Abstract
It was previously shown that pearl millet genotypes carrying a terminal drought tolerance quantitative trait locus (QTL) had a lower transpiration rate (Tr; g cm−2 d−1) under well-watered conditions than sensitive lines. Here experiments were carried out to test whether this relates to leaf abscisic acid (ABA) and Tr concentration at high vapour pressure deficit (VPD), and whether that leads to transpiration efficiency (TE) differences. These traits were measured in tolerant/sensitive pearl millet genotypes, including near-isogenic lines introgressed with a terminal drought tolerance QTL (NIL-QTLs). Most genotypic differences were found under well-watered conditions. ABA levels under well-watered conditions were higher in tolerant genotypes, including NIL-QTLs, than in sensitive genotypes, and ABA did not increase under water stress. Well-watered Tr was lower in tolerant than in sensitive genotypes at all VPD levels. Except for one line, Tr slowed down in tolerant lines above a breakpoint at 1.40–1.90 kPa, with the slope decreasing >50%, whereas sensitive lines showed no change in that Tr response across the whole VPD range. It is concluded that two water-saving (avoidance) mechanisms may operate under well-watered conditions in tolerant pearl millet: (i) a low Tr even at low VPD conditions, which may relate to leaf ABA; and (ii) a sensitivity to higher VPD that further restricts Tr, which suggests the involvement of hydraulic signals. Both traits, which did not lead to TE differences, could contribute to absolute water saving seen in part due to dry weight increase differences. This water saved would become critical for grain filling and deserves consideration in the breeding of terminal drought-tolerant lines.
Keywords: ABA, pearl millet, terminal drought stress, transpiration rate, transpiration efficiency, vapour pressure deficit
Introduction
Water deficit is one of the major factors limiting global crop production. Much research on drought tolerance has focused on the characterization of plants during water deficit (Farquhar and Richards, 1984; Howell, 2001; Condon et al., 2004; Blum, 2005). In pearl millet, stomata play an important role in minimizing crop water use in pre-anthesis water deficit (Winkel et al., 2001). However, controlling leaf water losses when water is non-limiting for plant development may also be a suitable adaptation strategy. It was recently shown that pearl millet genotypes carrying a terminal drought tolerance quantitative trait locus (QTL) have a lower rate of water loss per unit leaf area under well-watered (WW) conditions [transpiraion rate (Tr), in g cm−2 d−1] (Kholová et al., 2008, 2009). This water-saving mechanism operating under non-stressed conditions could leave water available in the soil profile for grain filling and could be beneficial for terminal stress conditions. Yet, how certain pearl millet plants achieve low Tr is still unclear. This water-saving mechanism from a lower leaf conductance could relate to high leaf abscisic acid (ABA) differences or to lower Tr at high vapour pressure deficit (VPD) levels, and these both may be related (Thompson et al., 2007). Lower leaf conductance could then lead to differences in transpiration efficiency (TE, g biomass kg−1 water transpired) (Condon et al., 2004).
The ABA content in the leaves could be involved in lowering the Tr differences. Indeed, ABA is known to be part of a complex mechanism of stomata regulation. Large inter/intraspecific variation in ABA levels has been reported (Conti et al., 1994; Mugo, 1999; Chandrasekar et al., 2000; Li and Wang, 2003; Yin et al., 2005; Zhang et al., 2005). In wheat and some woody plants, a higher ABA level was correlated with drought tolerance (Chandrasekar et al., 2000; Li and Wang, 2003; Yin et al., 2005; Zhang et al., 2005), although no such correlation was reported in maize and sunflower (Conti et al., 1994; Mugo, 1999; Cellier et al., 1998, 2000). Therefore, the ABA–tolerance link is, as expected, highly crop and environment specific. Here, leaf ABA contents in pearl millet genotypes differing in terminal drought tolerance and Tr are compared.
The Tr previously found to discriminate drought-tolerant and drought-sensitive pearl millet genotypes (Kholová et al., 2009) ‘integrates’ the regulation of stomata over a substantial length of time, i.e. 1–2 d. However, it may not pinpoint transient genotypic differences in stomata regulation occurring during the course of the day. As such, the Tr assessment does not indicate whether Tr differences between genotypes are constant during the day or whether transient changes in environmental conditions lead to transient larger Tr differences between genotypes. One such possibility are the changes in VPD. In areas where pearl millet is grown, a high VPD during the hotter hours of the day leads to a closure of stomata even under WW conditions (Black and Squire, 1979; Squire et al., 1979; Henson and Mahalakshmi, 1985). However, no attention was paid to possible genetic variation in this response to VPD. This phenomenon has only recently been reported in soybean (Sinclair et al., 2007) and it was assessed here in pearl millet genotypes that differ in Tr.
Several studies have reported a relationship between an enhanced ABA level and TE in gene-manipulated plants (Ashwart et al., 2005, Thompson et al., 2007). Zhang et al. (2005) subjected wheat genotypes originating from ‘wet’ and ‘dry’ regions to drought stress and found that plants with ‘dry land origin’ had higher ABA levels and subsequently better water use efficiency. In addition, modelling data show that a limited Tr at high VPD would indeed contribute to saving water in the soil profile and might increase the TE (Sinclair et al., 2005). So, putative differences in the Tr response to VPD and in leaf ABA could lead to differences in TE.
Two objectives of this study were to investigate leaf ABA content and Tr under different VPDs in pearl millet lines differing in Tr under WW conditions. A third objective was then to assess whether putative differences in leaf ABA and Tr at high VPD could lead to differences in TE. Whether water-saving mechanisms should be looked at from the angle of water productivity and/or absolute plant water use is discussed.
Materials and methods
Plant material
Parental genotypes
Two pairs of inbred pearl millet [Pennisetum glaucum (L.) R. Br.] lines, the parents of mapping populations, differing in terminal drought tolerance [PRLT 2/89-33 (tolerant) and H 77/833-2 (sensitive), 863B-P2 (tolerant) and ICMB 841-P3 (sensitive)] were selected for the study. This contrast was based on previous experiments (Yadav et al., 2002; Serraj et al., 2005), where tolerance/sensitivity was assessed on testcross hybrids of these inbred parental lines. These hybrids were developed by crossing the PRLT 2/89-33 and H 77/833-2 inbred parental lines to a male-sterile line tester, seed parent 843A, whereas hybrids of 863B-P2 and ICMB 841-P3 were developed by pollinating them with H 77/833-2 (Stegmeier et al., 1998). Tolerance of these hybrids was based on grain and stover yield maintenance under managed terminal drought stress in several years of field trials, and on panicle harvest index (PNHI), an index that proxies for the success of spikelet fertility and the degree of grain filling (Bidinger et al., 1987). The drought-sensitive parental genotypes (H 77/833-2 and ICMB 841-P3) are parents of F1 hybrid cultivars that were commercially important in northwestern India. The drought-tolerant parental genotype PRLT 2/89-33 was derived from the ICRISAT Bold Seeded Early Composite, which is an elite breeding population based largely on Iniadi landrace germplasm from West Africa (Andrews and Anand Kumar, 1996), while 863B-P2 was bred by inbreeding within a sample of this landrace (Rai et al., 2008). This Iniadi germplasm is generally known for better grain filling under terminal drought stress conditions.
Near-isogenic lines (NIL-QTLs)
Using the two crosses described above, a major QTL for terminal drought tolerance was identified on pearl millet linkage group 2 (Yadav et al., 2002; Bidinger et al., 2007). To develop the QTL introgression lines in the background of sensitive parent H 77/833-2 (recurrent parent), it was crossed to the drought-tolerant donor parent PRLT 2/89-33. The resulting F1 was backcrossed to recurrent parent H 77/833-2 for four generations. At each backcross, the presence or absence of the terminal drought tolerance QTL was determined using flanking RFLP (restriction fragment length polymorphism) markers on pearl millet linkage group 2. At the end of fourth generation, two steps of selfing and marker-assisted selection using flanking simple sequence repeat (SSR) markers Xpsmp2059, Xpsmp2066, and Xpsmp2237 were performed to generate a set of NILs (ICMR 1029, ICMR 1031, ICMR 2041, ICMR 2042, and ICMR 2044) transferred with a drought tolerance QTL (hereafter referred to as NIL-QTLs). Testcross hybrids were produced for each of these lines by using them as pollinator lines on the same male-sterile line 843A. Hybrids involving ICMR 01029, ICMR 01031, and ICMR 02041 were previously found to be superior to the testcross hybrid developed with the sensitive parent H 77/833-2 for their yield maintenance under terminal drought in the field; hybrids of ICMR 02042 had a yield response in the field that was more like the testcross hybrid from the drought-sensitive parent H 77/833-2; hybrids of ICMR 02044 had intermediate yield response (Serraj et al., 2005).
Plant growth and exposure to drought (drydown)
Description of experiments
Leaf ABA, the transpiration response to different VPD conditions, and TE were measured in seven experiments conducted during either the vegetative stage (25 days after sowing prior to panicle emergence) in Experiments 1, 3, 5, 6, and 7, or the reproductive stage [40 days after sowing (DAS) when panicles were fully emerged] in Experiments 2 and 4 (Table 1). Leaf ABA was analysed in water-stressed (WS) and WW plants in Experiments 1, 2, and 5. TE was assessed in WS and WW plants in Experiments 3, 4, and 6. The transpiration response to VPD was tested in WW plants in Experiments 3, 6, and 7. Parental lines were tested in Experiments 1–4, while parental lines plus NIL-QTLs were tested in Experiments 5–7 (Table 1). Plants were grown in pots with Alfisol mixed with sand and manure (5:3:1) (5/9 kg pot−1 for the vegetative/reproductive stage) with one plant per pot in the glasshouse under optimal conditions (day/night temperature 35/25 °C, relative humidity oscillated between 50% and 80% during the day and the resulting VPD ranged between 2.81 kPa and 0.63 kPa).
Table 1.
Description of the experiments, treatments used, material tested, stage of evaluation, measurement carried out, and date of sowing
| Experiment | Treatment | Additional sets | Plant material | Growth stage | Trait measured | Date |
| 1 | WW–WS | Parents | Vegetative | ABA | February 2007 | |
| 2 | WW–WS | Parents | Reproductive | ABA | February 2007 | |
| 3 | WW–WS | Pre-drydown set | Parents | Vegetative | TE | |
| Tr-VPD | May 2007 | |||||
| 4 | WW–WS | Pre-drydown set | Parents | Reproductive | TE | May 2007 |
| 5 | WW–WS | Parents | Vegetative | ABA | July 2007 | |
| ICMR 1029 | ||||||
| ICMR 1031 | ||||||
| ICMR 1041 | ||||||
| 6 | WW–WS | Pre-drydown set | Parents | Vegetative | TE | July 2007 |
| ICMR 1029 | Tr-VPD | |||||
| ICMR 1031 | ||||||
| ICMR 1041 | ||||||
| 7 | WW | Parents | Vegetative | Tr-VPD | February 2009 | |
| ICMR 1029 | ||||||
| ICMR 1031 | ||||||
| ICMR 2042 | ||||||
| ICMR 2044 |
Protocol for water stress imposition (drydown)
Eighteen pots of each genotype were grown under WW conditions until the time of imposing the water treatment (25 or 40 DAS depending on the experiment). Six pots of each genotype were harvested at the time of treatment imposition for biomass assessment (pre-drydown biomass). The remaining 12 pots of each genotype were saturated with water and allowed to drain overnight. The following morning, the pots were bagged with a plastic bag wrapped around the stem and pots were subsequently weighed. Pots were weighed thereafter every day in the morning. Half of the plants were maintained under WW conditions by daily re-watering up to 80% field capacity, by bringing the pot weight to that level [100/200 g (vegetative/reproductive stage) below the saturated weight] every day. The other half of the plants were gradually exposed to WS by partially compensating water loss from transpiration, i.e. plants were allowed to lose no more than 70/100 g each day at the vegetative/reproductive stage (see the experimental design below). Therefore, any transpiration in excess of 70/100 g was added back to the pots, as previously described (Vadez and Sinclair, 2001). The experiment was terminated when, for a given genotype, the transpiration of WS plants was <10% of that of WW plants. The duration of the experiment was ∼16–20 d, short enough to ignore the fresh weight increase in the pot weight. Plants were then harvested and leaf area and the dry weights of their parts were measured. After harvest, the fraction of transpirable soil water (FTSW) for each day of the experiment was calculated. The FTSW values that represented the portion of remaining transpirable soil water were used as the indicator of stress, so that experiments could be rigorously compared. FTSW of day n was calculated as:
The component ‘initial pot weight–final pot weight’ did not vary between genotypes (all extracted similar quantities of water from the pots). Since all plants transpired >70/100 g water d−1 (vegetative/reproductive stage), all the genotypes were at a similar FTSW throughout the experimental period and then exposed to similar stress intensities, at least from the viewpoint of the soil water content. The experimental design for Experiments 1–6 was a completely randomized design with water treatment as the main factor (WW and WS) and genotypes as subfactor in six replications.
Transpiration efficiency estimation
TE was measured in WW and WS plants of Experiments 3, 4, and 6, and was calculated as the production of biomass per amount of water transpired during the drydown as:
Water transpired was the sum of daily transpiration measured in the drydown, assessed by regular weighing of pots and recording of water added. The final harvested biomass was that of WW and WS at the end of the drydown.
ABA assessment
Free ABA was estimated in the last fully expanded leaf of every plant (WW and WS), using two replicated samples per plant in Experiments 1 and 2 and one sample per plant in Experiment 5. The first sampling was at the beginning of the drydown when the soil of WS plants was still wet (FTSW=0.75/0.9 in Experiments 1 and 2) and the second when FTSW had reached 0.25/0.30. In Experiment 5, based on the results from Experiments 1 and 2, samples were collected only once when the FTSW of WS plants had reached 0.25 The ethyl acetate fractionation technique of Ryu and Li (1994) was used for sample preparation; 150 mg of deep-frozen tissue, collected as a small strip from the middle portion of the leaf, avoiding the mid-rib, was ground with 2 ml of 80% methanol containing butylated hydroxy toluene (0.001%, w/v). The extract was mechanically shaken for 1 h at 4 °C, briefly centrifuged (13 000 g), and passed through a Sep-Pak column (C-18 cartridge). Clear extract was pressure-evaporated, the residue dissolved in water, and ethyl acetate was added. This mixture was partitioned into aqueous and organic phases to separate free ABA from inert ABA conjugates. The ethyl acetate fraction was collected, evaporated, and the residue dissolved in 0.5 ml of sample buffer. ABA was assayed by competitive enzyme-linked immunosorbent assay (cELISA) according to Weiler (1982) with modifications. Polyclonal antibodies were raised against antigen –(±) cis-trans ABA (Sigma Aldrich) previously conjugated through its C1 carboxyl site to a carrier protein bovine serum albumin (BSA). Cross-reaction of polyclonal antibodies with BSA to avoid their non-specific reaction was carried out before its use for ELISA. After coating ELISA plates with antigen, plates were loaded with 1% milk powder to minimize non-specific binding. The antigen coating dilution (300 ng ml−1), primary antibody dilution (1:50 000), and alkaline phosphatase-linked secondary antibody dilution (1:8000) were chosen so that under assay conditions, absorbance A405=1.0 for the blank was obtained after ∼1 h incubation with p-NPP substrate (0.1% w/v). Simultaneously with the samples, reference standards (1000 to 3.9 ng ABA ml−1) were loaded in every ELISA plate. Several spiking experiments to detect recovery of known ABA concentrations were conducted to validate this technique (not shown).
Transpiration rate under different VPD regimes
This was assessed on WW plants, either under natural variations in VPD during the course of the day in the glasshouse or outdoors, or by setting VPD conditions at constant light intensities in controlled-environment growth chambers. The design for the transpiration response to VPD in Experiments 3, 6, and 7 was a completely randomized design with one factor only (WW), genotypes as subfactors, and six replications.
Response of Tr to natural conditions
The transpiration of WW plants of Experiment 3 was measured hourly over an entire day in the glasshouse 1 d prior to harvest. At harvest, the leaf area was measured and Tr expressed as water loss per unit of leaf area and time (g H2O cm−2 h−1). A similar assessment was made with WW plants from Experiment 7 using the NILs ICMR 01029, ICMR 01031, ICMR 02042, and ICMR 02044, and parental genotypes PRLT 2/89-33 and H 77/833-2. That experiment was conducted outdoors and the VPD data were recorded hourly.
Response of Tr to increasing VPD in growth chambers
Under constant light and VPD in the growth chamber, the transpiration of WW plants showed little variation during the course of the light period, except for the first 2 h when there was an ∼15% increase in the Tr (data not shown). This interval in the light period showing constant transpiration was used to assess the changes in Tr of WW plants caused by a ladder of increasing VPD regimes starting from 0.85 kPa up to 3.43 kPa.
This method was applied to six plants per genotype (‘pre-drydown’ set), grown under WW conditions and at the same time as Experiments 3 and 6. At 23 DAS, these plants were saturated with water, allowed to drain overnight, bagged around the stem, and transferred to a growth chamber for 1 d of acclimation. The day following acclimation, their transpiration response to VPD was assessed.
To analyse the data, a broken stick analysis was done using the split line regression of Genstat (9.0), which provides a breakpoint value where the slope of the fitted regression changes and values of the different slopes.
Statistical analysis
Analyses of variance (ANOVAs) were done with the statistical program package CoStat version 6.204 (CoHort Software, Monterey, CA, USA). One-way ANOVA was carried out to test for genotypic differences within treatment, and to compare the treatment effect for each genotype. Means were analysed using the Tukey–Kramer test and LSD (at P=0.05).
Results
Leaf ABA content
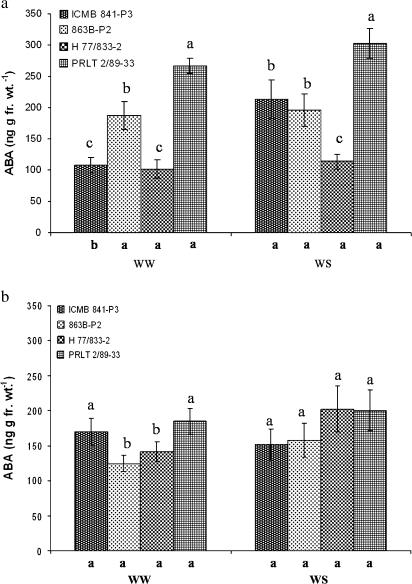
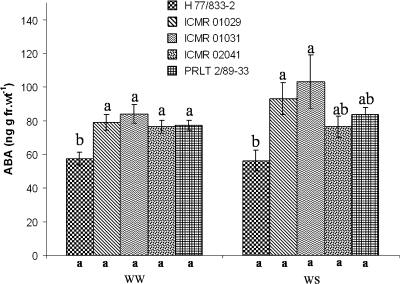
Across the two experiments, PRLT 2/89-33 (tolerant) generally had significantly higher leaf ABA than H 77/833-2 [Fig. 1a, b (P <0.1 under WS in Experiment 5; Fig. 2)], except under WS conditions during the reproductive stage in Experiment 1 (Fig. 1b). The ABA level of all three NIL-QTLs under WW conditions was significantly higher than in H 77/833-2 and similar to that of PRLT 2/89-33 (Fig. 2). Under WS conditions, the ABA content of drought-stressed plants did not discriminate NIL-QTLs from sensitive H 77/833-2 as well as it did under WW conditions, and ABA content was higher in ICMR 01029 and ICMR 01031 than in H 77/833-2.
Fig. 1.
ABA content of four pearl millet testcross hybrids [H 77/833-2, ICMB 841-P3 (sensitive), PRLT 2/89-33, 863B-P2 (tolerant)] grown in well-watered (WW) and water stress (WS) conditions and sampled at FTSW=0.25 at the vegetative stage (a) and at FTSW=0.30 at the reproductive stage (b). Experiments were conducted in January 2007 (Experiments 1 and 2). Values are the means (±SE) of six replicate plants per treatment and genotype. Genotypic means with the same letters above the bar within a treatment are not significantly different. Letters below the x-axis are for the genotypic comparison of means between treatments.
Fig. 2.
ABA content of two pearl millet testcross hybrids of parental lines [H 77/833-2 (sensitive), PRLT 2/89-33 (tolerant)] and the testcross hybrids of their NIL-QTLs (ICMR 01029, ICMR 01031, and ICMR 02041) grown in well-watered conditions and under drought and sampled at FTSW=0.25 in the vegetative stage (Experiment 5, July 2007). Values are the means (±SE) of six replicate plants per treatment and genotype. Genotypic means with the same letters above the bar within a treatment are not significantly different. Letters below the x-axis are for the genotypic comparison of means between treatments.
For the pair 863B-P2 (tolerant) and ICMB 841-P3 (susceptible), results were less consistent. Under WW conditions, 863B-P2 had significantly higher ABA than ICMB 841-P3 during the vegetative period, but a significantly lower ABA during the reproductive period. Under WS, differences were non-significant.
At both the vegetative and reproductive stages in Experiments 1 and 2, the drought treatment caused no significant increase in the ABA level in any of the genotypes except for ICMB 841-P3 at the vegetative stage (Fig. 1a, b). In Experiment 5, progressive exposure to water stress also did not cause any significant ABA increase in any of these genotypes.
Transpiration rate under different VPD conditions
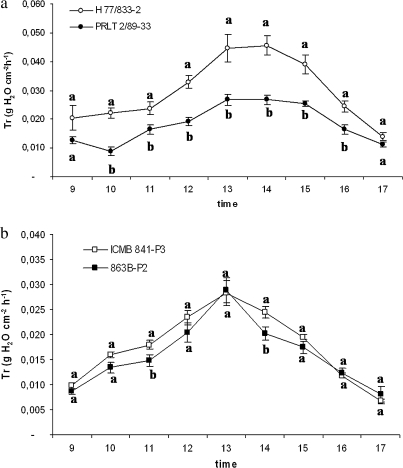
The transpiration of WW plants in Experiment 3 was measured under VPD conditions that varied between 0.63 kPa and 2.81 kPa. The Tr was higher in sensitive H 77/833-2 than in tolerant genotype PRLT 2/89-33 at all times of measurement except the initial and final measurements. In addition, these differences were largest between 12.15 h and 14.15 h, i.e. the time of the day with the highest VPD values (Fig. 3a). The other pair showed a similar trend, with 863B-P2 generally having a lower Tr than ICMB 841-P3, although differences were not always significant (Fig. 3b).
Fig. 3.
Transpiration rate (Tr, g cm−2 h−1) under well-watered conditions of two pearl millet testcross hybrids pairs: H 77/833-2 and PRLT 2/89-33 (a), and ICMB 841-P3 and 863B-P2 (b) [H 77/833-2, ICMB 841-P3 (sensitive), PRLT 2/89-33, 863B-P2 (tolerant)] over the course of a day. Plants were assessed at the vegetative stage (Experiment 3, May 2007). Each point expresses hourly Tr mean values. Values are the means (±SE) of six plants per treatment and genotype. Genotypic means with the same letters are not significantly different.
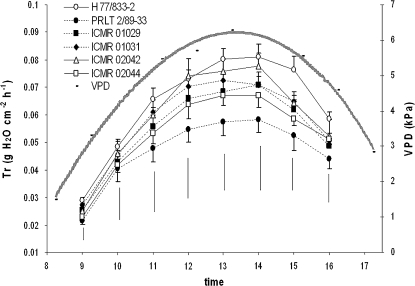
A similar transpiration response to the natural VPD cycle was tested outdoors (Experiment 7; Fig. 4). The daily pattern of Tr closely followed the daily pattern of VPD. Genotypic differences in Tr did not occur until the VPD was above ∼3 kPa. Hence, at 10.00 h, differences were non-significant. Above a VPD of 3 kPa, the increase in Tr was lower in PRLT than in H77 and the largest Tr differences were found at the time of highest VPD, between 11.00 h and 15.00 h, when the VPD was >4 kPa. The Tr curves as a function of time of all four NIL-QTLs were in between those of the parental genotypes, i.e. always below that of H 77/833-2 (Tr differences at each time point were significant at P <0.1). In particular, drought-tolerant NIL-QTLs ICMR 01029 and ICMR 01031 were closer to the pattern of PRLT 2/89-33. Drought-sensitive NIL-QTL ICMR 02042 showed a pattern of Tr closer to that of H 77/833-2.
Fig. 4.
Transpiration rate (Tr, g cm−2 h−1) under well-watered conditions and VPD of pearl millet testcross hybrids of parental lines: H 77/833-2 (drought sensitive) and PRLT 2/89-33 (drought tolerant) and the testcross hybrids of their four NILs (ICMR 01029, ICMR 01031, ICMR 02042, and ICMR 02044) over the course of a day. Plants were assessed at the vegetative stage (Experiment 7, February 2009). Values are the means (± 1/2 SE) of five plants per treatment and genotype. The bar at each measurement time indicates the LSD for genotypic means.
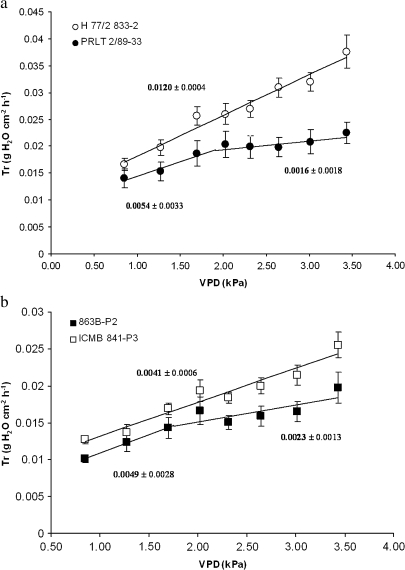
In the growth chamber in Experiment 3, the slope of the transpiration response to VPD was unchanged in H 77/833-2 (Fig. 5a) and ICMB 841-P3 (Fig. 5b) across the whole range of VPD. In contrast, this slope showed a breakpoint at 1.91 kPa in PRLT 2/89-33 and at 1.75 kPa in 863B-P2. For these two tolerant genotypes, the slope significantly decreased past their respective VPD breakpoints, indicating a slowdown in the transpiration response to VPD. Tr was also lower in PRLT 2/89-33 than in H77/833-2 even at VPD <2 kPa.
Fig. 5.
Transpiration rate (Tr, g cm−2 h−1) under well-watered conditions of four pearl millet testcross hybrids [H 77/833-2, ICMB 841-P3 (sensitive), PRLT 2/89-33, 863B-P2 (tolerant)] exposed to increasing VPD regimes. Each point expresses hourly Tr mean values. The experiment used plants at the vegetative stage (Experiment 3, May 2007). Values are the means (±SE) of six plants per treatment and genotype. Numbers above/below the regression curves are the slopes (±SE) of the Tr response. The breakpoint of the slope was 1.91±0.75 kPa for PRLT 2/89-33 (a) and 1.75±0.95 for 863B-P2 (b). The breakpoint of the slope was not significant for H 77/833-2 and ICMB 841-P3.
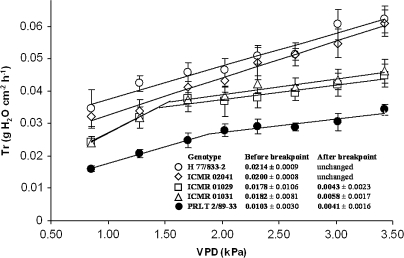
A similar assessment repeated with WW plants in Experiment 6 again showed the absence of a breakpoint in the transpiration response to VPD for H 77/833-2, whereas the Tr showed a breakdown in the response to VPD at 1.89 kPa in PRLT 2/89-33, and a significantly lower slope past this breakdown than before (Fig. 6). The NILs (ICMR 01029 and ICMR 01031) showed a breakdown in the Tr response similar to PRLT 2/89-33. One NIL showed no breakdown in the transpiration response (ICMR 02041). The reduction in the slope of the transpiration response to VPD above the breakpoint was in the order of 50–65%.
Fig. 6.
Transpiration rate (Tr, g cm−2 h−1) under well-watered conditions of two pearl millet testcross hybrids of parental lines H 77/833-2 (sensitive), PRLT 2/89-33 (tolerant) and the testcross hybrids of their drought-tolerant NIL-QTLs (ICMR 01029, ICMR 01031, and ICMR 02041) exposed to increasing VPD regimes. Each point expresses hourly Tr mean values of a particular genotype. Plants were tested at the vegetative stage (Experiment 6, July 2007). Values are the means (±SE) of six plants per treatment and genotype. The slope of the Tr response before and after putative breakpoints is indicated on the graph. The breakpoint of the slope was 1.45±0.40 kPa for ICMR 01029, 1.54±0.35 kPa for ICMR 01031, and 1.89±0.41 kPa for PRLT 2/89-33. The breakpoint of the slope was not significant for H 77/833-2 and ICMR 02041.
Transpiration efficiency
At the vegetative stage under WW conditions, TE was higher in H 77/833-2 than in PRLT 2/89-33 (Table 2), although that was not confirmed in Experiment 6 (Table 3). Tolerant genotype 863B-P2 had higher TE values than sensitive ICMB 841-P3, although that might have been due to experimental artefact. Surprisingly, the drought treatment did not cause any significant changes in TE (Table 2). At the vegetative stage under WS conditions and at the reproductive stage under both WW and WS conditions, there were no significant TE differences between any of the tolerant and sensitive genotypes.
Table 2.
Transpiration efficiency (TE, in g biomass kg−1 water transpired) of four pearl millet testcross hybrids [H 77/833-2, ICMB 841-P3 (sensitive), PRLT 2/89-33, 863B-P2 (tolerant)] measured at the vegetative and reproductive stage (Experiments 3 and 4, May 2007), under well-watered (WW) and water stress (WS) conditions, and TE measured across WW and WS treatments
| Genotype | Vegetative stage | Reproductive stage | ||||||
| 843A×PRLT 2-89/33 | 843A×H 77/833-2 | 863B-P2×H 77/833-2 | ICMB 841-P3×H 77/833-2 | 843A×PRLT 2-89/33 | 843A×H 77/833-2 | 863-P2×H 77/833-2 | ICMB 841-P3×H 77/833-2 | |
| TE WW | 10.06±0.82 b | 12.85±0.96 a | 13.80±0.78 a | 8.05±0.44 b | 9.28±2.77 a | 9.24±2.69 a | 11.72±2.76 a | 8.75±2.74 a |
| TE WS | 13.13±0.81 a | 12.87±0.96 a | 13.37±0.90 a | 10.01±0.81 a | 7.41±1.13 a | 7.06±1.03 a | 9.11±1.04 a | 11.30±1.03 a |
| LSD WW/WS | 4.51 | 4.13 | 2.84 | 4.65 | 5.51 | 5.01 | 5.53 | 4.95 |
| TE WW+WS | 11.60±1.15 a | 12.86±0.88 a | 13.58±0.6 a | 9.03±0.53 b | 8.35±0.59 a | 8.15±0.84 a | 10.42±1.38 a | 10.03±1.68 a |
Values are the means (±SE) of six replicate plants per treatment and genotype. Genotypic means followed by the same letter within a treatment are not significantly different. LSD is for treatment differences within each genotype
Table 3.
Transpiration efficiency (TE, in g biomass kg−1 water transpired) in two parental pearl millet genotypes (H 77/833-2, PRLT 2/89-33) and their three drought-tolerant NIL-QTLs in hybrid form under well-watered (WW) and water stress (WS) conditions, and TE measured across WW and WS treatments, measured at the vegetative stage (Experiment 6, July 2007)
| Genotype | 843A×PRLT 2/89-33 | 843A×H 77/833-2 | 843A×ICMR 01029 | 843A×ICMR 01031 | 843A×ICMR 02041 |
| TE WW | 6.19±0.54 a | 6.01±0.36 a | 7.19±0.37 a | 6.92±0.48 a | 6.19±0.52 a |
| TE WS | 6.16±0.39 a | 6.67±0.21 a | 7.31±0.28 a | 6.92±0.28 a | 6.01±0.25 a |
| LSD WW/WS | 1.16 | 0.95 | 1.10 | 1.45 | 1.79 |
| TE WW+WS | 6.18±0.32 a | 6.34±0.24 a | 7.24±0.24 a | 6.92±0.27 a | 6.10±0.28 a |
Values are the means (±SE) of six replicate plants per treatment and genotype. Genotypic means followed by same letter within a treatment are not significantly different. LSD is for treatment differences within each genotype.
TE was also examined in the parental genotypes and the NIL-QTLs during the vegetative growth stage (Experiment 6, Table 3). There was no TE change due to water stress and no TE differences between genotypes.
Discussion
Three important results were obtained, and all under WW conditions: (i) the drought-tolerant genotypes, including NIL-QTLs, had higher leaf ABA contents than the drought-sensitive parent H 77/833-2; (ii) tolerant genotypes were responsive to VPD and restricted transpiration at high VPD compared with sensitive genotypes, by slowing down the transpiration response to VPD at high VPD; and (iii) the Tr (g cm−2 d−1) were lower in tolerant genotypes even under low VPD conditions. Leaf ABA and the transpiration response to VPD under WW conditions discriminated tolerant and sensitive materials, although to a lesser extent in the 863B-P2–ICMB 841-P3 pair. Overall, TE did not differ between genotypes.
Differences in ABA content
During vegetative growth drought-tolerant genotypes (both the QTL donor parent and the three NIL-QTLs in testcross form) had higher leaf ABA content than the drought-sensitive genotypes under WW conditions. These results suggest a likely constitutive role for ABA in the drought tolerance QTL that plays most of its role when water is still non-limiting. The differences in leaf ABA are not due to a dilution effect at the leaf level since specific leaf area was similar in both pairs of parents (Kholová et al., 2009), and also because genotypes with high ABA had a leaf size similar to or larger than sensitive lines. These data would agree well with the lower Tr in the tolerant genotype, given that ABA is closely involved in the control of stomata aperture (Henson et al., 1983; Morison and Gifford, 1984; Cure and Acock, 1986). Whether the differences in ABA content have a causal effect on Tr and subsequently on yield under drought was not the purpose of the study and would need to be investigated further. The present analysis also showed that genotypic differences in leaf ABA were less marked at the reproductive stage. This might be the consequence of differential sensitivity to ABA between developmental stages (Henson et al., 1983; Winkel et al., 2001). Therefore, overall, the hypothesis is of a role for a constitutive higher production of ABA in tolerant lines to limit leaf water loss under WW conditions, which would save water for the later stage of plant development, hence turning out to be an important aspect of plant adaptation to water-limited conditions, as previously hypothesized (Mortlock and Hammer, 2001; Condon et al., 2002; Serraj et al., 2004; Sinclair et al., 2005; Kholová et al., 2008).
Surprisingly, differences in ABA level between control and stress-treated plants were not found in any of the experiments. Leaf ABA level was even higher in drought-sensitive genotype ICMB 841-P3 during the vegetative growth stage in stress conditions. This is contrary to previous reports in other species where ABA content significantly increased under drought stress conditions (Asch et al., 1995; Chandrasekar et al., 2000; Li and Wang, 2003; Yin et al., 2005; Zhang et al., 2005). It could be argued that genotypes may not have been at a similar leaf water potential where WS ABA was assessed, although they were at a similar FTSW level and so experienced the same stress intensity on the basis of soil water content.
Differences in VPD response
Transpiration was sensitive to VPD in drought-tolerant NIL-QTLs and in the drought-tolerant QTL donor parent, suggesting a direct involvement of the QTL in the manifestation of this trait. The lack of breakdown in the response of ICMR 02041 could be explained by a possible recombination in this large QTL region (∼30 cM), which would have excluded a putative portion involved in the VPD response of that particular NIL-QTL. This would indicate that only a portion of the QTL may be responsible for this trait. Current efforts to fine-map this QTL should help to generate the material needed to test this hypothesis, i.e. that the QTL region is underlying a cluster of traits contributing to water saving and that are held by different portions of the QTL. The fact that Tr differed between tolerant and sensitive genotypes even at low VPD indicates that the low Tr of tolerant genotypes (Kholová et al., 2010) is not a consequence of the Tr differences only at high VPD. The finding of genotypic differences in the transpiration response to VPD in pearl millet agrees with similar results in soybean (Sinclair et al., 2007), where a ‘slow-wilting’ genotype of soybean showed a linear increase in transpiration only until ∼2 Pa. Above these VPD levels, Tr remained essentially constant, whereas other genotypes maintained a linear increase in transpiration up to VPD values of ∼3.5 kPa. The reasons for the rapid change in Tr with a VPD increase are unclear and would probably require rapid control of the stomatal conductivity. Hydraulic signals (Zwieniecky et al., 2001; Sperry et al., 2002; Cochard et al., 2004) are more likely to mediate such a signal than drought signalling cascades (including ABA-dependent and ABA-independent pathway). In fact, previous work (Kholová et al., 2010) showed that Tr could be increased on a short-term basis with defoliation, providing evidence of the likelihood of non-hormone-related signals for the regulation of stomata in pearl millet too. Therefore, these results indicate clearly that in terminal drought-tolerant pearl millet, two distinct water-saving mechanisms operate under WW conditions: (i) a low Tr, which acts across VPD conditions; and (ii) a sensitivity to VPD in tolerant material that further limits the Tr when the VPD is high, >2 kPa. Both these traits would contribute to saving water in the soil profile, even if soil water is not limiting. This water would then be available and crucial for grain filling, as previously indicated (Turner, 2004; Manschadi et al., 2006; Ratnakumar et al., 2009). Thus, both traits are important to consider for the breeding of pearl millet lines with terminal drought tolerance. The data indicate the possibility to phenotype these traits using relatively simple Tr measurements at low and high VPD under natural conditions.
TE analysis
Large differences in TE were not found in different growth conditions and different genotypes. Part of the reason was the higher variability observed in TE values during the reproductive stage and under drought. This is contrary to what was suggested earlier (Sinclair et al., 2005), namely that Tr restriction at high VPD would normally increase TE. A possibility for the lack of differences is that the method used to assess TE was not sensitive enough to pinpoint TE differences arising from differences in sensitivity to VPD. Furthermore, the plants used for TE assessment were grown in the glasshouse at relatively low mean VPD and only rarely faced VPD conditions >1.5–2.0 kPa that would trigger the VPD response of transpiration and the expected transient increase in TE. Another possibility for the lack of TE differences could have been the differences in biomass partitioning to roots, since roots were not included in the TE measurement. Therefore, more work is needed to assess whether gravimetric TE differences could be found in conditions where plants are exposed to higher VPD, or simply whether intrinsic TE increases upon VPD increase in VPD-sensitive materials. In fact, the dry weight increase during the experimental period was lower in some of the tolerant material (data not shown), which suggests that the water-saving mechanisms could simply be reflected in differences in total water use. This lack of TE differences could also be linked to how individual stomata respond to VPD. In work reporting transpiration sensitivity to VPD (Sinclair et al., 2005; Devi et al., 2009; this work) mean stomatal conductance is partially reduced. This is assumed to be a consequence of a reduction of the aperture of all stomata. If that was the case, the intrinsic TE should indeed increase. However, it could be speculated that a decrease in stomatal conductance could be the mean of certain stomata having their conductance unchanged and other stomata that would fully close. Such a situation would normally not modify the intrinsic TE while it would decrease the Tr. Although that explanation may appear speculative, it would fit with reports that stomata are organized in patches (Pospíšilová and Šantrůček, 1994; Mott and Buckley, 2000) and may not all respond in the same way to external stimuli. In any case, the absence of TE differences stresses that the advantage of the VPD sensitivity trait, along with the lower Tr trait, probably related to ABA, needs to be considered in terms of total water use (lower) rather that in terms of water productivity.
Conclusion
It was found that the terminal drought tolerance QTL on pearl millet linkage group 2, previously found to correlate to a lower Tr, also correlated to higher ABA levels in the leaves of WW plants, and to the sensitivity of transpiration to a high VPD level under WW conditions. The low Trs previously found were a consequence not only of genotypic differences in the sensitivity of Tr to high VPD but of two separate water-saving mechanism, i.e. a low Tr at low VPD, which might be related to differences in the leaf ABA content, and a sensitivity to VPD leading to restrained Tr at high VPD. The major trait differences were all found under WW conditions, pointing to constitutive mechanisms underlying the QTL. The rapid response of the Tr to VPD points to a possible role for plant hydraulics in mediating such a rapid response. These traits would contribute to water saving in the soil profile when water is non-limiting. This ‘extra’ water, available for the later stage of the crop, would become critical to guarantee water supply to the plants at the time of grain filling and therefore for grain yield under terminal drought.
Acknowledgments
Ths senior author was supported by a grant from DFID-BBSRC, Research Contract BB/F004133/1, and by a grant for drought investigation No. MSM0021620858 of the Ministry of Education, Youth and Sports of the Czech Republic.
Glossary
Abbreviations
- ABA
abscisic acid
- FTSW
fraction of transpirable soil water
- NIL-QTLs
near-isogenic lines containing quantitative trait loci, TE, transpiration efficiency
- Tr
transpiration rate
- VPD
vapour pressure deficit
References
- Andrews DJ, Anand Kumar K. Use of the West African pearl millet landrace Iniadi in cultivar development. Plant Genetics Resource Newsletter. 1996;105:15–22. [Google Scholar]
- Asch F, Dörfling K, Dingkuhn M. Response of rice varieties to soil salinity and air humidity: a possible involvement of root-borne ABA. Plant and Soil. 1995;177:11–19. [Google Scholar]
- Ashwart CR, Kim SH, Mo SY, Kim DH. Transgenic plants of creeping bent grass harboring the stress inducible gene, 9-cis-epoxycarotenoid dioxygenase, are highly tolerant to drought and NaCl stress. Plant Growth Regulation. 2005;47:129–139. [Google Scholar]
- Bidinger FR, Mahalakshimi V, Durga Prasada Rao G. Assessment of drought resistance in pearl millet [ Pennisetum americanum (L.) Leeke]: II. Estimation of genotype response to stress. Australian Journal of Agricultural Research. 1987;38:49–59. [Google Scholar]
- Bidinger FR, Nepolean T, Hash CT, Yadav RS, Howarth CJ. Identification of QTLs for grain yield of pearl millet [ Pennisetum glaucum (L.) R. Br.] in environments with variable moisture during grain filling. Crop Science. 2007;47:969–980. [Google Scholar]
- Black CR, Squire GR. Effects of atmospheric saturation deficit on the stomatal conductance of pearl millet (Pennisetum typhoides S. and H.) and groundnut (Arachis hypogaea L. Journal of Experimental Botany. 1979;30:935–945. [Google Scholar]
- Blum A. Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Australian Journal of Agricultural Research. 2005;56:1159–1168. [Google Scholar]
- Cellier F, Conèjèro G, Breitler JC, Casse F. Molecular and physiological responses to water deficit in drought-tolerant and drought-sensitive sunflower lines (Helianthus annuus L.): accumulation of dehydrin transcripts correlates with tolerance. Plant Physiology. 1998;116:319–328. doi: 10.1104/pp.116.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier F, Conèjèro G, Casse F. Dehydrin transcript fluctuations during a day/night cycle in drought-stressed sunflower. Journal of Experimental Botany. 2000;343:299–300. doi: 10.1093/jexbot/51.343.299. [DOI] [PubMed] [Google Scholar]
- Chandrasekar V, Sairam RK, Srivastava GC. Physiological and biochemical responses of hexaploid and tetraploid wheat to drought stress. Journal of Agronomy Crop Science. 2000;185:219–227. [Google Scholar]
- Cochard H, Froux F, Mayr S, Coutand C. Xylem wall collapse in water-stressed pine needles. Plant Physiology. 2004;134:401–408. doi: 10.1104/pp.103.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. Improving intrinsic water-use efficiency and crop yield. Crop Science. 2002;42:122–131. doi: 10.2135/cropsci2002.1220. [DOI] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. Breeding for high water use efficiency. Journal of Experimental Botany. 2004;55:2447–2460. doi: 10.1093/jxb/erh277. [DOI] [PubMed] [Google Scholar]
- Conti S, Landi P, Sanguineti MC, Stafanelli S, Tuberosa R. Genetic and environmental effects on abscisic acid accumulation in leaves of field grown maize. Euphytica. 1994;78:81–89. [Google Scholar]
- Cure JD, Acock B. Crop responses to carbon dioxide doubling: a literature survey. Agriculture and Forest Meteorology. 1986;28:127–145. [Google Scholar]
- Devi JM, Sinclair TR, Vadez V. Genotypic variation in peanut (Arachis hypogaea L.) for transpiration sensitivity to atmospheric vapor pressure deficit. Crop Science. 2009 (in press) [Google Scholar]
- Farquhar GD, Richards RA. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology. 1984;11:539–552. [Google Scholar]
- Henson IE, Mahalakshmi V. Evidence for panicle control of stomatal behaviour in water stressed plants of pearl millet. Field Crops Research. 1985;11:281–290. [Google Scholar]
- Henson IE, Mahalakshmi V, Alagarswamy G, Bidinger FR. An association between flowering and reduced stomatal sensitivity to water stress in pearl millet (Pennisetum americanum (L.) Leeke) Annals of Botany. 1983;52:641–648. [Google Scholar]
- Howell TA. Enhancing water use efficiency in irrigated agriculture. Agronomy Journal. 2001;93:281–289. [Google Scholar]
- Kholová J, Vadez V, Hash CT. Jeju, South Korea: Book of abstracts; 2008. Mechanisms underlying drought tolerance in pearl millet (Pennisetum americanum L.). 5th International Crop Science Congress, March 13–18, 2008. [Google Scholar]
- Kholová J, Hash CT, Kakkera A, Kočová M, Vadez V. Constitutive water conserving mechanisms are correlated with the terminal drought tolerance of pearl millet [ Pennisetum glaucum (L.) R. Br.] Journal of Experimental Botany. 2010;61:369–377. doi: 10.1093/jxb/erp314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang K. Difference in drought responses of three contrasting Eucalyptus microtheca F. Muell. populations. Forest Ecology Management. 2003;179:377–385. [Google Scholar]
- Manschadi AM, Christopher J, deVoil P, Hammer GL. The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology. 2006;33:823–837. doi: 10.1071/FP06055. [DOI] [PubMed] [Google Scholar]
- Morison JIL, Gifford RM. Plant growth and water use with limited water supply in high CO2 concentrations. II. Plant dry weight, partitioning and water use efficiency. Australian Journal of Plant Physiology. 1984;11(5):375–384. [Google Scholar]
- Mortlock MY, Hammer GL. Genotype and water limitation effects on transpiration efficiency in sorghum. Journal of Crop Production. 2001;2:265–286. [Google Scholar]
- Mott KA, Buckley TN. Patchy stomatal conductance: emergent collective behaviour of stomata. Trends in Plant Science. 2000;5:258–262. doi: 10.1016/s1360-1385(00)01648-4. [DOI] [PubMed] [Google Scholar]
- Mugo SN. Ithaca, NY: Ph.D. thesis, Cornell University; 1999. Drought tolerance at seedling and flowering growth stages in maize: abscisic acid and other traits as selection tools. [Google Scholar]
- Pospíšilová J, Šantrůček J. Stomatal patchiness. Biologia Plantarum. 1994;36:481–510. [Google Scholar]
- Rai KN, Hash CT, Singh AK, Velu G. Adaptation and quality traits of a germplasm-derived commercial seed parent of pearl millet. Plant Genetic Resources Newsletter. 2008;154:20–24. [Google Scholar]
- Ratnakumar P, Vadez V, Nigam SN, Krishnamurthy L. Assessment of transpiration efficiency in peanut (Arachis hypogaea L.) under drought by lysimetric system. Plant Biology. 2009;11:124–130. doi: 10.1111/j.1438-8677.2009.00260.x. [DOI] [PubMed] [Google Scholar]
- Ryu SB, Li PH. Potato cold hardiness development and abscisic acid. I. Conjugated abscisic acid is not the source of the increase in free abscisic acid during potato (Solanum commersonii) cold acclimation. Physiologia Plantarum. 1994;90:15–20. [Google Scholar]
- Serraj R, Hash CT, Rivzi SMH, Sharma A, Yaday RS, Bidinger FR. Recent advances in marker-assisted selection for drought tolerance in pearl millet. Plant Production Science. 2005;8:334–337. [Google Scholar]
- Serraj R, Krishnamurthy L, Kashiwagi J, Kumar J, Chandra S, Crouch JH. Variation in root traits of chickpea (Cicer arietinum L.) grown under terminal drought. Field Crops Research. 2004;88:115–127. [Google Scholar]
- Sinclair TR, Hammer GL, van Oosterom EJ. Potential yield and water-use efficiency benefits in sorghum from limited maximum transpiration rate. Functional Plant Biology. 2005;32:945–952. doi: 10.1071/FP05047. [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Zwieniecki MA, Holbrook MN. Drought tolerance in soybean associated with limiting leaf hydraulic conductance. Physiologia Plantarum. 2007;132:446–451. doi: 10.1111/j.1399-3054.2007.01028.x. [DOI] [PubMed] [Google Scholar]
- Sperry JS, Hacke UG, Oren R, Comstock JP. Water deficits and hydraulic limits to leaf water supply. Plant, Cell and Environment. 2002;25:251–263. doi: 10.1046/j.0016-8025.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- Squire GR. The response of stomata of pearl millet (Pennisetum typhoides S. and H.) to atmospheric humidity. Journal of Experimental Botany. 1979;118:925–933. [Google Scholar]
- Stegmeier WD, Andrews DJ, Rai KN, Hash CT. Pearl millet parental lines 843A and 843B. International Sorghum and Millets Newsletter. 1998;39:129–130. [Google Scholar]
- Thompson AJ, Andrews J, Mulholland BJ, et al. Over-production of abscisic acid in Solanum lycopersicum L. increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiology. 2007;143:1905–1917. doi: 10.1104/pp.106.093559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner NC. Agronomic options for improving rainfall-use efficiency of crops in dryland farming systems. Journal of Experimental Botany. 2004;55:2413–2427. doi: 10.1093/jxb/erh154. [DOI] [PubMed] [Google Scholar]
- Vadez V, Sinclair TR. Leaf ureide degradation and N2 fixation tolerance to water deficit in soybean. Journal of Experimental Botany. 2001;52:153–159. [PubMed] [Google Scholar]
- Weiler EW. An enzyme immunoassay for cis (+) abscisic acid. Physiologia Plantarum. 1982;54:510–514. [Google Scholar]
- Winkel T, Payne W, Renno JF. Ontogeny modifies the effects of water stress on stomatal control, leaf area duration and biomass partitioning of Pennisetum glaucum. New Phytologist. 2001;149:71–82. doi: 10.1046/j.1469-8137.2001.00008.x. [DOI] [PubMed] [Google Scholar]
- Yadav RS, Hash CT, Bidinger FR, Cavan GP, Howarth CJ. Quantitative trait loci associated with traits determining grain and stover yield in pearl millet under terminal drought-stress conditions. Theoretical and Applied Genetics. 2002;104:67–83. doi: 10.1007/s001220200008. [DOI] [PubMed] [Google Scholar]
- Yin C, Peng Y, Zhang R, Zhu Y, Li C. Adaptive responses of Pupulus kangdingensis to drought stress. Physiologia Plantarum. 2005;122:445–451. [Google Scholar]
- Zhang X, Wang T, Li C. Different responses of two contrasting wheat genotypes to abscisic acid application. Biologia Plantarum. 2005;49:613–616. [Google Scholar]
- Zwieniecky MA, Melcher PJ, Holbrook NM. Hydrogel control of xylem hydraulic resistance in plants. Science. 2001;291:1059–1062. doi: 10.1126/science.1057175. [DOI] [PubMed] [Google Scholar]