Abstract
Estrogen receptor-alpha (ERα) is a major therapeutic target of hormonal therapies in breast cancer and its expression in tumors is predictive of clinical response. Protein levels of ERα are tightly controlled by the 26S proteasome, yet how the clinical proteasome inhibitor, bortezomib, impacts ERα regulation has not been studied. Bortezomib selectively inhibits the chymotrypsin-like activity of the proteasome. Unlike other laboratory proteasome inhibitors, bortezomib failed to stabilize ERα protein at a dose exceeding 90% inhibition of the chymotrypsin-like activity. Unexpectedly, however, chronic bortezomib exposure caused a reduction of ERα levels in multiple ER+ breast cancer cell lines. This response can be explained by the fact that bortezomib induced a dramatic decrease in ERα mRNA due to direct transcriptional inhibition and loss of RNA polymerase II recruitment on the ERα gene promoter. Bortezomib treatment resulted in promoter-specific changes in estrogen-induced gene transcription that related to occupancy of ERα and RNA PolII on endogenous promoters. In addition, bortezomib inhibited estrogen-dependent growth in soft agar. These results reveal a novel link between proteasome activity and expression of ERα in breast cancer and uncover distinct roles of the chymotrypsin-like activity of the proteasome in the regulation of the ERα pathway.
Keywords: nuclear receptor, proteolysis, hormone-dependent cancer, bortezomib, transcription
Introduction
The efficacy of any cancer therapy is dependent on the expression of the molecular targets of those therapies in tumors. One of the most important molecular markers guiding therapy decisions in breast cancer is estrogen receptor-alpha (ERα), a nuclear receptor which mediates the proliferative actions of estrogen (DeNardo, et al., 2007; O'Donnell, et al., 2005). ERα is expressed in approximately 70% of all breast cancers and is increased in both premalignant and malignant lesions (Clarke, et al., 1997; Fabris, et al., 1987; Shaaban, et al., 2002). It is the major target of current hormonal therapies, and, thus, determination of ERα expression by immunohistochemistry is common in clinical practice to gauge the differentiation state of the tumor and to predict response to therapies such as aromatase inhibitors, tamoxifen and fulvestrant (Robertson, et al., 2009; Viale, et al., 2007). Evidence also suggests that expression of ERα in tumors is dynamic and can change during the course of tumor progression and following therapy (Liedtke, et al., 2009; Nomura, et al., 1985).
A major regulatory pathway governing ERα protein expression is the ubiquitin-proteasome pathway. This pathway controls the stability of ERα protein through the covalent attachment of ubiquitin moieties to the receptor which then targets it to the 26S proteasome for degradation. Multiple signals, including estrogen binding, result in increased ubiquitination of ERα and its subsequent proteolysis (Alarid, et al., 1999; El Khissiin and Leclercq, 1999; Nawaz, et al., 1999; Wijayaratne and McDonnell, 2001). The loss of this response is associated with increased risk of breast cancer and hormone-insensitivity associated with metastasis (Harrell, et al., 2007; Khan, et al., 1999).
The 26S proteasome is a multi-subunit protease that functions as the major regulator of all short-lived proteins in cells (Rock, et al., 1994). It is comprised of two 19S regulatory complexes and a 20S core complex. The 20S core constitutes the major proteolytic activity of the proteasome, and is comprised of α and β subunits, which are organized into 4 rings of 7 subunits each. The β subunits (β1, β2, and β5) possess distinct protease activities based upon the cleavage site in the protein substrate (Orlowski and Wilk, 1981). The β1 and β2 subunits possess the PGH-like and trypsin-like activities, respectively. The β5 subunit contains the chymotrypsin-like activity, which is considered the dominant protease activity (Heinemeyer, et al., 1997).
Although the 26S proteasome is a critical regulator in all cells, cancer cells may be more dependent on proteasome activity. Proteasome subunits are up-regulated in gastric, breast, and ovarian cancers as well as leukemia (Bazzaro, et al., 2006; Bossola, et al., 2003; Chen and Madura, 2005; Kumatori, et al., 1990). In breast cancer, a “proteasome signature” was identified that is predictive of poor outcome (Wong, et al., 2008). In addition to changes in proteasome gene expression, the activity of the 26S proteasome was shown to be increased in primary breast tumors relative to normal adjacent tissue (Chen and Madura, 2005) and in invasive breast cancer cell lines relative to non-tumorigenic MCF10 cells (Xu, et al., 2008). Cumulatively, this evidence has suggested the possible benefit of inhibitors of 26S proteasome as anti-cancer agents.
In an effort to develop clinical proteasome inhibitors, drugs have been developed that target individual protease activities of the proteasome. Common proteasome inhibitors, such as MG132, target all three catalytic activities (Taggart, et al., 2002). Bortezomib is an FDA approved proteasome inhibitor that selectively inhibits the chymotrypsin-like activity (Kisselev, et al., 2006). It is currently in clinical use and shows efficacy in the treatment of multiple myeloma and mantle cell lymphoma as a single agent and in combination with other drugs for both refractory and relapsed disease (Richardson, et al., 2006). Given the critical role of the 26S proteasome in ERα protein regulation and the importance of ERα in therapy decisions for the treatment of breast cancer, we sought to examine the effects of bortezomib on ERα regulation and function in hormone-responsive tumor cells.
Results
The chymotrypsin-like activity of the 26S proteasome is not required for ligand-inducible degradation of ERα protein
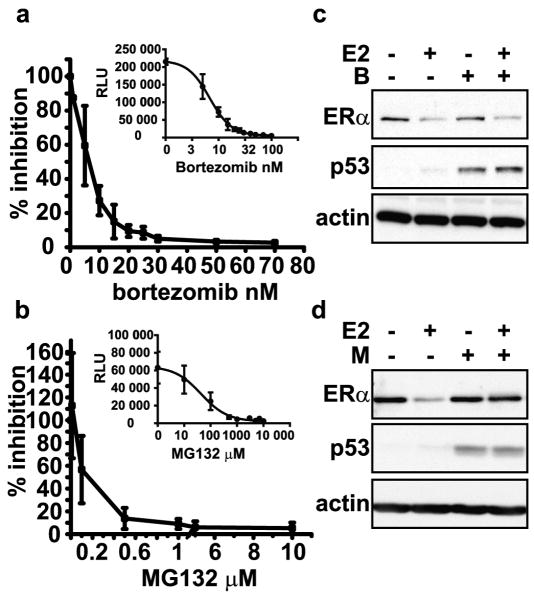
Dose-response curves were conducted initially to establish the doses of bortezomib that are necessary to inhibit the chymotrypsin-like activity in a panel of cells derived from estrogen-responsive tissues. The cell lines tested include representative lines of the major classes of breast cancer; luminal A (MCF7, T47D), luminal B (BT474), and triple negative (MDA-MB-231). Additional ER+ cells derived from endometrial cancer (ECC1) and pituitary hyperplasia (PR1) were also included. Cells were treated with bortezomib ranging from 0 to 70 nM for 4 hours and chymotrypsin-like activity was determined by luminescent enzyme assay that reports cleavage of a luminescent Suc-LLVY peptide. Table 1 shows that bortezomib inhibits 50% of the chymotrypsin-like activity at doses below 10 nanomolar in the cell lines tested. Comparison of the inhibitory activity of bortezomib and MG132 in MCF7 cells shows that inhibition of 95% of the chymotrypsin-like activity is achieved with 30 nM bortezomib (Figure 1a), while 10 μM MG132 is necessary to reach equivalent levels of inhibition (Figure 1b). These data show that bortezomib is more efficacious than MG132 and are consistent with the increased selectivity of bortezomib against the chymotrypsin-like activity of the proteasome relative to general proteasome inhibitors.
Table 1. Bortezomib inhibits the chymotrypsin-like activity of the proteasome in multiple cell lines.
Cells were incubated in the presence of increasing concentrations of bortezomib for 4 hours. Luminescent signal resulting from the cleavage of a luminescent Suc-LLVY peptide by the chymotrypsin-like activity of the proteasome was measured. IC50 values were calculated with a nonlinear regression analysis using the relative luminescent units (RLU) and the log of inhibitor concentration. Data are representative of a minimum of two experiments performed in duplicate.
| Cell Line | IC50 (nM) |
|---|---|
| BT474 | 4.1 |
| ECC1 | 1.8 |
| MCF7 | 6.8 |
| MDA-MB-231 | 6.3 |
| PR-1 | 1.4 |
| T47D | 2.9 |
Figure 1. Bortezomib and MG132 inhibit the chymotrypsin-like activity of the proteasome in MCF7 cells, but have different effects on estrogen-induced ERα proteolysis.
a & b) Chymotrypsin-like activity was measured as in Table 1. Data are representative of three independent experiments performed in duplicate, and are graphed as percent proteasome inhibition vs. concentration of bortezomib or MG132. Insets present the data as the relative luminescent units vs. inhibitor concentration. Representative Western blots from whole cells lysates of MCF7 cells pre-treated for 30 minutes with c) 30 nM bortezomib (B) or d) 10 μM MG132 (M) and then treated with 10 nM 17-β-estradiol (E2) or ethanol vehicle (-) for 4 hours. Blots were probed with antibodies for ERα, actin, as a loading control, and p53, as a control for proteasome inhibition.
We, and others, previously showed that ERα protein is rapidly degraded in response to 17β-estradiol (E2) treatment via a proteasome-dependent pathway. Proteasome inhibitors, including ALLnL, MG132, and lactacystin, were shown to abrogate ligand-induced receptor degradation in a dose-dependent manner. (Alarid, et al., 1999; El Khissiin and Leclercq, 1999; Nawaz, et al., 1999; Reid, et al., 2003). To establish the role of the chymotrypsin-like activity in estrogen-induced regulation of ERα protein, MCF7 cells were pretreated for 30 minutes with 30 nM bortezomib or 10 μM MG132, followed by treatment with 10 nM E2 or vehicle for four hours. In agreement with previous reports, estrogen treatment resulted in a loss of ERα protein that was prevented by MG132 (Figure 1d). Surprisingly, bortezomib treatment was without effect on estrogen-treated cells (Figure 1c). p53 protein levels were increased in the presence of MG132 and bortezomib, indicating that both inhibitors were active and prevented the constitutive degradation of p53 (Maki, et al., 1996). Similar results were observed in other estrogen-responsive cells, T47D, ECC1, and PR1 (Supplemental Figure 1 a-c).
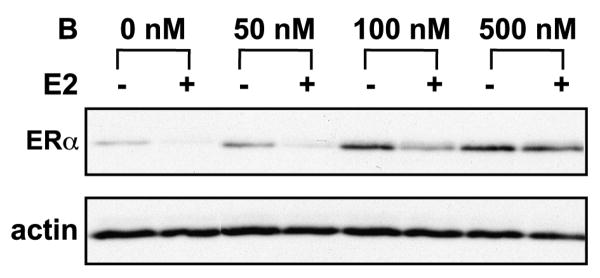
Dose response studies were then extended to determine if higher doses of bortezomib were required to inhibit ERα proteolysis. MCF7 cells were treated with bortezomib ranging from 0-500 nM in the presence or absence of E2 as above. Figure 2 shows that bortezomib partially prevents estrogen-induced proteolysis at 100 nM but complete inhibition is achieved at 500 nM bortezomib. At higher doses, the inhibitory activity of bortezomib expands beyond the chymotrypsin-like site. Indeed, 500 nM bortezomib was shown to also inhibit the caspase-like and trypsin-like activities (Kisselev, et al., 2006). Thus, it is unlikely that the stabilization of ERα protein at high concentrations of bortezomib is due to blockade of chymotrypsin-like activity alone. Rather, a more plausible explanation is that stabilization of ERα protein requires multiple enzymatic activities of the proteasome, and that the inhibition of the chymotrypsin-like activity is insufficient to block the rapid degradation of receptor induced upon ligand binding.
Figure 2. Dose response curve of bortezomib inhibition of ERα proteolysis.
MCF7 cells were pre-treated for 30 minutes with 50-500 nM bortezomib (B) and then treated for 4 hours with 10 nM E2 (+) or ethanol (-). Western blots were performed on whole cell lysates and probed with antibodies for ERα and actin.
Chronic chymotrypsin-like inhibition results in the loss of ERα in ER+ cells
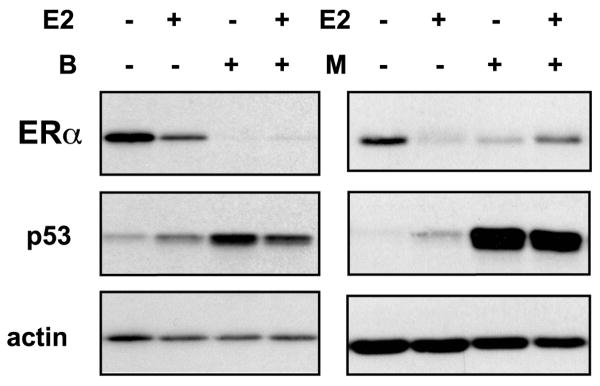
Proteasome-dependent regulation of ERα is a time-dependent process with observable differences between short and chronic treatments with estrogen (Valley, et al., 2008). Bortezomib is administered chronically, as patients are treated with bortezomib every 3 days and proteasome inhibition in the blood persists 24 hours after treatment (Shah, et al., 2004). The impact of long-term exposure to bortezomib was thus examined. MCF7 cells were treated with 30 nM bortezomib for 24 hours in the presence and absence of estrogen. Surprisingly, by 24 hours, ERα protein was dramatically diminished in both the presence of bortezomib and MG132 (Figure 3) relative to vehicle controls. Estrogen treatment similarly decreased ERα levels and this loss could be partially stabilized by MG132, but not by bortezomib. p53 levels were increased with both inhibitors and were induced by estrogen, consistent with results shown above and previous published reports, respectively (Qin, et al., 2002). Western blot analysis of ERα levels in other ER+ cells under identical conditions showed that ERα protein loss induced by bortezomib was a generalized response (Supplemental Figure 1d-g).
Figure 3. Chronic chymotrypsin-like inhibition of the proteasome results in the depletion of ERα protein.
Western blot analysis was performed on whole cell lysates from MCF7 cells pre-treated for 30 minutes with 30 nM bortezomib (B) or 10 μM MG132 (M) followed by vehicle (-) or 10 nM E2 treatment for 24 hours. Blots were probed with antibodies for ERα, actin, and p53.
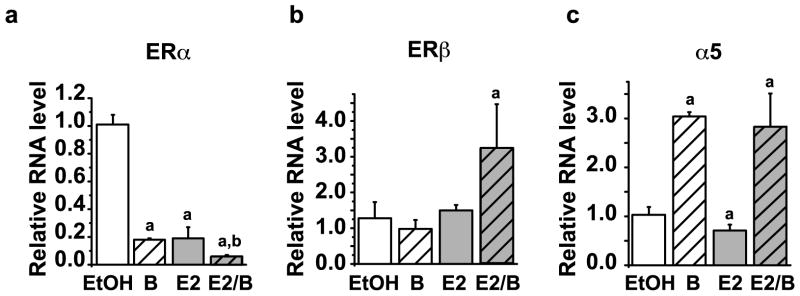
ERα protein levels under conditions of constant exposure to estrogen are largely controlled at the level of receptor synthesis (Valley, et al., 2008). ERα mRNA levels were therefore examined following bortezomib treatment. Evaluation of ERα mRNA levels by quantitative real-time PCR (qRT-PCR) showed that treatment with bortezomib caused a decrease in ERα mRNA to 18% ± 0.08 of control levels. E2 also reduced ERα mRNA and combined treatment with bortezomib and E2 results in an approximate 95% ± 0.01 decrease in ERα mRNA expression relative to vehicle-treated controls (Figure 4a). ERβ mRNA levels were not significantly altered by either E2 or bortezomib alone, but in combination, estrogen and bortezomib increased ERβ mRNA (Figure 4b). Bortezomib also increased expression of the α5 subunit of the proteasome (Figure 4c) providing further evidence that the effect of bortezomib on ERα gene expression was specific and not due to general inhibition of transcription.
Figure 4. ERα mRNA expression requires the chymotrypsin-like activity of the proteasome.
MCF7 cells were treated for 24 hours with 30 nM Bortezomib (B), 10 nM E2 or in combination (E2/B). RNA was isolated, reverse transcribed, and qRT-PCR was performed to assess relative levels of a) total ERα mRNA, b) ERβ or, c) proteasome subunit PSMA5 (α5). Values were normalized relative to P0 gene and fold changes were calculated relative to the average of a minimum of 3 independent vehicle ethanol (EtOH) treated samples. Error bars represent standard error of the mean. Statistically significant differences (p<0.05) compared with EtOH control and bortezomib are marked a and b, respectively.
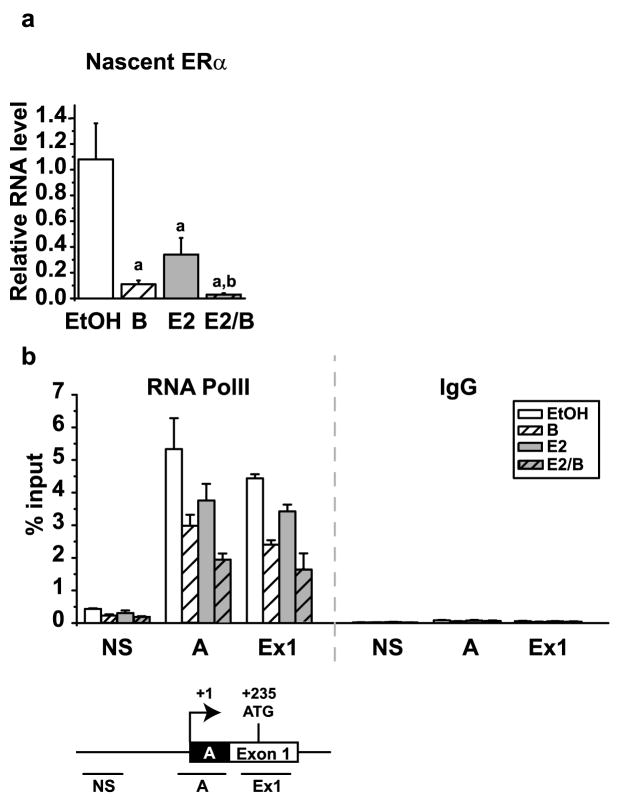
To probe further into the mechanism of bortezomib-induced decrease in ERα, levels of nascent unspliced ERα mRNA were evaluated by qRT-PCR. Similar to total ERα mRNA, the nascent transcript was decreased approximately 90% ± 0.03 by bortezomib alone and was further reduced by an additional 7% ± 0.01 by E2. In total, ERα transcription was reduced to 3% of the levels in control cells by bortezomib in the presence of estrogen (Figure 5a). Chromatin immunoprecipitation (ChIP) was performed to examine RNA polymerase II (RNA PolII) occupancy as an independent measure of transcription. Previous studies in our laboratory and those of others demonstrated that the major promoter governing ERα gene expression in MCF7 breast cancer cells is the A promoter located near the transcription start site (Denger, et al., 2001; Ellison-Zelski, et al., 2009). Studies in Figure 5b show that RNA PolII is present on the A promoter and Exon 1, with negligible occupancy at a non-specific site at -940 basepairs upstream of the transcription start site. These results are consistent with the active transcription of the ERα gene in MCF7 cells. Paralleling the loss of nascent transcript, bortezomib and estrogen treatments both resulted in a decrease in RNA PolII occupancy at both the A promoter and Exon 1. These data indicate that bortezomib regulates ERα directly at the transcriptional level by reducing RNA PolII occupancy at the proximal promoter.
Figure 5. Bortezomib results in a decrease in the nascent ERα mRNA transcript by decreasing RNA PolII on the ERα promoter.
MCF7 cells were treated for 30 minutes with 30 nM bortezomib and then for 24 hours with 10 nM E2. a) RNA isolation and qRT-PCR were performed as described in Figure 4 for the nascent ERα transcript. The data were analyzed relative to the average EtOH treatment and the mean of three independent experiments is shown. Statistics were performed to demonstrate significant differences (p< 0.05) between vehicle control and estrogen and are marked a and b, respectively. b) Chromatin immunoprecipitation (ChIP) was performed using antibodies for RNA PolII and IgG. Primers for non-specific (NS), the A promoter (A) and exon 1 (Ex1) regions of the ERα promoter were used with qRT-PCR to examine the occupancy at each site. Data are presented as the mean percent input of three independent experiments. Error bars represent the standard error of the mean.
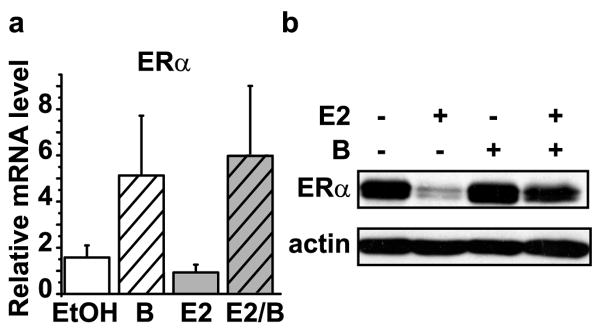
The data suggest that bortezomib regulation of ERα gene transcription is dependent on the endogenous ERα promoter. To test this further, C4-12 breast cancer cells, an ER- derivative of the MCF7 cells, were engineered to express wild-type ERα (wt-ER) under the control of a cytomegalovirus (CMV) promoter. Quantitative RT-PCR for ERα mRNA driven by a heterologous promoter showed that, in contrast to the endogenous gene, CMV-driven ERα mRNA levels were increased by bortezomib (Figure 6a). The lack of effect by estrogen is expected since estrogen regulation of ERα requires the native chromatin environment (Ellison-Zelski, et al., 2009). Analysis of wt-ER protein levels showed that chronic bortezomib also did not deplete ERα protein under these conditions (Figure 6b). These data reveal that bortezomib inhibits transcription of ERα mRNA through mechanisms involving the endogenous regulatory region. Moreover, they suggest that the loss of ERα protein observed in cell lines upon chronic bortezomib exposure can be explained by the inhibition of ERα mRNA transcription.
Figure 6. Transcriptional repression of the ERα gene is responsible for the decrease in ERα.
Stable cell lines in C4-12, an ER- derivative of MCF7 cells, expressing wild type ERα under the control of the CMV promoter (CMV-ER) were treated as in Figure 4a. a) Quantitative RT-PCR was performed, as described above, using primers for total ERα. b) Representative Western blot of CMV-ERα cells treated for 24 hours was probed with antibodies to assess ERα and actin expression.
The loss of ERα protein with chronic proteasome inhibition alters ERα functional activity
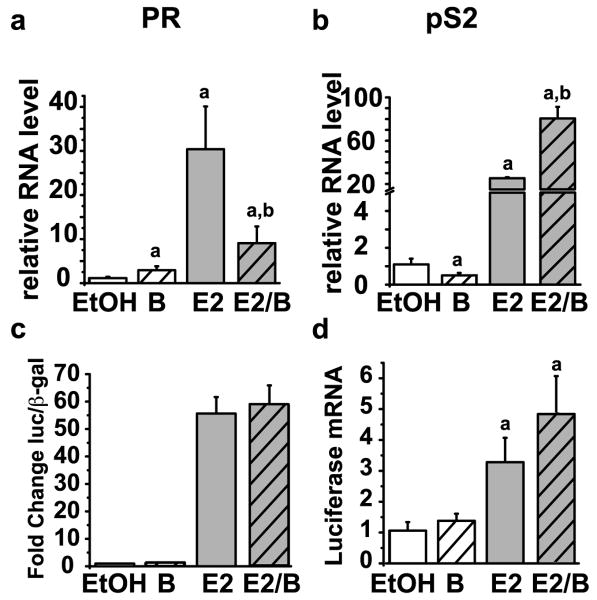
Next, the impact of bortezomib on ERα functional activity was assessed. The transcriptional activity of ERα was evaluated by analysis of endogenous progesterone receptor (PR) and pS2 gene expression. PR and pS2 are classical estrogen-responsive genes that are dependent on ERα for expression and are induced by estrogen (Feng, et al., 2007; Metivier, et al., 2003; Valley, et al., 2005). Chronic bortezomib treatment had opposite effects on these two estrogen receptor target genes. While bortezomib treatment resulted in a significant inhibition of estrogen-dependent activation of PR, it resulted in an enhancement of estrogen-dependent pS2 gene transcription (Figure 7a-b). In the absence of estrogen, bortezomib decreased pS2 and increased PR gene expression. An artificial reporter gene consisting of tandem estrogen response elements and a minimal thymidine kinase promoter was also analyzed to directly test the impact of bortezomib on ERα-dependent transcription. Luciferase protein and gene expression were significantly increased by estrogen treatment and this was unaffected by co-treatment with bortezomib (Figure 7c-d). Bortezomib also did not affect basal luciferase levels indicating that the thymidine kinase promoter was not sensitive to proteasome inhibition. These data suggest that bortezomib alters ERα-dependent gene transcription in a promoter-specific manner.
Figure 7. Proteasome inhibition results in gene specific effects on ERα target gene transcription.
MCF7 cells were treated for 30 minutes with or without 30 nM bortezomib and then treated for 24 hours with or without 10 nM E2. RNA isolation and qRT-PCR were performed as previously described using primers for a) PR or b) pS2. Data represent the means of a minimum of three independent experiments and error bars represent the standard error of the mean. Statistically significant differences (p< 0.05) relative to vehicle control (EtOH) or estrogen and are indicated with a or b, respectively. MCF7 cells were transfected with a 3× ERE-tk luciferase reporter plasmid and CMV-β-galactosidase (β-gal) plasmid. The next day the cells were treated for 24 hours with or without 30 nM bortezomib and 10 nM E2. c) Luciferase and β-galactosidase assays were performed on whole cell lysates. d) RNA isolation and qPCR was performed as previously described for luciferase mRNA. Data are presented relative to the EtOH control and error bars were calculated from the standard error of the mean of three independent experiments.
ChIP analysis was undertaken to determine whether the differences observed between PR and pS2 were related to the level of ERα occupancy on the promoters. In Table 2, the occupancy of RNA PolII and ERα on the pS2 and PR promoters can be compared with the changes in expression levels in each treatment group. RNA PolII occupancy on the two promoters follows the transcription of the genes. Occupancy of RNA PolII on both PR and pS2 promoters increased in response to estrogen. RNA PolIl remains elevated on the pS2 promoter in the presence of bortezomib, but is decreased on the PR promoter. This is consistent with high levels of expression of pS2 and loss of expression of PR in the presence of bortezomib. Similarly, ERα occupancy increased on both promoters in response to estrogen. Most striking, however, is the ERα occupancy on the two promoters in the presence of bortezomib. Despite a severe reduction in ERα protein levels in the presence of bortezomib (Figure 3), ERα occupancy on the pS2 gene is unaffected, whereas ERα occupancy on PR is decreased. These results demonstrate that the promoter-specific actions of bortezomib on ERα-mediated gene transcription relate to ERα occupancy on the promoters and the sensitivity of individual target genes to changes in ERα levels.
Table 2. The recruitment of RNA PolII and ERα on the pS2 and PR promoters in the presence of estrogen and bortezomib corresponds to the transcriptional response.
MCF7 cells were treated for 30 minutes with 30 nM bortezomib followed by 10 nM E2 for 24 hours. Cells were fixed, lysed, sonicated, and immunoprecipitated with antibodies for RNA polymerase II (RNA PolII), ERα, and IgG as a specificity control. Data are presented as the mean percent input +/- the standard error of the mean of three independent experiments.
| RNA level | RNA PolII | ERα | Ig G | ||
|---|---|---|---|---|---|
| Gene | Treatment | fold change | Promoter occupancy (relative ChIP value [% input]) | ||
| PR | |||||
| EtOH | 1.15 +/- 0.29 | 0.17 +/- 0.02 | 0.11 +/- 0.01 | 0.04 +/- 0.00 | |
| B | 2.93 +/-0.90 | 0.17 +/- 0.04 | 0.06 +/- 0.01 | 0.03 +/- 0.01 | |
| E2 | 30.40 +/- 9.70 | 1.41 +/- 0.15 | 0.18 +/- 0.02 | 0.04 +/- 0.01 | |
| E2/B | 9.08 +/- 3.78 | 0.32 +/- 0.06 | 0.06 +/- 0.01 | 0.04 +/- 0.01 | |
| pS2 | |||||
| EtOH | 1.10 +/- 0.31 | 0.40 +/- 0.04 | 0.94 +/- 0.19 | 0.03 +/- 0.00 | |
| B | 0.50 +/- 0.14 | 0.27 +/- 0.09 | 0.77 +/- 0.57 | 0.03 +/- 0.00 | |
| E2 | 25.37 +/- 0.91 | 0.94 +/- 0.09 | 3.97 +/- 0.41 | 0.04 +/- 0.01 | |
| E2/B | 80.54 +/- 10.76 | 0.68 +/- 0.17 | 3.09 +/-1.34 | 0.03 +/- 0.01 | |
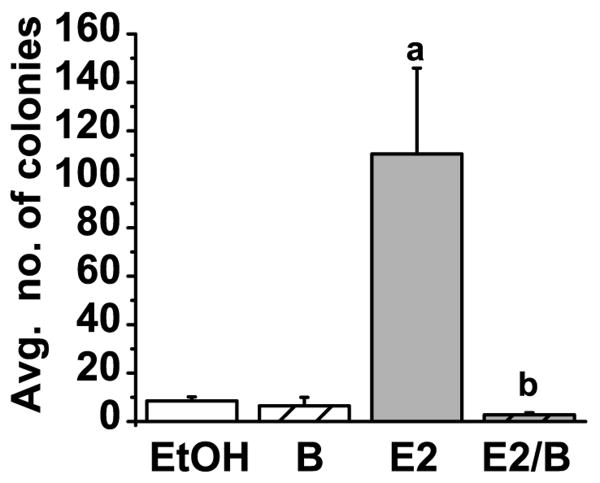
ERα is a major regulator of growth in estrogen-dependent tumor cells. Inhibitors of ERα activity are effective therapies in breast cancer and inhibit proliferation both in vitro and in vivo (Wakeling, et al., 1991). In particular, fulvestrant is a potent anti-estrogen therapeutic that depletes cells of receptor by targeting ERα protein for degradation (Dauvois, et al., 1993). Given the loss of ERα protein with bortezomib, it could be predicted that bortezomib would likewise inhibit estrogen-induced growth. Soft agar proliferation assays were conducted to establish the effect of bortezomib on estrogen-induced anchorage-independent colony formation. As expected, estrogen stimulated anchorage-independent growth. However, in the presence of estrogen and bortezomib colony formation was reduced to approximately 3% of that seen with estrogen alone (Figure 8). Thus, bortezomib, in the presence of estrogen, decreased anchorage independent growth, and antagonizes estrogen-dependent proliferation similar to other ERα antagonists (DeFriend, et al., 1994; Hui, et al., 2002).
Figure 8. Bortezomib decreases estrogen-induced colony formation.
A graph representing mean colony formation in soft agar assays performed on MCF7 cells treated with EtOH vehicle, 10 nM E2 and 30 nM bortezomib (B) as indicated. Colony formation was visualized after 14 days by staining with crystal violet. Data are representative of four independent experiments and error bars represent the standard error of the mean. Statistically significant differences (p<0.05) were determined using a student t-test and are denoted with an a (relative to EtOH) or b (relative to E2).
Discussion
Control of ERα levels and activity is of paramount importance in breast cancer. It is established that this control is in part mediated through the regulation of receptor protein stability by the 26S proteasome. The data presented here indicate that the role of the proteasome in ERα signaling extends beyond the control of protein turnover. Our data reveal that the inhibition of the chymotrypsin-like activity of the proteasome is not necessary for estrogen-induced degradation of ERα protein, but instead directly regulates the expression of ERα gene. This results in downstream consequences on ERα-mediated transcription. Further, inhibition of the proteasome prevents estrogen-dependent growth of breast cancer cells. These findings reveal that specific enzymatic activities have differential roles in the control of ERα protein and estrogen-dependent responses.
Bortezomib was developed as a selective inhibitor of the chymotrypsin-like activity of the 26S proteasome. This specificity allowed us to probe more deeply into the activities of the proteasome that contribute to hormone-inducible degradation of ERα. A surprising finding was the inability of bortezomib to inhibit ligand-activated ERα degradation. This is in contrast with other proteasome inhibitors, such as MG132, which has been used extensively by us and others to show stabilization of ERα protein (Alarid, 2006; Alarid, et al., 1999; El Khissiin and Leclercq, 1999; Nawaz, et al., 1999). Doses that are two orders of magnitude greater than those required to inhibit 95% of the chymotrypsin-like activity were required to prevent receptor degradation. Therefore, it is likely that other activities of the 26S proteasome play a larger role in ERα protein stability, and that the chymotrypsin-like activity is not required. Inhibitors developed to target the chymotrypsin-like activity will not prevent the rapid degradation of ERα protein that constitutes the autoregulatory negative feedback loop controlling sensitivity to estrogen, but they will have long term effects on ERα signaling in cells that depend on ERα expression.
Bortezomib had dramatic effects on ERα protein and function at 24 hours. Indeed, ERα protein was significantly depleted in bortezomib-treated cells. The loss of protein following chronic bortezomib is due to greater than 90% repression of ERα gene transcription. This is a direct transcriptional effect of bortezomib on ERα gene expression. ERα gene, ESR1, is 450 kilobases in size and is regulated by seven different promoters, A-E2, that yield different transcripts, making it one of the most complex genes in the genome (Kos, et al., 2001). How the different promoters interact and coregulate ERα expression has not been elucidated. Moreover, the molecular mechanisms that repress ERα gene regulation are only beginning to be elucidated (Adams, et al., 2007; Dhasarathy, et al., 2007; Ellison-Zelski, et al., 2009; Han, et al., 2008; Kondo, et al., 2008; Pandey and Picard, 2009; Wang, et al., 2009). Regulation of ERα by bortezomib involves loss of RNA PolII at the proximal promoter region. Our laboratory reported that estrogen repression of ERα involves specific chromatin modifications in both the A promoter and Exon 1 (Ellison-Zelski, et al., 2009). We observed that the combined effects of bortezomib and estrogen on ERα gene transcription is greater than either treatment alone (Figure 5a), and thus bortezomib is unlikely to repress ERα through the same mechanism. Elucidating the mechanism of proteasome-dependent ERα gene transcription is a subject of on-going experimentation.
Like ERα protein, the effects of bortezomib on ERα-mediated transcription are delayed. Activated genes, PR and pS2, were inversely affected by bortezomib, but only after prolonged bortezomib treatment. Estrogen-induction of PR and pS2 were not altered by bortezomib at 4 hours (data not shown). Similar variable effects on ERα-mediated gene transcription have been reported following MG132 treatment at various time points (Fan, et al., 2004; Lonard, et al., 2000; Reid, et al., 2003). The delayed transcriptional response suggested that the actions of bortezomib on ERα-mediated transcription are indirect and are most likely secondary to effects on ERα protein. ChIP analysis of RNA PolII and ERα occupancy on these genes revealed that indeed the relative expression of these genes is related to the occupancy of ERα and RNA PolII on these promoters. An interesting observation is that loss of ERα protein did not result in a uniform decrease in occupancy of ERα at all ERα target genes. ERα occupancy on the pS2 promoter was not diminished despite the severe depletion of ERα from the cells. This suggests that pS2 is less sensitive than PR to changes in ERα levels. Moreover, it points to the potential for promoter-specific requirements for different amounts of ERα protein for sustained activation.
The results of these studies have implications in breast cancer therapy and progression. Hormonal therapies antagonize the actions of ERα. Current clinical antagonists include aromatase inhibitors, tamoxifen and fulvestrant (Chia, et al., 2008; Coates, et al., 2007). Each functions through a distinct mechanism providing increased patient options and the potential for sequential therapeutic approaches. Aromatase inhibitors block ligand synthesis, tamoxifen inhibits ERα transcriptional function and fulvestrant degrades ERα protein (Dowsett, et al., 1985; Jordan, 1976; Parker, 1993; Viale, et al., 2007). Bortezomib functions through yet another distinct mechanism, i.e. inhibition of ERα gene expression. Anchorage-independent growth of breast cancer cells is inhibited by bortezomib, consistent with reports in other in vitro and in vivo models (Marx, et al., 2007; Teicher, et al., 1999). These studies expand on the previous studies with focus on estrogen-dependent growth. The data indicate that bortezomib can significantly decrease growth in presence of estrogen, similar to tamoxifen and ICI182780 (DeFriend, et al., 1994). The effectiveness of bortezomib as a single agent in solid tumors, however, has thus far been disappointing. (Engel, et al., 2007; Shah, et al., 2004; Yang, et al., 2006). Nevertheless these data, along with that from other preclinical models (Cardoso, et al., 2006; Marx, et al., 2007; Wong, et al., 2008), support the potential for proteasome inhibition as a viable route for development of new therapeutics for ER+ breast cancer.
In addition to its role as a predictive marker for therapy, ERα expression is also a marker for other changes associated with cancer progression. The percentage and intensity of ERα expression are increased in premalignant and malignant lesions relative to the normal mammary gland. ERα protein and mRNA is elevated in hyperplastic enlarged lobular units, a potential precursor to breast cancer (Lee, et al., 2007; Lee, et al., 2006). ERα expression is also increased in atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), ductal carcinoma in situ (DCIS), and invasive carcinomas (Shaaban, et al., 2002; Shoker, et al., 1999). The mechanism underlying the expansion of ER+ cells is unknown. Studies in Figure 3 and supplemental data suggest that proteasome activity sustains ERα expression in multiple estrogen responsive cells as inhibition of this activity leads to a loss of ERα mRNA. This suggests the possibility that increased ERα expression in early lesions may result from changes in proteasome activity. This notion is supported by evidence that protein levels of proteasome subunits and chymotrypsin-like activity are increased in tumor samples relative to normal adjacent tissue (Chen and Madura, 2005). In addition, proteasome activity in ER+ cell lines is approximately twice that found in ER- cell lines (Codony-Servat, et al., 2006). The association between proteasome activity and ERα expression in breast cancer, as revealed by this study, suggests the potential that proteasome function could contribute to multiple levels of breast cancer progression including induction of differentiation of ER- cells and/or driving the selective advantage of ER+ cells in malignancy. Examination of proteasome activity in early premalignant lesions would lend insight into this possibility.
In conclusion, this study shows that bortezomib, an FDA-approved anti-cancer agent, has significant and broad effects on the ERα pathway in breast cancer cells. Bortezomib does not interfere with the rapid response of estrogen-induced proteolysis of the receptor by the 26S proteasome, but chronically, it inhibits expression of ERα and PR genes as well as ERα protein. In addition, bortezomib was found to inhibit estrogen-dependent colony formation in breast cancer cells. These studies highlight the complexity of ERα regulation by the 26S proteasome and reveal a new link between the proteasome pathway and ER+ breast cancer.
Materials and Methods
Cell culture
Cells were maintained in media containing phenol red and L-glutamine supplemented with 10% fetal bovine serum (FBS; Biowest, Miami, FL, USA) and 100 units/mL of penicillin and 100 μg/mL streptomycin unless otherwise indicated. Reagents were from Gibco/Invitrogen (Carlsbad, CA, USA) unless indicated. MCF7, PR1, and MDA-MB-231 were cultured in high glucose DMEM (Mediatech, Inc Herndon, VA, USA). T47D cells were maintained in RPMI 1640 (Mediatech). ECC-1 and BT474 cells were cultured in DMEM/F12. BT474 cells were supplemented with 2 mM L-glutamine, 0.1 mM non-essential amino acids, and 6 ng/mL insulin (Sigma-Aldrich Corp., St, Louis, Mo, USA). Wt-ER cell lines were generated and maintained as previously described (Oesterreich, et al., 2001).
Hormone and proteasome inhibitor treatments
Three days before experiments cells were transferred to phenol red free media supplemented with 10% charcoal dextran-stripped FBS, penicillin/streptomycin, and 4mM L-Glutamine. Cells were pre-treated 30 minutes with proteasome inhibitors MG132 (Calbiochem, Gibbstown, NJ, USA) or Bortezomib (gift from Dr. Shigeki Miyamoto) followed by vehicle (0.1% ethanol) or 10 nM 17β-estradiol (E2; Steraloids, Inc., Newport, RI, USA) as indicated.
Determination of the IC50
50,000 cells/well were plated in a 96 well plate. Increasing concentrations of bortezomib from 0-70 nM or MG132 0-10 μM were incubated with the cells for 4 hours. Chymotrypsin-like activity of the proteasome was measured with a luminescent enzyme assay as per the manufacturer's instructions (Promega, Madison, WI, USA) and read on a Victor plate reader (PerkinElmer, Waltham, MA, USA). Luminescent signal was assessed relative to untreated cells. Data are representative of a minimum of two experiments done in duplicate. IC50 calculations were completed using nonlinear regression analysis in Graphpad Prism version 5.0 (San Diego, CA, USA).
Western blots
Western blots were performed as described previously (Valley, et al., 2008). Blots were probed with antibodies for ERα (6F11, Vector Laboratories Inc., Burlingame, CA, USA), p53 (DO 1, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and β-actin (Sigma-Aldrich Corp.). Anti-mouse horse radish peroxidase conjugated secondary antibody was used after primary antibody incubation (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). Enhanced chemiluminescence (GE) was used for visualization. Representative blots of a minimum of two independent experiments, demonstrating consistent results, are shown in figures.
Luciferase Assay
MCF7 cells were transfected using lipofectamine 2000 (Invitrogen) with ERE-tk-luciferase and CMV-βgalactosidase plasmids for 24 hours and then treated as indicated. Luciferase and β-galactosidase assays were performed as described previously (Fowler, et al., 2006).
qRT PCR
Total RNA isolation, reverse transcription, and qRT-PCR were performed as previously described (Valley, et al., 2008). Ribosomal protein P0 mRNA served as the internal control. Primer sequences are shown in the table below.
| α5 | 5′-GGAGGAGTTGATGAGAAAGG 5′TTGAGGATGATGAGTGAAGAC |
| Nascent ERα | 5′- TACCGTCCGTGCGAGAGG 5′- GCCAAGAGCGAGACCTTCC |
| ERα | 5′- CCTGATGATTGGTCTCGTCTG 5′-, GGCACACAAACTCCTCTCC |
| ERβ | 5′-AGAGTCCCTGGTGTGAAGCAAG 5′-GACAGCGCAGAAGTGAGCATC |
| Luciferase | 5′-GCAGCCTACCGTAGTGTTTG 5′-CGACTGAAATCCCTGGTAATCC |
| P0 | 5′-GACAATGGCAGCATCTACAAC 5′-GCAGACAGACACTGGCAAC |
| PR | 5′-TGACACCTCCAGTTCTTTGC; 5′-AACACCATTAAGCTCATCCAAG |
| pS2 | 5′-CGCCTTTGGAGCAGAGAG 5′-ACCACAATTCTGTCTTTCACG |
Relative RNA levels were calculated using the delta Ct method (Livak and Schmittgen, 2001). Statistics were performed using the MStat program with the Wilcoxon signed rank test (Yuan, et al., 2006).
ChIP
Chromatin immunoprecipitation (ChIP) and qRT-PCR were performed as previously described (Ellison-Zelski, et al., 2009). The antibodies used were ERα (HC-20 sc-543) and immunoglobulin G (sc 2027) from Santa Cruz and RNA PolII (PolII 8WG16) from Covance (Emeryville, CA). Primer sequences are listed below.
| NS | AGCTGGACCAGACCGACAATG GCCTTCCACAGGTTGGTTATGC |
| A | TCCTCCAGCACCTTTGTAATG AAGTGCAGCTCCCAGGAC |
| Ex1 | CTCTAACCTCGGGCTGTG CTTGGATCTGATGCAGTAGG |
| PR | GGCTTTGGGCGGGGCCTCC TCTGCTGGCTCCGTACTGCGG |
| pS2 | GGCCATCTCTCACTATGAATCACTTCTGC GGCAGGCTCTGTTTGCTTAAAGAGCG |
Soft agar assay
Two mL of 0.8% sea plaque agarose (Cambrex; Rockland, ME) were added to a 6 well plate. The following day 250,000 estrogen-deprived MCF7 cells were suspended in 0.4% agarose/media with 10 nM estrogen +/-30 nM bortezomib. Cells were plated on top of the previously poured layers. Treatments were replaced every four days. After 14 days colonies were stained with crystal violet, imaged and counted using a gel doc XR (Bio-rad, Hercules, CA, USA).
Supplementary Material
Representative Western blots from whole cells lysates of the indicated cell line pre-treated for 30 minutes with 30 nM bortezomib (B) and then treated with 10 nM 17-β-estradiol (E2) or ethanol vehicle (-) for 4 hours a) T47D, b) PR1, and c) ECC1 or 24 hours on d) T47D, e) BT474, f) ECC1, and g) PR1 cells. Blots were probed with antibodies for ERα and actin as a loading control.
Acknowledgments
This work was supported by grants NIH DK64034 (ETA), DAMD-17-02-1-0286 (AVL), T32CA009135 (GLP), T32GM08688 (GLP), and W81XWH-06-1-0714 (AJC). We would like to thank Drs. Shigeki Miyamoto and Michael Fritsch for reagents.
Footnotes
Disclosure/Conflict of Interest The authors declare no conflict of interest.
References
- Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERalpha) and represses ERalpha messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol. 2007;21:1132–1147. doi: 10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- Alarid ET. Lives and times of nuclear receptors. Mol Endocrinol. 2006;20:1972–1981. doi: 10.1210/me.2005-0481. [DOI] [PubMed] [Google Scholar]
- Alarid ET, Bakopoulos N, Solodin N. Proteasome-mediated proteolysis of estrogen receptor: a novel component in autologous down-regulation. Mol Endocrinol. 1999;13:1522–1534. doi: 10.1210/mend.13.9.0337. [DOI] [PubMed] [Google Scholar]
- Bazzaro M, Lee MK, Zoso A, Stirling WL, Santillan A, Shih Ie M, et al. Ubiquitin-proteasome system stress sensitizes ovarian cancer to proteasome inhibitor-induced apoptosis. Cancer Res. 2006;66:3754–3763. doi: 10.1158/0008-5472.CAN-05-2321. [DOI] [PubMed] [Google Scholar]
- Bossola M, Muscaritoli M, Costelli P, Grieco G, Bonelli G, Pacelli F, et al. Increased muscle proteasome activity correlates with disease severity in gastric cancer patients. Ann Surg. 2003;237:384–389. doi: 10.1097/01.SLA.0000055225.96357.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F, Durbecq V, Laes JF, Badran B, Lagneaux L, Bex F, et al. Bortezomib (PS-341, Velcade) increases the efficacy of trastuzumab (Herceptin) in HER-2-positive breast cancer cells in a synergistic manner. Mol Cancer Ther. 2006;5:3042–3051. doi: 10.1158/1535-7163.MCT-06-0104. [DOI] [PubMed] [Google Scholar]
- Chen L, Madura K. Increased proteasome activity, ubiquitin-conjugating enzymes, and eEF1A translation factor detected in breast cancer tissue. Cancer Res. 2005;65:5599–5606. doi: 10.1158/0008-5472.CAN-05-0201. [DOI] [PubMed] [Google Scholar]
- Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–1670. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- Codony-Servat J, Tapia MA, Bosch M, Oliva C, Domingo-Domenech J, Mellado B, et al. Differential cellular and molecular effects of bortezomib, a proteasome inhibitor, in human breast cancer cells. Mol Cancer Ther. 2006;5:665–675. doi: 10.1158/1535-7163.MCT-05-0147. [DOI] [PubMed] [Google Scholar]
- Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106(Pt 4):1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- DeFriend DJ, Anderson E, Bell J, Wilks DP, West CM, Mansel RE, et al. Effects of 4-hydroxytamoxifen and a novel pure antioestrogen (ICI 182780) on the clonogenic growth of human breast cancer cells in vitro. Br J Cancer. 1994;70:204–211. doi: 10.1038/bjc.1994.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Cuba VL, Kim H, Wu K, Lee AV, Brown PH. Estrogen receptor DNA binding is not required for estrogen-induced breast cell growth. Mol Cell Endocrinol. 2007;277:13–25. doi: 10.1016/j.mce.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Denger S, Reid G, Kos M, Flouriot G, Parsch D, Brand H, et al. ERalpha gene expression in human primary osteoblasts: evidence for the expression of two receptor proteins. Mol Endocrinol. 2001;15:2064–2077. doi: 10.1210/mend.15.12.0741. [DOI] [PubMed] [Google Scholar]
- Dhasarathy A, Kajita M, Wade PA. The transcription factor snail mediates epithelial to mesenchymal transitions by repression of estrogen receptor-alpha. Mol Endocrinol. 2007;21:2907–2918. doi: 10.1210/me.2007-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett M, Santner SJ, Santen RJ, Jeffcoate SL, Smith IE. Effective inhibition by low dose aminoglutethimide of peripheral aromatization in postmenopausal breast cancer patients. Br J Cancer. 1985;52:31–35. doi: 10.1038/bjc.1985.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khissiin A, Leclercq G. Implication of proteasome in estrogen receptor degradation. FEBS Lett. 1999;448:160–166. doi: 10.1016/s0014-5793(99)00343-9. [DOI] [PubMed] [Google Scholar]
- Ellison-Zelski SJ, Solodin NM, Alarid ET. Repression of ESR1 through actions of estrogen receptor alpha and Sin3A at the proximal promoter. Mol Cell Biol. 2009;29:4949–4958. doi: 10.1128/MCB.00383-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel RH, Brown JA, Von Roenn JH, O'Regan RM, Bergan R, Badve S, et al. A phase II study of single agent bortezomib in patients with metastatic breast cancer: a single institution experience. Cancer Invest. 2007;25:733–737. doi: 10.1080/07357900701506573. [DOI] [PubMed] [Google Scholar]
- Fabris G, Marchetti E, Marzola A, Bagni A, Querzoli P, Nenci I. Pathophysiology of estrogen receptors in mammary tissue by monoclonal antibodies. J Steroid Biochem. 1987;27:171–176. doi: 10.1016/0022-4731(87)90307-4. [DOI] [PubMed] [Google Scholar]
- Fan M, Nakshatri H, Nephew KP. Inhibiting proteasomal proteolysis sustains estrogen receptor-alpha activation. Mol Endocrinol. 2004;18:2603–2615. doi: 10.1210/me.2004-0164. [DOI] [PubMed] [Google Scholar]
- Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci U S A. 2007;104:14718–14723. doi: 10.1073/pnas.0706933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler AM, Solodin NM, Valley CC, Alarid ET. Altered target gene regulation controlled by estrogen receptor-alpha concentration. Mol Endocrinol. 2006;20:291–301. doi: 10.1210/me.2005-0288. [DOI] [PubMed] [Google Scholar]
- Han Y, Yang L, Suarez-Saiz F, San-Marina S, Cui J, Minden MD. Wilms' tumor 1 suppressor gene mediates antiestrogen resistance via down-regulation of estrogen receptor-alpha expression in breast cancer cells. Mol Cancer Res. 2008;6:1347–1355. doi: 10.1158/1541-7786.MCR-07-2179. [DOI] [PubMed] [Google Scholar]
- Harrell JC, Dye WW, Harvell DM, Pinto M, Jedlicka P, Sartorius CA, et al. Estrogen insensitivity in a model of estrogen receptor positive breast cancer lymph node metastasis. Cancer Res. 2007;67:10582–10591. doi: 10.1158/0008-5472.CAN-07-1655. [DOI] [PubMed] [Google Scholar]
- Heinemeyer W, Fischer M, Krimmer T, Stachon U, Wolf DH. The active sites of the eukaryotic 20 S proteasome and their involvement in subunit precursor processing. J Biol Chem. 1997;272:25200–25209. doi: 10.1074/jbc.272.40.25200. [DOI] [PubMed] [Google Scholar]
- Hui R, Finney GL, Carroll JS, Lee CS, Musgrove EA, Sutherland RL. Constitutive overexpression of cyclin D1 but not cyclin E confers acute resistance to antiestrogens in T-47D breast cancer cells. Cancer Res. 2002;62:6916–6923. [PubMed] [Google Scholar]
- Jordan VC. Antiestrogenic and antitumor properties of tamoxifen in laboratory animals. Cancer Treat Rep. 1976;60:1409–1419. [PubMed] [Google Scholar]
- Khan SA, Sachdeva A, Naim S, Meguid MM, Marx W, Simon H, et al. The normal breast epithelium of women with breast cancer displays an aberrant response to estradiol. Cancer Epidemiol Biomarkers Prev. 1999;8:867–872. [PubMed] [Google Scholar]
- Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- Kondo N, Toyama T, Sugiura H, Fujii Y, Yamashita H. miR-206 Expression is down-regulated in estrogen receptor alpha-positive human breast cancer. Cancer Res. 2008;68:5004–5008. doi: 10.1158/0008-5472.CAN-08-0180. [DOI] [PubMed] [Google Scholar]
- Kos M, Reid G, Denger S, Gannon F. Minireview: genomic organization of the human ERalpha gene promoter region. Mol Endocrinol. 2001;15:2057–2063. doi: 10.1210/mend.15.12.0731. [DOI] [PubMed] [Google Scholar]
- Kumatori A, Tanaka K, Inamura N, Sone S, Ogura T, Matsumoto T, et al. Abnormally high expression of proteasomes in human leukemic cells. Proc Natl Acad Sci U S A. 1990;87:7071–7075. doi: 10.1073/pnas.87.18.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Medina D, Tsimelzon A, Mohsin SK, Mao S, Wu Y, et al. Alterations of gene expression in the development of early hyperplastic precursors of breast cancer. Am J Pathol. 2007;171:252–262. doi: 10.2353/ajpath.2007.061010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Mohsin SK, Mao S, Hilsenbeck SG, Medina D, Allred DC. Hormones, receptors, and growth in hyperplastic enlarged lobular units: early potential precursors of breast cancer. Breast Cancer Res. 2006;8:R6. doi: 10.1186/bcr1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke C, Broglio K, Moulder S, Hsu L, Kau SW, Symmans WF, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009 doi: 10.1093/annonc/mdp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lonard DM, Nawaz Z, Smith CL, O'Malley BW. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell. 2000;5:939–948. doi: 10.1016/s1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- Maki CG, Huibregtse JM, Howley PM. In vivo ubiquitination and proteasome-mediated degradation of p53(1) Cancer Res. 1996;56:2649–2654. [PubMed] [Google Scholar]
- Marx C, Yau C, Banwait S, Zhou Y, Scott GK, Hann B, et al. Proteasome-regulated ERBB2 and estrogen receptor pathways in breast cancer. Mol Pharmacol. 2007;71:1525–1534. doi: 10.1124/mol.107.034090. [DOI] [PubMed] [Google Scholar]
- Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, et al. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- Nawaz Z, Lonard DM, Dennis AP, Smith CL, O'Malley BW. Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci U S A. 1999;96:1858–1862. doi: 10.1073/pnas.96.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y, Tashiro H, Shinozuka K. Changes of steroid hormone receptor content by chemotherapy and/or endocrine therapy in advanced breast cancer. Cancer. 1985;55:546–551. doi: 10.1002/1097-0142(19850201)55:3<546::aid-cncr2820550313>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- O'Donnell AJ, Macleod KG, Burns DJ, Smyth JF, Langdon SP. Estrogen receptor-alpha mediates gene expression changes and growth response in ovarian cancer cells exposed to estrogen. Endocr Relat Cancer. 2005;12:851–866. doi: 10.1677/erc.1.01039. [DOI] [PubMed] [Google Scholar]
- Oesterreich S, Zhang P, Guler RL, Sun X, Curran EM, Welshons WV, et al. Re-expression of estrogen receptor alpha in estrogen receptor alpha-negative MCF-7 cells restores both estrogen and insulin-like growth factor-mediated signaling and growth. Cancer Res. 2001;61:5771–5777. [PubMed] [Google Scholar]
- Orlowski M, Wilk S. A multicatalytic protease complex from pituitary that forms enkephalin and enkephalin containing peptides. Biochem Biophys Res Commun. 1981;101:814–822. doi: 10.1016/0006-291x(81)91823-4. [DOI] [PubMed] [Google Scholar]
- Pandey DP, Picard D. miR-22 inhibits estrogen signaling by directly targeting the estrogen receptor alpha mRNA. Mol Cell Biol. 2009;29:3783–3790. doi: 10.1128/MCB.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MG. Action of “pure” antiestrogens in inhibiting estrogen receptor action. Breast Cancer Res Treat. 1993;26:131–137. doi: 10.1007/BF00689686. [DOI] [PubMed] [Google Scholar]
- Qin C, Nguyen T, Stewart J, Samudio I, Burghardt R, Safe S. Estrogen up-regulation of p53 gene expression in MCF-7 breast cancer cells is mediated by calmodulin kinase IV-dependent activation of a nuclear factor kappaB/CCAAT-binding transcription factor-1 complex. Mol Endocrinol. 2002;16:1793–1809. doi: 10.1210/me.2002-0006. [DOI] [PubMed] [Google Scholar]
- Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D, et al. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell. 2003;11:695–707. doi: 10.1016/s1097-2765(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu Rev Med. 2006;57:33–47. doi: 10.1146/annurev.med.57.042905.122625. [DOI] [PubMed] [Google Scholar]
- Robertson JF, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, Macpherson E, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27:4530–4535. doi: 10.1200/JCO.2008.21.1136. [DOI] [PubMed] [Google Scholar]
- Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Shaaban AM, Sloane JP, West CR, Foster CS. Breast cancer risk in usual ductal hyperplasia is defined by estrogen receptor-alpha and Ki-67 expression. Am J Pathol. 2002;160:597–604. doi: 10.1016/s0002-9440(10)64879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MH, Young D, Kindler HL, Webb I, Kleiber B, Wright J, et al. Phase II study of the proteasome inhibitor bortezomib (PS-341) in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2004;10:6111–6118. doi: 10.1158/1078-0432.CCR-04-0422. [DOI] [PubMed] [Google Scholar]
- Shoker BS, Jarvis C, Clarke RB, Anderson E, Hewlett J, Davies MP, et al. Estrogen receptor-positive proliferating cells in the normal and precancerous breast. Am J Pathol. 1999;155:1811–1815. doi: 10.1016/S0002-9440(10)65498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart CC, Greene CM, McElvaney NG, O'Neill S. Secretory leucoprotease inhibitor prevents lipopolysaccharide-induced IkappaBalpha degradation without affecting phosphorylation or ubiquitination. J Biol Chem. 2002;277:33648–33653. doi: 10.1074/jbc.M203710200. [DOI] [PubMed] [Google Scholar]
- Teicher BA, Ara G, Herbst R, Palombella VJ, Adams J. The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res. 1999;5:2638–2645. [PubMed] [Google Scholar]
- Valley CC, Metivier R, Solodin NM, Fowler AM, Mashek MT, Hill L, et al. Differential regulation of estrogen-inducible proteolysis and transcription by the estrogen receptor alpha N terminus. Mol Cell Biol. 2005;25:5417–5428. doi: 10.1128/MCB.25.13.5417-5428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valley CC, Solodin NM, Powers GL, Ellison SJ, Alarid ET. Temporal variation in estrogen receptor-alpha protein turnover in the presence of estrogen. J Mol Endocrinol. 2008;40:23–34. doi: 10.1677/JME-07-0067. [DOI] [PubMed] [Google Scholar]
- Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell'Orto P, Rasmussen BB, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J Clin Oncol. 2007;25:3846–3852. doi: 10.1200/JCO.2007.11.9453. [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- Wang X, Belguise K, O'Neill CF, Sanchez-Morgan N, Romagnoli M, Eddy SF, et al. RelB NF-kappaB represses estrogen receptor alpha expression via induction of the zinc finger protein Blimp1. Mol Cell Biol. 2009;29:3832–3844. doi: 10.1128/MCB.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayaratne AL, McDonnell DP. The human estrogen receptor-alpha is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J Biol Chem. 2001;276:35684–35692. doi: 10.1074/jbc.M101097200. [DOI] [PubMed] [Google Scholar]
- Wong DJ, Nuyten DS, Regev A, Lin M, Adler AS, Segal E, et al. Revealing targeted therapy for human cancer by gene module maps. Cancer Res. 2008;68:369–378. doi: 10.1158/0008-5472.CAN-07-0382. [DOI] [PubMed] [Google Scholar]
- Xu H, Ju D, Jarois T, Xie Y. Diminished feedback regulation of proteasome expression and resistance to proteasome inhibitors in breast cancer cells. Breast Cancer Res Treat. 2008;107:267–274. doi: 10.1007/s10549-007-9553-4. [DOI] [PubMed] [Google Scholar]
- Yang CH, Gonzalez-Angulo AM, Reuben JM, Booser DJ, Pusztai L, Krishnamurthy S, et al. Bortezomib (VELCADE) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Ann Oncol. 2006;17:813–817. doi: 10.1093/annonc/mdj131. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative Western blots from whole cells lysates of the indicated cell line pre-treated for 30 minutes with 30 nM bortezomib (B) and then treated with 10 nM 17-β-estradiol (E2) or ethanol vehicle (-) for 4 hours a) T47D, b) PR1, and c) ECC1 or 24 hours on d) T47D, e) BT474, f) ECC1, and g) PR1 cells. Blots were probed with antibodies for ERα and actin as a loading control.