Abstract
In leaves of most C4 plants, the biochemistry of photosynthesis is partitioned between mesophyll and bundle sheath cells. In addition, their cell biology and development also differs from that in C3 plants. We have a poor understanding of the mechanisms that generate the cell-specific accumulation of proteins used in the C4 pathway, and there are few genes that have been shown to be important for the cell biology and development of C4 leaves. To facilitate functional analysis of C4 photosynthesis, and to enable knowledge from Arabidopsis thaliana to be translated to C4 species, an Agrobacterium tumefaciens-mediated transformation protocol was developed for the C4 species Cleome gynandra. A. tumefaciens, harbouring the binary vector SLJ1006, was used to transfer the uidA gene under the control of the CaMV 35S promoter into C. gynandra. Co-incubation of hypocotyls or cotyledons with SLJ1006 allowed efficient transfer of DNA into C. gynandra, and media that allowed callus production and then shoot regeneration were identified. Stable transformants of C. gynandra with detectable amounts of β-glucuronidase (GUS) were produced at an efficiency of 14%. When driven by the CaMV 35S promoter, GUS was visible in all leaf cells, whereas uidA translationally fused to a CgRbcS gene generated GUS accumulation specifically in bundle sheath cells. This transformation procedure is the first for an NAD-ME type C4 plant and should significantly accelerate the analysis of mechanisms underlying C4 photosynthesis.
Keywords: Agrobacterium tumefaciens, Arabidopsis thaliana, C4 photosynthesis, Cleome gynandra, transformation
Introduction
C4 photosynthesis has evolved independently multiple times within the angiosperms and allows CO2 to be concentrated around Ribulose Bisphosphate Carboxylase Oxygenase (RuBisCO) (Sage, 2004). In tropical and subtropical regions the increased supply of CO2 to RuBisCO represses its oxygenase reaction, increases the rate of photosynthesis, and allows higher rates of growth compared to C3 species. In fact, the most productive native vegetation and domesticated crops all use the C4 pathway (Brown, 1999) and, as a consequence, it has been proposed that placing characteristics of C4 photosynthesis into crops such as rice could be used to increase yields (Matsuoka et al., 2001; Surridge, 2002; Hibberd et al., 2008).
In most plants, the C4 pathway involves photosynthesis proteins being compartmented between mesophyll (M) and bundle sheath (BS) cells (Hatch, 1987; Brown et al., 2005), although single-celled C4 photosynthesis has been reported in aquatic and terrestrial plants (Bowes and Salvucci, 1984; Reiskind et al., 1989; Magnin et al., 1997; Voznesenskaya et al., 2001, 2002) as well as in a marine diatom (Reinfelder et al., 2000). In the two-celled version of the C4 pathway (Hatch, 1987), CO2 enters M cells and is converted into bicarbonate by carbonic anhydrase. Phosphoenolpyruvate carboxylase (PEPC) then combines with phosphoenolpyruvate to generate the C4 oxaloacetic acid (OAA), which is rapidly converted into either aspartate or malate. These C4 acids then diffuse into BS cells through abundant plasmodesmata, where one of three separate C4 acid decarboxylases releases CO2. In different lineages of C4 plants, the three distinct decarboxylases known as NADP-dependent malic enzyme (NADP-ME), NAD-dependent malic enzyme (NAD-ME) and phosphoenolpyruvate carboxykinase (PEPCK) have been co-opted into this process. In many cases one of the enzymes is believed to act as the primary decarboxylase with another fulfilling a secondary role (Wingler et al., 1999). The three carbon compound released after decarboxylation then diffuses back to the M, and in the case of pyruvate, rephosphorylation to PEP occurs, catalysed by pyruvate, orthophosphate dikinase (PPDK) (Hatch and Slack, 1968).
Despite the basics of the C4 pathway having been defined for decades, there still is a relatively poor understanding of the mechanisms that generate the accumulation of proteins used in C4 photosynthesis in the M or BS cells (Brown et al., 2005). For example, although an element known as the mesophyll enhancing module1 (MEM1) has been identified in the PPC promoter of Flaveria bidentis (Gowik et al., 2004; Akyildiz et al., 2007) and gel-retardation assays showed that a region about 200 bp upstream of the maize PPDK gene generates mesophyll specific expression of GUS (Matsuoka and Numazawa, 1991), in neither case have trans-factors responsible for generating M-specific expression been isolated. In the C4 plants studied to date, RbcS seems to be regulated at multiple levels; including transcriptional (Viret et al., 1994; Purcell et al., 1995), post-transcriptional, and translational elongation (Berry et al., 1985, 1986, 1987, 1990) but again, no trans-factors have been isolated. For many of the other genes encoding enzymes (e.g. carbonic anhydrase, malate dehydrogenase, phosphoenolpyruvate carboxykinase) that have been recruited into the C4 pathway, there is no information on mechanisms generating cell-specific expression.
Our understanding of the genetic basis associated with the alterations in cell biology and development of a C4 leaf is also poor. For example, there have been no genes identified to date that control the expansion, proliferation or polarized positioning of chloroplasts in BS cells, the increased plasmodesmatal connectivity between M and BS cells, nor Kranz anatomy itself. This is despite the fact that genes have been isolated in Arabidopsis thaliana that are involved in many of these processes. For example, the Min, FtsZ, and GC1 genes are known to control chloroplast division (Colletti et al., 2000; Vitha et al., 2001; Maple et al., 2004), CHUP1 is involved in controlling chloroplast movement (Oikawa et al., 2003) and glucan 1,3-β-glucosidases are implicated in plasmodesmatal connectivity (Levy et al., 2007). To date, it has not been possible to determine whether alterations to any of these genes are associated with the modifications to C4 cell biology. This is partly because transformation systems for the main NADP-ME-type C4 models, maize and Flaveria, are relatively difficult (Ishida et al., 1996; Chitty et al., 1999). To our knowledge, there are no transformation systems available for NAD-ME or PEPCK-type C4 species, and the phylogenetic distance of transformable C4 species from A. thaliana also means that direct comparison with the most widely studied model plant is unlikely to be meaningful.
The most closely related genus to A. thaliana that is known to contain C4 species is Cleome L. (Hall et al., 2002; Brown et al., 2005). Cleome belongs to the NAD-ME C4 subtype and contains C3 species and some that appear to be intermediate between the two forms of photosynthesis as well as bone fide C4 species (Marshall et al., 2007; Voznesenskaya et al., 2007). Although new sequencing technologies will generate insight into C4 photosynthesis, functional analysis will still be needed to validate candidates. An efficient transformation system in a C4 plant that is closely related to A. thaliana, would possibly help address areas where our knowledge of mechanisms underlying C4 photosynthesis is poor because it would allow gene reporter studies and candidate genes to be mis-expressed and knocked down. It would also allow meaningful comparative analysis of genes and proteins recruited into C4 photosynthesis in both A. thaliana and a closely related C4 plant. This will help to determine how gene expression and protein function have altered during the evolution of the C4 pathway. Therefore, a variety of approaches were tested to allow the stable transfer of genes into C. gynandra L., and a simple method is reported here that generates transformants of an NAD-ME type C4 plant and the most closely related C4 species to A. thaliana.
Materials and methods
Plant material
Cleome gynandra was grown in a greenhouse set at 22 °C with supplementary lighting from metal halide lamps for a 16 h photoperiod to produce seed for use in transformation experiments. Surface-sterilization of seeds involved rinsing in 70% ethanol followed by a 10 min soak in 5% sodium hypochlorite and three rinses in sterile water or by microwaving (Franco, 1993). Seed was also sown directly from freshly harvested intact pods, and placed in sterile germination plates of filter paper moistened with water, which were wrapped in Parafilm and incubated at 30 °C for 24 h. Healthy seed produced roots 1–5 mm in length after approximately 24 h, and these plants were transferred to sterile plastic pots containing MS medium (Murashige and Skoog, 1962) with B5 vitamins (Gamborg et al., 1968) (Duchefa Biochimie BV), 10 g l−1 sucrose, 8 g l−1 agar (Melford), pH 6.0). These pots were kept in a tissue culture room at 24 °C with a 16 h photoperiod and a light intensity of 50 μmol m−2 s−1. After 6–8 d the expanded cotyledons and hypocotyls were used for transformation experiments.
Generation of uidA constructs for transformation
The binary plasmid SLJ1006 contains the uidA reporter gene under the CaMV 35S promoter and Nos terminator (Jones et al., 1992). The CgRbcS construct consisted of the CgRbcS promoter (2.8 kb) and an unspliced coding region translationally fused to GFP and uidA, with 1 kb of the CgRbcS 3' region downstream of uidA. The promoter, together with the coding region, was amplified from genomic DNA by PCR with primers (Table 1) including restriction enzyme sites, to allow the translational fusion to be generated. The 3' region was also amplified from genomic DNA with primers, including restriction sites (Table 1). The uidA gene was amplified, together with gfp, from pKGWFS7 (Plant Systems Biology, http://www.psb.ugent.be/gateway/). The reporter gene fragment was then ligated into pUC19 using T4 DNA ligase, amplified by PCR together with the pUC19 MCS using M13 primers and inserted into pENTR/D-TOPO (Invitrogen, http://www.invitrogen.com/). The CgRbcS promoter and unspliced coding region were ligated into the entry plasmid upstream of the reporter genes using T4 DNA ligase; the CgRbcS 3' region was ligated downstream. The assembled construct was recombined into the Gateway destination vector pGWB1 (Nakagawa et al., 2007) using LR clonase (Invitrogen).
Table 1.
Primers used in polymerase chain reactions to generate the translational fusion between the RbcS gene from C. gynandra and uidA Restriction enzymes sites are shown in bold.
| Primer | Nucleotide sequence |
| CgRbcS promoter F | 5′-TCTGCAGCAAACACGCATTTATGGCTG |
| CgRbcS coding R | 5′-TACTACTCATCTCTTTCTTCTTTGCTC |
| gfp uidA F | 5′-CGTCTAGAGTGAGCAAGGG |
| gfp uidA R | 5′-GCCGAGCTCATTGTTTGCC |
| CgRbcS 3' F | 5′-TGAGCTCTTAAGCCATCCCTCTTTGC |
| CgRbcS 3' R | 5′-TGAATTCAAATATCCCCTCACTTCACT |
Bacterial stocks
A fresh plate of YEP medium (An et al., 1988) (10 g l−1 yeast extract, 10 g l−1 Bacto-peptone, 5 g l−1 NaCl, 15 g l−1 Bacto-agar, pH 7.0) with streptomycin sulphate (100 μg ml−1) and tetracycline (1 μg ml−1) was streaked with LBA4404 containing SLJ1006 (Jones et al., 1992) and grown at 30 °C overnight. A loop was used to inoculate 50 ml of liquid YEP medium (as above but without Bacto-agar) plus antibiotics in a sterile 250 ml flask and the suspension was grown for 18 h at 30 °C with shaking. The bacteria were pelletted and resuspended in 6 ml of l-glycerol (50% glycerol, 10 g l−1 Bacto-tryptone, 5 g l−1 yeast extract, 5 g l−1 NaCl, pH 7.0); 0.5 ml amounts were aliquoted into 1.5 ml microfuge tubes and stored at –80 °C for future use. A stock of LBA4404 with the CgRbcS construct was made in the same way but with the antibiotics kanamycin sulphate (50 μg ml−1) and streptomycin sulphate (100 μg ml−1) added to the growth medium.
Regeneration
Seedlings were removed individually from pots and placed on a filter paper wetted with sterile water; with a sharp scalpel blade the hypocotyl was cut into 1–2 pieces 5–8 mm long and the cotyledons were excised at least 1 mm away from the apical meristem. Shoot regeneration from hypocotyl and cotyledon explants was assessed initially on medium Z (Yan You, 1995) (MS medium, 30 g l−1 sucrose, 2 mg l−1 BAP, 0.1 mg l−1 NAA, pH 5.8, and 8 g l−1 agar) with 0 or 20 mg l−1 AgNO3 and A5 (De Block et al., 1989) solidified with 8 g l−1 agar containing 0, 10, or 20 mg l−1 AgNO3, added after the medium had been autoclaved.
Transformation
One day before the transformation, 200 μl of bacterial stock stored at –80 °C were transferred to 50 ml of YEP liquid medium plus antibiotics in a 250 ml flask and grown overnight at 30 °C with shaking (c. 18–20 h). Bacteria were pelletted in a 50 ml centrifuge tube for 10 min at 3500 rpm and resuspended in 40 ml liquid inoculation medium. Three liquid inoculation media were tested for their transformation efficiency: MSO+AS (MS medium with MS vitamins (Duchefa Biochimie BV), 30 g l−1 sucrose, 0.2 mM acetosyringone, pH 5.5); A3 (De Block et al., 1989) with 0.2 mM acetosyringone, pH 5.5; SIM (Alt-Mörbe et al., 1989), consisting of 20 g l−1 sucrose, 5.9 g l−1 Tri-sodium citrate, and 0.2 mM acetosyringone at pH 5.5. All the media tested were set at pH 5.5 to improve transformation by A. tumefaciens (Alt-Mörbe et al., 1989). Hypocotyl and cotyledon explants were cut as described above and transferred to dishes containing 10 ml of bacterial inoculum, placing cotyledons abaxial surface down into the inoculum. Twenty seedlings were inoculated in each 10 ml batch. After immersion for a total of 30 min the explants were transferred to co-culture plates [MS medium at 1/10 normal concentration, 30 g l−1 sucrose, 1 mg l−1 benzylaminopurine (BAP), 0.1 mg l−1 naphthalene acetic acid (NAA), 8 g l−1 agar, pH 5.5, covered with 1 ml MSO liquid medium without acetosyringone and a sterile filter paper], again placing cotyledons abaxial surface down. Co-culture plates were incubated for 2 d, after which the explants were transferred to regeneration medium. Two regeneration media, as well as several levels of kanamycin sulphate or paromomycin sulphate for the selection of transformed tissue, were tested for their effects on regeneration and transformation. Explants were transferred to medium A5 (Alt-Mörbe et al., 1989) solidified with 8 g l−1 agar or CR (modified from medium C of Shepard and Totten, 1977) as follows: MS major salts but with NH4NO3 replaced with NH4Cl (107 mg l−1), MS minor salts, Nitsch and Nitsch vitamins (Nitsch and Nitsch, 1969), all other addenda as in A5 (De Block et al., 1989) including 20 g l−1 sucrose, 1.0 mg l−1 BAP, 0.1 mg l−1 NAA, 0.5 g l−1 MES, 40 mg l−1 adenine sulphate, 0.5 g l−1 PVP, 8 g l−1 agar, pH 5.7; 10 mg l−1 AgNO3 was added to the medium after autoclaving. Media also contained the antibiotics carbenicillin (500 mg l−1) for unselected tissue or carbenicillin (500 mg l−1) and kanamycin sulphate (20, 40, 60, 80 mg l−1) or paromomycin sulphate (20, 30, 40 mg l−1) for the selection of transformed tissue. In one experiment, cotyledons were placed on A5 medium containing a matrix of BAP (0.5, 1.0, 2.0 mg l−1) and NAA (0.1, 0.5, 1.0, 1.5 mg l−1) concentrations to determine the optimum levels of these growth regulators.
After 3 weeks, explants were transferred to fresh selective medium; cotyledon pieces were placed on medium of the same composition, while hypocotyl explants that had already developed small shoots were transferred to medium A6 (De Block et al., 1989) solidified with 8 g l−1 agar+carbenicillin (250 mg l−1)+kanamycin sulphate (60 mg l−1). After a further 3 weeks cotyledon explants that had developed small shoots were transferred to A6 medium+agar+carbenicillin+kanamycin, and hypocotyl explants were transferred to A7 (De Block et al., 1989) solidified with 8 g l−1 agar+carbenicillin (250 mg l−1)+kanamycin sulphate. Three weeks later the cotyledon explants were transferred to A7+agar+carbenicillin+kanamycin. Groups of shoots from cotyledons or hypocotyls could be maintained on this medium in the presence of the selective agent; once individual shoots had grown enough to be cut off for rooting they were placed on A8 medium (De Block et al., 1989) solidified with 8 g l−1 agar+carbenicillin (100 mg l−1).
Seed production
Small shoots from tissue culture were used as scions for grafting onto 8–10-week-old Cleome gynandra stock plants grown in a growth chamber at 23 °C, with a 16 h photoperiod and light intensity of 350 μmol m−2 s−1. Grafting was carried out in the growth chamber and the stock plant was placed in a plastic bag to produce high humidity around the graft. Once the graft had established and the scion was producing new growth, the bag was removed slowly and the plant grown on for seed production.
Histochemical assay for β-glucuronidase
Tissue was assayed histochemically for β-glucuronidase activity (Jefferson et al., 1987) at various stages in the regeneration process. Pieces of callus, leaves or whole seedlings were immersed in 5-bromo-4-chloro-3-indolyl glucuronide (X-gluc), vacuum infiltrated, and incubated at 37 °C for 4–16 h, then destained in 70% ethanol. Leaf tissues destined for detailed microscopic examination of bundle sheath expression were treated further with 5% (w/v) NaOH solution at 37 °C for 4–20 h, washed several times in 70% ethanol, and clarified by immersing in a 75% solution of chloral hydrate for 20–24 h prior to examining microscopically. To make the chloral hydrate solution, 80 g chloral hydrate was stirred into 30 ml H2O to give a saturated solution; to this was added 6 ml H2O and 4 ml 10% glycerol.
Results and discussion
Sterilization of C. gynandra and incubation with A. tumefaciens
It was found that collecting mature unopened seed pods of C. gynandra, and sprinkling seeds from these onto moistened sterile filter paper prior to the transfer of germinated seedlings to agar plates led to very low levels of contamination and maximized the regeneration potential. Rates of seed germination of 80–90% were regularly obtained from fully-formed mature pods. Other more common methods of seed sterilization were problematic. For example, seed sterilization with ethanol and sodium hypochlorite reduced germination to 0–5% of controls. Microwaving of seed has reportedly been successful for tobacco and Arabidopsis (Franco, 1993) and so this was tested with seed of C. gynandra. Whilst microwaving for up to 10 min did not reduce the percentage of germination, it did not prevent low-level fungal contamination. Furthermore, explants derived from seeds that had been microwaved for longer than 5 min showed little or no callus development and regeneration during the following stages of tissue culture.
The composition of media used to suspend A. tumefaciens prior to, and during, incubation with plant tissue can alter transformation efficiency (Alt-Mörbe et al., 1989; Godwin et al., 1991; Humara et al., 1999). The influence of three separate liquid suspension media (MSO, A3, and SIM) on the transfer of the uidA gene encoding β-glucuronidase (GUS) into C. gynandra was therefore assessed. Both A. tumefaciens LBA4404 and EHA105 containing the binary plasmids SLJ1006 and pVDH (Jones et al., 1992) with uidA under the control of the CaMV 35S promoter was used for this purpose. β-glucuronidase assays carried out on inoculated explants 8 d or 14 d after transfer to selective or non-selective regeneration medium showed more transformed tissue in those explants treated with LBA4404 compared with EHA105, therefore further experiments were conducted with LBA4404 as it appeared to give the best chance for the regeneration of transformed shoots. LBA4404 continues to be used for Brassica transformation (Chen et al., 2008). A greater number of areas with GUS activity were found when explants were incubated with LBA4404 in SIM compared to either MSO or A3 (Table 2), and transformed areas were larger when SIM was used (Fig. 1). Future experiments were therefore conducted with SIM medium. It was discovered that subsequent explant growth was poor if tissue was left submerged in A. tumefaciens SIM inoculum for too long and so incubation was limited to 30 min.
Table 2.
Influence of Agrobacterium suspension media on pCaMV35S::uidA::Tnos transfer from LBA4404 containing SLJ1006 (Jones et al., 1992) into hypocotyls and cotyledons of C. gynandra
| Inoculum medium | MSO | A3 | SIM | ||||
| Regeneration medium | A5+C | A5+CK | A5+C | A5+CK | A5+C | A5+CK | |
| Hypocotyl | A | 20% (20) | 24% (46) | 15% (20) | 27% (44) | 90% (20) | 86% (42) |
| B | 10% (22) | 2% (46) | 5% (20) | 2% (46) | 40% (20) | 50% (46) | |
| Cotyledon | A | 43% (21) | 81% (31) | 48% (21) | 53% (38) | 100% (17) | 100% (26) |
| B | 47% (15) | 100% (16) | 60% (15) | 100% (16) | 100% (15) | 100% (6) | |
Two independent experiments (A and B) are reported in which three media were tested: MSO, A3, and SIM. The regeneration and selection medium was A5 and contained either 500 mg l-1 carbenicillin (A5+C) or 500 mg l−1 carbenicillin and 20 mg l−1 kanamycin (A5+CK). Details of media are reported in Materials and methods. Data are reported as the percentage of β-glucuronidase positive explants 8 d after incubation, with the total number of explants assessed in parentheses.
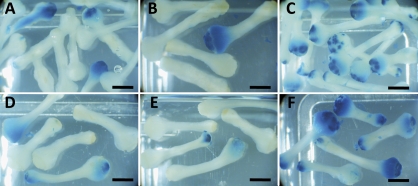
Fig. 1.
Hypocotyl explants of C. gynandra assayed for β-glucuronidase activity 8 d after transfer to regeneration medium A5 with (A–C) or without (D–F) 20 mg l−1 kanamycin. Hypocotyls were initially inoculated with LBA4404 containing the construct SLJ1006 in MSO (A, D), A3 (B, E) or SIM (C, F). Scale bars=2 mm.
Regeneration and selection of transformed C. gynandra
Regeneration of callus and then shoots from explants can vary between species, and is strongly influenced by factors such as the tissue used (van Wordragen and Dons, 1992; Akasaka-Kennedy et al., 2005). The production of callus from both hypocotyls and cotyledons after incubation with A. tumefaciens was therefore assessed. After transfer to regeneration medium, hypocotyl explants became swollen at both ends, and tiny calli or shoot initials appeared at one or both ends as early as 10 d. These appeared to be produced from the cut ends of the vascular tissue as has been reported for Brassica napus (Akasaka-Kennedy et al., 2005). By 4 weeks, well-formed shoots were visible (Fig. 2A) and these developed to produce clumps of shoots (Fig. 2B). Cotyledon explants developed more slowly and callus formation usually started after approximately 3 weeks following transfer to regeneration medium, with a few small shoots appearing after 4 weeks (Fig. 2C). Well-developed clumps were evident 8 weeks after incubation with A. tumefaciens (Fig. 2D).
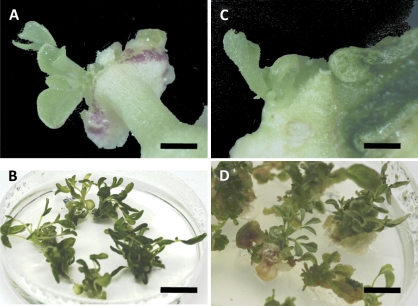
Fig. 2.
Regeneration of hypocotyl and cotyledon explants of C. gynandra. Hypocotyl explants after 4 weeks (A) and 8 weeks (B) on regeneration medium CR. Cotyledon explants after 4 weeks (C) and 8 weeks (D) on regeneration medium CR. Scale bars: (A, C) 2 mm, (B) 14 mm, (D) 12 mm.
To determine which medium gave the best regeneration efficiencies, the frequency of regeneration of cotyledon and hypocotyl explants was assessed 4 weeks after incubation with A. tumefaciens (Table 3). Hypocotyl explants showed the highest level (79%) of regeneration on A5 medium containing 10 mg l−1 AgNO3 and A5 without AgNO3 the lowest. AgNO3 has previously been used for Brassica transformation (De Block et al., 1989; Akasaka-Kennedy et al., 2005). After 4 weeks, cotyledon explants showed callusing and incipient shoot formation frequency of 52% on A5 plus 20 mg l−1 AgNO3 and 44% on medium Z. Thus, hypocotyls gave the most efficient regeneration, and addition of AgNO3 to the regeneration medium enhanced regeneration particularly in hypocotyl explants, but addition of more than 10 mg l−1 did not appear to be beneficial (Table 3). The choice of these media was informed by previous work. For example, Z has previously been used for tissue culture and propagation of C. gynandra (Yan You, 1995) and, because of the phylogenetic proximity of the Cleomaceae and Brassicaceae (Shepard and Totten, 1977; De Block et al., 1989), media previously used for Brassica transformation and regeneration were also tested.
Table 3.
Regeneration efficiencies from hypocotyl and cotyledon explants of C. gynandra after 4 weeks on regeneration medium A5 (De Block et al., 1989) or Z (Yan You, 1995) Regeneration was determined on four media with varying amounts of AgNO3, and data are presented as the percentage of explants with callus or shoots and the total number of explants assessed in parentheses. For details of media constituents see Materials and methods.
| A5 | A5+10 mg 1−1 AgNO3 | A5+ 20 mg 1−1 20 mg−1 | Z | |
| Hypocotyl | 31% (167) | 79% (67) | 71% (56) | 39% (137) |
| Cotyledon | 52% (90) | 44% (337) |
Following inoculation with A. tumefaciens LBA4404 harbouring the binary plasmid SLJ1006, hypocotyl and cotyledon explants were placed on regeneration medium with the addition of carbenicillin and kanamycin sulphate. Hypocotyl and cotyledon explants were tested for regeneration on medium A5 (De Block et al., 1989) and CR (modified from medium C; Shepard and Totten, 1977) and four levels of kanamycin sulphate. Regeneration from hypocotyls or cotyledons was assessed after 4 weeks (Table 4). The addition of antibiotics to plant regeneration media, which prevent the growth of A. tumefaciens, has been shown to affect the regeneration process (De Block et al., 1989; Lin et al., 1995; Nauerby et al., 1997). Addition of carbenicillin alone to A5 or CR led to higher regeneration levels from C. gynandra explants (Table 4) compared with the same medium lacking carbenicillin (Table 3). The presence of kanamycin in the medium reduced the levels of regeneration from both explant types but was not sufficient to prevent regeneration altogether. Medium CR was superior in terms of explant appearance and quantity of regenerable tissue or shoots and was therefore adopted for future experiments. Kanamycin sulphate at 60 mg l−1 was chosen as the best concentration to use because, in addition to the selection of GUS positive explants, tissue was healthy. Both hypocotyl and cotyledon explants were also cultured on medium containing paromomycin sulphate as the selective agent at 20, 30 and 40 mg l−1 and although regeneration frequencies were similar to those on kanamycin sulphate (data not shown), the tissue appeared vitreous and this approach was discontinued.
Table 4.
Regeneration from hypocotyl and cotyledon explants of C. gynandra after 4 weeks on regeneration medium containing carbenicillin and kanamycin sulphate after inoculation with LBA4404 containing SLJ1006 Material was selected on carbenicillin alone (C), or carbenicillin and kanamycin (CK) with kanamycin concentrations of 20, 40, 60, and 80 mg l−1. Data are shown as the percentage of explants with shoots, and in parentheses the total number of explants assessed.
| C | CK-20 | CK-40 | CK-60 | CK-80 | |
| Hypocotyls on A5 | 89% (160) | 12% (107) | 28% (167) | 43% (156) | 40% (116) |
| Hypocotyls on CR | 100% (43) | – | 42% (116) | 59% (114) | 60% (118) |
| Cotyledons on A5 | 100% (25) | 42% (50) | 43% (112) | 43% (116) | 45% (77) |
| Cotyledons on CR | 100% (11) | – | 54% (76) | 56% (75) | 46% (74) |
The efficiency of shoot regeneration after transformation can be modified by the relative amounts of benzylaminopurine (BAP) and naphthalene acetic acid (NAA). To investigate the best concentrations of these compounds for C. gynandra, a matrix of cotyledon and hypocotyl explants was set up on BAP at 0.5, 1.0, and 2.0 mg l−1 and NAA at 0.1, 0.5, 1.0, and 1.5 mg l−1 (Table 5). Hypocotyl explants seemed less responsive to this matrix of BAP and NAA than cotyledons. Although there was a relatively broad range of BAP and NAA concentrations that have reasonable regeneration efficiencies (Table 5), when the quality of the tissue was also assessed it was decided that combining 1 or 2 mg l−1 BAP with 0.1 or 0.5 mg l−1 NAA gave the best results. Therefore, a medium composition of 1.0 mg l−1 BAP + 0.1 mg l−1 NAA was adopted for future experiments.
Table 5.
Callus production from hypocotyls and cotyledons on a matrix of benzylaminopurine (BAP) and naphthalene acetic acid (NAA) Explants were scored for callus production after 5 weeks growth on regeneration medium A5 with 500 mg l−1 carbenicillin and 60 mg l−1 kanamycin sulphate. The effect of varying concentrations of BAP and NAA on callus production was assessed. Data are presented from two experiments as the percentage of explants that produced callus, and the total number of calli assessed in each experiment are in parentheses.
| Hypocotyls | BAP 0.5 mg−1 | BAP 1.0 mg l−1 | BAP 2.0 mg l−1 |
| NAA 0.1 mg l−1 | 53% (40) | 40% (40) | 28% (40) |
| 45% (40) | 40% (40) | 73% (40) | |
| NAA 0.5 mg l−1 | 28% (40) | 30% (40) | 40% (40) |
| 18% (40) | 35% (40) | 63% (40) | |
| NAA 1.0 mg l−1 | 15% (40) | 20% (40) | 28% (40) |
| 10% (40) | 25% (40) | 43% (40) | |
| NAA 1.5 mg l−1 | 5% (40) | 25% (40) | 15% (40) |
| 3% (40) | 50% (40) | 18% (40) | |
| Cotyledons | BAP 0.5 mg−1 | BAP 1.0 mg−1 | BAP 2.0 mg l−1 |
| NAA 0.1 mg 1−1 | 8% (24) | 15% (13) | 28% (25) |
| 31% (19) | 53% (19) | 89% (19) | |
| NAA 0.5 mg 1−1 | 0% (13) | 84% (19) | 15% (13) |
| 74% (19) | 20% (20) | 20% (39) | |
| NAA 1.0 mg 1−1 | 47% (19) | 68% (19) | 31% (19) |
| NAA 1.5 mg 1−1 | 12% (8) | 20% (10) | 16% (19) |
| 10% (20) | 5% (20) | 0% (6) |
Transformed shoots could only be rooted in the presence of kanamycin at an overall frequency of 0.4%. Different combinations of media, growth regulators, omission of antibiotics, addition of charcoal, use of vermiculite (De Block et al., 1989) all had little or no effect. For example, medium A8 (De Block et al., 1989) was tested with reduced levels of organic additions, different levels of IAA or IBA, the addition of activated charcoal, and the removal of kanamycin from the medium. In addition, incorporating a rooting medium previously used for Brassica (Bidney et al., 1983) with IBA or IAA, plus or minus activated charcoal, or kanamycin was also tested. Small shoots were also transferred to vermiculite with rooting media as in De Block et al. (1989). Overall, no consistent effects on rooting were seen. Consequently, shoots were grafted onto 8–10-week-old C. gynandra stock plants in order to produce seed of transgenic lines (Fig. 3A). Grafting was successful as long as the scion was kept in a humid atmosphere and held securely in place while the tissues made a connection. 80% of grafts were successful if healthy scions were used.
Fig. 3.
Stable transformation of C. gynandra. (A) Graft of a transformed shoot onto a wild-type scion allows seed set. (B–E) Regenerating hypocotyl and cotyledon explants after 4 weeks (B, D) or cotyledon explants at 8 weeks (C, E) of culture on regeneration medium containing 500 mg l−1 carbenicillin and 60 mg l−1 kanamycin. (B–D) Regenerants that show partial β-glucuronidase (GUS) staining. (E) Regenerants stained for GUS throughout the explant. Scale bars=2 mm.
Histochemical assays for GUS
To confirm that putative transformants were expressing transgenes, analysis of GUS activity was carried out on shoots resistant to kanamycin as well as T1 plants. Shoots from hypocotyl explants incubated with A. tumefaciens harbouring the pCaMV 35S::uidA::Tnos transgene developed quickly and some were visible as early as 10 d after the hypocotyl was placed on the regeneration medium. Assays of GUS activity after 4 weeks of culture, when quite large shoots were present, often showed areas of GUS at the base of shoots but not in the apical meristem (Fig. 3B). The same results were found for 4- and 8-week-old regenerating shoots from cotyledon explants (Fig. 3C, D). This was particularly frequent in those explants selected at a low level of kanamycin such as 20 mg l−1. However, it was possible to recover plants that stained for GUS in all tissues (Fig. 3E). Thus in order to optimize the production of transgenic shoots it was necessary to maximize the areas of GUS at the ends of the hypocotyl segments, so that fast-growing calli would be more likely to incorporate transgenic cells. Use of SIM medium as the inoculation medium appeared to give the most opportunity for the regeneration of transgenic shoots.
To confirm that the transformation procedure could be used to investigate mechanisms underlying the patterns of gene expression associated with C4 photosynthesis, GUS accumulation in lines of C. gynandra containing the pCaMV35S::uidA::Tnos construct was compared to lines with uidA translationally fused to a C. gynandra RbcS gene. In the latter lines, we would expect GUS to be restricted to BS cells (Marshall et al., 2007). Imaging of T1 seedlings of plants expressing pCaMV35S::uidA::Tnos showed that GUS was present in leaves and roots (Fig. 4A). Bright-field microscopy of leaves showed that GUS was present in both M and BS cells (Fig. 4B), consistent with the constitutive expression associated with the CaMV35S promoter. By contrast, when uidA was translationally fused to a CgRbcS gene, it accumulated in a strictly BS specific manner (Fig. 4C).
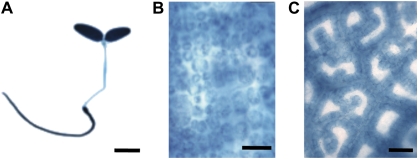
Fig. 4.
Constitutive and bundle sheath specific accumulation of β-glucuronidase (GUS) in C. gynandra plants. (A) Seedling expressing uidA under the control of the CaMV 35S promoter. (B) Mature leaf expressing uidA under the control of the CaMV 35S promoter, GUS is visible in mesophyll cells. (C) Mature leaf expressing uidA translationally fused to a C. gynandra RbcS gene, GUS is restricted to bundle sheath cells. Scale bars: (A) 5 mm, (B) 50 μm, (C) 100 μm.
Overall, suspending the inoculum in SIM medium and regenerating hypocotyls and cotyledons on CR medium, followed by media A5–A8 as detailed in De Block et al. (1989), led to a transformation efficiency (the number of independent transgenic shoots produced relative to the total number of explants that were cultured) of 14%. This compares reasonably with transformation efficiencies in the C3 species oilseed rape (De Block et al., 1989) as well as the NADP-ME type C4 plants F. bidentis (Chitty et al., 1999). Although the time taken to generate transgenic shoots of C. gynandra, and F. bidentis appear similar (this study; Chitty et al., 1999), because the life cycle of C. gynandra is faster, analysis should be faster with Cleome. The approach described here should therefore facilitate analysis of NAD-ME-type C4 photosynthesis, and through comparative analysis with A. thaliana, the identification of genes that determine the cell biology and development associated with leaves of C4 dicotyledons.
Acknowledgments
We thank the Leverhulme Trust and Brooks Fund for financial support.
References
- Akasaka-Kennedy Y, Yoshida H, Takahata Y. Efficient plant regeneration from leaves of rapeseed (Brassica napus L.): the influence of AgNO3 and genotype. Plant Cell Reports. 2005;24:649–654. doi: 10.1007/s00299-005-0010-8. [DOI] [PubMed] [Google Scholar]
- Akyildiz M, Gowik U, Engelmann S, Koczor M, Streubel M, Westhoff P. Evolution and function of a cis-regulatory module for mesophyll-specific gene expression in the C4 dicot Flaveria trinervia. The Plant Cell. 2007;19:3391–3402. doi: 10.1105/tpc.107.053322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt-Mörbe J, Kühlmann H, Schröder J. Differences in induction of Ti plasmid virulence genes virG_and virD, and continued control of virD expression by four external factors. Molecular Plant–Microbe Interactions. 1989;2:301–308. [Google Scholar]
- An G, Ebert PR, Mitra A, Ha SB. Binary vectors. In: Gelvin SB, Schilperoort RA, Verma DPS, editors. Plant molecular biology manual. Dordrecht: Kluwer; 1988. pp. 1–19. [Google Scholar]
- Berry JO, Breiding DE, Klessig DF. Light-mediated control of translational initiation of ribulose-1,5-bisphosphate carboxylase in Amaranth cotyledons. The Plant Cell. 1990;2:795–803. doi: 10.1105/tpc.2.8.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JO, Carr JP, Klessig DF. Translational control of ribulose 1,5-bisphosphate carboxylase synthesis in Amaranth. Journal of Cellular Biochemistry. 1987;33:48–56. [Google Scholar]
- Berry JO, Niklou BJ, Carr JP, Klessig DF. Transcriptional and post-transcriptional regulation of ribulose 1,5-bisphosphate carboxylase gene expression in light and dark grown Amaranth cotyledons. Molecular and Cellular Biology. 1985;5:2238–2246. doi: 10.1128/mcb.5.9.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JO, Nikolau BJ, Carr JP, Klessig DF. Translational regulation of light-induced ribulose 1,5-bisphosphate carboxylase gene expression in Amaranth. Molecular and Cellular Biology. 1986;6:2347–2353. doi: 10.1128/mcb.6.7.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidney DL, Shepard JF, Kaleikau E. Regeneration of plants from mesophyll protoplasts of Brassica oleracea. Protoplasma. 1983;117:89–92. [Google Scholar]
- Bowes G, Salvucci ME. Hydrilla: inducible C4-type photosynthesis without Kranz anatomy. In: Sybesma C, editor. Advances in photosynthesis research. Vol. 3. The Hague: Martinus Nijhoff/Junk Publishers; 1984. pp. 829–832. [Google Scholar]
- Brown HA. Agronomic implications of C4 photosynthesis. In: Sage RF, Monson RK, editors. C4 plant biology. San Diego: Academic Press; 1999. pp. 473–507. [Google Scholar]
- Brown NJ, Parsley K, Hibberd JM. The future of C4 research: maize, Flaveria or Cleome? Trends in Plant Science. 2005;10:215–221. doi: 10.1016/j.tplants.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Chen LFO, Lin CH, Kelkar SM, Chang YM, Shaw JF. Transgenic broccoli (Brassica oleracea var. italica) with antisense chlorophyllase (BoCLH1) delays postharvest yellowing. Plant Science. 2008;174:25–31. [Google Scholar]
- Chitty JA, Furbank RT, Marshall JS, Chen Z, Taylor WC. Genetic transformation of the C4 plant, Flaveria bidentis. The Plant Journal. 1999;6:949–956. [Google Scholar]
- Colletti KS, Tattersall EA, Pyke KA, Froelich JE, Stokes KD, Osteryoung KW. A homologue of the bacterial cell division site-determining factor MinD mediates placement of the chloroplast division apparatus. Current Biology. 2000;10:507–516. doi: 10.1016/s0960-9822(00)00466-8. [DOI] [PubMed] [Google Scholar]
- De Block M, De Brouwer D, Tenning P. Transformation of Brassica napus and Brassica oleracea using Agrobacterium tumefaciens and the expression of the bar and neo genes in transgenic plants. Plant Physiology. 1989;91:694–701. doi: 10.1104/pp.91.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AR. Seed sterilization. 1993 http://www.bio.net/bionet/mm/arab-gen. [Google Scholar]
- Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Experimental Cell Research. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Godwin I, Todd G, Ford-Lloyd B, Newbury HJ. The effects of acetosyringone and pH on Agrobacterium-mediated transformation vary according to plant species. Plant Cell Reports. 1991;9:671–675. doi: 10.1007/BF00235354. [DOI] [PubMed] [Google Scholar]
- Gowik U, Burscheidt J, Akyildiz M, Schlue U, Koczor M, Streubel M, Westhoff P. cis-regulatory elements for mesophyll-specific gene expression in the C4 plant Flaveria trinervia, the promoter of the C4 phospho enolpyruvate carboxylase gene. The Plant Cell. 2004;16:1077–1090. doi: 10.1105/tpc.019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Sytsma K, ltis H. Phylogeny of Capparaceae and Brassicaceae based on chloroplast sequence data. American Journal of Botany. 2002;89:1826–1842. doi: 10.3732/ajb.89.11.1826. [DOI] [PubMed] [Google Scholar]
- Hatch MD. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta. 1987;895:81–106. [Google Scholar]
- Hatch MD, Slack CR. A new enzyme for interconversion of pyruvate and phosphopyruvate and its role in C4 dicarboxylic acid pathway of photosynthesis. Biochemical Journal. 1968;106:141–146. doi: 10.1042/bj1060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd JM, Sheehy JE, Langdale JA. Using C4 photosynthesis to increase the yield of rice: rationale and feasibility. Current Opinion in Plant Biology. 2008;11:228–231. doi: 10.1016/j.pbi.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Humara JM, López M, Ordás RJ. Agrobacterium tumefaciens-mediated transformation of Pinus pinea L. cotyledons: an assessment of factors influencing the efficiency of uidA gene transfer. Plant Cell Reports. 1999;19:51–58. doi: 10.1007/s002990050709. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Saito H, Ohta S, Hiei Y, Komari T, Kumashiro T. High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nature Biotechnology. 1996;14:745–750. doi: 10.1038/nbt0696-745. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JDG, Shlumukov L, Carland F, English J, Scofield SR, Bishop GJ, Harrison K. Effective vectors for transformation, expression of heterologous genes, and assaying transposon excision in transgenic plants. Transgenic Research. 1992;1:285–297. doi: 10.1007/BF02525170. [DOI] [PubMed] [Google Scholar]
- Levy A, Erlanger M, Rosenthal M, Epel BL. A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. The Plant Journal. 2007;49:669–682. doi: 10.1111/j.1365-313X.2006.02986.x. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Assad-Garcia N, Kuo J. Plant hormone effect of antibiotics on the transformation efficiency of plant tissues by Agrobacterium tumefaciens cells. Plant Science. 1995;109:171–177. [Google Scholar]
- Magnin NC, Cooley BA, Reiskind JB, Bowes G. Regulation and localization of key enzymes during the induction of Kranz-less, C4-type photosynthesis in Hydrilla verticillata. Plant Physiology. 1997;115:1681–1689. doi: 10.1104/pp.115.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple J, Fujiwara MT, Kitahata N, Lawson T, Baker NR, Yoshida S, Moller SG. GIANT CHLOROPLAST 1 is essential for correct plastid division in Arabidopsis. Current Biology. 2004;14:776–781. doi: 10.1016/j.cub.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Marshall DM, Muhaidat R, Brown NJ, Liu Z, Stanley S, Griffiths HG, Sage RF, Hibberd JM. Cleome, a genus closely related to Arabidopsis, contains species spanning a developmental progression from C3to C4 photosynthesis. The Plant Journal. 2007;51:886–896. doi: 10.1111/j.1365-313X.2007.03188.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Furbank RT, Fukayama H, Miyao M. Molecular engineering of C4 photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:297–314. doi: 10.1146/annurev.arplant.52.1.297. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Numazawa T. Cis-acting elements in the pyruvate, orthophosphate dikinase gene from maize. Molecular and General Genetics. 1991;228:143–152. doi: 10.1007/BF00282459. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. Journal of Bioscience and Bioengineering. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- Nauerby B, Billing K, Wyndaele R. Influence of the antibiotic timentin on plant regeneration compared to carbenicillin and cefotaxime in concentrations suitable for elimination of Agrobacterium tumefaciens. Plant Science. 1997;123:169–177. [Google Scholar]
- Nitsch JP, Nitsch C. Haploid plants from pollen grains. Science. 1969;169:85–87. doi: 10.1126/science.163.3862.85. [DOI] [PubMed] [Google Scholar]
- Oikawa K, Kasahara M, Kiyosue T, Kagawa T, Suetsugu N, Takahashi F, Kanegae T, Niwa Y, Kadota A, Wada M. CHLOROPLAST UNUSUAL POSITIONING1 Is essential for proper chloroplast positioning. The Plant Cell. 2003;15:2805–2815. doi: 10.1105/tpc.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell M, Mabrouk YM, Bogorad L. Red/far red and blue light-responsive regions of maize rbcS-m3 are active in bundle sheath and mesophyll cells, respectively. Proceedings of the National Academy of Sciences, USA. 1995;92:11504–11508. doi: 10.1073/pnas.92.25.11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinfelder JR, Kraepiel AML, Morel FMM. Unicellular C4 photosynthesis in a marine diatom. Nature. 2000;407:996–999. doi: 10.1038/35039612. [DOI] [PubMed] [Google Scholar]
- Reiskind JB, Berg RH, Salvucci ME, Bowes G. Immunogold localization of primary carboxylases in leaves of aquatic and a C3–C4 intermediate species. Plant Science Letters. 1989;61:43–52. [Google Scholar]
- Sage RF. The evolution of C4 photosynthesis. New Phytologist. 2004;161:341–370. doi: 10.1111/j.1469-8137.2004.00974.x. [DOI] [PubMed] [Google Scholar]
- Shepard JF, Totten RE. Mesophyll cell protoplasts of potato. Isolation, proliferation, and plant regeneration. Plant Physiology. 1977;60:313–316. doi: 10.1104/pp.60.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surridge C. The rice squad. Nature. 2002;416:576–578. doi: 10.1038/416576a. [DOI] [PubMed] [Google Scholar]
- van Wordragen MF, Dons HJM. Agrobacterium tumefaciens-mediated transformation of recalcitrant crops. Plant Molecular Biology Reports. 1992;10:12–36. [Google Scholar]
- Viret J-F, Mabrouk Y, Bogorad L. Transcriptional photoregulation of cell-type preferred expression of maize rbcS-m3: 3′ and 5′ sequences are involved. Proceedings of the National Academy of Sciences, USA. 1994;91:8577–8581. doi: 10.1073/pnas.91.18.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitha S, McAndrew RS, Osteryoung KW. FtsZ ring formation at the chloroplast division site in plants. Journal of Cell Biology. 2001;153:111–120. doi: 10.1083/jcb.153.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Kiirats O, Artyusheva EG, Freitag H, Edwards GE. Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae) The Plant Journal. 2002;31:649–662. doi: 10.1046/j.1365-313x.2002.01385.x. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Kiirats O, Freitag H, Edwards GE. Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature. 2001;414:543–546. doi: 10.1038/35107073. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Chuong SDX, Ivanova AN, Barroca J, Craven LA, Edwards GE. Physiological, anatomical and biochemical characterization of photosynthetic types in genus Cleome (Cleomaceae) Functional Plant Biology. 2007;34:247–267. doi: 10.1071/FP06287. [DOI] [PubMed] [Google Scholar]
- Wingler A, Walker RP, Chen Z-H, Leegood RC. Phosphoenolpyruvate carboxykinase is involved in the decarboxylation of aspartate in the bundle sheath of maize. Plant Physiology. 1999;120:539–545. doi: 10.1104/pp.120.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan You W Tissue culture and propagation of Cleome gynandra. Plant Physiology Communications (China) 1995;5:359. [Google Scholar]