Abstract
The function of NMDA receptors in primary afferents remains controversial, in particular regarding their ability to evoke substance P release in the spinal cord. The objective of this study was, first, to confirm that substance P release evoked by NMDA is mediated by NMDA receptors in primary afferent terminals. Second, we investigated whether these NMDA receptors are inactivated in some conditions, which would explain why their effect on substance P release was not observed in some studies. Substance P release was induced in spinal cord slices and measured as NK1 receptor internalization in lamina I neurons. NMDA (combined with D-serine) induced NK1 receptor internalization with an EC50 of 258 nM. NMDA-induced NK1 receptor internalization was abolished by the NK1 receptor antagonist L-703,606, confirming that is was caused by substance P release, by NMDA receptor antagonists (MK1801 and ifenprodil), showing that it was mediated by NMDA receptors containing the NR2B subunit, and by preincubating the slices with capsaicin, showing that the substance P release was from primary afferents. However, it was not affected by lidocaine and ω-conotoxin MVIIA, which block Na+ channels and voltage-dependent Ca2+ channels, respectively. Therefore, NMDA-induced substance P release does not require firing of primary afferents or the opening of Ca2+ channels, which is consistent with the idea that NMDA receptors induce substance P directly by letting Ca2+ into primary afferent terminals. Importantly, NMDA-induced substance P release was eliminated by preincubating the slices for one hour with the Src family kinase inhibitors PP1 and dasatinib, and was substantially increased by the protein tyrosine phosphatase inhibitor BVT948. In contrast, PP1 did not affect NK1 receptor internalization induced by capsaicin. These results show that tyrosine-phosphorylation of these NMDA receptors is regulated by the opposite actions of Src family kinases and protein tyrosine phosphatases, and is required to induce substance P release.
Keywords: C-fiber, dorsal horn, internalization, nociceptor, neurokinin-1 receptor, protein tyrosine phosphatase
The function of NMDA receptors in primary afferents is still largely unknown and controversial. In 1994, Liu et al. (Liu et al., 1994) reported the presence of NMDA receptors in the central terminals of primary afferent. Subsequent studies using in situ hybridization (Sato et al., 1993), immunohistochemistry and real time PCR (Ma and Hargreaves, 2000; Marvizon et al., 2002) established that most primary afferent neurons express the NR1 and NR2B subunits of the NMDA receptor. The presence of functional NMDA receptors in primary afferent neurons was demonstrated with patch-clamp and Ca2+ imaging studies (Lovinger and Weight, 1988; McRoberts et al., 2001; Li et al., 2004).
NMDA receptors in primary afferents terminals appear to induce substance P release and subsequent activation of its receptor, the neurokinin 1 receptor (NK1R). Thus, Liu et al. (Liu et al., 1997) found that intrathecal injections of NMDA induced NK1R internalization in dorsal horn neurons, a measure of substance P release. Similarly, incubating spinal cord slices with NMDA induced NK1R internalization (Marvizon et al., 1997; Marvizon et al., 1999; Lao et al., 2003) and substance P release (Malcangio et al., 1998). In addition, NMDA receptor antagonists decreased substance P release evoked by electrical stimulation of the dorsal root (Marvizon et al., 1997; Malcangio et al., 1998; Marvizon et al., 1999) or by capsaicin (Malcangio et al., 1998; Afrah et al., 2001; Lao et al., 2003).
However, other studies have casted doubt on the idea that NMDA receptors in primary afferents induce substance P release. Lu et al. (Lu et al., 2003), using an anti-NR1 subunit antibody, found that this subunit colocalized with A-fiber markers but not with CGRP, which labels substance P-containing C-fibers. Bardoni et al. (Bardoni et al., 2004) reported that NMDA decreased monosynaptic EPSCs in dorsal horn neurons evoked by dorsal root stimulation, which suggests that NMDA receptors inhibit, rather than facilitate, glutamate release from primary afferents. This is surprising, because glutamate release was expected to parallel substance P release. Finally, Nazarian et al. (Nazarian et al., 2007) found that intrathecal NMDA did not induce NK1R internalization in anesthetized rats, in contradiction to the findings of Liu et al. (Liu et al., 1997) in awake rats.
These disparities suggest that NMDA receptors in primary afferents may be regulated, so that they induce substance P release in some conditions but not others. Indeed, Zeng et al. (Zeng et al., 2006) found that in naïve rats NMDA decreased EPSCs in dorsal horn neurons, just like it was reported by Bardoni et al. However, in morphine tolerant rats NMDA increased these EPSCs, and there was also an increased expression of the NR1 subunit in primary afferents. Other studies (Li et al., 2006; McRoberts et al., 2007) found that NMDA receptor currents in primary afferent neurons were increased by 17-β-estradiol, a steroid hormone, and by sodium vanadate, an inhibitor of protein tyrosine phosphatases (PTPs). Importantly, these effects were reversed by lavendustin, an inhibitor of tyrosine kinases, and by PP2 an inhibitor Src family kinases (SFKs) (Hanke et al., 1996). These findings suggest that NMDA receptors in primary afferents are modulated by tyrosine phosphorylation of the NR2B subunit, as has been demonstrated in a variety of other systems (Yu and Salter, 1999; Kalia et al., 2004; Kato et al., 2006; Sato et al., 2008; Xu et al., 2008; Zhang et al., 2008).
To test this hypothesis, we investigated whether the ability of NMDA to induce substance P release is affected by inhibitors of SFKs and PTPs, the enzymes that phosphorylate and dephosphorylate, respectively, tyrosine residues in NMDA receptors. We used NK1R internalization to measure substance P release in terms of the activation of its receptor, an approach that has been validated by a number of studies (Mantyh et al., 1995; Abbadie et al., 1997; Allen et al., 1997; Liu et al., 1997; Marvizon et al., 1997; Allen et al., 1999; Honore et al., 1999; Wang and Marvizon, 2002; Lao et al., 2003; Marvizon et al., 2003a; Adelson et al., 2009).
Experimental Procedures
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Veteran Affairs Greater Los Angeles Healthcare System, and conform to NIH guidelines. Efforts were made to minimize the number of animals used and their suffering. Rats used to prepare spinal cord slices were male Sprague-Dawley (Harlan, Indianapolis, IND), 3–5 weeks old.
Chemicals and solutions
Artificial cerebrospinal fluid (aCSF) contained, in mM: 124 NaCl, 1.9 KCl, 26 NaHCO3, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2 and 10 glucose, and was bubbled with 95% O2 / 5% CO2. Sucrose-aCSF was the same medium with 5 mM KCl and 215 mM sucrose instead of NaCl.
BVT948, ω-conotoxin MVIIA, PP1 and PP3 were from Tocris (Ellisville, MO). Dasatinib was from ChemieTek (Indianapolis, IN). Capsaicin, D-serine, ifenprodil, L-703,606, lidocaine, MK801, NMDA and other chemicals were from Sigma. Drugs were prepared as stock solutions of 10–100 mM in the appropriate solvent and then diluted in aCSF. NMDA was dissolved in 100 mM NaOH. BVT948, dasatinib, PP1 and PP3 were dissolved in DMSO. L-703,606 was dissolved in methanol. Capsaicin was dissolved in ethanol. Other compounds were dissolved in water.
Spinal cord slices
For a detailed description of the slice preparation see (Lao et al., 2003; Marvizon et al., 2003a; Song and Marvizon, 2003; Lao and Marvizon, 2005; Song and Marvizon, 2005; Adelson et al., 2009). Briefly, the spinal cord was extracted from 3–5 weeks old rats under isoflurane anesthesia (Halocarbon Laboratories, River Edge, NJ). Coronal slices (400 µm) were cut from the lumbar spinal cord (L2–L4) with a vibratome (Integraslice 7550PSDS, Lafayette Instruments, Lafayette, IN) using low advance speed and fast vibration. Slices were kept in aCSF, except that vibratome cutting was done in sucrose-aCSF. Slices were left to recover in oxygenated aCSF at 35°C for 1 hr, and were used within 3 hr of preparation.
Incubation of the slices with drugs
Slices were incubated with drugs using established procedures (Marvizon et al., 1997; Marvizon et al., 1999; Lao et al., 2003; Marvizon et al., 2003a; Marvizon et al., 2003b). The slices were placed on a nylon net glued to a plastic ring inserted halfway down a plastic tube containing 5 ml aCSF. The aCSF was superficially gassed with 95% O2/5% CO2 delivered through a needle inserted through the cap of the tube. This arrangement ensured access of oxygenated aCSF and drugs from both sides of the slice. To change solutions, the ring and net with the slice was transferred to another tube. To avoid possible excitotoxic effects, NMDA and capsaicin were applied to the slices for 2 min or less. This was followed by incubation in aCSF at 35 °C for 10 min to allow enough time for NK1R internalization (Wang and Marvizon, 2002). At the end of the experiment, slices were immersed in ice-cold fixative (4 % paraformaldehyde, 0.18 % picric acid).
Characterization of the NK1R antiserum
The NK1R antiserum # 94168 was made at CURE: Digestive Diseases Research Center, University of California Los Angeles, under the sponsorship of Dr. Nigel Bunnett, University of California San Francisco. It was generated in rabbits using a peptide corresponding to the C-terminus of the rat NK1R (amino acids 393–407, KTMTESSSFYSNMLA) coupled to KLH (Grady et al., 1996). It labeled cells transfected with rat NK1R and did not label nontransfected cells. Staining of the transfected cells was eliminated by preadsorption with its immunizing peptide. In Western blots from NK1R transfected cells the antiserum produced a single band corresponding to a molecular weight of 100 kDa (Grady et al., 1996).
Immunohistochemistry
Spinal cord slices were fixed, cryoprotected, frozen and re-sectioned at 25 µm in a cryostat as described (Lao et al., 2003; Marvizon et al., 2003a; Lao and Marvizon, 2005; Adelson et al., 2009). Sections were washed four times and then incubated overnight at room temperature with the NK1R antiserum diluted 1:3000 in phosphate-buffered saline containing 0.3 % Triton X-100, 0.001% thimerosal and 5% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA). After three washes, the secondary antibody (1:2000, Alexa Fluor 488 goat anti-rabbit, Molecular Probes-Invitrogen, Eugene, OR) was applied at for 2 hours at room temperature. Sections were washed four more times, mounted on glass slides, and coverslipped with Prolong Gold (Molecular Probes-Invitrogen).
Quantification of NK1R internalization
The amount of NK1R internalization was quantified using a standard method (Mantyh et al., 1995; Abbadie et al., 1997; Riley et al., 2001; Trafton et al., 2001) with minor modifications (Marvizon et al., 1997; Marvizon et al., 1999; Lao et al., 2003; Lao and Marvizon, 2005; Adelson et al., 2009). NK1R neurons in lamina I were visually counted while classifying them as with or without internalization. A Zeiss Axio-Imager A1 (Carl Zeiss, Inc., Thornwood, NY) fluorescence microscope with a 63× (1.40 numerical aperture) objective was used. The criterion for having internalization was the presence in the neuronal soma of ten or more NK1R endosomes, defined as a small region of bright staining separated from the cell surface. The person counting the neurons was blinded to the treatment. Four sections per slice were used, counting all lamina I NK1R neurons in each section. Results were expressed as the percentage of the NK1R neurons in lamina I with NK1R internalization.
Confocal microscopy
Confocal images were acquired using a Zeiss LSM 710 confocal microscope (Carl Zeiss, Inc., Thornwood, NY), with objectives of 10× (numerical aperture 0.3) and 63× oil (numerical aperture 1.4). Excitation light for the Alexa Fluor 488 fluorophore (emission peak 519 nm) was provided by the 488 nm line of an argon laser. The emission window was 500–570 nm. The pinhole was 1.0 Airy unit: 38.2 µm for the 10× objective and 51.5 µm for the 63× objective, as determined by the confocal microscope software. Images were acquired in grayscale as confocal stacks of sections of 1024×1024 pixels. Each section was averaged 4 times to reduce noise. The separation between confocal sections, optimized by the confocal microscope software using the Nyquist formula, was 5.98 µm for the 10× objective and 0.38 µm for the 63× objective.
Image processing
Images of the entire dorsal horn obtained with the 10× objective were used to show the location of the neurons imaged with the 63× objective. Confocal stacks acquired with the 10× objective were processed using adaptive point spread function (‘blind’) deconvolution to reduce blur (Wallace et al., 2001; Cannell et al., 2006; Holmes et al., 2006), using the program AutoQuant × 2.0.1 (Media Cybernetics, Inc., Bethesda, MD). Images taken with the 63× objective were not deconvolved because their native low blur made this unnecessary. The program Imaris 6.1.5 (×64, Bitplane AG, Zurich, Switzerland) was used to crop the images in three dimensions. Images at 10× were cropped into the two brightest optical sections. Often, images of several NK1R neurons were acquired in a single 63× confocal stack, in which case Imaris was used to crop each cell out of the stack into 3–5 optical sections through the middle of the cell. After cropping, a two-dimension projection picture was generated in Imaris and imported into Adobe Photoshop 5.5 (Adobe Systems Inc., Mountain View, CA), which was used to make slight adjustments in the gamma. Adobe Photoshop was also used to compose the multipanel figures and to add text and arrows.
Data analysis
Data were analyzed using Prism 5 (GraphPad Software, San Diego, CA). Statistical analyses consisted of one-way ANOVA followed by Bonferroni’s post-hoc test. Statistical significance was set at 0.05. Concentration-response data were fitted using non-linear regression by a sigmoidal dose-response function: Y = bottom + (top-bottom) / (1 + 10^(Log EC50-Log X)), where the EC50 is the concentration of drug that produces half of the effect. Baseline measures (zero concentration of drug) were included in the non-linear regression by assigning them a concentration value three log units lower than the estimated EC50. Parameter constraints were: 0% < top < 100%, 0% < bottom. The statistical error of the EC50 was expressed as 95% confidence intervals (CI).
Results
NMDA receptors induce substance P release
Incubating rat spinal cord slices with NMDA induced NK1R internalization in the superficial dorsal horn. In control slices, NK1Rs in lamina I neurons were present at the cell surface (Fig. 1 A). In slices incubated with NMDA (30 µM), NK1Rs were internalized forming endosomes (Fig. B) in about half of the lamina I neurons with NK1Rs. In this and subsequent experiments, NMDA was combined with D-serine (10 µM), a selective agonist of the NMDA receptor co-agonist site, which is required for its activation (Dingledine et al., 1999). Incubations with NMDA and D-serine were limited to 2 min to avoid excitotoxic effects. NK1R internalization induced by NMDA was eliminated by the NMDA receptor antagonist/channel blocker MK801 (10 µM, Fig. 2), showing that it was mediated by NMDA receptors. It was also eliminated by the NK1R antagonist L-703,606 (10 µM, Fig. 2), indicating that it was caused by binding to the NK1R of substance P released from the slices.
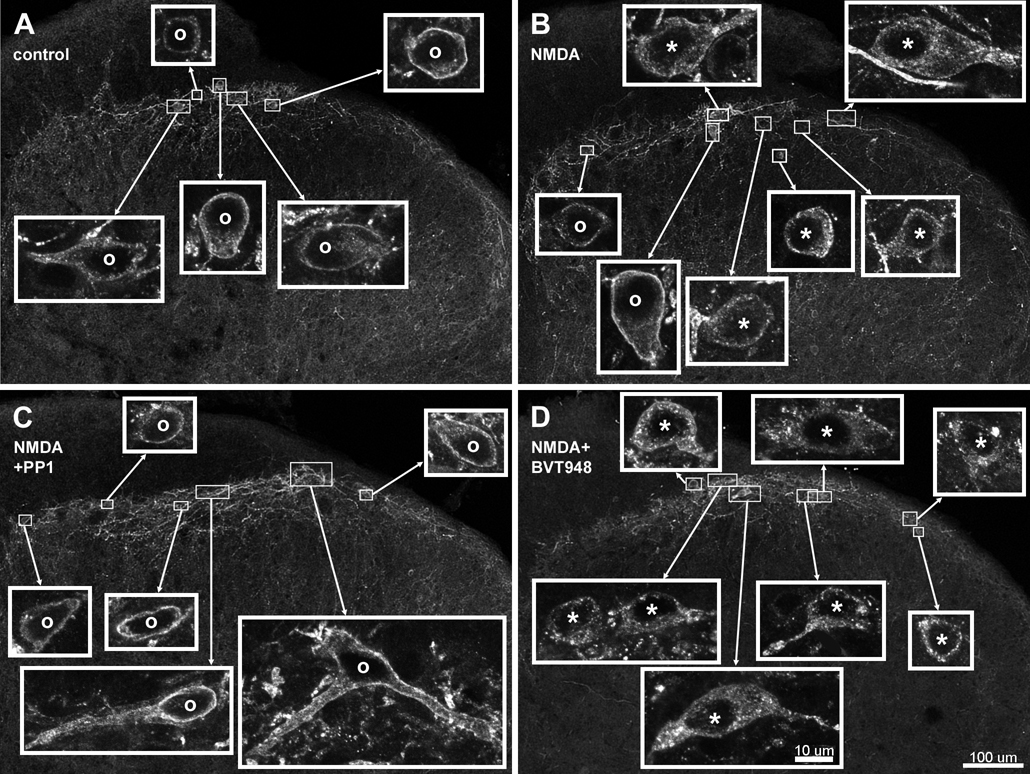
Fig. 1. Confocal images of NK1R neurons in lamina I.
Spinal cord slices were untreated (control, A), or incubated with 30 µM NMDA + 10 µM D-Ser (NMDA, B), NMDA, D-Ser and 10 µM PP1 (NMDA+PP1, C) or NMDA, D-Ser and 10 µM BVT948 (NMDA+BVT948, D). Images in the main panels were taken with a 10x objective consist of 2 optical sections separated 6 µm. They were deblurred using deconvolution. Images in the insets were taken with a 63× objective and consist of 3–5 optical sections separated 0.38 µm. Neurons with NK1R internalization are indicated with “*” andneurons without internalization by “o”. Scale bars (in panel D) are 100 µm for the main panels and 10 µm for the insets.
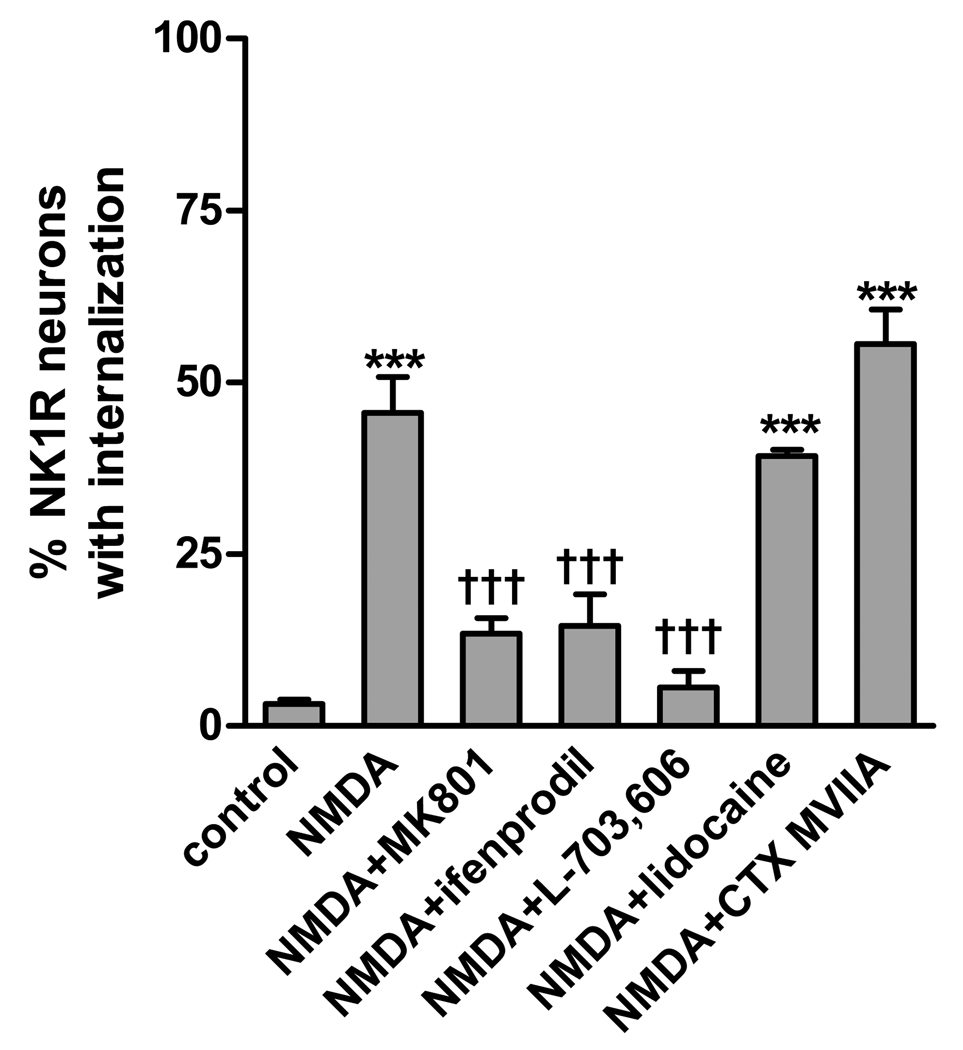
Fig. 2. NK1R internalization induced by NMDA was blocked by NMDA receptor and NK1R antagonists, but not by lidocaine.
Spinal cord slices were incubated at 35 °C with no drugs (control) or 10 µM NMDA + 10 µM D-Ser and the following compounds: none (NMDA), 10 µM MK801 (NMDA receptor channel blocker), 10 µM ifenprodil (NR2B subunit-selective antagonist), 10 µM L-703,606 (NK1R antagonist), 1 mM lidocaine (Na+ channel blocker) or 100 nM ω-conotoxin MVIIA (CTX MVIIA, Ca(V) channel blocker). The incubation consisted in a 10 min preincubation with the compounds (40 min for L-703,606) followed by the addition of NMDA plus D-Ser for 2 min more. ANOVA revealed a significant effect of the drug combinations (p<0.0001). Bonferroni’s post-tests: ***, p<0.001 compared to control; †††, p<0.001 compared with NMDA.
NMDA receptor-induced substance P release does not require firing of action potentials
The most likely explanation for this effect of NMDA is that NMDA receptors located presynaptically in primary afferent terminals directly induce substance P release. However, it is also possible that NMDA receptors induce the firing of action potentials in the primary afferents, which then drives substance P release. To investigate this possibility, we explored whether NMDA-induced NK1R internalization was eliminated by the Na+ channel blocker lidocaine (1 mM). Lidocaine had no effect (Fig. 2), showing that NMDA is able to induce substance P release in the absence of primary afferent firing.
NMDA receptor-induced substance P release does not require opening of voltage-dependent Ca2+ [Ca(V)] channels
Given that NMDA receptors form Ca2+ permeable channels, it is likely that they induce substance P release by letting Ca2+ into the terminal. If this is true, then NMDA-induced substance P would not require the activation of Ca(V) channels. To investigate this possibility, we used ω-conotoxin MVIIA, which blocks N-type and P-type Ca(V) channels with IC50s of 18 nM and 50 nM, respectively (McDonough et al., 1996). At a concentration of 100 nM, ω-conotoxin MVIIA did not affect NMDA-induced NK1R internalization (Fig. 2), showing that NMDA-induced substance P release is independent of the opening of Ca(V) channels.
NMDA receptors release substance P from primary afferents
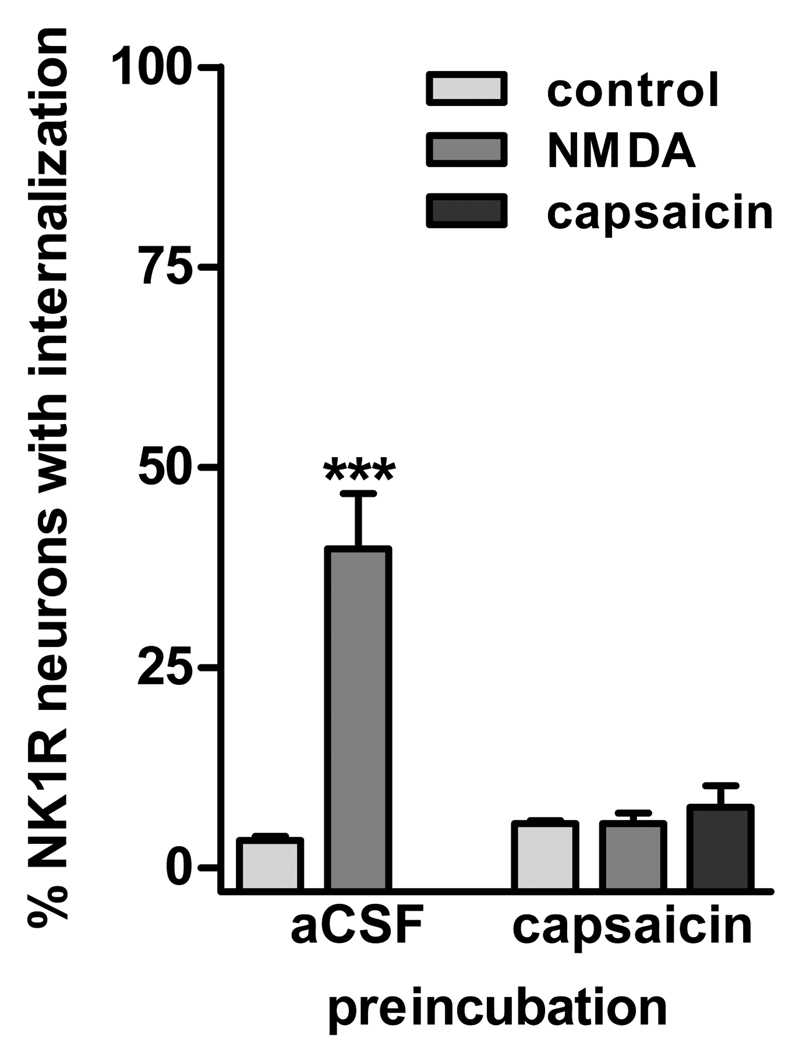
To determine whether NMDA released substance P from primary afferents, we used the strategy of depleting primary afferent terminals with a preincubation with capsaicin (10 µM for 10 min at 35 °C). This should produce abundant NK1R internalization. Hence, in order to use NK1R internalization to measure subsequent substance P release, we waited 90 min, a time long enough to allow NK1Rs to cycle back to the cell surface (Wang and Marvizon, 2002). Indeed, no NK1R internalization was detected after this time (Fig. 3, control after capsaicin preincubation). Incubating the capsaicin-treated slices a second time with capsaicin (1 µM for 2 min) failed to induce NK1R internalization, confirming that primary afferents were depleted of substance P. Incubating the capsaicin-treated slices with NMDA (10 µM, with 10 µM D-Ser) for 2 min also failed to induce NK1R internalization (Fig. 3), showing that NMDA induced substance P release from capsaicin-sensitive primary afferents.
Fig. 3. Preincubation with capsaicin eliminated NMDA-induced NK1R internalization.
Spinal cord slices were preincubated for 10 min with aCSF or 10 µM capsaicin to deplete primary afferents of substance P. The slices were then kept in aCSF for 90 min to allow NK1R recycling to the cell surface. After that, the slices were incubated 2 min with no drugs (control), 10 µM NMDA + 10 µM D-Ser, or 1 µM capsaicin. All incubations were done at 35 °C. Two-way ANOVA revealed a significant effect of capsaicin preincubation (p=0.0019), incubation with NMDA/capsaicin (p=0.0009) and interaction of both variables (p=0.0009). Bonferroni’s post-tests: ***, p<0.001 compared to control.
NMDA receptors that induce substance P release contain the NR2B subunit
Most primary afferent neurons express NMDA receptors and in particular the NR2B subunit (Ma and Hargreaves, 2000; Marvizon et al., 2002; Li et al., 2004). To determine whether the NMDA receptors that induced substance P release contained the NR2B subunit, we used ifenprodil, a selective antagonist of NR2B-containing NMDARs (Avenet et al., 1996; Gallagher et al., 1996). Ifenprodil (10 µM) eliminated NMDA-induced NK1R internalization (Fig. 2), confirming the involvement of NR2B-containing NMDA receptors.
The induction of NK1R internalization by NMDA was dose-dependent
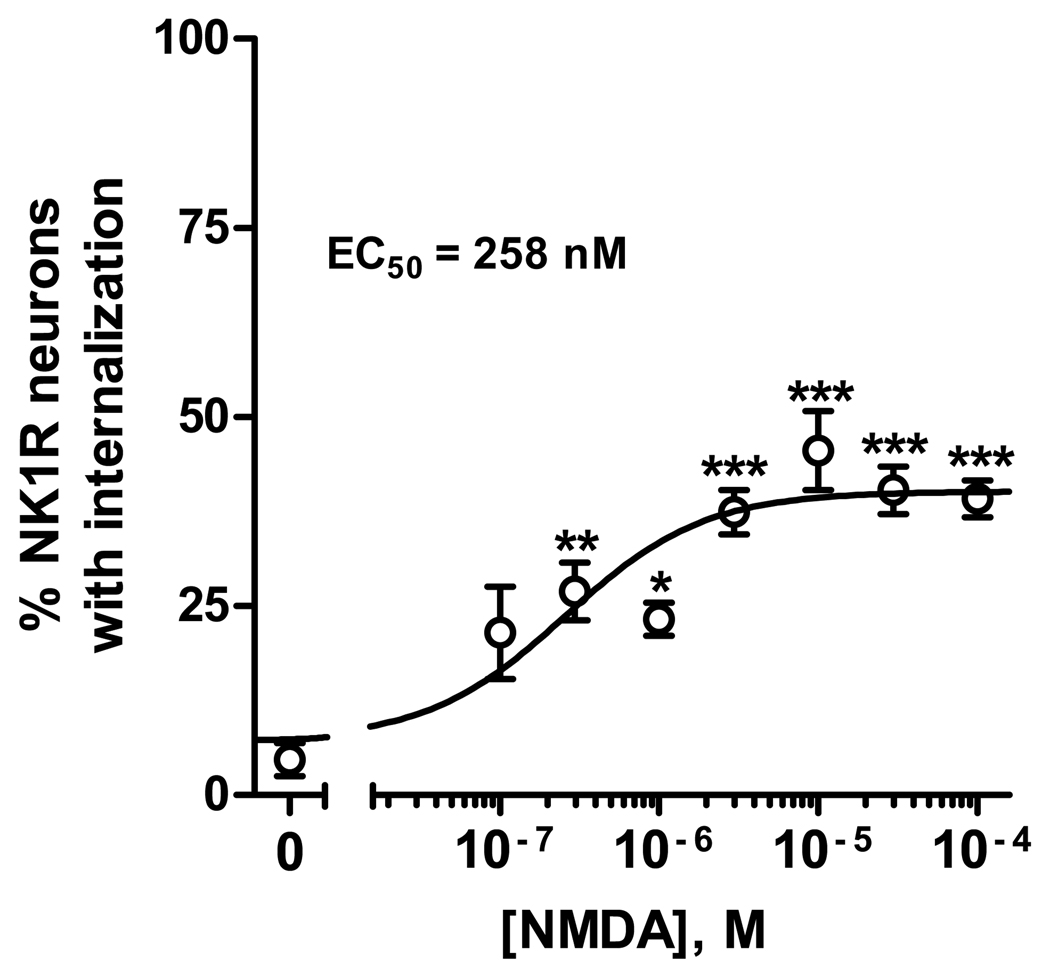
As shown in Fig. 4, NMDA induced NK1R internalization dose-dependently and with relatively high potency, with an EC50 of 258 nM (95% CI: 91–735 nM). D-Serine (10 µM) was used with all the concentrations of NMDA. The control was D-serine without NMDA (Fig. 4), which did not induce NK1R internalization. The efficacy of NMDA was only moderate, with a maximum effect of 40±2 % NK1R neurons with internalization in lamina I. ANOVA revealed a significant effect of NMDA concentration (p<0.0001).
Fig. 4. Concentration-response for NMDA.
Spinal cord slices were incubated for 2 min at 35 °C with NMDA at the concentrations indicated and 10 µM D-Ser (included also in the control, [NMDA] = 0). N = 4 slices per concentration of NMDA. ANOVA revealed a significant effect of NMDA (p<0.0001). Bonferroni’s post-tests, compared to control: *, p<0.05; **, p<0.01; ***, p<0.001. The curve represent fitting a dose-response function to the data, which yielded EC50 = 258 nM (95% CI = 91–735 nM), top = 40±2 %, bottom = 7±4 %.
SFK inhibitors abolished NMDA-induced NK1R internalization
We hypothesized that NMDA receptors induce substance P release only when they are Tyr-phosphorylated by SKFs. To test this hypothesis, we determined whether the SFK inhibitors PP1 and dasatinib decreased NMDA-induced NK1R internalization. PP1 is a widely-used inhibitor of SFKs, particularly of Lck and Fyn (Hanke et al., 1996; Liu et al., 1999). It inhibits Tyr phosphorylation in T-cells and lymphocyte proliferation with IC50 of 0.5–5 µM (Hanke et al., 1996). PP3 is an inactive analog of PP1 that was be used as control. Dasatinib is a SFK inhibitor unrelated to PP1, and that also inhibits Abl, Btk and Tec kinases (Schittenhelm et al., 2006; Hantschel et al., 2007).
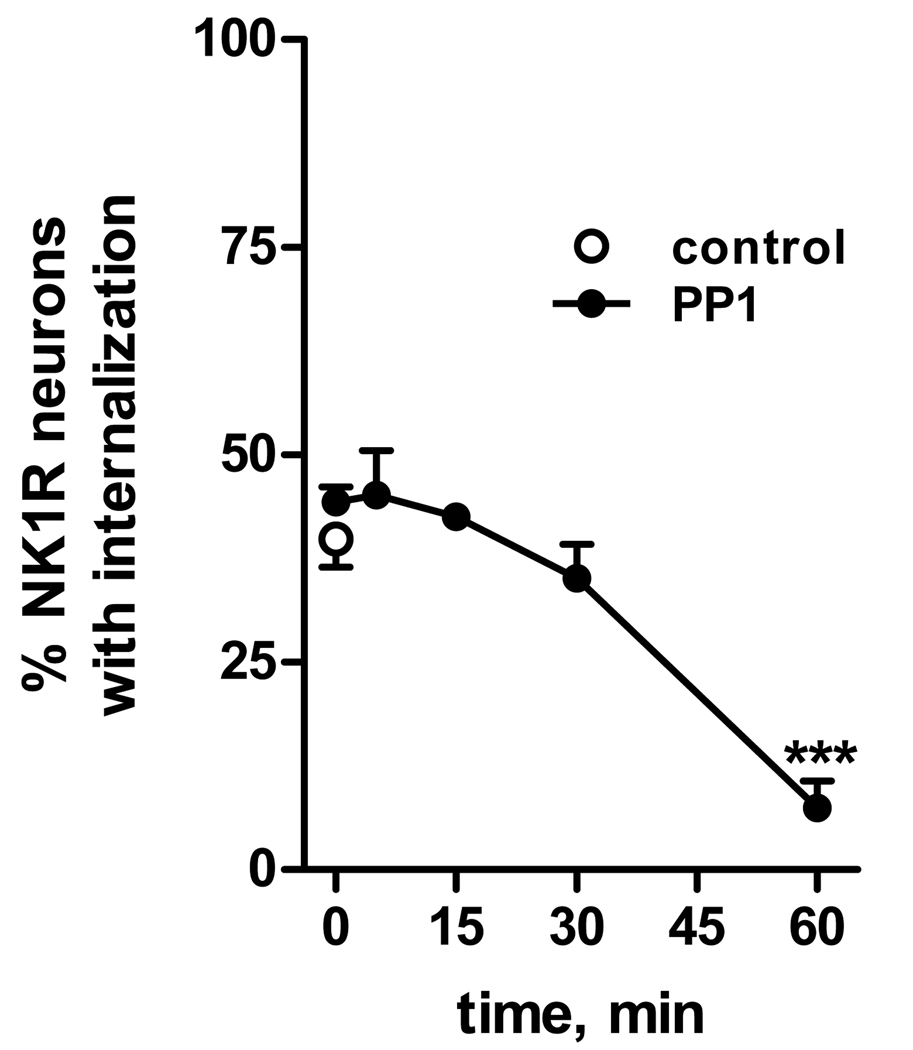
Since tyrosine phosphorylation of NMDA receptors in primary afferents appears to proceed quite slowly (Li et al., 2006), we first performed a time course of the effect of PP1. Slices were preincubated at 35 °C with 10 µM PP1 for times up to 1 hr (Fig. 5). Then 10 µM NMDA and 10 µM D-serine were added for 2 min, still in the presence of PP1. PP1 abolished NMDA-induced NK1R internalization when added 60 min before NMDA, but not with shorter preincubation times (Fig. 5). Fig. 1 shows examples of NK1R neurons in lamina I after treatment with PP1 and NMDA: whereas NMDA induced NK1R internalization (Fig. 1 B), a 60 min treatment with PP1 eliminated the NK1R internalization (Fig. 1 C).
Fig. 5. Time-course of the inhibition of the effect of NMDA by the SFK inhibitor PP1.
Spinal cord slices were incubated for 2 min at 35 °C with NMDA (10 µM) and D-Ser (10 µM), without any pretreatment (control), or after preincubation with PP1 (10 µM) for the indicated times. ANOVA revealed a significant effect of time of preincubation with PP1 (p<0.0001). Bonferroni’s post-tests: ***, p<0.001 compared to control.
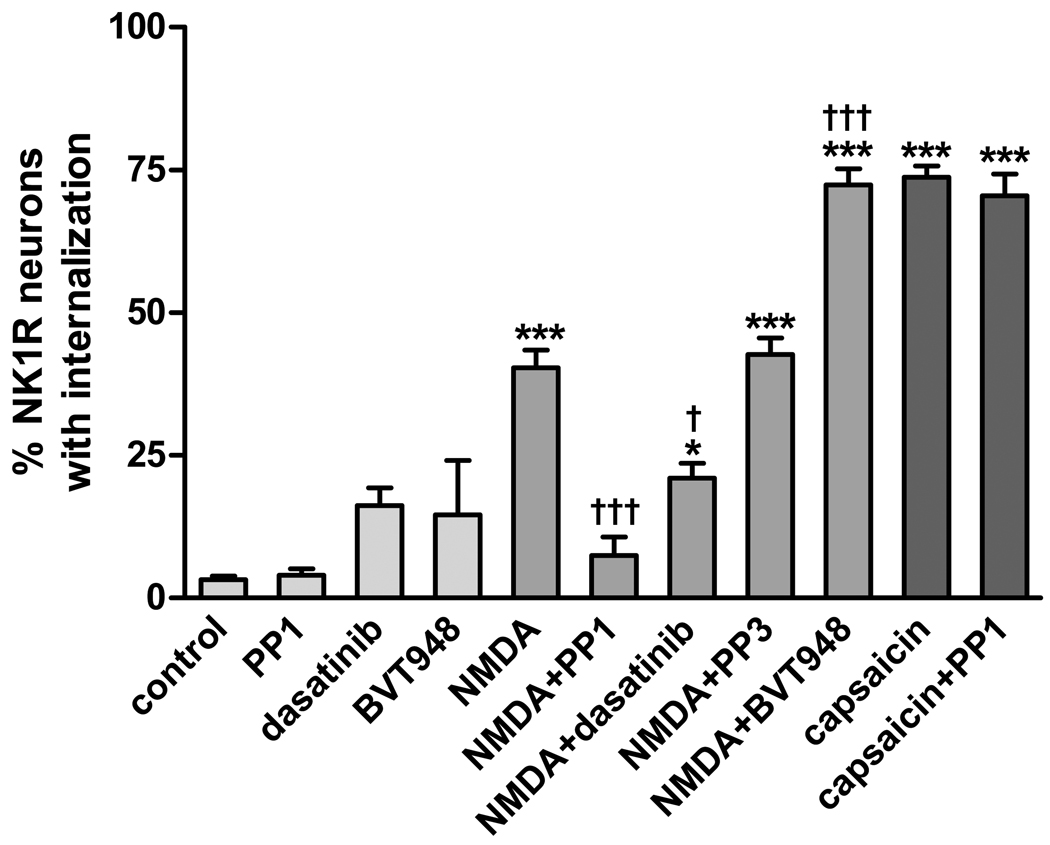
Dasatinib, also preincubated with the slices for 60 min, significantly decreased NMDA-induced NK1R internalization (Fig. 6). PP3, the inactive analog of PP1, produced no effect. In the absence of NMDA, preincubating the slices with PP1 and dasatinib did not affect NK1R internalization (Fig. 6).
Fig. 6. Effect of inhibitors of SFKs and PTPs on the induction of substance P release by NMDA.
Spinal cord slices were incubated for 2 min at 35 °C with the indicated drug combinations (control: no drugs). Drug concentrations were: 10 µM PP1, 1 µM dasatinib, 10 µM BVT948, 30 µM NMDA (with 10 µM D-Ser), and 1 µM capsaicin. Treatments with PP1, dasatinib, PP3 and BVT948 were preceded by a 60 min preincubation with these compounds. ANOVA revealed a significant effect of the drug combinations (p<0.0001). Bonferroni’s post-tests: ***, p<0.001 compared to control; †††, p<0.001 compared to NMDA.
A PTP inhibitor increased NMDA-induced NK1R internalization
PTPs are enzymes that catalyze the opposite reaction of SFKs: they de-phosphorylate tyrosine residues that are phosphorylated by SFKs. It is likely that the extent of tyrosine phosphorylation of these NMDA receptors is determined by the relative activity of SFKs and PTPs. If so, inhibiting PTPs should increase the tyrosine phosphorylation of the NMDA receptors and thus their ability to induce substance P release. Accordingly, we treated spinal cord slices with the PTP inhibitor BVT948 (10 µM) (Liljebris et al., 2004) before and during the addition of NMDA. We assumed that the increase in NMDA phosphorylation once PTPs were inhibited would proceed slowly, so we preincubated the slices with BVT948 fro 60 min, as in the case of PP1. A subsequent 2 min incubation with NMDA (30 µM) plus D-Ser (10 µM) resulted in a much larger induction of NK1R internalization that in slices not preincubated with BVT948 (Fig. 6). The increase in NK1R internalization produced by BVT948 was statistically significant (p<0.001, Bonferroni’s post-hoc test). Fig. 1 D illustrates the extensive NK1R internalization in lamina I neurons induced by the combination of BVT948 and NMDA. These results indicate that in the basal state the NMDA receptors that induce substance P release are only partially tyrosine-phosphorylated. When these NMDA receptors are fully tyrosine-phosphorylated, they are very efficacious in inducing substance P release.
Induction of substance P release by capsaicin is not affected by SFK inhibition
Next, we determined whether tyrosine phosphorylation affects NMDA receptors or other mechanisms involved in substance P release. Capsaicin is a powerful stimulus to induce substance P release, an effect mediated by the influx of Ca2+ into primary afferent terminals through TRPV1 channels (Lao et al., 2003). However, preincubating spinal cord slices with PP1 (10 µM for 60 min) did not affect the ability of capsaicin (1 µM for 2 min) to evoke substance P release and NK1R internalization (Fig. 6). Therefore, unlike NMDA receptors, TRPV1 channels do not require phosphorylation by SFKs to induce substance P release.
Discussion
This study shows that NMDA receptors induce substance P release from primary afferent terminals only when they are phosphorylated by SFKs.
NMDA receptors in primary afferent terminals induce substance P release
Confirming our previous results (Marvizon et al., 1997; Marvizon et al., 1999), this study shows that NMDA applied to rat spinal cord slices induced NK1R internalization, a measure of substance P release (Marvizon et al., 2003a) and subsequent NK1R activation (Trafton et al., 1999; 2001). We have extended these previous observations with more evidence that NMDA-induced NK1R internalization is indeed mediated by presynaptic NMDA receptors that evoke substance P release from primary afferent terminals. First, the effect of NMDA was blocked by NMDA receptor antagonists, demonstrating that is mediated by NMDA receptors. Second, it was also blocked by a NK1R antagonist, consistent with the idea that NK1R internalization was elicited by substance P release. Third, the effect of NMDA was not affected by lidocaine, a Na+ channel blocker that prevents the firing of action potentials (Courtney et al., 1987). Therefore, NMDA induced substance P release by acting on NMDA receptors located in the primary afferent terminals themselves. Fourth, the effect of NMDA was not affected by the Ca(V) channel blocker ω-conotoxin MVIIA at a concentration sufficient to block N-type and P-type Ca(V) channels (McDonough et al., 1996), the main types present in primary afferent neurons (Rusin and Moises, 1995, 1998; Raingo et al., 2007). Therefore, the influx of Ca2+ into the terminal through the NMDA receptor Ca2+-permeable channels (Yamakura and Shimoji, 1999) is sufficient to trigger substance P release (Fig. 7). Fifth, the effect of NMDA was eliminated by preincubating the slices with capsaicin to deplete primary afferent terminals of substance P, indicating that NMDA receptors released substance P from primary afferents.
Fig. 7. Diagram indicating the proposed function of NMDA receptors in primary afferent terminals.
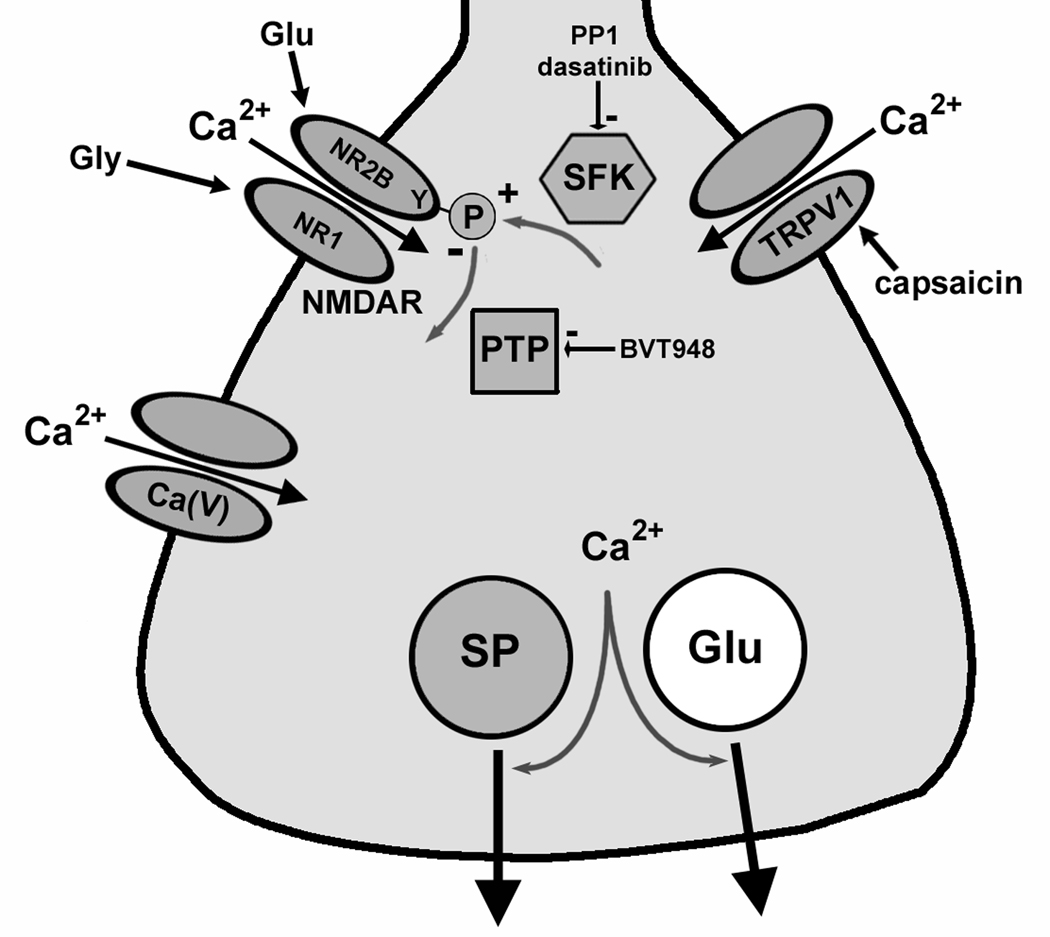
Substance P (SP) and glutamate (Glu) are released when Ca2+ enters the terminal through voltage-gated Ca2+ channels [Ca(V)], TRPV1 channels or NMDA receptors (NMDAR). NMDA receptors are upregulated (+) by tyrosine (Y) phosphorylation of the NR2B subunit by SFKs, and downregulated (−) by dephosphorylation by PTPs.
Practically all primary afferents express NMDA receptors (Sato et al., 1993; Ma and Hargreaves, 2000; Marvizon et al., 2002). Agonist binding to these NMDA receptors produces a Ca2+ influx (Lovinger and Weight, 1988; McRoberts et al., 2001; Li et al., 2004). The central terminals of primary afferents contain the NR1 subunit of the NMDA receptor, as determined by electron microscopic immunohistochemistry (Liu et al., 1994). However, Lu et al. (Lu et al., 2003) reported that the NR1 subunit was not found in primary afferent terminals containing CGRP and substance P. This could have been due to the fact that the anti-NR1 antibody used by Lu et al. recognized only NR1 splice variants with the C2 cassette of exon 22 (Lu et al., 2003), i.e. NR1-1 and NR1-2 (Dingledine et al., 1999). However, primary afferents also express NMDA receptors formed by NR1 splice variants without the C2 cassette, i.e. NR1-3 and NR1-4 (Li et al., 2006). It is possible, therefore, that the NMDA receptors in substance P-continuing terminals have the NR1-3 and NR1-4 splice variants.
The NMDA receptors that induce substance P release have the NR2B subunit
We found that NMDA-induced NK1R internalization was eliminated by ifenprodil, a selective antagonist of NR2B subunit-containing NMDA receptors (Williams, 1993). Therefore, the NMDA receptors that induce substance P release contain this subunit, which is consistent with the fact that the NR2B subunit is expressed by most primary afferent neurons (Ma and Hargreaves, 2000; Marvizon et al., 2002; Li et al., 2006). The NR2B subunit is also found in many synapses throughout laminae I and II (Nagy et al., 2004; Zhang et al., 2009) and is involved in pain modulation, as evidenced by the fact that ifenprodil and other selective NR2B antagonists induce antinociception (Boyce et al., 1999; Zhang et al., 2009). NR2B-selective antagonists also attenuated neuropathic pain (Abe et al., 2005).
NMDA receptors that induce substance P release are upregulated by SFK-mediated tyrosine phosphorylation
Extensive evidence shows that NR2B-containing NMDA receptors are upregulated by SFK-mediated phosphorylation of tyrosine residues in the NR2B subunit (Dingledine et al., 1999). This upregulation plays a critical role in synaptic plasticity and other physiological processes. For example, SFKs upregulate the NMDA receptors that mediate hippocampal long-term potentiation (LTP) (Kalia et al., 2004; Xu et al., 2008). More specifically, SFKs phosphorylate tyrosine Y1472 of the NR2B subunit, which is part of a YEKL motif involved in NMDA receptor internalization. Phosphorylation of Y1472 inhibits the binding of the clathrin adaptor protein AP-2 to the YEKL motif. This reduced coupling to clathrin prevents the internalization of the NMDA receptors, increasing the presence of functional NMDA receptors at the cell surface (Zhang et al., 2008; Goebel-Goody et al., 2009).
In the dorsal horn, phosphorylation of tyrosine Y1472 of the NR2B subunit of NMDA receptors was found to take place in a neuropathic pain model (Abe et al., 2005). The SFK that phosphorylates NR2B was probably Fyn, because phosphorylation of Y1472 was lost in mice lacking Fyn. However, these NMDA receptors are probably not the ones that induce substance P release, because the NR2B subunit phosphorylated during neuropathy was localized postsynaptically.
Our results show that NMDA receptors in primary afferents need to be phosphorylated by SFKs to be able to induce substance P release (Fig. 7). Thus, NMDA-induced NK1R internalization was prevented by inhibition of SFKs and dramatically increased by inhibition of PTPs. In addition, this last result shows that NMDA receptor activation is able to release large amounts of substance P, comparable with those released by capsaicin. It also indicates that in the basal state these NMDA receptors are only partially phosphorylated.
There is some prior evidence that NMDA receptors in primary afferents are upregulated by SFKs. Thus, Li et al. (Li et al., 2006) found that in a colitis model there was a three-fold increase in NMDA receptor currents in primary afferent neurons, and that this increase was eliminated by the SFK inhibitor PP2. In another study in primary afferent neurons, the same group (McRoberts et al., 2007) found that 17-β-estradiol, a estrogen receptor agonist, increased NMDA receptor currents and that this was prevented by the SFK inhibitor lavendustin A.
We found that the effect of the SFK inhibitor was slow: spinal cord slices needed to be preincubated with PP1 for one hour in order to eliminate NMDA-induced NK1R internalization. The amount of tyrosine phosphorylation of these NMDA receptors is probably determined by the balance of the activity of SFKs and PTPs (Fig. 7), so that when the SFKs are inhibited the receptors are de-phosphorylated by the PTPs. If so, the slow effect of the SFK inhibitor indicates that the de-phosphorylation of the NR2B subunit by PTPs is a slow process. This idea is consistent with previous results: Li et al. (Li et al., 2006), measuring NMDA receptor currents in primary afferent neurons, found that inhibition by the SFK inhibitor PP2 took one hour to reach its maximum.
Capsaicin-induced substance P release was not affected by SFK inhibition
Like NMDA receptors, capsaicin-activated TRPV1 channels induce substance P release by letting Ca2+ into primary afferent terminals (Fig. 7). Evidence for this was provided by a previous study (Lao et al., 2003). In it we found, first, that substance P release (NK1R internalization) evoked by incubating spinal cord slices with capsaicin was not affected by lidocaine. This indicates that the effect of capsaicin did not require the firing of action potentials in primary afferents, and therefore was mediated by TRPV1 channels in the presynaptic terminals. Second, capsaicin-induced substance P release was not affected by the GABAB receptor agonist baclofen. Since GABAB receptors inhibit Ca(V) channels (Raingo et al., 2007), Ca2+ entry through TRPV1 channels was sufficient to evoke substance P release.
We found that NK1R internalization induced by capsaicin was not affected by the SFK inhibitor PP1. Two conclusions can be derived from this finding. First, it shows that, unlike NMDA receptors, TRPV1 channels are not regulated by phosphorylation by SFKs. Second, it indicates that SFK act on the NMDA receptors themselves and not on release mechanisms downstream from Ca2+ entry, i.e., those regulating the fusion of the dense-core vesicles with the plasma membrane.
Can SFK upregulation of NMDA receptors resolve contradictions between previous studies?
NMDA-induced substance P release was demonstrated in vivo by Liu et al. (Liu et al., 1997) and in spinal cord slices by us (Marvizon et al., 1997; Marvizon et al., 1999) and by Malcangio et al. (Malcangio et al., 1998). In contrast, Afrah et al. (Afrah et al., 2001) and Nazarian et al. (Nazarian et al., 2007), both working in vivo, could not detect NMDA-induced substance P release. Importantly, both Afrah et al. and Nazarian et al. measured substance P release in anesthetized rats, whereas Liu et al. did so in non-anesthetized rats. Spinal cord slices are routinely preincubated in aCSF long enough to eliminate the anesthetic. The present results suggest that anesthesia inhibits these NMDA receptors by causing the dephosphorylation of the NR2B subunit.
Physiological relevance of NMDA receptor-induced substance P release
Dorsal horn neurons expressing NK1Rs receive synapses from primary afferents and project directly to the brain (Todd et al., 2002; Todd et al., 2005). Therefore, changes in these neurons would be expected to have a major impact on the intensity of nociceptive signals sent to the brain. The excitability of these neurons is increased by the NK1Rs, which should result in hyperalgesia. Indeed, there is ample evidence that NK1Rs modulate pain in rodents: hyperalgesic responses are decreased by NK1R antagonists (Traub, 1996; Henry et al., 1999), by eliminating NK1R-expressing neurons with substance P-saporin (Mantyh et al., 1997) and in NK1R knockout mice (De Felipe et al., 1998; Laird et al., 2000; Laird et al., 2001).
It was originally suggested that NMDA receptors in primary afferent terminals act as autoreceptors. As such, they would amplify incoming signals in a positive feedback loop in which glutamate released by the primary afferents would induce Ca2+ entry into the terminal through the NMDA receptors, leading to more glutamate and substance P release. This idea was supported by the finding that NMDA receptor antagonists inhibit substance P release in spinal cord slices (Marvizon et al., 1997; Malcangio et al., 1998; Marvizon et al., 1999; Lao et al., 2003) and in vivo (Afrah et al., 2001; Nazarian et al., 2007). However, Bardoni et al. (Bardoni et al., 2004), using whole-cell recordings from dorsal horn neurons, reported that NMDA receptors actually inhibit glutamate release from primary afferents. This inhibition was attributed to axon depolarization by the NMDA receptors, which would block the propagation of action potentials to the terminal. Comparing the study by Bardoni et al. with our results is complicated by the fact that they used neonatal rats and recorded EPSCs in the presence of antagonists of GABAA and glycine receptors. A later study by Zeng et al. (Zeng et al., 2006), also using neonatal rats, found that morphine tolerance produced an increase of EPSCs in dorsal horn neurons that was eliminated by NMDA receptor antagonists. This is consistent with a facilitatory effect of NMDA receptors on neurotransmitter release from primary afferents.
NMDA receptors in primary afferents may be upregulated by SFK phosphorylation in hyperalgesic states. Indeed, the fact that the PTP inhibitor BVT948 considerably increased NMDA-induced substance P release indicates that NMDA receptors are mostly dephosphorylated and downregulated in the basal state. Further support for this idea is the observation by Li et al. (2006) that during colitis there is a generalized increase in NMDA receptor currents in primary afferents, which was suppressed by the SFK inhibitor PP2 and mimicked by the PTP inhibitor sodium vanadate.
In conclusion, NMDA receptors in primary afferent terminals may be “silent” in normal conditions and become upregulated during morphine tolerance and hyperalgesia. This upregulation possibly involves phosphorylation by SFKs and trafficking from extrasynaptic to synaptic sites.
Acknowledgements
Supported by grant B4766I from the Rehabilitation Research & Development Service, Department of Veteran Affairs to J.C.M.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- Ca(V)
voltage-dependent calcium channel
- DMSO
dimethyl-sulfoxide
- EC50
half of the effective concentration
- NK1R
neurokinin 1 receptor
- NMDA
N-methyl-D-aspartate
- PTP
protein tyrosine phosphatase
- SFK
Src family kinase
- sucrose-aCSF
artificial cerebrospinal fluid containing 215 mM sucrose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci. 1997;17:8049–8060. doi: 10.1523/JNEUROSCI.17-20-08049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, Nakazawa T, Yamamoto T, Mishina M, Nakai Y, Ito S. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci. 2005;22:1445–1454. doi: 10.1111/j.1460-9568.2005.04340.x. [DOI] [PubMed] [Google Scholar]
- Adelson DW, Lao L, Zhang G, Kim W, Marvizón JC. Substance P release and neurokinin 1 receptor activation in the rat spinal cord increases with the firing frequency of C-fibers. Neuroscience. 2009;161:538–553. doi: 10.1016/j.neuroscience.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrah AW, Stiller CO, Olgart L, Brodin E, Gustafsson H. Involvement of spinal N-methyl-D-aspartate receptors in capsaicin-induced in vivo release of substance P in the rat dorsal horn. Neurosci Lett. 2001;316:83–86. doi: 10.1016/s0304-3940(01)02380-1. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA. Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. J Neurophysiol. 1999;81:1379–1390. doi: 10.1152/jn.1999.81.3.1379. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Rogers SD, Ghilardi JR, Menning PM, Kuskowski MA, Basbaum AI, Simone DA, Mantyh PW. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. J Neurosci. 1997;17:5921–5927. doi: 10.1523/JNEUROSCI.17-15-05921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenet P, Leonardon J, Besnard F, Graham D, Frost J, Depoortere H, Langer SZ, Scatton B. Antagonist properties of the stereoisomers of ifenprodil at NR1A/NR2A and NR1A/NR2B subtypes of the NMDA receptor expressed in Xenopus oocytes. Eur J Pharmacol. 1996;296:209–213. doi: 10.1016/0014-2999(95)00700-8. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Torsney C, Tong CK, Prandini M, MacDermott AB. Presynaptic NMDA Receptors Modulate Glutamate Release from Primary Sensory Neurons in Rat Spinal Cord Dorsal Horn. J Neurosci. 2004;24:2774–2781. doi: 10.1523/JNEUROSCI.4637-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce S, Wyatt A, Webb JK, O'Donnell R, Mason G, Rigby M, Sirinathsinghji D, Hill RG, Rupniak NM. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999;38:611–623. doi: 10.1016/s0028-3908(98)00218-4. [DOI] [PubMed] [Google Scholar]
- Cannell MB, McMorland A, Soeller C. Image Enhancement by Deconvolution. In: Pawley JB, editor. Handbook of Biological Confocal Microscopy. Third Edition. New York, NY: Springer; 2006. pp. 488–500. [Google Scholar]
- Courtney KR, Strichartz GR, Strichartz GR. Structural elements which determine local anesthetic activity. In: Born GVR, Farah A, Herken H, Welch AD, editors. Local Anesthetics. New York: Springer-Verlag; 1987. pp. 53–94. [Google Scholar]
- De Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Gallagher MJ, Huang H, Pritchett DB, Lynch DR. Interactions between ifenprodil and the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1996;271:9603–9611. doi: 10.1074/jbc.271.16.9603. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Grady EF, Baluk P, Bohm S, Gamp PD, Wong H, Payan DG, Ansel J, Portbury AL, Furness JB, McDonald DM, Bunnett NW. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J Neurosci. 1996;16:6975–6986. doi: 10.1523/JNEUROSCI.16-21-06975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Hantschel O, Rix U, Schmidt U, Burckstummer T, Kneidinger M, Schutze G, Colinge J, Bennett KL, Ellmeier W, Valent P, Superti-Furga G. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci U S A. 2007;104:13283–13288. doi: 10.1073/pnas.0702654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JL, Yashpal K, Pitcher GM, Chabot J, Coderre TJ. Evidence for tonic activation of NK-1 receptors during the second phase of the formalin test in the rat. J Neurosci. 1999;19:6588–6598. doi: 10.1523/JNEUROSCI.19-15-06588.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes TJ, Biggs D, Abu-Tarif A. Blind Deconvolution. In: Pawley JB, editor. Handbook of Biological Confocal Microscopy. Third Edition. New York, NY: Springer; 2006. pp. 468–487. [Google Scholar]
- Honore P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal cord substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J Neurosci. 1999;19:7670–7678. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Gingrich JR, Salter MW. Src in synaptic transmission and plasticity. Oncogene. 2004;23:8007–8016. doi: 10.1038/sj.onc.1208158. [DOI] [PubMed] [Google Scholar]
- Kato H, Narita M, Miyoshi K, Asato M, Hareyama N, Nozaki H, Takagi T, Suzuki M, Suzuki T. Implication of Src family kinase-dependent phosphorylation of NR2B subunit-containing NMDA receptor in the rewarding effect of morphine. Nihon Shinkei Seishin Yakurigaku Zasshi. 2006;26:119–124. [PubMed] [Google Scholar]
- Laird JM, Roza C, De Felipe C, Hunt SP, Cervero F. Role of central and peripheral tachykinin NK1 receptors in capsaicin-induced pain and hyperalgesia in mice. Pain. 2001;90:97–103. doi: 10.1016/s0304-3959(00)00394-8. [DOI] [PubMed] [Google Scholar]
- Laird JM, Olivar T, Roza C, De Felipe C, Hunt SP, Cervero F. Deficits in visceral pain and hyperalgesia of mice with a disruption of the tachykinin NK1 receptor gene. Neuroscience. 2000;98:345–352. doi: 10.1016/s0306-4522(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Lao L, Marvizon JCG. GABAA receptor facilitation of neurokinin release from primary afferent terminals in the rat spinal cord. Neuroscience. 2005;130:1013–1027. doi: 10.1016/j.neuroscience.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Lao L, Song B, Marvizon JCG. Neurokinin release produced by capsaicin acting on the central terminals and axons of primary afferents: relationship with NMDA and GABAB receptors. Neuroscience. 2003;121:667–680. doi: 10.1016/s0306-4522(03)00501-3. [DOI] [PubMed] [Google Scholar]
- Li J, McRoberts JA, Nie J, Ennes HS, Mayer EA. Electrophysiological characterization of N-methyl-d-aspartate receptors in rat dorsal root ganglia neurons. Pain. 2004;109:443–452. doi: 10.1016/j.pain.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Li J, McRoberts JA, Ennes HS, Trevisani M, Nicoletti P, Mittal Y, Mayer EA. Experimental colitis modulates the functional properties of NMDA receptors in dorsal root ganglia neurons. Am J Physiol Gastrointest Liver Physiol. 2006;291:G219–G228. doi: 10.1152/ajpgi.00097.2006. [DOI] [PubMed] [Google Scholar]
- Liljebris C, Baranczewski P, Bjorkstrand E, Bystrom S, Lundgren B, Tjernberg A, Warolen M, James SR. Oxidation of protein tyrosine phosphatases as a pharmaceutical mechanism of action: a study using 4-hydroxy-3,3-dimethyl-2H-benzo[g]indole-2,5(3H)-dione. J Pharmacol Exp Ther. 2004;309:711–719. doi: 10.1124/jpet.103.062745. [DOI] [PubMed] [Google Scholar]
- Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Sheng M, Jan LY, Jan YN, Basbaum AI. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proc Natl Acad Sci U S A. 1994;91:8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bishop A, Witucki L, Kraybill B, Shimizu E, Tsien J, Ubersax J, Blethrow J, Morgan DO, Shokat KM. Structural basis for selective inhibition of Src family kinases by PP1. Chem Biol. 1999;6:671–678. doi: 10.1016/s1074-5521(99)80118-5. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Weight FF. Glutamate induces a depolarization of adult rat dorsal root ganglion neurons that is mediated predominantly by NMDA receptors. Neurosci Lett. 1988;94:314–320. doi: 10.1016/0304-3940(88)90037-7. [DOI] [PubMed] [Google Scholar]
- Lu CR, Hwang SJ, Phend KD, Rustioni A, Valtschanoff JG. Primary afferent terminals that express presynaptic NR1 in rats are mainly from myelinated, mechanosensitive fibers. J Comp Neurol. 2003;460:191–202. doi: 10.1002/cne.10632. [DOI] [PubMed] [Google Scholar]
- Ma QP, Hargreaves RJ. Localization of N-methyl-D-aspartate NR2B subunits on primary sensory neurons that give rise to small-caliber sciatic nerve fibers in rats. Neuroscience. 2000;101:699–707. doi: 10.1016/s0306-4522(00)00419-x. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Fernandes K, Tomlinson DR. NMDA receptor activation modulates evoked release of substance P from rat spinal cord. Br J Pharmacol. 1998;125:1625–1626. doi: 10.1038/sj.bjp.0702260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Martinez V, Grady EF, Bunnett NW, Mayer EA. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J Neurosci. 1997;17:8129–8136. doi: 10.1523/JNEUROSCI.17-21-08129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Grady EF, Stefani E, Bunnett NW, Mayer EA. Substance P release in the dorsal horn assessed by receptor internalization: NMDA receptors counteract a tonic inhibition by GABA(B) receptors. Eur J Neurosci. 1999;11:417–426. doi: 10.1046/j.1460-9568.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Wang X, Matsuka Y, Neubert JK, Spigelman I. Relationship between capsaicinevoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience. 2003a;118:535–545. doi: 10.1016/s0306-4522(02)00977-6. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, Corneliussen B, Mayer EA. Two N-methyl-D-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446:325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- Marvizon JCG, Wang X, Lao L, Song B. Effect of peptidases on the ability of exogenous and endogenous neurokinins to produce neurokinin 1 receptor internalization in the rat spinal cord. Br J Pharmacol. 2003b;140:1389–1398. doi: 10.1038/sj.bjp.0705578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough SI, Swartz KJ, Mintz IM, Boland LM, Bean BP. Inhibition of calcium channels in rat central and peripheral neurons by omega-conotoxin MVIIC. J Neurosci. 1996;16:2612–2623. doi: 10.1523/JNEUROSCI.16-08-02612.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRoberts JA, Li J, Ennes HS, Mayer EA. Sex-dependent differences in the activity and modulation of N-methyl-d-aspartic acid receptors in rat dorsal root ganglia neurons. Neuroscience. 2007;148:1015–1020. doi: 10.1016/j.neuroscience.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- Nagy GG, Watanabe M, Fukaya M, Todd AJ. Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur J Neurosci. 2004;20:3301–3312. doi: 10.1111/j.1460-9568.2004.03798.x. [DOI] [PubMed] [Google Scholar]
- Nazarian A, Gu G, Gracias NG, Wilkinson K, Hua XY, Vasko MR, Yaksh TL. Spinal NMDA receptors and nociception-evoked release of primary afferent substance P. Neuroscience. 2007;152:119–127. doi: 10.1016/j.neuroscience.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingo J, Castiglioni AJ, Lipscombe D. Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat Neurosci. 2007;10:285–292. doi: 10.1038/nn1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RC, Trafton JA, Chi SI, Basbaum AI. Presynaptic regulation of spinal cord tachykinin signaling via GABA(B) but not GABA(A) receptor activation. Neuroscience. 2001;103:725–737. doi: 10.1016/s0306-4522(00)00571-6. [DOI] [PubMed] [Google Scholar]
- Rusin KI, Moises HC. µ-Opioid receptor activation reduces multiple components of highthreshold calcium current in rat sensory neurons. J Neurosci. 1995;15:4315–4327. doi: 10.1523/JNEUROSCI.15-06-04315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusin KI, Moises HC. Mu-opioid and GABAB receptors modulate different types of Ca2+ currents in rat nodose ganglion neurons. Neuroscience. 1998;85:939–956. doi: 10.1016/s0306-4522(97)00674-x. [DOI] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Tae Park H, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport. 1993;4:1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- Sato Y, Tao YX, Su Q, Johns RA. Post-synaptic density-93 mediates tyrosine-phosphorylation of the N-methyl-d-aspartate receptors. Neuroscience. 2008;153:700–708. doi: 10.1016/j.neuroscience.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittenhelm MM, Shiraga S, Schroeder A, Corbin AS, Griffith D, Lee FY, Bokemeyer C, Deininger MW, Druker BJ, Heinrich MC. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–481. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- Song B, Marvizon JCG. Dorsal horn neurons firing at high frequency, but not primary afferents, release opioid peptides that produce µ-opioid receptor internalization in the rat spinal cord. J Neurosci. 2003;23:9171–9184. doi: 10.1523/JNEUROSCI.23-27-09171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Marvizon JCG. NMDA receptors and large conductance calcium-sensitive potassium channels inhibit the release of opioid peptides that induce µ-opioid receptor internalization in the rat spinal cord. Neuroscience. 2005;136:549–562. doi: 10.1016/j.neuroscience.2005.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, Spike RC, Young S, Puskar Z. Fos induction in lamina I projection neurons in response to noxious thermal stimuli. Neuroscience. 2005;131:209–217. doi: 10.1016/j.neuroscience.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Puskar Z, Spike RC, Hughes C, Watt C, Forrest L. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance p-containing afferents and respond to noxious stimulation. J Neurosci. 2002;22:4103–4113. doi: 10.1523/JNEUROSCI.22-10-04103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Basbaum AI. Differential contribution of substance P and neurokinin A to spinal cord neurokinin-1 receptor signaling in the rat. J Neurosci. 2001;21:3656–3664. doi: 10.1523/JNEUROSCI.21-10-03656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marchand S, Mantyh PW, Basbaum AI. Spinal opioid analgesia: how critical is the regulation of substance P signaling? J Neurosci. 1999;19:9642–9653. doi: 10.1523/JNEUROSCI.19-21-09642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RJ. The spinal contribution of substance P to the generation and maintenance of inflammatory hyperalgesia in the rat. Pain. 1996;67:151–161. doi: 10.1016/0304-3959(96)03076-X. [DOI] [PubMed] [Google Scholar]
- Wallace W, Schaefer LH, Swedlow JR. A workingperson's guide to deconvolution in light microscopy. BioTechniques. 2001;31:1076–1078. doi: 10.2144/01315bi01. [DOI] [PubMed] [Google Scholar]
- Wang X, Marvizon JC. Time-course of the internalization and recycling of neurokinin 1 receptors in rat dorsal horn neurons. Brain Res. 2002;944:239–247. doi: 10.1016/s0006-8993(02)02918-9. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Xu J, Weerapura M, Ali MK, Jackson MF, Li H, Lei G, Xue S, Kwan CL, Manolson MF, Yang K, Macdonald JF, Yu XM. Control of Excitatory Synaptic Transmission by C-terminal Src Kinase. J Biol Chem. 2008;283:17503–17514. doi: 10.1074/jbc.M800917200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Shimoji K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol. 1999;59:279–298. doi: 10.1016/s0301-0082(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Yu XM, Salter MW. Src, a molecular switch governing gain control of synaptic transmission mediated by N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA. 1999;96:7697–7704. doi: 10.1073/pnas.96.14.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Thomson LM, Aicher SA, Terman GW. Primary afferent NMDA receptors increase dorsal horn excitation and mediate opiate tolerance in neonatal rats. J Neurosci. 2006;26:12033–12042. doi: 10.1523/JNEUROSCI.2530-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Edelmann L, Liu J, Crandall JE, Morabito MA. Cdk5 regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors. J Neurosci. 2008;28:415–424. doi: 10.1523/JNEUROSCI.1900-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Shi CX, Gu XP, Ma ZL, Zhu W. Ifenprodil induced antinociception and decreased the expression of NR2B subunits in the dorsal horn after chronic dorsal root ganglia compression in rats. Anesth Analg. 2009;108:1015–1020. doi: 10.1213/ane.0b013e318193ffd2. [DOI] [PubMed] [Google Scholar]