Abstract
Objective
To determine the incidence of vasospasm in children who have suffered moderate to severe traumatic brain injury.
Methods
A prospective observational pilot study in a 24-bed pediatric intensive care unit was performed. Twenty-two children aged 7 months to 14 years with moderate to severe traumatic brain injury as indicated by Glasgow Coma Score ≤12 and abnormal head imaging were enrolled. Transcranial Doppler ultrasound was performed to identify and follow vasospasm. Patients with a flow velocity in the middle cerebral artery (MCA) >120 cm/s were considered to have vasospasm by criterion A. If flow velocity in the MCA was >120 cm/s and the Lindegaard ratio was >3, vasospasm was considered to be present by criterion B. Patients with basilar artery (BA) flow velocity >90 cm/s met criteria for vasospasm in the posterior circulation (criterion C).
Results
In the MCA, 45.5% of patients developed vasospasm based on criterion A and 36.3% developed vasospasm based on criterion B. A total of 18.2% of patients developed vasospasm in the BA by criterion C. Typical day of onset of vasospasm was hospital day 2–3. Duration of vasospasm in the anterior circulation was 4 ± 2 days based on criteria A and 3 ± 1 days based on criteria B. Vasospasm in the posterior circulation persisted for 2 ± 1 days.
Conclusions
Using the adult criteria outlined above to diagnose vasospasm, a significant proportion of pediatric patients who have suffered moderate to severe traumatic brain injury develop vasospasm during the course of their treatment.
Keywords: Vasospasm, Transcranial doppler ultrasound, Traumatic brain injury, Pediatric
Introduction
Head trauma is an important factor contributing to childhood morbidity and mortality. Injury is suffered in two distinct phases. The primary injury occurs at the moment of impact and results from mechanical forces that cause direct disruption of the brain parenchyma. The second phase of injury is a sequelae of events triggered by the primary injury and includes endogenous factors such as metabolic, cellular, and biochemical derangements and exogenous factors such as hypoxia and hypotension. Vasospasm may be an unrecognized phenomenon contributing to secondary brain injury in pediatric head trauma patients.
Over the last 2 decades, several studies have evaluated for vasospasm in adult head trauma patients. In these small series, vasospasm was identified in 25–60% of patients [1–4]. A much larger study involving 299 adult patients with traumatic brain injury was recently published. These patients underwent transcranial Doppler ultrasound (TCD) screening for vasospasm in their middle cerebral and basilar arteries at regular intervals for 15 days. A 36% incidence of middle cerebral artery (MCA) and a 19% incidence of basilar artery (BA) vasospasm were reported [5].
There is a paucity of literature evaluating the epidemiology of vasospasm in pediatric patients who have suffered traumatic brain injury. In a study evaluating changes in cerebral hemodynamcis after head injury, Mandera et al. [6] did not diagnose vasospasm in any participant. In a study comparing the TCD pulsatility index and intracranial pressure (ICP) in children with traumatic brain injury, Figaji et al. [7] reported an 11% incidence of vasospasm in their study population. In a recent study involving 32 severely head-injured patients, at least some of whom were children, Ojha et al. [8] noted a 38% incidence of vasospasm in the anterior circulation. Therefore, the primary aim of this study was to evaluate the incidence of vasospasm in pediatric head trauma patients using transcranial Doppler ultrasound. Secondarily, time to onset and duration of vasospasm were assessed.
Materials and methods
Study population
We performed a prospective, observational study in a 24-bed tertiary care pediatric intensive care unit (PICU) between 1 May 2007 and 31 March 2009. The study was approved by the institutional review board. Informed consent was obtained for all patients before enrollment. Children zero to 17 years old admitted with a diagnosis of moderate to severe head trauma [Glasgow Coma Score (GCS) ≤12] and abnormal head imaging were recruited. Children were excluded if they had experienced non-traumatic intracranial hemorrhage.
Procedures
All patients received routine ICU care. Patients with moderate head trauma underwent frequent neurologic checks and had sodium levels maintained in a normal range. Patients with severe head trauma were treated following the Society of Critical Care Medicine guidelines [9]. This included tracheal intubation, sedation, elevation of the head of the bed to 30 degrees, and placement of an ICP monitor with cerebrospinal fluid (CSF) drainage if ventriculostomy was present. Ongoing elevation in ICP (>20 mmHg for children and adolescents, >10–15 mmHg for infants <1 year of age) was treated with osmolar therapy. Patients received 3% sodium chloride boluses (5 ml/kg) followed by continuous drips to maintain sodium levels in a range where ICP was adequately controlled. Mannitol therapy (0.5–1 g/kg) was used to treat continued ICP elevation despite therapy with 3% sodium chloride. Mild hyperventilation with a PCO2 goal of 30–35 mm mercury (mmHg) was undertaken in patients requiring osmolar therapy. More aggressive hyperventilation, barbiturate coma, and decompressive craniectomy were used for refractory elevations in ICP. Age-appropriate cerebral perfusion pressure (CPP) (neonates 40–50 mmHg, children 50–60 mmHg, adolescents >60 mmHg) was maintained using fluid boluses, dopamine, or epinephrine.
Transcranial Doppler ultrasonography
TCD was performed at the bedside by two sonographers using a 2-MHz pulsed probe and commercially available TCD ultrasonography unit (Companion III, Nicolet Biomedical, Madison, WI). MCAs, extracranial internal carotid arteries (EC-ICAs), and BAs were insonated using the method described by Aaslid [10, 11]. Measurements of mean flow velocities were recorded in 2-mm increments along the length of each vessel. Participants underwent one baseline ultrasound within the first 48 h of admission. Subjects then underwent ultrasound every other day. If vasospasm was not identified by hospital day 8, no further ultrasounds were performed. If vasospasm was identified, subjects underwent daily ultrasound from the day of onset of vasospasm forward. Daily ultrasound was continued through hospital day 8 if vasospasm had completely resolved. If vasospasm had not resolved by hospital day 8, daily ultrasound was continued until the vasospasm resolved. If the patient died or was discharged, no further ultrasounds were performed. Physicians caring for study participants were blinded to the findings of the TCD examinations, and no specific interventions to treat vasospasm were carried out based on the results.
Diagnostic criteria
Aaslid found that adult patients with vasospasm on conventional angiography had blood flow velocities in the MCA greater than 120 cm/s and blood flow velocities in the BA greater than 90 cm/s on transcranial Doppler ultrasound [10, 11]. Later, Lindegaard developed a ratio of flow velocity in the MCA to flow velocity in the EC-ICA to help differentiate hyperemia from vasospasm [13]. Under the assumption that increased flow velocity in the EC-ICA is secondary to increased blood flow rather than vasospasm, a MCA/EC-ICA ratio <3 represents hyperemia. A MCA/EC-ICA ratio >3 is diagnostic of vasospasm. This ratio is known as the Lindegaard ratio (LR). In the current study, criteria for the presence of vasospasm included flow velocity in the middle cerebral artery (Vmca) > 120 cm/s (criterion A), Vmca > 120 cm/s and LR > 3 (criterion B), and flow velocity in the basilar artery (Vba) > 90 cm/s (criterion C). Vasospasm was considered to be present as long as flow velocity in one area of circulation met at least one criterion.
Data analysis
Patient’s demographic, clinical, and laboratory data were collected in an electronic database (Excel 2003, Microsoft, Redmond, WA). Statistical analysis was performed using the chi-squared or Fisher’s exact t test for categorical variables and Mann–Whitney test for continuous variables. Data analysis was carried out using the SAS 9.1 program (SAS Institute Inc, SAS statistical software, Cary, NC).
Results
Twenty-three children met inclusion criteria. Twenty-two children were enrolled, and one child’s parents declined entry into the study. All children completed participation and were included in the data analysis.
Demographic data such as age, gender, mechanism of injury, GCS, initial head imaging findings, and Glasgow Outcome Score (GOS) at the time of discharge from the hospital are noted for all study participants in Table 1. Enrolled patients were managed as outlined in the Materials and Methods section. Of note, the sodium level was maintained in the range of 145–155 milliequivalents/liter (meq/l) for most patients. Seven patients experienced ongoing elevations in ICP despite this sodium level and required intermittent mannitol therapy as well as an increase in the sodium level to 160–165 meq/l for adequate ICP control. Four patients (patients 1, 10, 11, and 14) had refractory elevations in ICP despite this and received increased osmolar therapy with multiple doses of mannitol and boluses of 3% sodium chloride. This resulted in extremely elevated sodium levels in these patients (176, 178, 178, and 192 meq/l respectively). In addition, while most patients underwent mild hyperventilation with a goal PCO2 of 30–35 mmHg, these four patients required moderate to significant hyperventilation with PCO2 goals of 23–29 mmHg. During significant hyperventilation, all patients underwent brain tissue oxygen monitoring, and regional saturation indexes (rSO2) were maintained in an acceptable range (53–81%). In addition, patients 1 and 10 underwent decompressive craniectomy as part of their management for elevated ICP.
Table 1.
Demographic information of all study patients
| Patient | Sex | Age (months) | Mechanism | GCS | Initial head imaging | GOS |
|---|---|---|---|---|---|---|
| 1 | F | 72 | MVA | 3 | Diffuse edema, EAH,SAH, SDH | 1 |
| 2 | M | 36 | Peds vs. auto | 10 | DAI, IPH, SDH | 5 |
| 3 | F | 60 | Peds vs. auto | 12 | IPH, SDH | 5 |
| 4 | M | 9 | AHT | 10 | EDH, SDH | 5 |
| 5 | M | 108 | Peds vs. auto | 9 | EDH | 5 |
| 6 | M | 144 | Motocross | 11 | DAI, IPH, SDH | 4 |
| 7 | M | 156 | Peds vs. auto | 3 | IPH, C1fx | 3 |
| 8 | F | 24 | MVA | 4 | Diffuse edema, DAI, SAH, SDH | 3 |
| 9 | M | 36 | AHT | 3 | Diffuse edema, SAH, SDH | 5 |
| 10 | M | 108 | Peds vs. auto | 3 | IPH | 2 |
| 11 | F | 19 | TV to head | 7 | Diffuse edema, IPH, SAH | 4 |
| 12 | F | 11 | AHT | 9 | SDH | 5 |
| 13 | M | 7 | Fall | 10 | EDH | 5 |
| 14 | F | 11 | TV to Head | 6 | Diffuse edema, EDH, IVH, SAH, SDH | 1 |
| 15 | F | 72 | Fall | 11 | IPH, SAH, SDH | 5 |
| 16 | M | 48 | MVA | 8 | Diffuse edema, IPH, SDH | 4 |
| 17 | M | 36 | MVA | 4 | DAI, IPH, SAH | 5 |
| 18 | M | 84 | Fall | 11 | IPH, SDH | 5 |
| 19 | F | 7 | AHT | 12 | DAI, IPH, SDH | 5 |
| 20 | F | 84 | Fall | 7 | IPH | 5 |
| 21 | M | 168 | Fall | 3 | EDH | 2 |
| 22 | F | 7 | AHT | 3 | Diffuse edema, SDH | 5 |
AHT Abusive head trauma, MVA motor vehicle accident, Peds vs Auto pedestrian/cyclist versus automobile, TV television
GCS Glasgow Coma Score
DAI Diffuse axonal injury, EAH extra-axial hemorrhage, SAH subarachnoid hemorrhage, SDH subdural hemorrhage, IPH intraparenchymal hemorrhage, EDH epidural hemorrhage, C1fx cervical spine 1 fracture, IVH intraventricular hemorrhage
GOS Glasgow Outcome Score, 1 dead, 2 vegetative state, 3 severe disability, 4 moderate disability, 5 good recovery
A total of 45.5% of patients developed vasospasm in the MCA based on criterion A and 36.3% developed vasospasm in the MCA based on criterion B. A total of 18.2% of the patients developed vasospasm in the basilar artery based on criterion C (Table 2). Six patients had vasospasm of the MCA only. Four patients experienced vasospasm in both the MCA and the BA. No patient was found to have isolated BA vasospasm. Of note, the incidence of vasospasm by at least one criterion was higher in the subset of patients with severe brain injury (58.3%) as compared to those with moderate brain injury (30%).
Table 2.
Results of transcranial doppler ultrasound examinations
| Vasospasm location/criterion | No. who met criterion | No. of tested | With criteriona (%) | 95% confidence interval |
|---|---|---|---|---|
| Anterior circulation | ||||
| Criterion A | 10 | 22 | 45.5 | 23.2–65.5 |
| Criterion B | 8 | 22 | 36.4 | 17.2–59.3 |
| Posterior circulation | ||||
| Criterion C | 4 | 22 | 18.2 | 5.2–40.3 |
aThe majority of patients in whom one criterion for vasospasm was identified met more than one criterion
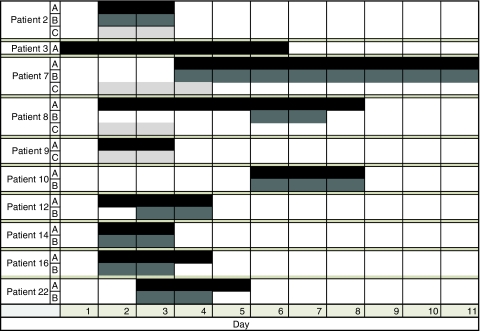
Mean time to onset of vasospasm in the MCA was 3 ± 1 days based on criterion A and 4 ± 1 days based on criterion B. Mean time to onset of vasospasm in the BA was 2 ± 1 days (Fig. 1). Vasospasm persisted in the MCA for an average of 4 ± 2 days based on criteria A and 3 ± 1 days based on criteria B. Sixty percent of patients who met criterion A had resolution of vasospasm by post-injury day 5. All patients had resolution by post-injury day 11. By criterion B, 63% of patients had resolution of spasm by post-injury day 5. Again, all patients had resolution by post-injury day 11. Basilar artery vasospasm, based on criterion C, persisted for 2 ± 1 days with all patients having resolution by post-injury day 4 (Fig. 1).
Fig. 1.
Day of onset (from time of injury) and duration (in days) of vasospasm by criteria A, B, and C in each patient who met at least one criterion for vasospasm
Severity of vasospasm in both the anterior and posterior circulation was variable from patient to patient. Table 3 represents the peak flow velocity in the MCA and BA, the post-injury day the peak flow velocity was recorded, and the distribution of abnormalities on initial head imaging of patients who met criteria for vasospasm. In addition, PCO2, mean arterial blood pressure (MABP), ICP, CPP, and sodium level at the time peak velocity was recorded are noted in Table 4.
Table 3.
Peak flow velocity in the middle cerebral and basilar artery and day of onset of peak velocity in patients who met criteria for vasospasm
| Patient | Criteria met | MCA affected | Peak flow velocity MCA (cm/s) | LR | Day (post-injury) of peak velocity in MCA | Peak flow velocity BA (cm/s) | Day (post-injury) of peak velocity in BA | Initial head imaging |
|---|---|---|---|---|---|---|---|---|
| 2 | A, B, C | Left | 144 | 3.4 | 2 | 97 | 2 | L lateral SDH |
| L parietal IPH | ||||||||
| R temporal IPH | ||||||||
| DAI | ||||||||
| 3 | A | Right | 158 | 5 | L frontal SDH | |||
| R frontal IPH | ||||||||
| 7 | A, B, C | Left | 177 | 6.1 | 5 | 136 | 3 | B frontal IPH |
| B occipital IPH | ||||||||
| C1fx | ||||||||
| 8 | A, B, C | Left | 157 | 3.1 | 5 | 91 | 4 | B lateral SDH |
| B frontal SAH | ||||||||
| B lateral SAH | ||||||||
| Diffuse edema | ||||||||
| DAI | ||||||||
| 9 | A, C | Left | 161 | 2 | 100 | 2 | B frontal SDH | |
| B lateral SDH | ||||||||
| R lateral SAH | ||||||||
| Diffuse edema | ||||||||
| 10 | A, B | Bilateral | 162/164 | 4.1 | 7 | R lentiform nucleus | ||
| IPH L temporal | ||||||||
| IPH L parietal | ||||||||
| IPH | ||||||||
| 12 | A, B | Left | 132 | 3.1 | 3 | L lateral SDH | ||
| 14 | A, B | Right | 146 | 4 | 3 | L lateral SDH | ||
| R frontal EDH | ||||||||
| R lateral SAH | ||||||||
| R IVH | ||||||||
| Diffuse edema | ||||||||
| 16 | A, B | Bilateral | 184/179 | 3.6 | 3 | B frontal SDH | ||
| B frontal IPH | ||||||||
| B temporal IPH | ||||||||
| B parietal IPH | ||||||||
| Diffuse edema | ||||||||
| 22 | A, B | Right | 164 | 3.1 | 3 | L SDH with herniation | ||
| Diffuse edema |
Initial head imaging findings are also included
MCA Middle cerebral artery, LR Lindegaard ratio, BA basilar artery
L Left, R right, B bilateral
DAI diffuse axonal injury, SDH subdural hemorrhage, IPH intraparenchymal hemorrhage, C1fx cervical spine 1 fracture, SAH subarachnoid hemorrhage, EDH epidural hematoma, IVH intraventricular hemorrhage
Table 4.
Carbon dioxide, mean arterial blood pressure, intracranial pressure, cerebral perfusion pressure, and sodium level at the time of measurement of peak flow velocity in patients who met criteria for vasospasm
| Patient | PCO2 (mmHg) | MABP (mmHg) | ICP (mmHg) | CPP (mmHg) | Na (meq/l) |
|---|---|---|---|---|---|
| 2 | 42 | 75 | 11 | 64 | 148 |
| 3 | 35 | 80 | 11 | 69 | 150 |
| 7a | 35/29 | 97/87 | NA | NA | 146/156 |
| 8a | 44/33 | 77/66 | 15/4 | 52/62 | 163/160 |
| 9 | 35 | 79 | 10 | 69 | 155 |
| 10 | 28 | 88 | 27 | 61 | 178 |
| 12 | 32 | 70 | 13 | 57 | 149 |
| 14 | 28 | 75 | 25 | 50 | 192 |
| 16 | 37 | 82 | 6 | 76 | 153 |
| 22 | 38 | 79 | 10 | 69 | 148 |
aPatients 7 and 8 had maximum flow velocity in the middle cerebral and basilar arteries on different days. The carbon dioxide, mean arterial blood pressure, intracranial pressure, cerebral perfusion pressure, and sodium levels on each of the 2 days when peak flow velocity occurred are noted as level at time of MCA peak velocity/level at time of BA peak velocity
PCO2 Partial pressure carbon dioxide, MABP mean arterial blood pressure, ICP intracranial pressure, CPP cerebral perfusion pressure, Na sodium, mmHg millimeters mercury, meq/l milliequivalents/liter, NA not available
Discussion
Several findings emerged from this study: (1) using the current accepted adult criteria to diagnose vasospasm, there is a significant incidence of vasospasm in pediatric patients who have suffered moderate to severe traumatic brain injury; (2) time to onset of vasospasm in pediatric patients appears to peak at day 2–3, but some patients may have earlier onset; (3) duration of vasospasm in pediatric head trauma patients is 2–4 days.
In this study, there was a 45.5% incidence of vasospasm in the anterior circulation based on criterion A and a 36.3% incidence based on criterion B. Basilar artery vasospasm was noted 18.2% of the time based on criterion C. The incidence of vasospasm diagnosed in this study is similar to several studies in adult patients with traumatic brain injury [1–5]. Two pediatric studies involving children with traumatic brain injury noted a much smaller incidence of vasospasm than was found here [6, 7]. Several possibilities may explain this difference. The other pediatric studies were performed for reasons other than examining the epidemiology of vasospasm in children. Therefore, the protocols used for TCD examinations may have been different than would be required to diagnose all cases of vasospasm. For example, Figaji et al. [7] performed unilateral MCA examinations (as opposed to bilateral) and did so at undefined intervals and as late as post-injury day 10. In addition, the patient population studied by Mandera et al. [6] included patients with only minor head injury, and the incidence of vasospasm is likely much lower in these patients compared to those with more significant injury. However, it should be noted that the diagnostic criteria used in this study were developed from well-conducted studies in adult patients with subarachnoid hemorrhage or traumatic brain injury [10–12]. In these studies, the criteria for vasospasm were developed by correlating TCD flow velocity to the degree of vasospasm measured on conventional angiography. No controlled studies comparing TCD flow velocity to angiographic data in pediatric patients suspected of having vasospasm exist. Of concern is that baseline flow velocities in children are elevated compared to adults [13–17]. There may also be gender differences for normal flow velocities in children, with pre-pubescent girls experiencing increased velocities compared to boys [18, 19]. Since normal flow velocities are higher in children than adults, the diagnostic criteria used in this study may have indeed overestimated the true incidence of vasospasm in pediatric head trauma patients. In a recent study evaluating cerebral blood flow in pediatric head trauma patients, Philip et al. [21] defined high Vmca as a mean middle cerebral artery flow velocity >2 standard deviations above the age and gender normal value. Rather than using a set value of a mean flow velocity above which vasospasm is diagnosed as was done in our study, a more appropriate approach to diagnose vasospasm in children of different ages and gender may be to use a similar definition as was used by Philip et al. [20].
In addition, one should note that while criteria A and B are both used currently to diagnose vasospasm, there is increasing concern about using criterion A alone for this purpose. Criterion A evaluates the mean flow velocity in the MCA without consideration of the flow velocity in other vessels. Criterion B evaluates both the mean flow velocity in the MCA as well as in the EC-ICA. An increased ratio of the flow velocity in the MCA as compared to the flow velocity in the EC-ICA better differentiates hyperemia from vasospasm. Therefore, when interpreting the findings of this study, a 45.5% incidence based on criterion A alone likely overestimates the true incidence of vasospasm by including some patients who were actually experiencing hyperemia. The 36.3% incidence noted in this study based on criterion B is likely a better representation of the true incidence of vasospasm in pediatric patients.
Time to onset of vasospasm was 3 ± 1 days by criterion A and 4 ± 1 days by criterion B. In the posterior circulation, onset of vasospasm was 2 ± 1 days by criterion C. These findings are consistent with previous literature describing the time to onset of vasospasm in adult head trauma patients [5]. A limitation of this study that should be noted, however, is that due to adult studies reporting a very small incidence of onset of vasospasm prior to post-injury day 2, study protocol was written to perform the initial ultrasound during the first 48 h of hospitalization. Therefore, several patients underwent initial ultrasound evaluation on post-injury day 2. Seven patients who met criteria for vasospasm in this study did so on the first transcranial Doppler ultrasound that was performed. Therefore, the onset of vasospasm in these patients may actually have occurred up to 1 day prior to what was recorded. If this was the case, mean time to onset of vasospasm in pediatric head trauma patients may be earlier than is reported. Ideally, a baseline ultrasound would have been performed on admission to the hospital followed by daily ultrasounds thereafter in order to more completely evaluate time of onset of vasospasm. In fact, recent Italian guidelines for the management of severe pediatric head injury recommend performing an initial TCD as soon as possible after head trauma followed by twice daily ultrasounds thereafter [21].
Vasospasm duration in the MCA persisted for an average of 4 ± 2 days from the time of onset based on criteria A and 3 ± 1 days based on criteria B. Sixty percent of patients who met criterion A had resolution of vasospasm by post-injury day 5. All patients had resolution by post-injury day 11. By criterion B, 63% of patients had resolution of spasm by post-injury day 5, and all patients had resolution by post-injury day 11. Basilar artery vasospasm, based on criterion C, persisted for 2 ± 1 days from the time of onset with 100% of patients having resolution by post-injury day 4. In the paper by Oertel et al. [5], 50% of adults had resolution of vasospasm in the anterior circulation by day 5 and by day 3.5 in the posterior circulation. All patients had resolution of vasospasm by hospital day 14. Therefore, pediatric vasospasm after head injury is of similar duration but may be slightly abbreviated compared to vasospasm in adult head-injured patients.
The intention of this study was not to determine the clinical significance of vasospasm in pediatric patients with traumatic brain injury. Glasgow Outcome Score at the time of discharge from the hospital was gathered on each patient, but no long-term evaluation of neurologic function was available. There was insufficient power in this study to detect differences in GOS between patients who did and did not meet criteria for vasospasm. Future studies with larger numbers of patients and with long-term outcomes of patients with and without vasospasm should be undertaken. Additionally, this study was not designed or powered to determine risk factors for development of vasospasm. Again, future studies should be carried out to evaluate this.
Conclusion
Using current adult guidelines, vasospasm occurs in a significant proportion of pediatric patients who have suffered traumatic brain injury. Further studies validating these criteria or developing new criteria for the diagnosis of vasospasm in children need to be performed. In addition, exploration of the clinical significance of vasospasm in regards to long-term neurologic outcomes of these children should be undertaken. Finally, if warranted, potential therapeutic measures to prevent or treat vasospasm in pediatric head trauma patients should be examined.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Nicole Fortier O’Brien, Phone: +1-614-7223432, FAX: +1-614-7223443, Email: Nicole.Obrien@nationwidechildrens.org.
Karin E. Reuter-Rice, Phone: +1-858-9665863, FAX: +1-858-9665864, Email: kreuterrice@rchsd.org
Sandeep Khanna, Phone: +1-858-9665863, FAX: +1-858-9665864, Email: skhanna@rchsd.org.
Bradley M. Peterson, Phone: +1-858-9665863, FAX: +1-858-9665864, Email: bpeterson@rchsd.org
Kenneth B. Quinto, Phone: +1-858-9665863, FAX: +1-858-9665864, Email: kbquinto@ucsd.edu
References
- 1.Compton JS. Cerebral arterial vasospasm following severe head injury: a transcranial Doppler study. Br J Neurosurg. 1987;1:435–439. doi: 10.3109/02688698708999633. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH. Hemodynamically significant cerebral vasospasm and outcome after headinjury: a prospective study. J Neurosurg. 1997;2:221–233. doi: 10.3171/jns.1997.87.2.0221. [DOI] [PubMed] [Google Scholar]
- 3.Martin N, Doberstein C, Zane C. Posttraumatic vasospasm: transcranial Doppler ultrasound, cerebral blood flow and angiographic findings. J Neurosurg. 1992;77:575–583. doi: 10.3171/jns.1992.77.4.0575. [DOI] [PubMed] [Google Scholar]
- 4.Weber M, Grolimund P, Seiler RW. Evaluation of post-traumatic cerebral blood flow velocities by transcranial Doppler ultrasonography. Neurosurgery. 1990;27:106–112. doi: 10.1097/00006123-199007000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Oertel M, Boscardin WJ, Obrist WD, Glenn TC, McArthur DC, Gravori T, Lee JH, Martin NA. Posttraumatic vasospasm: the epidemiology, severity, and time course of an underestimated phenomenon: a prospective study performed in 299 patients. J Neurosurg. 2005;103:812–824. doi: 10.3171/jns.2005.103.5.0812. [DOI] [PubMed] [Google Scholar]
- 6.Mandera M, Larysz D, Wojtacha M. Changes in cerebral hemodynamics assessed by transcranial Doppler ultrasonography in children after head injury. Childs Nerv Syst. 2002;18:124–128. doi: 10.1007/s00381-002-0572-5. [DOI] [PubMed] [Google Scholar]
- 7.Figaji AA, Zwane E, Fieggen AG, Siesjo P, Peter JC. Transcranial Doppler pulsatility index is not a reliable indicator of intracranial pressure in children with severe traumatic brain injury. Surg Neurol. 2009;4:389–394. doi: 10.1016/j.surneu.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Ojha BK, Jha DK, Kale SS, Mehta VS. Transcranial Doppler in severe head injury: evaluation of pattern of changes in cerebral blood flow velocity and its impact on outcome. Surg Neurol. 2005;2:174–179. doi: 10.1016/j.surneu.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Society of Critical Care Medicine (2003) Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Crit Care Med 31(6 suppl):S407–S491 [PubMed]
- 10.Aaslid R, Huber P, Nornes H. Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg. 1984;60:37–41. doi: 10.3171/jns.1984.60.1.0037. [DOI] [PubMed] [Google Scholar]
- 11.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 12.Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P. Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir. 1989;100:12–24. doi: 10.1007/BF01405268. [DOI] [PubMed] [Google Scholar]
- 13.Bode H, Wais U. Age dependence of flow velocities in basal cerebral arteries. Arch Dis Childhood. 1988;63:606–611. doi: 10.1136/adc.63.6.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bode H, Eden A. Transcranial Doppler sonography in children. J Child Neurol. 1989;4:S68–S76. doi: 10.1177/0883073889004001s11. [DOI] [PubMed] [Google Scholar]
- 15.Schoning M, Staab M, Walter J. Transcranial color duplex sonography in childhood and adolescence. Stroke. 1993;24:1305–1309. doi: 10.1161/01.str.24.9.1305. [DOI] [PubMed] [Google Scholar]
- 16.Bode H, Harders A. Transient stenosis and occlusions of main cerebral arteries in children—diagnosis and control of therapy by transcranial Doppler sonography. Eur J Pediatr. 1989;148:406–411. doi: 10.1007/BF00595898. [DOI] [PubMed] [Google Scholar]
- 17.Wintermark M, Lepori D, Cotting J. Brain perfusion in children: evolution with age assessed by quantitative perfusion computed tomography. Pediatrics. 2004;113:1642–1652. doi: 10.1542/peds.113.6.1642. [DOI] [PubMed] [Google Scholar]
- 18.Vavilala MS, Kinkaid MS, Muangman SL. Gender differences in cerebral blood flow velocity and autoregulation between anterior and posterior circulation in healthy children. Pediatr Res. 2005;58:574–578. doi: 10.1203/01.PDR.0000179405.30737.0F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tontisirin N, Muangman SL, Suz P. Early childhood gender differences in anterior and posterior cerebral blood flow velocity and autoregulation. Pediatrics. 2007;119:610–615. doi: 10.1542/peds.2006-2110. [DOI] [PubMed] [Google Scholar]
- 20.Philip S, Chaiwait O, Udomphorn Y, Moore A, Zimmerman J, Armstead W, Vavilala M. Variation in cerebral blood flow velocity with cerebral perfusion pressure >40 mmHg in 42 children with severe traumatic brain injury. Crit Care Med. 2009;37:2973–2978. doi: 10.1097/CCM.0b013e3181a963f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietrini D, Savioli A, Grossetti R, Barbieri MA, Buscalferri A. SIAARTI-SARNePI guidelines for the management of severe pediatric head injury. Minerva Anestesiol. 2004;70:549–604. [PubMed] [Google Scholar]