Abstract
Bz-423 is a pro-apoptotic 1,4-benzodiazepine with potent therapeutic properties in murine models of lupus and psoriasis. Bz-423 modulates the F1F0-ATPase, inducing the formation of superoxide within the mitochondrial respiratory chain, which then functions as a second messenger initiating apoptosis. Herein, we report the signaling pathway activated by Bz-423 in mouse embryonic fibroblasts containing knockouts of key apoptotic proteins. Bz-423-induced superoxide activates cytosolic ASK1 and its release from thioredoxin. A mitogen activated protein kinase cascade follows leading to the specific phosphorylation of JNK. JNK signals activation of Bax and Bak which then induces mitochondrial outer membrane permeabilization to cause the release of cytochrome c and a commitment to apoptosis. The response of these cells to Bz-423 is critically dependent upon both superoxide and JNK activation as antioxidants and the JNK inhibitor SP600125 prevent Bax translocation, cytochrome c release, and cell death. These results demonstrate that superoxide generated from the mitochondrial respiratory chain as a consequence of a respiratory transition can signal a sequential and specific apoptotic response. Collectively, these data suggest that the selectivity of Bz-423 observed in vivo results from cell-type specific differences in redox balance and signaling by ASK1 and Bcl-2 proteins.
Keywords: benzodiazepine, apoptosis, Bz-423, superoxide, mitochondria, Bcl-2, Bax, Bak, JNK
Introduction
Bz-423 is a pro-apoptotic 1,4-benzodiazepine with potent therapeutic properties against murine lupus and psoriasis [1–3]. The absence of either general toxicities or significant effects on normal immune responses in treated mice indicates that Bz-423 has selective effects on pathogenic cells. Affinity-based screening of a phage-display human cDNA expression library identified the oligomycin-sensitivity conferring protein (OSCP), a component of the mitochondrial F1F0-ATPase, as the molecular target of Bz-423 [4]. Binding of Bz-423 to the OSCP modulates the enzyme and induces a state 3 to state 4 respiratory transition, leading to the formation of superoxide by the mitochondrial respiratory chain (MRC).
To gain an understanding of the cellular response to Bz-423, we previously characterized the general features of apoptosis in a Burkitt lymphoma cell line (Ramos) [1]. In these cells, Bz-423 induced increase in superoxide is followed by caspase activation, mitochondrial electrochemical gradient (ΔΨm) collapse and the release of cytochrome c into the cytoplasm nearly simultaneously, consistent with mitochondrial outer membrane permeabilization (MOMP) and the release of cytochrome c from the mitochondrial inter-membrane space [5]. Following these events, morphological and biochemical evidence of apoptosis is detected. In rat liver isolated mitochondria, Bz-423 induces reactive oxygen species (ROS), but does not cause gradient collapse or swelling. These data show that Bz-423-induced superoxide does not directly trigger opening of the permeability transition pore, and implicates extra-mitochondrial factors in the mechanism coupling Bz-423-induced ROS to apoptosis.
To identify factors that couple Bz-423 generated superoxide to apoptosis, the response to Bz-423 was studied in detail in mouse embryonic fibroblasts (MEFs) [6]. Although MEFs are significantly less sensitive to Bz-423 induced killing than either primary B cells or B cell lymphoma derived cell lines (i.e. longer incubation times and higher Bz-423 concentrations are required), and in vivo data has yet to identify cytotoxic effects on non-lymphoid cells, MEFs were selected to exploit the use of well-characterized single gene knock-outs [6], to identify signaling molecules that are part of the Bz-423 response and to identify factors that potentially explain the relative resistance of fibroblasts. Using these cells, we show that cytosolic factors, including pro-apoptotic Bcl-2-family proteins and mitogen-activated protein (MAP) kinases, couple Bz-423 induced ROS to an apoptotic cascade that is reflected back to the mitochondria to release cytochrome c. The release of cytochrome c commits the cell to apoptosis. The events described following Bz-423 treatment of MEFs demonstrate how ROS generated by modulation of the mitochondrial F1F0-ATPase can induce a sequential and specific apoptotic signal transduction pathway.
Materials and Methods
Reagents
Bz-423 was synthesized as previously described [7]. Dihydroethidium (DHE) and 5-(and -6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) were obtained from Invitrogen Corp. (Carlsbad, CA, USA). Manganese (III) tetrakis (4-benzoic acid)porphyrin (MnTBAP) was purchased from Alexis Biochemicals (Lausen, Switzerland). Unless otherwise specified, all additional reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell lines and culture
SV40 transformed WT, Bad−/−, Bax−/−, Bak−/−, and DKO (Bax−/−Bak−/−) MEFs (a gift from S. Korsmeyer) were maintained in DMEM supplemented with heat-inactivated FBS (10%), 1× non-essential amino acids (Invitrogen), 2-mercaptoethanol (100 μM), penicillin (100 U/mL), streptomycin (100 μg/mL), and L-glutamine (290 μg/mL). In vitro experiments were conducted in media containing 2% FBS unless otherwise noted. Organic compounds were dissolved in media containing 0.5% DMSO.
Immunofluorescence
Cells were cultured on glass chamber slides (Nalge Nunc International, Rochester, NY, USA). Cells were fixed (0.25 h, RT) with PBS containing paraformaldehyde (2%). To remove the fixative, cells were washed five times with PBS containing saponin (10% w/v) and heat inactivated FBS (5%). Cells were incubated (overnight, 4 °C, 1 μg/mL) with antibodies for detection of activated Bax (catalog # 06-499, Millipore, Charlottesville, VA, USA) and Bak (06-536, Millipore). Following six washes, the cells were incubated (0.5 h, RT, 5 μg/mL) with biotinylated goat anti-rabbit IgG (BA-1000, Vector Laboratories, Burlingame, CA, USA). Following six washes, the cells were incubated (0.5 h, RT, 5 μg/mL) with fluorescein-conjugated avidin D (A-2001, Vector Laboratories). Samples were examined by microscopy using a Leica DM-LB microscope. Images (630X) were captured using a SPOT RS slider digital camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA) interfaced to a Macintosh PC. Fluorescence microscopy of isolated mitochondria was performed as previously described [1].
Detection of intracellular superoxide, ΔΨm, cell death, and hypodiploid DNA
Detection of intracellular superoxide formation was performed monitoring the oxidation of DHE to oxyethidium by flow cytometry using the FL2 channel (585 nm) [8]. DHE (4 μM) was added to cells 30 min prior to flow cytometric analysis. Measurement of intracellular peroxide formation by the oxidation of CM-H2DCFDA to dichlorodihydrofluorescein (DCF) was performed by flow cytometry in the FL1 channel (530 nm). Cells were pre-loaded (30 min) with CM-H2DCFDA (3 μM) prior to media exchange and Bz-423 treatment. Measurement of ΔΨm with DiOC6(3) was performed by flow cytometry as previously described [1]. Cell viability and hypodiploid diploid DNA content was assessed by staining with propidium iodide (PI) using flow cytometry as previously described [1].
Preparation of whole cell extracts
Cells (20 × 106) were pelleted and washed with PBS prior to lysis with WCE lysis buffer (25 mM Hepes pH 7.7, 150 mM NaCl, 2.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 20 mM β-glycerophosphate, 0.5 mM DTT containing 1 mM phenylmethylsulphonylfluoride (PMSF), complete protease inhibitor cocktail tablet (Roche), 3.3 mM NaF, and 0.1 mM sodium orthovanadate). Following incubation on ice (30 min), the lysate was centrifuged (16,000 g, 0.5 h, 4 °C) to pellet insoluble cellular debris. Total protein content in the supernatant was quantified by the Bradford protein assay (Bio-rad Laboratories, Hercules, CA, USA).
Mitochondria isolation
Cells (107) were harvested and washed with ice cold PBS. Cells were resuspended in ice-cold buffer A (200 μL, 20 mM Hepes-KOH, pH 7.5, 10 mM KCl, 10 mM β-glycerophosphate, 5 mM NaF, 1.5 mM MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA, 1 mM DTT, 1 mM sodium orthovanadate, 250 mM sucrose, complete protease inhibitor cocktail tablet and 0.1 mM PMSF). The cell suspension was allowed to sit on ice (20 min) and then was disrupted by 10 strokes through a 28.5 G needle. The homogenate was centrifuged (1000 g, 10 min, 4 °C) to pellet nuclei. The resulting supernatant was centrifuged (10,000 g, 30 min, 4 °C) to obtain the mitochondrial fraction. The supernatant from this centrifugation was harvested as the cytosolic fraction. The purity of fractions was tested by immunoblotting with antibodies specific for the cytosolic proteins β-tubulin or glyceradehyde-3-phosphate dehydrogenase (GAPDH), or the mitochondrial proteins cytochrome c oxidase (Complex IV, Cox IV) or the β-subunit of complex V (V-β).
Immunoblot Analysis
Cell lysates were separated by SDS-PAGE, and transferred to polyvinylidene difluoride membranes as previously described [1]. The membranes were incubated with primary antibodies for proteins of interest, including: cytochrome c (556433, BD Biosciences, Franklin Lakes, NJ, USA), Bax (06-499, Millipore), Bak (06-536, Millipore), Bad (610392, BD Biosciences), ASK1 (sc-7931, Santa Cruz Biotechnology, Santa Cruz, CA, USA), thioredoxin (sc-20146, Santa Cruz Biotechnology), MKK7 (4172, Cell Signaling Technology, Danvers, MA, USA), phospho-MKK7 Ser271/Thr275 (4171, Cell Signaling Technology), MKK4 (9152, Cell Signaling Technology), phospho-MKK4 Ser257/Thr261 (9156, Cell Signaling Technology), JNK (9252, Cell Signaling Technology), phospho-JNK Thr183/Tyr185 (9251, Cell Signaling Technology), p38 (9212, Cell Signaling Technology), phospho-p38 Thr180/Tyr182 (9215, Cell Signaling Technology), c-Jun (9162, Cell Signaling Technology), phospho-c-Jun Ser63 (9261, Cell Signaling Technology), ATF2 (9226, Cell Signaling Technology), phospho-ATF2 Thr69/Thr71 (9225, Cell Signaling Technology), GAPDH (MAB374, Millipore), β-tubulin (T4026, Sigma-Aldrich), cytochrome c oxidase (A6403, Invitrogen), and β-subunit of complex V (A21351, Invitrogen). Blots were then incubated with horseradish peroxidase conjugated secondary antibodies (NA931 or NA934, GE Healthcare Bio-sciences, Piscataway, NJ, USA) and reacted with chemiluminescence reagents (GE Healthcare Biosciences).
Immunoprecipitations
Cytoplasmic protein (500 μg) was incubated (2 h, 4 °C) with agarose conjugated anti-ASK1 (15 μg, sc-7931-AC, Santa Cruz Biotechnology) or anti-thioredoxin (15 μg, sc-20146-AC, Santa Cruz Biotechnology) while rotating. The immunocomplexes were isolated by centrifugation (1000 g, 1 min). The immunoprecipitate was washed three times with buffer A and once with buffer B (50 mM Tris, pH 7.5, 10 mM MgCl2, 0.02% BSA) prior to analysis via immunoblot.
Statistical analysis
Where indicated, statistical significance was assessed by a student’s t-test. P-values are two-tailed, and all data is presented as mean ± one standard deviation (SD), unless otherwise mentioned. Figures contain representative data of experiments performed in triplicate.
Results
Isolated mitochondria respond to Bz-423
Incubating mitochondria isolated and purified from MEFs with Bz-423 under conditions supporting state 3 respiration results in increased superoxide within the mitochondria (Figure S1A). This response is consistent with inhibition of the F1F0-ATPase, and demonstrates that mitochondria respond to Bz-423 independent of other components of the cell. In this cell-free system, however, Bz-423 does not cause ΔΨm collapse or trigger cytochrome c release (Figure S1A–B). Together, these data show that Bz-423 does not directly induce opening of the mitochondrial permeability transition (MPT) pore and reveals that extra-mitochondrial factors couple mitochondrial-generated superoxide to eventual cytochrome c release (and MOMP) at which point the cell is irreversibly committed to die [9].
Bz-423 induces apoptosis in MEFs
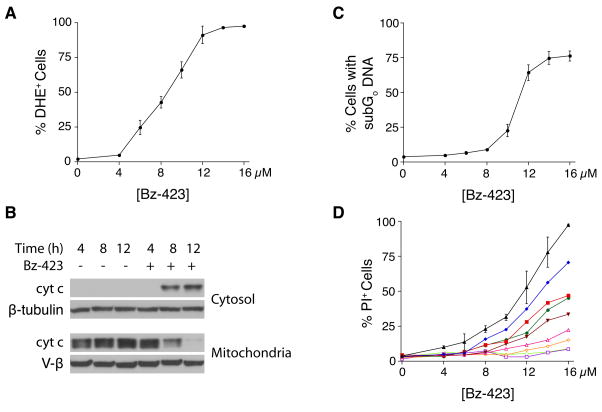
As with isolated mitochondria, Bz-423 rapidly increases superoxide levels in MEFs within 1 h and the magnitude of the increase is concentration-dependent (Figure 1A). Consistent with the activation of an intrinsic apoptotic pathway [5], release of cytochrome c into the cytosol is detected at 8 h (Figure 1B). By 12 h, mitochondria are depleted of cytochrome c (Figure 1B) and ΔΨm has collapsed (data not shown). Activation of caspases-9 and 3 between 8 h and 12 h is observed consistent with activation of the apoptosome by cytochrome c (data not shown). These events are followed by apoptotic DNA fragmentation and cell death (24 h, Figure 1C–D). Of note, the EC50 values for apoptotic DNA changes is similar to the EC50 for changes in plasma membrane permeability indicating that Bz-423 induced cell death results from apoptosis.
Fig. 1.
Bz-423 induces Apoptosis in MEFs. (A) Superoxide production (1 h) was measured using dihydroethidium (DHE). (B) Cytochrome c release into the cytosol was determined by western blot following Bz-423 treatment (12 μM) for the indicated times. (C) Apoptosis (24 h) was measured by identifying cells with subG0 DNA content. (D) MEFs were incubated with the indicated concentrations of Bz-423 and then Bz-423 was washed out of the media at various time points (1 h: light green open circles, 3 h: purple open squares, 5 h: orange open diamonds, 7 h: pink open triangles, 8 h, brown inverted triangles, 10 h: red squares, 12 h: blue diamonds, no wash: black triangles). Cell viability was determined by propidium iodide (PI) exclusion after 24 h of total culture.
To investigate the kinetics of cell death, Bz-423 was washed out of the culture media at multiple time points, and cell viability measured at 24 h (Figure 1D). While incubation of MEFs with Bz-423 for 1 h is sufficient to generate superoxide, it is insufficient, irrespective of Bz-423 concentration, to cause cell death. Treatment of MEFs with Bz-423 for 8 h, the point at which cytochrome c release is first observed, causes modest cell death. The maximal death response requires exposure of MEFs to Bz-423 for at least 12 h, the point at which we observe ΔΨm collapse and near complete release of cytochrome c from the mitochondria. By comparison, lymphocytes show apoptotic DNA changes by 5 h and require less drug to induce apoptosis [1].
Bz-423-induced apoptosis dependents upon superoxide
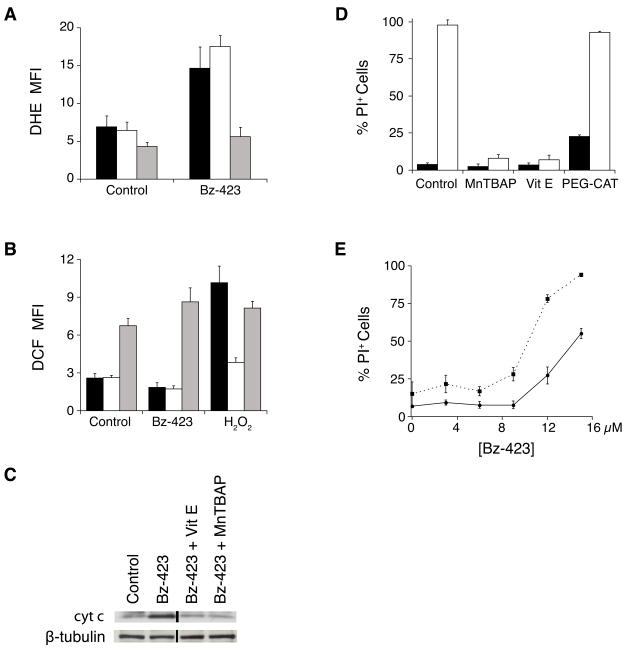
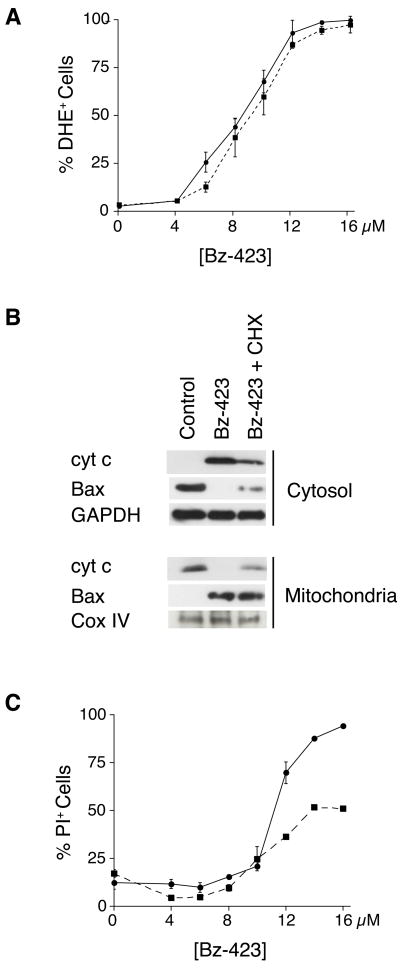
To examine the nature of the ROS signal generated by Bz-423, MEFs were pre-treated with various antioxidants prior to the detection of ROS with DHE and DCF. As seen in Figure 2A, pre-treatment with MnTBAP dramatically decreases the DHE response induced by Bz-423 (P = 0.001). While Bz-423 does not induce a DCF response on its own, pre-treatment with MnTBAP significantly augments this signal (P < 0.001, Figure 2B). These results are consistent with the actions of MnTBAP as a superoxide dismutase mimetic (see review by Patel and Day [10]). In contrast, pre-treatment with a cell permeant form of catalase conjugated to polyethylene glycol (PEG-CAT, [11]) does not inhibit the Bz-423 DHE or DCF responses (P = 0.12 and P = 0.62, respectively), despite reducing the DCF response induced by hydrogen peroxide (P = 0.001, Figure 2A–B). Taken together, these results are consistent with the specific production of superoxide by Bz-423 via its ability to modulate the F1F0-ATPase
Fig. 2.
Apoptosis by Bz-423 requires superoxide. (A–B) Cells were pre-treated with MnTBAP (30 min, 100 μM, grey bars), PEG-CAT (overnight, 200 U/mL, white bars) or vehicle (black bars), prior to the addition of Bz-423 (12 μM). Superoxide production (1 h, panel A) was determined by recording the FL2 channel median fluorescence intensity (MFI) as a marker of the oxidation of DHE to oxyethidium. Peroxide formation (1 h, panel B) was determined by recording the FL1 channel MFI as a marker of the oxidation of DCF. (C) Cytochrome c release into the cytosol was measured in MEFs following Bz-423 treatment (12 μM) with or without pre-treatment (30 min) with MnTBAP (100 μM) or vitamin E (100 μM). The black vertical line indicates that non-adjacent lanes from the same gel were used to compile this figure. (D) MEFs were pre-treated with vitamin E (30 min, 100 μM), MnTBAP (30 min, 100 μM), PEG-CAT (overnight, 300 U/mL) or vehicle (black bars), prior to the addition of Bz-423 (15 μM, white bars). Cell viability (24 h) was determined by PI exclusion. P < 0.001 for vehicle vs MnTBAP, P < 0.001 for vehicle vs vitamin E, P = 0.45 for vehicle vs PEG-CAT. (E) After 24 h of pre-treatment with vehicle (solid line) or BSO (1 mM, dashed line), MEFs were incubated with Bz-423 and cell viability determined by PI exclusion at 24 h. P < 0.05 for vehicle vs BSO at all [Bz-423] ≥ 3 μM.
To determine the importance of Bz-423-induced superoxide in the MEF death response, MEFs were pre-treated with the antioxidants vitamin E or MnTBAP. Each of these agents prevents both cytochrome c release (Figure 2C) and Bz-423-induced cell death (P < 0.001, Figure 2D). In contrast, pre-treatment of MEFs with PEG-CAT, which does not inhibit Bz-423 superoxide production, fails to inhibit Bz-423-induced cell death (P = 0.45, Figure 2D). These findings confirm that Bz-423 induced apoptosis requires superoxide. Furthermore, the correlation observed between the amount of inhibition of cytochrome c release and inhibition of cell death supports the hypothesis that cytochrome c release is a key checkpoint in this response [9].
Glutathione (GSH) is a major component of the cellular defense to oxidants and also functions as a regulator of oxidant-sensitive enzymes [12]. To assess the importance of GSH in determining the cellular response to Bz-423, MEFs were treated with L-buthionine sulfoximine (BSO), an inhibitor of γ-glutamylcysteine synthetase, the rate-limiting step in GSH synthesis [13]. Treatment of MEFs with BSO (1 mM, 24 h) decreases both cytoplasmic and mitochondrial GSH stores by greater than 99% relative to those in untreated cells (data not shown). After pre-treatment, MEFs were incubated with Bz-423 for 24 h, and cell viability determined (Figure 2E). Glutathione-depleted cells are more sensitive to Bz-423 demonstrating the importance of endogenous GSH levels in determining cellular sensitivity to Bz-423. The magnitude of this sensitization is similar to what is observed with other pro-oxidants [14, 15].
Bz-423-induced apoptosis depends upon Bcl-2 proteins
The next series of experiments focused on determining the signaling mechanism intervening between Bz-423 induced ROS and MOMP, reflected by cytochrome c release. MOMP results from either (i) activation and homo-oligomerization of pro-apoptotic multi-domain Bcl-2 proteins Bax and/or Bak or (ii) opening of the MPT pore leading to mitochondrial swelling-induced rupture of the outer membrane [9]. Since we do not observe cytochrome c release when isolated mitochondria are exposed to Bz-423 (Figure S1), we focused on experiments to determine whether Bax and/or Bak is required for Bz-423 induced cytochrome c release. In their inactive states, Bax is primarily a cytoplasmic protein, while Bak is associated with mitochondria [16]. Upon activation, both proteins undergo conformational changes and, in the case of Bax translocate to the mitochondria, causing MOMP and allowing cytochrome c release from the inter-membrane space.
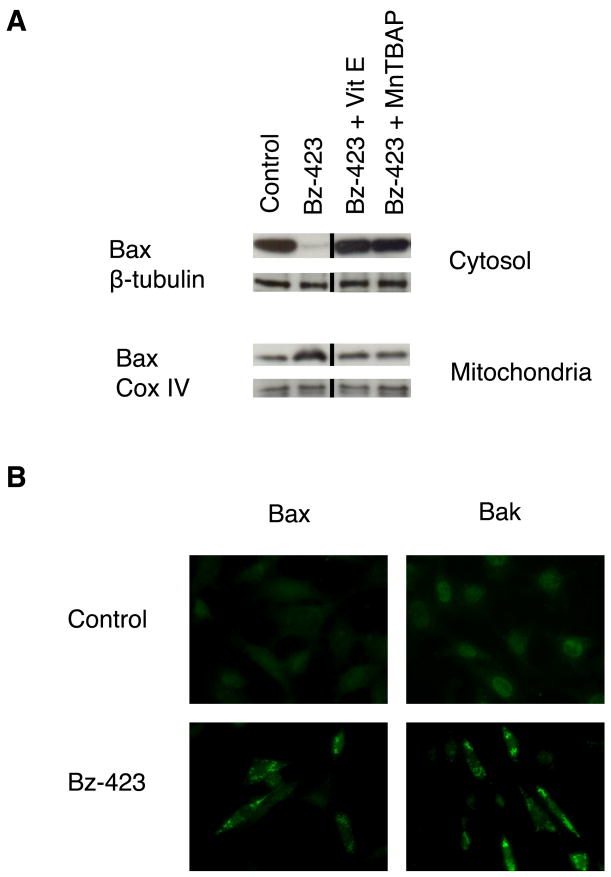
In control MEFs, Bax is detected almost exclusively in the cytosolic fraction, whereas treatment with Bz-423 for 12 h increases the amount of Bax in the mitochondrial fraction (Figure 3A). These findings are consistent with Bz-423 treatment causing Bax activation and translocation. Pre-treating cells with MnTBAP or vitamin E inhibits Bax translocation indicating that this response depends upon Bz-423 triggered superoxide (Figure 3A). To confirm that Bax undergoes conformational activation and assess whether Bak is similarly activated in response to Bz-423, MEFs were incubated with antibodies to Bax and Bak that recognize an N-terminal epitope that is only accessible upon activation. Using immunofluorescence microscopy, we find that cells treated with Bz-423 for 12 h display a bright, punctate staining pattern with each antibody (Figure 3B). This pattern is not seen in control cells, and these results are consistent with conformational activation and mitochondrial localization of these proteins following treatment with Bz-423.
Fig. 3.
Bz-423 activates Bak and Bax. (A) MEFs were pre-treated with MnTBAP (100 μM) or vitamin E (100 μM) for 30 min prior to treatment with Bz-423 (12 μM, 12 h) and cell fractionation. Cytosolic or mitochondrial Bax was detected by immunoblot. The black vertical line indicates where non-adjacent lanes from the same gel were used to compile this figure. (B) MEFs were treated with vehicle or Bz-423 (12 μM, 12 h) followed by detection of activated N-terminal Bax or Bak by imunofluorescence microscopy.
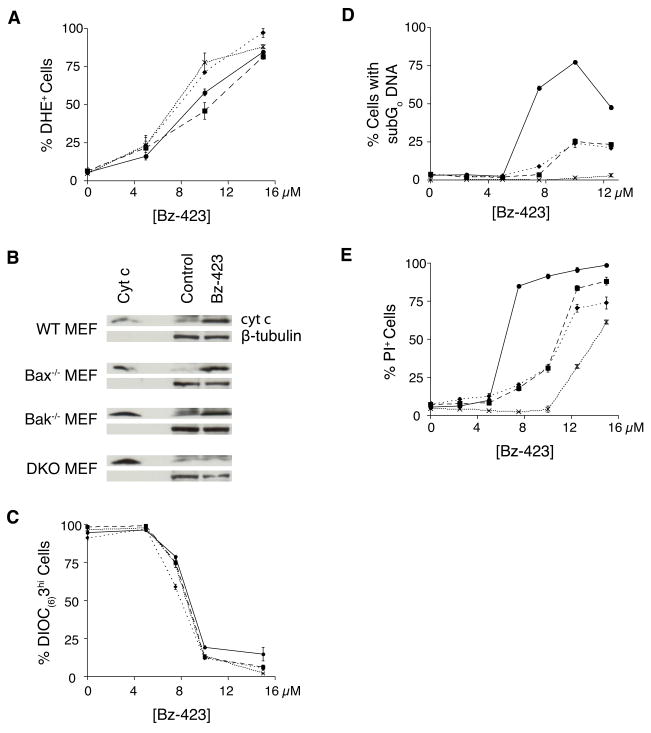
MEFs derived from Bax−/−, Bak−/−, or double knockout-mice (DKO) were used to determine if these proteins are required for the apoptotic response to Bz-423. Wild type MEFs, along with the three knockout strains increase superoxide in response to Bz-423, consistent with these proteins being involved downstream of the initial ROS signal (Figure 4A). The cells were then analyzed for cytochrome c release and ΔΨm after 12 h of treatment, a time point at which Bax and Bak activation and Bax translocation are readily detectable. Knock-out of either Bax or Bak has little if any effect on Bz-423-induced cytochrome c release, whereas cytochrome c release is blocked in DKO MEFs (Figure 4B). In contrast to the results with cytochrome c release, ΔΨm collapse following Bz-423 is not inhibited in any of the knock out cell lines (Figure 4C). These results show that Bz-423-induced MOMP is mediated by Bax and Bak, which have redundant functions in these cells.
Fig. 4.
Bak and Bax knockout block Bz-423 induced death. (A) Superoxide was measured using DHE in WT MEF (circles, solid line), Bax−/− MEF (squares, long dashes), Bak−/− MEF (diamonds, short dashes), and DKO MEF (“X”, dotted line) after treatment with Bz-423 (1 h). (B) Cytosolic fractions prepared from WT, Bax−/−, Bak−/−, and DKO MEF following treatment (12 h) with Bz-423 (12 μM) or control were analyzed for cytochrome c. (C) ΔΨm was measured using DIOC6(3) in WT, Bax−/−, Bak−/−, and DKO MEF following Bz-423 (12 h). (D) Apoptosis was determined by hypodiploid DNA content in WT (circles, solid line), Bax−/− (squares, long dashes), Bak−/− (diamonds, short dashes), and DKO MEFs (“X”, dotted line) following Bz-423 treatment (48 h). P < 0.01 for WT vs Bak−/−, Bax−/−, or DKO MEFs at all [Bz-423] > 5 μM, P < 0.01 for Bak−/− vs DKO MEFs at all [Bz-423] > 5 μM, P < 0.01 for Bax−/− vs DKO MEFs at all [Bz-423] > 7.5 μM. (E) Cell viability was determined by PI exclusion in WT, Bax−/−, Bak−/−, and DKO MEF following Bz-423 treatment (48 h). P < 0.05 for WT vs Bak−/− or Bax−/− MEFs at all [Bz-423] > 5 μM, P < 0.02 for WT vs DKO MEFs at all [Bz-423] ≥ 2.5 μM, P < 0.02 for Bak−/− vs DKO at [Bz-423] between 2.5 μM and 12.5 μM, P < 0.01 for Bax−/− vs DKO at all [Bz-423] > 2.5 μM.
Differences in Bz-423-induced apoptotic DNA fragmentation are observed between control, single knock out and DKO cells that are consistent with the differences in cytochrome c release (Figure 4D). In particular, DKO MEFs do not undergo apoptosis as indicated by the absence of hypodiploid DNA content even after 48 h of culture. Supporting the involvement of both Bax and Bak in the mechanism, the single knock out cell lines show an intermediate level of apoptotic DNA changes compared to WT and DKO MEFs. Similar differences are also observed when overall cell viability based on plasma membrane integrity is assessed in these four cell lines (Figure 4E). Higher concentrations of Bz-423 (>10 μM) are able to overcome the protection afforded by knock out of Bak and Bax against cell death measured by PI exclusion. This finding suggests that in the absence of effective MOMP, higher concentrations of Bz-423 eventually induce non-apoptotic cell death, likely via secondary necrosis from prolonged alterations in redox balance and ΔΨm collapse [17].
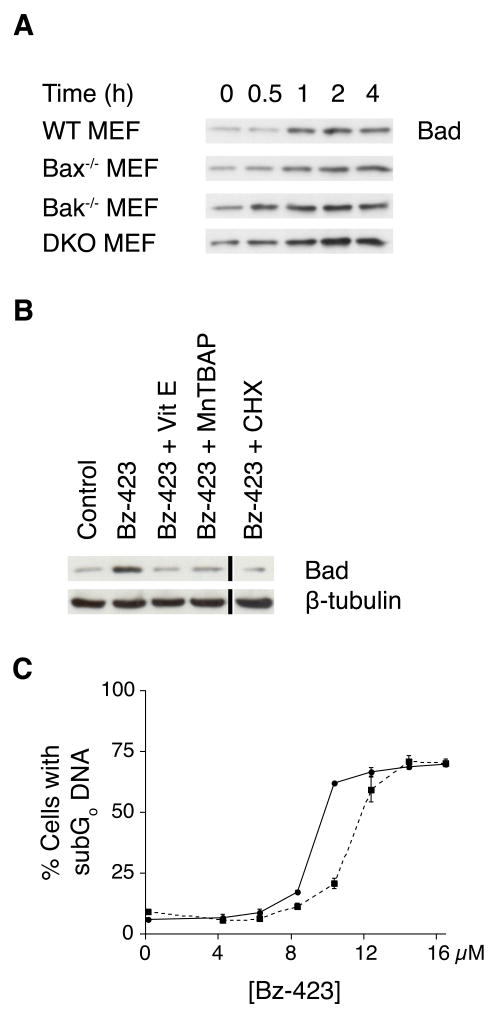
Bax and Bak activation is regulated by the balance between anti-apoptotic Bcl-2 proteins (e.g., Bcl-2, Bcl-xL, Mcl-1) and pro-apoptotic BH3-only proteins [16]. Because increased expression or in some cases, post-translational modification of BH3-only proteins can activate the pro-apoptotic functions of Bax and Bak [18], we screened cellular lysates to detect changes in selected BH3-only proteins following Bz-423 treatment. We find that Bad levels increase within 1 h of Bz-423 treatment (Figure 5A), whereas no changes in the expression level of other BH3-only proteins (Puma, Bim, Bid, Bik, Bmf, or Blk) are observed (data not shown). The increase in Bad expression is blocked in cells pre-treated with antioxidants (vitamin E or MnTBAP) while knock out of Bax and/or Bak has no effect on Bad expression (Figure 5A–B). Pre-treatment with the protein synthesis inhibitor cycloheximide (CHX) also prevents the increase in Bad levels (Figure 5B). These results indicate that Bz-423 induced superoxide increases Bad levels independent of Bak and Bax and also indicates that Bz-423 signals de novo protein synthesis upstream of Bax and Bak activation. To determine the relative importance of Bad in Bz-423 induced apoptosis, we tested MEFs from Bad−/− mice. We find that knock out of this BH3-only protein inhibits, but does not prevent apoptosis (Figure 5C). From these results, we conclude that Bad is not the sole signal through which Bax and Bak are activated following Bz-423 treatment.
Fig. 5.
Bad expression increases in response to Bz-423. (A) After treatment with Bz-423 (10 μM), lysates were prepared and immunoblotted to detect Bad protein in WT, Bax−/−, Bak−/−, and DKO MEF. (B) WT MEF were pre-treated with MnTBAP (100 μM), vitamin E (100 μM), CHX (1 μg/mL), or vehicle for 30 min prior to treatment with Bz-423 (10 μM, 2 h) then immunoblotted for Bad. The black vertical line indicates where non-adjacent lanes from the same gel were used to compile this figure. (C) Following treatment with Bz-423 for 48 h, WT (solid line) and Bad−/− (dashed line) MEFs were analyzed to detect cells with subG0 DNA content. P < 0.05 for WT vs Bad−/− MEFs at 8 and 10 μM Bz-423.
Because Bz-423 increased Bad expression is blocked by CHX, we next examined the effect of CHX on the overall Bz-423 response mechanism. Consistent with the direct interaction of Bz-423 with the F1F0-ATPase, superoxide generation (Figure 6A) is not inhibited by CHX. CHX partially inhibits Bax translocation, cytochrome c release, and Bz-423 triggered cell death (Figure 6B–C). These results, when combined with partial protection afforded by knock out of Bad, suggest that the commitment to cell death as indicated by MOMP and mediated by Bax/Bak activation is controlled by two separate signal-response couples, one dependent upon and one independent of de novo protein synthesis.
Fig. 6.
CHX inhibits Bz-423 induced apoptosis. (A) Following pre-treatment (30 min) with CHX (1 μg/mL, dashed line) or vehicle (solid line), MEFs were incubated with Bz-423 and superoxide production (1 h) was monitored with DHE. (B) MEFs were pre-treated with CHX (1 μg/mL) or vehicle for 30 min prior to incubation with Bz-423 (12 μM, 12 h), fractionated as indicated, and immunoblotted to detect cytochrome c and Bax. (C) Following pre-treatment with CHX (1 μg/mL, dashed line) or vehicle (solid line), MEFs were incubated with Bz-423 and cell viability (24 h) determined by PI exclusion. P < 0.02 for vehicle vs CHX at [Bz-423] > 10 μM.
Bz-423 activates MAP kinases
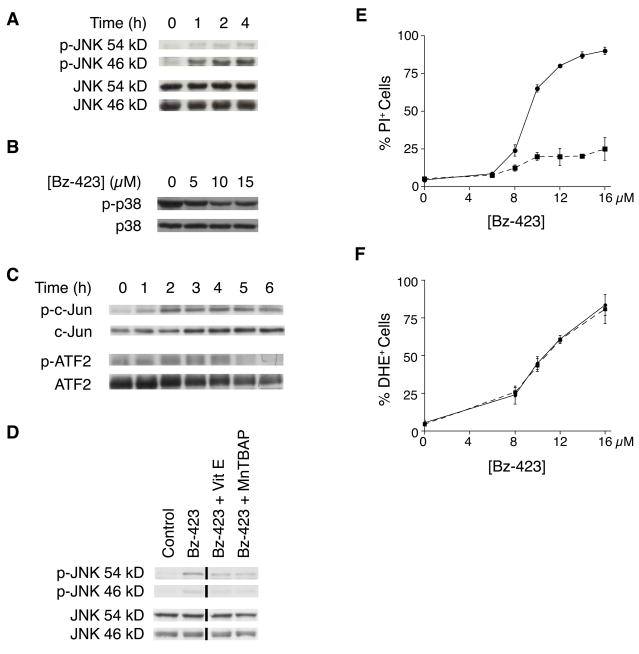
The partial inhibition of Bz-423 induced apoptosis by CHX suggests that in MEFs additional non-protein synthesis dependent cellular signals are activated by Bz-423 downstream of superoxide and upstream of Bax, and Bax. The MAP kinases, JNK and p38, are good candidates to link these responses because activation of JNK and p38 often occurs following changes in redox balance, and both can link cellular stress to activation of Bax and Bak and to changes in gene transcription [18–20]. Treatment of MEFs with Bz-423 induces phosphorylation of JNK (Figure 7A) but not p38 (Figure 7B). To determine the functional consequence of JNK activation, we assayed the phosphorylation state of two of its substrates, the transcription factors c-Jun and ATF2 [21]. Treatment with Bz-423 causes sustained phosphorylation of both of these proteins (Figure 7C), consistent with JNK activation effecting changes in gene transcription [22].
Fig. 7.
Bz-423 activates JNK. (A) Lysates prepared from MEFs treated with Bz-423 (10 μM) were immunoblotted to detect total and phosphorylated JNK. (B) Following treatment (2 h) with the indicated concentrations of Bz-423 (in μM), total cellular lysates were immunoblotted for total and phospho-p38. (C) Lysates prepared from MEFs treated with Bz-423 (10 μM) were immunoblotted to detect total and phosphorylated c-Jun and ATF2. (D) MEF were pre-treated with MnTBAP (100 μM), vitamin E (100 μM), or vehicle for 30 min prior to treatment with Bz-423 (10 μM, 2 h). Whole cell lysates were immunoblotted for JNK and phospho-JNK expression. The black vertical line indicates where non-adjacent lanes from the same gel were used to compile this figure. (E) Following pre-treatment with SP600125 (10 μM, dashed line) or vehicle (solid line), MEFs were incubated with Bz-423 and viability (24 h) was determined by PI exclusion. P < 0.01 for vehicle vs SP600125 at [Bz-423] > 8 μM. (F) Following pre-treatment with SP600125 (10 μM, dashed line) or vehicle (solid line), MEFs were incubated with Bz-423 and superoxide was detected by DHE (1 h).
Pre-treating MEFs with antioxidants inhibits JNK activation (Figure 7D), placing JNK downstream of Bz-423 induced superoxide. We used SP600125, a kinase inhibitor selective for JNK [23], to determine if JNK is required for the Bz-423 apoptotic response. As seen in Figure 7E, pre-treatment with SP600125 almost completely prevents Bz-423 killing of MEFs implying that Bz-423 induced JNK activation is central to the death mechanism. Indeed, pre-treatment with SP600125 blocks the Bz-423-induced increase in Bad levels, Bax activation, and cytochrome c release (data not shown), but does not inhibit Bz-423-induced superoxide (Figure 7F). These results indicate that JNK activation is required for Bz-423-induced apoptosis in MEFs, and that this kinase is activated at a proximal point in the signaling cascade triggered by Bz-423, prior to the activation of Bax/Bak.
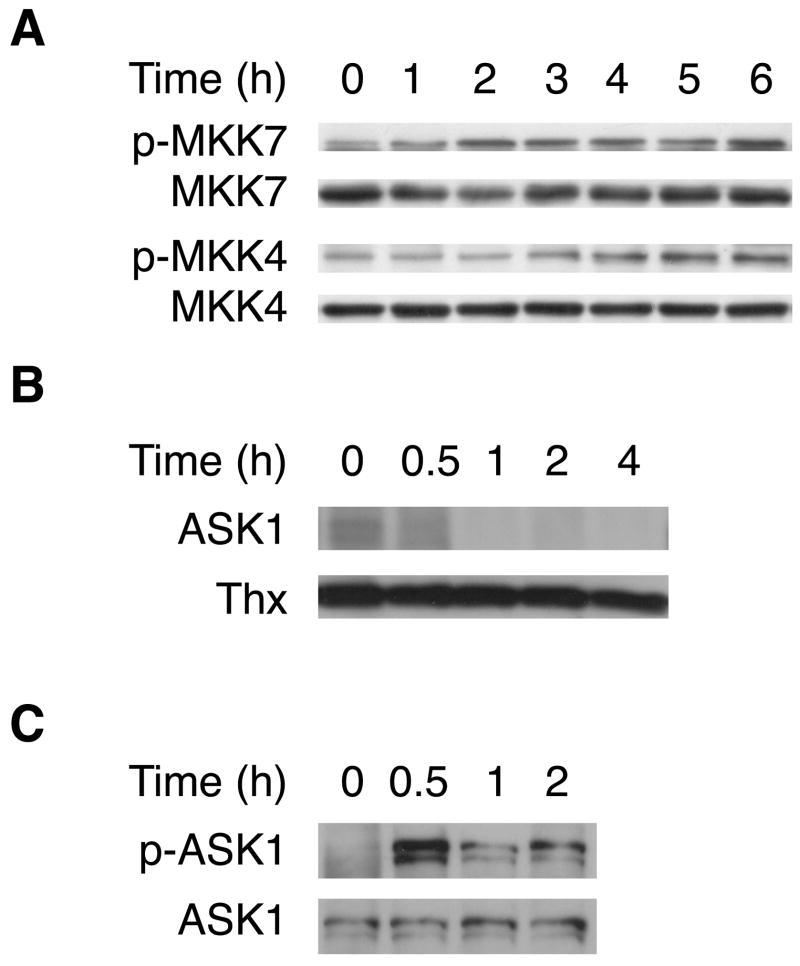
As part of the MAP kinase signaling cascade, JNK is phosphorylated by the upstream kinases MKK4 and MKK7 [19]. As expected, we find that MKK4 and MKK7 are phosphorylated following treatment with Bz-423 (Figure 8A). MKK4/7 are in turn substrates of apoptosis signaling-regulating kinase-1 (ASK1) that couples changes in cellular redox balance with activation of JNK [20]. ASK1 is essential for sustained activation of JNK in apoptosis induced by oxidants, including hydrogen peroxide and TNF-α [19, 22]. In its inactive state, ASK1 is a cytosolic protein complexed with reduced thioredoxin-1 (Thx1). When Thx1 is oxidized, it dissociates from ASK1, enabling ASK1 to autoactivate [19]. Therefore, we tested whether Bz-423 induces Thx1-ASK1 dissociation and ASK1 phosphorylation. In control cells, an intact Thx1-ASK1 complex co-immunoprecipitates, whereas treatment with Bz-423 causes a time-dependent dissociation of ASK1 from Thx1 (Figure 8B). Moreover, within 30 min of treatment, increasing amounts of phosphorylated ASK1 are detected (Figure 8C). Taken together, these results are consistent with a mechanism in which Bz-423-induced ROS activates a sequential protein kinase cascade that involves ASK1 and JNK that in turn leads to activation of Bak and Bax through protein synthesis dependent and independent signals.
Fig. 8.
Bz-423 activates upstream MAP kinases. (A) Lysates prepared from MEFs treated with Bz-423 (10 μM) were immunoblotted to detect total and phosphorylated MKK4 and MKK7. (B) Co-immunoprecipitation of Thx and ASK1 after treatment with Bz-423 (12 μM) shown by immunoprecipitating Thx and then blotting the immune complexes for ASK1 and Thx. (C) Lysates prepared from cells treated with Bz-423 (12 μM) were immunoprecipitated for ASK1 and then blotted to detect total ASK1 and phospho-ASK1.
Discussion
Inhibition of F1F0-ATPase induces a state 3 to state 4 transition leading to formation of reduced intermediates in mitochondria [4, 24, 25]. These reactive intermediates (e.g., reduced ubisemiquinones) can form at both the matrix and inter-membrane sides of the inner membrane and release superoxide into both compartments [26]. Superoxide can also be formed on the matrix side at complex I most likely via the transfer of an electron from a half-reduced flavin mononucleotide to molecular oxygen [27, 28]. Superoxide levels in cells are limited by dismutation to hydrogen peroxide through the action of Mn-superoxide dismutase (SOD) in the mitochondrial matrix or Cu, Zn-SOD in the inter-membrane space and cytoplasm [26]. As part of cellular antioxidant defenses, reduced glutathione present both in the mitochondrial matrix and cytoplasm can also react with (and detoxify) ROS including superoxide [29]. Depletion of GSH with BSO sensitizes cells to Bz-423 supporting the hypothesis that ROS generated by Bz-423 is critical for its apoptosis. Moreover, these findings suggest that part of the selectivity of Bz-423 for different cell types may result from variation in cellular antioxidant defenses. Similarly, such differences underlie the selectivity of several redox active anti-cancer agents [30].
Bz-423 rapidly activates ASK1 in MEFs. This MAP kinase kinase kinase is increasingly recognized as a cytosolic redox sensor that triggers apoptosis [20]. ASK1 is found as a homooligomer and may also complex with several other proteins, including thioredoxin, 14-3-3 proteins, and calcineurin in untreated cells [19, 31]. Thioredoxin binds to and inhibits ASK1 by interfering with the homophilic interaction of ASK1 via its N-terminal coiled-coil domain [32]. Oxidation of cysteine residues in thioredoxin to cystine releases thioredoxin from this complex and activates ASK1 through trans-autophosphorylation [32]. This activation is triggered by pro-oxidants including hydrogen peroxide, the heavy metals arsenic, cadmium, and mercury, the complex I inhibitor rotenone, as well as the oxidative burst that accompanies TNF-α signaling [33–35].
Following expsoure to various prooxidants, activation of ASK1 is generally associated with activation of both p38 and JNK [20]. In contrast, we only detect activation of JNK following treatment with Bz-423. The interactions among kinases and substrates in the MAPK cascade are highly regulated. Treatment specific and cell-type specific responses are in part mediated by the differential involvement of scaffold proteins in the regulation of ASK1 [36]. One example is JNK/stress-activated protein kinase-associated protein 1 (JSAP1) that is activated by ASK1 in response to oxidants, and in turn leads to the preferential recruitment of MKK7-JNK3 as opposed to p38 as ASK1 substrates [36]. Gemin5 is another recently described scaffolding protein that is required for JNK1 activation following pro-oxidant treatment with hydrogen peroxide and TNF-α [37]. Gemin5 specifically interacts with ASK1, MKK4, and JNK1. Gemin5 potentiates ASK1 homooligomerization, and does not interact with MKK7, MKK3, MKK6, or p38. Thus, the observed MKK4/7-JNK specific recruiting function of Bz-423-activated-ASK1 may depend on the specific expression and activation of stimulus-specific adaptors. Alternatively, specific MAP kinase activation is regulated by the action of dual specificity phosphatases [38]. For example, oxidative stress-mediated hepatotoxicity following treatment with carbon tetrachloride is associated with activation of the phosphatase MKP-1 that dephosphorylates p38 and ERK, and is activated by JNK [39]. Treatment with Bz-423 may also activate this phosphatase, as we observe dephosphorylation of p38 (Figure 7B) and ERK (unpublished observations).
The identity of the signal that leaves the mitochondria to trigger extra-mitochondrial apoptotic pathways following Bz-423 treatment is unclear. The ability of the superoxide dismutase mimetic MnTBAP to prevent Bz-423 induced apoptosis (and not PEG-CAT) implies that superoxide, and not hydrogen peroxide, is the relevant ROS. However, recent reports have questioned the dismutase functions of commerical lots of this compound, and suggested that MnTBAP may instead be acting as a xanthine oxidase inhibitor [40]. In support of its role as a superoxide dismutase mimetic, we find that MnTBAP inhibits DHE oxidation and induces a prominent DCF response in cells treated with Bz-423. In addition, MnTBAP does not significantly inhibit the DCF response in cells treated with hydrogen peroxide (Figure 2B). We have also previously shown that allopurinol, a specific inhibitor of xanthine oxidase, does not inhibit Bz-423 induced DHE oxidation [1]. Taken together, these findings indicate that MnTBAP is acting as a superoxide dismutase mimetic and supports our hypothesis that treatment with Bz-423 specifically generates superoxide. Recent studies indicate that this reaction occurs via a sulfenic acid intermediate that does not involve hydrogen peroxide [41, 42]. Collectively, these findings suggest the possibility that superoxide is the direct link between the initial mitochondrial response to Bz-423 and activation of ASK1. Indeed, ASK1 is activated by other superoxide sources including methylglyoxal and NADPH oxidase [43–45]. For this reaction with thioredoxin to occur, superoxide must be exported from mitochondria into the cytosol. Although it does not readily diffuse through lipid membranes, superoxide can be transported from the mitochondria through via the voltage-dependent anion channel located in the mitochondrial outer membrane [46].
The precise mechanism by which JNK activates Bax and Bak activation following Bz-423 treatment in MEFs is not yet clear. Both SP600125 and CHX prevent increased levels of Bad, suggesting that the observed increase results from JNK-regulated de novo protein synthesis. Because knockout of Bad or pre-treatment with CHX only provide modest protection against Bz-423-induced apoptosis, other non-protein synthesis dependent mechanisms by which Bax and Bak are activated must be involved. Indeed, JNK can activate Bax or Bak by mechanisms independent of protein synthesis that include the direct phosphorylation of Bax, Bcl-2, and various BH3-only proteins [18, 47, 48]. Based on our results showing limited protection by CHX and greater protection by SP600125, we hypothesize that JNK orchestrates both protein synthesis-dependent and independent effects.
Cytochrome c release
A key checkpoint in Bz-423 induced apoptosis is the release of cytochrome c from mitochondria. The mechanisms through which this molecule is released are still debated [9]. One long standing hypothesis proposes that opening of the MPT pore leads to swelling of the inner mitochondrial membrane that ruptures the outer mitochondrial membrane, releasing pro-apoptotic proteins from the inter-membrane space [9]. Oxidizing agents induce MPT pore opening, suggesting that Bz-423 may be acting along these lines [49–51]. However, experiments with isolated mitochondria derived from MEFs or rat liver confirm that Bz-423 does not directly induce the release of cytochrome c from the inter-membrane space nor does it cause large amplitude mitochondrial swelling seen following MPT [1]. These findings argue that Bz-423 generated superoxide does not directly induce the MPT. An alternative hypothesis is that cytochrome c is selectively released by MOMP triggered by activation of pro-apoptotic multidomain pro-apoptotic Bcl-2 proteins [9, 16]. Our data are consistent with this hypothesis - Bax and Bak are essential for cytochrome c release and apoptosis following treatment with Bz-423. Therefore, Bax and Bak represent the apoptotic signal that returns to the mitochondria to cause the release of this key mediator.
Comparison to other MRC inhibitors
ROS-mediated apoptosis is caused by other agents that modulate the MRC including antimycin A, rotenone, thenoyltrifluoroacetone, and trogliatzone [52, 53]. Cyanoaziridines like imexon also induce oxidant-dependent apoptosis via the direct reaction with and depletion of glutathione [54–56]. Oligomycin, the best characterized F1F0-ATPase inhibitor, can give rise to a respiratory transition and generate ROS [4, 25]. However, the binding site for this compound is located within the membrane spanning F0 component [57]. In addition to the mitochondrial F1F0-ATPase, oligomycin inhibits the Na+/K+- and H+/K+-ATPases [58, 59], suggesting that these enzymes contain similar binding pockets. In contrast to Bz-423, oligomycin induces time-dependent inhibition of the F1F0-ATPase [60], and is associated with decreases in total cellular ATP [52]. Because apoptosis requires sufficient levels of ATP to allow caspase activation through apoptosome formation [61], ATP synthesis inhibitors that result in a large decrease in intracellular ATP levels would not be able to engage apoptosis process like Bz-423, and instead induce necrosis [62]. Hence, Bz-423 is not directly comparable to oligomycin. However, some other agents that inhibit the F1F0-ATPase seem to have similar properties to Bz-423. For example, 3,3′-diindolylmethane induces redox-regulated apoptosis via inhibition of the F1F0-ATPase [63, 64]. This compound has antitumor properties in animal models of cancer [65, 66], and taken with our data, suggest that modulation of the F1F0-ATPase may be a valuable approach for new drug development [67].
Conclusions
Our data show that redox balance modulates ASK1-JNK-Bax/Bak signaling in response to Bz-423. Because antioxidants inhibit all downstream signals, superoxide is positioned at a proximal point in the response mechanism engaged following modulation of F1F0-ATPase activity by Bz-423. However, the reduction in cellular glutathione levels with BSO only modestly sensitizes MEFs to this compound, which suggests that the variation in glutathione existing across cell types may only be a minor factor in determining the sensitivity of cells to Bz-423, and is consistent with data showing that the apoptotic response of cells to other pro-oxidants does not strictly correlate with total cellular glutathione [14, 68–71].
In contrast to the limited role for glutathione, inhibition of JNK signaling profoundly inhibits the cellular response to Bz-423 by preventing activation of the pro-apoptotic Bcl-2, as well as inhibiting apoptosis and overall cell death. Since members of the MAP kinase family are differentially regulated across cell types (and stages of development) [72], the ability of a cell to activate these kinases in response to Bz-423 may be a key factor in modulating the selective response of Bz-423. Furthermore, the partial requirement for de novo protein synthesis (which follows MAP kinase activation) in the apoptotic response in MEFs helps to explain why these cells require prolonged exposure to Bz-423 relative to lymphoid cell types where Bz-423 induced death is independent of de novo protein synthesis [73]. These findings suggest that a different signal transduction pathway might link Bz-423 triggered superoxide to MOMP and apoptosis in lymphocytes. Such differences likely are key elements that underlie the favorable selectivity observed in vivo.
Supplementary Material
Bz-423 interactions with isolated mitochondria. (A) Images (630x) of mitochondria isolated from WT MEFs following incubation (30 min, 37 °C) with Bz-423 (10 μM) or control. Mitochondria were stained with DHE to detect superoxide, DIOC6(3) to detect ΔΨm, and mitotracker red to label all mitochondria. The uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP, 50 μM) was included as a positive control for membrane depolarization. (B) Cytochrome c release (1 h) from MEF isolated mitochondria was determined by western blot. Lane 1, purified cytochrome c (1 ng); Lane 2, vehicle control; Lane 3, Bz-423 (10 μM), Lane 4, Ca2+ (150 μM).
Acknowledgments
This work was supported by grants from the NIH (R01-AI 47450 to G.D.G, R01-CA 10456 to A.W.O.). N.B.B. is supported by a training grant from the NIH (T32 DK065517).
List of abbreviations
- JNK
c-Jun N-terminal kinase
- ASK1
apoptosis signal-regulating kinase 1
- OSCP
oligomycin-sensitivity conferring protein
- MRC
mitochondrial respiratory chain
- ROS
reactive oxygen species
- ΔΨm
mitochondrial electrochemical gradient/transmembrane potential
- MOMP
mitochondrial outer membrane permeabilization
- MEF
mouse embryonic fibroblast
- MAP
mitogen-activated protein
- DHE
dihydroethidium
- CM-H2DCFDA
5-(and -6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester
- DCF
dichlorodihydrofluorescein
- MnTBAP
Manganese (III) tetrakis (4-benzoic acid)porphyrin
- DKO
Bax/Bak double knockout
- PI
propidium iodide
- PMSF
phenylmethylsulphonylfluoride
- GAPDH
glyceradehyde-3-phosphate dehydrogenase
- Cox IV
cytochrome c oxidase
- V-β
the β-subunit of complex V
- MPT
mitochondrial permeability transition
- GSH
glutathione
- BSO
L-buthionine sulfoximine
- MKK
MAP kinase kinase
- CHX
cycloheximide
- SOD
superoxide dismutase
- MFI
median fluorescence intensity
- PEG-CAT
polyethylene glycol-catalase conjugate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blatt NB, Bednarski JJ, Warner RE, Leonetti F, Johnson KM, Boitano A, Yung R, Richardson BC, Johnson KJ, Ellman JA, Opipari AW, Glick GD. Benzodiazepine-induced superoxide signals B cell apoptosis: mechanistic insight and potential therapeutic utility. J Clin Invest. 2002;110:1123–1132. doi: 10.1172/JCI16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bednarski JJ, Warner RE, Rao T, Leonetti F, Yung R, Richardson BC, Johnson KJ, Ellman JA, Opipari AW, Glick GD. Attenuation of autoimmune disease in Fas-deficient mice by treatment with a cytotoxic benzodiazepine. Arthritis Rheum. 2003;48:757–766. doi: 10.1002/art.10968. [DOI] [PubMed] [Google Scholar]
- 3.Bhagavathula N, Nerusu KC, Hanosh A, Aslam MN, Sundberg TB, Opipari AW, Johnson K, Kang S, Glick GD, Varani J. Bz-423, a benzodiazepine, suppresses keratinocyte proliferation and has anti-psoriatic activity in the human skin-SCID mouse transplant model. J Pharmacol Exp Ther. 2008;324:938–947. doi: 10.1124/jpet.107.130955. [DOI] [PubMed] [Google Scholar]
- 4.Johnson KM, Chen X, Boitano A, Swenson L, Opipari AW, Glick GD. Identification and validation of the mitochondrial F1F0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423. Chem Biol. 2005;12:485–496. doi: 10.1016/j.chembiol.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Green DR, Kroemer G. Pharmacological manipulation of cell death: clinical applications in sight? J Clin Invest. 2005;115:2610–2617. doi: 10.1172/JCI26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei MC, Zong W-X, Cheng EH-Y, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunin BA, Plunkett MJ, Ellman JA. The combinatorial synthesis and chemical and biological evaluation of a 1,4-benzodiazepine library. Proc Natl Acad Sci USA. 1994;91:4708–4712. doi: 10.1073/pnas.91.11.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 9.Bouchier-Hayes L, Lartrigue L, Newmeyer DD. Mitochondria: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2640–2647. doi: 10.1172/JCI26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel M, Day BJ. Metalloporphyrin class of therapeutic catalytic antioxidants. Trends Pharmacol Sci. 1999;20:359–364. doi: 10.1016/s0165-6147(99)01336-x. [DOI] [PubMed] [Google Scholar]
- 11.Beckman JS, Minor RL, White CW, Repine JE, Rosen GM, Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988;263:6884–6892. [PubMed] [Google Scholar]
- 12.Giles GI. The redox regulation of thiol dependent signaling pathways in cancer. Curr Pharm Des. 2006;12:4427–4443. doi: 10.2174/138161206779010549. [DOI] [PubMed] [Google Scholar]
- 13.Bailey HH. L-S, R-buthionine sulfoximine: historical development and clinical issues. Chem Biol Interact. 1998;111–112:239–254. doi: 10.1016/s0009-2797(97)00164-6. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Chan R, Waxman S, Jing Y. Buthionine sulfoximine enhancement of arsenic trioxide-induced apoptosis in leukemia and lymphoma cells is mediated via activation of c-Jun NH2-terminal kinase and up-regulation of death receptors. Cancer Res. 2006;66:11416–11423. doi: 10.1158/0008-5472.CAN-06-0409. [DOI] [PubMed] [Google Scholar]
- 15.Morales M, Perez-Yarza G, Nieto-Rementeria N, Boyano MD, Jangi M, Atencia R, Asumendi A. Intracellular glutathione levels determine cell sensitivity to apoptosis induced by the antineoplastic agent N-(4-hydroxyphenyl) retinamide. Anticancer Res. 2005;25:1945–1951. [PubMed] [Google Scholar]
- 16.Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 2006;13:1396–1402. doi: 10.1038/sj.cdd.4401963. [DOI] [PubMed] [Google Scholar]
- 17.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascade, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2006;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Shen H-M, Liu Z. JNK signaling is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbagen.2007.1012.1011. [DOI] [PubMed] [Google Scholar]
- 21.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 22.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygens species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 25.Tirosh O, Aronis A, Melendez JA. Mitochondrial state 3 to 4 respiration transition during Fas-mediated apoptosis controls cellular redox balance and rate of cell death. Biochem Pharmacol. 2003;66:1331–1334. doi: 10.1016/s0006-2952(03)00481-7. [DOI] [PubMed] [Google Scholar]
- 26.Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol. 2006;291:C1082–1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 27.Skulachev VP. Bioenergetic aspects of apoptosis, necrosis, and mitoptosis. Apoptosis. 2006;11:473–485. doi: 10.1007/s10495-006-5881-9. [DOI] [PubMed] [Google Scholar]
- 28.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q, Wilkins BJ, Lee YJ, Ichijo H, Molkentin JD. Direct interaction and reciprocal regulation between ASK1 and calcineurin-NFAT control cardiomyocyte death and growth. Mol Cell Biol. 2006;26:3785–3797. doi: 10.1128/MCB.26.10.3785-3797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujino G, Noguchi T, Matsuzawa A, Yamauchi S, Saitoh M, Takeda K, Ichijo H. Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol Cell Biol. 2007;27:8152–8163. doi: 10.1128/MCB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsukawa J, Matsuzawa A, Takeda K, Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J Biochem (Tokyo) 2004;136:261–265. doi: 10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- 34.Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med. 2006;40:138–145. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh C-C, Papaconstantinou J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J. 2006;20:259–268. doi: 10.1096/fj.05-4376com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuura H, Nishitoh H, Takeda K, Matsuzawa A, Amagasa T, Ito M, Yoshioka K, Ichijo H. Phosphorylation-dependent scaffolding role of JSAP1/JIP3 in the ASK1-JNK signaling pathway. J Biol Chem. 2002;277:40703–40709. doi: 10.1074/jbc.M202004200. [DOI] [PubMed] [Google Scholar]
- 37.Kim EK, Noh K-T, Yoon J-H, Cho J-H, Yoon KW, Dreyfuss G, Choi E-J. Positive regulation of ASK1-mediated c-Jun NH2-terminal kinase signaling pathway by the WD-repeat protein Gemin5. Cell Death Differ. 2007;14:1518–1528. doi: 10.1038/sj.cdd.4402157. [DOI] [PubMed] [Google Scholar]
- 38.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 39.Mendelson KG, Contois L-R, Tevosian SG, Davis RJ, Paulson KE. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc Natl Acad Sci USA. 1996;93:12908–12913. doi: 10.1073/pnas.93.23.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rebouças JS, Spasojevic I, Batinic-Haberle I. Pure manganse(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: a case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. J Biol Inorg Chem. 2008;13:289–302. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- 41.Benrahmoune M, Therond P, Abendinzadeh Z. The reaction of superoxide radical with N-acetylcysteine. Free Radic Biol Med. 2000;29:775–782. doi: 10.1016/s0891-5849(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 42.Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med. 1999;27:322–328. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 43.Du J, Suzuki H, Nagase F, Akhand AA, Ma X-Y, Yokoyama T, Miyata T, Nakashima I. Superoxide-mediated early oxidation and activation of ASK1 are important for initiating methylglyoxal-induced apoptosis process. Free Radic Biol Med 2001. 2001 doi: 10.1016/s0891-5849(01)00611-6. [DOI] [PubMed] [Google Scholar]
- 44.Chiang E, Dang O, Anderson K, Matsuzawa A, Ichijo H, David M. Apoptosis-regulating signal kinase 1 is required for reactive oxygen species-mediated activation of IFN regulatory factor 3 by lipopolysaccharide. J Immunol. 2006;176:5720–5724. doi: 10.4049/jimmunol.176.10.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H, Zhang H, Iles KE, Rinna A, Merrill G, Yodoi J, Torres M, Forman HJ. The ADP-stimulated NADPH oxidase activates the ASK-1/MKK4/JNK pathway in alveolar macrophages. Free Radic Res. 2006;40:865–874. doi: 10.1080/10715760600758514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 47.Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2004;23:1889–1899. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim B-J, Ryu S-W, Song B-J. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 49.Zoratti M, Szabo I. The mitochondrial permability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 50.Madesh M, Hajnóczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costantini P, Belzacq A-S, Vieira HLA, Larochette N, de Pablo MA, Zamzami N, Susin SA, Brenner C, Kroemer G. Oxidation of a critical thiol residue of the adenine nucleotide translocator enforces Bcl-2-independent permeability transition pore opening and apoptosis. Oncogene. 2000;19:307–314. doi: 10.1038/sj.onc.1203299. [DOI] [PubMed] [Google Scholar]
- 52.Watabe M, Nakaki T. ATP depletion does not account for apoptosis induced by inhibition of mitochondrial electron transport chain in human dopaminergic cells. Neuropharmacology. 2007;52:536–541. doi: 10.1016/j.neuropharm.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 53.Lim PL, Liu J, Boelsterli UA. The mitochondrial superoxide/thioredoxin-2/Ask1 signaling pathway is critically involved in troglitazone-induced cell injury to human hepatocytes. Toxicol Sci. 2008;101:341–349. doi: 10.1093/toxsci/kfm273. [DOI] [PubMed] [Google Scholar]
- 54.Dvorakova K, Payne CM, Tome ME, Briehl MM, McClure T, Dorr RT. Induction of oxidative stress and apoptosis in myeloma cells by the aziridine-containing agent imexon. Biochem Pharmacol. 2000;60:749–758. doi: 10.1016/s0006-2952(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 55.Dvorakova K, Waltmire CN, Payne CM, Tome ME, Briehl MM, Dorr RT. Induction of mitochondrial changes in myeloma cells by imexon. Blood. 2001;97:3544–3551. doi: 10.1182/blood.v97.11.3544. [DOI] [PubMed] [Google Scholar]
- 56.Iyengar BS, Dorr RT, Remers WA. Chemical basis for the biological activity of imexon and related cyanoaziridines. J Med Chem. 2004;47:218–223. doi: 10.1021/jm030225v. [DOI] [PubMed] [Google Scholar]
- 57.Shchepina LA, Pletjushkina OY, Avetisyan AV, Bakeeva LE, Fetisova EK, Izyumov DS, Sparunova VB, Vyssokikh MY, Chernyak BV, Skulachev VP. Oligomycin, inhibitor of the F0 part of H+-ATP-synthase, suppresses the TNF-induced apoptosis. Oncogene. 2002;21:8149–8157. doi: 10.1038/sj.onc.1206053. [DOI] [PubMed] [Google Scholar]
- 58.Swarts HGP, Koenderink JB, Willems PHGM, De Pont JJHHM. The non-gastric H, K-ATPase is oligomycin-sensitive and can function as an H+, NH4+-ATPase. J Biol Chem. 2005;280:33115–33122. doi: 10.1074/jbc.M504535200. [DOI] [PubMed] [Google Scholar]
- 59.Homareda H, Ishii T, Takeyasu K. Binding domain of oligomycin on Na(+), K(+)-ATPase. Eur J Pharmacol. 2000;400:177–183. doi: 10.1016/s0014-2999(00)00411-8. [DOI] [PubMed] [Google Scholar]
- 60.Johnson KM, Cleary J, Fierke CA, Opipari AW, Glick GD. Mechanistic basis for therapeutic targeting of the mitochondrial F1F0-ATPase. ACS Chem Biol. 2006;1:304–308. doi: 10.1021/cb600143j. [DOI] [PubMed] [Google Scholar]
- 61.Saito Y, Nishio K, Ogawa Y, Kimata J, Kinumi T, Yoshida Y, Noguchi N, Niki E. Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic Res. 2006;40:619–630. doi: 10.1080/10715760600632552. [DOI] [PubMed] [Google Scholar]
- 62.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 63.Gong Y, Sohn H, Xue L, Firestone GL, Bjeldanes LF. 3,3′-diindolylmethane is a novel mitochondrial H+-ATP synthase inhibitor that can induce p21Cip1/Waf1 expression by induction of oxidative stress in human breast cancer cells. Cancer Res. 2006;66:4880–4887. doi: 10.1158/0008-5472.CAN-05-4162. [DOI] [PubMed] [Google Scholar]
- 64.Nachshon-Kedmi M, Yannai S, Fares FA. Induction of apoptosis in human prostate cancer cell line, PC3, by 3,3′-diindolylmethane through the mitochondrial pathway. Br J Cancer. 2004;91:1358–1363. doi: 10.1038/sj.bjc.6602145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen I, McDougal A, Wang F, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19:1631–1639. doi: 10.1093/carcin/19.9.1631. [DOI] [PubMed] [Google Scholar]
- 66.Wattenberg LW, Loub WD. Inhibition of polycyclic aromatic hydrocarbon-induced neoplasia by naturally occurring indoles. Cancer Res. 1978;38:1410–1413. [PubMed] [Google Scholar]
- 67.Armstrong JS. Mitochondrial medicine: pharmacological targeting of mitochondria in disease. Br J Pharmacol. 2007;151:1154–1165. doi: 10.1038/sj.bjp.0707288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Remmen H, Salvador C, Yang H, Huang TT, Epstein CJ, Richardson A. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch Biochem Biophys. 1999;363:91–97. doi: 10.1006/abbi.1998.1060. [DOI] [PubMed] [Google Scholar]
- 69.Armstrong JS, Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002;16:1263–1265. doi: 10.1096/fj.02-0097fje. [DOI] [PubMed] [Google Scholar]
- 70.Davison K, Cote S, Mader S, Miller WH. Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia. 2003;17:931–940. doi: 10.1038/sj.leu.2402876. [DOI] [PubMed] [Google Scholar]
- 71.Maeda H, Hori S, Ohizumi H, Segawa T, Kakehi Y, Ogawa O, Kakizuka A. Effective treatment of advanced solid tumors by the combination of arsenic trioxide and L-buthionine-sulfoximine. Cell Death Differ. 2004;11:737–746. doi: 10.1038/sj.cdd.4401389. [DOI] [PubMed] [Google Scholar]
- 72.Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F. Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie. 2006;88:1091–1098. doi: 10.1016/j.biochi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 73.Bednarski JJ, Lyssiotis CA, Roush R, Boitano A, Glick GD, Opipari AW. A novel benzodiazepine increase the sensitivity of B cells to receptor stimulation with synergistic effects on calcium signaling and apoptosis. J Biol Chem. 2004;279:29615–29621. doi: 10.1074/jbc.M403507200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bz-423 interactions with isolated mitochondria. (A) Images (630x) of mitochondria isolated from WT MEFs following incubation (30 min, 37 °C) with Bz-423 (10 μM) or control. Mitochondria were stained with DHE to detect superoxide, DIOC6(3) to detect ΔΨm, and mitotracker red to label all mitochondria. The uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP, 50 μM) was included as a positive control for membrane depolarization. (B) Cytochrome c release (1 h) from MEF isolated mitochondria was determined by western blot. Lane 1, purified cytochrome c (1 ng); Lane 2, vehicle control; Lane 3, Bz-423 (10 μM), Lane 4, Ca2+ (150 μM).