Abstract
PI 3-kinase enhancer A (PIKE-A) is critical for the activation of Akt signalling, and has an essential function in promoting cancer cell survival. However, its physiological functions are poorly understood. Here, we show that PIKE-A directly associates with both signal transducer and activator of transcription 5a (STAT5a) and prolactin (PRL) receptor, which is essential for PRL-provoked STAT5a activation and the subsequent gene transcription. Depletion of PIKE-A in HC11 epithelial cells diminished PRL-induced STAT5 activation and cyclin D1 expression, resulting in profoundly impaired cell proliferation in vitro. To confirm the function of PIKE-A in PRL signalling in vivo, we generated PIKE knockout (PIKE−/−) mice. PIKE−/− mice displayed a severe lactation defect that was characterized by enhanced apoptosis and impaired proliferation of mammary epithelial cells. At parturition, STAT5 activation and cyclin D1 expression were substantially reduced in the mammary epithelium of PIKE−/− mice. The defective mammary gland development in PIKE−/− mice was rescued by overexpression of a mammary-specific cyclin D1 transgene. These data establish a critical function for PIKE-A in mediating PRL functions.
Keywords: mammary gland, PIKE, prolactin receptor, STAT5
Introduction
PI 3-kinase enhancer (PIKE) is a family of GTPases that regulate PI 3-kinase (PI3K)/Akt signalling pathway. It has three family members (PIKE-S, PIKE-L and PIKE-A), which are generated by differential transcription and alternative splicing of the gene CENTG1 (Chan and Ye, 2007). PIKE-S is a nuclear protein that enhances the kinase activity of PI3K and executes the anti-apoptotic function of NGF (Ye et al, 2000, 2002). In hippocampal neurons, PIKE-L binds to Homer, an adaptor protein known to link mGluR I to multiple intracellular targets including IP3 receptor. Activation of mGluR I enhances the formation of an mGluR I-Homer-PIKE-L complex, leading to activation of PI3K activity and prevention of neuronal apoptosis (Rong et al, 2003). Recently, we showed that PIKE-L exerted its neuroprotective action through inhibition of SET proteolytic cleavage by AEP, an asparagine endopeptidase (Liu et al, 2008). PIKE-L also binds to Unc5B and mediates the prosurvival effect of netrin 1 in neurons (Tang et al, 2008).
Although PIKE-S and -L associates with PI3K, PIKE-A interacts with Akt, but not PI3K (Ahn et al, 2004b). PIKE-A is co-amplified with CDK4 in human glioblastomas, which specifically binds to active Akt in a guanine nucleotide-dependent manner, and stimulates Akt-kinase activity (Ahn et al, 2004a). Human cancer cells with PIKE amplification are more resistant to apoptosis than those with normal PIKE copy number. Knockdown of PIKE-A diminishes Akt activity and, therefore, enhances apoptosis (Ahn et al, 2004a, 2004c). We also reported that PIKE-A acted as a proto-oncogene, and promoted cell transformation through Akt activation (Liu et al, 2007). Indeed, overexpression of PIKE-A has been observed in a variety of tumours including prostate and breast cancers (Liu et al, 2007; Cai et al, 2009). These observations indicate that PIKE-A amplification promotes cancer cell growth by inhibiting apoptosis through stimulation of Akt.
Prolactin (PRL) is a pituitary hormone with numerous physiological functions (Ben-Jonathan et al, 2008). It provokes multiple signalling cascades including the Janus-kinase 2 (JAK2)/signal transducer and activator of transcription 5 (STAT5) (Hennighausen et al, 1997) and the Ras-MAPK (Erwin et al, 1995). Binding of PRL to its receptor (PRLR) leads to receptor dimerization and autophosphorylation of the receptor-associated JAK2 (Rui et al, 1994). JAK2 phosphorylates the PRLR, thereby creating docking sites for Src homology 2-domain proteins such as STAT5, Src, Fyn and Tec. Subsequently, Jak2 phosphorylates both STAT5 forms (STAT5a and STAT5b) and triggers the formation of STAT5 dimers, which translocate into the nucleus, tether to specific DNA sequence motifs and activate the transcription of target genes such as WAP, β-casein and cyclin D1 (Liu et al, 1997; Miyoshi et al, 2001; Brockman et al, 2002; Brockman and Schuler, 2005; Sakamoto et al, 2007). Among the many functions ascribed to PRL, its involvement in mammary gland development has been best characterized. Mammary gland development is a highly regulated process (Hennighausen and Robinson, 2001). The tissue begins to develop during embryogenesis as a rudimentary ductal system. Under the influence of systemic hormones at puberty, the ducts begin to expand into the surrounding fat pad. With repeated estrous cycles and during pregnancy, the complexity of the ductal system increases through the addition of side branches (Brisken, 2002). The last stage of mammary gland morphogenesis, alveologenesis, is closely intertwined with the functional differentiation of the mammary epithelium called lactogenesis (Brisken and Rajaram, 2006). Lactogenesis contains two stages. Stage 1 begins at mid-pregnancy and involves increased expression of genes involved in the synthesis of different milk proteins such as β-casein, lactalbumin and WAP (whey acidic protein) (Neville et al, 2002), which are also transiently increased during the estrous cycles (Robinson et al, 1998). The second stage of lactogenesis occurs around parturition; expressions of milk protein genes are further increased and cytoplasmic lipid droplets and casein are moved to the alveolar lumen (Jensen et al, 2001; Neville and Morton, 2001). PRL exerts only minor effects on morphological changes that occur in the mammary gland during peripubertal life, but it is heavily involved in lobuloalveolar differentiation, lactogenesis galactopoiesis (maintenance of milk secretion) and involution (a return to a nonlactating state) (Oakes et al, 2006).
In this report, we show that PIKE-A forms a complex with STAT5 and couples it to PRLR, which is regulated by PRL. Ablation of the PIKE gene cripples PRL/JAK2/STAT5 and Akt signalling leading to substantial apoptosis and defective epithelial cell proliferation in mammary gland at postpartum, resulting in underdeveloped lobuloalveolar network and failed lactation. Cyclin D1 expression is decreased in vitro and in vivo and forced Cyclin D1 expression in vivo can rescue these defects. Thus, PIKE-A is a critical factor in mediating PRL function during lactation by promoting mammary epithelial cell proliferation and differentiation.
Results
PIKE-A specifically interacts with STAT5a and PRLR
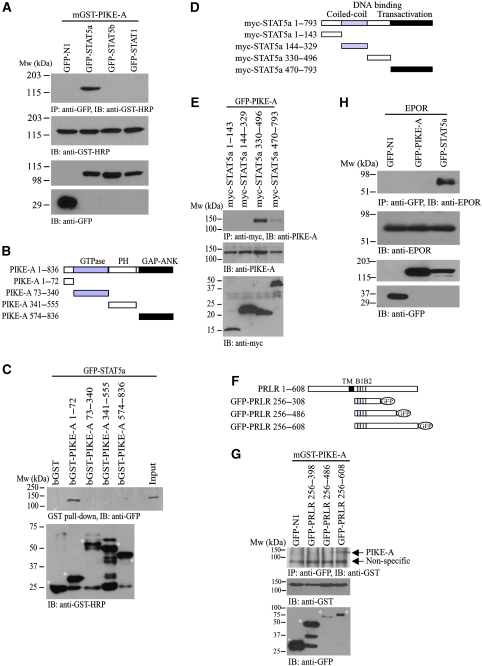
PIKE-A is overexpressed in breast cancer (Liu et al, 2007). Aberrant STAT activities highly correlate with breast cancer progression (Clevenger, 2004; Liu et al, 2007). These observations lead us to test whether PIKE-A directly interacts with STAT proteins. We conducted an immunoprecipitation assay from HEK293 cells that were co-transfected with mammalian GST-PIKE-A and various GFP-STAT proteins. Immunoblotting analysis revealed that GST-PIKE-A selectively interacted with STAT5a, but not STAT5b, STAT1 or GFP alone (Figure 1A). To determine which portion of PIKE-A associated with STAT5a, we conducted truncation assays. This deletion mapping experiment demonstrated that the N-terminal 1–72 residues in PIKE-A were necessary and sufficient for the interaction between PIKE-A and STAT5a (Figure 1B and C). We also examined the PIKE-A interaction domain in STAT5a by co-transfecting HEK293 cells with GFP-PIKE-A and different myc-tagged STAT5a truncations mutants (Figure 1D). PIKE-A strongly interacted with the C-terminal DNA-binding domain of STAT5a (Figure 1E). Weak but detectable interaction was also found between the trans-activation domain of STAT5a and PIKE-A. In contrast, we did not observe any interaction between PIKE-A and JAK2 (data not shown).
Figure 1.
PIKE-A interacts with STAT5a and PRLR. (A) PIKE-A specifically binds STAT5a. HEK293 cells were co-transfected with various GFP-STAT plasmids and mGST-PIKE-A. The GFP proteins were immunoprecipitated and the bound PIKE-A proteins were detected using anti-GST-HRP antibody (top panel). The expression of mGST-PIKE-A (middle panel) and different GFP-STAT proteins (bottom panels) were verified. (B) Diagrammatic representation of GST-PIKE truncation constructs. (C) PIKE-A N-terminus associates with STAT5a. In vitro mapping of PIKE-A domains that associate with STAT5a. Purified GST-tagged PIKE-A proteins were incubated with lysates of HEK293 cells transfected with GFP-STAT5a. The N-terminal end (1–72 a.a.) of PIKE-A, but not the C-terminal domain associates with STAT5a (middle panel). The GST-fused PIKE-A fragments (as indicated with asterisk) used in the in vitro binding were detected using anti-GST-HRP antibody (bottom panel). (D) Diagrammatic representation of myc-STAT5a truncation constructs. (E) STAT5a C-terminal associates with PIKE-A. HEK293 cells were co-transfected with GFP-PIKE-A and various deletion mutants of myc-STAT5a as shown in (D). The expressed STAT5a mutants were immunoprecipitated and the bound GFP-PIKE-A was detected using anti-GFP antibody (second panel). The expression of GFP-PIKE-A (third panel) and myc-STAT5a mutants (fourth panel) were also examined. (F) Diagrammatic representation of GFP-PRLR truncation constructs. (G) PIKE-A binds C-terminal PRLR intracellular-domain. HEK293 cells were co-transfected with mGST-PIKE-A and various deletion mutants of GFP-PRLR intracellular domains. The expressed PRLR mutants were immunoprecipitated and the bound mGST-PIKE-A was detected using anti-GST-HRP antibody (top panel). The expression of mGST-PIKE-A (bottom panel) and GFP-PRLR mutants (middle panel, indicated with asterisk) were also examined. (H) PIKE-A does not interact with other STAT5a-associated cytokine receptor. HEK293 cells were transfected with mouse EPO receptor (EPOR) plasmid and various GFP constructs as indicated. After 24 h of serum starvation, the transfected cells were stimulated with 3 ng/ml EPO for 15 min. The GFP-tagged proteins were immunoprecipitated and the associated EPOR was examined by using anti-EPOR antibody (top panel). The expression of EPOR (middle panel) and GFP-tagged proteins (bottom panel) were also verified.
PRLR is one of the well-studied receptors that trigger STAT5a activation. We thus tested whether PIKE-A associated with the PRLR. We co-transfected HEK293 cells with GST-PIKE-A and different truncated constructs of mouse GFP-tagged PRLR intracellular domains (Figure 1F). We found that PIKE-A interacted with the C-terminus of the PRLR intracellular domain between a.a. 486–608 (Figure 1G). Moreover, this interaction is receptor specific, as PIKE-A does not associate with other STAT5-activating receptors such as the erythropoietin (EPO) receptor (Figure 1H).
PRL provokes the formation of PIKE-A/STAT5/PRLR complex
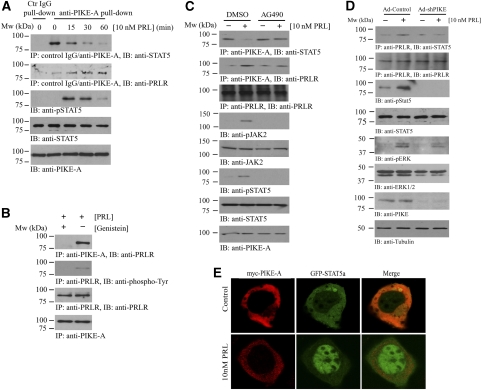
Binding of PRL to PRLR induces receptor dimerization and activation of JAK2 (Darnell et al, 1994; Schindler and Darnell, 1995), which subsequently phosphorylates STAT5 and triggers its dimerization and nuclear translocation (Ihle and Kerr, 1995). To determine whether the binding of STAT5 to PIKE-A is regulated by PRL, we treated mammary epithelial HC11 cells with PRL and monitored the interaction at various time points. Coimmunoprecipitation assays revealed that the tight association between PIKE-A and STAT5 was gradually disrupted by PRL in a time-dependent manner (Figure 2A, first panel). Concomitantly, the PIKE-A/PRLR association was initially increased (Figure 2A, second panel) and returned to basal level after 2 h (data not shown). Accordingly, phosphorylation of STAT5 was evident at 15 min, reached a maximal value at 30 min and partially decayed by 60 min after PRL stimulation. The PIKE-A/STAT5/PRLR binding was specific, as PIKE-A antibody, but not control IgG selectively pulled down STAT5 and PRLR from HC11 cells (Figure 2A, first and second panels). To determine whether phosphorylation of PRLR is necessary for PIKE-A/PRLR interaction, we pre-treated the PIKE-A-expressing HC11 cells with tyrosine-kinase inhibitor genistein, which blocked the PRLR phosphorylation by JAK2 on PRL treatment. The strong association of PIKE-A and PRLR in control cells was abolished in genistein-treated cells (Figure 2B), suggesting phosphorylation of PRLR is a prerequisite to PIKE-A docking. The PRLR/PIKE-A association was also abolished when HC11 cells were pre-treated with JAK2-specific inhibitor AG490 (Figure 2C, second panel), which further support the notion that PRLR phosphorylation is required for their interaction.
Figure 2.
PRL stimulation provokes the formation of PRLR/PIKE-A/STAT5a complex. (A) PRL stimulation interferes with the PIKE-A and Stat5 interaction, but enhances the formation of PRLR/PIKE-A complex. HC11 cells infected with adenovirus-expressing wild-type PIKE-A were stimulated with 10 nM recombinant mouse PRL for various time intervals as indicated. PIKE-A was then immunoprecipitated by control IgG or anti-PIKE-A antibody and the bound STAT5 and PRLR were detected using anti-Stat5 (first panel) and anti-PRLR antibodies (second panel). The expression of STAT5 (fourth panel) and PIKE-A (fifth panel) were examined. Phosphorylation of total STAT5 was also verified to confirm the activation of HC11 by PRL (third panel). (B) Phosphorylation of PRLR is essential for PIKE-A/PRLR interaction. HC11 cells were infected with adenovirus-expressing wild-type PIKE-A for 48 h followed by serum starvation for 24 h. Before 10 mM PRL stimulation, the cells were pre-treated with tyrosine-kinase inhibitor genistein (1 μM) for 45 min. The expressed PIKE-A was immunoprecipitated and the associated PRLR was detected using an anti-PRLR antibody (first panel). Phosphorylation of PRLR was examined using anti-phospho-tyrosine antibody on immunoprecipitated PRLR (second panel). The expression of PRLR (third panel) and PIKE-A (fourth panel) was also verified. (C) PIKE-A/PRLR interaction is JAK2-kinase dependent. HC11 cells were infected with adenovirus-expressing wild-type PIKE-A for 48 h followed by serum starvation for 24 h. Before 10 mM PRL stimulation for 15 min, the cells were pre-treated with JAK2-kinase inhibitor AG490 (50 μM) for 1 h. PIKE-A was immunoprecipitated and the associated STAT5 and PRLR was detected using specific antibody (first and second panels). Phosphorylation of JAK2 and STAT5 were determined to examine the effect of JAK2 inhibition (fourth and sixth panels). The expressions of PRLR, JAK2, STAT5 and PIKE were also verified (third, fifth, seventh and eighth panels). (D) PRL-provoked STAT5 phosphorylation is abolished in PIKE-A-depleted HC11 cells. HC11 cells were infected with either control adenovirus or adenovirus-expressing shPIKE. Two days after infection, the cells were serum-starved for 24 h and stimulated with 10 nM recombinant mouse PRL for 15 min. PRLR was immunoprecipitated and the associated STAT5 were determined by anti-STAT5 antibody (first panel). Phosphorylation of STAT5 was determined using specific antibody against phosphorylated STAT5 (third panel). Total PRLR (second panel), STAT5 (fourth panel) and PIKE-A (seventh panel) expressions was examined. Phosphorylation of ERK (fifth panel) by PRL was not affected in PIKE-depleted HC11 cells. Total ERK and tubulin were also determined (sixth and eighth panels). (E) PRL triggers nuclear translocation of STAT5a, but not PIKE-A. GFP-STAT5a and myc-PIKE-A were co-transfected in HC11 cells, serum-starved for 24 h and stimulated with 10 nM recombinant mouse PRL for 45 min. Cellular localization of PIKE-A and STAT5a was examined by confocal microscopy. Exclusive cytoplasmic localization of myc-PIKE-A was detected in both control and PRL-stimulated HC11 cells, whereas GFP-STAT5a accumulated in the nucleus after PRL stimulation.
To test whether PIKE-A is essential for STAT5 activation induced by PRL, we depleted PIKE-A from HC11 epithelial cells using adenovirus-expressing-specific shRNAs. PRL elicited potent STAT5 activation in control adenovirus-infected cells, whereas STAT5 activation was completely abrogated in PIKE-A knockdown cells (Figure 2D, third panel). This reduction of STAT5 phosphorylation might be a result of disconnection between PRLR and STAT5 interaction as PRL failed to induce STAT5 docking to PRLR (Figure 2D, first panel). However, PRL-induced ERK phosphorylation was intact (Figure 2D, fifth panel), underscoring that PIKE-A selectively mediates STAT5 in PRL signalling.
Immunofluorescent staining revealed that co-transfected PIKE-A and STAT5 co-localized in the cytoplasm. PRL stimulation-provoked STAT5 nuclear translocation, whereas PIKE-A remained in the cytoplasm (Figure 2E). These data suggest a model in which PRL triggers the PIKE-A/STAT5 complex to bind PRLR. STAT5 is subsequently phosphorylated by Jak2, which provokes STAT5 to dissociate from PIKE-A and leads to its nuclear translocation.
Depletion of PIKE abolishes cyclin D1 expression and decreases epithelial cell growth
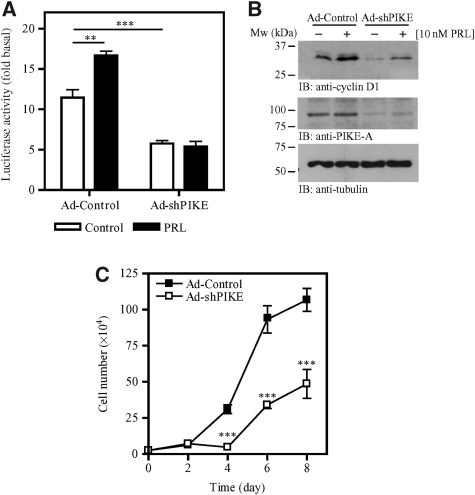
PIKE-A is essential for PRL-induced STAT5 phosphorylation, conceivably, depletion of PIKE-A might abolish STAT5-induced gene transcription. Cyclin D1 is a downstream target of PRL signalling, and transcriptional expression and post-translational modification are mediated through PRL-provoked JAK2/STAT5, Ras/MAP kinase and PI3K/Akt signalling cascades (Fantl et al, 1995; Sicinski et al, 1995). PRL activates the cyclin D1 promoter via the JAK2/STAT pathway (Brockman et al, 2002). To explore whether PIKE-A regulates cyclin D1 transcription, we conducted a luciferase assay with construct containing GAS sites (γ-interferon activation sites) isolated from the cyclin D1 promoter (Brockman et al, 2002). PRL stimulated the activation of cyclin D1 promoter in control HC11 cells, but not PIKE-A-ablated cells (Figure 3A). Immunoblotting analysis further showed that expression of cyclin D1 was significantly diminished in PIKE-A-depleted HC11 cells (Figure 3B), confirming the observation made using the luciferase assay. As depletion of cyclin D1 in mice leads to impaired mammary epithelial proliferation (Sicinski et al, 1995), we assessed the effect of PIKE-A depletion on cell growth. Cell proliferation assays revealed that depletion of PIKE-A in HC11 cells substantially diminished cell growth (Figure 3C). These findings indicate that PIKE-A is indispensable for cyclin D1 expression and mammary epithelial cell proliferation.
Figure 3.
PIKE-A is essential for epithelial cell proliferation. (A) PIKE-A is required for PRL-stimulated cyclin D1 promoter activity. After transient transfection with the PRE3-luciferase vector, PIKE-A expression in HC11 cells was suppressed by infecting the cells with adenovirus-expressing sh-PIKE. After 48 h, the infected cells were then serum-starved for 24 h and treated with (solid bar) or without (open bar) 10 nM PRL in serum-free medium for another 24 h, and luciferase activity was then determined (**P<0.01, ***P<0.001, Student's t-test). (B) PIKE-A is critical for basal and PRL-induced cyclin D1 expression in HC11 cells. HC11 cells were infected with either control adenovirus or adenovirus-expressing sh-PIKE. Two days after infection, the cells were serum-starved for 24 h and stimulated with 10 nM recombinant mouse PRL for 24 h. Expression of cyclin D1 was examined by immunoblotting (top panel). PIKE-A expression in HC11 after shRNA infections was confirmed (middle panel). Tubulin level was also examined to show equal input of the proteins (bottom panel). (C) Diminished proliferation in PIKE-depleted mammary gland epithelial cells. HC11 cells were infected with control adenovirus or adenovirus-expressing sh-PIKE. Three days after infection, cell proliferations of the infected cells were examined by trypan blue exclusion assay (***P<0.001, two-way ANOVA).
PIKE is essential for lactation and lobuloalveolar development during lactation
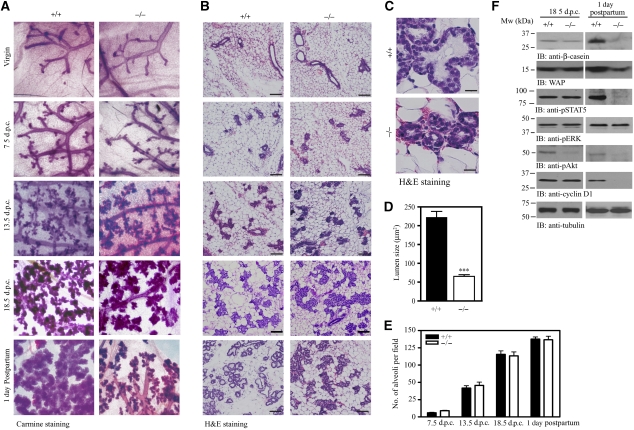
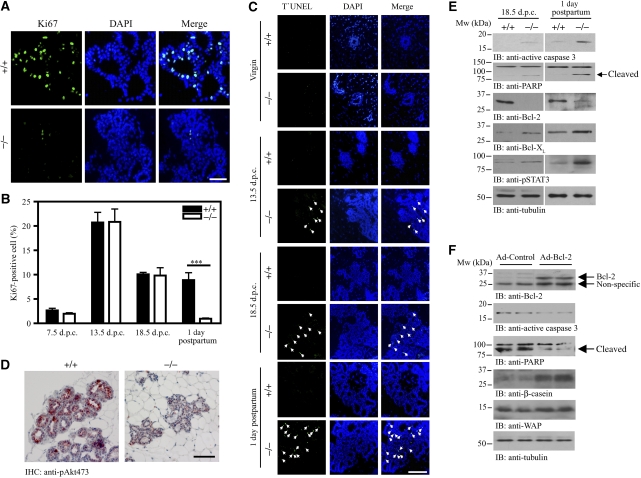
PIKE-A is essential for PRL-induced STAT5a activation and consequential gene transcription. To study the physiological functions of PIKE-A on STAT5 function, we generated PIKE−/− mice (Supplementary data; Supplementary Figure 1). As PRL signalling has a critical function in alveologenesis and lactogenic differentiation during mammary gland development (Ormandy et al, 1997), we focused on the function of PIKE-A in mammary gland function. To determine the effect of PIKE ablation on mammary gland development, we examined the histology of mammary glands from PIKE−/− mice. Whole-mount analysis of mammary glands showed that PIKE−/− virgin females completed normal ductal development. In age-matched PIKE−/− and wild-type females, formation of the mammary gland anlage, elongation, extension of the ductal tree and ductal side branching were similar (Figure 4A, first panel), indicating that normal development occurs before pregnancy. Pregnancy hormones induced comparable proliferation of ductal epithelium and sprouting of alveolar buds in early, mid and late pregnancy (7.5, 13.5 and 18.5 days post-coitus (dpc)) in both wild-type and PIKE−/− mice (Figure 4A, second to fourth panels). At postpartum, fully developed lobuloalveolar structures and dilated primary ducts were evident in wild-type mice; however, the expansion of the alveolar buds into mature lobuloalveolar mammary structures was significantly attenuated in PIKE−/− females (Figure 4A, fifth panel). Histological analysis of sectioned specimens confirmed that the lobuloalveolar development in wild-type and PIKE−/− mice remained similar until 18.5 dpc. At parturition, the mammary gland from multiparous wild-type mice was filled with distended lobuloalveoli, indicating that lactation was successfully engaged. In contrast, PIKE−/− mice alveoli were small and condensed. The lumina were either closed or had accumulated residual luminal secretory lipids (Figure 4B and C). Quantitative analysis of the number of alveoli in various gestation stages revealed no significant difference between PIKE−/− and wild-type dams (Figure 4E); however, the size of the alveolar lumina in PIKE−/− mice during lactation was reduced to 29.2% of the wild-type control (Figure 4D). These results suggest that secretory epithelium in PIKE−/− mammary tissue fails to undergo proliferation at parturition. In addition to the defect in lobuloalveolar development, expression of the STAT5-induced milk proteins β-casein and WAP were also decreased in PIKE−/− mothers at parturition, but not in late gestation (Figure 4F, first and second panels). Similarly, phosphorylation of STAT5 was only diminished in lactation, but not in gestation (Figure 4F, third panel; Supplementary Figure 2A and B). Although Akt phosphorylation was reduced in both late gestation and early lactation, phosphorylation of ERK was not changed (Figure 4F, fourth and fifth panels). The expression of cyclin D1 was also decreased in 1 day postpartum, but not 18.5 dpc (Figure 4F, sixth panel). In contrast to the milk protein production, lipid droplets were present in PIKE−/− mammary alveoli in late gestation and during lactation (Supplementary Figure 2), suggesting that gestational lipid metabolism was not aborgated.
Figure 4.
Lobuloalveolar hypoplasia in PIKE knockout mice. (A) Impaired lobuloalveolar development in PIKE−/− mammary glands. Carmine alum-stained whole mount of wild-type and PIKE−/− mammary glands (fourth inguinal) collected from 14-week-old virgin, pregnant (7.5, 13.5 and 18.5 days post-coitus) and lactating mice (1 day postpartum). Representative results of three individual animals from each genotype were shown. (B) Histological analysis (H&E staining) of mammary glands is shown in (A). Scale bar represents 100 μm. (C) Structure of mammary alveolus of lactating wild-type and PIKE−/− mice. Scale bars represent 10 μm. Representative results from of three individual animals from each genotype were shown. (D) Alveolar lumen size of lactating mammary gland (1 day postpartum). The results are expressed as mean±s.e.m. of three mice (>30 alveoli in each animal) from each genotype. Scar bars represent 10 μm. (E) Alveoli density of pregnant and lactating mammary gland (1 day postpartum). The results are expressed as mean±s.e.m. of three mice (four individual fields in each sample) of each genotype. (F) Impaired protein expression in PIKE−/− mammary gland. Mammary tissues (fourth inguinal) from late gestation (18.5 dpc) and lactating (1 day postpartum) animals were collected and various milk proteins (β-casein and WAP), phosphorylation of signal transduction molecules (STAT5, Akt and ERK) and cyclin D1 levels were analysed by immunoblotting. Expression of β-tubulin was also performed to show equal loading. The results are representative blot of three individual animals from each genotype.
PIKE depletion leads to impaired mammary epithelial cell proliferation and apoptosis
To further explore the effect of PIKE ablation on epithelial proliferation from lactating mice, we conducted Ki67 staining. Compared with wild-type control, substantially less Ki67 positive staining was found in PIKE−/− mammary glands during lactation (Figure 5A). In contrast, no significant difference in cell proliferation, as revealed by positive Ki67 staining, was found between wild-type and PIKE−/− mice during pregnancy (Figure 5B). Similar results were obtained when the lactating mammary tissues were stained with proliferating cell nuclear antigen(Supplementary Figure 3). As PIKE has an essential function in promoting cell survival (Rong et al, 2003; Ahn et al, 2004a, 2004b; Tang et al, 2008), it is possible that ablation of PIKE-A might also trigger apoptosis in mammary epithelial cells, leading to impaired development. To test this possibility, we performed TUNEL assays on mammary gland tissue. No apoptotic epithelial cells were detected in ductal and alveolar tissues of wild-type mice during pregnancy. In contrast, apoptosis was readily detectable among epithelial cells from PIKE−/− females at 13.5 dpc, 18.5 dpc and postpartum. The pronounced apoptosis occurred primarily in epithelial cells of the alveolar buds (Figure 5C). PI3K/Akt signalling has a critical function in suppressing apoptosis in most cell types and tissues, and inactivation of Akt significantly enhances apoptosis. PIKE is essential for the activation of PI3K and Akt in response to various stimuli (Ye et al, 2000; Rong et al, 2003; Ahn et al, 2004b). Thus, in the absence of PIKE, Akt may fail to activate in the mammary gland. To examine Akt activation status in alveolar epithelial cells, we conducted immunohistochemistry with anti-phospho-Akt S473 antibody. Akt phosphorylation was substantially increased in wild-type mice during the postpartum period, as reported (Fata et al, 2000). In contrast, no Akt activation was detectable in the epithelial cells of PIKE−/− mice (Figure 5D). Therefore, loss of PIKE-A results in defective Akt activation in alveolar epithelial cells. We further studied the molecular mechanism of the apoptosis found in PIKE−/− mammary tissues by examining the expression of various apoptotic signalling components. In agreement with the results from the TUNEL assay, apoptotic signals including enhanced caspase 3 and PARP cleavage (Figure 5E, first and second panels) and STAT3 phosphorylation (Figure 5E, fifth panel) were detected in PIKE−/− mammary tissue in both 18.5 dpc and 1 day postpartum. The expression of the pro-apoptotic factor Bcl-2 was also reduced (Figure 5E, third panel). However, the levels of another anti-apoptotic protein, Bcl-XL, were increased (Figure 5E, fourth panel), perhaps as a compensatory response to the increasing cell death in PIKE−/− tissue. Together, these results indicate that PIKE-A-mediated Akt activation is important for epithelial cell proliferation and survival in mammary gland.
Figure 5.
Defective proliferation and enhanced apoptosis in lactating mammary epithelial cells from PIKE−/− mice. (A) Ki67 staining of lactating mammary glands (1 day postpartum, fourth inguinal) from wild-type and PIKE−/− mice. Representative results of three individual animals from each genotype are shown. Scale bar represents 50 μm. (B) Quantitative measurement of cells showing positive Ki67 staining from different gestation stage (n=3, ***P<0.001, Student's t-test). (C) Apoptosis occurs in PIKE−/− mammary gland. Paraffin-embedded fourth inguinal mammary glands from wild-type or PIKE−/− mice at virgin, mid-pregnancy (13.5 dpc), late pregnancy (18.5 dpc) and lactation (1 day postpartum) were examined by TUNEL (green) and the total nuclei were stained with DAPI (blue). Apoptotic nuclei (as indicated by arrows) were found in PIKE−/− mammary gland, but not in the wild-type control. Representative results of three individual animals from each genotype at specific time point are shown. (D) Akt phosphorylation in lactating mammary epithelial cells (fourth inguinal, 1 day postpartum) was determined by immunohistochemical staining using phosphor-Ser473 antibody. Diminished Akt phosphorylation was found in PIKE−/− mice. Representative results of three individual animals from each genotype are shown. Scale bar represents 50 μm. (E) Apoptotic signalling in PIKE−/− mammary gland. Expression of various apoptotic signalling components was examined in late gestation (18.5 dpc) and lactating mammary gland (1 day postpartum) collected from PIKE−/− and wild-type mice. Expression of β-tubulin was also performed to show equal loading. Shown are representative blots from three individual animals from each genotype. (F) Overexpression of Bcl-2 in PIKE−/− mammary gland. PIKE−/− mice in mid-gestation (12.5 dpc) were administrated with control adenovirus (Ad-control) or adenovirus carrying Bcl-2 (Ad-Bcl-2) through intaductal injection. Mammary glands were collected at lactation (1 day postpartum) and proteins were extracted for western blot analysis as indicated.
To confirm that the impaired mammary gland development during lactation resulted, at least in part, from increased apoptosis in PIKE-null tissue, we attempted to rescue the defective phenotype by overexpressing the anti-apoptotic factor Bcl-2 in vivo. Adenovirus overexpressing Bcl-2 (Ad-Bcl2) was delivered to gestating (12.5 dpc) PIKE−/− mammary gland through intraductal injection (Russell et al, 2003). As shown in Figure 5F, the cleavage of caspase 3 and PARR was significantly diminished in Ad-Bcl2-injected mammary gland (second and third panels). Further, differentiation of mammary epithelial cells, as revealed by increased WAP and β-casein expressions (Figure 5F, fifth and sixth panels), was enhanced when Bcl-2 was overexpressed. These results strongly support the conclusion that enhanced apoptosis is responsible for the defective mammary gland development in PIKE−/− mice.
STAT5 activation is diminished in PIKE−/− mammary gland
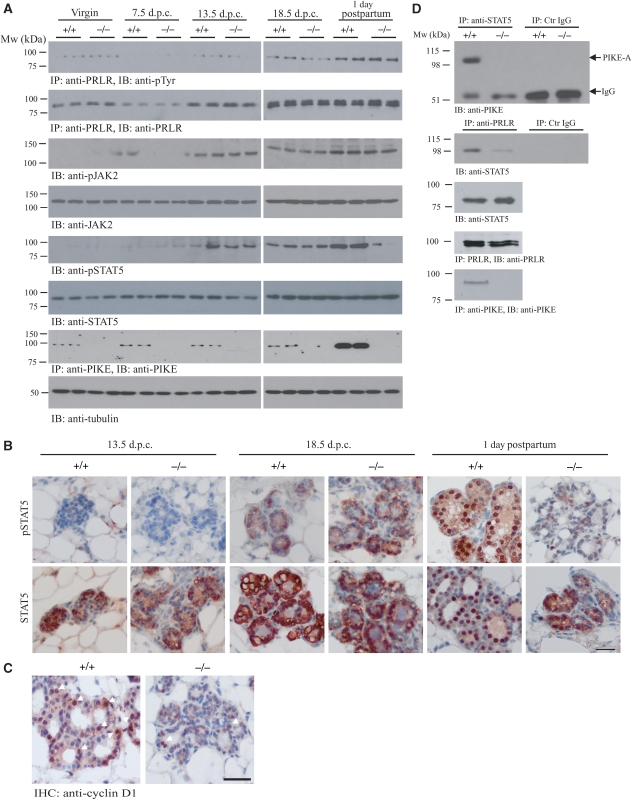
As impaired mammary gland development in lactating PIKE−/− mice occurred only postpartum (Figure 4A), we hypothesized that defective STAT5 activation might be confined to lactation, but not gestation, in PIKE−/− mammary tissue. To test this notion, we monitored JAK2 and STAT5 phosphorylation in mammary gland tissue from virgin, pregnant and lactating mice. Immunoblotting analysis revealed that PRLR tyrosine phosphorylation was strong at 18.5 days of pregnancy and sustained during the postpartum period in both wild-type and PIKE−/− mice. Expression of PRLR increased in both genotypes during gestation, but a reduction of receptor expression was observed at 7.5 dpc. This finding is consistent with earlier report that mRNA of PRLR decreases during middle pregnancy (Hovey et al, 2001) (Figure 6A, first and second panels). The specificity of the anti-PRLR antibody was confirmed as no signal was detected in immunoprecipitation using mammary gland from PRLR−/− mice (Supplementary Figure 4). In alignment with these observations, STAT5 phosphorylation was negligible in virgins and at 7.5 days of pregnancy, but demonstrable at 13.5 and 18.5 days of pregnancy. Significant phosphorylation of JAK2 could be detected at 13.5 dpc. in both genotypes. At parturition, high JAK2 activation was detectable in both wild-type and PIKE−/− mice; however, in some virgin (PIKE−/−) or 7.5 dpc (PIKE+/+) mammary samples, faint but detectable JAK2 phosphorylation was observed, possibly because of the individual variation among different mice. The total expression level of JAK2 was comparable in all mice (Figure 6A, fourth panel). Although STAT5 activation was substantially increased in wild-type mice during lactation, it was severely reduced in PIKE−/− mice (Figure 6A, fifth panel). The total STAT5 protein levels remained constant in mice regardless of genotype (Figure 6A, sixth panel). These findings are consistent with earlier reports that phosphorylation of JAK2 and STAT5 is very low in the mammary gland of virgins and during early pregnancy, but rises sharply around mid-pregnancy and persists throughout lactation and involution (Liu et al, 1996, 1997). Interestingly, mammary PIKE-A expression was significantly increased during lactation, but not during gestation (Figure 6A, seventh panel), which further supports the critical function of PIKE-A in mammary gland development during lactation.
Figure 6.
STAT5 phosphorylation is diminished in PIKE−/− mammary gland. (A) Wild-type (+/+) and PIKE (−/−) mammary gland (fourth inguinal) at different times of pregnancy (14-week-old virgin, 7.5 days, 13.5 days, 18.5 post-coitus and 1 day postpartum) were collected and homogenized. The phosphorylation of JAK2 (third panel) and STAT5 (fifth panel) were determined using specific antibodies as indicated. Phosphorylation of PRLR was determined by immunoprecipitation followed by phospho-tyrosine determination (first panel). The expression of PRLR (second panel), JAK2 (fourth panel), total STAT5 (sixth panel) and PIKE-A (seventh panel) were also examined. Tubulin expression of each sample was determined to show equal loading (eighth panel). (B) Phosphorylation of STAT5 was impaired in lactating PIKE−/− mammary gland epithelial cells. Phosphorylation of STAT5 was determined by immunohistochemical analysis in mammary gland (fourth inguinal) collected at mid-gestation (13.5 dpc), late gestation (18.5 dpc) and lactation day 1 (upper panel). Total STAT5 levels were also determined (lower panel). Representative results from three individual mice of each genotype were shown. Scale bar represents 50 μm. (C) Expression of cyclin D1 was impaired in PIKE−/− mammary gland epithelial cells. Cyclin D1 expression was determined by immunohistochemical analysis in lactating mammary gland (1 day postpartum). Positive cells showing nuclear cyclin D1 staining are indicated by arrows. Representative results from three individual mice of each genotype were shown. Scale bar represents 50 μm. (D) Association of endogenous STAT5 and PIKE-A in fourth inguinal mammary gland (1 day postpartum). Endogenous STAT5 was immunoprecipitated using specific antibody against total STAT5 and the bound PIKE-A was detected using anti-PIKE-A antibody. Association of STAT5 and PIKE-A was detected in wild type, but not PIKE−/− mammary gland (first panel). PRLR bound to STAT5 in wild type, but not PIKE−/− mice (third panel). PRLR in the cell lysates was immunoprecipitated using anti-PRLR antibody and the associated STAT5 was detected using anti-STAT5 antibody (second panel). The presence of STAT5 (third panel), PRLR (fourth panel) and PIKE-A (fifth panel) in the cell lysates was verified.
Defective STAT5 activation was further supported by immunohistochemical analysis. Robust STAT5 phosphorylation accumulated in the nuclei of lactating mammary epithelial cells from wild type, but not PIKE−/− mice (Figure 6B, upper panel). Although total STAT5 staining revealed that most STAT5 proteins resided in the nucleus of wild-type mammary, STAT5 was mostly localized to the cytoplasm of the epithelial cells in lactating PIKE−/− mice, consistent with the notion that phosphorylation is essential for STAT5 nuclear translocation (Figure 6B, lower panel). Nuclear cyclin D1 expression was also substantially reduced in PIKE−/− mammary epithelial cells at 1 day postpartum (Figure 6C). This observation concurred with the reduced cyclin D1 protein levels as assessed by western blot in lactating mammary tissue from PIKE−/− females compared with wild-type control (Figure 4F, sixth panel). The expression pattern of cyclin D1 tightly correlated with STAT5 activation status, confirming that STAT5 activation is required for cyclin D1 expression. These results suggest that reduced cyclin D1 expression might underlie the proliferation defect in PIKE−/− mammary epithelium.
Our studies in HC11 cells also showed the formation of PIKE-A/STAT5/PRLR complex during PRL stimulation (Figure 2A). To assess whether this complex exists in vivo, we performed coimmunoprecipitation assays in mammary gland tissues collected from lactating mice, when the circulating PRL concentration is high (Supplementary Figure 5). STAT5 strongly and selectively associated with PIKE-A in wild type, but not PIKE−/− mice (Figure 6C, first panel), verifying our in vitro binding results. Interestingly, the association of STAT5 with PRLR was also diminished in PIKE−/− mammary tissues (Figure 6C, second panel), further indicating that PIKE-A is important for the interaction between STAT5 and PRLR.
Defective mammary epithelial development in PIKE−/− mice is cell autonomous and could be restored by a cyclin D1 transgene
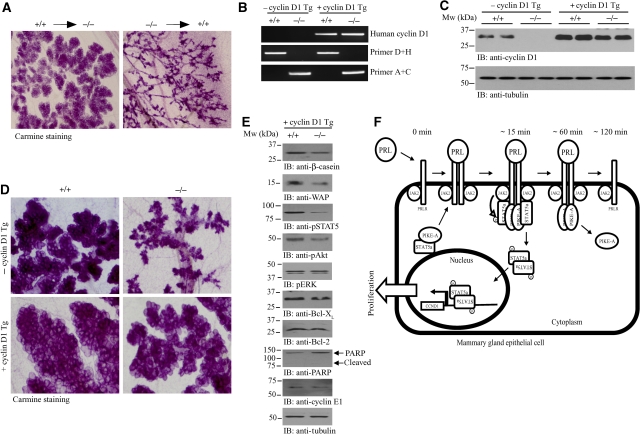
To determine whether the impaired development of PIKE−/− mammary epithelial cells is mammary cell autonomous, we performed transplantation experiments as described earlier (Mori et al, 2000; Roarty et al, 2009). A primary mammary gland network could be regenerated in wild-type recipient mice injected with PIKE−/− mammary epithelia, indicating that PIKE-A is dispensable for pubertal mammary gland development. Although successful lobuloalveolar development was detected in PIKE−/− mice (1 day postpartum) receiving wild-type mammary epithelial cells (Figure 7A, left panel), PIKE−/− epithelial cells failed to produce lobuloalveolar structure in wild-type recipient (Figure 7A, right panel), showing that PIKE−/− mammary epithelial cells cannot respond to the normal developmental cues of late pregnancy, whereas PIKE−/− hosts can competently provide these cues.
Figure 7.
Rescue of PIKE−/− mammary gland defect by a cyclin D1 transgene. (A) Cell autonomous defect in PIKE−/− mammary gland. Whole-mount analysis of transplanted mammary glands were stained with carmine alum on lactation day 1. Directions of transplantation are indicated. (B) PCR screening of mice from heterozygous mating of PIKE+/−MMTV-cyclinD1+/− mice. (C) Western blot analysis of cyclin D1 expression in the mammary gland (fourth inguinal) of lactating mice as shown in (B). (D) Carmine alum-stained whole mount of cyclin D1 transgene bearing wild-type and PIKE−/− mammary glands (fourth inguinal) collected from lactating mice (1 day postpartum). Representative results from two individual animals of each genotype were shown. (E) Characterization of mammary gland from PIKE−/−MMTV-cyclin D1 mice. Proteins were extracted from lactating mammary gland (1 day postpartum) and used for western blot analysis as indicated. (F) Proposed function of PIKE-A in mammary PRL signalling cascade.
Our data suggest that the defect in PIKE−/− mammary glands during lactation was caused by the failure of PRL-induced STAT5a phosphorylation, which results in impaired transcription of cyclin D1 and a reduction in cell proliferation. If this is true, the functional defect of PIKE−/− mammary tissue should be rescued by overexpressing cyclin D1. To test whether overexpression of cyclin D1 could restore cell proliferation during lactation, we crossed PIKE−/− mice with the MMTV-cyclin D1 transgenic line, which overexpresses cyclin D1 in mammary tissue (Wang et al, 1994) to produce PIKE−/−MMTV-cyclin D1 mice (Figure 7B and C). After delivery, PIKE−/−MMTV-cyclin D1 dams exhibited functional rescue of lactation, as shown by the presence of milk in the stomach of newborns (data not shown) and the weight gain of the pups, which was indistinguishable from those suckled by the control PIKE+/+MMTV-cyclin D1 dams (Supplementary Figure 6A). Whole-mount and histological analysis of mammary gland at 1 day postpartum revealed normal lobuloalveolar development and proliferation in PIKE−/−MMTV-cyclin D1 dams (Figure 7D; Supplementary Figure 6B–D), Apoptotic signals including Bcl-2 and Bcl-XL expressions (Figure 7E, sixth and seventh panels), PPAR cleavage (Figure 7E, eighth panel) and caspase 3 activation (data not shown) were relieved in lactating PIKE−/−MMTV-cyclin D1 mammary gland, indicating that apoptosis was not initiated. This result was further confirmed by the lack of TUNEL positive cells in PIKE−/− mammary gland with cyclin D1 overexpression (Supplementary Figure 6). Mammary STAT5 and Akt phosphorylations were not fully restored in PIKE−/−MMTV-cyclin D1 mice (Figure 7E, third and fourth panels), and there was a slight reduction of β-casein and WAP expressions (Figure 7E, first and second panels; Supplementary Figure 6E). Together, these data show that overexpression of cyclin D1 in PIKE-null mammary gland can rescue lactation, via full rescue of epithelial proliferation and near complete restoration of cell signalling and milk protein production.
Discussion
In this study, we show that PIKE-A is a novel component in the PRLR/STAT5 signalling. PIKE-A directly associates with both STAT5a and PRLR, which is affected by PRL stimulation. PIKE-A might thus serve as an adapter mediating the interaction between STAT5 and PRLR. During PRL stimulation, PIKE-A couples STAT5a to the PRLR. This receptor-associated PIKE-A/STAT5 complex is disrupted by Jak2 phosphorylation on STAT5, leading to STAT5 nuclear translocation (Figure 7F). This model is strongly supported by the defective mammary gland development during lactation in PIKE−/− mice, when PRL/STAT5 signalling executes its predominant function in promoting mammary gland proliferation and differentiation. Depletion of PIKE-A in mammary epithelial, therefore, impairs the interaction between STAT5a and PRLR (Figure 6D), and abolishes the PRL-induced gene transcriptions such as cyclin D1, β-casein and WAP (Figure 4F). It is noteworthy that the interaction between PIKE-A and PRLR is transient, which dissociates after 2 h PRL stimulation. The mechanism leading to PIKE-A/PRLR dissociation is currently unknown, but we have shown that phosphorylation of PRLR is essential for PRLR/PIKE-A association (Figure 2B and C). Presumably, dephosphorylation of PRLR by phosphatase or interaction with inhibitory proteins such as suppressor of cytokine signalling (Sutherland et al, 2007) might serve as the disassembly signal for PRLR/PIKE association.
Our results show that PIKE-A is essential for epithelial cell proliferation in mammary gland during lobuloalveolar development. This notion is supported by the fact that PIKE-A expression is not changed during gestation, but increased drastically on lactation, which correlates well with the temporal pattern of STAT5 phosphorylation (Figure 6A). As such, the virgin, early and late-pregnant mammary gland differentiations of mature PIKE−/− females were similar to their wild-type counterparts. During lactation, however, when high JAK2/STAT5 activation is required for milk protein production and alveolar epithelial cell proliferation, PIKE-A ablation disrupts STAT5 activation resulting in lobuloalveolar and lactogenic defects. It is tempting to speculate that PIKE-A may be involved in the mechanisms that suppress lactation during gestation.
Knockdown of PIKE-A in cancer cells diminishes Akt activity and enhances apoptosis (Ahn et al, 2004b). Here, we show that ablation of PIKE-A in mammary epithelial HC11 cells inhibits cell proliferation in vitro (Figure 3C). In addition to reduced STAT5 activation, we also show that ablation of PIKE-A in mammary gland in vivo provokes spontaneous apoptosis in epithelial cells in pregnant and lactating PIKE−/− females (Figure 5), further confirming that PIKE-A is indispensable for promoting cell survival in different cell types. Moreover, we found that Akt activation was prominently diminished in PIKE−/− mammary gland (Figures 4F and 5D), indicating that PIKE-A stimulates cell survival by inhibiting apoptosis through upregulating Akt. On the other hand, PRL/JAK2/STAT5 also mediates the transcription anti-apoptotic genes such as Bcl-2 (Dumon et al, 1999). Diminished expression of Bcl-2 in PIKE−/− mammary gland (Figure 5E) might further contribute to the higher apoptotic activity in PIKE-A-deficient epithelial cells.
Interestingly, PIKE−/− mice displayed a noticeable defect in mammary epithelial cell proliferation, which is reminiscent of observations in several lines of mice carrying mutations in different genes, including ERBB4, STAT5a and cyclin D1. These mice exhibit substantially reduced lobuloalveolar outgrowth and severely underdeveloped alveolar buds with small and condensed lumen. They display impaired STAT5/cyclin D1 signalling, which was also observed in our PIKE−/− mice. Loss of ERBB4 or STAT5A expression results in the accumulation of histologically identical lobuloalveolar defects during pregnancy, and a failure of lactation. It is interesting to note that PRLR signalling remains intact in mammary glands of ERBB4 Flox/FloxWap-Cre mice (Long et al, 2003), but PRL-mediated STAT5 signalling was disrupted in PIKE−/− epithelia, which was further supported by the results from in vitro PIKE-A knockdown in HC11 cells. Impairment of STAT5 activation begins at mid-pregnancy (13.5 dpc) in ERBB4−/− mammary and remains inactivated until postpartum (Long et al, 2003; Tidcombe et al, 2003), but mammary STAT5 phosphorylation is diminished only after delivery in PIKE−/− dams (Figure 6A). Furthermore, intracellular domain of ERBB4 (4ICD) functions as the nuclear chaperone of STAT5 by enhancing its nuclear translocation (Williams et al, 2004), but PIKE-A serves as the adaptor that anchors STAT5a to the PRLR in the cytoplasm (Figure 7). These data propose the mechanistic and temporal discrepancies between PIKE and ERBB4 in mammary gland development. Nevertheless, the phenotypic outcomes of PIKE−/− and ERBB4−/− in mammary tissue highlight the functional significance of STAT5, which serves as a converging point between PRLR and ERBB4 pathways during mammogenesis. As such, independent signals from different stimuli are coupled at STAT5 activation, leading to the functional differentiation of mammary gland.
PIKE−/− mice exhibited markedly reduced cyclin D1 expression and epithelial cell proliferation (Figures 3, 4 and 6). Cyclin D1 is transcriptionally regulated by STAT5; however, its nuclear localization and cytoplasmic degradation are controlled by Akt/GSK-3 signalling (Diehl et al, 1998). Ablation of PIKE-A not only cripples STAT5 activation but also inhibits Akt activation, which might further reduce cyclin D1 expression in the mammary gland. Consequently, the defective lobuloalveolar development in PIKE−/− females resembles the impaired mammary gland development in cyclin D1−/− mice. Most importantly, restoration of mammary cyclin D1 to its normal level in PIKE−/− mice fully rescued the mammary gland development (Figure 7B) and milk production after delivery. The normal lactation activity in PIKE−/−MMTV-cyclin D1 dams, therefore, strongly support that impaired PIKE-A/STAT5/cyclin D1 signalling is the major cause of the defective lactogenesis observed in the PIKE−/− mice.
In summary, our results indicate that PIKE-A is a new member of the PRL signalling cascade, and PIKE-A serves as an adaptor protein coupling STAT5a to PRLR during lactation. PIKE-A/STAT5a association is essential for PRL to provoke STAT5a tyrosine phosphorylation and activation, and this interaction is indispensable for lobuloalveolar development of mammary gland.
Materials and methods
Generation of knockout animals and genotyping
Heterozygous PIKE knockout C57BL/6 mice with a targeted deletion of exon 3 to 6 of CENTG1 were generated by Ozgene (Australia). Mice were then bred to homozygosity. Genotyping of offspring was performed by PCR using genomic DNA from tail biopsies. Tail samples were digested overnight in nuclear lysis solution containing 0.4 × SSC, 10 mM Tris pH 7.5, 1 mM EDTA, 1% SDS and 0.5 mg/ml proteinase K. Genomic DNA was then purified by ethanol precipitation. PCR was performed using a combination of primer D (5′-ACAGGATCAGTGCATCATCTC-3′) and H (5′-CTGCCCAGCTACAGGAGTAG-3′), and primer A (5′-TCAGTTGACTGGAAGCTCTG-3′) and C (5′-CCAGAGCCTATCTATGCCTAG-3′).
Genotyping of MMTV-cyclin D1 transgene (Wang et al, 1994) was performed by PCR using forward (5′-GAACAAACAGATCATCCGCAAA-3′) and reverse (5′-TCGATCTGCTCCTGGCAGGCC-3′) primers.
Cell culture and transfection
Mouse mammary epithelial HC11 cells were cultured as reported earlier (Neilson et al, 2007). HEK293 cells were maintained in DMEM containing 10% FBS and 1 × Penicillin-Streptomycin (Invitrogen, USA). Transfections on HEK293 and HC11 cells were performed using Lipofectamine 2000 (Invitrogen, USA) as instructed.
Histology, whole-mount analysis and immunohistochemistry staining
Mammary tissues were fixed in 4% paraformaldehyde overnight followed by paraffin embedding. Sections of 8–10 μm were cut, rehydrated in graded ethanol and stained with H&E by standard protocol. Whole-mount analysis of intact mammary gland was performed as reported earlier (Seagroves et al, 1998). For immunohistochemical staining, mammary gland sections were deparaffinized in xylene and rehydrated in graded alcohols. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 5 min and all slides were boiled in 10 mM sodium citrate buffer (pH 6.0) for 10 min. Phosphorylated Akt, JAK2 and Stat5 were detected using specific antibodies from Cell Signaling (USA) and Zymed Histo-SP AEC kit (Invitrogen, USA). Slides were then counterstained with haematoxylin.
TUNEL assay
Mammary gland apoptosis was detected using an in situ cell death detection kit (Roche Diagnostics, USA).
Luciferase assay
HC11 cells transfected with the PRE3-Luc plasmid consists of three copies of the consensus sequence for the Stat5-binding site (TTCTTGGAA) from the cyclin D1 promoter (PRL response element, PRE), upstream of a luciferase reporter (Brockman et al, 2002). Luciferase activity of cell lysates was then determined using Dual Luciferase Assay Kit (Promega, USA).
Immunoprecipitation and western blotting
Tissue extracts were prepared by homogenizing the tissues in buffer containing 50 mM Tris, pH 7.4, 40 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1.5 mM Na3VO4, 50 mM NaF, 10 mM Na4P2O7 and 10 mM sodium β-glycerol phosphate and protease inhibitor cocktail. Cell debris was removed by centrifugation, and the supernatants were collected and used for further analysis. Immunoprecipitation using specific antibodies as indicated was performed as reported (Ye et al., 2000). Antibodies used in the western blot analysis were obtained from Santa Cruz Biotechnology, USA (Akt, β-casein, WAP, cyclin D1, Bcl-2, Bcl-XL and β-tubulin), Thermo Fisher Scientific Inc., USA (PRLR) and Cell Signaling Technology, USA (phosphor-Ser473 of Akt, phosphor-JAK2, phospho-STAT5, STAT5, phospho-ERK, active caspase 3 and PARP).
Intraductal adenovirus injection
Adenovirus overexpressing mouse Bcl-2 gene (1 × 108 pfu in PBS) (Vector Biolabs, USA) was injected into the teat canal of pregnant (12.5 dpc) PIKE−/− mice as described earlier (Russell et al, 2003). The mammary glands were collected at 1 day postpartum and proteins were extracted for western blot analysis as indicated.
Mammary gland transplantation
Transplantations were carried out as described (Roarty et al, 2009). Briefly, mammary glands were obtained from 8-week-old wild-type or PIKE−/− mice and subjected to collagenase digestion to isolate the mammary epithelial cells. Mammary organiods were then prepared by differential centrifugation, washed win PBS and resuspended in DMEM. A 50-μl volume of organoids was injected into the clear fat pad of the fourth mammary gland on 3-week-old wild-type or PIKE−/− mice. Successful delivery of organoid suspension was confirmed by visible engorgement of the fat pad on injection. Six weeks after transplantation, the mice were subjected to mating and the transplanted mammary glands were collected at 1 day postpartum.
Statistical analysis
The results were expressed as mean±s.e.m. and were considered significant when P<0.05. Statistical analysis of the data was performed using either t-test or two-way ANOVA followed by Tukey's multiple comparison test or Bonferroni post-tests by the computer program GraghPad Prism (GraphPad Software, USA).
Supplementary Material
Acknowledgments
This work is supported by grant RO1 (NS045627) from NIH to K Ye. We thank Dr D Weinshenker (Emory University, Atlanta, USA) for the critical reading and the helpful comments on this manuscript. We also thank Eileen Breding and Evan Dessasau III (Yerkes National Primate Research Center, Emory University, Atlanta, USA) for their technical assistance in preparing the mammary gland tissue sections. We are grateful to Dr A Arnold (University of Connecticut School of Medicine, Farmington, USA) for providing the MMTV-cyclin D1 transgenic mice; Dr NC Reich (Stony Brook University, NY, USA) for the GFP-STAT5a; Dr DJ Donoghue (University of California at San Diego, La Jolla, USA) for the GFP-STAT5b; Dr M David (University of California at San Diego, La Jolla, USA) for the GFP-STAT1; Dr K Ikuta (Kyoto University, Kyoto, Japan) for various myc-STAT5 constructs; Dr LA Schuler (University of Wisconsin-Madison, Madison, USA) for the PRE-Luciferase plasmid; Dr H Rui (Thomas Jefferson University, Philadelphia, USA) for the HC11 cells and Dr AF Parlow (National Hormone and Peptide Program, NIH, USA) for the recombinant mouse prolactin.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahn JY, Hu Y, Kroll TG, Allard P, Ye K (2004a) PIKE-A is amplified in human cancers and prevents apoptosis by up-regulating Akt. Proc Natl Acad Sci USA 101: 6993–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JY, Rong R, Kroll TG, Van Meir EG, Snyder SH, Ye K (2004b) PIKE (phosphatidylinositol 3-kinase enhancer)-A GTPase stimulates Akt activity and mediates cellular invasion. J Biol Chem 279: 16441–16451 [DOI] [PubMed] [Google Scholar]
- Ahn JY, Rong R, Liu X, Ye K (2004c) PIKE/nuclear PI 3-kinase signaling mediates the antiapoptotic actions of NGF in the nucleus. EMBO J 23: 3995–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N, LaPensee CR, LaPensee EW (2008) What can we learn from rodents about prolactin in humans? Endocr Rev 29: 1–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C (2002) Hormonal control of alveolar development and its implications for breast carcinogenesis. J Mammary Gland Biol Neoplasia 7: 39–48 [DOI] [PubMed] [Google Scholar]
- Brisken C, Rajaram RD (2006) Alveolar and lactogenic differentiation. J Mammary Gland Biol Neoplasia 11: 239–248 [DOI] [PubMed] [Google Scholar]
- Brockman JL, Schroeder MD, Schuler LA (2002) PRL activates the cyclin D1 promoter via the Jak2/Stat pathway. Mol Endocrinol 16: 774–784 [DOI] [PubMed] [Google Scholar]
- Brockman JL, Schuler LA (2005) Prolactin signals via Stat5 and Oct-1 to the proximal cyclin D1 promoter. Mol Cell Endocrinol 239: 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Wang J, Li R, Ayala G, Ittmann M, Liu M (2009) GGAP2/PIKE-a directly activates both the Akt and nuclear factor-kappaB pathways and promotes prostate cancer progression. Cancer Res 69: 819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CB, Ye K (2007) PIKE GTPase are phosphoinositide-3-kinase enhancers, suppressing programmed cell death. J Cell Mol Med 11: 39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger CV (2004) Roles and regulation of stat family transcription factors in human breast cancer. Am J Pathol 165: 1449–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE Jr, Kerr IM, Stark GR (1994) Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264: 1415–1421 [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ (1998) Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12: 3499–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumon S, Santos SC, Debierre-Grockiego F, Gouilleux-Gruart V, Cocault L, Boucheron C, Mollat P, Gisselbrecht S, Gouilleux F (1999) IL-3 dependent regulation of Bcl-xL gene expression by STAT5 in a bone marrow derived cell line. Oncogene 18: 4191–4199 [DOI] [PubMed] [Google Scholar]
- Erwin RA, Kirken RA, Malabarba MG, Farrar WL, Rui H (1995) Prolactin activates Ras via signaling proteins SHC, growth factor receptor bound 2, and son of sevenless. Endocrinology 136: 3512–3518 [DOI] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C (1995) Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev 9: 2364–2372 [DOI] [PubMed] [Google Scholar]
- Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM (2000) The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103: 41–50 [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW (2001) Signaling pathways in mammary gland development. Dev Cell 1: 467–475 [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW, Wagner KU, Liu W (1997) Prolactin signaling in mammary gland development. J Biol Chem 272: 7567–7569 [DOI] [PubMed] [Google Scholar]
- Hovey RC, Trott JF, Ginsburg E, Goldhar A, Sasaki MM, Fountain SJ, Sundararajan K, Vonderhaar BK (2001) Transcriptional and spatiotemporal regulation of prolactin receptor mRNA and cooperativity with progesterone receptor function during ductal branch growth in the mammary gland. Dev Dyn 222: 192–205 [DOI] [PubMed] [Google Scholar]
- Ihle JN, Kerr IM (1995) Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet 11: 69–74 [DOI] [PubMed] [Google Scholar]
- Jensen EV, Cheng G, Palmieri C, Saji S, Makela S, Van Noorden S, Wahlstrom T, Warner M, Coombes RC, Gustafsson JA (2001) Estrogen receptors and proliferation markers in primary and recurrent breast cancer. Proc Natl Acad Sci USA 98: 15197–15202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hu Y, Hao C, Rempel SA, Ye K (2007) PIKE-A is a proto-oncogene promoting cell growth, transformation and invasion. Oncogene 26: 4918–4927 [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Hennighausen L (1996) Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol Endocrinol 10: 1496–1506 [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L (1997) Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 11: 179–186 [DOI] [PubMed] [Google Scholar]
- Liu Z, Jang SW, Liu X, Cheng D, Peng J, Yepes M, Li XJ, Matthews S, Watts C, Asano M, Hara-Nishimura I, Luo HR, Ye K (2008) Neuroprotective actions of PIKE-L by inhibition of SET proteolytic degradation by asparagine endopeptidase. Mol Cell 29: 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long W, Wagner KU, Lloyd KC, Binart N, Shillingford JM, Hennighausen L, Jones FE (2003) Impaired differentiation and lactational failure of Erbb4-deficient mammary glands identify ERBB4 as an obligate mediator of STAT5. Development 130: 5257–5268 [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Shillingford JM, Smith GH, Grimm SL, Wagner KU, Oka T, Rosen JM, Robinson GW, Hennighausen L (2001) Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J Cell Biol 155: 531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Nishikawa SI, Yokota Y (2000) Lactation defect in mice lacking the helix-loop-helix inhibitor Id2. EMBO J 19: 5772–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson LM, Zhu J, Xie J, Malabarba MG, Sakamoto K, Wagner KU, Kirken RA, Rui H (2007) Coactivation of janus tyrosine kinase (Jak)1 positively modulates prolactin-Jak2 signaling in breast cancer: recruitment of ERK and signal transducer and activator of transcription (Stat)3 and enhancement of Akt and Stat5a/b pathways. Mol Endocrinol 21: 2218–2232 [DOI] [PubMed] [Google Scholar]
- Neville MC, McFadden TB, Forsyth I (2002) Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia 7: 49–66 [DOI] [PubMed] [Google Scholar]
- Neville MC, Morton J (2001) Physiology and endocrine changes underlying human lactogenesis II. J Nutr 131: 3005S–3008S [DOI] [PubMed] [Google Scholar]
- Oakes SR, Hilton HN, Ormandy CJ (2006) The alveolar switch: coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium. Breast Cancer Res 8: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA (1997) Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 11: 167–178 [DOI] [PubMed] [Google Scholar]
- Roarty K, Baxley SE, Crowley MR, Frost AR, Serra R (2009) Loss of TGF-beta or Wnt5a results in an increase in Wnt/beta-catenin activity and redirects mammary tumour phenotype. Breast Cancer Res 11: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GW, Johnson PF, Hennighausen L, Sterneck E (1998) The C/EBPbeta transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev 12: 1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K (2003) PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci 6: 1153–1161 [DOI] [PubMed] [Google Scholar]
- Rui H, Kirken RA, Farrar WL (1994) Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem 269: 5364–5368 [PubMed] [Google Scholar]
- Russell TD, Fischer A, Beeman NE, Freed EF, Neville MC, Schaack J (2003) Transduction of the mammary epithelium with adenovirus vectors in vivo. J Virol 77: 5801–5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Creamer BA, Triplett AA, Wagner KU (2007) The Janus kinase 2 is required for expression and nuclear accumulation of cyclin D1 in proliferating mammary epithelial cells. Mol Endocrinol 21: 1877–1892 [DOI] [PubMed] [Google Scholar]
- Schindler C, Darnell JE Jr (1995) Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem 64: 621–651 [DOI] [PubMed] [Google Scholar]
- Seagroves TN, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington GJ, Rosen JM (1998) C/EBPbeta, but not C/EBPalpha, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev 12: 1917–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA (1995) Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82: 621–630 [DOI] [PubMed] [Google Scholar]
- Sutherland KD, Lindeman GJ, Visvader JE (2007) Knocking off SOCS genes in the mammary gland. Cell Cycle 6: 799–803 [DOI] [PubMed] [Google Scholar]
- Tang X, Jang SW, Okada M, Chan CB, Feng Y, Liu Y, Luo SW, Hong Y, Rama N, Xiong WC, Mehlen P, Ye K (2008) Netrin-1 mediates neuronal survival through PIKE-L interaction with the dependence receptor UNC5B. Nat Cell Biol 10: 698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP (2003) Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci USA 100: 8281–8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV (1994) Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369: 669–671 [DOI] [PubMed] [Google Scholar]
- Williams CC, Allison JG, Vidal GA, Burow ME, Beckman BS, Marrero L, Jones FE (2004) The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol 167: 469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K, Aghdasi B, Luo HR, Moriarity JL, Wu FY, Hong JJ, Hurt KJ, Bae SS, Suh PG, Snyder SH (2002) Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature 415: 541–544 [DOI] [PubMed] [Google Scholar]
- Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong JJ, Blackshaw S, Ferris CD, Snyder SH (2000) Pike. A nuclear gtpase that enhances PI3kinase activity and is regulated by protein 4.1N. Cell 103: 919–930 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.