Abstract
Hyaluronan is an oligosaccharide found in the pericellular matrix of numerous cell types and hyaluronan induced signaling is known to facilitate fibrosis and cancer progression in some tissues. Hyaluronan is also commonly instilled into the eye during cataract surgery to protect the corneal endothelium from damage. Despite this, little is known about the distribution of hyaluronan or its receptors in the normal ocular lens. In this study, hyaluronan was found throughout the mouse lens, with apparently higher concentrations in the lens epithelium. CD44, a major cellular receptor for hyaluronan, is expressed predominately in mouse secondary lens fiber cells born from late embryogenesis into adulthood. Surgical removal of lens fiber cells from adult mice resulted in a robust upregulation of CD44 protein which preceded the upregulation of α-smooth muscle actin expression typically used as a marker of epithelial-mesenchyme transition in this model of lens epithelial cell fibrosis. Mice lacking the CD44 gene had morphologically normal lenses with a response to lens fiber cell removal similar to wildtype, although they exhibited an increase in cell associated hyaluronan. Overall, these data suggest that lens cells have a hyaluronan containing pericellular matrix whose structure is partially regulated by CD44. Further, these data indicate that CD44 upregulation in the lens epithelium may be an earlier marker of lens injury responses in the mouse lens than the upregulation of α-smooth muscle actin.
Introduction
The ocular lens is a transparent, cellular tissue whose major function is to focus light onto the retina (1). The anterior surface of the lens is composed of a monolayer of flattened, polarized cells denoted the lens epithelium, while the remainder of the lens is made up of the lens fibers, elongated polarized cells which are formed from lens epithelial precursors (2). The lens is surrounded by the lens capsule, a thickened basement membrane which provides basal attachment sites for both lens epithelial and fiber cells (3). The precise arrangement of lens cells is important for lens transparency and loss of this organization is one of many causes of cataract (4), the major cause of blindness worldwide (5-7).
Over the past thirty years, phacoemulsification has become the standard of care for cataract treatment. In this method, the central lens epithelium and lens fiber cells are removed, leaving the bulk of the lens capsule and peripheral lens epithelium behind. Refraction is then restored by the implantation of a foldable intraocular lens (IOL) into the empty capsular bag (8). While current surgical methods have great benefits over prior cataract treatments, the residual lens epithelial cells often change phenotype into a mixture of dysgenic lens fibers and migratory myofibroblasts (9,10). If these cells migrate into the visual axis, posterior capsular opacification (PCO) develops, and the patient’s vision is again impaired. While the overall rate of PCO requiring medical treatment has declined in recent years due to improved surgical methods and new IOLs which trap migrating lens cells at the periphery, it still occurs in 3-40% of operated eyes (11,12). Further, accommodating intraocular lenses (IOL) implanted after cataract surgery can rapidly lose their function since the entrapped lens epithelial cells nearly always form new lens fibers at the periphery of the capsular bag (Soemmering’s ring) which prevents force transmission to the accommodating IOL. Thus, understanding the molecular mechanisms mediating lens responses to cataract surgery leading to both PCO and Soemmering’s ring is important to improve visual outcomes including the restoration of accommodation following cataract surgery (13).
Most evidence suggests that PCO forms due to a combination of increased proliferation of the residual lens epithelial cells, the epithelial-mesenchymal transition (EMT) of these cells to migratory myofibroblasts followed in some cases by the partial transition of these cells to a lens fiber cell phenotype (9,10) which can swell to form Elschnig’s pearls (10). It is likely that TGFβ induced signaling is important for the EMT response of lens cells (9). However, it is apparent that the signaling leading to PCO is complex and requires cross talk between several pathways including those induced by fibroblast growth factor (14,15), osteopontin (16), hepatocyte growth factor (17), epidermal growth factor (18), integrins (19), and Wnts (20). Overall, it is likely that the full extent of the pathways regulating PCO is not known.
CD44 was originally described as a cellular receptor for hyaluronan (21), a complex oligosaccharide found in the pericellular matrix of numerous cell types (22) as well as both the aqueous humor (23) and vitreous gel (24) of the eye. CD44 signaling induced by hyaluronan engagement has been implicated in numerous fibrotic conditions (25-27) as well as the loss of epithelial markers and enhanced cell migration in cancer (28). CD44 also functions as a platform to assemble growth factors and MMPs at the cell surface and can serve as a co-receptor for several receptor tyrosine kinases including TGFβ receptor type I, cMet, and ERBB (21,29-31). Notably, CD44 expression has been detected in both human lens epithelial cells obtained from cataractous lenses and PCO material (32) while function blocking CD44 antibodies reduced human lens epithelial cell proliferation/migration in vitro (33). However, the distribution and function of CD44 in the normal lens has not been previously assessed. This study describes the developmental expression pattern of CD44 and its ligand hyaluronan in the mouse lens, the dynamics of its expression in the mouse lens following lens fiber cell removal and whether deletion of the CD44 gene leads to any alterations in lens architecture or its response to injury.
Materials and Methods
Animals
All experiments using animals were approved by the University of Delaware Institutional Animal Care Committee and conform to the ARVO statement on the use of animals in ophthalmic research. The wild type mice used in these experiments are derived from strain C57Bl/6NHsd mice obtained from Harlan Laboratories (Indianapolis, Indiana) and were produced in house at the University of Delaware animal facility. Homozygous null CD44 null STOCK Cd44tm1Hbg/J mice (34) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and bred in house. Mice with β1-integrin conditionally deleted from the lens were created by mating mice harboring a floxed β1-integrin allele (35) with mice transgenic for MLR10-CRE (36) as described (37). Embryos were staged by defining noon of the day on which the semen plug was observed as 0.5 days post-coitum (dpc).
Immunofluorescence
All immunofluorescence experiments were carried out as previously described (38). Briefly, tissues were removed from mice that were sacrificed by carbon-dioxide inhalation, and embedded in OCT (Sakura). Sections were obtained on a cryostat, mounted on slides and fixed in 1:1 acetone:methanol at −20 °C for 20 minutes. The sections were air dried and blocked for one hour at room temperature in 1% bovine serum albumin/phosphate buffered saline (PBS). Primary antibodies were then applied to the slide and incubated for one hour at room temperature. Following three room temperature washes in PBS, the slides were incubated in a 1:2000 dilution of Draq5 (Biostatus, Leicestershire, United Kingdom) with the addition of appropriate AlexaFluor 568 labeled secondary antibodies (Invitrogen, Carlsbad, California) for one hour at room temperature. The slides were washed, cover slipped and imaged with a Zeiss LSM 510 VIS Confocal Microscope (Carl Zeiss, Inc., Gottingen, Germany) configured with Argon/Krypton (488 and 568 nm excitation lines) and Helium Neon (633 nm excitation line) lasers. All comparisons of staining intensity between specimens were done on sections stained simultaneously and the imaging for each antibody was performed using identical laser power and software settings to ensure validity of intensity comparisons.
Antibodies
The fluorescein labeled mouse monoclonal anti-α-smooth muscle actin antibody (clone 1A4, #F3777, Sigma-Aldrich (St. Louis, Missouri) was used at a concentration of 10 μg/ml. CD44 was detected with a 1:400 dilution of a rat monoclonal antibody raised against CD44 (clone IM7, #14-0441, eBioscience, San Diego, CA).
Hyaluronan detection
Hyaluronan was detected by a method similar to that previously described (39). Frozen sections of unfixed eyes were prepared and fixed in 1:1 acetone:methanol as for immunofluorescence. The sections were first blocked for non-specific protein binding with 1% BSA in PBS for one hour at room temperature then the tissue bound biotin was blocked with a strepavidin/biotin blocking kit (Vector Laboratories, Burlingame, CA) using the manufacturer’s protocol. Biotinylated hyaluronic acid binding protein (2μg/mL in PBS, #0361C1703, Sigma-Aldrich, St. Louis, Missouri) was overlaid onto the sections and incubated for one hour at room temperature. The sections were washed twice for 10 minutes each in PBS, then overlaid with a 1:200 dilution of streptavidin conjugated to AlexaFluor 488 (Invitrogen, #S11223) in PBS with Draq5 (Biostatus) added at a 1:2000 dilution. Following a one hour room temperature incubation, the slides were washed twice in PBS, the sections cover slipped and imaged as above. Control slides were treated similarly except that biotinylated hyaluronic acid binding protein was omitted.
Surgical removal of lens fiber cells
Lens fiber cell extraction was performed on living mice using a modification of a protocol first described by Call et al. (40). Pupils were dilated with 1% tropicamide and 2.5% phenylephrine hydrochloride ophthalmic solution (Schein), then the mice were anesthetized with ketamine/xylazine. The eye to be operated on was rinsed with balanced salt solution (BSS) and an ophthalmic knife was used to make a 2-3 mm incision in the center of the cornea reaching to the anterior lens capsule. The lens fiber cells were separated from the lens capsule with BSS, then pressure was applied on both sides of the eye to express the lens fiber mass. The remaining lens capsule was washed with BSS to remove residual cells and to inflate the anterior chamber to its original depth. The corneal incision was closed with a single 10-0 nylon suture, and BSS was used to bring the anterior chamber back to its original depth. Erythromycin ophthalmic ointment was applied topically and the mouse allowed to awaken. For analysis, mice were sacrificed by carbon-dioxide inhalation 0, 6, 12, 24, and 48 hours after surgery and the operated eyes removed for immunofluorescence and RNA isolation. At least three independent animals were analyzed for each experiment.
Reverse transcription PCR (rt-PCR)
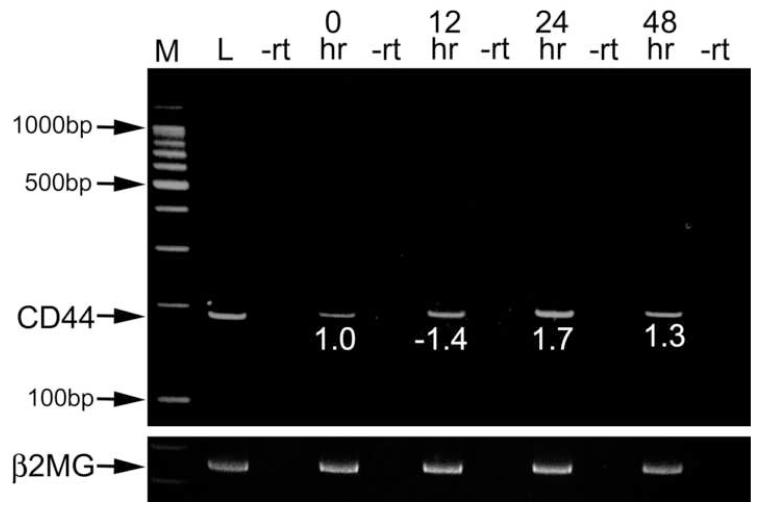
RNA was isolated from the cells remaining in the capsular bag following fiber cell removal (four capsular bags were pooled for each sample) using the SV total RNA Isolation System (Promega, Madison WI). Conventional rt-PCR was performed using the Qiagen One-Step RT-PCR kit (Qiagen, Valencia, CA) while controls lacking reverse transcriptase were performed using Hot Star DNA Polymerase (Qiagen). The CD44 forward primer used was derived from exon 5 of the CD44 gene (5′- AGC AGC GGC TCC ACC ATC GAG A-3)′ while the CD44 reverse primer was from exon 16 (5′- TCG GAT CCA TGA GTC ACA GTG-3′). The cycling conditions were 30 minutes at 50 °C, 15 minutes at 95 °C then 22 cycles of 94 °C, 30 seconds, 65 °C, 30 seconds; 72 °C, 90 seconds. RNA concentration was normalized against the expression level of β2-microglobulin (βMG2) by rt-PCR analysis using primers 5′ TGG TGC TTG TCT CAC TGA CC 3′ and 5′ TCA CAT GTC TCG ATC CCA GT 3′.
For quantitative, real-time rt-PCR, the mRNA was converted into cDNA using the RT2 PCR array first strand synthesis kit using the protocol provided by the manufacturer (SuperArray Bioscience, Frederick, MD). Real time PCR was performed on this cDNA with the above primers for CD44, while the primers for four housekeeping genes: Hprt1 (hypoxanthine guanine phosphoribosyl transferase 1), Gusb (beta glucuronidase) and Tbp (TATA box binding protein) were obtained from SuperArray Bioscience (Frederick, MD). A total volume of 25 μl of PCR mixture, which included 12.5 μl of RT2 Real-Time SYBR Green/ROX PCR master mix from SuperArray Bioscience (containing HotStart DNA polymerase, SYBR Green dye, and the ROX reference dye), 1 μl of each primers, 1 μl of template cDNA, and 9.5 μl of nuclease-free H2O was added to each well of a 96-well plate. All reactions were performed in triplicate with appropriate negative controls. The real time PCR was performed using an ABI7000 PCR machine (Applied Biosystems, Foster City, CA) with an initial 10minute step at 95 °C followed by 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute. The cycle threshold (Ct) data were imported into an Excel database and analyzed using the comparative ΔCt method with normalization of the raw data to housekeeping genes. Statistical significance of the data was analyzed with a two-tailed, non-paired Student’s t test. The presence of a single product was confirmed by melting curve analysis and gel electrophoresis.
Results
CD44 protein is a late lens fiber cell marker in the normal mouse lens
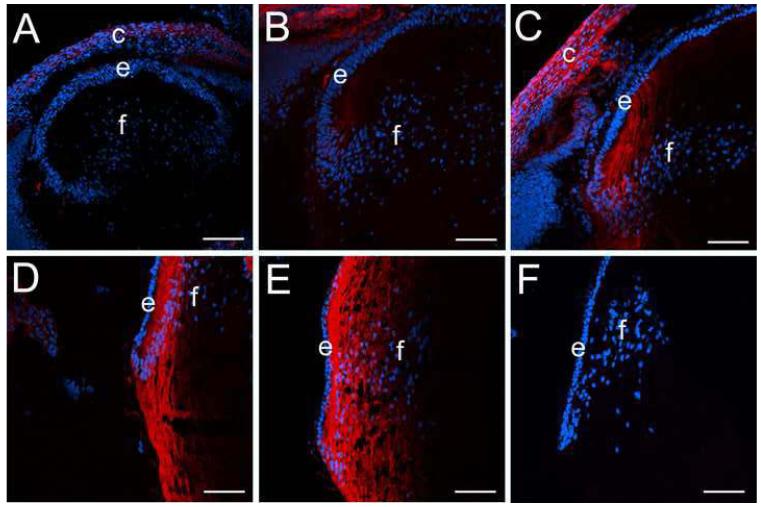
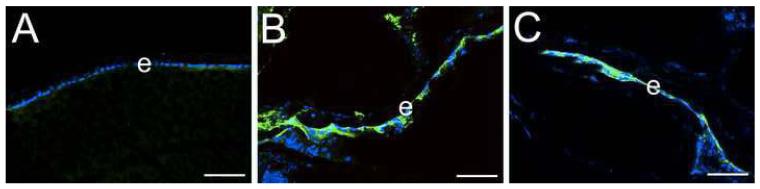
Previously, CD44 mRNA and protein have been detected in adult human lens epithelial cells (32,33,41,42), however, the expression pattern of CD44 during lens development has not been previously reported. In the mouse lens, the first detectable CD44 protein was seen in newly forming cortical lens fiber cells at 16.5 dpc (Figure 1B) while it was absent from the lens prior to that point (Figure 1A, data not shown for earlier stages). CD44 protein levels remain high in lens cortical fibers from birth (Figure 1C) into adulthood (Figure 1D, E) while it is not detected in the lens epithelium (Figure 1B-E; Figure 3B) or embryonic lens nucleus (Figure 1C, D, E). The specificity of the antibody for CD44 was confirmed by the lack of staining in adult lenses from CD44 null mice (Figure 1F).
Figure 1.
CD44 expression initiates in cortical lens fiber cells at around 16.5 dpc in the mouse lens and is absent from the embryonic lens nucleus A) 14 dpc eye showing a lack of CD44 staining (red) in both the primary and early secondary lens fibers as well as the lens epithelium B) 16.5 dpc lens showing the early onset of CD44 expression in the newly forming secondary fibers. C) newborn lens showing the further upregulation of CD44 protein expression in cortical lens fibers and its absence in the lens epithelium D) 2 weeks postnatal lens showing maintained CD44 expression in cortical lens fibers and its absence in the lens epithelium E) 7 weeks postnatal lens showing maintained CD44 expression in cortical lens fibers and its absence in the lens epithelium F) 7 weeks postnatal CD44 null stained as in panel E demonstrating the specificity of the CD44 antibody used in this study. Abbreviations: c, cornea; e, lens epithelium; f, lens fibers. Blue, nucleic acid stain; Red, CD44. Scale bars A=108 μm; B,C,D,E,F=77 μm
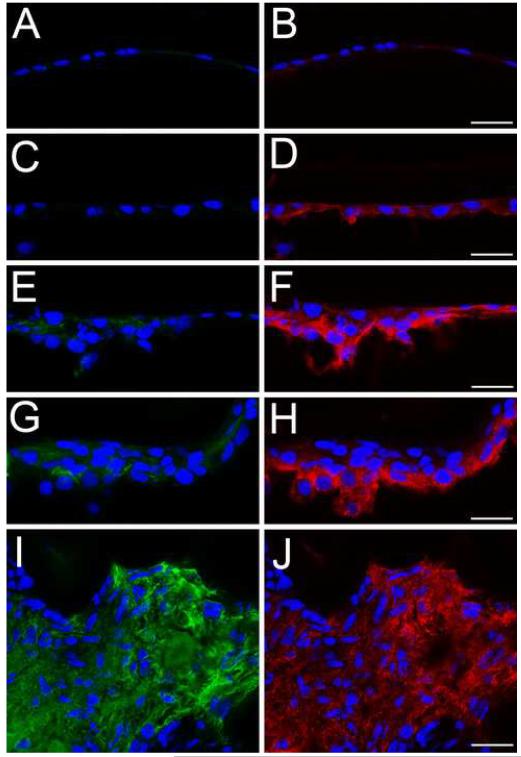
Figure 3.
CD44 protein levels upregulate in the lens epithelium prior to α smooth muscle actin after fiber cell removal. A, B- the lens epithelium time zero after fiber cell removal showing a little CD44 (B) or α-smooth muscle actin staining (A); C, D- lens epithelial cells remaining in the eye 12 hours after fiber cell removal showing CD44 staining (D) but no upregulation of α-smooth muscle actin protein expression(C) ; E, F-lens epithelial cells 24 hours after fiber cell removal showing increased levels of CD44 (F) and α-smooth muscle actin protein (E) ; G, H-Lens epithelial cells 48 hours after fiber cell removal showing robust CD44 (H) and α-smooth muscle actin protein (G) expression; I, J lens capsular bag five days after lens fiber cell removal showing a fibrotic mass of cells staining positive for both α-smooth muscle actin (I) and CD44 protein (J) Red, CD44; Blue, nucleic acid stain; Green, α-smooth muscle actin; Scale bars- 24 μm
Hyaluronan is found in the lens and is increased following deletion of CD44
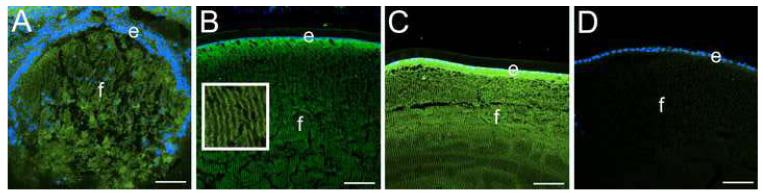
CD44 is a major receptor for hyaluronan, an oligosaccharide found in both extracellular and pericellular matrices (22). Thus, we used hyaluronan binding protein affinity histochemistry to determine the localization of hyaluronan in the lens. In the developing mouse lens, we detected hyaluronan in/on all lens cells, even at stages prior to the onset of CD44 expression (Figure 2A, data not shown). In the adult lens, hyaluronan was associated with both the lens epithelium and fibers in a distribution consistent with its presence in the pericellular matrix (see inset Figure 2B). In CD44 null mice, lens hyaluronan staining was consistently more intense in both lens epithelial and fiber cells (Figure 2C) as compared to the wildtype lens (Figure 2B).
Figure 2.
Hyaluronic acid is found in the pericellular matrix of the lens and increases in the absence of CD44. A) 14.5 dpc mouse lens showing hyluronan staining in all lens cells prior to the onset of CD44 expression B) Wildtype adult lens showing hyaluronan staining in both the lens epithelium and fibers. Inset shows a higher magnification of the lens fibers showing staining along the lateral lens fiber cell membrane. C) CD44 null adult lens showing elevated hyaluronan staining in both the lens epithelium and fibers D) CD44 null lens with hyaluronic acid binding protein omitted from the staining reaction as a negative control. Blue, nucleic acid stain; Green, hyaluronic acid. Scale bars all equal 77 μm.
CD44 protein levels upregulate prior to α smooth muscle actin in the lens epithelium following fiber cell removal
In other tissues, CD44 is involved in the pathogenesis of fibrosis (26,27). Further, CD44 can serve both as a scaffold for TGFβ activation by MMPs (30) and as a modulator of TGFβRI phosphorylation (29,31). However, our immunolocalization data suggested that CD44 protein was not found at appreciable levels in the mouse lens epithelium (Figure 1), although it has been reported in human lens epithelium obtained from cataract surgeries (32,33). Thus, we investigated whether CD44 protein levels upregulate in the lens epithelium during epithelial-mesenchymal transition in vivo using a mouse cataract surgery model. Directly after lens fiber cell removal, neither CD44 (Figure 3B) nor αSMA (Figure 3A) were detected in the lens epithelial cells remaining in the eye. However, CD44 protein levels were consistently upregulated strongly by 12 hours after fiber cell removal (Figure 3D) and remained high at 24 (Figure 3F) and 48 hours (Figure 3H) and 5 days (Figure 3J). In contrast, we never detected an upregulation of αSMA protein levels prior to 24 hours after fiber cell removal (Figure 3C). At 24 hours after fiber cell removal, elevated levels of αSMA protein were detected in many cases (Figure 3E) although this upregulation was not consistently detected until 48 hours after fiber cell removal (Figure 3G). At five days post fiber cell removal, high levels of both αSMA (Figure 3I) and CD44 (Figure 3J) protein are found throughout the capsular plaque.
CD44 protein levels upregulate in the lens epithelium after αSMA in the β1-integrin null lens
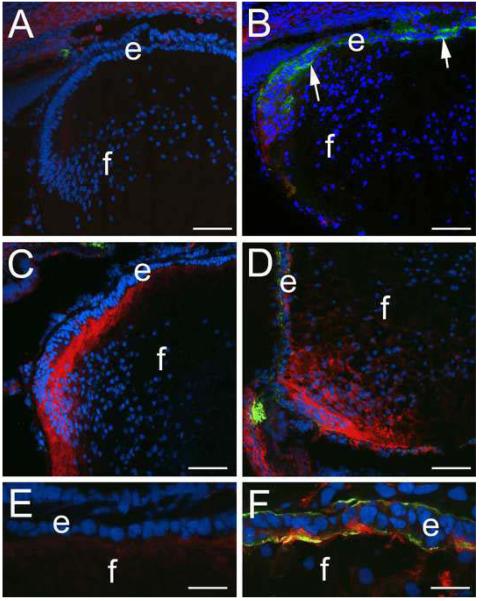
We have previously shown that deletion of β1-integrin from the mouse lens using a CRE-LOX approach results in the upregulation of αSMA expression in the lens epithelium followed by destruction of these cells by apoptosis (37). Since the dynamics of CD44 expression in the lens following fiber cell removal (Figure 3) suggested that CD44 upregulation could be an early marker of lens EMT, we investigated the expression of CD44 in the β1-integrin null lens (Figure 4). As previous reported (37), the first expression of αSMA in the β1-integrin null lens epithelium occurs at around 16.5 dpc (figure 4B), however the only CD44 expression detected in the β1-integrin null lens at that stage was in the newly forming cortical fibers similar to that detected in the wildtype lens (Figure 1B, 4A). At birth, most of the CD44 detected in the β1-integrin null lens is in the cortical fibers (Figure 4D) similar to the wildtype lens (Figure 4C), however, elevated levels of CD44 protein were detected in the β1-integrin null lens epithelium that also expresses elevated levels of αSMA (Figure 4D, F) while no CD44 nor αSMA protein was detected in the wildtype newborn lens epithelium (Figure 4C, E).
Figure 4.
CD44 protein levels increase after the upregulation of α-smooth muscle actin in lens epithelial cells lacking β1-integrin. A) 16.5 dpc wildtype mouse lens showing little CD44 or αSMA staining B) 16.5 dpc β1-integrin null lens showing upregulation of αSMA but little to no D44 staining in the lens epithelium; C) Transition zone of a mewborn wildtype lens showing upregulation of CD44 expression in cortical fibers. D) Transition zone of a newborn β1-integrin null lens showing CD44 in both the cortical fibers and in lens epithelial cells also expressing αSMA. E) high magnification view of the lens epithelium of the wildtype newborn lens shown in C showing a lack of both CD44 and αSMA staining in the lens epithelium. F) high magnification view of the β1-integrin null lens epithelium shown in D showing both CD44 and αSMA staining; Red, CD44; Blue, nucleic acid stain; Green, α-smooth muscle actin; Scale bars-Panels A-D=77 μm, E,F=24 μm
CD44standard (CD44s) is the major CD44 splice form found in the lens
The CD44 gene can produce a large diversity of mRNA transcripts. The CD44 standard (CD44s) isoform directly splices exon 5 to exon 16, while most of the other isoforms include one or more of the 10 variant exons that are spliced out of the CD44s form (43). Since the inclusion of these variant exons alters the structure of the CD44 cytoplasmic domain changing its affinity for hyaluronan and other ligands, we investigated the structure of the CD44 mRNA found in the normal mouse lens and following lens fiber cell removal (Figure 5). Conventional reverse transcriptase PCR using primers generated from exon 5 and exon 16 and a long extension time generated a single product of 180 bp consistent with the CD44s isoform being the sole variant expressed in all samples studied including the intact lens, the lens epithelium immediately after fiber cell removal as well as lens cells remaining attached to the lens capsule 24 and 48 hours after fiber cell removal. Quantitative real time rt-PCR analysis of CD44 mRNA levels 24 and 48 hours after fiber cell removal detected small, but statistically significant changes in CD44 levels compared to the lens epithelium immediately following fiber cell removal (Figure 5).
Figure 5.
CD44 splicing is not altered following fiber cell removal. rt-PCR analysis of CD44 expression in the whole lens and the lens epithelium after lens fiber extraction using primers that amplify across the major region of CD44 alternate splicing. The canonical form of CD44 which does not utilize the variable exons gives a product of 180 bp (arrow). The differences in CD44 level in the lens epithelium following surgery were then determined by quantitative real time rt-PCR by the ΔΔcT method and are expressed as fold differences in expression level from samples collected immediately after surgery (0 hr). All of the post surgery samples were found to be significantly different than the 0 hr time point with p≤0.02. The conventional rt-PCR was controlled by performing controls lacking reverse transcriptase (-rt) and amplification of the samples with primers for β2-microglobulin (β2MG). M- marker; L- entire adult mouse lens; 12 hr- twelve hours following fiber cell extraction; 24 hr- 24 hours following fiber cell extraction; 48 hr- 48 hours following fiber cell extraction.
CD44 is not essential for the induction of αSMA expression in the lens epithelium following fiber cell removal
Lenses from CD44 null mice remained transparent past one year of age and did not exhibit any detectable morphological alterations (data not shown). However, it was previously shown that antibodies against CD44 can inhibit human lens epithelial cell proliferation and/or migration in vitro (33), while we found that CD44 expression upregulates in the lens epithelium following fiber cell removal in mice (Figure 2). Thus, we removed the fibers from the CD44 null mouse lens and followed the induction of αSMA expression in response to this procedure (Figure 6). Overall, the kinetics of αSMA induction in the CD44 null lens were similar overall to that observed in the normal lens (Figure 2). We were able to consistently detect an upregulation of αSMA expression in CD44 null lens epithelia 48 hours after lens fiber cell removal (Figure 6C), while we often also detected αSMA expression as early as 24 hours after surgery (Figure 6B) although that finding was not consistent animal to animal.
Figure 6.
CD44 is not essential for α-smooth muscle actin expression in the lens following injury. A) CD44 null lens epithelium immediately following fiber cell removal; B) CD44 null lens epithelium 24 hours after fiber cell removal; C) CD44 null lens epithelium 48 hours after fiber cell removal showing robust αSMA upregulation. Green- α-smooth muscle actin; Blue-nucleic acid stain; Scale bars= 77 μm
Discussion
Hyaluronan (hyaluronic acid or sodium hyaluronate; HA) is a linear glycosaminoglycan comprised of 2,000-25,000 repeating units of the disaccharide D-glucuronic acid/N-acetylglucosamine. This molecule was first recognized as a component of extracellular matrices whose ability to interact with water is important for the physical properties of numerous structures including the dermis and vitreous body (44). Further, the viscoelastic behavior of HA along with its biocompatibility have led to its widespread use as a protectant for the corneal endothelium during both extracapsular lens extraction and phacoemulsification based cataract surgeries (45).
However, HA is also a component of the pericellular matrix of many cell types that facilitates the reorganization of this matrix during cell migration and division (22). Further, HA can interact with cell surface hyaluronan receptors (hyaladherins) leading to diverse direct and indirect effects on the signal transduction status of cells (44). HA is produced in vertebrates by a family of transmembrane enzymes, the hyaluronan synthases (HAS), which synthesize HA on the cytoplasmic face of the plasma membrane then extrude this molecule to the pericellular matrix (46). Deletion of the major HAS expressed in embryos (HAS2) from the mouse genome leads to lethality at 9.5-10.5 dpc, partially due to a lack of epithelial-mesenchymal transition (EMT) during heart morphogenesis (47). The role of HA in EMT responses is further supported by the observation that overexpression of HASes in epithelial cells sufficient to drive EMT while the ability of β-catenin and HGF overexpression to drive EMT is HA dependent (48).
CD44 is probably the best characterized HA receptor in vertebrates and it is known to mediate many of the effects of HA on cellular phenotype (21,49). While CD44 mRNA and protein have been described in the diseased human lens (32) and high concentrations of anti-CD44 antibodies block the proliferation and migration of cultured human lens epithelia (33), little was known about the normal expression pattern or function of either CD44 or HA in the in vivo lens. Thus, it was not possible to ascertain whether CD44 expression was constitutive or a marker of lens pathology. Here we show that CD44 protein expression initiates in newly forming cortical lens fiber cells of the mouse lens during late embryogenesis and it is maintained in cortical fiber cells until adulthood. Its absence from both the primary lens fibers derived from elongation of the cells of the posterior lens vesicle and most secondary fibers formed prenatally is consistent with other observations that nuclear lens fibers formed during embryogenesis have a very different pattern of gene expression than cortical fiber cells forming later in development (50-54).
Since CD44 protein is expressed in lens fiber cells, we then investigated the distribution of its major ligand, HA in the lens using hyaluronan affinity histochemistry (Figure 2). We found that HA was present in embryonic lenses and evenly distributed between epithelial and fiber cells. This is consistent with the high levels of hyaluronan synthase 2 (HAS2) mRNA detected in the lens pit of mouse embryos (55). In the normal adult lens, HA is still found throughout the lens in a distribution consistent with its location in the pericellular matrix although lens epithelial cells stained more intensely than fibers. It is possible that the presence of HA in the lens prior to the onset of CD44 expression and in the adult lens epithelium reflects HA association with the extracellular domain of HAS2 or conversely, the embryonic lens may express other hyaladherins besides CD44 (22). HA has been found to be an important support for membrane extrusions on epithelial cells (56) raising the possibility that one function of HA in the lens could be to support the membrane extrusions found at the lateral surfaces of the fiber cell plasma membrane. In contrast to our results, it had been previously reported that HA was absent from lenses of aged mice (57). The most likely explanation for this discrepancy is that the fluorescent assay used in this study was more sensitive than the technique used previously. Alternatively, it is possible that HA is found at higher levels in the embryonic and young adult lenses used in this study compared to the aged (15 month old) lenses investigated previously.
Despite the constitutive expression of CD44 in lens fiber cells, lenses from CD44 null mice remained clear until at least one year of age and did not show any histological defects (data not shown) suggesting either that CD44 is not important for lens morphogenesis/homeostasis or that this function in compensated for in the CD44 null lens. It was previously hypothesized that functional compensation for CD44 loss does not occur by inducing the expression of other hyaladherins; instead cells lacking CD44 accumulate hyaluronan on their cell surfaces leading to enhanced signaling through other hyaladherins preexisting in the tissue (58). Notably, we do detect elevated levels of hyaluronan in the CD44 null lens (Figure 2) consistent with this hypothesis. It is currently unclear which hyaladherins besides CD44 are present in the lens, however, prior microarray analysis has indicated that RHAMM (receptor for hyaluronan mediated motility) is preferentially expressed in human lens fiber cells compared to the lens epithelium (41) while stabilin-2, a hyaladherin highly expressed in the liver, was also found to be highly expressed in the adult mouse lens epithelium by immunohistochemistry (59).
While we did not detect high levels of CD44 protein in the normal lens epithelium, in other tissues, CD44 mediates the pathogenesis of fibrosis (27,60), while in cancer, high levels CD44 protein on the cell surface are associated with the loss of epithelial characteristics and increased tumor aggressiveness (28,61,62). Further, in metastatic cancer, the CD44 gene is alternately spliced leading to the production of variant isoforms with differing affinity for hyaluronan and other CD44 ligands as compared to the CD44s form produced by normal cells (43). Thus, we investigated CD44 expression and splicing in the lens epithelium as it undergoes EMT in response to fiber cell removal. Notably, we found a robust upregulation of CD44 protein expression in the lens epithelium following lens fiber cell removal which occurred 12-24 hours earlier than observable increases in αSMA expression. It appears that this upregulation of CD44 protein levels is controlled posttranscriptionally, since we actually detected a modest downregulation of CD44 mRNA levels in the lens epithelium at 12 hours after fiber cell removal (Figure 5). Further, we did not detect any CD44 splice variants in either the intact lens or in lens epithelial cells following lens fiber cell removal suggesting that CD44 in the lens epithelium is mostly modulated by increases in protein expression. Overall, our data do not allow us to definitively state whether CD44 expression changes in the lens epithelium in response to the onset of EMT, fiber cell differentiation responses or both. This, along with the known complex mechanisms regulating CD44 expression and splicing (63,64) will make it difficult to use CD44 expression as an injury response marker in the human lens.
We then took advantage of the availability of the CD44 null mouse to investigate whether CD44 was essential for the elevation in αSMA actin expression commonly associated with EMT responses in the lens. However, we did not note any observable differences in the extent or timing of αSMA expression in the CD44 null lens as compared to wildtype animals following lens fiber cell extraction. However, it is still possible that hyaladherins are important for lens EMT since we did detect highly elevated levels of hyaluronan in the CD44 null lens epithelium. Additional work is necessary to determine whether communication between hyaluronan receptors and growth factor receptors is important for lens EMT or not.
Since these data suggested that CD44 protein upregulation in the lens epithelium could be an earlier marker for the injury response of the rodent lens epithelium than αSMA upregulation, we then investigated CD44 expression in the β1-integrin null lens which we previously reported disintegrates around birth due to EMT then apoptosis of the lens epithelium (37). Increased CD44 levels were detected in newborn β1-integrin null lens epithelial cells expressing αSMA, however, in the 16.5 dpc lens, we detected elevated αSMA levels but not CD44 in the β1-integrin null lens epithelium. This could reflect differences in the signaling pathways activated in response to lens fiber cell removal versus the loss of β1-integrins from the lens epithelium. Alternatively, since CD44 expression is only just initiating in the lens fiber cells of the 16.5 dpc lens, the CD44 gene may be epigenetically silenced in the embryonic lens epithelium at this age. While it is tempting to suggest that elevation of CD44 protein expression is a robust, early marker of EMT responses in the mouse lens epithelium, it should be emphasized that CD44 protein is abundant in normal mouse lens fiber cells. Further work will be necessary to determine whether CD44 expression is an early EMT marker for lens epithelial cells or a marker of the bipotential of lens epithelial cells to produce both fiber cells and myofibroblasts following lens fiber cell removal (9,13,40)
Overall, this study demonstrates that CD44 protein is present in normal mouse lens fiber cells and its ligand hyaluronan is found in the lens as well. We also demonstrated that CD44 protein levels upregulate quickly in the mouse lens epithelium following fiber cell removal suggesting that understanding the molecular mechanisms of this observation would give insight into the early injury responses of the lens epithelium. However, it should be emphasized that the regulation of CD44 gene expression and mRNA splicing is complex (63,64) which may make it difficult to elucidate the mechanistic connection between lens injury and alterations in CD44 expression in the human lens. Further work is necessary to determine the importance of hyaluronan/hyaladherins in lens biology and the pathogenesis of posterior capsular opacification following cataract surgery.
Acknowledgements
We thank Drs Carlton Cooper and Robert Sikes, University of Delaware for valuable discussions.
Funding: National Eye Institute grant EY015279 to MKD, INBRE program grant P20 RR16472 supported the University of Delaware Core Imaging facility, VDD was supported on a Beckman Scholars Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banh A, Bantseev V, Choh V, Moran KL, Sivak JG. Prog Retin Eye Res. 2005 doi: 10.1016/j.preteyeres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Wride MA. Differentiation. 1996;61:77–93. doi: 10.1046/j.1432-0436.1996.6120077.x. [DOI] [PubMed] [Google Scholar]
- 3.Danysh BP, Duncan MK. Exp Eye Res. 2008 doi: 10.1016/j.exer.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulombre AJ. Ophthalmology. 1979;86:1559–1570. doi: 10.1016/s0161-6420(79)35347-7. [DOI] [PubMed] [Google Scholar]
- 5.Limburg H, von-Bischhoffshausen F. Barria, Gomez P, Silva JC, Foster A. Br J Ophthalmol. 2008;92:315–319. doi: 10.1136/bjo.2007.125906. [DOI] [PubMed] [Google Scholar]
- 6.Khandekar R, Mohammed AJ, Raisi AA. Ophthalmic Epidemiol. 2007;14:9–15. doi: 10.1080/09286580600864802. [DOI] [PubMed] [Google Scholar]
- 7.West S. Ophthalmic Epidemiol. 2007;14:173–178. doi: 10.1080/09286580701423151. [DOI] [PubMed] [Google Scholar]
- 8.Riaz Y, Mehta JS, Wormald R, Evans JR, Foster A, Ravilla T, Snellingen T. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD001323.pub2. CD001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- 10.Marcantonio JM, Vrensen GF. Eye. 1999;13(Pt 3b):484–488. doi: 10.1038/eye.1999.126. [DOI] [PubMed] [Google Scholar]
- 11.Bertelmann E, Kojetinsky C. Curr Opin Ophthalmol. 2001;12:35–40. doi: 10.1097/00055735-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Chen X, Zhang J, Zhou Y, Yao X, Du L, Wei M, Liu Y. Ophthalmology. 2008;115:830–838. doi: 10.1016/j.ophtha.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 13.Dewey S. Curr Opin Ophthalmol. 2006;17:45–53. doi: 10.1097/01.icu.0000193074.24746.e6. [DOI] [PubMed] [Google Scholar]
- 14.Mansfield KJ, Cerra A, Chamberlain CG. Mol Vis. 2004;10:521–532. [PubMed] [Google Scholar]
- 15.Symonds JG, Lovicu FJ, Chamberlain CG. Exp Eye Res. 2006;82:693–699. doi: 10.1016/j.exer.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Saika S, Shirai K, Yamanaka O, Miyazaki K, Okada Y, Kitano A, Flanders KC, Kon S, Uede T, Kao WW, Rittling SR, Denhardt DT, Ohnishi Y. Lab Invest. 2007;87:130–138. doi: 10.1038/labinvest.3700508. [DOI] [PubMed] [Google Scholar]
- 17.Choi J, Park SY, Joo CK. Invest Ophthalmol Vis Sci. 2004;45:2696–2704. doi: 10.1167/iovs.03-1371. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Q, Zhou C, Bi Z, Wan Y. J Ocul Pharmacol Ther. 2006;22:93–102. doi: 10.1089/jop.2006.22.93. [DOI] [PubMed] [Google Scholar]
- 19.Walker J, Menko AS. Exp Eye Res. 2008 [Google Scholar]
- 20.Chong CC, Stump RJ, Lovicu FJ, McAvoy JW. Exp Eye Res. 2008 doi: 10.1016/j.exer.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponta H, Sherman L, Herrlich PA. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 22.Evanko SP, Tammi MI, Tammi RH, Wight TN. Adv Drug Deliv Rev. 2007;59:1351–1365. doi: 10.1016/j.addr.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navajas EV, Martins JR, Melo LA, Jr., Saraiva VS, Dietrich CP, Nader HB, Belfort R., Jr. Exp Eye Res. 2005;80:853–857. doi: 10.1016/j.exer.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Theocharis DA, Skandalis SS, Noulas AV, Papageorgakopoulou N, Theocharis AD, Karamanos NK. Connect Tissue Res. 2008;49:124–128. doi: 10.1080/03008200802148496. [DOI] [PubMed] [Google Scholar]
- 25.Svee K, White J, Vaillant P, Jessurun J, Roongta U, Krumwiede M, Johnson D, Henke C. J Clin Invest. 1996;98:1713–1727. doi: 10.1172/JCI118970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura K, Nagaki M, Matsuura T, Moriwaki H, Kakimi K. Hepatol Res. 2008 doi: 10.1111/j.1872-034X.2008.00409.x. [DOI] [PubMed] [Google Scholar]
- 27.Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, Haudek S, Thakker G, Frangogiannis NG. J Immunol. 2008;180:2625–2633. doi: 10.4049/jimmunol.180.4.2625. [DOI] [PubMed] [Google Scholar]
- 28.Heldin P, Karousou E, Bernert B, Porsch H, Nishitsuka K, Skandalis SS. Connect Tissue Res. 2008;49:215–218. doi: 10.1080/03008200802143323. [DOI] [PubMed] [Google Scholar]
- 29.Wakahara K, Kobayashi H, Yagyu T, Matsuzaki H, Kondo T, Kurita N, Sekino H, Inagaki K, Suzuki M, Kanayama N, Terao T. J Cell Biochem. 2005;94:995–1009. doi: 10.1002/jcb.20364. [DOI] [PubMed] [Google Scholar]
- 30.Acharya PS, Majumdar S, Jacob M, Hayden J, Mrass P, Weninger W, Assoian RK, Pure E. J Cell Sci. 2008;121:1393–1402. doi: 10.1242/jcs.021683. [DOI] [PubMed] [Google Scholar]
- 31.Bourguignon LY, Singleton PA, Zhu H, Zhou B. J Biol Chem. 2002;277:39703–39712. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- 32.Saika S, Kawashima Y, Miyamoto T, Okada Y, Tanaka S, Yamanaka O, Ohnishi Y, Ooshima A, Yamanaka A. J Cataract Refract Surg. 1998;24:1266–1270. doi: 10.1016/s0886-3350(98)80025-4. [DOI] [PubMed] [Google Scholar]
- 33.Nishi O, Nishi K, Akaishi T, Shirasawa E. Invest Ophthalmol Vis Sci. 1997;38:579–585. [PubMed] [Google Scholar]
- 34.Protin U, Schweighoffer T, Jochum W, Hilberg F. J Immunol. 1999;163:4917–4923. [PubMed] [Google Scholar]
- 35.Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H, Yang Y, Rizo CM, Overbeek PA, Robinson ML. Invest Ophthalmol Vis Sci. 2004;45:1930–1939. doi: 10.1167/iovs.03-0856. [DOI] [PubMed] [Google Scholar]
- 37.Simirskii VN, Wang Y, Duncan MK. Dev Biol. 2007;306:658–668. doi: 10.1016/j.ydbio.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed NA, Oh DJ, Czymmek KJ, Duncan MK. J Immunol Methods. 2001;253:243–252. doi: 10.1016/s0022-1759(01)00374-x. [DOI] [PubMed] [Google Scholar]
- 39.Simpson MA, Wilson CM, McCarthy JB. Am J Pathol. 2002;161:849–857. doi: 10.1016/S0002-9440(10)64245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Call MK, Grogg MW, Del Rio-Tsonis K, Tsonis PA. Exp Eye Res. 2004;78:297–299. doi: 10.1016/j.exer.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 41.Hawse JR, DeAmicis-Tress C, Cowell TL, Kantorow M. Mol Vis. 2005;11:274–283. [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta V, Galante A, Soteropoulos P, Guo S, Wagner BJ. Mol Vis. 2005;11:1018–1040. [PubMed] [Google Scholar]
- 43.Lesley J, Hyman R. Front Biosci. 1998;3:d616–630. doi: 10.2741/a306. [DOI] [PubMed] [Google Scholar]
- 44.Laurent TC, Laurent UB, Fraser JR. Immunol Cell Biol. 1996;74:A1–7. doi: 10.1038/icb.1996.32. [DOI] [PubMed] [Google Scholar]
- 45.Storr-Paulsen A, Norregaard JC, Farik G, Tarnhoj J. Acta Ophthalmol Scand. 2007;85:183–187. doi: 10.1111/j.1600-0420.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 46.Weigel PH, DeAngelis PL. J Biol Chem. 2007;282:36777–36781. doi: 10.1074/jbc.R700036200. [DOI] [PubMed] [Google Scholar]
- 47.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr., Kubalak S, Klewer SE, McDonald JA. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zoltan-Jones A, Huang L, Ghatak S, Toole BP. J Biol Chem. 2003;278:45801–45810. doi: 10.1074/jbc.M308168200. [DOI] [PubMed] [Google Scholar]
- 49.Ito T, Williams JD, Fraser DJ, Phillips AO. J Biol Chem. 2004;279:25326–25332. doi: 10.1074/jbc.M403135200. [DOI] [PubMed] [Google Scholar]
- 50.Duncan MK, Cvekl A, Kantorow M, Piatigorsky J. Lens crystallins. In: Lovicu FJ, Robinson ML, editors. Development of the Ocular Lens. Cambridge University Press; 2004. p. 416pp. [Google Scholar]
- 51.Hejtmancik JF, Beebe DC, Ostrer H, Piatigorsky J. Dev. Biol. 1985;109:72–81. doi: 10.1016/0012-1606(85)90347-1. [DOI] [PubMed] [Google Scholar]
- 52.Carper D, Smith-Gill SJ, Kinoshita JH. Dev. Biol. 1986;113:104–109. doi: 10.1016/0012-1606(86)90112-0. [DOI] [PubMed] [Google Scholar]
- 53.Van Leen RW, Breuer ML, Lubsen NH, Schoenmakers JG. Dev. Biol. 1987;123:338–345. doi: 10.1016/0012-1606(87)90392-7. [DOI] [PubMed] [Google Scholar]
- 54.Ueda Y, Duncan MK, David LL. Invest. Ophthalmol. Vis. Sci. 2002;43:205–215. [PubMed] [Google Scholar]
- 55.Tien JY, Spicer AP. Dev Dyn. 2005;233:130–141. doi: 10.1002/dvdy.20328. [DOI] [PubMed] [Google Scholar]
- 56.Rilla K, Tiihonen R, Kultti A, Tammi M, Tammi R. J Histochem Cytochem. 2008;56:901–910. doi: 10.1369/jhc.2008.951665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaarniranta K, Ihanamaki T, Sahlman J, Pulkkinen H, Uusitalo H, Arita M, Tammi R, Lammi MJ, Helminen HJ. Exp Eye Res. 2006;83:297–303. doi: 10.1016/j.exer.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 58.Nedvetzki S, Gonen E, Assayag N, Reich R, Williams RO, Thurmond RL, Huang JF, Neudecker BA, Wang FS, Turley EA, Naor D. Proc Natl Acad Sci U S A. 2004;101:18081–18086. doi: 10.1073/pnas.0407378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Falkowski M, Schledzewski K, Hansen B, Goerdt S. Histochem Cell Biol. 2003;120:361–369. doi: 10.1007/s00418-003-0585-5. [DOI] [PubMed] [Google Scholar]
- 60.Kasper M, Gunthert U, Dall P, Kayser K, Schuh D, Haroske G, Muller M. Am J Respir Cell Mol Biol. 1995;13:648–656. doi: 10.1165/ajrcmb.13.6.7576702. [DOI] [PubMed] [Google Scholar]
- 61.Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Semin Cancer Biol. 2008;18:260–267. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 62.Bourguignon LY. Semin Cancer Biol. 2008;18:251–259. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Isacke CM, Yarwood H. Int J Biochem Cell Biol. 2002;34:718–721. doi: 10.1016/s1357-2725(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 64.Gee K, Kryworuchko M, Kumar A. Arch Immunol Ther Exp (Warsz) 2004;52:13–26. [PubMed] [Google Scholar]