Abstract
Genetic knockout mice studies suggested ABCG2/Abcg2 translocates nitrofurantoin at the mammary – blood barrier resulting in drug accumulation in milk. The purpose of this study was to establish the role of Abcg2 in nitrofurantoin accumulation in rat milk using GF120918 as a “chemical knockout” equivalent. The inhibitory effect of GF120918 was verified in MDCKII cells stably expressing rat Abcg2 with Hoechst 33342 and nitrofurantoin flux in transwells. Nitrofurantoin was infused (0.5mg/h) in the absence and presence GF120918 (10mg/kg in DMSO) to Sprague Dawley lactating female rats using a balanced crossover design. Administration of GF120918 increased nitrofurantoin concentration in serum (from 443±51 to 650±120ng/ml) and decreased concentration in milk (from 18.1±0.9 to 1.9±1.2μg/ml), resulting in corresponding mean values for M/S of 41.4±19.1 vs. 3.04±2.27 in the absence and presence of GF120918 (p<0.05), respectively. There was a decrease in systemic clearance with GF120918 (2.8±0.5 L/h/kg) compared to vehicle controls (4.1±0.5L/h/kg; p<0.05). Western blot analysis revealed good expression of Abcg2 and no P-gp expression in mammary gland while immunohistochemistry confirmed the apical expression of Abcg2 in lactating mammary gland epithelia. Nitrofurantoin active transport into rat milk can be inhibited by GF120918 resulting in a 10-fold lower M/S. Although GF120918 inhibits both Abcg2 and P-gp, the high expression of Abcg2 and the absence of detectable P-gp expression in lactating mammary gland validates an important role for Abcg2 in nitrofurantoin accumulation in rat milk. GF120918 is particularly useful as a rat “chemical knockout” model to establish ABCG2’s role in drug transfer into milk during breastfeeding.
Introduction
Most xenobiotics are transferred into milk by passive diffusion and the extent of their accumulation in milk (as measured by milk to serum concentration ratio, (M/S)) can be predicted by in vitro measurements of drug binding and ionization in milk and serum as well as lipid partitioning into milk (Fleishaker et al., 1987; Fleishaker and McNamara, 1988a). Such an approach successfully predicted the M/S for a number of drugs (Fleishaker and McNamara, 1988b); however, this approach was unable to estimate the large M/S observed for drugs like nitrofurantoin (Gerk et al., 2001a). In fact the observed ratios for nitrofurantoin in the human and rat were 20 and 100 times greater (Gerk et al., 2001a; Oo et al., 2001), respectively than could be accounted for by passive diffusion alone, clearly implicating an active transport process.
The transport of nitrofurantoin across a murine mammary epithelial cell culture model has been shown to be saturable and sodium dependent in the basal-to-apical direction (Toddywalla et al., 1997; Gerk et al., 2002). Merino et al described that nitrofurantoin was efficiently transported by murine Bcrp1 and human BCRP by using polarized cell lines (Merino et al., 2005). Importantly, they also demonstrated that the milk-to-plasma ratio of nitrofurantoin was almost 80 times higher in wild-type compared with Bcrp1 knockout lactating female mice (Merino et al., 2005). Nitrofurantoin, therefore, was identified as an excellent BCRP/Bcrp1 substrate that is translocated at the blood–mammary barrier resulting in its accumulation in milk.
A growing number of studies have shown the compensatory mechanism in genetic knockout mouse models. The most impressive evidence was from recent literature. In Bcrp (-/-) mice, several transporters, like Abca5, Abcb4, Abcc2, and Abcc4, were significantly up- or down-regulated; with the most pronounced differential expression observed with abcg5 and abcg8, which showed a 20- to 100-fold up-regulation in Bcrp (-/-) mice (Huls et al., 2008). Therefore, compensatory mechanisms in transport function in genetic knockout mice may complicate the interpretation of drug metabolism and disposition studies as well as active transport into milk.
In present study, we systematically evaluated the role of Abcg2 (Bcrp) in nitrofurantoin accumulation in rat milk. GF120918 pretreatment was administered to generate a “chemical knockout” equivalent of Abcg2 in lactating rat and nitrofurantoin was employed as the prototypical Abcg2 substrate. The results obtained from these studies provide new insights into the utility of the rat model for ABCG2 substrates in drug active transport into milk and other drug metabolism and disposition studies.
Materials and Methods
Chemicals
Nitrofurantoin and furazolidone were purchased from Sigma Chemical Co. (St. Louis, MO). N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918) was a gift from GlaxoSmithKline (Research Triangle Park, NC). Hoechst-33342 was from Invitrogen (Carlsbad, CA). All organic solvents (HPLC grade) and PEG400 were purchased from Fisher (Pittsburgh, PA) and all other chemicals were obtained from Sigma (St. Louis, Missouri) unless specified otherwise.
Animals
Five adult female lactating Sprague-Dawley rats (250 to 350 g) with 1 or 3 day old pups were purchased from Harlan Laboratories (Indianapolis, IN). Animals were maintained under a 12 h/12 h light/dark cycle and had access to food and water during the experiments. The rats were acclimatized for at least 1 week before the experiment. All procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Expression and Functional Characterization of Rat Abcg2 in MDCK-II Cells
Total RNA was isolated from a frozen liver of a Sprague Dawley rat using RNAeasy kit (Qiagen, Valencia, CA) and reverse transcribed to cDNA using oligo dT primers and Thermoscript RT PCR kit (Invitrogen, Carlsbad, CA). Rat Abcg2 cDNA was amplified using forward (5’-CCGCTCGAGGCATAGATCCTAAAGATGTCTC-3’) and reverse 5’-CTAGTCTAGAGGAGTACTATCAATAGTCCTTTC-3’) primers and Phusion DNA polymerase enzyme (NEB, Ipswich, MA). The PCR product was cloned into the pcDNA3.1 vector (Invitrogen, Carlsbad, CA) between XhoI and XbaI restriction sites. The Abcg2 sequence in this vector was confirmed (Davis Sequencing, Davis, CA) and it had 100% homology with the published rat Abcg2 sequence (Gene Bank accession number AB105817). MDCKII cells were maintained in MEM with Earle’s salts (Mediatech, Manassas, VA) with 5% FBS (GIBCO/Invitrogen, Carlsbad, CA) and 1X Penicillin Streptomycin (Invitrogen, Carlsbad, CA) at 37°C in 5% CO2. Cells were transfected with pcDNA3.1 or pcDNA3.1-rAbcg2 using Fugene 6 transfection reagent (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions. After 48 hours, cells were selected with 800 μg/ml G418 (Invitrogen, Carlsbad, CA) for 10 days.
Abcg2 expression was confirmed by Western blot analysis with polyclonal anti-ABCG2 antibody from Kamiya Biomedical Company (Seattle, WA). Rat Abcg2 function was assessed using the Hoechst-33342 (10μM) accumulation assay (Leggas et al., 2006). Single clones were isolated by fluorescence-activated cells sorting (FACS) (MoFlo™, DakoCytomation, Fort Collins, CO) on the basis of reduced accumulation of the Hoechst 33342 dye. The clone with the highest functional activity was chosen for in-vitro studies.
Inhibition of rAbcg2 by GF120918
To assess the utility of GF120918 as a chemical inhibitor of rat Abcg2, first, we incubated MDCKII-rAbcg2 cells with Hoechst 33342 in the presence and absence of 1μM GF120918. Briefly, cells were incubated with dye-containing medium at 37°C for 45 minutes. GF120918 was added at the indicated concentrations 10 minutes before the dye incubation. All flow cytometry assays with 10μM Hoechst 33342(Leggas et al., 2006). Secondly, the effect of GF120918 on nitrofurantoin directional transport was evaluated using MDCKII cells expressing rat Abcg2 or empty vector (pcDNA3.1). Cells were seeded on microporous membrane filters (3.0-μm pore size, 24-mm diameter; Transwell 3414; Corning Glassworks, Corning, NY) at a density of 1.0×106 cells per well. Cells were grown for 4 days to achieve TEER>200 Ω·cm2 and medium was replaced every other day. Prior to the experiment, the medium at both the apical and basolateral side of the monolayer was replaced with 2ml of OpitiMEM medium (Invitrogen, Carlsbad, CA) without serum, and either 1μM GF120918 or vehicle-only (0.1% DMSO) with 10μM nitrofurantoin containing 0.2μCi/ml [3H]-Mannitol. Cells were incubated at 37°C in 5% CO2. To ensure the tightness of each monolayer, 50 μl aliquots were collected to assess the paracellular flux of [3H]-Mannitol into the opposite compartment. We required that layers restricted mannitol transport to <1% of the total radioactivity per hour. For nitrofurantoin transport, 140μl aliquots were taken to at 0.5, 1, 2 and 3 hours. Samples were stored at -80°C until the time of analysis by HPLC assay.
In-vivo Studies
The jugular and femoral veins of Sprague-Dawley lactating female rats were cannulated under ketamine/xylazine anesthesia on day 10 to 12 post partum. After a day of recovery, each dam (n = 5) was randomized to receive an intravenous infusion of nitrofurantoin in PEG400 (0.5 mg/0.25ml/h) for 5 hours either with pretreatment of the inhibitor (GF120918, 10 mg/kg in DMSO) or with equivalent volume (about 0.15 ml DMSO) of vehicle administered intravenously over 10 min and crossed over on the second day to complete both phases (Gerk et al., 2001b; Edwards et al., 2005). One ml normal saline was given hourly to prevent dehydration during infusion. The dams were separated from the pups prior to the infusion. Blood samples were drawn hourly for the last 4 h of the infusion. The blood samples were protected from light, allowed to clot, centrifuged to harvest serum, and frozen at -80°C until analysis. Milk samples were obtained by manual manipulation under light anesthesia (ketamine) at the end of the infusion, protected from light, and also frozen at−80°C until analysis. Mammary gland and kidney samples were harvested from a separate group of lactating rats on day 16 to18 post partum, frozen in liquid nitrogen, and stored at -80°C until analysis.
Nitrofurantoin HPLC Analysis
To each aliquot (70 μl) of serum, 15 μl of a 5 μg/ml furazolidone HCl was added as an internal standard and was precipitated with 85 μl of cold methanol, vortexed for 5 min, and then centrifuged at 16,000g for 10 min at 4°C. Fifty microliters of the supernatant plus 50 μl mobile phase was mixed for 10 seconds and injected onto the HPLC. To each aliquot of milk, 25 μl of a 25 μg/ml furazolidone HCl was added as an internal standard to 50 μl milk sample and was precipitated with 200 μl of cold methanol, vortexed for 5 min, and then centrifuged at 16,000g for 10 min at 4°C. Then 50 μl of the supernatant mixed with 50 μl mobile phase and injected onto the HPLC. The HPLC system consists of a Lichrosorb 5 RP18 125 × 4.0-mm column (Phenomenex, Torrance, CA) and eluted with 20% acetonitrile: 80% 25 mM potassium phosphate buffer (pH 3.0) at 1.0 ml/min. UV absorbance was measured at 366 nm. Peak height ratios (nitrofurantoin/furazolidone) were used for comparison with the standard curve. The standard curve range for nitrofurantoin in serum was 62.5 to 4000 ng/ml. The milk samples were diluted if the concentration was greater than the linear range for the standard curve (0.725 to 29 μg/ml). Aliquots of nitrofurantoin in OptiMEM medium were directly injected onto the HPLC. The standard curve range was 7.8 to 2000 ng/ml. All standard curves demonstrated an intra- and inter-day variability of less than 10% and r2 >0.99.
Western Blot Analysis for P-gp and Abcg2
Crude membrane fractions were prepared from lactating mammary gland and kidney. Briefly, the tissue samples were homogenized in Dounce Buffer (Tris buffer pH 7.6 at 4°C, 0.5 mM magnesium chloride) with protease inhibitors (Complete Mini EDTA-free tablets, Roche Diagnostics, Indiapolis, IN) and the tonicity restored to 150 mM with sodium chloride. Following centrifugation at 1200 g for 5 min at 4°C, the supernatant was removed and EDTA was added to a final concentration of 5 mM. The sample was then centrifuged further at 100,000 g for 1 hour at 4°C to pellet the crude membrane fractions. Pellets were resuspended in resuspension buffer (0.2 M mannitol, 0.07 M sucrose, 50 μM Tris HCl, 1 μM EDTA). Rat Abcg2- and pcDNA3.1 (empty vector)-MDCKII cell pellets were resuspended in RIPA buffer (50mM Tris pH 8.0, 150mM NaCl, 1% NP40, 0.5% deoxycholate, 0.1% SDS, protease inhibitor cocktail), rotated for 30 minutes at 4°C, then centrifuged at 13000 rpm for 20 minutes at 4°C. Supernatants were collected for immunoblot. Protein concentrations were measured using the BCA Protein Assay kit (Pierce, Rockford, IL). PNGase F (New England Biolabs, Ipswich, MA) was used for deglycosylation of rat Abcg2 following manufacturer instructions. Briefly, 60 μg (lactating mammary gland), 100 μg (kidney) and 7 μg (pcDNA3.1 expressed MDCKII cell and Abcg2 expressed MDCKII cell) of membrane fractions were incubated with 10× denaturing buffer supplied and PNGase F (4 μl) at 37 °C for 2.5 h. Then the samples were mixed with 2× SDS-loading buffer and reducing buffer at 37°C for 30 minutes as the non-PNGase treatment group. Western blotting was performed as described previously (Edwards, 2005). P-gp expression was determined using the C219 (mouse anti-human P-gp; 1:100) monoclonal antibody which was obtained from Signet Laboratories (Dedham, MA) and Abcg2 was determined using the PC-138 (1:1000) antibody which was obtained from Kamiya Biomedical (Seattle, WA). Antibody binding was detected using a horseradish peroxide linked IgG (H+L) (goat anti-rabbit) and IgG (H+L) (rabbit anti-mouse) antibodies (1:20000) obtained from Pierce, Inc. (Rockford, IL). The bands were visualized using a SuperSignal Pico Chemiluminescent substrate detection kit (Pierce, Rockford, IL).
Immunohistochemistry for Abcg2 localization in lactating mammary gland
Six-micrometer sections of paraffin blocks were cut using microtomy. After deparaffinization and rehydration, endogenous peroxidase was blocked with H2O2 in methanol for 20 min. The sections were blocked using an avidin biotin blocking kit (Vector Laboratories, Burlingame, CA), and PC-138 (1:1000) was applied for kidney and lactating mammary gland. For non-specific staining control, rabbit IgG (Invitrogen, Carlsbad, CA) was applied to the slide instead of PC-138 (1:1000). The sections were incubated overnight at 4°C with amplifier. Subsequently, the slides were washed in PBS-T and then incubated with biotinylated anti-rabbit antibody (1:600 dilutions) and HRP-conjugated avidin-biotin-complex (Vector Laboratories, Burlingame, CA). The antibody binding sites were visualized by incubation with DAB-H2O2 solution to develop a brown color and counterstained with hematoxylin, dehydrated, and coverslipped.
Pharmacokinetic calculations and statistical analysis
Systemic clearance was calculated using the following formula.
| (1) |
Where Cs is the average concentration of nitrofurantoin in serum from 3 to 5 hours.
M/S was calculated by:
| (2) |
Where Cm is concentration of nitrofurantoin in milk at 5 hours.
All data are expressed as mean ± standard deviation (SD). Analyses were performed to estimate intergroup differences using the One-way ANOVA. A p value of <0.05 was considered statistically significant.
Results
Functional characterization of rat Abcg2 expressed in MDCKII cells and GF120918 as an inhibitor of rat Abcg2
The full-length rat Abcg2 complementary DNA was expressed in MDCK-II cells. The functional activity of Abcg2 was characterized and high activity clones were selected using FACS based on the ability to limit the accumulation of the prototypical human and mouse ABCG2 substrate, Hoechst 33342. Rat Abcg2 clone was selected and validated as (Figure 1A). The phenotype of this clone could be reversed with GF120918 (Figure 1B). Furthermore, the effect of GF120918 on the transport of nitrofurantoin by rat Abcg2 was investigated in MDCKII-rat Abcg2 and MDCKII-empty cells. These MDCKII-rat Abcg2 cells demonstrated 7.5-fold increase in apical directed transport and a 55% decrease in basolateral transport of nitrofurantoin (Figure 1C), which was largely reversed by GF120918 treatment (Figure 1D). These results show the creation of a MDCKII–rat Abcg2 expressing cell line was successful and that GF120918 is an inhibitor of rat Abcg2.
Figure 1.
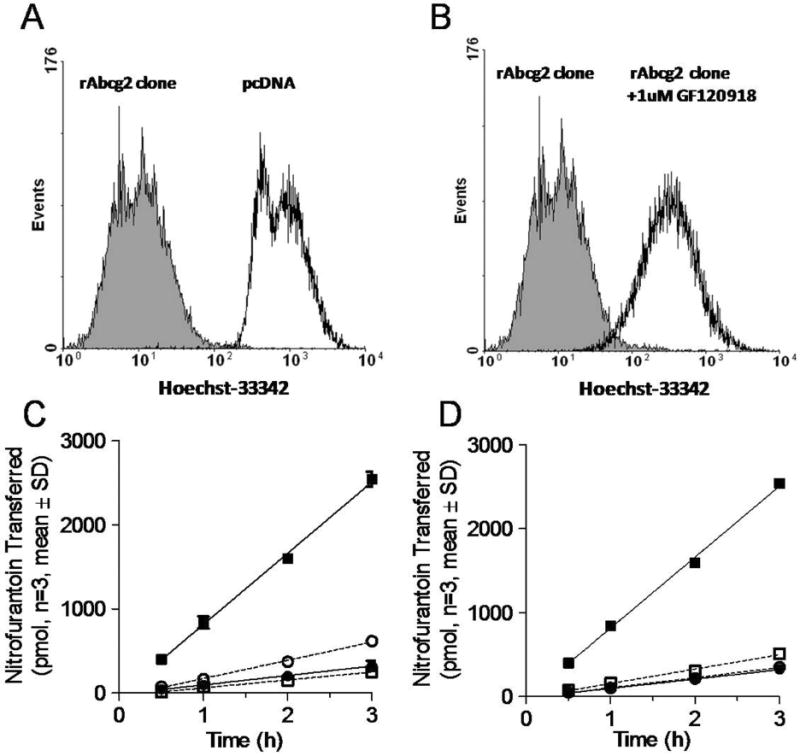
Functional characterization of MDCKII-rat Abcg2 expressed cells. The Hoechst 33342 accumulation of MDCKII cells with either empty vector (black unfilled) or rAcbg2 clone cells (shaded) (Panel A). Cells were analyzed by flow cytometry following incubation of the ABCG2 substrate Hoescht 33342 in presence (black unfilled) and absence (shaded) of 1 μM of the ABCG2 inhibitor, GF120918 (Panel B). The amount of nitrofurantoin appearing in the receiver compartment across MDCKII-empty vector and MDCKII-rAbcg2 monolayers grown on Transwells (Panels C and D). Panel C depicts the flux of nitrofurantoin from the basolateral to apical (●■); apical to basolateral (○□) in MDCKII-empty (●○) and MDCKII –Abcg2 (□■); Panel D depicts the transport of nitrofurantoin from the basolateral to apical in MDCKII-empty (●○) and MDCKII –Abcg2 (□■) in MDCKII-rAbcg2 absence (●■) or presence (○□) of 1 μM GF120918.
Expression of rAbcg2 and P-gp in lactating mammary gland and rAbcg2 expressed MDCKII cells
Abcg2 and P-gp expression in rat lactating mammary gland were assayed by Western blot. In contrast to the kidney (positive control), P-gp was not detected in lactating mammary gland (Figure 2A). Abcg2 expression was detected in lactating mammary gland, kidney and Abcg2 expressing MDCKII cell line (Figure 2 B and C); however, the native Abcg2 bands were different sizes in the mammary gland (~72kd), rat Abcg2 expressing in MDCKII cell line (~80kd) and the kidney (~80kd). Following deglycosylation (Figure 2 B and C), all of the native bands were reduced to approximately 60 kDa. Immunohistochemistry result showed the localization of Abcg2 in rat lactating mammary gland (Figure 3). Abcg2 was strongly expressed on the apical side of epithelial cells in lactating mammary gland. As a positive control, Abcg2 staining was mainly observed in the renal proximal tubules in kidney. Abcg2 staining was not found in the nonspecific staining control.
Figure 2.
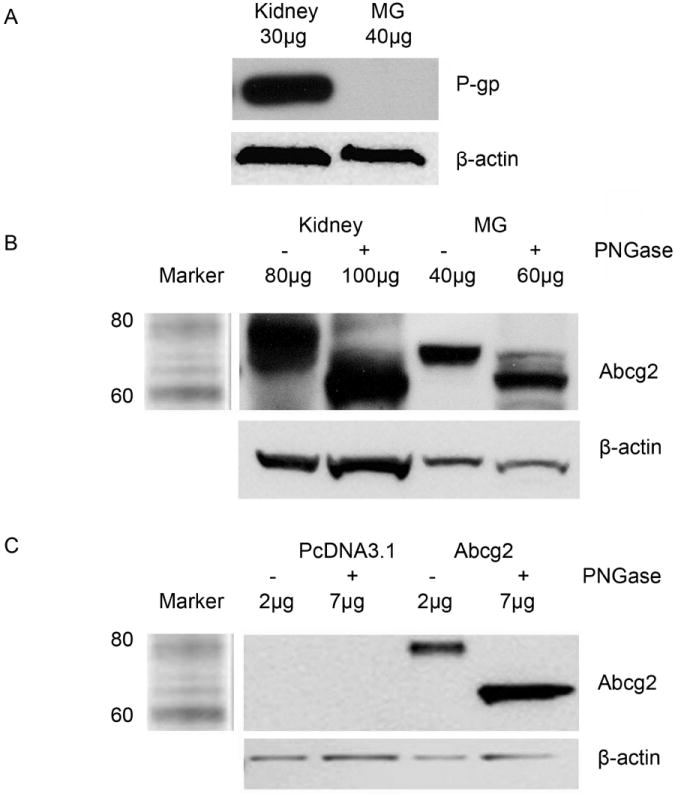
Western blot analysis of P-gp and Abcg2 expression. Crude membrane fractions were prepared for all the samples. P-gp expression in rat kidney and lactating mammary gland (Panel A). The expression of native and deglycosylated rAbcg2 in lactating mammary gland, kidney (Panel B), vector (pcDNA3.1), and rAbcg2 expressed MDCKII cell line (Panel C).
Figure 3.
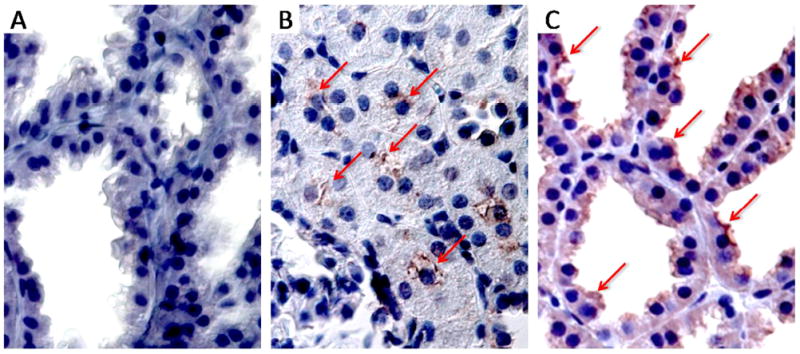
Immunohistochemical detection of Abcg2 (magnification, 63x) in rat lactating mammary gland (nonspecific staining control) (Panel A), rat kidney (positive control) (Panel B) and rat lactating mammary gland (Panel C).
Infusion Studies
Results from the nitrofurantoin infusion study in lactating rats are shown in Figure 4. The concentration of nitrofurantoin in serum reached steady-state at 2 hours following the initiation of the infusion and was maintained through 5 hours. Linear regression revealed that the slope (plasma concentration versus time) was not significantly (p<0.05) different from zero over this timeframe, however there was a trend for the GF120918 treatment to increase with time. The presence of GF120918 resulted in a higher concentration of nitrofurantoin in serum (Figure 4A). The systemic clearance of nitrofurantoin in lactating rats was 4.12 ± 0.5 L/h/kg and was significantly decreased to 2.78 ± 0.5 L/h/kg (p<0.05, Figure 4B) by coadministration with GF120918. Higher nitrofurantoin serum concentrations and lower milk concentrations (Figure 4A) in GF120918 treated group resulted in a substantial reduction (93%) in the M/S of nitrofurantoin from 41.5 ± 19.1 to 3.04 ± 2.27 (Figure 4C).
Figure 4.
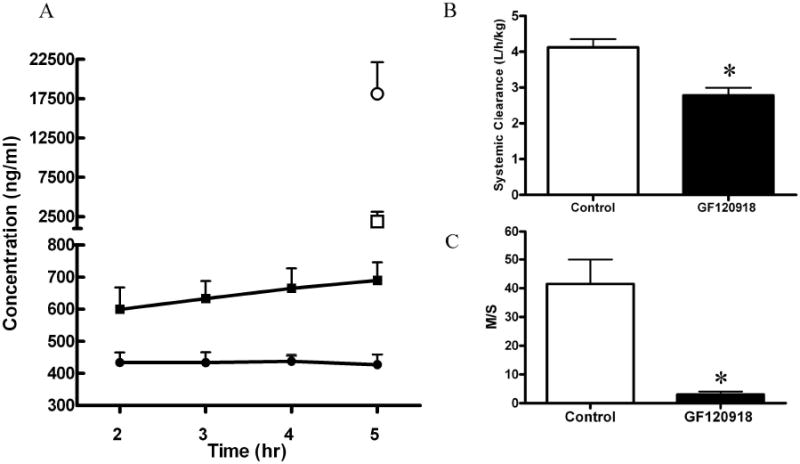
Concentration versus time profile (Panel A) for serum (●■) and milk (○□) following nitrofurantoin infusion (0.5mg/hr) in GF120918 (10mg/kg) treated (□■) or control lactating rats (●○). Nitrofurantoin systemic clearance (Panel B) and M/S (Panel C). Asterisks represent data points that are significantly different from control. * P<0.05. Data is presented as mean ± SD).
Discussion
In the present study, we have utilized GF120918 as an inhibitor of Abcg2 in order to assess its role in drug transfer into rat milk. GF120918 was originally developed as a second-generation P-gp inhibitor (Tan et al., 2000). It belongs to the family of acridone carboxamide derivatives (Warmann et al., 2002; Boumendjel et al., 2007) and has an IC50 value of ~30 to 300nM on the cellular accumulation of phosphine technetium complexes by human P-gp in Sf9 cells (Luker et al., 1997). GF120918 is also a potent ABCG2 inhibitor with IC50 values of ~300nM on efflux or accumulation in cell lines transfected with human ABCG2 (Ahmed-Belkacem et al., 2005; Pan et al., 2007). Based on our results, GF120918 (1 μM) appeared to effectively inhibit the efflux of Hoechst 33342 and nitrofurantoin from rAbcg2 expressing MDCKII cell line, thus confirming that GF120918 is an effective in vitro inhibitor of rat Abcg2.
Following a 10 mg/kg dose, GF120918 significantly increased the serum concentrations of nitrofurantoin by decreasing its systemic clearance. Previous investigators have noted the systemic clearance of nitrofurantoin to be mediated, in part, by Abcg2-dependent processes (Merino et al., 2005; Wang and Morris, 2007). Following oral and intravenous administration of nitrofurantoin, the area under the concentration time curve in Bcrp1 knockout mice was 4-fold and 2-fold higher, respectively, than in wild-type mice (Merino et al., 2005), due to reduced first pass and hepatobiliary excretion of nitrofurantoin. Zhang et al have also shown that Bcrp1 limited the fetal distribution of nitrofurantoin and had a minor effect on the systemic clearance of the drug in the pregnant mouse (Zhang et al., 2007).
In previous work in our lab, the P-gp substrate, nelfinavir, was dosed to lactating rats with GF120918 (Edwards et al., 2005). GF120918 had no effect on M/P (the ratio of drug concentration in milk to in plasma) but did change brain to plasma ratio. Nelfinavir, like other human immunodeficiency virus protease inhibitors are well established substrates of P-gp (Kim et al., 1998; Lee et al., 1998; Washington et al., 1998). These protease inhibitors have been shown to be inhibitors, but not substrates of Abcg2 (Gupta et al., 2004). This evidence suggests that P-gp does not play a role in drug transfer into rat milk. By contrast, nitrofurantoin has not been shown to be a substrate of other major efflux transporters, such as P-gp, Mrp1 and Mrp2 (Merino et al., 2005; Wang and Morris, 2007). Hence, the affect GF120918 on the clearance and M/S of nitrofurantoin is not mediated by P-gp, but due largely to Abcg2 inhibition. It should be noted, however, a study of membrane transporters in MDCKII-hMDR1 using GF120918 to inhibit digoxin and loperamide efflux implicated a possible basolateral transporter(s) which could be inhibited by GF120918 (Acharya et al., 2008). The identity or function of basolateral transporter(s) on lactating mammary epithelial cells and their potential impact on M/S has not been established.
Consistent with mammary gland expression in other species (Merino et al., 2005), our western blotting results show Abcg2 is highly expressed in the lactating rat. P-gp appears to be transcriptional down-regulated during lactation (Alcorn et al., 2002) and protein expression was not detectable in the lactating rat mammary gland (Figure 2A). All of the native Abcg2 bands were reduced to approximately 60 kDa following deglycosylation. This observation is consistent with the result previous work suggesting that ABCG2 contains N-linked oligosaccharides, probably at both the putative glycosylation sites (Litman et al., 2002). The native Abcg2 bands in kidney and rAbcg2 - MDCKII cell line had a larger size (approximately 80 kd) than the mammary gland (approximately 70kd), suggesting rat Abcg2 may have tissue or cell specific patterns of glycosylation.
Consistent with the Western blot analysis, immunohistochemistry clearly indicated that Abcg2 is strongly expressed on the apical side of epithelial cells in lactating mammary gland. Apical expression also confirms the role of Abcg2 in actively transporting nitrofurantoin out of the epithelial cells and into rat milk.
The rat is a frequently used animal model in drug development and may be a better animal model under certain experimental paradigms (e.g., multiple samples, crossover study design). Furthermore, drugs identified as accumulating in rat milk by active transport are consistent with human literature (McNamara et al., 1991; McNamara et al., 1992). Hence, evidence gathered to date suggest that the rat is a good model for identifying which drugs might undergo active transport into human milk.
As noted in the introduction, genetic knockout models are useful tools to demonstrate transporter function with the assumption that the only difference from wild-type is the abolishment of expression of the intended protein. However, knockout models have been found to undergo additional changes which might further complicate interpretation. For example, brain microvessels from Mdr1a deficient mice express 3 times more Abcg2 RNA than microvessels of wild-type mice (Cisternino et al., 2004). Although the accumulation of Abcg2 substrates, mitoxantrone and prazosin was significantly increased in the presence of GF120918 in both wild-type and P-gp deficient mice there was significant difference between them (Cisternino et al., 2004). Whether or not this increased expression and activity of Abcg2 is a compensatory mechanism for the lack of Mdr1a at the mouse blood-brain barrier remains to be elucidated. There are other compensatory functions which also may be present in genetic knockout animals. In Mrp2 (-/-) mice, Mrp4 mRNA and protein expression in liver and kidney were increased approximately 6- and 2-fold, respectively (Chu et al., 2006). In Mrp2-deficient rats [TR (-)], Mrp3 was significantly up-regulated in the liver (approximately 6-fold) and kidney (approximately 3.5-fold) compared with wild-type controls. Likewise, the expression of the Ugt1 enzyme was increased in the liver and kidney of TR (-) rats by approximately 3.5- and 5.5-fold (Johnson et al., 2006). Therefore, alterations in drug disposition using knockout animals must be interpreted cautiously because of the possibility of compensatory changes in other transport proteins.
The inhibition of Abcg2-associated nitrofurantoin accumulation by GF120918 in the lactating rat (“chemical knockout”) provides support for the use of this alternative to the genetic knockout model for establishing the role of Abcg2 in drug active transport into milk. GF120918 has been tested in phase I clinical trials and was confirmed to have little toxicity (Planting et al., 2005; Kuppens et al., 2007). Various studies have shown that GF120918 can be tolerated in humans and animals at concentrations sufficient to inhibit ABCG2. Hence, GF120918 is suitable for in vivo animal and perhaps even clinical studies (Mao and Unadkat, 2005).
In summary, Abcg2, but not P-gp is expressed in lactating mammary epithelia and appears to be responsible for nitrofurantoin active transport into rat milk. The rat appears to be a good animal model for identifying those drugs which would accumulate in human milk. GF120918 appears to be an effective in vivo inhibitor (“chemical knockout”) tool for confirming the role of Abcg2 in milk transfer. These data are especially valuable for the design of studies of ABCG2 substrates during breastfeeding using the rat as animal model.
Abbreviations
- ABCG2
ATP binding cassette transporter family G member 2
- P-gp
p-glycoprotein
- MDCKII
Madin-Darby canine kidney II
- GF120918
N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide
References
- Acharya P, O’Connor MP, Polli JW, Ayrton A, Ellens H, Bentz J. Kinetic identification of membrane transporters that assist P-glycoprotein-mediated transport of digoxin and loperamide through a confluent monolayer of MDCKII-hMDR1 cells. Drug Metab Dispos. 2008;36:452–460. doi: 10.1124/dmd.107.017301. [DOI] [PubMed] [Google Scholar]
- Ahmed-Belkacem A, Pozza A, Munoz-Martinez F, Bates SE, Castanys S, Gamarro F, Di Pietro A, Perez-Victoria JM. Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. Cancer Res. 2005;65:4852–4860. doi: 10.1158/0008-5472.CAN-04-1817. [DOI] [PubMed] [Google Scholar]
- Alcorn J, Lu X, Moscow JA, McNamara PJ. Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction. J Pharmacol Exp Ther. 2002;303:487–496. doi: 10.1124/jpet.102.038315. [DOI] [PubMed] [Google Scholar]
- Boumendjel A, Macalou S, Ahmed-Belkacem A, Blanc M, Di Pietro A. Acridone derivatives: design, synthesis, and inhibition of breast cancer resistance protein ABCG2. Bioorg Med Chem. 2007;15:2892–2897. doi: 10.1016/j.bmc.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Chu XY, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, Wang RW, Yabut J, Hartley DP, Evans DC, Evers R. Characterization of mice lacking the multidrug resistance protein MRP2 (ABCC2) J Pharmacol Exp Ther. 2006;317:579–589. doi: 10.1124/jpet.105.098665. [DOI] [PubMed] [Google Scholar]
- Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann JM. Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res. 2004;64:3296–3301. doi: 10.1158/0008-5472.can-03-2033. [DOI] [PubMed] [Google Scholar]
- Edwards JE, Alcorn J, Savolainen J, Anderson BD, McNamara PJ. Role of P-glycoprotein in distribution of nelfinavir across the blood-mammary tissue barrier and blood-brain barrier. Antimicrob Agents Chemother. 2005;49:1626–1628. doi: 10.1128/AAC.49.4.1626-1628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleishaker JC, Desai N, McNamara PJ. Factors affecting the milk-to-plasma drug concentration ratio in lactating women: physical interactions with protein and fat. J Pharm Sci. 1987;76:189–193. doi: 10.1002/jps.2600760302. [DOI] [PubMed] [Google Scholar]
- Fleishaker JC, McNamara PJ. Effect of altered serum protein binding on propranolol distribution into milk in the lactating rabbit. J Pharmacol Exp Ther. 1988a;244:925–928. [PubMed] [Google Scholar]
- Fleishaker JC, McNamara PJ. In vivo evaluation in the lactating rabbit of a model for xenobiotic distribution into breast milk. J Pharmacol Exp Ther. 1988b;244:919–924. [PubMed] [Google Scholar]
- Gerk PM, Hanson L, Neville MC, McNamara PJ. Sodium dependence of nitrofurantoin active transport across mammary epithelia and effects of dipyridamole, nucleosides, and nucleobases. Pharm Res. 2002;19:299–305. doi: 10.1023/a:1014495018640. [DOI] [PubMed] [Google Scholar]
- Gerk PM, Kuhn RJ, Desai NS, McNamara PJ. Active transport of nitrofurantoin into human milk. Pharmacotherapy. 2001a;21:669–675. doi: 10.1592/phco.21.7.669.34574. [DOI] [PubMed] [Google Scholar]
- Gerk PM, Oo CY, Paxton EW, Moscow JA, McNamara PJ. Interactions between cimetidine, nitrofurantoin, and probenecid active transport into rat milk. J Pharmacol Exp Ther. 2001b;296:175–180. [PubMed] [Google Scholar]
- Gupta A, Zhang Y, Unadkat JD, Mao Q. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2) J Pharmacol Exp Ther. 2004;310:334–341. doi: 10.1124/jpet.104.065342. [DOI] [PubMed] [Google Scholar]
- Huls M, Brown CD, Windass AS, Sayer R, van den Heuvel JJ, Heemskerk S, Russel FG, Masereeuw R. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73:220–225. doi: 10.1038/sj.ki.5002645. [DOI] [PubMed] [Google Scholar]
- Johnson BM, Zhang P, Schuetz JD, Brouwer KL. Characterization of transport protein expression in multidrug resistance-associated protein (Mrp) 2-deficient rats. Drug Metab Dispos. 2006;34:556–562. doi: 10.1124/dmd.105.005793. [DOI] [PubMed] [Google Scholar]
- Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens IE, Witteveen EO, Jewell RC, Radema SA, Paul EM, Mangum SG, Beijnen JH, Voest EE, Schellens JH. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res. 2007;13:3276–3285. doi: 10.1158/1078-0432.CCR-06-2414. [DOI] [PubMed] [Google Scholar]
- Lee CG, Gottesman MM, Cardarelli CO, Ramachandra M, Jeang KT, Ambudkar SV, Pastan I, Dey S. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry. 1998;37:3594–3601. doi: 10.1021/bi972709x. [DOI] [PubMed] [Google Scholar]
- Leggas M, Panetta JC, Zhuang Y, Schuetz JD, Johnston B, Bai F, Sorrentino B, Zhou S, Houghton PJ, Stewart CF. Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo. Cancer Res. 2006;66:4802–4807. doi: 10.1158/0008-5472.CAN-05-2915. [DOI] [PubMed] [Google Scholar]
- Litman T, Jensen U, Hansen A, Covitz KM, Zhan Z, Fetsch P, Abati A, Hansen PR, Horn T, Skovsgaard T, Bates SE. Use of peptide antibodies to probe for the mitoxantrone resistance-associated protein MXR/BCRP/ABCP/ABCG2. Biochim Biophys Acta. 2002;1565:6–16. doi: 10.1016/s0005-2736(02)00492-3. [DOI] [PubMed] [Google Scholar]
- Luker GD, Rao VV, Crankshaw CL, Dahlheimer J, Piwnica-Worms D. Characterization of phosphine complexes of technetium(III) as transport substrates of the multidrug resistance P-glycoprotein and functional markers of P-glycoprotein at the blood-brain barrier. Biochemistry. 1997;36:14218–14227. doi: 10.1021/bi971931z. [DOI] [PubMed] [Google Scholar]
- Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. Aaps J. 2005;7:E118–133. doi: 10.1208/aapsj070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara PJ, Burgio D, Yoo SD. Pharmacokinetics of acetaminophen, antipyrine, and salicylic acid in the lactating and nursing rabbit, with model predictions of milk to serum concentration ratios and neonatal dose. Toxicol Appl Pharmacol. 1991;109:149–160. doi: 10.1016/0041-008x(91)90198-n. [DOI] [PubMed] [Google Scholar]
- McNamara PJ, Burgio D, Yoo SD. Pharmacokinetics of caffeine and its demethylated metabolites in lactating adult rabbits and neonatal offspring. Predictions of breast milk to serum concentration ratios. Drug Metab Dispos. 1992;20:302–308. [PubMed] [Google Scholar]
- Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol. 2005;67:1758–1764. doi: 10.1124/mol.104.010439. [DOI] [PubMed] [Google Scholar]
- Oo CY, Paxton EW, McNamara PJ. Active transport of nitrofurantoin into rat milk. Adv Exp Med Biol. 2001;501:547–552. doi: 10.1007/978-1-4615-1371-1_68. [DOI] [PubMed] [Google Scholar]
- Pan G, Giri N, Elmquist WF. Abcg2/Bcrp1 mediates the polarized transport of antiretroviral nucleosides abacavir and zidovudine. Drug Metab Dispos. 2007;35:1165–1173. doi: 10.1124/dmd.106.014274. [DOI] [PubMed] [Google Scholar]
- Planting AS, Sonneveld P, van der Gaast A, Sparreboom A, van der Burg ME, Luyten GP, de Leeuw K, de Boer-Dennert M, Wissel PS, Jewell RC, Paul EM, Purvis NB, Jr, Verweij J. A phase I and pharmacologic study of the MDR converter GF120918 in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2005;55:91–99. doi: 10.1007/s00280-004-0854-6. [DOI] [PubMed] [Google Scholar]
- Tan B, Piwnica-Worms D, Ratner L. Multidrug resistance transporters and modulation. Curr Opin Oncol. 2000;12:450–458. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Toddywalla VS, Kari FW, Neville MC. Active transport of nitrofurantoin across a mouse mammary epithelial monolayer. J Pharmacol Exp Ther. 1997;280:669–676. [PubMed] [Google Scholar]
- Wang X, Morris ME. Effects of the flavonoid chrysin on nitrofurantoin pharmacokinetics in rats: potential involvement of ABCG2. Drug Metab Dispos. 2007;35:268–274. doi: 10.1124/dmd.106.011684. [DOI] [PubMed] [Google Scholar]
- Warmann S, Gohring G, Teichmann B, Geerlings H, Fuchs J. MDR1 modulators improve the chemotherapy response of human hepatoblastoma to doxorubicin in vitro. J Pediatr Surg. 2002;37:1579–1584. doi: 10.1053/jpsu.2002.36188. [DOI] [PubMed] [Google Scholar]
- Washington CB, Duran GE, Man MC, Sikic BI, Blaschke TF. Interaction of anti-HIV protease inhibitors with the multidrug transporter P-glycoprotein (P-gp) in human cultured cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:203–209. doi: 10.1097/00042560-199811010-00001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Unadkat JD, Mao Q. Breast cancer resistance protein 1 limits fetal distribution of nitrofurantoin in the pregnant mouse. Drug Metab Dispos. 2007;35:2154–2158. doi: 10.1124/dmd.107.018044. [DOI] [PubMed] [Google Scholar]


