Abstract
Objective
Few studies have examined the impact of viral hepatitis on bone mineral density (BMD), and none have done so among HIV-infected patients. Our objective was to determine if viral hepatitis was associated with low BMD in HIV.
Design
Cross-sectional study among 1,237 HIV-infected subjects (625 with viral hepatitis).
Methods
Dual-energy x-ray absorptiometry (DXA) scans of the lumbar spine and femoral neck were obtained. Clinical data, hepatitis B and C status, and markers of bone metabolism were determined at DXA scanning. Multivariable logistic regression examined the association between hepatitis and low BMD (Z-score ≤−2.0 at the lumbar spine and/or femoral neck).
Results
Mean BMD Z-scores were lower among hepatitis-coinfected women at the lumbar spine (−0.15 versus +0.29; difference=−0.44 [95% CI, −0.65 to −0.23]; p<0.001) and femoral neck (−0.64 versus −0.39; difference=−0.25 [95% CI, −0.44 to −0.06]; p=0.009) compared to HIV-monoinfected women. No differences in mean BMD Z-scores were observed between coinfected and monoinfected men. After adjustment for age, body mass index, duration of HIV, antiretroviral use, physical activity, and smoking, viral hepatitis was associated with low BMD among women (adjusted OR, 2.87; 95% CI, 1.31 – 6.29) but not men (adjusted OR, 1.19; 95% CI, 0.74 – 1.91). Coinfected women had lower mean parathyroid hormone (60.1 versus 68.1 pg/mL; p=0.02) but similar mean 25-hydroxyvitamin D (19.1 versus 19.6 ng/mL; p=0.6) and osteocalcin (3.0 versus 3.2 ng/mL; p=0.8) concentrations than HIV-monoinfected women.
Conclusions
Viral hepatitis was associated with a higher risk of low BMD among HIV-infected women but not men.
Keywords: Viral hepatitis, HBV, HCV, bone mineral density, DXA, vitamin D, bone
INTRODUCTION
While advances in antiretroviral therapy (ART) have led to a substantial decline in AIDS-related opportunistic infections and increased longevity of HIV-infected patients, complications of chronic viral hepatitis have now emerged as a major cause of morbidity in the HIV population [1]. Existing studies among HIV-infected patients have mainly examined the impact of viral hepatitis coinfection on liver-related complications (e.g., cirrhosis, end-stage liver disease, and hepatocellular carcinoma) [2–4], but the effect of viral hepatitis on non-hepatic outcomes, in particular abnormalities in bone mineral density (BMD) and bone metabolism (termed “hepatic osteodystrophy”), remains unclear.
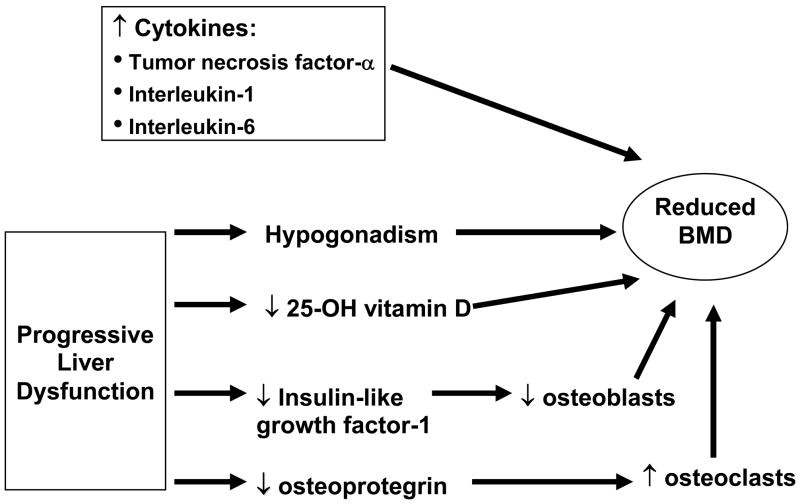
Viral hepatitis has been associated with reduced BMD among HIV-uninfected patients [5, 6], and a number of factors related to chronic viral hepatitis have been hypothesized to contribute to low BMD (Figure 1). Prior studies have suggested that elevated serum levels of cytokines associated with viral hepatitis (e.g., tumor necrosis factor-α, interleukin-1, interleukin-6) could inhibit bone formation and increase bone resorption, leading to low BMD [7, 8]. In addition, progressive hepatitis-induced liver dysfunction may be associated with reduced hepatic hydroxylation of vitamin D [9], hypogonadism [10], and impaired hepatic production of insulin-like growth factor-1 and osteoprotegerin, both of which promote bone formation [6], and these conditions can also contribute to low BMD. Moreover, HIV itself is associated with reduced BMD and increased fracture risk [11–13]. However, to date, no study has yet examined if viral hepatitis is an independent risk factor for low BMD in HIV. Evaluating the association between viral hepatitis and low BMD in HIV-infected patients is important because: 1) viral hepatitis is prevalent in HIV [14], and 2) low BMD is a predictor of both fragility fractures [15] and all-cause mortality [16, 17]. We therefore examined if viral hepatitis coinfection was associated with reduced BMD among HIV-infected individuals.
Figure 1.
Potential mechanisms for bone loss due to chronic viral hepatitis infection.
METHODS
Study Design and Subjects
We performed a cross-sectional study among subjects enrolled in the Modena HIV Metabolic Clinic Cohort, which was initiated in September 2004 to examine metabolic alterations among HIV-infected patients seen at the metabolic clinic of the University of Modena and Reggio Emilia School of Medicine (Modena, Italy) [18]. Subjects in the cohort have laboratory-confirmed HIV, provide informed consent, and complete a standardized questionnaire at enrollment that collects demographic, clinical, and HIV data. Dual-energy x-ray absorptiometry (DXA) scans are performed to assess BMD, and blood is drawn for hepatitis status, HIV RNA, immune function, and metabolic parameters.
For this study, all subjects enrolled in the Modena HIV Metabolic Clinic Cohort between September 1, 2004 and December 31, 2007 were eligible for inclusion. Approval for the study was obtained from the Institutional Review Boards of the University of Pennsylvania and University of Modena and Reggio Emilia.
Main Outcome Measures
The primary study outcome was the age- and sex-specific standard deviation score (Z-score) of BMD of the lumbar spine and femoral neck. This is the appropriate method to assess disease effects across a population of varied age and sex.[19] In addition, Z-scores of −2.0 or less identify subjects with BMD below the expected range for age and sex [19]. Thus, we also examined as study outcomes: 1) low lumbar spine BMD, defined as a spine BMD Z-score of −2.0 or less, 2) low femoral neck BMD, defined as a femoral neck BMD Z-score of −2.0 or less, and 3) low BMD, defined as a lumbar and/or femoral neck BMD Z-score of −2.0 or less.
Data Collection
All data were collected from the Modena HIV Metabolic Clinic Cohort’s electronic database. Data on age; sex; smoking (number of cigarettes/day); alcohol consumption (grams of alcohol/day); physical activity (none, mild [<4 hours weekly], intensive [≥4 hours weekly]); diabetes mellitus (based on self-reported physician’s diagnosis and/or use of anti-diabetic medications); use of corticosteroids, oral estrogens, and calcium and vitamin D supplements; amenorrhea (defined as the absence of menstrual periods for more than 6 months in women who previously had menses); HIV diagnosis date; risk factors for HIV transmission; nadir CD4 T lymphocyte count; and antiretroviral medications were collected from subjects at enrollment using a structured questionnaire. ART use was defined by receipt of three antiretroviral agents.
Body weight was measured using a digital scale to the nearest 0.1 kg with subjects wearing light clothes without shoes. Height was measured using a wall-mounted stadiometer to the nearest 0.1 cm. Measurements for body weight and height were completed in triplicate and the mean recorded.
Blood was drawn from all subjects for determination of hepatitis B virus (HBV) surface antigen (HBsAg; Elecsys 2010; Roche Diagnostics, Indianapolis, IN), hepatitis C virus (HCV) antibody (anti-HCV; Abbott HCV EIA 3.0 enzyme immunoassay; Abbott Laboratories), HIV RNA (Abbott RealTime™ HIV-1 assay; Abbott Laboratories; lower limit of detection: 50 copies/mL), CD4 cell count, thyroid stimulating hormone (TSH; Advia Centaur TSH assay, Bayer Healthcare, Tarrytown,. NY), serum creatinine, and biochemical parameters of bone metabolism, including intact parathyroid hormone (PTH; Liaison N-Tact PTH Assay; DiaSorin; Stillwater, MN), 25-hydroxyvitamin D (DiaSorin 25-hydroxyvitamin D chemiluminescence immunoassay; Stillwater, MN), and osteocalcin (Liaison Osteocalcin Assay; DiaSorin; Stillwater, MN).
All subjects enrolled in the cohort had BMD measured by DXA at the lumbar spine (i.e., L1-L4) and femoral neck. All subjects were scanned using the same single densitometer (Lunar DPX-MD; Lunar Corporation, Madison, WI). Values were expressed as Z-scores (number of standard deviations relative to age within males and females) compared with the manufacturer’s reference population. The instrument was calibrated daily with a hydroxyapatite phantom. The in vivo coefficients of variation of the DXA measures at the lumbar and femoral sites were less than 2%.
Statistical Analysis
Differences between HIV/viral hepatitis-coinfected and HIV-monoinfected subjects were assessed using Chi-square tests for categorical data and Wilcoxon rank-sum tests for continuous data. We first examined interactions between viral hepatitis, age, and sex. Next, multivariable linear regression was used to determine differences in the mean lumbar and femoral neck Z-scores between coinfected and monoinfected subjects, after adjusting for potential confounders. Multivariable logistic regression was then used to determine unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of low femoral neck and lumbar spine BMD between subjects with and without viral hepatitis. Potential confounders evaluated included age, sex, body mass index (BMI, calculated as weight/height2), duration of HIV infection, nadir CD4 count, HIV viral load and CD4 count at the time of DXA, receipt of ART, smoking status, alcohol consumption, physical activity, amenorrhea, and estimated glomerular filtration rate (eGFR, calculated using the formula developed by the Modification of Diet in Renal Disease Study [20]: 186 × [serum creatinine]−1.154 × [age]−0.203 × [0.742 if female]). We assessed the impact of confounding by examining the change in the beta coefficient for viral hepatitis as potential confounders were included in the model [21]. Additionally, since HBsAg-positive subjects may not have had active HBV replication due to treatment with antiretroviral medications that are also active against HBV, we performed sub-analyses excluding HBsAg-positive subjects and examined the association between chronic HCV alone and low BMD.
Variables of interest were examined for missing data, and the covariate with the highest proportion of missingness was HIV RNA (4%). To ensure that missing data did not affect the validity of our results, multiple imputation was performed to generate 10 data sets [22]. Multivariable linear and logistic regression analyses were then performed on each data set, and results were combined to arrive at appropriate variances that accounted for missing data [23]. Since renal function affects serum levels of bone turnover markers, we adjusted for eGFR when comparing these parameters between coinfected and monoinfected patients.
Assuming a type 1 error of 0.05, 80% power, a 5% prevalence of low BMD in HIV-monoinfected patients, and a 1:1 ratio of monoinfected:coinfected subjects, we estimated that 1,030 subjects (515 with viral hepatitis coinfection) was needed to detect an OR=2.0 of low BMD. This sample size also provided 80% power to detect a difference in Z-score of 0.15 between subjects with and without viral hepatitis. All data were analyzed using STATA 10.0 (Stata Corporation, College Station, TX). Multiple imputation analysis was done using SAS 9.1 (SAS Incorporated, Cary, NC).
RESULTS
Patient Characteristics
A total of 1,237 subjects (625 HIV/viral hepatitis-coinfected; 612 HIV-monoinfected) were included in the study. Demographic and clinical characteristics of the study groups are summarized in Table 1. Eighty-five (9%) were HBsAg-positive, and 572 (47%) were anti-HCV-positive. Coinfected subjects had lower BMIs, a longer median duration of HIV infection, lower nadir as well as total CD4 cell counts at the time of DXA, more commonly were physically inactive, less commonly drank alcohol, more commonly smoked cigarettes, and had lower serum 25-hydroxyvitamin D concentrations.
Table 1.
Subject characteristics, total and by viral hepatitis status.
| Characteristic | All Subjects (n=1,237) | Viral Hepatitis Negative (n=612) | Viral Hepatitis Positive (n=625) | P-Value |
|---|---|---|---|---|
| Median age (yrs, IQR) | 43 (40–48) | 44 (39–49) | 43 (41–47) | 0.8 |
| Sex (no., %) | ||||
| Male | 765 (62%) | 385 (63%) | 380 (61%) | 0.4 |
| Female | 472 (38%) | 227 (37%) | 245 (39%) | |
| History of injection drug use (no., %) | 433 (35%) | 22 (4%) | 411 (66%) | <0.001 |
| Body mass index (no., %) | <0.001 | |||
| <18.5 kg/m2 | 75 (6%) | 28 (4%) | 47 (8%) | |
| 18.5–24.9 kg/m2 | 841 (68%) | 392 (64%) | 449 (72%) | |
| 25–29.9 kg/m2 | 247 (20%) | 150 (25%) | 97 (15%) | |
| ≥30 kg/m2 | 74 (6%) | 42 (7%) | 32 (5%) | |
| Median duration of HIV diagnosis (mo, IQR) | 177 (126–229) | 145 (102–185) | 220 (164–244) | <0.001 |
| Median nadir CD4 cell count (cells/mm3, IQR) | 160 (60–258) | 177 (75–279) | 138 (50–225) | <0.001 |
| Median CD4 cell count (cells/mm3, IQR) | 490 (352–680) | 516 (381–690) | 470 (324–660) | <0.001 |
| Median HIV RNA level (copies/mL, IQR) | 50 (50–328) | 50 (50–425) | 50 (50–200) | 0.3 |
| Viral hepatitis | ||||
| Overall | 625 (51%) | 625 (100%) | ||
| HBsAg+ | 85 (9%) | 85 (14%) | ||
| Anti-HCV+ | 572 (47%) | 572 (92%) | ||
| HBsAg+ and anti-HCV+ | 32 (2.5%) | 32 (5%) | ||
| Physical activity (no., %) | <0.001 | |||
| None | 791 (64%) | 355 (58%) | 436 (70%) | |
| Mild (<4 hours weekly) | 303 (25%) | 163 (27%) | 140 (23%) | |
| Intensive (≥4 hours weekly) | 136 (11%) | 91 (15%) | 45 (7%) | |
| Alcohol consumption (no., %) | 0.001 | |||
| None | 687 (56%) | 314 (51%) | 373 (60%) | |
| <20 gm/d | 531 (43%) | 294 (48%) | 237 (38%) | |
| ≥20 gm/d | 13 (1%) | 3 (1%) | 10 (2%) | |
| Smoking status (no., %) | <0.001 | |||
| Non-smoker | 625 (51%) | 384 (63%) | 241 (39%) | |
| <10 cigarettes/d | 236 (19%) | 101 (17%) | 135 (22%) | |
| ≥10 cigarettes/d | 368 (30%) | 124 (20%) | 244 (39%) | |
| Diabetes mellitus (no., %) | 82 (7%) | 43 (7%) | 40 (6%) | 0.6 |
| On ART (no., %) | 977 (79%) | 488 (80%) | 489 (78%) | 0.5 |
| Corticosteroid use (no., %) | 23 (2%) | 10 (2%) | 13 (2%) | 0.6 |
| Use of calcium and vitamin D supplements (no., %) | 2 (0.2%) | 2 (0.3%) | 0 (0%) | 0.2 |
| Estrogen replacement, among women (no., %) | 3 (0.2%) | 3 (0.5%) | 0 (0%) | 0.1 |
| Amenorrhea (no., %)* | 59 (5%) | 26 (4%) | 33 (5%) | 0.5 |
| Median 25-hydroxyvitamin D (ng/mL, IQR) | 15.9 (9.6–24.9) | 17.1 (9.9–25.7) | 15.0 (9.1–23.8) | 0.02 |
| Hypovitaminosis D (no., %)‡ | 573 (46%) | 263 (43%) | 310 (50%) | 0.02 |
| Median osteocalcin (ng/mL, IQR) | 4 (3–7) | 4 (3–7) | 4 (3–7) | 0.2 |
| Median parathyroid hormone (pg/mL, IQR) | 57.7 (43.1–76.7) | 59.7 (46.1–81.3) | 55.3 (40.0–73.5) | <0.001 |
| Median thyroid stimulating hormone (μU/mL) | 1.87 (1.31–2.58) | 1.84 (1.27–2.51) | 1.89 (1.35–2.68) | 0.1 |
| Median eGFR (mL/min/1.73 m2)┼ | 84.9 (73.8–100.3) | 81.9 (71.6–96.8) | 88.8 (75.9–102.7) | <0.001 |
IQR=interquartile range; HIV=human immunodeficiency virus; HBsAg=hepatitis B surface antigen; anti-HCV=hepatitis C virus antibody ART=antiretroviral therapy
Amenorrhea defined as the absence of menstrual periods for more than 6 months in women who previously had menses.
Hypovitaminosis D defined as 25-hydroxyvitamin D concentration <15 ng/mL.
eGFR = Estimated glomerular filtration rate, calculated using the formula developed by the Modification of Diet in Renal Disease Study Group: 186 × (serum creatinine)−1.154 × (age)−0.203 × (0.742 if female).
Differences in BMD Z-scores by Viral Hepatitis Status
A statistically significant interaction between viral hepatitis and sex was identified at the lumbar spine (p=0.002) and femoral neck (p=0.04). As a result, all subsequent analyses were stratified by sex.
Differences in Lumbar Spine BMD Z-scores
Overall, men had lower mean Z-scores at the lumbar spine than women (−0.50 versus +0.06; difference = −0.56 [95% CI, −0.42 to −0.69]; p<0.001). However, viral hepatitis-coinfected women had significantly lower mean lumbar spine Z-scores compared to HIV-monoinfected women (−0.15 versus +0.29; difference = −0.44 [95% CI, −0.65 to −0.23]; p<0.001). After adjustment for age, BMI, duration of HIV infection, use of ART, physical activity, and smoking, deficits in lumbar BMD remained greater among coinfected women (difference = −0.51 [95% CI, −0.75 to −0.27]; p<0.001; Table 2a). In contrast, coinfected men had similar mean Z-scores at the lumbar spine compared to monoinfected men (−0.52 versus −0.47; difference = −0.05 [95% CI, −0.22 to +0.12]; p=0.5). Adjustment for confounders (Table 2a) yielded little change in the difference in lumbar BMD between these two groups (difference = −0.12 [95% CI, −0.32 to +0.08]; p=0.2). When we performed adjusted analyses excluding HBsAg-positive subjects, mean lumbar Z-scores remained lower in HCV-coinfected compared to HIV-monoinfected women (−1.31 versus −0.82; difference = −0.49 [95% CI, −0.74 to −0.25]; p<0.001) but not between coinfected and monoinfected men (−1.24 versus −1.14; difference = −0.10 [95% CI, −0.32 to −0.12]; p=0.4).
Table 2.
Differences (95% confidence intervals [CIs]) in lumbar (Table 2a) and femoral (Table 2b) mean bone mineral density Z-scores between subjects with and without viral hepatitis after adjustment for selected covariates, by sex.
| Table 2a. Lumbar spine, female | ||
|---|---|---|
| Regression Model | Differences (95% CI) in mean femoral Z-score | P-Value |
| Base model: adjusted for age | −0.44 (−0.65 to −0.23) | <0.001 |
| * Add BMI category | −0.46 (−0.68 to −0.26) | <0.001 |
| Add duration of HIV infection | −0.51 (−0.74 to −0.28) | <0.001 |
| Add HAART use | −0.51 (−0.74 to −0.28) | <0.001 |
| Add physical activity | −0.49 (−0.73 to −0.26) | <0.001 |
| Add active tobacco use | −0.51 (−0.75 to −0.27) | <0.001 |
| Table 2a. Lumbar spine, male | ||
|---|---|---|
| Regression Model | Differences (95% CI) in mean femoral Z-score | P-Value |
| Base model: adjusted for age | −0.05 (−0.22 to +0.12) | 0.6 |
| * Add BMI category | −0.02 (−0.20 to +0.14) | 0.7 |
| Add duration of HIV infection | −0.13 (−0.32 to +0.01) | 0.2 |
| Add HAART use | −0.13 (−0.33 to +0.06) | 0.2 |
| Add physical activity | −0.13 (−0.33 to +0.06) | 0.2 |
| Add active tobacco use | −0.12 (−0.32 to +0.08) | 0.2 |
| Table 2b. Femoral neck, female | ||
|---|---|---|
| Regression Model | Differences (95% CI) in mean lumbar Z-score | P-Value |
| Base: adjusted for age | −0.25 (−0.44 to −0.06) | 0.009 |
| * Add BMI category | −0.26 (−0.45 to −0.07) | 0.008 |
| Add duration of HIV infection | −0.28 (−0.49 to −0.07) | 0.009 |
| Add HAART use | −0.28 (−0.49 to −0.07) | 0.009 |
| Add physical activity | −0.26 (−0.47 to −0.05) | 0.02 |
| Add active tobacco use | −0.26 (−0.47 to −0.04) | 0.02 |
| Table 2b. Femoral neck, male | ||
|---|---|---|
| Regression Model | Differences (95% CI) in mean lumbar Z-score | P-Value |
| Base: adjusted for age | −0.07 (−0.21 to +0.07) | 0.3 |
| * Add BMI category | −0.02 (−0.15 to +0.12) | 0.8 |
| Add duration of HIV infection | −0.02 (−0.18 to +0.14) | 0.8 |
| Add HAART use | −0.03 (−0.18 to +0.13) | 0.7 |
| Add physical activity | −0.02 (−0.17 to +0.14) | 0.8 |
| Add active tobacco use | −0.02 (−0.18 to +0.14) | 0.8 |
Sequentially adding each factor to the model in the previous row.
Differences in Femoral Neck BMD Z-scores
Men also had lower mean Z-scores at the femoral neck than women (−0.68 versus −0.52; difference = −0.16 [95% CI, −0.05 to −0.28]; p=0.006). Mean femoral neck Z-scores were lower for coinfected versus monoinfected women (−0.64 versus −0.39; difference = −0.25 [95% CI, −0.44 to −0.06]; p=0.009). After adjustment for BMI, duration of HIV, nadir CD4 cell count, ART use, physical activity, and smoking, deficits in femoral BMD were again greater among coinfected women (difference = −0.26 [95% CI, −0.47 to −0.04]; p=0.02; Table 2b). Coinfected men had similar mean Z-scores at the femoral neck compared to monoinfected men (−0.72 versus −0.64; difference = −0.08 [95% CI, −0.22 to +0.06]; p=0.2). After adjustment for confounders (Table 2b), deficits in femoral neck BMD remained similar between coinfected and monoinfected men (difference = −0.02 [95% CI, −0.18 to +0.14); p=0.8). When we again excluded HBsAg-positive subjects, mean femoral Z-scores were lower in HCV-coinfected versus HIV-monoinfected women (−1.12 versus −0.84; difference = −0.28; 95% CI, −0.50 to −0.06; p=0.01) but not between coinfected and monoinfected men (−2.28 versus −2.27; difference = −0.01; 95% CI, −0.18 to +0.17; p=0.9).
Analyses using imputed data produced similar results.
Risk of Low BMD Associated with Viral Hepatitis Coinfection
Risk of Low Lumbar Spine BMD
Overall, 97 subjects (8%; 17 women [4%] versus 80 men [10%]; p<0.001) had lumbar spine BMD Z-scores ≤−2.0. Low lumbar BMD was more prevalent in coinfected subjects (58 [9%] versus 39 [6%]; OR, 1.50; 95% CI, 0.99 – 2.29). Adjustment for age, BMI, duration of HIV infection, ART use, physical activity, and smoking increased the odds of low lumbar BMD in viral hepatitis-coinfected versus monoinfected women (adjusted OR, 3.36; 95% CI, 0.96 – 11.75) and coinfected versus monoinfected men (adjusted OR, 1.34; 95% CI, 0.77 – 2.36).
Risk of Low Femoral Neck BMD
One hundred subjects (8%; 32 women [7%] versus 68 men [9%]; p=0.2) had femoral Z-scores ≤−2.0. Coinfected subjects more commonly had low femoral BMD Z-scores compared to monoinfected subjects (56 [9%] versus 44 [7%]; OR, 1.27; 95% CI, 0.84 – 1.92), but this difference was not statistically significant. After controlling for age, BMI, duration of HIV infection, ART use, physical activity, and smoking, the risk of low femoral BMD was greater but non-significant for viral hepatitis-coinfected versus HIV-monoinfected women (adjusted OR, 1.87; 95% CI, 0.81 – 4.34) and coinfected versus monoinfected men (adjusted OR, 1.03; 95% CI, 0.54–1.94).
Risk of Low Lumbar Spine and/or Femoral Neck BMD
A total of 170 subjects (14%; 43 women [9%] versus 127 men [17%]; p<0.001) were identified with low BMD, defined as either a lumbar or femoral Z-score ≤−2.0. This outcome was more common among coinfected subjects (100 [16%] versus 70 [11%]; p=0.02). After adjustment for age, BMI, duration of HIV infection, ART use, physical activity, and smoking, viral hepatitis was associated with low BMD in HIV-infected women (adjusted OR, 2.87; 95% CI, 1.31 – 6.29) but not men (adjusted OR, 1.19; 95% CI, 0.74 – 1.91). When we excluded HBsAg-positive subjects, the risk of low BMD remained greater in HCV-coinfected women versus women with HIV alone (adjusted OR, 2.99; 95% CI, 1.33 – 6.74), but no association was found between HCV coinfection and low BMD in men (adjusted OR, 1.26; 95% CI, 0.75 – 2.10).
Results are summarized in Table 3. Analyses using imputed data produced similar results.
Table 3.
Association between viral hepatitis coinfection and low bone mineral density at the lumbar spine and femoral neck among HIV-infected women and men.
| Sex | Site | Adjusted Odds Ratio (95% Confidence Interval)* |
|---|---|---|
| Women | Lumbar spine | 3.36 (0.96, 11.75) |
| Femoral neck | 1.87 (0.81, 4.34) | |
| Lumbar and/or femoral | 2.87 (1.31, 6.29) | |
| Men | Lumbar spine | 1.34 (0.77, 2.36) |
| Femoral neck | 1.03 (0.54, 1.94) | |
| Lumbar and/or femoral | 1.19 (0.74, 1.91) |
Odds ratios were adjusted for age, body mass index, duration of HIV infection, smoking status, physical activity, and use of combination antiretroviral therapy.
Biochemical Parameters of Bone Metabolism by Hepatitis Status
After adjusting for eGFR, HIV/viral hepatitis-coinfected women had lower mean intact PTH concentrations (60.1 versus 68.1 pg/mL; p=0.02) but similar mean 25-hydroxyvitamin D (19.1 versus 19.6 ng/mL; p=0.6) and osteocalcin (3.0 versus 3.2 ng/mL; p=0.8) concentrations compared to HIV-monoinfected women. Coinfected men also had lower mean intact PTH concentrations (66.1 versus 73.7 pg/mL; p=0.001) but similar mean 25-hydroxyvitamin D (21.4 versus 22.3 ng/mL; p=0.3) and osteocalcin (3.4 versus 4.0 ng/mL; p=0.13) concentrations compared with HIV-monoinfected men after adjusting for eGFR.
DISCUSSION
To our knowledge, this study is the first to examine if viral hepatitis coinfection was associated with reduced BMD Z-scores in HIV. We unexpectedly identified sex differences in the comparison of BMD Z-scores between coinfected and monoinfected subjects. HIV/viral hepatitis-coinfected women had significantly lower BMD Z-scores at both the lumbar spine and femoral neck compared to women with HIV alone. After adjusting for confounding variables, viral hepatitis remained associated with low BMD Z-scores in women. In contrast, we found no differences in lumbar or femoral BMD Z-scores between coinfected and monoinfected men, and viral hepatitis was not associated with reduced BMD Z-scores among HIV-infected men. Regardless of sex, coinfected subjects had lower intact PTH concentrations but similar 25-hydroxyvitamin D and octeocalcin levels compared to subjects with HIV alone.
Few published studies have exclusively examined the impact of viral hepatitis on BMD, and none have done so among HIV-infected patients. Among viral hepatitis-monoinfected patients, the prevalence of reduced lumbar and/or femoral BMD has been reported to range from 10% to 56% [8, 24–28]. However, these studies are difficult to interpret given their small sample sizes, inclusion of predominantly male subjects, and use of different BMD outcomes. Importantly, these studies primarily reported low T-scores as their main endpoint. Since T-scores represent the comparison of an individual’s BMD with a young adult at the time of peak bone mass, T-scores are significantly lower in older subjects due to the expected age-related declines in BMD. Therefore, studies reporting T-scores in subjects of varied age are likely confounded by age. Rather, in comparing subjects across the entire adult age range, Z-scores are the preferred outcome in which an individual’s BMD is compared to a reference population of the same age and sex. Using this outcome, we found that 16% of coinfected patients had low lumbar and/or femoral bone mass. Had we used T-scores, the proportion would have been much higher among older adults and would not have captured the independent effect of coinfection.
We examined several biochemical parameters of bone metabolism to examine potential mechanisms for low BMD due to viral hepatitis in HIV-infected patients. Serum levels of osteocalcin, a protein synthesized by osteoblasts that reflects bone formation, have been reported to be decreased among patients with viral hepatitis [28] and viral cirrhosis [8, 24, 25, 27] compared to healthy viral hepatitis-uninfected patients, suggesting impaired osteogenesis. However, after adjusting for eGFR, we found no difference in serum levels of osteocalcin according to hepatitis status. Moreover, the mean osteocalcin concentrations observed in coinfected and monoinfected subjects in our sample were similar to results found in HIV-uninfected men and women [29]. In addition, serum 25-hydroxyvitamin D concentrations have been reported to be lower among viral hepatitis-monoinfected patients compared to healthy controls [8, 24, 25, 27]. However, after controlling for eGFR, we found that 25-hydroxyvitamin D concentrations were similar between coinfected and monoinfected women and between coinfected and monoinfected men. We did not observe a compensatory increase in intact PTH concentration among the coinfected subjects that would have been expected given the generally low 25-hydroxyvitamin D concentrations observed. Reduced values of both serum PTH and 25-hydroxyvitamin D have been reported in studies of viral hepatitis-monoinfected patients [8, 23, 24, 26]. Cytokines associated with viral hepatitis infection (particularly, interleukins-1 and -6) have been shown in vitro to inhibit PTH secretion [30], which might explain this observation.
The precise mechanisms for the association between viral hepatitis and low BMD in HIV-infected women but not men remain unclear. It is unknown if there are clinically significant differences by sex in serum levels of hepatitis-associated cytokines, markers of bones resorption (e.g., serum pyridinoline, type 1 collagen cross-linked C-telopeptide), or other factors that maintain bone balance (e.g., insulin-like growth factor-1, osteoprotegerin) among HIV-infected patients, but such variables are not routinely measured by the Modena HIV Metabolic Clinic Cohort. Moreover, the effect of both HIV and viral hepatitis coinfection, particularly in the setting of hepatic dysfunction, on ovarian function and estrogen production in women is unknown. To elucidate the mechanisms for low BMD in coinfected women, future studies should evaluate serum levels of cytokines, markers of bone resorption, insulin-like growth factor-1, osteoprotegerin, estradiol, testosterone, follicle stimulating hormone, and sex hormone binding globulin.
The increased risk of low BMD among HIV/viral hepatitis-coinfected woman has important clinical implications. Since low BMD is a predictor of fragility fractures [15], our data suggest that viral hepatitis-coinfected women are at even greater risk for vertebral and hip fractures compared to those with HIV alone. Moreover, low BMD is also associated with increased mortality [16, 17]. Since liver disease due to viral hepatitis currently accounts for the second leading cause of death in HIV [1], low BMD in coinfected women might contribute further to mortality, particularly as this population ages. The impact of therapies to improve bone mass (e.g., calcium, vitamin D, and bisphosphonates) as well as the effect of chronic HBV and HCV treatments on BMD should also be evaluated.
Interestingly, we found that men had lower mean BMD Z-scores at the lumbar spine and femoral neck compared to women. This finding has been reported in other studies of HIV-infected patients [31, 32], but the reasons for these differences are unclear. Future studies should examine differences in BMD among HIV-infected women and men and evaluate hormonal, anthropometric, virologic, immunologic, and therapeutic factors that may account for these differences.
Our study had several limitations. First, since cross-sectional studies evaluate exposure and disease status at the same point in time, this study design is limited in its ability to determine whether exposure preceded or resulted from disease. However, with regard to this study, low BMD does not lead to hepatitis B or C virus infections. As such, a cross-sectional study design remains appropriate to determine the association between viral hepatitis and low BMD.
Second, identification of HCV status was based on anti-HCV testing alone, since the cohort does not routinely test for HCV RNA, but not all anti-HCV-positive patients are HCV viremic. However, in the case of HIV/HCV coinfection, 95% of HIV-infected patients who are anti-HCV-positive have HCV viremia [33, 34], minimizing the likelihood for misclassification. Similarly, HBsAg-positive subjects may not have had active HBV replication, especially if they were treated with antiretroviral medications that are also active against HBV. To eliminate the possibility of this affecting our results, we performed sub-analyses excluding HBsAg-positive subjects, given the small number of patients with HBV infection in the sample.
Third, a number of potential confounding variables were evaluated and controlled for analytically, but multivariable analyses may not entirely eliminate residual confounding from additional unmeasured factors, as is always true for all observational studies. We did not have information on the duration of viral hepatitis infection, stage of hepatic fibrosis, or hepatitis genotype and therefore could not examine the relationship between these variables and reduced BMD. In particular, advanced liver histologic disease stage could negatively impact BMD in viral hepatitis, but liver biopsy results were not collected by the cohort.
Fourth, subjects were only from Italy, a homogeneous population, potentially limiting the generalizability of our results.
Finally, this study was limited by the absence of concurrent, local non-HIV-infected controls for the generation of Z-scores. Rather, this study relied on the DXA manufacturer’s reference data in ambulatory Caucasian volunteers from the United States, Europe, and Australia [35]. However, potential differences in BMD between the manufacturer’s reference data and the Modena non-HIV-infected population would not be expected to explain the sex differences in the effects of viral hepatitis coinfection observed here.
In conclusion, we found that viral hepatitis increased the risk of low BMD among HIV-infected patients. Future studies should evaluate fracture rates and examine risk factors and potential mechanisms for low bone mass among HIV/viral hepatitis-coinfected patients.
Acknowledgments
The authors appreciate the reviews of drafts of the manuscript by Dr. Brian L. Strom, Dr. Nicola Squillace, and Ms. Valerie Teal. They would also like to acknowledge the efforts of all study coordinators for the Modena HIV Metabolic Clinical Cohort. Finally, the authors would like to thank all study subjects for their participation in the Modena HIV Metabolic Clinical Cohort.
This work was supported by NIH research grant K01-AI070001 (to V.L.R.), the Penn AIDS Clinical Trial Unit U01-IA069467 (to P.T.), and the Penn Center for AIDS Research grant P30-AI045008 (to P.T.).
List of Abbreviations
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- BMD
bone mineral density
- DXA
dual-energy x-ray absorptiometery
- ART
antiretroviral therapy
Footnotes
V.L.R., P.T., J.R.K., and G.G. participated in the conception and design of the study. G.G., G.O., L.Z., and V.R. enrolled all patients in the study and collected data. V.L.R., M.B.L., R.L., and J.L. participated in data cleaning, analysis, and interpretations of results. V.L.R., M.B.L., R.L., and P.T. collaborated on the initial drafts of the manuscript. All other authors contributed further to manuscript edits.
These results were presented at the 16th Conference on Retroviruses and Opportunistic Infections, February 8–11, 2009, Montreal, Canada [Abstract 820].
References
- 1.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 2.Giordano TP, Kramer JR, Souchek J, Richardson P, El-Serag HB. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: a cohort study, 1992–2001. Arch Intern Med. 2004;164:2349–2354. doi: 10.1001/archinte.164.21.2349. [DOI] [PubMed] [Google Scholar]
- 3.Merchante N, Giron-Gonzalez JA, Gonzalez-Serrano M, Torre-Cisneros J, Garcia-Garcia JA, Arizcorreta A, et al. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS. 2006;20:49–57. doi: 10.1097/01.aids.0000198087.47454.e1. [DOI] [PubMed] [Google Scholar]
- 4.Soto B, Sanchez-Quijano A, Rodrigo L, del Olmo JA, Garcia-Bengoechea M, Hernandez-Quero J, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 5.Leslie WD, Bernstein CN, Leboff MS. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology. 2003;125:941–966. doi: 10.1016/s0016-5085(03)01062-x. [DOI] [PubMed] [Google Scholar]
- 6.Rouillard S, Lane NE. Hepatic osteodystrophy. Hepatology. 2001;33:301–307. doi: 10.1053/jhep.2001.20533. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Calvin JL, Gallego-Rojo F, Fernandez-Perez R, Casado-Caballero F, Ruiz-Escolano E, Olivares EG. Osteoporosis, mineral metabolism, and serum soluble tumor necrosis factor receptor p55 in viral cirrhosis. J Clin Endocrinol Metab. 2004;89:4325–4330. doi: 10.1210/jc.2004-0077. [DOI] [PubMed] [Google Scholar]
- 9.Hay JE. Bone disease in cholestatic liver disease. Gastroenterology. 1995;108:276–283. doi: 10.1016/0016-5085(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 10.Pignata S, Daniele B, Galati MG, Esposito G, Vallone P, Fiore F, et al. Oestradiol and testosterone blood levels in patients with viral cirrhosis and hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 1997;9:283–286. doi: 10.1097/00042737-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Amorosa V, Tebas P. Bone disease and HIV infection. Clin Infect Dis. 2006;42:108–114. doi: 10.1086/498511. [DOI] [PubMed] [Google Scholar]
- 12.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 14.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 15.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 16.Mussolino ME, Gillum RF. Low bone mineral density and mortality in men and women: the Third National Health and Nutrition Examination Survey linked mortality file. Ann Epidemiol. 2008;18:847–850. doi: 10.1016/j.annepidem.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mussolino ME, Madans JH, Gillum RF. Bone mineral density and mortality in women and men: the NHANES I epidemiologic follow-up study. Ann Epidemiol. 2003;13:692–697. doi: 10.1016/s1047-2797(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 18.Guaraldi G, Orlando G, Squillace N, De Santis G, Pedone A, Spaggiari A, et al. Multidisciplinary approach to the treatment of metabolic and morphologic alterations of HIV-related lipodystrophy. HIV Clin Trials. 2006;7:97–106. doi: 10.1310/EYWJ-8B5K-X7VQ-9CPE. [DOI] [PubMed] [Google Scholar]
- 19.The International Society for Clinical Densitometry. [Accessed July 2, 2008];Official Positions and Pediatric Official Positions of the International Society for Clinical Densitometry. 2007 http://www.iscd.org/Visitors/pdfs/ISCD2007OfficialPositions-Combined--AdultandPediatric.pdf.
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.Rothman KJ, Greenland S. Modern Epidemiology. 2. Philadelphia: Lippincott Williams and Wilkins; 1998. [Google Scholar]
- 22.Royston P. Multiple imputation of missing values: update. The STATA Journal. 2005;5:188–201. [Google Scholar]
- 23.Freedman VA, Wolf DA. A case study on the use of multiple imputation. Demography. 1995;32:459–470. [PubMed] [Google Scholar]
- 24.Chen CC, Wang SS, Jeng FS, Lee SD. Metabolic bone disease of liver cirrhosis: is it parallel to the clinical severity of cirrhosis? J Gastroenterol Hepatol. 1996;11:417–421. doi: 10.1111/j.1440-1746.1996.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 25.Corazza GR, Trevisani F, Di Stefano M, De Notariis S, Veneto G, Cecchetti L, et al. Early increase of bone resorption in patients with liver cirrhosis secondary to viral hepatitis. Dig Dis Sci. 2000;45:1392–1399. doi: 10.1023/a:1005568406664. [DOI] [PubMed] [Google Scholar]
- 26.Duarte MP, Farias ML, Coelho HS, Mendonca LM, Stabnov LM, do Carmo d Oliveira M, et al. Calcium-parathyroid hormone-vitamin D axis and metabolic bone disease in chronic viral liver disease. J Gastroenterol Hepatol. 2001;16:1022–1027. doi: 10.1046/j.1440-1746.2001.02561.x. [DOI] [PubMed] [Google Scholar]
- 27.Gallego-Rojo FJ, Gonzalez-Calvin JL, Munoz-Torres M, Mundi JL, Fernandez-Perez R, Rodrigo-Moreno D. Bone mineral density, serum insulin-like growth factor I, and bone turnover markers in viral cirrhosis. Hepatology. 1998;28:695–699. doi: 10.1002/hep.510280315. [DOI] [PubMed] [Google Scholar]
- 28.Tsuneoka K, Tameda Y, Takase K, Nakano T. Osteodystrophy in patients with chronic hepatitis and liver cirrhosis. J Gastroenterol. 1996;31:669–678. doi: 10.1007/BF02347615. [DOI] [PubMed] [Google Scholar]
- 29.Polak-Jonkisz D, Zwolinska D. Osteocalcin as a biochemical marker of bone turnover. Nephrology. 1998;4:339–346. [Google Scholar]
- 30.Toribio RE, Kohn CW, Capen CC, Rosol TJ. Parathyroid hormone (PTH) secretion, PTH mRNA and calcium-sensing receptor mRNA expression in equine parathyroid cells, and effects of interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha on equine parathyroid cell function. J Mol Endocrinol. 2003;31:609–620. doi: 10.1677/jme.0.0310609. [DOI] [PubMed] [Google Scholar]
- 31.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 32.Madeddu G, Spanu A, Solinas P, Calia GM, Lovigu C, Chessa F, et al. Bone mass loss and vitamin D metabolism impairment in HIV patients receiving highly active antiretroviral therapy. Q J Nucl Med Mol Imaging. 2004;48:39–48. [PubMed] [Google Scholar]
- 33.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 34.Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology. 1999;29:908–914. doi: 10.1002/hep.510290311. [DOI] [PubMed] [Google Scholar]
- 35.Mazess RB, Barden H. Bone density of the spine and femur in adult white females. Calcif Tissue Int. 1999;65:91–99. doi: 10.1007/s002239900663. [DOI] [PubMed] [Google Scholar]