Abstract
Crassulacean acid metabolism (CAM) plants exhibit a complex interplay between CO2 fixation by phosphoenolpyruvate carboxylase (PEPC) and ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco), and carbon demand for CAM maintenance and growth. This study investigated the flux of carbon from PEPC and direct Rubisco fixation to different leaf carbon pools and to phloem sap over the diurnal cycle. Concentrations and carbon isotope compositions of starch, soluble sugars, and organic acids were determined in leaves and phloem exudates of Kalanchoë daigremontiana Hamet et Perr., and related to CO2 fixation by PEPC and Rubisco. Three types of leaf carbon pools could be distinguished. (i) Starch and malate pools were dominant and showed a pattern of reciprocal mobilization and accumulation (85/54 and 13/48 mg C g−1 DW, respective, at the beginning/end of phase I). The carbon isotope composition of these pools was compatible with predominant PEPC fixation (δ13C values of –13 and –11‰ for starch and malate compared to –11‰ of PEPC fixed carbon). (ii) Isotopic composition (–17‰ and –14‰) and concentration of glucose and fructose (2 and 3 mg C g−1 DW, respectively) were not affected by diurnal metabolism, suggesting a low turnover. (iii) Sucrose (1–3 mg C g−1 DW), in contrast, exhibited large diurnal changes in δ13C values (from –17‰ in the evening to –12‰ in the morning), which were not matched by net changes in sucrose concentration. This suggests a high sucrose turnover, fed by nocturnal starch degradation and direct Rubisco fixation during the day. A detailed dissection of the carbon fixation and mobilization pattern in K. daigremontiana revealed that direct fixation of Rubisco during the light accounted for 30% of phloem sucrose, but only 15% of fixed carbon, indicating that carbon from direct Rubisco fixation was preferentially used for leaf export.
Keywords: Compound-specific isotope analysis, malate, PEPC, Rubisco, starch, sucrose
Introduction
Crassulacean acid metabolism (CAM) is performed by about 6% of vascular plant species (Winter and Smith, 1996), mainly occurring in habitats that are affected by drought, such as deserts and tropical forest canopies. In CAM, CO2 uptake occurs at night when relative humidity is high, thus reducing water loss from open stomata and enabling the plant to survive dry conditions. The diurnal cycle of CAM can be divided into four phases (Osmond, 1978): The period of night-time CO2 fixation catalysed by phosphoenolpyruvate carboxylase (PEPC) and the accumulation of malate in the vacuole is denoted as phase I. With the onset of light, phase II follows, characterized by a decline in PEPC fixation and, eventually, a certain amount of direct C3-like fixation by Rubisco. Phase III starts with stomatal closure in the late morning, when malate is decarboxylated and the released CO2 is refixed by Rubisco. In the afternoon, stomata may re-open for direct CO2 fixation by Rubisco in phase IV. The duration of the phases and amplitudes of CO2 uptake differ widely between species and environmental conditions and, in particular, additional direct fixation of CO2 by Rubisco (phases II and IV) is highly variable (for a review see Dodd et al., 2002).
The balance of mobilization and accumulation of storage carbohydrates, which accompanies the mobilization and accumulation of malate, is an important feature of CAM. In phase I, CO2 is attached to the C3 molecule phosphoenolpyruvate (PEP), derived from the breakdown of storage carbohydrates (usually starch or soluble sugars), resulting in the formation of the C4 molecule malate. During the day, this carbohydrate pool has to be refilled for the subsequent night (Winter and Smith, 1996). CAM plants need to maintain a high pool of easily available carbohydrates for photosynthesis. A low level of carbohydrates for PEP generation may lead to a limited fixation of CO2 by PEPC (Dodd et al., 2003), which, in turn, would further reduce carbohydrate stocks. Thus, the maintenance of CAM, and, in consequence, the ability to cope with unfavourable environmental conditions, depends on the availability of these carbohydrates. This necessity to maintain large carbohydrate stocks, together with the high energy demand caused by conversions between metabolites and intracellular transport processes, is limiting the productivity of CAM plants. Nevertheless, some CAM plants exhibit growth rates close to C3 plants (Nobel, 1991) – therefore, highly co-ordinated control mechanisms have to be assumed that regulate the competition between carbohydrate storage and growth.
To investigate the proportions of PEPC and direct Rubisco fixation in CAM plants, the analysis of C isotope composition (usually expressed as δ13C) has become a useful tool. Rubisco discriminates between 12C and 13C, leading to strongly depleted δ13C values between –25‰ and –30‰ which are characteristic for C3 metabolism. Discrimination, of course, is also influenced by environmental conditions in C3 plants, mainly via limitations in CO2 diffusion. Thus, under drier conditions, i.e. when stomatal resistance increases, discrimination is less pronounced (Farquhar et al., 1989). PEPC on the other hand does not or only marginally discriminate against the heavier C isotope (Farquhar et al., 1989). The discrimination during re-fixation of CO2 from malate by Rubisco is only pronounced when CO2 is leaking from the leaf (Griffiths et al., 1990), which is usually considered to be low. Carbon originally fixed by PEPC (although re-fixed by Rubisco, assuming no leakage) therefore has δ13C values between –8‰ and –10‰, which is close to the atmospheric value of about –8‰. Thus, the proportions of both pathways can be estimated by the plant δ13C values (Winter and Holtum, 2002), provided that mesophyll conductance did not influence discrimination (Griffiths et al., 2007) and that PEPC and direct Rubisco fixation have contributed proportionally to the biomass in the analysed tissue. Improving the resolution of this approach, Deleens and Garnier-Dardart (1977) and Deleens et al. (1979) separated biochemical fractions of Kalanchoë species performing CAM, and demonstrated differences in δ13C values between different metabolites. Starch, organic acids, and phosphorylated compounds were generally 13C enriched compared to soluble sugars and cellulose, giving rise to the idea of a compartmentation of C derived from PEPC and direct Rubisco fixation. Furthermore, C from direct daytime fixation of atmospheric CO2 might be preferentially exported from the leaf compared to C from night-time fixation, both in Kalanchoë pinnata (Lam.) Pers. (Mayoral and Medina, 1985) and in Clusia rosea Jacq., which showed C3-like isotope composition of fruits in contrast to far more PEPC-like signals in leaves (Borland and Dodd, 2002).
Thus, it was hypothesized that the delicate balance of C partitioning between carbohydrate storage for maintenance of photosynthetic capacity and growth was facilitated by the separate use of C from PEPC and direct Rubisco fixation. C from PEPC fixation was dedicated to the maintenance of CAM, whereas C from direct Rubisco fixation in phases II and IV was exported to sink organs, for example, inflorescences, roots or new shoots. Although C partitioning between CAM maintenance and export has been estimated by calculating C budgets from changes in pool sizes between dawn and dusk, refined by isotope signatures (Borland, 1996; Borland and Dodd, 2002), the isotopic composition and metabolic origin of exported C has not yet been directly measured in the exported C (i.e. in the phloem sap). With the recent improvements in compound-specific stable isotope analysis, i.e. HPLC systems that are directly coupled to isotope ratio mass spectrometers, more detailed analyses of such C fluxes became feasible.
Against this background, the present study aimed to clarify carbon partitioning in K. daigremontiana, a well-investigated CAM plant that utilizes starch as a storage carbohydrate and malic enzyme as the major decarboxylase (Christopher and Holtum, 1996). It is hypothesized that carbon from direct Rubisco fixation in phases II and IV is preferentially exported to sink tissues, while carbon originating from phase I is predominantly used for replenishment of the carbohydrate pool for CAM maintenance.
Towards this goal, plants were subjected to environmental conditions favouring PEPC fixation, with direct Rubisco fixation accounting for only 15% of daily CO2 fixation. Gas exchange and instantaneous discrimination were measured over the diurnal cycle, as well as concentrations and δ13C values of organic acids, soluble sugars, and starch. Phloem exudates were collected and analysed for δ13C, providing the first direct measurement of phloem isotopic composition in a CAM plant.
Materials and methods
Plant material
Kalanchoë daigremontiana Hamet et Perr. plants were precultivated in a greenhouse and transferred to a climate chamber 5 months before the start of the experiment. Plants were watered daily and fertilized with 0.2% Wuxal top N (Kwizda Agro GmbH, Vienna, Austria) every 3–4 weeks. The light period lasted from 07.00 h to 23.00 h, with light intensity increasing from dawn to 11.00 h, reaching an average midday intensity of 550 μmol m−2 s−1 at plant height. Starting at 19.00 h, the light intensity was gradually reduced, simulating natural conditions. Air temperature was 25/18 °C during the light and dark periods, respectively, and relative air humidity was constant at 60%.
When harvested, plants had 4–5 mature leaf pairs. Leaves were excised, weighed, and scanned to determine leaf area. One leaf of each pair was used for phloem exudation, and the other for the determination of soluble sugars, organic acids, and starch, where the leaves of each plant were pooled. For starch analysis, discs (diameter 0.8 cm) were punched out using a cork borer, weighed, and dried at 60 °C. Tissue saps for the determination of soluble sugars and organic acids were produced from the residual leaves by transferring them into 20 ml polyethylene flasks, heating them in a microwave oven for 10 s at 900 W to stop enzymatic activity (Popp et al., 1996) and freezing them to destroy the cell membranes. Syringes containing discs of filter paper (Whatman GF/F Glass Microfibre Filters, Cat. No. 1825 025, Whatman International Ltd., Maidstone, UK) were placed in centrifuge vials, filled with the thawed samples, and centrifuged at 10 845 g for 10 min to collect tissue sap. Tissue saps were analysed for organic acids with HPLC-IRMS (see below). For sugars, tissue saps were treated with ion-exchange cartridges to remove ionic components (OnGuard II H 1cc cartridges and OnGuard II A 1cc, Dionex Corporation, Sunnyvale, CA, USA) before analysis with HPLC-IRMS.
To get phloem sap, leaves were recut with a razor blade at the base of the petiole. Each leaf was then inserted with the petiole into a vial containing 1.5 ml of 15 mM sodium hexametaphosphate (Fluka, Sigma-Aldrich, St Louis, MO, USA), taking care that the cut surface was fully immersed. The sodium hexametaphosphate solution represents a C-free polyphosphate buffer binding calcium ions and therefore reducing callose blocking of sieve tubes (Gessler et al., 2004). In pre-experiments, it was found that exudation was high during the first hour before reaching a lower, but constant rate. This initial maximum was likely to have been caused by leakage from wounded parenchyma cells during the first hour. Therefore, exudates from the first hour were discarded and the vials replaced by new ones, containing the same amount of solution. Collection of exudate was stopped after 5 h and the leaf area determined. Phloem samples from individual leaves were pooled for each plant and time point. To remove polyphosphate buffer and ionic components, samples were ion-exchanged as described above. Concentration and isotopic composition of the phloem sugars were determined with HPLC-IRMS (see below).
Compound-specific isotope analysis of sugars and organic acids
For compound-specific isotope analysis, an HPLC system (Dionex Corporation, Sunnyvale, CA, USA) was coupled to a Finnigan Delta V Advantage Mass Spectrometer by a Finnigan LC IsoLink Interface (Thermo Fisher Scientific, Waltham, MA, USA). In the first step, sample compounds were separated by the HPLC system, consisting of an ICS-3000 pump (directly supplied with deionized water by an ICW-2000 device, Millipore Corporation, Billerica, MA, USA), an AS50 autosampler with a 25 μl sample loop and an Ultimate 3000 column compartment (Dionex Corporation). Columns and eluents used for the separation of sugars and organic acids are described below. In the second step, the separated compounds were oxidized to CO2 in the Finnigan LC IsoLink Interface (Krummen et al., 2004). As oxidant and acid reagents, 0.5 M sodium persulphate (sodium peroxodisulphate purum p.a., ≥99%, Fluka, Sigma-Aldrich) and 1.7 M phosphoric acid (orthophosphoric acid puriss. p.a., crystallized, ≥99%, Fluka, Sigma-Aldrich), respectively, were used. Flow rates of both reagents were 50 μl min−1, and oxidation temperature was 99.9 °C. In the third step, excess O2 was removed as described by Hettman et al. (2007): Two ceramic tubes (length 30 cm, diameter 1 mm, Friatec AG, Mannheim, Germany) were filled with copper wire (30 wires each, diameter 50 μm, Goodfellow Cambridge Ltd., Huntington, UK), and heated to 580 °C in a furnace (MTF 10/15/130 with a Carbolite 301 temperature controller, Carbolite Ltd., London, UK). A two-position eight-port valve (EC8WE, VICI AG International, Schenkon, Switzerland) switched between the sample stream (He) and H2 (1% H2 in He), with one of the tubes being oxidized by the O2 in the sample stream and one reduced at a time. Finally, the O2-free gas stream was transferred to the mass spectrometer for stable isotope analysis.
For sugar separation, a HyperREZ XP Carbohydrate Pb2+ 8 μm column (Thermo Fisher Scientific) was used at 80 °C and with 0.6 ml min−1 of deionized water as eluent, which allowed baseline separation of sucrose, fructose, and an unidentified component X1 in the tissue saps. Glucose could not be fully separated from another unidentified component X2, potentially affecting glucose C isotope values. Samples were measured and compared with standard solutions to calculate the concentrations and correct for δ13C offset. Standards were referenced with EA/IRMS (EA 1110, CE Instruments, Milan, Italy, coupled to a Finnigan MAT Delta Plus IRMS, Thermo Fisher Scientific) as pure chemicals (n=3) before preparing the solutions. As standards, two stock solutions of sucrose, glucose, and fructose with δ13C values ranging from –27‰ to –10‰ were prepared, diluted to different concentrations (12.5–300 mg l−1), and analysed interspersed with the samples. Peak areas and concentrations of standards showed a linear correlation which was used to calculate the concentrations of the samples. Measurement of δ13C with HPLC-IRMS showed an offset from EA/IRMS, depending on sample concentration. To correct for the offset, a multiple regression equation of the following form was applied:
where δoffline and δonline represent the δ13C values of the standards measured by EA/IRMS and HPLC-IRMS, respectively. δ13C values of each sample compound were corrected by inserting a, b, c (calculated from the respective standards), peak area, and δonline (from HPLC-IRMS analysis of the sample) into the equation. Average standard deviations of δ13C values were 0.06‰ for sucrose, 0.10‰ for glucose, and 0.13‰ for fructose (n=3, for standards ranging from 25–200 mg l−1).
For organic acids, an ion pack AS11 4 mm column (Dionex Corporation) was selected, at a flow rate of 0.35 ml min−1 and 25 °C. As eluent, a KOH gradient from 2–25 mM was used. KOH was generated online by an EGC II KOH eluent generator cartridge, controlled by an RFC-30 device (Dionex Corporation), and removed after HPLC separation by an ASRS Ultra 2 mm suppressor (Dionex Corporation). As well as malate, samples contained high amounts of citrate and isocitrate, which could not be completely separated and are therefore represented as combined citrate/isocitrate values. As standards, a concentration series of malate and isocitrate (12.5–300 mg l−1) was analysed with the samples. Calculation of sample concentrations and correction for δ13C offset were performed as for sugars. Average standard deviations of δ13C values were 0.06‰ for malate, 0.08‰ for citrate, and 0.09‰ for isocitrate (n=3, for standards ranging from 12.5–300 mg l−1).
Determination of isotopic composition and concentration of starch
The determination of isotopic composition and amount of starch in leaves was based on an enzymatic digestion as previously described (Gottlicher et al., 2006; Richter et al., 2009) with the slight modification that only 50 mg of plant material was used.
Gas exchange and instantaneous discrimination
For the determination of net CO2 exchange, whole plant shoots were sealed into custom-built plexiglass chambers equipped with ventilators, the root system being outside the chambers. Dried ambient air (pCO2=409.4±2.5 ppm, n=113, and δ13C= –10.0±0.2‰, n=27, mean ±SE) was directed through the chambers and analysed by an infrared gas analyser (ADC 225-MK3, ADC Bioscientific Ltd., Herts, UK). A WA-161 device (ADC Bioscientific Ltd.) switched between air from the two chambers and the unaltered reference gas stream (ambient air). Flow rates were determined with mass flow meters installed in front of the chambers and leaf temperature was measured with thermo elements. After the end of the experiment, plants were harvested and leaves scanned to determine the leaf area. Net photosynthesis (NP; in μmol m−2 s−1) was calculated as
where V20 (l min−1) is the flow of gas through the chamber corrected for the deviation from standard conditions of 20 °C and 1000 mBar air pressure as
cr and cs represent the CO2 partial pressure of reference and sample gas (ppm), respectively, a is the leaf area (m2), MolVol20 denotes the volume of 1 mol gas under standard conditions, f is the flow of gas through the chamber measured by the mass flow meter (l min−1), T is the ambient temperature (°C), and p is the air pressure (mBar) inside the chamber. Transpiration was not measured.
For the determination of instantaneous discrimination (Δ13C), gas samples were taken by connecting a syringe to the IRGA outlet. The samples were immediately transferred into pre-evacuated gas containers (IVA 738W, IVA Analysentechnik, Meerbusch, Germany), stored at 4 °C and analysed within 7 d. δ13C of CO2 was measured on a Finnigan GasBench coupled to a Finnigan Delta V Advantage Mass Spectrometer (Thermo Fisher Scientific). Δ13C (‰) was calculated according to Evans et al. (1986), as
where ξ=cr/(cr–cs), and δr and δs are δ13C values of the reference and sample gas, respectively. Each data point represents the average of three consecutive measurements for a single plant.
Carbon budgets
Fixation of external CO2 (Mext) was calculated from the gas exchange measurements. Subtraction of Δ13C from δ13C of reference gas of the respective phase gave δ13C of CO2 taken up. The amount (Mresp) and δ13C (δresp) of respiratory CO2 refixed in phase I were calculated based on Borland (1996) from malate accumulation (Mm and δm), uptake of external CO2 (Mext and δext), and the amount of degraded starch necessary for C skeletons (Mm×3/4 and δst), assuming one C per malate coming from CO2 (either external or respiratory) and three from starch degradation, as
and
To estimate the proportions of PEPC and direct Rubisco fixation during phase II, a mass balance equation was applied, setting PEPC Δ13C to the night-time value and Rubisco Δ13C to the maximum reached at the end of phase II when pure C3 fixation was likely:
and
MC3, δC3, MC4, and δC4 represent amounts and δ13C values of C fixed by Rubisco and PEPC (calculated by subtraction of Δ13C from δ13C of phase II reference gas), respectively, and MII and δII of total C fixed during phase II.
For the analysis of cellular C partitioning, malate, starch, and sucrose were considered, which exhibited the largest changes in concentration (malate, starch) or isotopic composition (sucrose) and are thus supposed to have the highest turnover. If δ13C values changed significantly over a certain phase (P <0.1), the isotopic composition of the net flux was calculated with mass balance equations (Deleens and Garnier-Dardart, 1977).
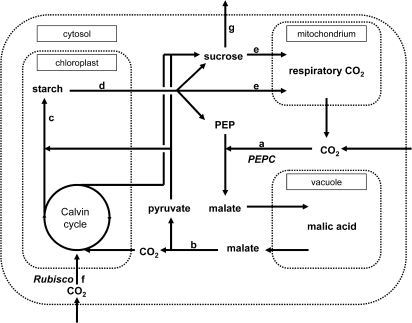
Starch was assumed to provide the C skeletons for malate synthesis in phase I (with three carbons from starch per carbon fixed). A scheme of the C fluxes that were considered (modified after Dodd et al., 2002) is presented in Fig. 1. The contribution of C fixed by PEPC and directly by Rubisco to phloem sucrose was calculated with a mixing model using the average δ13C values of C fixed by the respective enzyme, and the weighted average of phloem sucrose δ13C values. No isotopic discrimination during leaf export was assumed. δ13C values of phloem sucrose were compared with those of leaf sucrose at different time points (measured values plus several integrated values) to analyse the strength of correlation and, eventually, the time-lag for phloem exudation.
Fig. 1.
Proposed scheme of cellular carbon flow in K. daigremontiana, modified after Dodd et al. (2002), focusing on the sucrose pool. PEPC fixation, a; malate decarboxylation, b; starch accumulation, c; starch degradation, d; respiration, e; Rubisco fixation, f; export, g.
Statistical analysis
Statistical analysis was performed with Statistica 6.0 (StatSoft, Inc., Tulsa, OK, USA). Normal distribution of the data was checked with the Lilliefors test (95%). One-way ANOVA with the Tukey HSD test was applied to calculate levels of significance.
Results
Gas exchange and instantaneous discrimination
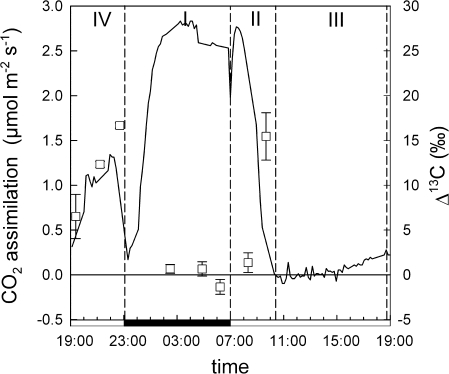
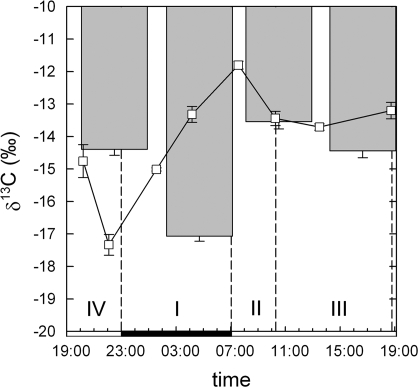
A typical four-phase gas exchange pattern was observed (Fig. 2). The major proportion of CO2 was fixed during phase I by PEPC. During this time, respiratory refixation accounted for 27% of total fixation (Table 1). With the onset of light and the start of phase II, CO2 uptake increased sharply, concurrent with an increase in the instantaneous discrimination from 1‰ to 15‰ due to a shift from PEPC to Rubisco fixation. After about 3 h of light, stomata closed, marking the onset of phase III. During this time, only minimal net gas exchange was observed. CO2 uptake increased in the late afternoon (phase IV), with instantaneous discrimination reaching 17‰ at the end of the phase, indicating a high proportion of direct Rubisco fixation. The lower discrimination in the beginning may have been due to stomatal limitation rather than to a temporary activation of PEPC. Altogether, PEPC fixation accounted for 85% of overall C fixation, while direct Rubisco fixation accounted for 15%.
Fig. 2.
Diurnal pattern of net CO2 uptake (solid line) and instantaneous discrimination Δ13C (squares) of an exemplary K. daigremontiana plant. CAM phases are denoted by dashed lines, the solid bar on the x-axis indicates the dark period. Δ13C is shown as the mean ±SE of three consecutive measurements.
Table 1.
CO2 fixation (mean, n=2), instantaneous discrimination (Δ13C; mean, n=3) and δ13C of fixed CO2
| Phase | Time | CO2 fixation (mg C g−1 DW) | Δ13C (‰) | δ13C (‰) |
| Phase I | 23:20–07:00 h | 8.92 | −11.28 | |
| External fixation | 6.54 | −0.01 | −10.22 | |
| Respiratory refixation | 2.38 | −14.22 | ||
| Phase II | 07:00–10:20 h | 1.88 | −11.88 | |
| Direct Rubisco fixation | 0.34 | 15.45 | −24.62 | |
| PEPC fixation | 1.55 | (–0.01) | −9.09 | |
| Phase III | 10:20–18:40 h | 0.14 | ||
| Phase IV | 18:40–23:20 h | 1.41 | 11.84 | −22.38 |
| Total external fixation | 9.98 | 2.11 | −12.28 | |
| Total fixation | 12.36 | −12.66 | ||
| Total direct Rubisco fixation | 1.89 | −22.81 | ||
| Total PEPC fixation | 10.46 | −10.96 |
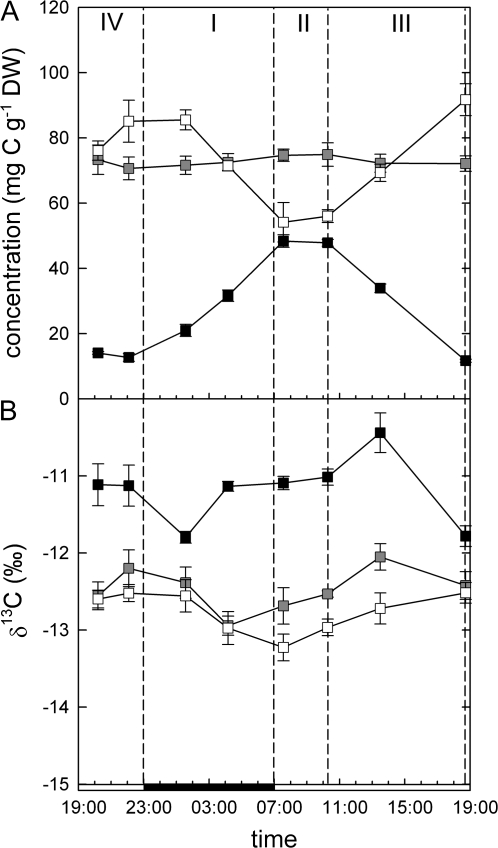
Concentration and isotopic composition of starch and organic acids
Concentrations of malate and starch changed significantly over the diurnal cycle (P <0.00001 and P <0.000001, respectively), whereas no significant differences could be observed for citrate/isocitrate (Fig. 3). As expected, degradation of starch and accumulation of malate occurred in phase I, and vice versa in phase III. In phases II and IV, no significant net change of malate or starch levels was observed (Table 2), although instantaneous discrimination during phase II indicated a high rate of PEPC fixation, suggesting decarboxylation of phase II malate immediately after its synthesis.
Fig. 3.
Diurnal changes in the concentration (A) and δ13C (B) of starch (open symbols), malate (closed symbols), and citrate/isocitrate (shaded symbols) of K. daigremontiana. CAM phases are denoted by dashed lines, the solid bar on the x-axis indicates the dark period. Data shown are the means ±SE of five replicate plants.
Table 2.
Change of size and isotopic composition of pools between start and end of each phase (P values were calculated by ANOVA, followed by Tukey HSD test)
| Phase | Pool | Net flux size | Net flux δ13C | Pool δ13C | ||
| (mg C g−1 DW) | P value | (‰) | P value | (‰) | ||
| I | Malate | 35.67 | 0.0001 | 1.0000 | −11.29 | |
| I | Starch | −30.98 | 0.0003 | −11.29 | 0.0789 | −12.82 |
| I | Sucrose | 0.54 | 0.1083 | 0.59 | 0.0001 | −14.37 |
| II | Malate | −0.52 | 1.0000 | 0.9994 | −11.02 | |
| II | Starch | 1.88 | 1.0000 | 0.9467 | −13.10 | |
| II | Sucrose | 0.82 | 0.0027 | −16.94 | 0.0027 | −12.62 |
| III | Malate | −36.15 | 0.0001 | −10.69 | 0.0493 | −11.06 |
| III | Starch | 35.68 | 0.0001 | 0.5412 | −12.73 | |
| III | Sucrose | −1.32 | 0.0001 | 0.9978 | −13.45 | |
| IV | Malate | 1.00 | 0.9995 | 0.2006 | −11.34 | |
| IV | Starch | −6.58 | 0.9421 | 1.0000 | −12.55 | |
| IV | Sucrose | −0.05 | 1.0000 | 97.37 | 0.0001 | −15.10 |
Net flux δ13C values were calculated by applying mass balance equations, if changes were marginally significant (P <0.1). Pool δ13C values are averages of the respective phase.
Malate δ13C values were close to the value of CO2 fixed by PEPC and only slightly varied over the course of the day (range 1.4‰, P=0.0003). Starch was depleted in 13C compared to malate by about 1.5‰ and showed only non-significant changes over the diurnal cycle that can be explained by a large, isotopically inhomogenous starch background.
Concentration and isotopic composition of soluble sugars
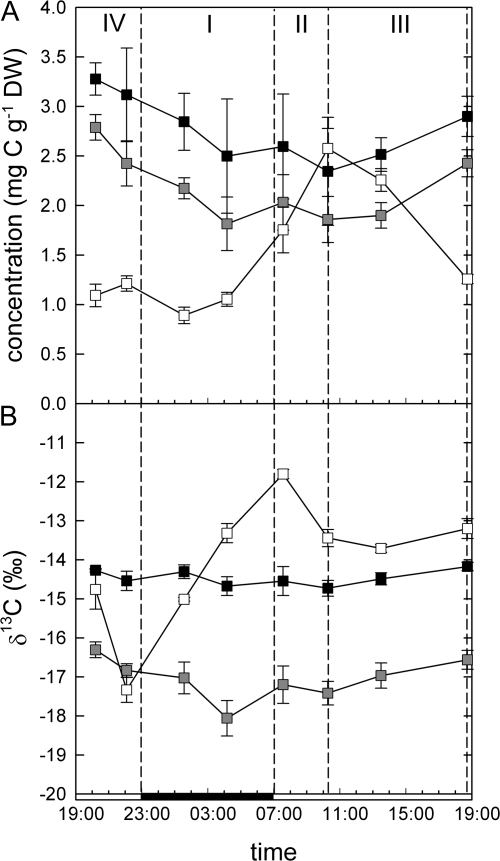
Compound-specific stable isotope analysis with HPLC-IRMS revealed remarkable differences between diurnal patterns of soluble sugars in both concentration and isotopic composition. Glucose and fructose concentrations were highly correlated, both exhibiting non-significant decreases during the night and increases during the day. Sucrose, on the other hand, showed strong and highly significant changes in concentration (P <0.000001). Sucrose concentration started to increase in the morning and reached a maximum at the end of phase II, then decreased to the initial value at the end of phase III (Fig. 4).
Fig. 4.
Diurnal changes in the concentration (A) and δ13C (B) of sucrose (open symbols), fructose (closed symbols), and glucose (shaded symbols) of K. daigremontiana. CAM phases are denoted by dashed lines, the solid bar on the x-axis indicates the dark period. Data shown are the means ±SE of five replicate plants. At the end of the phases I, II, III, and IV, carbon from direct Rubisco fixation accounted for 7, 21, 19, and 54% of sucrose, respectively.
δ13C values of glucose and fructose remained constant over the diurnal cycle. By contrast, mean sucrose δ13C values ranged between –17‰ and –12‰ (P <0.000001). The lowest δ13C values of sucrose were observed at the end of phase IV, representing the highest contribution of C derived from Rubisco fixation to the sucrose pool (Fig. 4). During phase I, δ13C of sucrose increased, reaching the maximum at the end of the phase, then slightly declined during phase II and remained constant until phase IV, when it started to decline again.
Sucrose accounted for about 70% of phloem sugars and was the only sugar with concentrations sufficient for δ13C analysis. δ13C values of phloem sucrose exhibited significant differences over the diurnal cycle (P <0.000001; Fig. 5). The lowest values were observed in phase I, several hours after the leaf sucrose pool had reached its lowest value. δ13C values of phloem and leaf sucrose (δp and δl, respectively) showed the best correlation when a time-lag of 5 h was assumed (r2=0.9973, P=0.0013; δp= 0.97×δl–1.30).
Fig. 5.
Diurnal changes in δ13C of sucrose in the leaf (squares) and in phloem exudates (shaded bars) in K. daigremontiana. Exudation lasted 5 h. The phases are denoted by dashed lines, the solid bar on the x-axis indicates the dark period. Data shown are the means ±SE of five replicate plants.
Carbon budgets
The gas exchange data (Table 1) and changes in pool size and isotopic composition of malate, starch, and sucrose (Table 2) were combined to model leaf C flows (as outlined in Fig. 1). Malate and starch net fluxes were much higher than sugar and CO2 net fluxes (Table 2). In phase I, 86% of C from starch degradation was necessary to provide the cell with C for malate synthesis (assuming that three-quarters of the C accumulated as malate came from starch degradation), and 10% for dark respiration (calculated from respiratory re-fixation; Table 1). The calculated δ13C value of dark respiration suggests that the C had passed through the sucrose pool before being respired (–14.22 versus –14.37‰; Tables 1, 2). In phase III, 99% of C from malate decarboxylation (including both released CO2 and pyruvate) were accumulated as starch. In phases II and IV, only minor net fluxes of malate and starch were observed.
Discussion
Based on their turnover rates, three types of metabolic C pools could be distinguished. First, malate and starch showed a diurnal pattern of reciprocal mobilization and accumulation (Fig. 3), with 99% of C from phase III malate decarboxylation used for starch accumulation and 86% of C from phase I starch degradation used to provide C skeletons. Average δ13C values of –11‰ and –13‰ (for malate and starch, respectively) demonstrated the pivotal contribution of PEPC fixation (average δ13C of PEPC fixation –11‰) to these pools. Second, glucose and fructose exhibited low turnover rates. Both concentration and isotopic composition remained constant over the diurnal cycle. Third, sucrose showed rapid and highly significant (P <0.000001) changes of δ13C values, ranging between –17‰ and –12‰ (Fig. 4). The strongest isotopic changes in sucrose occurred during phases I and IV, when concentrations were nearly constant (Table 2). If only influx into the sucrose pool was responsible for the 13C enrichment during phase I, C entering must have had a δ13C of 0.59‰ (Table 2); which is extremely unlikely. Therefore, a high flow through the sucrose pool has to be assumed where 13C-enriched C enters and comparatively 13C-depleted C leaves the pool, as supported by the depleted values of phloem sucrose in phase I (Fig. 5). The same can be concluded for phase IV, where a small efflux of sucrose with 97.37‰ would be necessary to explain the changes of isotopic composition, if no import into the pool was assumed. Both results point to a high turnover of the sucrose pool during the diurnal cycle.
Furthermore, from the changes in leaf sucrose δ13C values, it is obvious that C influx in phases II and IV predominantly originated from direct Rubisco fixation, which was also supported by the increase in sucrose concentration during phase II. During phase I, however, the C influx was coming from a PEPC-derived pool—presumably starch. Carbon flux from malate decarboxylation to sucrose in phase III must have been minimal, as sucrose δ13C values did not reach the comparatively enriched malate values. Comparing the δ13C values of dark respiration (–14.22‰), C derived from phase I starch degradation (–11.29‰), and the phase I sucrose pool (–14.37‰), one may speculate that the sucrose pool was the main substrate for dark respiration. It is therefore likely that the nocturnal starch degradation was not only a source of PEP for PEPC fixation, but was also fuelling the leaf sucrose pool, which, in turn, was the source of dark respiration and phloem export.
The demonstration of three pool types—CAM maintenance carbon, low turnover hexoses, and high turnover sucrose—in this study supports previous postulations of C partitioning within plant leaves: For Kalanchoë spp., two distinct glycolytic pathways during phase I were suggested by Deleens and Garnier-Dardart (1977) and Deleens et al. (1979), with starch as the source of C skeletons on the one hand and soluble sugars as the substrate for dark respiration on the other hand. A similar compartmentation was demonstrated for Sedum telephium L. (Borland and Griffiths, 1996). Two distinct sugar pools were suggested for Clusia spp., which uses soluble sugars instead of starch for CAM maintenance: A vacuolar sugar pool from PEPC fixation, used as a substrate for malate synthesis, as opposed to a high turnover cytosolic sugar pool from direct Rubisco fixation, fuelling phloem export (Borland and Dodd, 2002; Borland et al., 1994).
In this study, direct Rubisco fixation accounted for 30% of phloem sucrose C, but only for 15% of total CO2 fixation. Thus, a preference of C fixed directly by Rubisco from atmospheric CO2 during phases II and IV for export was demonstrated. It is unlikely that the time lag of 5 h between leaf and phloem sucrose δ13C values was caused by the distance for transport (about 2 cm), but rather by differences between cellular sucrose pools (e.g. vacuolar versus cytosolic pool), which are likely considering the high turnover of the total sucrose pool that was found (Fig. 4).
As both Rubisco and PEPC-derived C are contributing to the leaf sucrose pool, a shift in the relationship between PEPC and direct Rubisco fixation will strongly affect the isotopic composition of the leaf sucrose pool and, in consequence, of phloem sap and sink tissues. This relationship may depend on plant species, developmental stage and especially on environmental parameters: For K. daigremontiana, Griffiths et al. (2002) demonstrated that a reduced water supply resulted in an increase in nocturnal CO2 uptake, whereas phases II and IV almost disappeared. Temperature was also shown to affect CO2 exchange of K. daigremontiana with phase II fixation being reduced when temperature increased (Winter and Tenhunen, 1982). A higher proportion of direct Rubisco fixation might, therefore, result in a clear Rubisco signal of exported C, in spite of PEPC fixation.
By taking up CO2 at night, CAM plants manage to deal with low or intermittent water supply, but the associated elevated energy demand and the necessity of large carbohydrate stocks for CAM maintenance are limiting productivity. Thus, curtailing the competition between C storage for CAM maintenance and export for growth is of crucial importance for this type of plant. CAM plants have to store as much C as necessary to maintain a high capacity to fix CO2, but yet to export as much as possible for growth. Furthermore, as both the available amount of C and the nocturnal demand for C skeletons are influenced by environmental conditions, control mechanisms have to be highly adaptive. By using PEPC-fixed C preferentially for refilling leaf carbohydrate stocks, the amount of C stored for CAM maintenance is directly controlled by the amount of C fixed by CAM in the previous night—and thus by the expected C demand in the following night. While 99% of C from phase III malate decarboxylation was accumulated as starch in this study, only 86% of C from nocturnal starch degradation was required for malate synthesis. The remaining 14% of starch-derived C, as well as C from direct Rubisco fixation, were available for other purposes such as phloem export.
In summary, it was possible to demonstrate by direct measurements that carbon fixed in different CAM phases is not equally used for growth or maintenance. Carbon fixed directly by Rubisco during phases II and IV was preferentially, but not exclusively, utilized for export to the stem and roots.
Acknowledgments
Stable isotope analysis was performed in the SILVER facility (Stable Isotope Laboratory at the University of Vienna for Environmental Research). We thank Margarete Watzka for EA/IRMS and GasBench analysis, and Andreas Blöchl for assistance with the HPLC system.
References
- Borland AM. A model for the partitioning of photosynthetically fixed carbon during the C3-CAM transition in Sedum telephium. New Phytologist. 1996;134:433–444. [Google Scholar]
- Borland AM, Dodd AN. Carbohydrate partitioning in Crassulacean acid metabolism plants: reconciling potential conflicts of interest. Functional Plant Biology. 2002;29:707–716. doi: 10.1071/PP01221. [DOI] [PubMed] [Google Scholar]
- Borland AM, Griffiths H. Variations in the phases of Crassulacean acid metabolism and regulation of carboxylation patterns determined by carbon-isotope-discrimination techniques. Ecological Studies. 1996;114:230–249. [Google Scholar]
- Borland AM, Griffiths H, Broadmeadow MSJ, Fordham MC, Maxwell C. Carbon-isotope composition of biochemical fractions and the regulation of carbon balance in leaves of the C3-Crassulacean acid metabolism intermediate Clusia minor L growing in Trinidad. Plant Physiology. 1994;106:493–501. doi: 10.1104/pp.106.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher JT, Holtum JAM. Patterns of carbon partitioning in leaves of Crassulacean acid metabolism species during deacidification. Plant Physiology. 1996;112:393–399. doi: 10.1104/pp.112.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleens E, Garnier-Dardart J. Carbon isotope composition of biochemical fractions isolated from leaves of Bryophyllum daigremontianum Berger, a plant with Crassulacean acid metabolism: some physiological aspects related to CO2dark fixation. Planta. 1977;135:241–248. doi: 10.1007/BF00384896. [DOI] [PubMed] [Google Scholar]
- Deleens E, Garnier-Dardart J, Queiroz O. Carbon isotope composition of intermediates of the starch-malate sequence and level of the Crassulacean acid metabolism in leaves of Kalanchoë blossfeldiana Tom Thumb. Planta. 1979;146:441–449. doi: 10.1007/BF00380858. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Borland AM, Haslam RP, Griffiths H, Maxwell K. Crassulacean acid metabolism: plastic, fantastic. Journal of Experimental Botany. 2002;53:569–580. doi: 10.1093/jexbot/53.369.569. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Griffiths H, Taybi T, Cushman JC, Borland AM. Integrating diel starch metabolism with the circadian and environmental regulation of Crassulacean acid metabolism in Mesembryanthemum crystallinum. Planta. 2003;216:789–797. doi: 10.1007/s00425-002-0930-2. [DOI] [PubMed] [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. Carbon isotope discrimination measured concurrently with gas-exchange to investigate CO2diffusion in leaves of higher plants. Australian Journal of Plant Physiology. 1986;13:281–292. [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:503–537. [Google Scholar]
- Gessler A, Rennenberg H, Keitel C. Stable isotope composition of organic compounds transported in the phloem of European beech: evaluation of different methods of phloem sap collection and assessment of gradients in carbon isotope composition during leaf-to-stem transport. Plant Biology. 2004;6:721–729. doi: 10.1055/s-2004-830350. [DOI] [PubMed] [Google Scholar]
- Gottlicher S, Knohl A, Wanek W, Buchmann N, Richter A. Short-term changes in carbon isotope composition of soluble carbohydrates and starch: from canopy leaves to the root system. Rapid Communications In Mass Spectrometry. 2006;20:653–660. doi: 10.1002/rcm.2352. [DOI] [PubMed] [Google Scholar]
- Griffiths H, Broadmeadow MSJ, Borland AM, Hetherington CS. Short-term changes in carbon-isotope discrimination identify transitions between C3and C4carboxylation during Crassulacean acid metabolism. Planta. 1990;181:604–610. doi: 10.1007/BF00193017. [DOI] [PubMed] [Google Scholar]
- Griffiths H, Cousins AB, Badger MR, von Caemmerer S. Discrimination in the dark. Resolving the interplay between metabolic and physical constraints to phosphoenolpyruvate carboxylase activity during the Crassulacean acid metabolism cycle. Plant Physiology. 2007;143:1055–1067. doi: 10.1104/pp.106.088302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths H, Helliker B, Roberts A, Haslam RP, Girnus J, Robe WE, Borland AM, Maxwell K. Regulation of Rubisco activity in Crassulacean acid metabolism plants: better late than never. Functional Plant Biology. 2002;29:689–696. doi: 10.1071/PP01212. [DOI] [PubMed] [Google Scholar]
- Hettmann E, Brand WA, Gleixner G. Improved isotope ratio measurement performance in liquid chromatography/isotope ratio mass spectrometry by removing excess oxygen. Rapid Communications In Mass Spectrometry. 2007;21:4135–4141. doi: 10.1002/rcm.3304. [DOI] [PubMed] [Google Scholar]
- Krummen M, Hilkert AW, Juchelka D, Duhr A, Schluter HJ, Pesch R. A new concept for isotope ratio monitoring liquid chromatography/mass spectrometry. Rapid Communications In Mass Spectrometry. 2004;18:2260–2266. doi: 10.1002/rcm.1620. [DOI] [PubMed] [Google Scholar]
- Mayoral ML, Medina E. 14C-translocation in Kalanchoë pinnata at two different stages of development. Journal of Experimental Botany. 1985;36:1405–1413. [Google Scholar]
- Nobel PS. Achievable productivities of certain CAM plants: basis for high values compared with C3 and C4 plants. New Phytologist. 1991;119:183–205. doi: 10.1111/j.1469-8137.1991.tb01022.x. [DOI] [PubMed] [Google Scholar]
- Osmond CB. Crassulacean acid metabolism: curiosity in context. Annual Review of Plant Physiology and Plant Molecular Biology. 1978;29:379–414. [Google Scholar]
- Popp M, Lied W, Meyer AJ, Richter A, Schiller P, Schwitte H. Sample preservation for determination of organic compounds: microwave versus freeze-drying. Journal of Experimental Botany. 1996;47:1469–1473. [Google Scholar]
- Richter A, Wanek W, Werner RA, et al. Preparation of starch and soluble sugars of plant material for the analysis of carbon isotope composition: a comparison of methods. Rapid Communications In Mass Spectrometry. 2009;23:2476–2488. doi: 10.1002/rcm.4088. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. How closely do the δ13C values of Crassulacean acid metabolism plants reflect the proportion of CO2fixed during day and night? Plant Physiology. 2002;129:1843–1851. doi: 10.1104/pp.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Smith JAC. An introduction to Crassulacean acid metabolism. Biochemical principles and ecological diversity. In: Winter K, Smith JAC, editors. Crassulacean Acid Metabolism: Biochemistry, Ecophysiology and Evolution. Berlin: Springer; 1996. pp. 1–13. [Google Scholar]
- Winter K, Tenhunen JD. Light-stimulated burst of carbon-dioxide uptake following nocturnal acidification in the Crassulacean acid metabolism plant. Kalanchoë daigremontiana. Plant Physiology. 1982;70:1718–1722. doi: 10.1104/pp.70.6.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]