Abstract
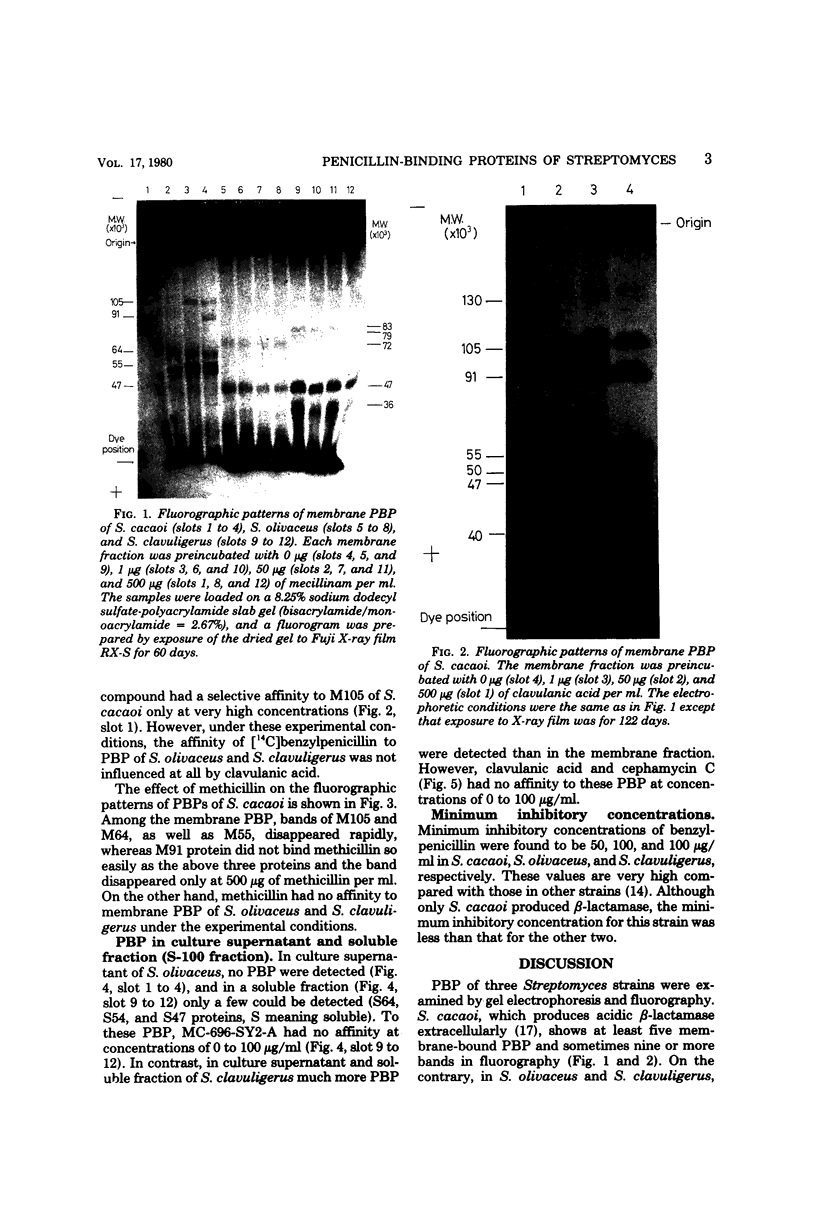
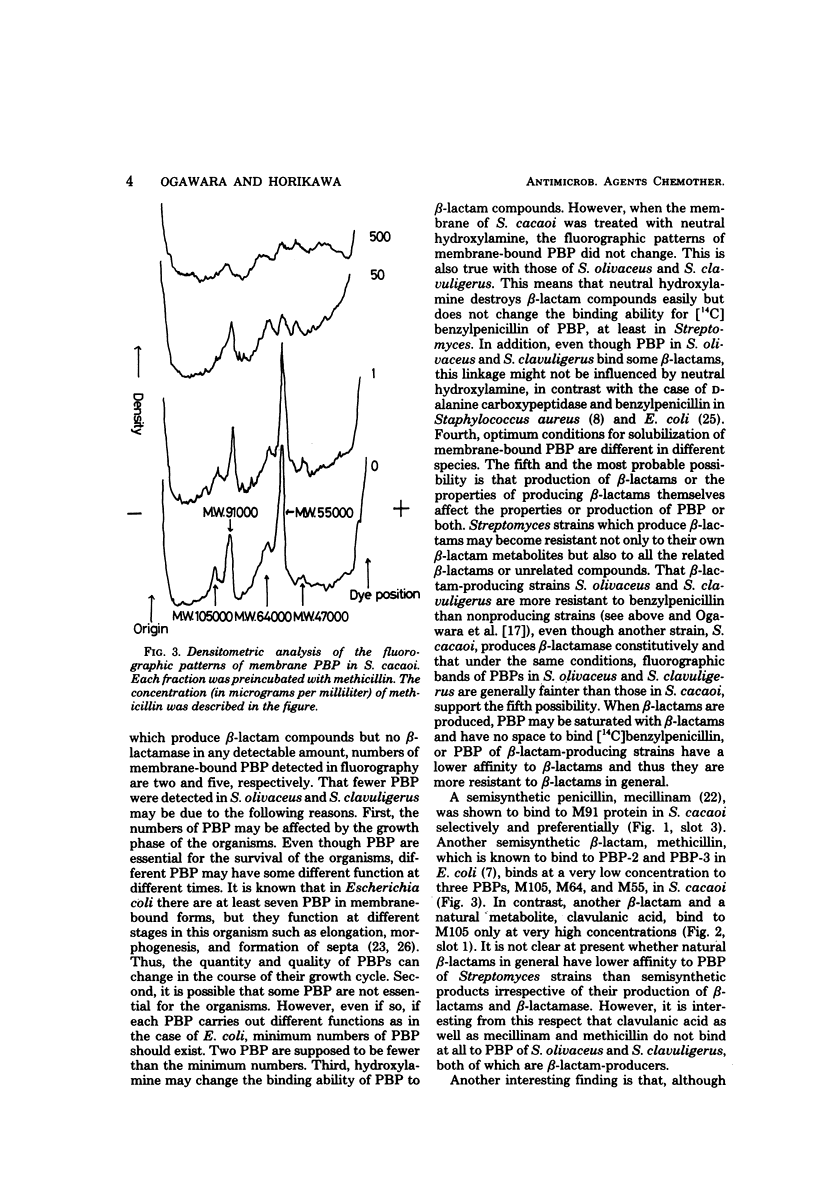
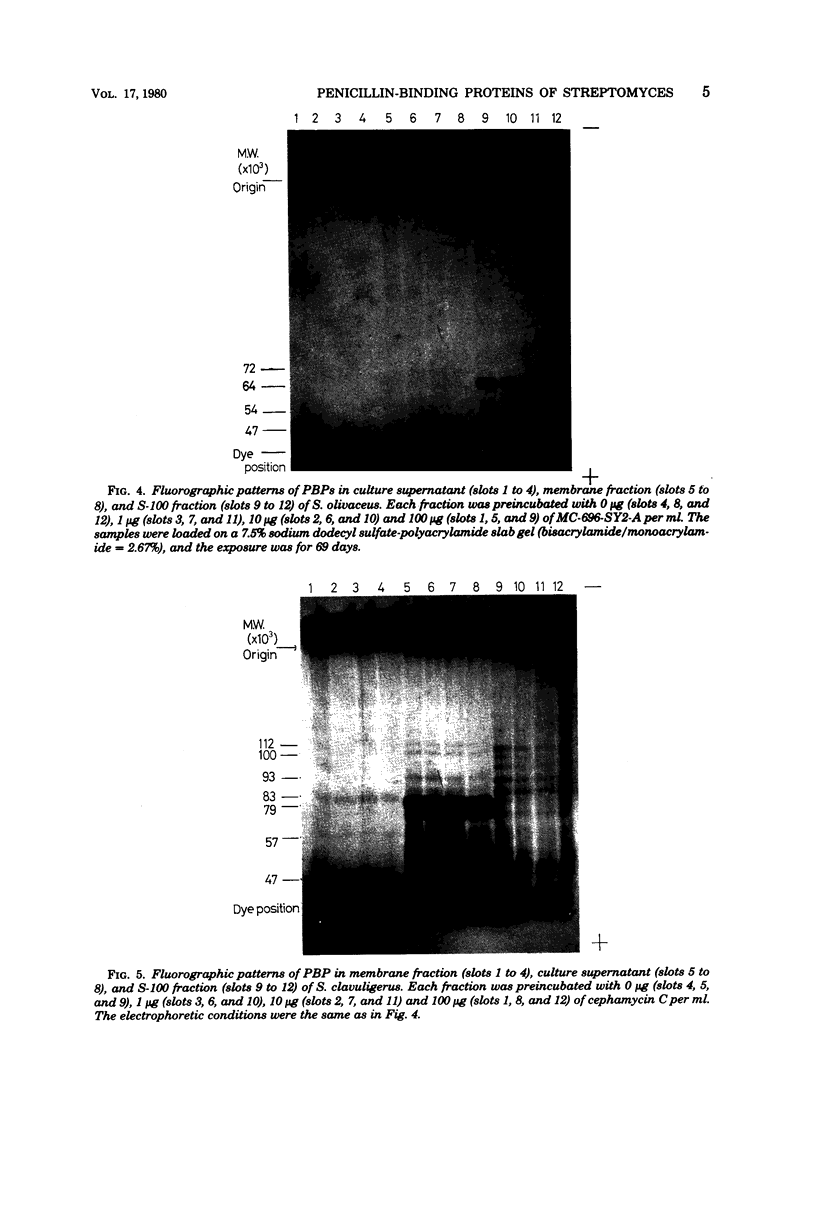
Penicillin-binding proteins of three Streptomyces strains, S. cacaoi, S. olivaceus, and S. clavuligerus, were examined by gel electrophoresis and fluorography. In a beta-lactamase producer, S. cacaoi, at least five membrane-bound penicillin-binding proteins were detected, but in two beta-lactam producers, S. olivaceus and S. clavuligerus, fewer penicillin-binding proteins were detected. Mecillinam and methicillin bound selectively to some penicillin-binding proteins in S. cacaoi, whereas they did not bind at all to those in S. olivaceus and S. clavuligerus. Clavulanic acid bound to penicillin-binding proteins only at a very high concentration in S. cacoi. This compound did not bind to those of S. olivaceus and S. clavuligerus. Penicillin-binding proteins in culture supernatant and cytoplasm and minimum inhibitory concentrations of these strains against benzylpenicillin were also examined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2276–2280. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Butterworth D., Cole M., Hanscomb G., Hood J. D., Reading C., Rolinson G. N. Naturally-occurring beta-lactamase inhibitors with antibacterial activity. J Antibiot (Tokyo) 1976 Jun;29(6):668–669. doi: 10.7164/antibiotics.29.668. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Strominger J. L. Degradation of penicillin G to phenylacetylglycine by D-alanine carboxypeptidase from Bacillus stearothermophilus. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3463–3467. doi: 10.1073/pnas.72.9.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa S., Ogawara H. Membrane-bound penicillin-binding proteins of Escherichia coli. Comparison of a strain carrying an R-factor and the parent strain. J Antibiot (Tokyo) 1978 Dec;31(12):1283–1291. doi: 10.7164/antibiotics.31.1283. [DOI] [PubMed] [Google Scholar]
- Kozarich J. W., Nishino T., Willoughby E., Strominger J. L. Hydroxylaminolysis of penicillin binding componenets is enzymatically catalyzed. J Biol Chem. 1977 Nov 10;252(21):7525–7529. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Leyh-Bouille M., Dusart J., Nguyen-Distèche M., Ghuysen J. M., Reynolds P. E., Perkins H. R. The peptidoglycan crosslinking enzyme system in Streptomyces strains R61, K15 and rimosus. Eur J Biochem. 1977 Nov 15;81(1):19–28. doi: 10.1111/j.1432-1033.1977.tb11922.x. [DOI] [PubMed] [Google Scholar]
- Maeda K., Takahashi S., Sezaki M., Iinuma K., Naganawa H., Kondo S., Ohno M., Umezawa H. Isolation and structure of a beta-lactamase inhibitor from Streptomyces. J Antibiot (Tokyo) 1977 Sep;30(9):770–772. doi: 10.7164/antibiotics.30.770. [DOI] [PubMed] [Google Scholar]
- Ogawara H. A specific cephalosporin-binding protein of Citrobacter freundii. Biochim Biophys Acta. 1976 Jan 20;420(1):155–164. doi: 10.1016/0005-2795(76)90354-8. [DOI] [PubMed] [Google Scholar]
- Ogawara H., Horikawa S., Shimada-Miyoshi S., Yasuzawa K. Production and property of beta-lactamases in Streptomyces: comparison of the strains isolated newly and thirty years ago. Antimicrob Agents Chemother. 1978 May;13(5):865–870. doi: 10.1128/aac.13.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara H., Maeda K., Umezawa H. A -lactamase of Escherichia coli. Biochim Biophys Acta. 1972 Nov 10;289(1):203–211. doi: 10.1016/0005-2744(72)90123-4. [DOI] [PubMed] [Google Scholar]
- Ogawara H., Nozaki S. Effect of acriflavine of the production of beta-lactamase in Streptomyces. J Antibiot (Tokyo) 1977 Apr;30(4):337–339. doi: 10.7164/antibiotics.30.337. [DOI] [PubMed] [Google Scholar]
- Ogawara H. Penicillin-binding proteins of Escherichia coli. Comparison of a strain carrying an R-factor and the parent strain. Biochim Biophys Acta. 1977 Mar 28;491(1):223–231. doi: 10.1016/0005-2795(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Ogawara H. Production and property of beta-lactamases in Streptomyces. Antimicrob Agents Chemother. 1975 Oct;8(4):402–408. doi: 10.1128/aac.8.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara H., Umezawa H. Bacillus cereus beta-lactamase. Reaction with N-bromosuccinimide and the properties of the product. Biochim Biophys Acta. 1975 Jun 24;391(2):435–447. doi: 10.1016/0005-2744(75)90268-5. [DOI] [PubMed] [Google Scholar]
- Park J. T., Burman L. FL-1060: a new penicillin with a unique mode of action. Biochem Biophys Res Commun. 1973 Apr 16;51(4):863–868. doi: 10.1016/0006-291x(73)90006-5. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]







