Abstract
Background and objective
The haemodynamics of crystalloid and colloid fluid loading may depend on underlying disease, i.e. sepsis versus non-sepsis.
Design and setting
A single-centre, single-blinded, randomized clinical trial was carried out on 24 critically ill sepsis and 24 non-sepsis patients with clinical hypovolaemia, assigned to loading with normal saline, gelatin 4%, hydroxyethyl starch 6% or albumin 5% in a 90-min (delta) central venous pressure (CVP)-guided fluid loading protocol. Transpulmonary thermodilution was done each 30 min, yielding, among others, global end-diastolic volume and cardiac indices (GEDVI, CI).
Results
Sepsis patients had hyperdynamic hypotension in spite of myocardial depression and dilatation, and greater inotropic/vasopressor requirements than non-sepsis patients. Independent of underlying disease, CVP and GEDVI increased more after colloid than saline loading (P < 0.018), so that CI increased by about 2% after saline and 12% after colloid loading (P = 0.029). The increase in preload-recruitable stroke work was also greater with colloids and did not differ among conditions.
Conclusion
Fluid loading with colloids results in a greater linear increase in cardiac filling, output and stroke work than does saline loading, in both septic and non-septic clinical hypovolaemia, in spite of myocardial depression and presumably increased vasopermeability potentially decreasing the effects of colloid fluid loading in the former.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-010-1776-x) contains supplementary material, which is available to authorized users.
Keywords: Colloids, Crystalloids, Sepsis, Preload-recruitable stroke work, Hypovolaemia, Global end-diastolic volume, Fluid challenge
Introduction
Hypovolaemia is common in septic and non-septic critical illness, and fluid resuscitation is aimed at rapid increase of cardiac output and tissue oxygenation. It is still controversial whether crystalloid or colloid (albumin) fluids should be used, since, among others, the clinical outcome may not differ according to fluid type or may even be somewhat worse for colloids (albumin) [1, 2]. Other studies [3, 4] suggest that resuscitation with albumin tends to benefit morbidity and survival over that with saline during sepsis. The crystalloid–colloid controversy includes the role of colloid osmotic pressure (COP) in plasma in retaining fluids intravascularly and in the speed at and extent to which colloids, maintaining COP, restore plasma volume and blood flow as opposed to crystalloids, which dilute plasma proteins, lower COP and rapidly leak into the interstitium [1, 5–9]. When using crystalloids, two to four times more fluid may be required to restore and maintain intravascular fluid volume compared with colloids [1, 5–8]. This is controversial, however, since, for instance, the ratio in the SAFE study comparing albumin with saline resuscitation was 1:1.3, whereas the rise in central venous and arterial blood pressures was only slightly greater with albumin [3]. A potential difference between fluid types may critically depend on underlying disease, so that, during sepsis, a decrease of cardiac function and an increase of vasopermeability may attenuate haemodynamic differences between fluids by decreasing the slope of the cardiac function curve and diminishing the contribution of plasma COP and thus the ability of colloids to retain fluids intravascularly, respectively [1, 8, 10–14]. If so, a potential survival benefit of albumin over saline in sepsis [4] may not relate to its colloid osmotic properties [15]. Finally, controversies may also stem in part from the monitored endpoints for fluid resuscitation, which, if imprecise, may mask haemodynamic differences. Absolute (rather than changes in) filling pressures of the heart may be poor indicators of cardiac preload and fluid responsiveness while global end-diastolic volume, assessed from transpulmonary thermodilution, may be superior [16–18]. In our study on cardiovascular surgery and fluid loading guided by (delta) filling pressures, colloid loading had more effects on plasma volume and cardiac filling and output than did saline loading [9].
For the current study, the hypothesis was that fluid loading with colloids results in greater increase in preload-recruitable cardiac output and stroke work than does saline loading, more so in patients with non-septic than in those with septic clinical hypovolaemia in the intensive care unit (ICU), because of differences in cardiac and vascular function. We thus compared saline with colloids and evaluated COP and cardiac output and function, using a standard (delta) central venous pressure-guided fluid challenge protocol over 90 min [9], verified by transpulmonary thermodilution [16], in septic and non-septic clinical hypovolaemia.
Patients and methods
This is a companion study on the same patients of a prospective study involving pulmonary [and few (t = 0–90 min) raw haemodynamic] data regarding fluid loading [19]. Further methods are described in the Electronic Supplemental Material (ESM).
Protocol
The protocol was started within 3 h after surgery or gastrointestinal haemorrhage and 12 h after meeting criteria for sepsis. At baseline, patient characteristics and clinical data were recorded, including acute physiology and chronic health evaluation (APACHE) II score. Doses of vasoactive drugs, ventilatory settings and haemodynamics were recorded. After baseline measurements (t = 0 min), fluids were given during 90 min on the basis of the response within predefined limits and changes in central venous pressure (CVP), according to a fluid challenge protocol as described [19]. Boluses of maximum 200 mL were given per 10 min, so that the maximum fluid challenge was 1,800 mL in 90 min. Concomitant treatment was unchanged. All measurements were repeated after completing the fluid challenge (t = 90 min). Every 30 min until t = 90 min, CVP, cardiac index (CI) and global end-diastolic volume index (GEDVI) were measured also.
Statistical analysis
The study had 80% power to detect a statistically significant difference between saline and colloid fluids (at α < 0.05) in fluid-loading-induced increases in CI, the primary study parameter, of 10% (at standard deviation of 10% of the increase). Data are expressed as mean ± standard deviation (SD), except in the figures, where mean ± standard error of mean (SEM) is shown. Data were normally distributed (Kolmogorov–Smirnov test), after logarithmic transformation where appropriate. We used generalized estimating equation (GEE) to test for effect of underlying disease and fluid type on baseline values and, taking repeated measurements in the same patients and first-order interactions into account, on changes in time with baseline values as covariates. Then, interactions were allowed to assess whether effects of fluid type in time were dependent on underlying disease. Fisher’s exact or χ 2 test was used for categorical variables. A similar analysis was done to compare colloid fluids (ESM). Values of P < 0.05 were considered statistically significant, and exact values >0.001 are reported.
Results
Groups were comparable, except for a higher APACHE II score, creatinine, positive end-expiratory pressure (PEEP), more inotropic/vasopressor treatment and less diuresis in sepsis (ESM Table 1). More colloid than saline fluid had been administered, irrespective of underlying disease. Sepsis patients had hyperdynamic hypotension, as indicated by higher heart rate (HR) and CI (after fluid loading) and lower mean arterial pressure (MAP), in spite of myocardial depression and dilatation as indicated by higher CVP and GEDVI, and lower global ejection fraction (GEF) and left ventricular stroke work index (LVSWI) (to GEDVI/4 ratio, indicative of preload-recruitable stroke work) compared with non-septic patients, respectively. Baseline SVRI was lower in sepsis than in non-sepsis (P = 0.013, data not shown). Albumin level was also lower (P < 0.001). Sepsis carried higher ICU mortality than non-sepsis (P = 0.017), irrespective of fluid type.
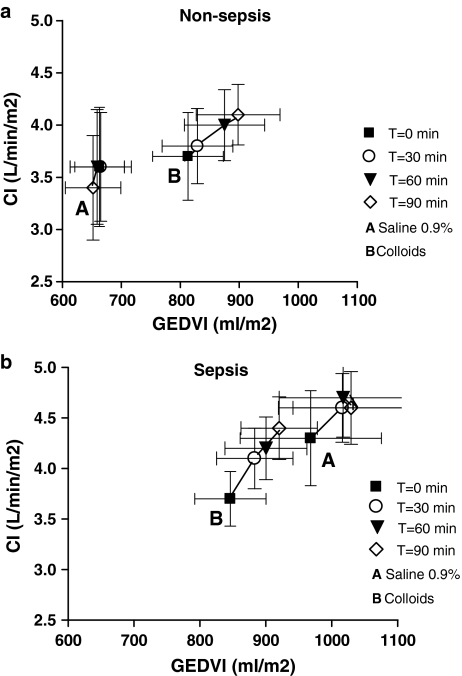
Baseline values were comparable among fluid types, except for slightly lower haemoglobin and higher PEEP and thus CVP in colloid- than in saline-loaded patients. Haemoglobin levels fell more in colloid than in saline loading, whereas COP increased in colloid-loaded patients only, irrespective of underlying disease (ESM Table 3). The rises in MAP, CVP, GEDVI and CI were greater with colloid than saline loading, independent of underlying disease and baseline values (Table 1). Indeed, CVP and CI increased with time (t = 0, 30, 60, 90 min), dependent on fluid type (P = 0.007 or lower), irrespective of underlying disease. Figure 1a and b shows that the increases of GEDVI (t = 0, 30, 60, 90 min) were greater with colloid than saline loading in septic and non-septic patients (P = 0.003). Indeed, the change in GEDVI predicted the change in CI (P < 0.001), irrespective of underlying disease or fluid type. However, the rise in SVI and LVSWI with saline loading was greater in sepsis than in non-sepsis patients, but greater in colloid- than in saline-loaded patients (ESM Fig. 1). ScvO2 increased, particularly in colloid-loaded patients with non-sepsis, while lactate levels did not change. DO2 increased and VO2 was unchanged, independent of underlying disease and fluid type.
Table 1.
Haemodynamics
| Non-sepsis | Sepsis | P value | |||||
|---|---|---|---|---|---|---|---|
| Saline (n = 6) | Colloid (n = 18) | Saline (n = 6) | Colloid (n = 18) | U | Ty | UxTy | |
| Heart rate (beats/min) | |||||||
| t = 0 | 72 ± 27 | 69 ± 19 | 93 ± 33 | 97 ± 22 | 0.004 | 0.964 | 0.721 |
| t = 90 | 72 ± 28 | 70 ± 17 | 89 ± 29 | 98 ± 18 | 0.411 | 0.165 | 0.115 |
| MAP (mmHg) | |||||||
| t = 0 | 82 ± 12 | 82 ± 15 | 74 ± 10 | 75 ± 10 | 0.033 | 0.889 | 0.804 |
| t = 90 | 84 ± 6 | 93 ± 15 | 83 ± 10 | 89 ± 16 | 0.212 | 0.004 | 0.398 |
| CI (L/min/m2) | |||||||
| t = 0 | 3.6 ± 1.4 | 3.8 ± 1.7 | 4.3 ± 1.2 | 3.7 ± 1.1 | 0.450 | 0.621 | 0.342 |
| t = 90 | 3.5 ± 1.2 | 4.1 ± 1.1 | 4.6 ± 0.9 | 4.4 ± 1.3 | 0.001 | 0.029 | 0.371 |
| CVP (mmHg) | |||||||
| t = 0 | 4 ± 4 | 6 ± 3 | 5 ± 2 | 8 ± 4 | 0.064 | 0.003 | 0.319 |
| t = 90 | 5 ± 3 | 9 ± 3 | 6 ± 4 | 13 ± 4 | 0.180 | <0.001 | 0.551 |
| SVI (mL/m2) | |||||||
| t = 0 | 51 ± 9 | 55 ± 15 | 52 ± 24 | 39 ± 12 | 0.147 | 0.434 | 0.114 |
| t = 90 | 49 ± 9 | 60 ± 13 | 56 ± 17 | 45 ± 12 | 0.330 | 0.059 | 0.009 |
| GEDVI (mL/m2) | |||||||
| t = 0 | 664 ± 102 | 813 ± 234 | 968 ± 264 | 846 ± 215 | 0.010 | 0.832 | 0.039 |
| t = 90 | 653 ± 117 | 898 ± 276 | 1,029 ± 215 | 921 ± 232 | 0.276 | 0.018 | 0.117 |
| LVSWI (gm/m2) | |||||||
| t = 0 | 55 ± 14 | 57 ± 15 | 50 ± 25 | 35 ± 10 | 0.022 | 0.248 | 0.139 |
| t = 90 | 54 ± 13 | 69 ± 15 | 60 ± 22 | 46 ± 13 | 0.706 | 0.040 | 0.005 |
| GEF (mL/mL) | |||||||
| t = 0 | 0.31 ± 0.05 | 0.28 ± 0.06 | 0.21 ± 0.06 | 0.19 ± 0.07 | <0.001 | 0.198 | 0.756 |
| t = 90 | 0.31 ± 0.06 | 0.28 ± 0.06 | 0.22 ± 0.05 | 0.20 ± 0.07 | 0.568 | 0.895 | 0.890 |
| DO2 (mL/min/m2) | |||||||
| t = 0 | 533 ± 204 | 495 ± 173 | 623 ± 217 | 474 ± 145 | 0.979 | 0.110 | 0.184 |
| t = 90 | 546 ± 149 | 498 ± 156 | 667 ± 272 | 505 ± 143 | 0.262 | 0.511 | 0.568 |
| VO2 (mL/min/m2) | |||||||
| t = 0 | 106 ± 50 | 140 ± 48 | 148 ± 68 | 127 ± 61 | 0.999 | 0.609 | 0.301 |
| t = 90 | 124 ± 38 | 138 ± 64 | 163 ± 78 | 125 ± 66 | 0.901 | 0.902 | 0.192 |
Mean ± SD
MAP mean arterial pressure, CI cardiac index, CVP central venous pressure, SVI stroke volume index, GEDVI global end-diastolic volume index, LVSWI left ventricular stroke work index, GEF global ejection fraction, DO 2 oxygen delivery, VO 2 oxygen consumption, P: U underlying disease (sepsis versus non-sepsis), Ty fluid type (saline versus colloids), U × Ty, interaction
Fig. 1.
a Mean ± SEM for cardiac index (CI) versus global end-diastolic volume index (GEDVI) according to fluid type (A saline; B colloid), at four time points of fluid loading, in non-sepsis patients. b Mean ± SEM for cardiac index (CI) versus global end-diastolic volume index (GEDVI) according to fluid type (A saline; B colloid), at four time points of fluid loading, in sepsis patients. For GEDVI and CI: increases differed between fluid types (P = 0.007 or lower), indicating greater rises in colloid than in saline loading, irrespective of underlying disease
Discussion
As expected, sepsis patients had lower baseline albumin levels, presumably following increased vasopermeability, and MAP, but higher HR and cardiac filling than non-sepsis patients. The latter may have resulted from myocardial depression, characteristic for severe sepsis, as shown by lower GEF and down- and rightward displacement of preload-recruitable stroke work [10]. Nevertheless, the haemodynamic response to fluid loading was similar to that in non-sepsis, in disagreement with the literature [10]. The slope of preload-recruitable stroke work did not differ among fluid types, suggesting unaltered cardiac function during fluid loading, so that the differences between fluid types in cardiac output responses were primarily caused by differences in filling. However, a rise in LVSWI that, in contrast to cardiac filling and output, seemed somewhat greater in saline loading in sepsis than in non-sepsis patients can be explained in part by a greater effect on SVI. The greater cardiac filling and output with colloid than with saline loading maintained in sepsis argue against increased vasopermeability that may increase (rapid) equilibration of infused proteins and artificial colloids with the extravascular space and thereby limit intravascular retention of fluids, but such effect in more severely ill septic patients with higher permeability cannot be excluded [1, 7, 8, 12]. Neither can we exclude slowly increased extravasation of colloids in sepsis, even though nearly complete equilibration between the intra- and extravascular space is expected within 90 min [7]. The similar COP in sepsis and non-sepsis after colloid fluid loading agrees with the literature showing that colloid/albumin solutions are able to increase, at least transiently, low COP/albumin in critically ill patients with sepsis and shock [6, 7, 12]. Our results may also help explain a potential survival benefit of albumin over saline resuscitation in sepsis [3]. In animal experiments, authors [13, 14] found that, even in sepsis and shock, colloids were effective, and even more so than crystalloids, in maintaining COP, cardiac filling and output. Otherwise, that colloids, per unit volume and time, are better able to recruit cardiac preload than are rapidly extravasating crystalloid solutions is in line with our previous study in cardiovascular surgery patients with less elevated permeability [9, 19]. When using crystalloids, two to four times more fluid may be required to restore and maintain intravascular fluid volume compared with colloids, although true evidence is scarce [1, 5–7, 9]. Our results agree with this idea, even in septic clinical hypovolaemia, since the difference in cardiac output increase multiplied by the difference in volume infused was three for colloids versus saline. The ratio in the SAFE study comparing albumin with saline resuscitation was 1:1.3 [3], however. This can be explained by either insufficient need for fluid resuscitation, severely increased permeability, poor monitoring and guidance of therapy, or combinations thereof. The current data finally indicate that our clinical criteria were useful in selecting patients with, on average, a linear increase in cardiac output upon fluid loading in the steep part of the cardiac function curve.
The limitations of our study include the coincidental imbalance in haemoglobin and CVP between fluid types at baseline. The latter can be explained by a coincidental imbalance in PEEP and the effect of transmitted airway pressure on atmospheric-pressure-referenced CVP. We did not measure mixed venous SO2, which may be lower than ScvO2. However, changes may be similar, so that the unchanged VO2 is probably true. The increase in DO2 did not differ among fluid types since higher cardiac output was offset by greater haemodilution after colloid than saline loading. The relatively high ScvO2 values and low lactate levels may otherwise imply adequate tissue oxygenation. Admittedly, the number of patients in this study was relatively small, but sufficient for analyses of fluid pathophysiology, the principal aim, rather than therapy, of our study. Finally, we cannot exclude that infusion of even more saline, for instance guided by GEDVI [18], would have resulted in greater rises in preload-recruitable CI and LVSWI. By comparing (and pooling) different, roughly iso-oncotic colloid fluids, our study carries the advantage over others, in which only one or two colloid fluid types were studied [3, 4, 6, 7, 12, 13, 18], of evaluating the contribution of COP independently of other fluid properties. Finally, most studies, unlike ours, did not separate effects in sepsis from those in non-sepsis [2, 3, 6].
In conclusion, fluid loading with colloids results in a greater linear increase in cardiac filling, output and stroke work than does saline loading, in both septic and non-septic clinical hypovolaemia, in spite of myocardial depression and presumably increased vasopermeability potentially decreasing the effects of colloid fluid loading in the former.
Electronic supplementary material
Below is the link to the electronic supplementary material
Acknowledgments
We thank Joanne Verheij for helping collecting the data and B. Braun Medical (Melsungen, Germany) for an unrestricted research grant.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00134-010-1939-9
References
- 1.Boldt J. Volume replacement in the surgical patient-does the type of solution make a difference? Br J Anaesth. 2000;84:783–793. doi: 10.1093/bja/84.6.783. [DOI] [PubMed] [Google Scholar]
- 2.Perel P, Roberts J (2007) Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev (4):CD000567 [DOI] [PubMed]
- 3.The SAFE Study investigators A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 4.Dubois M-J, Orellana-Jimenez C, Mélot C, De Backer D, Berré J, Leeman M, Brimioulle S, Appoloni O, Creteur J, Vincent JL. Albumin administration improves organ function in critically ill hypoalbuminemic patients: a prospective, randomized, controlled pilot study. Crit Care Med. 2006;23:2336–2540. doi: 10.1097/01.CCM.0000239119.57544.0C. [DOI] [PubMed] [Google Scholar]
- 5.Shoemaker WC, Schluchter M, Hopkins JA, Appel PL, Schwartz S, Chang PC. Comparison of the relative effectiveness of colloids and crystalloids in emergency resuscitation. Am J Surg. 1981;142:73–84. doi: 10.1016/S0002-9610(81)80015-3. [DOI] [PubMed] [Google Scholar]
- 6.Rackow EC, Falk JL, Fein IA, Siegel JS, Packman MI, Haupt MT, Kaufman BS, Putnam D. Fluid resuscitation in circulatory shock: a comparison of the cardiorespiratory effects of albumin, hetastarch, and saline in patients with hypovolemic and septic shock. Crit Care Med. 1983;11:839–850. doi: 10.1097/00003246-198311000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Ernest D, Belzberg AS, Dodek PM. Distribution of normal saline and 5% albumin infusions in septic patients. Crit Care Med. 1999;27:46–50. doi: 10.1097/00003246-199901000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Marx G. Fluid therapy in sepsis with capillary leakage. Eur J Anaesthesiol. 2003;20:429–442. doi: 10.1097/00003643-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Verheij J, van Lingen A, Beishuizen A, Christiaans HMT, de Jong JR, Girbes ARJ, Wisselink W, Rauwerda JA, Huybregts MAJM, Groeneveld ABJ. Cardiac response is greater for colloid than saline fluid loading after cardiac or vascular surgery. Intensive Care Med. 2006;32:1030–1038. doi: 10.1007/s00134-006-0195-5. [DOI] [PubMed] [Google Scholar]
- 10.Ognibene FP, Parker MM, Natanson C, Shelhamer JH, Parrillo JE. Depressed left ventricular performance: response to volume infusion in patients with sepsis and septic shock. Chest. 1988;93:903–910. doi: 10.1378/chest.93.5.903. [DOI] [PubMed] [Google Scholar]
- 11.Boldt J, Heesen M, Müller M, Pabsdorf M, Hempelman G. The effects of albumin versus hydroxyethyl starch solution on cardiorespiratory and circulatory variables in critically ill patients. Anesth Analg. 1996;83:254–261. doi: 10.1097/00000539-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Margarson MP, Soni NC. Effects of albumin supplementation on microvascular permeability in septic patients. J Appl Physiol. 2002;92:2139–2145. doi: 10.1063/1.1495889. [DOI] [PubMed] [Google Scholar]
- 13.Marx G, Pedder S, Smith L, Swaraj S, Grime S, Stockdale H, Leuwer M. Attenuation of capillary leakage by hydroxyethyl starch (130/0.42) in a porcine model of septic shock. Crit Care Med. 2006;34:3005–3010. doi: 10.1097/01.CCM.0000242755.74063.ED. [DOI] [PubMed] [Google Scholar]
- 14.Su F, Wang Z, Cai Y, Rogiers P, Vincent JL. Fluid resuscitation in severe sepsis and septic shock: albumin, hydroxyethyl starch, gelatin or Ringer’s lactate, does it really matter? Shock. 2007;27:520–526. doi: 10.1097/01.shk.0000248583.33270.12. [DOI] [PubMed] [Google Scholar]
- 15.Horstick G, Lauterbach M, Kempf T, Bhakdi S, Heimann A, Horstick M, Meyer J, Kempski O. Early albumin infusion improves global and local hemodynamics and reduces inflammatory response in hemorrhagic shock. Crit Care Med. 2002;30:851–855. doi: 10.1097/00003246-200204000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Gödje O, Peyerl M, Seebauer T, Dewald O, Reichart B. Reproducibility of double indicator dilution measurements of intrathoracic blood volume compartments, extravascular lung water, and liver function. Chest. 1998;113:1070–1077. doi: 10.1378/chest.113.4.1070. [DOI] [PubMed] [Google Scholar]
- 17.Combes A, Berneau JB, Luyt CE, Trouillet JL. Estimation of left ventricular systolic function by single transpulmonary thermodilution. Intensive Care Med. 2004;30:1377–1383. doi: 10.1007/s00134-004-2289-2. [DOI] [PubMed] [Google Scholar]
- 18.Molnár Z, Mikor A, Leiner T, Szakmány T. Fluid resuscitation with colloids of different molecular weight in septic shock. Intensive Care Med. 2004;30:1356–1360. doi: 10.1007/s00134-004-2278-5. [DOI] [PubMed] [Google Scholar]
- 19.Van der Heijden M, Verheij J, Van Nieuw Amerongen GP, Groeneveld ABJ. Crystalloid or colloid fluid loading and pulmonary permeability, edema and injury in septic and non-septic critically ill patients with hypovolemia. Crit Care Med. 2009;37:1275–1281. doi: 10.1097/CCM.0b013e31819cedfd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.