 slices in each direction to obtain scout views of the brain for positioning subsequent MRI slices. (ii) A double spin echo sequence [repetition time/echo time 1/echo time 2 (TR/TE1/TE2) = 5000/20/80 ms] to obtain proton density and T2-weighted MRIs, 51 contiguous axial slices (3 mm) covering the entire brain and angulated −10° from the AC-PC line; 1.0 × 1.25 mm2 in-plane resolution. (iii) Volumetric magnetization prepared rapid gradient echo MRI [MPRAGE, repetition time/echo time/inversion time (TR/TE/TI) = 10/4/300 ms] to obtain T1-weighted images of the entire brain, 15° flip angle, coronal orientation perpendicular to the double spin echo sequence, 1.0 × 1.0 mm2 in-plane resolution and 1.5 mm slab thickness.
Image Processing
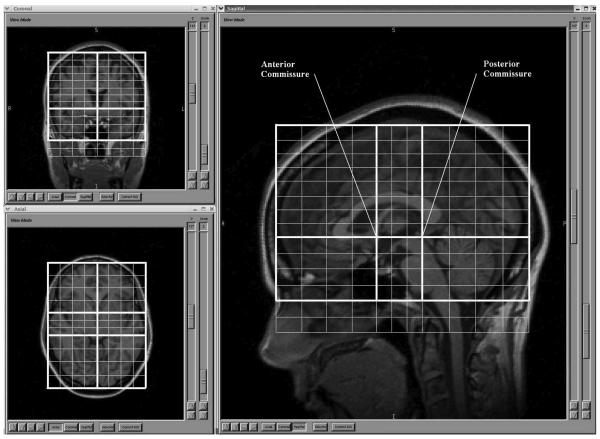
Magnetic resonance images were processed on Linux workstations using the BRAINS2 software package, which is developed and made available by the Mental Health – Clinical Research Center at the University of Iowa.26 BRAINS2 provides an approach for automatic parcellation of the major lobes of the brain, based on the Talairach coordinate system. In contrast to morphometric techniques such as VBM, each participant's brain is analyzed in its native space. This technique involves applying the Talairach coordinate system to each subjects' brain, based on the location of the anterior and posterior commissures, as well as the gross height, width and length.26 Regions of the Talairach grid are pre-assigned by the program to correspond to the major lobes of the brain, and so the lobes in each person's brain are identified based on the its location within the applied Talairach grid. The accuracy of this lobar parcellation method for measuring the targeted lobar volumes has been established by the developers using hand tracing as the gold standard26,35 and numerous studies have used this program to examine volume changes in the brain.27-34 Details of BRAINS2 processing are as follows.
First, the T1 weighted images are resampled to 1.0 mm3 voxels and reoriented so that the anterior-posterior axis of the brain is realigned parallel to the anterior commissure-posterior commissure line and the inter-hemispheric fissure is aligned on the other two axes. Next, the outermost boundaries of the cortex, as well as the anterior commissure and posterior commissure, are identified in order to warp the Talairach grid36 onto the current brain. The T2 and PD weighted images are then realigned to the spatially normalized T1 weighted image using an automated image registration program.37
Intracranial tissue is then classified into gray matter, white matter and CSF in order to identify brain vs. non-brain tissue, using information form the T1, T2 and PD images. This is accomplished using the co registered images and a discriminate analysis method based on automated training class selection,38 which uses a Bayesian classifier approach based on discriminate analysis in order to reduce the variability in signal intensity across individual image sets and to correct for partial voluming. This step requires the manual tracing of venous blood, but is able to perform “plug” selection for grey matter, white matter, and cerebrospinal fluid automatically. The brain mask is then generated using a previously trained artificial neural network (ANN), one of the features of the BRAINS2 software package. Then, lobar volumes are calculated using the validated, automated Talairach-based method of regional classification (see Figure 1).26,35 Tissue classification is visually checked. Because gray-white differentiation was not always accurate, and to increase the generalizability of the study, analyses for this study used whole lobes, including both grey and white matter. Final lobar identification by BRAINS2 was also visually inspected for each case. Major inaccuracy in lobar identification was not identified in any case, and there was no evidence of a systematic problem with lobar identification in any diagnostic group.
Figure 1. Talaraich Parameters of Regional Classification.
This figure illustrates the automated Talairach-based method of regional classification.
Although the technique is mostly automated, the initial stages for resizing of the Talairach grid require manual selection of the brain's boundaries and reorientation of the images. Inter-rater reliability for implementation of these procedures in our lab was established by having two raters complete the lobar identification procedure for 10 scans. The intraclass correlation coefficients across the two raters were .98-.99 for all lobar regions. Because the goal of this study was to assess longitudinal changes, all subjects served as their own controls, and correction for total intracranial volume (TIV) was not required.
Statistical Analysis
Percent change in volume per 12 months was calculated using the following formula: ((Time 2 volume − Time 1 volume)/Time 1 volume) × (12/# of months between scans). Annualized rates of change in lobar volumes were analyzed using mixed-model ANCOVA with diagnosis as the grouping variable and with lobe and hemisphere (right vs. left) as within-subject variables. Age was included as a covariate in all analyses. Post-hoc analyses for pairwise comparisons used the Tukey A (HSD) test.
RESULTS
Baseline lobar volumes across diagnoses
Table 2 shows the baseline volumes in each lobe for all groups. As would be expected, the FTD group had the smallest frontal lobes at baseline, while SD showed the smallest volumes in the temporal lobes. ANCOVA for the cross-sectional analysis revealed a main effect for lobe (F(3,189) = 45.80, p<.001) and diagnosis (F(3,63) = 10.22, p< .05), but not for hemisphere (p=.90). In addition, there was a significant diagnosis X lobe interaction (F(9,189) = 9.18, p<.001). This means that baseline lobar volumes are not consistent across diagnostic groups. The three way interaction of diagnosis by hemisphere by lobe was not significant (p=.14). Post hoc analyses revealed that differences were significant for FTD vs. controls in the left frontal lobe and for FTD vs. all other groups in the right frontal lobe. For SD, differences were significant vs. controls in the right temporal lobe and vs. controls and AD in the left temporal lobe. Baseline volumes were not significantly different between diagnostic groups in the parietal and occipital lobes.
Table 2.
Baseline lobar volumes in cubic centimeters (standard deviations in parentheses)
| Control | AD | FTD | SD | |
|---|---|---|---|---|
| LFrontal | 184.17 (26.5) | 174.58 (18.4) | 159.02 (24.9) | 172.90 (16.0) |
| RFrontal | 192.79 (26.0) | 188.97 (20.7) | 163.27 (22.2) | 186.40 (18.2) |
| LTemporal | 107.53 (11.6) | 100.01 (14.3) | 99.29 (12.9) | 88.07 (10.6) |
| RTemporal | 108.37 (10.8) | 101.36 (14.0) | 98.36 (12.3) | 96.33 (14.2) |
| LParietal | 110.71 (14.6) | 101.53 (15.6) | 105.52 (7.8) | 106.04 (11.8) |
| RParietal | 113.07 (14.4) | 103.98 (14.3) | 106.26 (8.7) | 111.84 (12.6) |
Changes in lobar volumes over time
Table 3 displays annualized rates of atrophy (both as a percent of the baseline volume and absolute volume loss in cc3) in patients with AD, FTD, SD and in normal controls. Regions with the highest rates of atrophy are highlighted in bold for each group. In FTD and SD, longitudinal rates of change were largest in the regions that showed the smallest volumes in that group relative to other groups. Specifically, FTD showed the greatest rate of change in the frontal lobes (6.3% on the left and 6.1% on the right), while SD showed the greatest volume loss in the temporal lobes (5.9% on the left and 4.8% on the right). As would be expected, the highest rate of parietal loss was found in the AD group (2.6% on the left and 2.2% on the right). The mixed-model ANCOVA revealed a significant main effect for diagnosis (F(3,63) = 7.76, p<.001), but not for lobe (p=.81) or hemisphere (p=.50). The three way interaction of diagnosis by hemisphere by lobe was not significant (p= .38). However, there was a significant diagnosis X lobe interaction (F(9,189) = 2.07, p<.05). This means that annualized rates of lobar atrophy are not consistent across diagnostic groups. In this sample, 27.0% of the variance in annual lobar atrophy rates was associated with diagnosis (Eta squared = .270). Different dementia syndromes exhibited disparate rates of atrophy in various lobar regions. Differences were significant for FTD vs. controls in the left frontal lobe and for FTD vs. all other groups in the right frontal lobe. For SD, differences were significant vs. controls in the right temporal lobe and vs. all other groups in the left temporal lobe. Atrophy rates in AD patients were not significantly faster than the other groups in any brain region. Notably, atrophy rates in the frontal lobes of FTD patients and in the temporal lobes of SD patients were twice than seen in AD patients these regions.
Table 3.
Annualized volume loss in cc3 and annualized atrophy rates as a percent of baseline volume (standard deviations in parentheses)
| Control (n=23) | AD (n=12) | FTD (n=13) | SD (n=20) | |||||
|---|---|---|---|---|---|---|---|---|
| Volume Δ | % Δ | Volume Δ | % Δ | Volume Δ | % Δ | Volume Δ | % Δ | |
| LFrontal | 2.40 |
−1.3% (3.1) |
4.69 | −2.8% (3.6) |
8.80 |
−6.3% (9.2) |
5.55 | −3.2% (2.9) |
| RFrontal | 1.39 |
−0.8% (2.8) |
3.29 | −1.8% (4.0) |
8.98 |
−6.1% (6.5) |
4.38 | −2.4% (2.5) |
| LTemporal | 0.78 | −0.7% (2.1) |
2.77 |
−2.9% (2.5) |
1.46 | −1.5% (4.3) |
5.16 |
−5.9% (3.0) |
| RTemporal | 1.00 | −0.9% (2.1) |
3.53 |
−3.5% (2.8) |
2.99 | −3.1% (3.5) |
4.59 |
−4.8% (2.9) |
| LParietal | 0.03 |
¥ (2.3) |
2.64 | −2.6% (1.9) |
0.87 | −0.9% (5.6) |
2.31 | −2.2% (1.8) |
| RParietal | 0.15 |
¥ (2.6) |
2.22 | −2.2% (2.7) |
1.96 | −1.7% (6.2) |
1.88 | −1.7% (1.9) |
Note:
<0.01% volume loss
DISCUSSION
Our analyses revealed greater annualized atrophy rates in the frontal lobes for FTD and in the temporal lobes for SD when compared with both age-matched controls and AD. These results are consistent with previous morphometric studies showing predominantly temporal changes over time in SD and frontal changes in FTD.5-6 These longitudinal changes are also consistent with cross-sectional studies showing that each dementia syndrome is associated with a specific pattern of abnormality in cerebral volume at clinical presentation. Similarly, rates of volume loss are disease-specific and are greater in the regions with the smallest volumes at baseline. Measurement of volume change over time in FTD and SD is likely to be most sensitive if the regions measured are chosen based on the clinical syndrome and the regions with the lowest volumes relative to controls at baseline.
Atrophy rates in both SD and FTD were twice than seen in AD, which is consistent with published data that FTD patients have a faster rate of ventricular expansion (brain atrophy) than AD patients.14,39 It is also consistent with clinical studies showing that rates of cognitive decline are about twice as fast in FTD as in AD.40 This suggests that changes in brain volume may progress at a similar rate as do clinical changes, increasing the potential for volumetric measures to predict clinical decline and making them more attractive for clinical trials. Rates of atrophy over time may be useful for differential diagnosis, and could be used to monitor disease progression, serving as an outcome measure for clinical trials. The ability to reliably detect disease-specific cerebral atrophy rates by serial neuroimaging may result in the use of these measurements as biomarkers in the clinical assessment of dementia.
Volume loss was not significantly faster in the AD group in this study in any region compared with the other diagnostic groups. This may be because regional volumetric abnormalities are more variable in AD than in FTD or SD. In our study, patients were grouped together because of their common diagnosis of AD. All cases had typical, probable AD (presenting with memory complaints, no progressive aphasia, and no posterior cortical atrophy). Nevertheless, AD is not a homogeneous disease and often reflects individual variability in patients. Thus, a patient with AD and prominent language difficulty will have more rapid volume loss in the left hemisphere, whereas an AD patient with prominent visuospatial deficits may have more rapid volume loss in the right hemisphere. This would be a question for future studies. Also, AD does not progress as rapidly as other types of dementia, such as FTD, so annualized rates of atrophy were not as high. Furthermore, the AD patients in this sample are somewhat young, so this may have impacted the results. Lastly, we did not measure volumes in the hippocampus and entorhinal cortex. Because these are the regions most impacted by AD, volume loss should be fastest in these regions, and it is likely that atrophy in these regions would be significantly faster than in other groups. However, the rates of atrophy in AD patients in the current study are similar to results found in other studies.41-46
One limitation to this study is the relatively small sample size; however, a within-subjects design was used and the sample size was comparable to other studies of this type. Also statistically significant differences were found indicating fairly large effect sizes. Another possible limitation is the fact that controls were significantly older than AD and FTD subjects. Nevertheless, age was controlled for in analyses in order to remove this possible confound. In addition, one might expect that the increased age in the controls would be associated with larger rates of atrophy, if it had any effect at all. More importantly, there were no significant differences in age between dementia groups.
Finally, the current study examined longitudinal changes in lobar volume. Longitudinal changes in brain volume may not be linear in these diseases; therefore, volume loss observed over a specific time period may not necessarily be generalizable. In addition, while this gross parcellation of the brain is already enough to generate region-specific atrophy in different dementia subtypes, cross sectional studies and longitudinal morphometric studies have indicated that the greatest changes occur in sub-lobar regions. AD is associated with atrophy in the left parietal lobe and bilaterally in the posterior cingulate/precuneus.9 FTD has significant regional gray matter tissue loss in the orbital frontal, insular, ventromedial, and anterior cingulate cortex.6,19,47 SD has been found to affect both left and right anterior temporal lobes, but specific regional volume loss involves the ventromedial frontal and anterior insular regions as well.20 Future studies should focus on these specific regions of interest in order to extend research in this area.
ACKNOWLEDGEMENTS
This work was supported by the National Institute on Aging (NIA) grants AG22983 and P50-AG05142, and the State of California Alzheimer's Disease Research Center of California (ARCC) grant 01-154-20. The information in this manuscript has never before been published either electronically or in print.
Footnotes
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Carey CL, Woods SP, Damon J, et al. Discriminant validity and neuroanatomical correlates of rule monitoring in frontotemporal dementia and Alzheimer's disease. Neuropsychologia. 2008;46:1081–1087. doi: 10.1016/j.neuropsychologia.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Harciarek M, Jodzio K. Neuropsychological differences between frontotemporal dementia and Alzheimer's disease: a review. Neuropsychol Rev. 2005;15:131–145. doi: 10.1007/s11065-005-7093-4. [DOI] [PubMed] [Google Scholar]
- 3.Hodges J, Miller B. The neuropsychology of frontal variant frontotemporal dementia and semantic dementia. Introduction to the special topic papers: part II. Neurocase. 2001;7:113–121. doi: 10.1093/neucas/7.2.113. [DOI] [PubMed] [Google Scholar]
- 4.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and alzheimer disease. Cog Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Mummery CJ, Patterson K, Price CJ, et al. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- 6.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 7.Whitwell JL, Anderson VM, Scahill RI, et al. Longitudinal patterns of regional change on volumetric MRI in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2004;17:307–310. doi: 10.1159/000077160. [DOI] [PubMed] [Google Scholar]
- 8.Boccardi M, Laakso MP, Bresciani L, et al. The MRI pattern of frontal and temporal brain atrophy in fronto-temporal dementia. Neurobiol Aging. 2003;24:95–103. doi: 10.1016/s0197-4580(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 9.Boxer AL, Rankin KP, Miller BL, et al. Cinguloparietal atrophy distinguishes Alzheimer disease from semantic dementia. Arch Neurol. 2003;60:949–956. doi: 10.1001/archneur.60.7.949. [DOI] [PubMed] [Google Scholar]
- 10.Rabinovici GD, Seeley WW, Kim EJ, et al. Distinct MRI atrophy patterns in autopsy-proven Alzheimer's disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen. 2007;22:474–488. doi: 10.1177/1533317507308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corey-Bloom J. The ABC of Alzheimer's disease: cognitive changes and their management in Alzheimer's disease and related dementias. Int Psychogeriatr. 2002;14:51–75. doi: 10.1017/s1041610203008664. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 13.Fox NC, Freeborough PA, Rossor MN. Visualisation and quantification of rates of atrophy in Alzheimer's disease. Lancet. 1996;348:94–97. doi: 10.1016/s0140-6736(96)05228-2. [DOI] [PubMed] [Google Scholar]
- 14.Whitwell JL, Jack CR, Jr, Parisi JE, et al. Rates of cerebral atrophy differ in different degenerative pathologies. Brain. 2007;130:1148–1158. doi: 10.1093/brain/awm021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes J, Foster J, Boyes RG, et al. A comparison of methods for the automated calculation of volumes and atrophy rates in the hippocampus. Neuroimage. 2008;40:1655–1671. doi: 10.1016/j.neuroimage.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Hua X, Leow AD, Lee S, et al. 3D characterization of brain atrophy in Alzheimer's disease and mild cognitive impairment using tensor-based morphometry. Neuroimage. 2008;41:19–34. doi: 10.1016/j.neuroimage.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avants B, Grossman M, Gee JC. The correlation of cognitive decline with frontotemporal dementia induced annualized gray matter loss using diffeomorphic morphometry. Alzheimer Dis Assoc Disord. 2005;19:S25–28. doi: 10.1097/01.wad.0000183083.14939.82. [DOI] [PubMed] [Google Scholar]
- 19.Brambati SM, Renda NC, Rankin KP, et al. A tensor based morphometry study of longitudinal gray matter contraction in FTD. Neuroimage. 2007;35:998–1003. doi: 10.1016/j.neuroimage.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brambati SM, Rankin KP, Narvid J, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: A tensor-based morphometry study. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes J, Godbolt AK, Frost C, et al. Atrophy rates of the cingulate gyrus and hippocampus in AD and FTLD. Neurobiol Aging. 2007;28:20–28. doi: 10.1016/j.neurobiolaging.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Avants B, Anderson C, Grossman M, et al. Spatiotemporal normalization for longitudinal analysis of gray matter atrophy in frontotemporal dementia. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2007;10:303–310. doi: 10.1007/978-3-540-75759-7_37. [DOI] [PubMed] [Google Scholar]
- 23.Giuliani NR, Calhoun VD, Pearlson GD, et al. Voxel-based morphometry versus region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophre Res. 2005;74:135–147. doi: 10.1016/j.schres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDAWork Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 26.Magnotta VA, Harris G, Andreasen NC, et al. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 27.Kramer JH, Quitania L, Dean D, et al. Magnetic resonance imaging correlates of set shifting. J Int Neuropsychol Soc. 2007;13:386–392. doi: 10.1017/S1355617707070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaendner NH, Reuner G, Pietz J, et al. MR imaging-based volumetry in patients with early-treated phenylketonuria. AJNR Am J Neuroradiol. 2005;26:1681–1685. [PMC free article] [PubMed] [Google Scholar]
- 29.Popple RA, Griffith HR, Sawrie SM, et al. Implementation of talairach atlas based automated brain segmentation for radiation therapy dosimetry. Technol Cancer Res Treat. 2006;5:15–21. doi: 10.1177/153303460600500103. [DOI] [PubMed] [Google Scholar]
- 30.Jatzko A, Rothenhofer S, Schmitt A, et al. Hippocampal volume in chronic posttraumatic stress disorder (PTSD): MRI study using two different evaluation methods. J Affect Disord. 2006;94:121–126. doi: 10.1016/j.jad.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Crespo-Facorro B, Roiz-Santianez R, Pelayo-Teran JM, et al. Caudate nucleus volume and its clinical and cognitive correlations in first episode schizophrenia. Schizophr Res. 2007;91:87–96. doi: 10.1016/j.schres.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Crespo-Facorro B, Roiz-Santianez R, Pelayo-Teran JM, et al. Reduced thalamic volume in first-episode non-affective psychosis: correlations with clinical variables, symptomatology and cognitive functioning. Neuroimage. 2007;35:1613–1623. doi: 10.1016/j.neuroimage.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 33.Crespo-Facorro B, Roiz-Santianez R, Pelayo-Teran JM, et al. Low-activity allele of Catechol-O-Methyltransferase (COMTL) is associated with increased lateral ventricles in patients with first episode non-affective psychosis. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1514–1518. doi: 10.1016/j.pnpbp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 34.McCormick LM, Ziebell S, Nopoulos P, et al. Anterior cingulate cortex: an MRI-based parcellation method. Neuroimage. 2006;32:1167–1175. doi: 10.1016/j.neuroimage.2006.04.227. [DOI] [PubMed] [Google Scholar]
- 35.Andreasen NC, Rajarethinam R, Cizadlo T, et al. Automatic atlas-based volume estimation of human brain regions from MR images. J Comput Assist Tomogr. 1996;20:98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical; New York: 1988. [Google Scholar]
- 37.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 38.Harris G, Andreasen NC, Cizadlo T, et al. Improving Tissue Classification in MRI: A Three-Dimensional Multispectral Discriminant Analysis Method with Automated Training Class Selection. Journal of Computer Assisted Tomography. 1999;23:144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- 39.Whitwell JL, Jack CR, Jr., Pankratz VS, et al. Rates of brain atrophy over time in autopsy-proven frontotemporal dementia and Alzheimer disease. Neuroimage. 2008;39:1034–1040. doi: 10.1016/j.neuroimage.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rascovsky K, Salmon DP, Lipton AM, et al. Rate of progression differs in frontotemporal dementia and Alzheimer disease. Neurology. 2005;65:397–403. doi: 10.1212/01.wnl.0000171343.43314.6e. [DOI] [PubMed] [Google Scholar]
- 41.Kaye JA, Swihart T, Howieson D, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 42.Fox NC, Scahill RI, Crum WR, et al. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology. 1999;52:1687–1689. doi: 10.1212/wnl.52.8.1687. [DOI] [PubMed] [Google Scholar]
- 43.Fox NC, Cousens S, Scahill R, et al. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects. Arch Neurol. 2000;57:339–344. doi: 10.1001/archneur.57.3.339. [DOI] [PubMed] [Google Scholar]
- 44.Silbert LC, Quinn JF, Moore MM, et al. Changes in premorbid brain volume predict Alzheimer's disease pathology. Neurology. 2003;61:487–492. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- 45.Fox NC, Schott JM. Imaging cerebral atrophy: normal ageing to Alzheimer's disease. Lancet. 2004;363:392–394. doi: 10.1016/S0140-6736(04)15441-X. [DOI] [PubMed] [Google Scholar]
- 46.Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imaging. 1997;16:623–629. doi: 10.1109/42.640753. [DOI] [PubMed] [Google Scholar]
- 47.Seeley WW, Carlin DA, Allman JM, et al. Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol. 2006;60:660–667. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]