Abstract
Nepenthes pitcher plant species differ in their prey capture strategies, prey capture rates, and pitcher longevity. In this study, it is investigated whether or not interspecific differences in nutrient sequestration strategy are reflected in the physiology and microstructure of the pitchers themselves. Using a non-invasive technique (MIFE), ion fluxes in pitchers of Nepenthes ampullaria Jack, Nepenthes bicalcarata Hook.f., and Nepenthes rafflesiana Jack were measured. Scanning electron microscopy was also used to characterize the distribution of glandular and other structures on the inner pitcher walls. The results demonstrate that nutrient sequestration strategy is indeed mirrored in pitcher physiology and microstructure. Species producing long-lived pitchers with low prey capture rates (N. ampullaria, N. bicalcarata) showed lower rates of NH4+ uptake than N. rafflesiana, a species producing short-lived pitchers with high capture rates. Crucially, species dependent upon aquatic commensals (N. ampullaria, N. bicalcarata) actively manipulated H+ fluxes to maintain less acid pitcher fluid than found in ‘typical’ species; in addition, these species lacked the lunate cells and epicuticular waxes characteristic of ‘typical’ insectivorous congeners. An unexpected finding was that ion fluxes occurred in the wax-covered, non-glandular zones in N. rafflesiana. The only candidates for active transport of aqueous ions in these zones appear to be the epidermal cells lying beneath the lunate cells, as these are the only sites not visibly coated with epicuticular waxes.
Keywords: Digestive glands, H+, ion flux, MIFE, Nepenthes, NH4+, phytotelm, pitcher plant, scanning electron microscopy
Introduction
Carnivorous pitcher plants of the palaeotropical genus Nepenthes (Nepenthaceae) use fluid-filled traps to sequester nutrients from animal prey (Adamec, 1997; Schulze et al., 1997; Moran and Moran, 1998; Pavlovič et al., 2009). A variety of digestive enzymes are secreted by Nepenthes pitchers, including peptidase, aspartic and cysteine proteases, ribonuclease, chitinase, phosphatase, and esterase (Jentsch, 1972; Heslop-Harrison, 1975; An et al., 2002a, b; Athauda et al., 2004; Takahashi et al., 2005; Eilenberg et al., 2006; Plachno et al., 2006; Stephenson and Hogan, 2006; Hatano and Hamada, 2008; Thornhill et al., 2008). Secretion of free radicals into the pitcher fluid may also facilitate digestion (Chia et al., 2004). Nitrogen (N) from digested prey is transported actively across the Nepenthes pitcher wall, and transporters have been identified for NH4+, amino acids and peptides (Schulze et al., 1999; Rischer et al., 2002); conversely, protons (H+) may be pumped into the pitcher fluid, to optimize pH for enzymatic degradation of prey material (Higashi et al., 1993; An et al., 2001).
The typical Nepenthes pitcher shows distinct zonation, with three functional zones (Hooker, 1875; Adams and Smith, 1977; Owen and Lennon, 1999). The ‘attractive zone’ consists of the pitcher lid and the peristome, a collar-like structure surrounding and overhanging the pitcher mouth. This zone is rich in nectaries, serving to attract and retain prey. Colour patterns and scent in this zone may also aid in attraction of anthophilous insects (Moran, 1996; Moran et al., 1999; DiGiusto et al., 2008; Bauer et al., 2009). The peristome itself is a wettable, anisotropic structure: when wetted, it becomes slippery, causing invertebrate visitors to lose traction and fall into the pitcher (Bonn and Federle, 2004; Bauer et al., 2008).
The ‘conductive zone’ comprises the upper inside surface of the pitcher and is often characterized by lunate cells and epicuticular waxes (Juniper and Burras, 1962; Pant and Bhatnagar, 1977; Gaume et al., 2002, 2004; Gorb and Gorb, 2006), both of which function to deny traction and conduct the prey downwards under gravity into the third, or ‘digestive’ zone. This encompasses the fluid-filled base of the pitcher, the inner walls of which are lined with digestive glands (Owen et al., 1999; Gorb et al., 2004; Plachno et al., 2006; Thornhill et al., 2008). These glands undergo an ontogenic shift in function: in immature pitchers, they secrete the pitcher fluid, but once the pitcher matures, secretion ceases and the function switches to the absorption of digestion products (Owen and Lennon, 1999; Owen et al., 1999). The pitcher fluid itself possesses viscoelastic qualities, which in species such as N. rafflesiana, may contribute to prey retention to a greater degree than does the waxy zone (Gaume and Forterre, 2007; DiGiusto et al., 2008; Gaume and DiGiusto, 2010).
Despite recent research undertaken into Nepenthes pitcher function, there have been no direct measurements of ion fluxes across the pitcher wall to date. The MIFE ion-selective electrode technique has been used successfully to measure ion fluxes in plant roots, as well as in bacteria and protists (Cuin et al., 2008; Hawkins et al., 2008; Shabala et al., 2009a, b). In this study, its use has been extended to investigate ion fluxes in the trap of a carnivorous plant. The MIFE technique was used to measure net fluxes of NH4+ and H+ across the pitcher walls of three Nepenthes species. These differ in their trapping ecology, rates of prey capture, pitcher morphology and longevity (see Discussion). The first aim of the current study was to determine whether or not interspecific differences in ecology are mirrored by differences in nutritional physiology, as quantified by ion fluxes. The second aim was to determine whether or not the microstructure of the inner pitcher wall could also be related to the nutrient sequestration strategy.
Materials and methods
Plant culture and pitcher preparation
Tissue-cultured Nepenthes specimens were obtained commercially (Hawaiian Botanicals, Richmond, BC, Canada). Three Bornean species were used: Nepenthes ampullaria Jack; Nepenthes bicalcarata Hook.f.; and Nepenthes rafflesiana Jack. Plants were grown in a terrarium at 25–30 °C and 70% relative humidity under a 12/12 light/dark cycle (600 μmol m−2 s−1). A fully-formed pitcher (terrestrial form: see Moran, 1996) was gently removed from the plant, emptied of liquid and sliced longitudinally into sections. The section including the ‘winged’ portion of the pitcher was discarded, and the remainder divided into four roughly equal sections. These were tied with sewing thread onto a Perspex® holder, which was placed vertically in a test tube filled with 95 ml aerated measurement solution (500 μM NH4NO3 and 200 μM CaSO4.2H2O, adjusted to pH 4.0 with 2 M HCl), and positioned under 300 μmol m−2 s−1 irradiance at 20 °C for a minimum of 30 min. Immediately prior to ion flux measurements, the holder with a pitcher section was removed from the test tube and placed horizontally in a Perspex® container filled with 40 ml fresh measurement solution. Sections remained in the container for at least 5 min before the measurement of net ion fluxes began.
Measurement of ion fluxes
Ion flux measurements were made on the inner surface of pitchers of each species (n=3 per species). Fluxes of H+ and NH4+ were measured using a non-invasive microelectrode ion flux measurement system (MIFE, Unitas Consulting, Hobart, Australia), as described in Shabala et al. (1997). Electrode blanks were pulled from 1.5 mm borosilicate glass capillaries, dried in an oven at 220 °C for 4 h, and silanized with tributylchlorosilane (Fluka, Seelze, Germany). Cooled microelectrodes were backfilled with 200 mM NH4Cl for NH4+, and 15 mM NaCl and 40 mM KH2PO4 (adjusted to pH 6.0 using 0.1 M NaOH) for H+. Electrode tips were then filled with commercially-available ion-selective H+ or NH4+ cocktails (Fluka). Electrodes were calibrated with a set of known standards. The slopes were 54–59 mV pIon−1.
The electrodes were mounted on an electrode holder (MMT-5, Narishige, Tokyo, Japan) providing three-dimensional positioning, and positioned in a line 20 μm above the inner surface of the pitcher with their tips spaced 3–4 μm apart. The chamber was attached to a computer-controlled micromanipulator (PatchMan NP2, Eppendorf AG, Hamburg, Germany). During flux measurements, the MIFE computer gently moved the chamber up and down, providing virtual movement of the electrode tips between two positions, 20 μm and 60 μm above the pitcher surface, in a 10 s square-wave cycle. The concentration of each ion was calculated from its electrochemical potential at each position. The flux of each ion was later calculated from the measurements of the difference in the electrochemical potential between these positions (Shabala et al., 1997). For analysis, the first 1 s of each half-cycle was ignored.
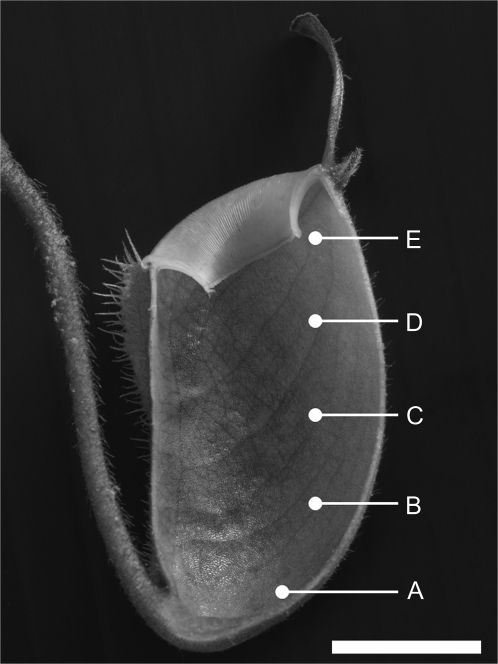
Fluxes were measured at five positions (A–E) on each pitcher section (Fig. 1). Position A was at the base of the pitcher, position C was immediately below the top of the digestive zone, and position B was half way between positions A and C. Position D was approximately 1 cm above the digestive zone in the conductive zone, and position E was approximately 1 cm below the peristome. The digestive zone was recognized by the dark-flecked, glandular appearance of the inner surface of the pitcher. In N. ampullaria pitchers, the entire inner surface appears dark-flecked and glandular; therefore, ion flux measurements were performed at the same proportional distances from the base as for the other species (Fig. 1). Ion fluxes were measured for an average of 5 min at each position and all measurements for one pitcher section were usually complete within 80 min. Ion concentrations declined by a maximum of 3 μM (or 0.3 pH units) during measurements.
Fig. 1.
Longitudinal section of N. ampullaria pitcher, showing zones at which ion fluxes were measured (A to E). Bar=1 cm.
Following flux measurements at pH 4.0, pitcher sections and their holders were removed from the Perspex® container, placed in a test tube in 500 μM NH4NO3 and 200 μM CaSO4 solution adjusted to pH 6.0, and put back under lights for 23 h. The next day, fluxes were re-measured in fresh solution at pH 6.0 using the procedure described above. In preliminary trials, fluxes were more consistent if measurements were made first at pH 4.0, followed by a 23 h acclimation to pH 6.0.
pH values of 4 and 6 were chosen as they fall within the naturally-occurring range of values found in pitchers of these species in the wild (mean ±SE, n: 2.55±0.17, 20; 3.72±0.09, 20; 4.33±0.14, 20 for N. rafflesiana, N. ampullaria, and N. bicalcarata, respectively; Clarke, 1997), and represent a 100-fold difference in proton concentration in the pitcher fluid. This would provide a sufficient range of acidity for us to differentiate between species that actively maintain moderately acidic pitcher conditions and those maintaining more highly acidic conditions.
Scanning electron microscopy (SEM) and image analyses
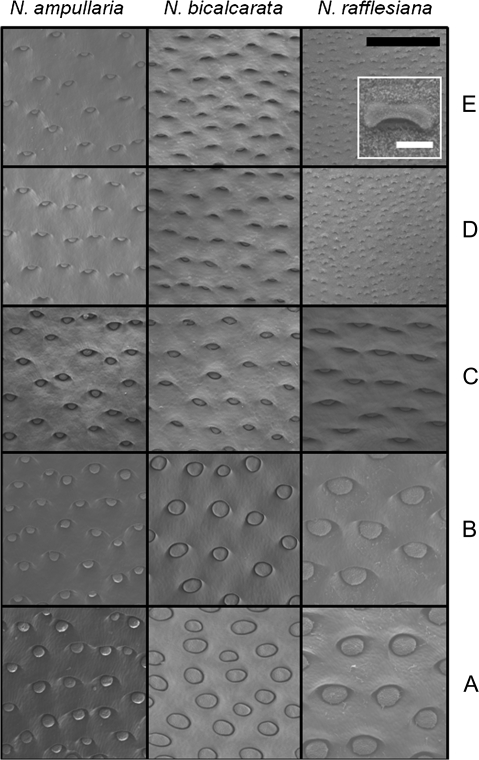
Pitcher wall samples were fixed in glutaraldehyde (4% in 0.1 M sodium phosphate buffer), then stained with osmium tetroxide, dehydrated via an ethanol dilution series and critical point dried, after which they were sputter coated with gold. Digital images of the specimens were recorded using a Hitachi S-3500 N scanning electron microscope (Hitachi Inc., Toronto, Canada) at 15 kV. Measurements of gland diameter and gland area/area of pitcher wall were recorded from the digital images using ImageJ v.1.38 software (Research Services Branch, National Institutes of Health, USA; http://rsbweb.nih.gov/ij/index.html). Mean and SE values were calculated for each species from 1 mm2 areas of pitcher wall (n=5 per zone, 25 per species). In N. ampullaria and N. bicalcarata, glands were present in all zones (Fig. 2). By contrast, N. rafflesiana lacked glands in zones D and E. Instead, the inner pitcher wall was covered with lunate cells and epicuticular wax crystals (Fig. 2, see inset). However, after it was found that ionic fluxes were occurring in these zones in this species (see Discussion), the width and area of the aperture beneath each lunate cell were measured and entered into the data as ‘gland’ diameter and ‘gland’ area, respectively.
Fig. 2.
Scanning electron microscope (SEM) images comparing the inner pitcher surface at zones A through E for N. ampullaria, N. bicalcarata, and N. rafflesiana. Inset outlined in white for N. rafflesiana shows individual lunate cell. Note pale epicuticular wax crystals on surface. Black bar=0.5 mm; white bar=25 μm.
Statistical analyses
For the ion flux measurements, three pitchers were used per species; for the gland distribution, five pitchers were used per species. Despite the small sample sizes, the effects of the factors on ion fluxes and gland measurements were found to be sufficiently robust to circumvent Type II errors at β=0.05 in the statistical models used. Data were analysed first by ANOVA, using the factors ‘Species’, ‘pH’ and ‘Zone’ for the ion flux data, and ‘Species’ and ‘Zone’ for the gland distribution data (SYSTAT v.13; Systat Software Inc., Chicago IL); however, since the interaction term of ‘Species’ and ‘Zone’ or ‘pH’ was usually significant, further analyses were conducted separately by species. Pairwise comparisons between means were determined using the Tukey test. All data were tested for normality and homoscedasticity; data sets violating the assumptions were transformed and re-tested prior to running parametric tests (Sokal and Rohlf, 1981). In cases where transformation failed to resolve the violation of assumptions, the non-parametric Student–Neuman–Keuls test was used instead of the Tukey test.
Results
Gland morphology and distribution
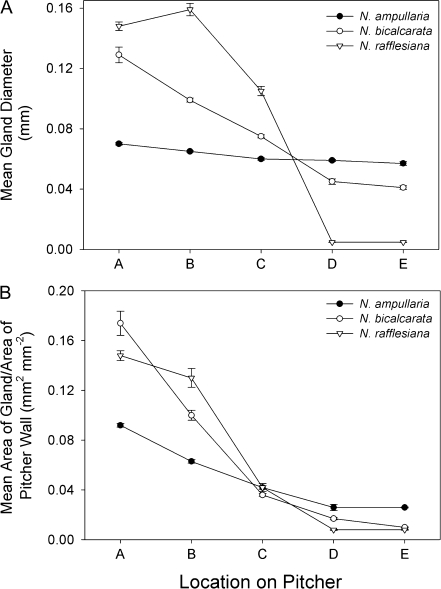
N. rafflesiana possessed lunate cells and epicuticular wax crystals in zones D and E; these structures were absent from N. ampullaria and N. bicalcarata (Fig. 2, inset). There was a general trend of decreasing gland diameter from zones A to E (i.e. from pitcher base towards pitcher mouth; P <0.001), although there were noticeable interspecific differences (Figs 2, 3A; Tables 1, 2). N. ampullaria differed from the other species in two respects. Firstly, there was the smallest absolute difference in gland size across the zones; secondly, and partly as a result, although N. ampullaria had the smallest glands at zone A, it had the largest at zones D and E (Figs 2, 3A). The relative ratio of area of gland to area of wall also decreased from zones A to E (P <0.001; Fig. 3B; Tables 1, 2). As with gland diameter, N. ampullaria showed the smallest change in gland area/area of wall with zone (Figs 2, 3B).
Fig. 3.
Gland distribution by zone for pitchers of N. ampullaria, N. bicalcarata, and N. rafflesiana. (A) Mean gland diameter (mm). (B) Mean area of gland/area of pitcher wall (mm2 mm−2). Mean and SE were calculated for each species from 5×1 mm2 areas of pitcher wall (n=5 per zone, 25 per species). In N. rafflesiana, zones D and E possess lunate cells rather than typical digestive glands. However, the width and area of the aperture beneath each lunate cell are represented here as ‘gland’ diameter and ‘gland’ area, respectively (see Discussion).
Table 1.
ANOVA table for gland measurements by ‘Species’ and ‘Zone’
| Measurement | Source | Type III SS | df | F | P |
| Width | Species | 0.005 | 2 | 36.301 | <0.001 |
| Zone | 0.178 | 4 | 636.833 | <0.001 | |
| Species×Zone | 0.026 | 8 | 46.329 | <0.001 | |
| Area | Species | 0.006 | 2 | 134.356 | <0.001 |
| Zone | 0.089 | 4 | 919.468 | <0.001 | |
| Species×Zone | 0.053 | 8 | 276.498 | <0.001 |
Table 2.
All-pairwise multiple comparisons for effect of ‘Zone’ on ion fluxes and gland measurements
| Species | Measurement | Zonea | Test used | ||||
| Ions | A | B | C | D | E | ||
| N. ampullaria | NH4+ | – | – | – | – | – | n/ab |
| H+ | d | b | c | abcd | a | Tukey | |
| N. bicalcarata | NH4+ | a | – | – | – | a | Tukey |
| H+ | – | – | – | – | – | n/a | |
| N. rafflesiana | NH4+ | abcd | b | c | d | a | Tukey |
| H+ | – | – | – | – | – | n/a | |
| Glands | A | B | C | D | E | ||
| N. ampullaria | Width | ab | ac | ad | a | bcd | Tukey |
| Area | abc | ad | a | b | cd | SNKc | |
| N. bicalcarata | Width | a | a | a | a | a | SNK |
| Area | ab | ac | ad | a | bcd | SNK | |
| N. rafflesiana | Width | ab | ac | ad | a | bcd | SNK |
| Area | ab | ac | ad | a | bcd | SNK | |
Zones sharing the same lower case letter are significantly different (i.e. P <0.05).
Not applicable, i.e. ANOVA P >0.05 for the effect of ‘Zone’ in this species.
Student–Neuman–Keuls test (non-parametric).
NH4+ fluxes
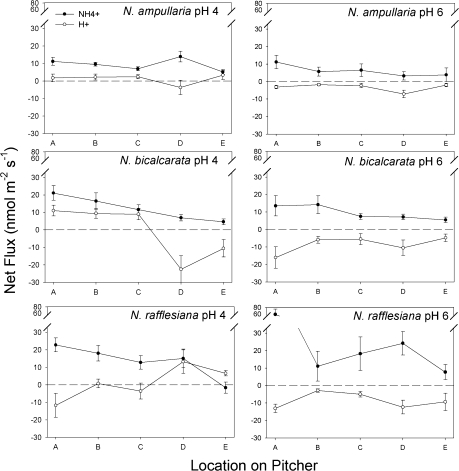
There was a significant effect of ‘Species’ on NH4+ uptake rate, in the relative order N. rafflesiana>N. bicalcarata>N. ampullaria (P <0.001; Fig. 4; Table 3). There was a general trend of decreasing uptake rates from zones A to E (P=0.018, P=0.002 for N. bicalcarata and N. rafflesiana, respectively, no significant effect of ‘Zone’ for N. ampullaria; Fig. 4; Table 4). NH4+ uptake occurred at all zones in all species (Fig. 4), indicating that zones A to E were capable of active transport of this ion, even the wax-covered upper zones (D and E) of N. rafflesiana, in which digestive glands were replaced by lunate cells (Fig. 2). There was no significant effect of pH on NH4+ uptake rate in any of the three species (Table 4).
Fig. 4.
Mean net ion fluxes (nmol m−2 s−1) by zone and pH for pitchers of N. ampullaria, N. bicalcarata, and N. rafflesiana. Closed circles, NH4+; open circles, H+. Positive values indicate net influx from lumen into pitcher tissue; negative values indicate net efflux into lumen from pitcher tissue. Bars indicate 1 SE (n=3 per species).
Table 3.
ANOVA table for NH4+ and H+ fluxes by ‘Species’, ‘pH’, and ‘Zone’
| Ion | Source | Type III SS | df | F | P |
| NH4+ | Species | 1 651.860 | 2 | 11.851 | <0.001 |
| pH | 33.229 | 1 | 0.477 | nsa | |
| Zone | 3 614.287 | 4 | 12.965 | <0.001 | |
| Species×pH | 746.293 | 2 | 5.354 | 0.008 | |
| Species×Zone | 2 054.347 | 8 | 3.685 | 0.002 | |
| pH×Zone | 591.515 | 4 | 2.122 | ns | |
| H+ | Species | 220.313 | 2 | 1.588 | ns |
| pH | 1 179.514 | 1 | 17.006 | <0.001 | |
| Zone | 711.349 | 4 | 2.564 | 0.047 | |
| Species×pH | 80.726 | 2 | 0.582 | ns | |
| Species×Zone | 1 614.789 | 8 | 2.910 | 0.008 | |
| pH×Zone | 77.162 | 4 | 0.278 | ns |
Not significant, i.e. P >0.05.
Table 4.
ANOVA summary results for ion fluxes by ‘pH’ and ‘Zone’, ‘Species’ treated separately
| Species | Ion | Effect of pH | Effect of Zone | Effect of pH×Zone | |||
| F | P | F | P | F | P | ||
| N. ampullaria | NH4+ | 2.825 | nsa | 1.248 | ns | 0.852 | ns |
| H+ | 23.761 | <0.001 | 5.292 | 0.004 | 0.137 | ns | |
| N. bicalcarata | NH4+ | 1.170 | ns | 3.858 | 0.018 | 0.445 | ns |
| H+ | 3.423 | ns | 2.745 | ns | 3.002 | 0.043 | |
| N. rafflesiana | NH4+ | 4.196 | ns | 7.348 | 0.002 | 2.910 | ns |
| H+ | 8.947 | 0.007 | 2.639 | ns | 2.560 | ns | |
Not significant, i.e. P >0.05.
H+ fluxes
pH exerted a significant effect on H+ flux (P <0.001; Table 3; Fig. 4). At pH 6.0, protons were pumped into the lumen side (i.e. into the pitcher fluid in an intact pitcher) in all zones, in the order N. rafflesiana≥N. bicalcarata>N. ampullaria (Fig. 4). At pH 4.0, both N. ampullaria and N. bicalcarata actively pumped protons out of the lumen side (i.e. increasing the pH of the fluid of an intact pitcher) in zones A to C. In N. rafflesiana, protons were pumped into the lumen side (i.e. decreasing the pH of the fluid of an intact pitcher) in zones A and C; the opposite occurred in zones B, D, and E (Fig. 4).
Discussion
Of the ∼100 species of Nepenthes identified to date, the ecology of perhaps less than a dozen has been studied in any great detail. Nonetheless, it is apparent that there exists a wide variety of N sequestration strategies employed by the Nepenthaceae. For example, in addition to producing scent, N. rafflesiana pitchers generate colour contrast signals that are ‘tuned’ to the visual sensitivity maxima of many anthophilous insect taxa, allowing exploitation of volant prey (Moran, 1996; Moran et al., 1999; DiGiusto et al., 2008); Nepenthes albomarginata T. Lobb ex Lindl. pitchers produce a lichen-mimicking tissue to attract termites (Moran et al., 2001; Merbach et al., 2002); in N. ampullaria, a significant proportion of foliar N is derived from leaf litter (Cresswell, 1998; Moran et al., 2003); and Nepenthes lowii Hook. f. deploys funnel-shaped pitchers to capture and utilize vertebrate faeces (Clarke et al., 2009).
There are also interspecific differences in pitcher longevity and prey capture rates. While some species employ a high prey capture rate/high pitcher turnover strategy, others produce longer-lived pitchers that harvest a slow but steady ‘trickle’ of prey. N. rafflesiana falls into the first category: although its pitchers are relatively short-lived at ≤3 months (Osunkoya et al., 2008; Bauer et al., 2009), its prey capture rate is high (Moran, 1996; Adam, 1997; Bauer et al., 2009). By contrast, N ampullaria pitchers are long-lived at ≥8 months (Clarke, 1997; Osunkoya et al., 2008) and exhibit a prey capture rate an order of magnitude less than that of N. rafflesiana (Adam, 1997). N. bicalcarata is another species characterized by long-lived pitchers (≥7 months; Clarke, 1997; Osunkoya et al., 2008) and a low prey capture rate, comparable to that of N. ampullaria (Adam, 1997).
Ion fluxes
Are interspecific differences in pitcher ion fluxes related to nutrient sequestration strategy? We will take each species in turn. Before doing so, however, it is important to note that, from a functional viewpoint, zones A to C are the most important in terms of ion fluxes, as they comprise the digestive zone of the pitcher (see Introduction). Pitchers of N. rafflesiana and N. bicalcarata are almost never completely filled with fluid, and only zones A to C are usually below the fluid level. Interpretation of Fig. 4 should therefore be undertaken with this in mind. By contrast, N. ampullaria pitchers are often filled with fluid up to zone D or even occasionally E (J Moran, personal observation), the implications of which will be dealt with in the next section.
As outlined above, N. rafflesiana is a ‘typical’ invertebrate-trapping species that deploys relatively short-lived pitchers to trap and utilize large numbers of prey over a brief period of time. It would be expected that a high rate of prey input to the pitcher might be reflected in a correspondingly high rate of absorption of the nitrogenous products of digestion. This strategy is confirmed by the observed rates of NH4+ uptake, which are the highest among the species in this study (Fig. 4). At both pH 4 and 6, N. rafflesiana actively pumps H+ into the pitcher fluid at zones A to C, presumably to maintain optimally acidic conditions for enzymatic activity. This corresponds to the finding of Clarke (1997): in its natural habitat, N. rafflesiana maintains a significantly lower pH in its pitchers than do N. ampullaria or N. bicalcarata (mean ±SE, n: 2.55±0.17, 20; 3.72±0.09, 20; 4.33±0.14, 20, respectively. P <0.001, F=43.3, ANOVA).
N. ampullaria is unique among the Nepenthaceae in that, although it catches invertebrate prey (albeit at a much slower rate than ‘typical’ species; Adam, 1997), it derives a significant amount of its N (c. 35%) from abscised leaves that have fallen into its pitchers from the forest canopy (Moran et al., 2003). N. ampullaria pitchers possess a suite of morphological adaptations to this unusual mode of nutrition (Moran et al., 2003); in addition, they are home to a rich assemblage of aquatic organisms: more than a dozen such species have been described, the majority of them dipteran larvae, mosquitoes in particular (Mogi and Yong, 1992; Clarke and Kitching, 1993; Clarke, 1998; Cresswell, 1998). Moran et al. (2003) hypothesized that N. ampullaria relies on these aquatic organisms to break down the litter and release assimilable nitrogen species such as NH4+, based on processes occurring in tree-holes, aquatic habitats analogous to Nepenthes pitchers (Carpenter, 1982; Fish and Carpenter, 1982; Bradshaw and Creelman, 1984; Yanoviak, 1999; Paradise, 2004; Verdonschot et al., 2008). A simple putative pathway from organically bound N in leaf litter to assimilable NH4+ within the N. ampullaria pitcher is as follows:
Leaf litter → Bacterial film on leaf → ‘Grazing’ mosquito larvae → Excreted NH4+
This pathway is comparable to that described for the unrelated North American pitcher plant Sarracenia purpurea L. (Sarraceniaceae), in which aquatic invertebrates, bacteria, and fungi mineralize organically bound N which is then assimilated by the plant (Bradshaw and Creelman, 1984; Mouquet et al., 2008). The results of the current study confirm that N. ampullaria is capable of the active uptake of NH4+, albeit at a slower rate than N. rafflesiana. Such a slow rate of uptake is not unexpected, given the slow but steady ‘trickle’ of abscised leaves into the pitcher, the increased time required for plant cell contents to be made available via bacterial breakdown of the cellulose cell wall, and the longevity of the pitchers themselves. The N. ampullaria pitcher appears to function to some extent as a tree-hole analogue, complete with leaf litter inputs from which it ultimately derives mineralized N. Although phytotelms such as tree holes (and N. ampullaria pitchers) are typically acidic environments, too low a pH can have negative effects on bacterial degradation of leaf litter (Kok and Van der Velde, 1994). Invertebrate pitcher inhabitants are also sensitive to very low pH: increased acidity in phytotelms has been shown to discourage egg-laying by some diptera, as well as having negative impacts on the survival of mosquito larvae and other aquatic invertebrates (Carpenter, 1982; Harrison, 2001; Kitching, 2001). Therefore, if N. ampullaria is dependent upon bacterial- and invertebrate-mediated release of N from leaf litter inputs, the plant would be expected to exert control on the pH of the pitcher fluid in order to prevent hyperacidic conditions from developing. Our results confirm this: at pH 6, N. ampullaria showed the lowest H+ efflux rate, i.e. the lowest degree of pitcher fluid acidification compared to N. bicalcarata and N. rafflesiana. At pH 4, N. ampullaria demonstrated H+ uptake in most zones, i.e. the pitchers were actively reducing acidity levels (Fig. 4).
It is also important to bear in mind that, in addition to leaf-litter inputs, a significant proportion of N. ampullaria foliar N is animal-derived (Moran et al., 2003). It is therefore likely that N. ampullaria must perform a balancing act between creating optimal conditions for micro-organism-derived N mineralization on the one hand, and digestion of animal-derived N species on the other.
Although N. bicalcarata is ‘typical’ in that its pitchers catch only invertebrate prey, it is also unique in that it has a mutualistic association with a species of swimming ant, Camponotus schmitzi Stärke. In return for the provision of a domatium, the ants benefit the plant by repelling pitcher-damaging weevils (Merbach et al., 2007), and entering the pitcher fluid to remove overly-large prey items, preventing putrefaction and consequent pitcher death (Clarke and Kitching, 1995). Like N. ampullaria, N. bicalcarata actively pumps H+ from the pitcher fluid under highly acidic conditions (pH 4; Fig. 4D), as might be expected from a species dependent upon a commensal that spends a significant portion of its time in the pitcher fluid (Harrison, 2001). Thus, both N. ampullaria and N. bicalcarata actively maintain less acidic pitcher conditions than sympatric congeners such as N. rafflesiana. This finding may help to explain the presence of acid-intolerant visitors. For example, in Brunei, Northwest Borneo, it is not uncommon to find terrestrial crabs (Geosesarma sp.; Ng and Lim, 1987) in N. ampullaria pitchers (J Moran, personal observation); whether the crabs are feeding on the detritus in the pitchers is unknown at present. Similarly, tree frogs (Philautus sp.; Dring, 1987) lay eggs in pitchers of N. bicalcarata (J Moran, personal observation). Both groups of animals are known to be intolerant of hyperacidic conditions (Diesel, 1992; Vatnick et al., 2006).
Gland distribution and morphology
With regard to gland distribution and characteristics, the species investigated fall into two categories (Figs 2, 3). The first comprises N. rafflesiana, which possesses a pattern typical of many invertebrate-trapping species. Zones A to C are lined with digestive glands that have the dual functions of secretion and absorption (Owen and Lennon, 1999; Owen et al., 1999). Zones D and E, lying above the fluid level in intact pitchers, possess large numbers of lunate cells and are coated with epicuticular wax crystals (Fig. 2). The lunate cells are modified stomatal guard cells, the orientation of which produces an anisotropic surface that denies traction to insects attempting to ascend the pitcher wall (Fig. 2; Adams and Smith, 1977; Gaume et al., 2002, 2004; Thornhill et al., 2008). In addition, the epicuticular wax crystals clog claws, and the waxes themselves create a surface of low free surface energy, preventing traction via capillarity for insects with hairy pulvillae (Juniper and Burras, 1962; Gaume et al., 2004; Gorb et al., 2004).
The second category comprises N. ampullaria and N. bicalcarata, from which lunate cells and epicuticular waxes are absent. Digestive glands line the entire inner surface of the pitcher (Fig. 2). Why do these two species lack structures with demonstrated roles in the capture and retention of prey? In N. ampullaria, the pitcher lid (which prevents entrance of rainwater in other species) is reflexed away from the mouth, and the pitchers are often filled with fluid slightly below or even up to the level of the peristome (J Moran, personal observation). This appears to be a strategy to maximize the volume of fluid, and thus the effective size of the aquatic habitat available for colonization by commensals: the larger the volume of a phytotelm, the more organisms can be supported (Schmidl et al., 2008). Since almost the entire inner wall of the pitcher is submerged, the lunate cells and epicuticular waxes would be of little use, and the entire surface has been turned over to the uptake of nutrients via the digestive glands. This is demonstrated by the fact that there is no significant effect of Zone on NH4+ uptake rates in this species (Table 4). N. bicalcarata also relies on mutualism, in this case with C. schmitzi, which often takes up station inside the pitcher, beneath the overhanging peristome. The ant frequently enters the pitcher fluid to hunt dipteran larvae and also to remove oversized prey items (Clarke and Kitching, 1995; Clarke, 1997). A conductive zone possessing lunate cells and epicuticular waxes would possibly prevent easy movement of C. schmitzi around the inner surface of the pitcher.
A surprising finding of the study is that in N. rafflesiana, zones D and E exhibit ion fluxes (NH4+ and H+; Fig. 4). Active transport of NH4+ was also found to occur across wax-covered zones D and E in Nepenthes fusca Danser pitchers (data not presented). At first glance, this finding is highly counterintuitive, as these zones do not possess the multicellular glands typical of the lower zones. Even more problematically, they are covered in epicuticular waxes, primarily very long chain aldehydes (Riedel et al., 2003, 2007) that present a surface of low free surface energy, and are thus effectively unwettable (Gorb et al., 2004; Gorb and Gorb, 2006). The only possible portal for fluxes of aquatic ions must be the epidermal cell that lies within the depression beneath each overhanging lunate cell, as there is no pore analogous to a stoma (Pant and Bhatnagar, 1977; Owen and Lennon, 1999) and all other surfaces possess epicuticular waxes (Fig. 2, inset). There is evidence that these epidermal cells are capable of active transport: MacFarlane (1893) reported exudation of fluid from the wax-covered conductive zone and identified the depressions beneath the lunate cells as the source of these secretions. Therefore, it appears that, although the conductive zone is rarely submerged beneath the pitcher fluid, it is nonetheless capable of active transport of aqueous ions. This raises the intriguing possibility that the capability of ion exchange in the epidermal cells beneath the lunate cells represents an atavism, and that such structures may represent a primitive form of the digestive glands, which are known to be of epidermal origin (Pant and Bhatnagar, 1977; Owen and Lennon, 1999; Thornhill et al., 2008). However, exploring this possibility is beyond the scope of the current study.
Acknowledgments
We thank Alison Moran and two anonymous referees for significantly improving earlier drafts of the manuscript, Brendan Porter for help with the MIFE measurements, and Jeanie and Jack Wootton at Hawaiian Botanicals for help in obtaining specimens.
References
- Adam JH. Prey spectra of Bornean Nepenthes species (Nepenthaceae) in relation to their habitat. Pertanika Journal of Tropical Agricultural Science. 1997;20:121–134. [Google Scholar]
- Adamec L. Mineral nutrition of carnivorous plants: a review. Botanical Review. 1997;63:273–299. [Google Scholar]
- Adams RM, Smith GW. An SEM survey of the five carnivorous plant genera. American Journal of Botany. 1977;64:265–272. [Google Scholar]
- An C-I, Fukusaki E-I, Kobayashi A. Plasma-membrane H+-ATPases are expressed in pitchers of the carnivorous plant Nepenthes alata Blanco. Planta. 2001;212:547–555. doi: 10.1007/s004250000455. [DOI] [PubMed] [Google Scholar]
- An C-I, Fukusaki E-I, Kobayashi A. Aspartic proteinases are expressed in pitchers of the carnivorous plant Nepenthes alata Blanco. Planta. 2002a;214:661–667. doi: 10.1007/s004250100665. [DOI] [PubMed] [Google Scholar]
- An C-I, Takekawa S, Okazawa A, Fukusaki E-I, Kobayashi A. Degradation of a peptide in pitcher fluid of the carnivorous plant Nepenthes alata Blanco. Planta. 2002b;215:472–477. doi: 10.1007/s00425-002-0768-7. [DOI] [PubMed] [Google Scholar]
- Athauda SBP, Matsumoto K, Rajapakshe S, Kuribayashi M, Kojima M, Kubomura-Yoshida N, Iwamatsu A, Shibata C, Inoue H, Takahashi K. Enzymic and structural characterization of nepenthesin, a unique member of a novel subfamily of aspartic proteinases. Biochemical Journal. 2004;381:295–306. doi: 10.1042/BJ20031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proceedings of the National Academy of Sciences, USA. 2004;101:14138–14143. doi: 10.1073/pnas.0405885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer U, Bohn HF, Federle W. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar. Proceedings of the Royal Society B. 2008;275:259–265. doi: 10.1098/rspb.2007.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer U, Willmes C, Federle W. Effect of pitcher age on trapping efficiency and natural prey capture in carnivorous Nepenthes rafflesiana plants. Annals of Botany. 2009;103:1219–1226. doi: 10.1093/aob/mcp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw WE, Creelman RA. Mutualism between the carnivorous purple pitcher plant and its inhabitants. American Midland Naturalist. 1984;112:294–304. [Google Scholar]
- Carpenter SR. Stemflow chemistry: effects on population dynamics of detritivorous mosquitoes in tree-hole ecosystems. Oecologia. 1982;53:1–6. doi: 10.1007/BF00377128. [DOI] [PubMed] [Google Scholar]
- Chia TF, Aung HH, Osipov AN, Goh NK, Chia LS. Carnivorous pitcher plant uses free radicals in the digestion of prey. Redox Report. 2004;9 doi: 10.1179/135100004225006029. DOI: 10.1179/135100004225006029. [DOI] [PubMed] [Google Scholar]
- Clarke CM. Nepenthes of Borneo. Kota Kinabalu, Malaysia: Natural History Publications; 1997. [Google Scholar]
- Clarke CM. A re-examination of geographical variation in Nepenthes food webs. Ecography. 1998;21:430–436. [Google Scholar]
- Clarke CM, Bauer U, Lee CC, Tuen AA, Rembold K, Moran JA. Tree shrew lavatories: a novel nitrogen sequestration strategy in a tropical pitcher plant. Biology Letters. 2009;5:632–635. doi: 10.1098/rsbl.2009.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CM, Kitching RL. The metazoan food webs from six Bornean Nepenthes species. Ecological Entomology. 1993;18:7–16. [Google Scholar]
- Clarke CM, Kitching RL. Swimming ants and pitcher plants: a unique ant–plant interaction from Borneo. Journal of Tropical Ecology. 1995;11:589–602. [Google Scholar]
- Cresswell JE. Morphological correlates of necromass accumulation in the traps of an Eastern tropical pitcher plant, Nepenthes ampullaria Jack, and observations on the pitcher infauna and its reconstitution following experimental removal. Oecologia. 1998;113:383–390. doi: 10.1007/s004420050390. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Betts SA, Chalmandrier R, Shabala S. A root's ability to retain K+correlates with salt tolerance in wheat. Journal of Experimental Botany. 2008;59:2697–2706. doi: 10.1093/jxb/ern128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesel R. Managing the offspring environment: brood care in the bromeliad crab, Metopaulias depressus. Behavioural Ecology and Sociobiology. 1992;30:125–134. [Google Scholar]
- DiGiusto B, Grosbois V, Fargeas E, Marshall DJ, Gaume L. Contribution of pitcher fragrance and fluid viscosity to high prey diversity in a Nepenthes carnivorous plant from Borneo. Journal of Biosciences. 2008;33:121–136. doi: 10.1007/s12038-008-0028-5. [DOI] [PubMed] [Google Scholar]
- Dring JCM. Bornean treefrogs of the genus Philautus (Rhacophoridae) Amphibia-Reptilia. 1987;8:19–47. [Google Scholar]
- Eilenberg H, Pnini-Cohen S, Schuster S, Movtchan A, Zilberstein A. Isolation and characterization of chitinase genes from pitchers of the carnivorous plant Nepenthes khasiana. Journal of Experimental Botany. 2006;57:2775–2784. doi: 10.1093/jxb/erl048. [DOI] [PubMed] [Google Scholar]
- Fish D, Carpenter SR. Leaf litter and larval mosquito dynamics in tree-hole ecosystems. Ecology. 1982;63:283–288. [Google Scholar]
- Gaume L, DiGiusto B. Adaptive significance and ontogenetic variability of the waxy zone in Nepenthes rafflesiana. Annals of Botany. 2010 doi: 10.1093/aob/mcp238. doi: 101093/aob/mcp238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume L, Forterre Y. A viscoelastic deadly fluid in carnivorous pitcher plants. PLoS ONE. 2007;11:e1185. doi: 10.1371/journal.pone.0001185. doi: 10.1371/journal.pone.0001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume L, Gorb S, Rowe N. Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytologist. 2002;156:479–489. doi: 10.1046/j.1469-8137.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- Gaume L, Perret P, Gorb E, Gorb S, Labat J-J, Rowe N. How do plant waxes cause flies to slide? Experimental tests of wax-based trapping mechanisms in three pitfall carnivorous plants. Arthropod Structure and Development. 2004;33:103–111. doi: 10.1016/j.asd.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gorb EV, Gorb SN. Physicochemical properties of functional surfaces in pitchers of the carnivorous plant Nepenthes alata Blanco (Nepenthaceae) Plant Biology. 2006;8:841–848. doi: 10.1055/s-2006-923929. [DOI] [PubMed] [Google Scholar]
- Gorb E, Kastner V, Peressadko A, Arzt E, Gaume L, Rowe N, Gorb S. Structure and properties of the glandular surface in the digestive zone of the pitcher in the carnivorous plant Nepenthes ventrata and its role in insect trapping and retention. Journal of Experimental Biology. 2004;207:2947–2963. doi: 10.1242/jeb.01128. [DOI] [PubMed] [Google Scholar]
- Harrison JF. Insect acid–base physiology. Annual Review of Entomology. 2001;46:221–250. doi: 10.1146/annurev.ento.46.1.221. [DOI] [PubMed] [Google Scholar]
- Hatano N, Hamada T. Proteome analysis of pitcher fluid of the carnivorous plant Nepenthes alata. Journal of Proteome Research. 2008;7:809–816. doi: 10.1021/pr700566d. [DOI] [PubMed] [Google Scholar]
- Hawkins BJ, Boukcim H, Plassard C. A comparison of ammonium, nitrate and proton net fluxes along seedling roots of Douglas-fir and lodgepole pine grown and measured with different inorganic nitrogen sources. Plant, Cell and Environment. 2008;31:278–287. doi: 10.1111/j.1365-3040.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison Y. Enzyme release in carnivorous plants. Frontiers in Biology. 1975;43:525–578. [PubMed] [Google Scholar]
- Higashi S, Nakashima A, Ozaki H, Abe M, Uchiumi T. Analysis of feeding mechanism in a pitcher of Nepenthes hybrida. Journal of Plant Research. 1993;106:47–54. [Google Scholar]
- Hooker JD. Address to the Department of Zoology and Botany. Report to the British Association for the Advancement of Science: Report of the Forty-Fourth Meeting, Belfast. 1875;1874:102–116. [Google Scholar]
- Jentsch J. Enzymes from carnivorous plants (Nepenthes): isolation of the protease nepenthacin. FEBS Letters. 1972;21:273–276. doi: 10.1016/0014-5793(72)80181-9. [DOI] [PubMed] [Google Scholar]
- Juniper BE, Burras J. How pitcher plants trap insects. New Scientist. 1962;13:75–77. [Google Scholar]
- Kitching RL. Food webs in phytotelmata: ‘bottom-up’ and ‘top-down’ explanations for community structure. Annual Review of Entomology. 2001;46:729–760. doi: 10.1146/annurev.ento.46.1.729. [DOI] [PubMed] [Google Scholar]
- Kok CJ, Van der Velde G. Decomposition and macroinvertebrate colonization of aquatic and terrestrial leaf material in alkaline and acid still water. Freshwater Biology. 1994;31:65–75. [Google Scholar]
- MacFarlane JM. Observations on pitchered insectivorous plants (part II) Annals of Botany. 1893;7:403–441. [Google Scholar]
- Merbach MA, Merbach DJ, Maschwitz U, Booth WE, Fiala B, Zizka G. Mass march of termites into the deadly trap. Nature. 2002;415:37. doi: 10.1038/415036a. [DOI] [PubMed] [Google Scholar]
- Merbach MA, Zizka G, Fiala B, Merbach D, Booth WE, Maschwitz U. Why a carnivorous plant cooperates with an ant- selective defense against pitcher-destroying weevils in the myrmecophytic pitcher plant Nepenthes bicalcarata Hook.f. Ecotropica. 2007;13:45–56. [Google Scholar]
- Mogi M, Yong HS. Aquatic arthropod communities in Nepenthes pitchers: the role of niche differentiation, aggregation, predation and competition in community organization. Oecologia. 1992;90:172–184. doi: 10.1007/BF00317174. [DOI] [PubMed] [Google Scholar]
- Moran JA. Pitcher dimorphism, prey composition and the mechanisms of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. Journal of Ecology. 1996;84:515–525. [Google Scholar]
- Moran JA, Booth WE, Charles JK. Aspects of pitcher morphology and spectral characteristics of six Bornean Nepenthes pitcher plant species: implications for prey capture. Annals of Botany. 1999;83:521–528. [Google Scholar]
- Moran JA, Clarke CM, Hawkins BJ. From carnivore to detritivore? Isotopic evidence for leaf litter utilization by the tropical pitcher plant Nepenthes ampullaria. International Journal of Plant Sciences. 2003;164:635–639. [Google Scholar]
- Moran JA, Merbach MA, Livingston NJ, Clarke CM, Booth WE. Termite prey specialization in the pitcher plant Nepenthes albomarginata: evidence from stable isotope analysis. Annals of Botany. 2001;88:307–311. [Google Scholar]
- Moran JA, Moran AJ. Foliar reflectance and vector analysis reveal nutrient stress in prey-deprived pitcher plants (Nepenthes rafflesiana) International Journal of Plant Sciences. 1998;159:996–1001. [Google Scholar]
- Mouquet N, Daufresne T, Gray SM, Miller TE. Modelling the relationship between a pitcher plant (Sarracenia purpurea) and its phytotelma community: mutualism or parasitism? Functional Ecology. 2008;22:728–737. [Google Scholar]
- Ng PKL, Lim RP. The taxonomy and biology of the nepenthophilous freshwater sesarmine crab, Geosesarma malayanum Ng and Lim, 1986 (Crustacea, Decapoda, Brachyura, Grapsidae) from Peninsular Malaysia. Malayan Nature Journal. 1987;41:393–402. [Google Scholar]
- Osunkoya OO, Daud SD, Wimmer FL. Longevity, lignin content and construction cost of the assimilatory organs of Nepenthes species. Annals of Botany. 2008;102:845–853. doi: 10.1093/aob/mcn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen TP, Lennon KA, Santo MJ, Anderson AN. Pathways for nutrient transport in the pitchers of the carnivorous plant Nepenthes alata. Annals of Botany. 1999;4:459–466. [Google Scholar]
- Owen TP, Lennon KA. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae) American Journal of Botany. 1999;86:1382–1390. [PubMed] [Google Scholar]
- Pant DD, Bhatnagar S. Morphological studies in Nepenthes (Nepenthaceae) Phytomorphology. 1977;27:13–34. [Google Scholar]
- Paradise CJ. Relationship of water and leaf litter variability to insects inhabiting treeholes. Journal of the North American Benthological Society. 2004;23:793–805. [Google Scholar]
- Pavlovič A, Singerová L, Demko V, Hudák J. Feeding enhances photosynthetic efficiency in the carnivorous pitcher plant Nepenthes talangensis. Annals of Botany. 2009;104:307–314. doi: 10.1093/aob/mcp121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachno BJ, Adamec L, Lichsteidl IK, Peroutka M, Adlassnig W, Vrba J. Fluorescence labelling of phosphatase activity in digestive glands of carnivorous plants. Plant Biology. 2006;8:813–820. doi: 10.1055/s-2006-924177. [DOI] [PubMed] [Google Scholar]
- Riedel M, Eichner A, Jetter R. Slippery surfaces of carnivorous plants: composition of epicuticular wax crystals in Nepenthes alata Blanco pitchers. Planta. 2003;218:87–97. doi: 10.1007/s00425-003-1075-7. [DOI] [PubMed] [Google Scholar]
- Riedel M, Eichner A, Meimberg H, Jetter R. Chemical composition of epicuticular wax crystals on the slippery zone in pitchers of five Nepenthes species and hybrids. Planta. 2007;225:1517–1534. doi: 10.1007/s00425-006-0437-3. [DOI] [PubMed] [Google Scholar]
- Rischer H, Hamm A, Bringmann G. Nepenthes insignis uses a C2-portion of the carbon skeleton of l-alanine acquired via its carnivorous organs, to build up the allelochemical plumbagin. Phytochemistry. 2002;59:603–609. doi: 10.1016/s0031-9422(02)00003-1. [DOI] [PubMed] [Google Scholar]
- Schmidl J, Sulzer P, Kitching RL. The insect assemblage in water-filled tree-holes in a European temperate deciduous forest: community composition reflects structural, trophic and physicochemical factors. Hydrobiologia. 2008;598:285–303. [Google Scholar]
- Schulze W, Frommer WB, Ward JM. Transporters for ammonium, amino acids and peptides are expressed in pitchers of the carnivorous plant Nepenthes. The Plant Journal. 1999;17:637–646. doi: 10.1046/j.1365-313x.1999.00414.x. [DOI] [PubMed] [Google Scholar]
- Schulze W, Schulze ED, Pate JS, Gillison AN. The nitrogen supply from soils and insects during growth of the pitcher plants Nepenthes mirabilis, Cephalotus follicularis and Darlingtonia californica. Oecologia. 1997;112:464–471. doi: 10.1007/s004420050333. [DOI] [PubMed] [Google Scholar]
- Shabala L, Bowman J, Brown J, Ross T, McMeekin T, Shabala S. Ion transport and osmotic adjustment in Escherichia coli in response to ionic and non- ionic osmotica. Environmental Microbiology. 2009a;11:137–148. doi: 10.1111/j.1462-2920.2008.01748.x. [DOI] [PubMed] [Google Scholar]
- Shabala L, McMeekin T, Shabala S. Osmotic adjustment and requirement for sodium in marine protist thraustochytrid. Environmental Microbiology. 2009b;11:1835–1843. doi: 10.1111/j.1462-2920.2009.01908.x. [DOI] [PubMed] [Google Scholar]
- Shabala SN, Newman IA, Morris J. Oscillations in H+ and Ca2+ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiology. 1997;113:111–118. doi: 10.1104/pp.113.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 2nd edn. New York, USA: WH Freeman and Company; 1981. [Google Scholar]
- Stephenson P, Hogan J. Cloning and characterization of a ribonuclease, a cysteine proteinase, and an aspartic proteinase from pitchers of the carnivorous plant Nepenthes ventricosa Blanco. International Journal of Plant Sciences. 2006;167:239–248. [Google Scholar]
- Takahashi K, Athauda SBP, Matsumoto K, Rajapakshe S, Kuribayashi M, Kojima M, Kubomura-Yoshida N, Iwamatsu A, Shibata C, Inoue H. Nepenthesin, a unique member of a novel subfamily of aspartic proteinases: enzymatic and structural characteristics. Current Protein and Peptide Science. 2005;6:513–525. doi: 10.2174/138920305774933259. [DOI] [PubMed] [Google Scholar]
- Thornhill AH, Harper IS, Hallam ND. The development of the digestive glands and enzymes in the pitchers of three Nepenthes species: N. alata, N. tobaica, and N. ventricosa (Nepenthaceae) International Journal of Plant Sciences. 2008;169:615–624. [Google Scholar]
- Vatnick I, Andrews J, Colombo M, Madhoun H, Rameswaran M, Brodkin MA. Acid exposure is an immune disruptor in adult Rana pipiens. Environmental Toxicology and Chemistry. 2006;25:199–202. doi: 10.1897/05-324r1.1. [DOI] [PubMed] [Google Scholar]
- Verdonschot RCM, Febria CM, Williams DD. Fluxes of dissolved organic carbon, other nutrients and microbial communities in a water-filled treehole ecosystem. Hydrobiologia. 2008;596:17–30. [Google Scholar]
- Yanoviak SP. Effects of leaf litter species on macroinvertebrate community properties and mosquito yield in neotropical tree hole microcosms. Oecologia. 1999;120:147–155. doi: 10.1007/s004420050843. [DOI] [PubMed] [Google Scholar]