Abstract
The sustained differentiation of T cells in the thymus cannot be maintained by resident intrathymic (IT) precursors and requires that progenitors be replenished from the bone marrow (BM). In patients with severe combined immunodeficiency (SCID) treated by hematopoietic stem cell transplantation, late T-cell differentiation defects are thought to be due to an insufficient entry of donor BM progenitors into the thymus. Indeed, we find that the intravenous injection of BM progenitors into nonconditioned ζ-chain–associated protein kinase 70 (ZAP-70)–deficient mice with SCID supports short- but not long-term thymopoiesis. Remarkably, we now show that the IT administration of these progenitors produces a significant level of donor-derived thymopoiesis for more than 6 months after transplantation. In contrast to physiologic thymopoiesis, long-term donor thymopoiesis was not due to the continued recruitment of progenitors from the BM. Rather, IT transplantation resulted in the unique generation of a large population of early c-Kithigh donor precursors within the thymus. These ZAP-70–deficient mice that received an IT transplant had a significantly increased prothymocyte niche compared with their untreated counterparts; this phenotype was associated with the generation of a medulla. Thus, IT administration of BM progenitors results in the filling of an expanded precursor niche and may represent a strategy for enhancing T-cell differentiation in patients with SCID.
Introduction
Patients with severe combined immunodeficiency (SCID) present with opportunistic infections that are fatal in infancy. Allogeneic hematopoietic stem cell (HSC) transplantation has long been the mainstay treatment of patients with SCID. The generation of T cells from intravenously administered donor HSCs occurs in the thymus and, as such, requires that they home to this organ before differentiation. In humans, it is not clear whether intravenously injected HSCs or progenitors with T-lineage potential first home to the bone marrow (BM) and are then exported to the thymus or, alternatively, directly enter the thymus. At least in mice, it is known that the latter scenario exists. Specifically, Spangrude and Weissman1 showed that intravenously injected BM-derived progenitor cells enter the thymus within 4 hours after their infusion. Nevertheless, it appears that the murine thymus is not continuously receptive to the import of hematopoietic progenitors, alternating between refractory and responsive periods.2 During refractory periods, donor progenitor cells differentiate into T cells only if they are injected directly into the thymus.2
The intrathymic (IT) transfer of thymocyte progenitors results in thymopoiesis, but this process does not continue long term.3–5 As a consequence, it is thought that long-term thymocyte differentiation requires ongoing migration of donor progenitors from the BM to the thymus. In the case of patients with SCID who receive a HSC transplant, recent studies suggest that this long-term thymopoiesis may, in fact, not occur. This conclusion is based on several findings. (1) Only a few naive T cells are detected 10 years after transplantation, correlating with a skewing of the T cell receptor repertoire.6 (2) The number of T-cell receptor excision circles, representing thymic export, decreases to extremely low levels within 18 years after transplantation, whereas in healthy persons, this process occurs over 80 years.7 (3) T-cell function declines at late time points after transplantation.8 Although it was once assumed that all transplanted SCID patients with long-term peripheral T cells had donor stem cell engraftment, we now know that T cells can have a life span of longer than 10 years.9 Thus, the presence of peripheral T cells does not necessarily reflect an ongoing thymopoiesis. The lack of long-term thymopoiesis is probably due to an absence of donor HSC engraftment in the BM after the intravenous administration of donor HSCs,10 a problem that is compounded by the lack of conditioning in many patients with SCID.
We hypothesized that early T-cell reconstitution of patients with SCID might be enhanced if HSCs/progenitor cells were injected directly into the thymus. To test this hypothesis, we previously performed studies in nonconditioned neonatal ζ-chain–associated protein kinase 70 (ZAP-70)–deficient mice with SCID and found that IT injection of wild-type (WT) HSCs results in a more rapid and diverse T-cell reconstitution, requiring 10-fold fewer donor HSCs.11 Moreover, to evaluate the clinical potential of an IT-based treatment strategy, we assessed whether this approach was feasible in macaques. Using thoracoscopy, we determined that this technique could be easily and safely applied to anesthetized macaques, with accurate targeting of the thymus, requiring a total procedure time of less than 15 minutes.12
However, our initial experiments did not address the question of stem/progenitor cell engraftment and long-term donor-derived thymopoiesis. Previous conclusions that the thymus cannot sustain the maintenance of a progenitor cell with self-renewal capacity were based on experiments wherein thymic or BM precursors were injected into the thymus in a noncompetitive setting, and, in most cases, the thymus was perturbed by irradiation before transplantation.3–5,13–16 As such, the question of whether the thymus environment is capable of providing a niche for a progenitor cell with long-term T-cell differentiation potential remained open.
Here, we initiated studies to assess the long-term fate of WT donor cells after their IT administration into nonconditioned ZAP-70−/− mice. We now demonstrate that thymopoiesis is maintained for longer than 6 months after IT transplantation. Although donor-derived thymopoiesis was detected in most of the IT-reconstituted mice, it was not observed in any of the mice that received an intravenous transplant. Notably, this long-term thymopoiesis was not due to the continuous migration of donor cells from the BM into the thymus, but it was due to increased occupancy of thymic precursor niches. After IT transplantation, the absolute numbers of c-Kithigh early T-cell progenitors (ETPs) in mice that received an IT transplant approached that detected in WT mice. Moreover, the massive increase in precursors after IT transplantation was associated with the generation of a normal medullary structure. Thus, the present study shows increased occupancy of thymic precursor niches after the forced entry of BM progenitors into a severe combined immunodeficient thymus.
Methods
BM transplantation protocols
ZAP-70−/− mice (CD45.2+), kindly provided by A. Singer and R. Bosselut (National Institutes of Health), were bred and maintained under pathogen-free conditions. Donor BM cells, harvested from femurs and tibias of WT CD45.1+ C57Bl/6J mice, were first incubated with a cocktail of specific antibodies (Abs) directed against lineage markers (TER119, B220, MAC-1, GR-1, CD4, CD8, all rat α mouse hybridoma Abs) and then with α-rat immunoglobulin G (IgG) magnetic beads (Dynal) to remove differentiated hematopoietic cells. Alternatively, T cell–depleted BM was isolated by removing T cells with anti-CD4 and anti-CD8 Abs. These lineage-negative (lin−) progenitor cells (2 × 105) or T cell–depleted BM (9 × 106) were either injected intravenously into the tail vein (volume, 150 μL) or directly into the thymus (total volume, 20 μL) by insertion of a 0.3-mL 28-gauge 8-mm insulin syringe through the skin into the thoracic cavity immediately above the sternum. Mice were anesthetized with isoflurane before the latter procedure. For transplantation into lethally irradiated recipients, mice were irradiated at 9 Gy (900 rad) 16 hours before administration of T cell–depleted BM. All experiments were approved by the animal facility institutional review board of Institut de Genetique Moleculaire de Montpellier in accordance with national guidelines.
Immunophenotyping and flow cytometric analyses
Cells isolated from lymph nodes, spleen, BM, and thymus were stained with the appropriate conjugated αCD3, αCD25, αCD45.1, αCD62L, αCD4, αCD8, αCD44, αc-Kit, αSca-1, αlineage cocktail mouse mAbs (BD Biosciences), as indicated. Stained cells were analyzed by flow cytometry (FACSCanto; BD Biosciences).
Histology
Thymic tissues were removed after killing, embedded with OCT embedding matrix (CellPath) and directly frozen with iso-Pentane (Prolabo) and liquid nitrogen. Transverse cryosections of 10-μm thickness were prepared for hematoxylin and eosin staining. Thymic tissue sections were scanned with a slide scanner NanoZoomer (Hamamatsu, 20×/0.7 NA objective lens). Images were acquired with a Hamamatsu Photonics camera with time delay and integration scanning and NDP viewer 0.2.2 software.
Statistical analyses
Statistical significance was determined by using a Mann-Whitney test with a 2-tailed distribution (GraphPad Software Inc). Data were considered to be statistically different for P values equal to or less than .05. All data are presented as means plus or minus standard deviations.
Results
IT injection of WT BM progenitor cells into ZAP-70−/− mice results in an increased number of peripheral naive T cells
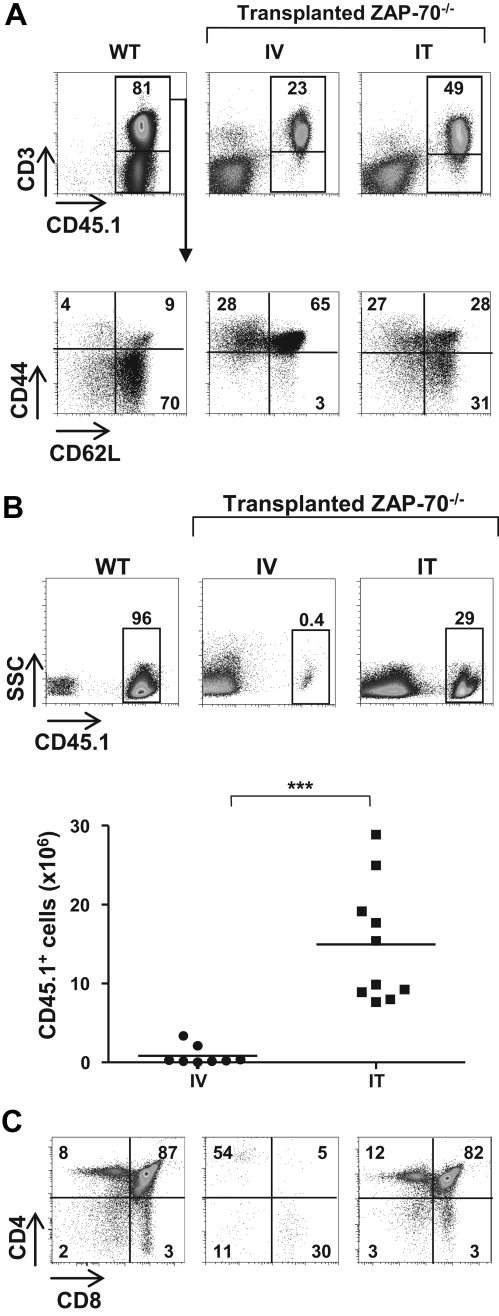
To determine whether the mode of administration of BM progenitors modulates long-term T-cell reconstitution in SCID in the absence of any conditioning before transplantation, BM progenitors (2 × 105 lineage-negative [lin−] cells) were administered to 3- to 4-week-old ZAP-70−/− mice by intravenous or IT injection. Long-term reconstitution was assessed 20 to 25 weeks later, and T cells were detected in the spleens and lymph nodes of all animal that received a transplant. However, there was a higher level of peripheral T cells in IT- (45% ± 11.4%) than in intravenously reconstituted (17.8% ± 8.9%) animals (Figure 1A). Even more striking, the percentage of naive T cells was significantly higher after IT transplantation, with 31.6% plus or minus 16.2% lymphocytes in mice that received an IT transplant and only 6.4% plus or minus 4.6% CD62L+/CD44lo cells in mice that received an intravenous transplant (P < .001; Figure 1A). Thus, the naive T-cell pool was only maintained in mice that received an IT transplant.
Figure 1.
IT but not intravenous injection of WT BM progenitors into nonconditioned ZAP-70–deficient mice results in long-term thymopoiesis. (A) CD45.2+ ZAP-70−/− mice were injected with lineage negative (lin−) BM progenitor cells (2 × 105) isolated from CD45.1+ WT mice by either intravenous (IV) or IT routes. Animals were killed 20 to 25 weeks later, and the percentages of CD45.1+ cells in the lymph nodes were assessed. Dot plots showing representative CD45.1 and CD3 staining of WT and ZAP-70−/− mice reconstituted by intravenous and IT administration of BM progenitors are shown (top). The percentages of naive (CD44intCD62Lhi), central memory (CD44hiCD62Lhi), and effector (CD44hiCD62Llo) T cells in these mice were monitored by assessing CD62L and CD44 expression in gated donor CD3+ lymphocytes. Representative dot plots are shown, and the percentages of each population are indicated (bottom). (B) Representative dot plots showing the percentages of CD45.1+ cells in the thymi of CD45.1 WT and CD45.2 ZAP-70−/− mice reconstituted by IV or IT injection of WT CD45.1+ progenitors. The accompanying graph shows the absolute numbers of CD45.1+ donor thymocytes in ZAP-70−/− mice reconstituted by intravenously (n = 8) and intrathymically (n = 10) administered progenitors. ***P < .001. (C) CD4/CD8 profiles of thymic donor cells were assessed after gating on CD45.1+ thymocytes. Representative dot plots from a control WT mouse and intravenously and intrathymically reconstituted ZAP-70−/− mice are shown. The percentages of cells in each gate are indicated.
Long-term thymopoiesis of donor progenitors distinguishes IT and intravenous transplant recipients
On the basis of the results presented in the first paragraph of “Results,” it was important to determine whether the increased number of peripheral naive T cells in mice that received an IT transplant compared with mice that received an intravenous transplant was due to differences in thymopoiesis. Notably, at 25 weeks after transplantation, we found that there was a significant number of donor (CD45.1+) cells in the thymi of intrathymically reconstituted mice (7.7-28.6 × 106; n = 10), whereas the presence of donor cells in intravenously injected mice was low (0.04-3.4 × 106; n = 8; Figure 1B). To determine whether the high percentage of donor cells represented a continued thymopoiesis or, alternatively, resulted from a recirculation of mature T cells from the periphery, we assessed thymocyte profiles. In intrathymically reconstituted mice, the CD4/CD8 phenotype of donor thymocytes showed a relatively robust thymopoiesis with 76% to 88% of CD45.1+ cells at the double positive (DP) stage of differentiation (81.2 ± 4.0; n = 11). In marked contrast, the low numbers of donor cells detected in the thymi of mice that received an intravenous transplant probably resulted from the recirculation of lymphocytes because most CD45.1+ cells were mature single positive (SP) thymocytes (> 80%; Figure 1C). These data show that robust long-term thymopoiesis is characteristic of nonconditioned ZAP-70–deficient mice that received a transplant by IT but not intravenous administration of WT BM progenitors.
Donor-derived BM progenitors contribute to all stages of thymopoiesis after their IT administration
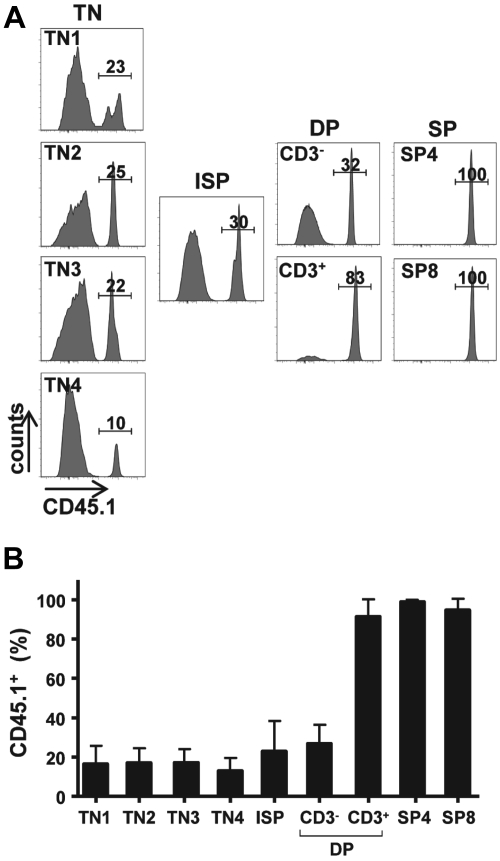
The ongoing thymopoiesis detected in mice that receive an IT transplant represented a significant number of donor thymocytes, with a mean of 14.9 plus or minus 7.5 × 106 (range, 7.7-28.6 × 106). This number is remarkable given that only 2 × 105 progenitors had been intrathymically injected 25 weeks earlier and that ZAP-70 deficiency does not result in a striking decrease in thymocyte numbers. The absence of the ZAP-70 protein kinase in the host mice is associated with a block in T-cell differentiation beyond the DP stage and as such the relative distribution of triple negative (TN; CD3−CD4−CD8−) and CD3− DP thymocytes is relatively normal.17–19 Indeed, at 25 to 30 weeks of age, the time at which the ZAP-70–deficient mice that received a transplant were analyzed, total thymocyte numbers ranged from 20 to 130 × 106 (mean, 59 ± 33 × 106). It was therefore of interest to determine the relative percentage of donor cells at each stage of thymocyte maturation.
As expected from the finding that ZAP-70 deficiency results in a block in positive selection,17–19 most CD3+ DP cells as well as the more mature CD4 SP and CD8 SP thymocytes were of donor origin (> 90%; Figure 2). More surprising, donor cells also accounted for greater than 27% plus or minus 9% of CD3− DP thymocytes, a stage before positive selection. Furthermore, this trend was detected in all TN progenitors, including the most immature TN1 subset (Figure 2). Indeed, the percentage of donor cells remained relatively constant between TN1 and CD3− DP thymocytes at approximately 25%. Thus, the significant donor-derived thymopoiesis detected after IT transplantation could be traced to an early stage of differentiation.
Figure 2.
Donor-derived BM progenitors contribute to all stages of thymopoiesis after their IT administration. (A) CD45.1+ WT lin− BM cells (2 × 105) were injected intrathymically in ZAP-70−/− mice. Thymi were harvested between 20 and 25 weeks after injection, and the percentages of donor CD45.1+ thymocytes in each thymocyte subset were analyzed. Representative histograms show the percentages of donor TN1, TN2, TN3, TN4, immature single-positive (ISP), CD3− DP, CD3+ DP, CD4 SP, as well as CD8 SP thymocytes. (B) Bar graph quantification showing the percentages of CD45.1+ donor thymocytes at each maturation stage are presented as means ± SDs (n = 11).
IT administration of WT BM progenitors does not sustain the differentiation of non–T-lineage hematopoietic lineages
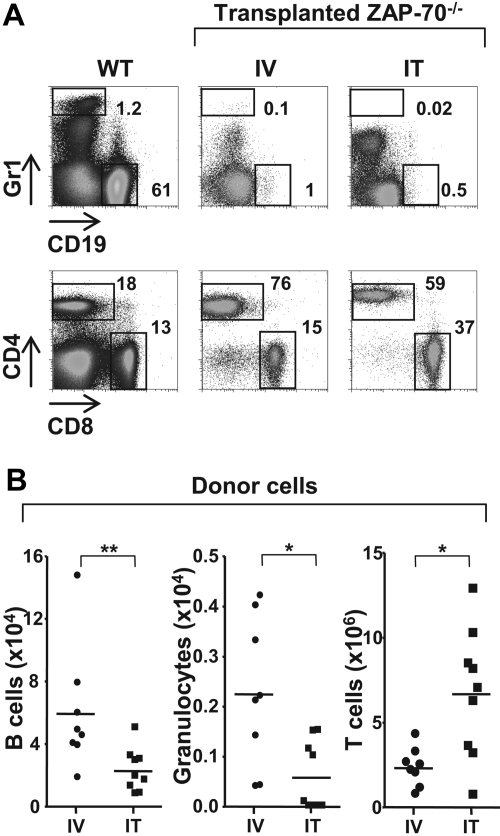
On the basis of the data presented in the previous section, it was critical to determine whether the IT administration of BM progenitors was associated with an enhanced differentiation of other hematopoietic lineages. As such, the level of donor B cells and donor granulocytes in mice that received an IT transplant and mice that received an intravenous transplant were compared 25 weeks after transplantation. Non–T-lineage cells of donor origin were rare in both intrathymically and intravenously treated animals (Figure 3), because the recipient mice were not conditioned before transplantation. However, they were significantly lower in mice that received an IT transplant than mice that received an intravenous transplant with the relative number of donor B lymphocytes accounting for 2.3 plus or minus 1.4 × 104 and 6.0 plus or minus 3.9 × 104 of cells, respectively. CD45.1+ granulocyte cells (Gr1high) were even lower in mice that received an IT transplant (0.06 ± 0.07 × 104) and in mice that received an intravenous injection (0.22 ± 0.15 × 104). One subset, characterized by intermediate Gr1 staining, was present at higher levels in intrathymically injected mice than in intravenously injected mice (17% vs 5%), but these cells represented a subpopulation of CD8+ lymphocytes expressing Ly6C as previously described20,21 (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These data indicate that the IT mode of progenitor administration is not in itself responsible for an augmented engraftment of progenitors in the BM or an enhanced nonspecific lineage differentiation. Rather, IT administration of donor progenitors results in an extensive thymopoiesis without significant involvement of other hematopoietic lineages, probably because of an absence of appropriate stimuli in the thymus microenvironment.
Figure 3.
Differentiation of non–T-lineage cells is significantly lower after IT administration of WT BM progenitors. (A) ZAP-70−/− mice were injected with WT CD45.1+ lin− BM progenitor cells (2 × 105) by either intravenous (IV) or IT routes. Animals were killed 20 to 25 weeks later, and donor cells in the spleen were assessed by CD45.1 staining. Within the CD45.1+ gate, the relative percentages of granulocyte (Gr1high) and B-lineage (CD19+) donor cells (top) as well as CD4 and CD8 T cells (bottom) in representative WT mice as well as ZAP-70−/− mice reconstituted by intravenous (IV) and IT administration of donor progenitors are indicated. (B) The graphs show the absolute numbers of LN donor B lymphocytes, granulocytes, and T cells in ZAP-70−/− mice reconstituted by IV (n = 8) and IT (n = 9) injection of donor progenitors. *P = .01-.015; **P = .008.
IT transplantation does not result in a significant BM engraftment of donor hematopoietic progenitors
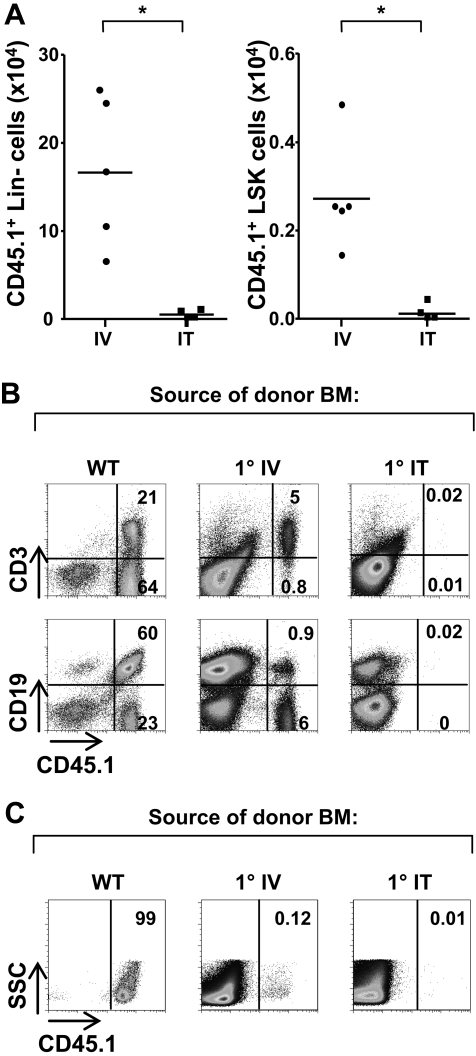
One hypothesis to explain the significant donor-derived thymopoiesis that we observed 25 weeks after IT transplantation is that a subset of the intrathymically injected BM progenitors with T-lineage potential migrates from the thymus to the BM and then reenters the thymus during “receptive” periods.2,22 We were therefore interested in assessing whether donor BM engraftment was enhanced in ZAP-70–deficient mice after IT administration of progenitor cells. Notably, engraftment of lineage-negative donor cells in the BM of mice that receive an IT transplant was significantly lower, with 4-fold lower levels of progenitors in mice that received an IT transplant than in mice that received an intravenous transplant (0.4 ± 0.5 × 104 versus 17 ± 8.5 × 104, respectively; P = .02; Figure 4A). Moreover, the engraftment of the more primitive lin− population expressing both the Sca-1 and c-Kit markers was significantly lower in intrathymically injected group (0.01 ± 0.02 × 104) than in the group that received an intravenous transplant (0.27 ± 0.12 × 104; P = .02). The route of administration therefore significantly modulates the fate of progenitor cells, with significantly fewer precursors homing to the BM after their IT, compared with intravenous, transplantation.
Figure 4.
Long-term thymic differentiation of donor progenitors in IT-injected mice does not result in the engraftment of BM progenitors with secondary repopulating ability. (A) ZAP-70−/− mice were injected intravenously or intrathymically with CD45.1+ WT lin− BM cells (2 × 105). Bone marrow was harvested 20 to 25 weeks after transplantation, and the absolute numbers of lineage-negative (negative for Ter119, B220, Mac-1, Gr-1, and CD3 staining) CD45.1+ cells in the BM were assessed in mice who received an intravenous (IV) transplant (n = 5) and mice that received an IT transplant (n = 4). Absolute numbers of engrafted lin−/Sca-1+/c-Kit+ cells were also determined. *P = .02. (B) Secondary repopulation ability was assessed by transplanting T cell–depleted BM (9 × 106) from these intravenously and intrathymically reconstituted ZAP-70−/− mice (25 weeks after transplant) into lethally irradiated CD45.2+ ZAP-70−/− mice (9 Gy [900 rad]). As a control, lethally irradiated CD45.2+ ZAP-70−/− mice received a transplant with CD45.1+ T cell-depleted BM (9 × 106) from WT mice. The presence of CD45.1+ cells in the spleen was assessed 13 weeks later. Representative dot plots showing the percentages of splenic CD45.1+ donor B/T lymphocytes are presented. (C) The relative levels of CD45.1+ donor cells in the thymi of secondary reconstituted mice are shown in representative dot plots. Results represent data obtained in 3 independent mice.
Our finding that donor progenitor cells were present in the BM of intrathymically treated mice at significantly lower levels than in intravenously treated mice suggested that the long-term thymopoiesis observed in mice that received an IT transplant did not result from the continuous recruitment of donor BM cells with T-lineage potential. However, it was possible that a specific subset of progenitors that are able to sustain thymopoiesis was present in the BM of intrathymically but not intravenously treated mice. Indeed, Flt3, interleukin-7 receptor α (IL-7Rα), and CCR9 markers specifically identify progenitor cells with T-cell differentiation potential.23–28 Although we did not detect marked differences in the phenotypes of donor progenitors in the BM of mice that received an intravenous transplant compared with mice that received an IT transplant by flow cytometry, we were concerned that differences might have been obscured by the low levels of donor cell engraftment in the BM of host mice (R.V., V.S.Z., and N.T., unpublished data, October 2007).
We therefore addressed this hypothesis in a functional manner by performing secondary BM transplantations. T-depleted BM from primary ZAP-70–deficient mice that received an IT transplant and primary ZAP-70–deficient mice that received an intravenous transplant, isolated 25 weeks after transplantation, was injected into lethally irradiated secondary ZAP-70–deficient recipients. Notably, we detected low but observable donor reconstitution after secondary BM transplantation of intravenously reconstituted mice but detected no donor lymphocytes in secondary recipients of transplants with BM from intrathymically reconstituted ZAP-70–deficient mice (Figure 4B). This difference may reflect the higher engraftment of donor progenitors in primary mice that received an intravenous transplant (see first paragraph of this section). Nonetheless, this WT donorprogenitor engraftment did not represent stem cells, neither in the mice that received an intravenous transplant nor in the mice that received an IT transplant, because thymopoiesis was not observed in any of the secondary recipients (Figure 4C). As expected, transplantation of control lethally irradiated ZAP-70–deficient mice with T-depleted BM from CD45.1 WT mice resulted in near-complete donor chimerism, both in the periphery and in the thymus (Figure 4B-C). These data show that IT transplantation does not result in the engraftment of donor stem cells or early lymphoid potential progenitors in the BM.
IT transplantation of BM progenitors results in increased filling of thymic precursor niches
The absence of stem cell engraftment in the BM of mice that receive an IT transplant and the inability of BM from these mice to support any lymphopoiesis in secondary recipients provide strong evidence that the long-term thymopoiesis in primary recipients of IT transplants is not due to the migration and recruitment of thymocyte precursors from the BM. Thus, we hypothesized that WT progenitors occupy an available stromal niche, thereby sustaining long-term thymopoiesis.
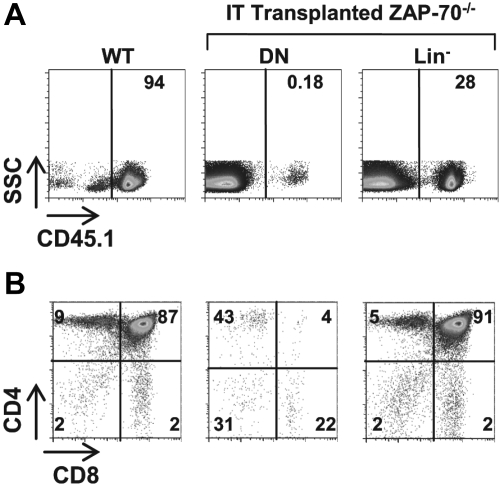
As discussed in the Introduction, BM precursors that naturally migrate to the thymus, even immature TN1 thymocytes, are not capable of maintaining long-term thymocyte differentiation, at least in WT mice.13–15 In ZAP-70–deficient mice, we also found that long-term thymopoiesis could not be reproduced by IT administration of WT thymic precursors; no donor-derived thymopoiesis was detected by 8 weeks after IT injection of WT TN progenitors (Figure 5). Thus, it is not the presence of a WT thymocyte precursor per se that sustains long-term thymopoiesis in ZAP-70–deficient mice. These results indicate that it is the forced entry of a WT BM precursor into a thymic niche, not accessible to circulating host precursors, that allows for long-term thymocyte development.
Figure 5.
WT thymic precursors do not sustain long-term thymopoiesis in ZAP-70–deficient hosts. (A) ZAP-70−/− mice were intrathymically transplanted with DN thymocytes or lineage-negative (lin−) BM progenitor cells (2 × 105) isolated from CD45.1+ WT mice. Eight weeks later, donor engraftment was assessed by monitoring the percentages of cells expressing the CD45.1 allele. A thymus from a CD45.1+ WT mouse is shown as a control. (B) The phenotype of donor cells was assessed by CD4 and CD8 staining of gated CD45.1+ thymocytes. A representative dot plot from a control CD45.1+ WT mouse is shown. The percentage of cells in each gate is indicated.
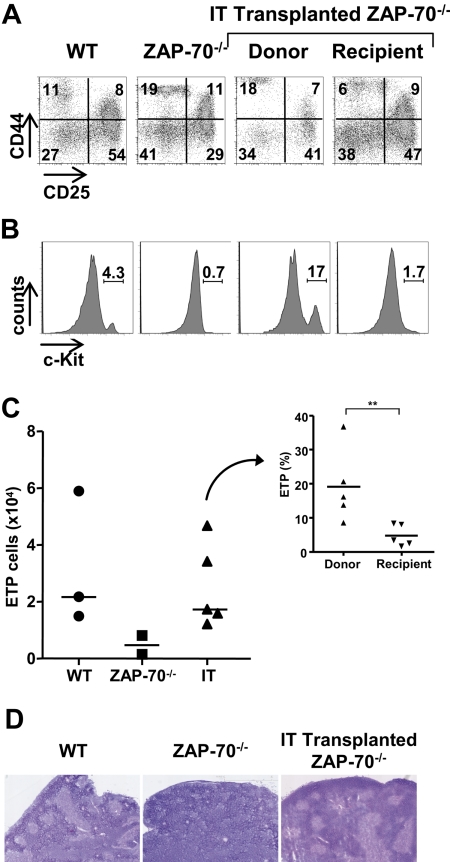
We therefore assessed the phenotype of the immature donor thymocytes persisting in ZAP-70–deficient mice after IT BM administration. As shown in Figure 6, a comparison of the donor and recipient TN subsets in mice that received an IT transplant 25 weeks after transplantation shows a significantly higher percentage of TN1 (CD44+/CD25−) thymocytes within the donor pool compared with the host pool (Figure 6A). Because this could be the result of a more immature subset, we assessed c-Kit expression within the TN1 population. We found that the percentage as well as the absolute number of c-Kithigh thymocytes within the CD44+CD25− population in ZAP-70–deficient mice increased massively after IT transplantation (Figure 6B-C). Indeed, the absolute numbers of c-Kithigh ETPs in mice that received an IT transplant approached that detected in WT mice (Figure 6C). Notably though, the ability of donor and recipient progenitors to fill this c-Kithigh ETP niche after IT transplantation was not equivalent; the relative proportion of c-Kithigh precursors within the donor TN1 pool was more than 4-fold higher than that detected in the recipient TN1 pool (19.1% ± 10.8% vs 4.8% ± 3.2%; P = .008; Figure 6C inset).
Figure 6.
IT BM transplantation results in increased WT c-Kithigh thymic precursors and a high progenitor niche occupancy. (A) The phenotype of immature donor thymocytes was assessed in ZAP-70−/− mice 25 weeks after IT administration of WT CD45.1+ BM progenitor cells. CD25/CD44 profiles of donor CD45.1+ and recipient CD45.1− TN thymocytes were evaluated to distinguish TN1, TN2, TN3, and TN4 populations, and representative dot plots are shown. Control dot plots of TN thymocytes from CD45/1+ WT and ZAP-70−/− mice not receiving a transplant are shown. (B) The presence of early progenitors within gated CD44+/CD25− TN1 thymocytes was assessed by c-Kit staining. (C) Graph shows the absolute numbers of c-Kithigh thymocytes within the TN1 subset of WT, ZAP-70−/−, and intrathymically reconstituted ZAP-70−/− mice. The graph in the inset shows the relative percentages of c-Kithigh thymocytes within the donor and recipient populations from ZAP-70−/− mice that received an IT transplant (n = 5). **P = .008. (D) Hematoxylin and eosin staining of transverse thymus sections from WT, ZAP-70−/−, and intrathymically reconstituted ZAP-70−/− mice. Thymi from WT, but not ZAP-70−/−, mice are normally structured with densely packed cortical regions and less dense medullary regions, whereas thymi from intrathymically reconstituted mice show the formation of an extensive medulla.
The prominent increase in the progenitor precursor pool after IT transplantation raised the question of whether there was also a change in the host thymic microenvironment. We therefore evaluated the thymic architecture of these mice, and, as previously reported,29 we found that thymi of ZAP-70–deficient mice were not normally compartmentalized. They lacked medullary regions defined by low cell density and medullary stromal cells (Figure 6D). Strikingly, an apparently normal thymic architecture was restored by IT transplantation, with clearly demarcated cortical and medullary regions (Figure 6D). Thus, the forced homing of progenitors into the thymus resulted in a higher occupancy of available stromal niches with c-Kithigh thymic precursors and an associated change in the thymic architecture.
Discussion
Under physiologic conditions, ongoing thymocyte differentiation requires a sustained periodic colonization of the thymus by precursors from the BM.3–5 On the basis of these data, the current thinking is that stem cells with self-renewing capacity cannot take up residence in the thymus. Indeed, the absence of hematopoietic stem cells in the thymus has been one of the holy grails in the field of thymocyte differentiation. However, we now show that there are conditions under which hematopoietic progenitors with long-term renewal potential can be maintained within the thymus. In nonconditioned mice, this requires that BM precursors be directly implanted into the thymus and cannot be recapitulated by intravenous injection of the same cell populations. Thus, a thymic niche, not reached by circulating host precursors, allows for long-term T-cell development.
Ongoing thymopoiesis in our intrathymically reconstituted mice was first suggested by the presence of a large number of peripheral naive T lymphocytes, a condition not recapitulated in the intravenously reconstituted mice. The memory phenotype shared by most peripheral T lymphocytes in intravenously reconstituted mice was probably due to a homeostatic expansion of donor T cells without thymopoiesis. This was indeed the case because thymopoiesis was not sustained in mice that received an intravenous transplant, consistent with observations made in transplanted SCID patients who display a dearth of naive T cells with a decline in T-cell function at long-term follow-up.7,8 The absence of long-term thymopoiesis after intravenous transplantation was not due to a paucity of injected progenitors but was directly associated with the nonconditioned context of the recipient mice; intravenous transplantation of progenitors into irradiated/chemotherapy-treated ZAP-70–deficient mice was associated with an extensive and extended donor thymopoiesis.
As indicated in the Introduction, ongoing thymopoiesis requires that the thymus be replenished with BM progenitors.3–5 Indeed, even the earliest thymic precursors do not sustain thymopoiesis for more than 4 weeks.13–15 We therefore explored the hypothesis that a subset of intrathymically injected BM progenitors engrafted in the BM of recipient mice and then periodically reentered the thymus at later periods.2,22 A previous study showed that 0.1% to 1% of BM stem cell niches are available for engraftment after intravenous stem cell transplantation in the absence of conditioning.30 In agreement with these data, we found that engraftment of donor cells in the BM of ZAP-70–deficient mice that received an intravenous transplant was 0.16% plus or minus 0.09%. Intriguingly though, the BM engraftment of intrathymically injected donor cells was 10-fold lower (0.018% ± 0.02%). This extensive difference in BM engraftment translated to an ability of donor BM from mice that received an intravenous transplant but not mice that received an IT transplant to sustain short-term T-cell differentiation in lethally irradiated secondary ZAP-70–deficient recipients. As such, the observed thymopoiesis in mice that received an IT transplant was not due to the BM engraftment of T progenitor cells. Moreover, the differentiation of nonlymphoid lineage cells, such as short-lived granulocytes, was significantly higher in mice that received an intravenous transplant that in mice that received an IT transplant. Taken together, these data show that an IT mode of progenitor administration does not result in a broad progenitor engraftment, but rather it results in a specific thymus engraftment with enhanced differentiation of T-lineage cells.
Because ZAP-70 deficiency results in a block in positive selection,17–19 it was expected that most mature CD3+ thymocytes would be of donor origin (> 90%). However, it was not clear as to whether there would be a selective advantage for WT thymocytes at an earlier stage of differentiation. Palacios and Weiss31 have detected a role for ZAP-70 in the differentiation of CD3− DP thymocytes and WT donor cells accounted for greater than 27% plus or minus 9% of CD3− DP thymocytes. However, this did not appear to be due to a selective advantage for ZAP-70–expressing thymocytes per se because the percentage of donor cells within the most immature TN1 subset was also at this level, in the range of 25%. Because ZAP-70 does not appear to play a role at this early stage and is up-regulated later at the DN2 stage,31,32 these data indicate that it is the “forcing” of BM precursors into the thymus that results in the increased filling of the thymus with donor precursor cells.
Many previous studies have assessed thymopoiesis under conditions of irradiation. However, note that those studies differ from those reported here in that the irradiation itself, by decreasing stromal cell populations secreting cytokines such as interleukin-7 (gp38+) and modulating the relative percentages of DC subsets, can influence the thymic fate of both intravenously and intrathymically administered progenitors. Indeed, although both HSCs and more downstream multipotent progenitors (MPPs) have been shown to generate thymocytes in irradiated recipients,14 elegant recent studies performed in the absence of conditioning have found that only intravenously administered MPPs can directly settle in the thymus and give rise to thymocyte progeny.27 Although MPPs have not been found to sustain long-term hematopoietic reconstitution after their intravenous administration,33,34 we assessed whether their IT administration might facilitate their potential thymopoiesis-repopulating ability. Significantly, we were able to detect long-term thymopoiesis (25 weeks) in a ZAP-70–deficient recipient reconstituted with MPPs, at levels similar to that detected after IT injection of total lineage-negative progenitors (R.V., V.S.Z., and N.T., unpublished observations, October 2008).
The extended thymopoiesis that we observed on forcing WT hematopoietic precursors into the ZAP-70–deficient thymus was associated with a massive expansion of c-Kithigh ETPs. However, only the WT BM precursors directly transplanted into ZAP-70–deficient mice, and not naturally colonizing IT progenitors also proceeding through an ETP stage of differentiation,35 were able to foster this long-term T-cell differentiation. In this regard, it is notable that ZAP-70–deficient mice differed significantly from their WT counterparts as regards the “basal” level of ETPs, with 7-fold lower levels in the former (0.5 ± 0.5 × 104 versus 3.6 ± 2.6 × 104, respectively). Because the “natural” entry of thymic progenitors expressing P-selectin glycoprotein ligand 1 and the CCL25 receptor CCR9 has been shown to be facilitated by expression of P-selectin and CCL25 on thymic endothelium,26,36,37 we assessed the relative levels of these molecules in WT and ZAP-70–deficient thymi by quantitative real-time polymerase chain reaction (qRT-PCR). Strikingly, we found that both P-selectin and CCL25 are expressed at 40% lower levels in thymi from ZAP-70–deficient mice compared with WT controls (R.V., V.S.Z., and N.T., unpublished observations, September 2009). Thus, the decreased expression of these 2 homing molecules may represent at least one mechanism accounting for the decreased thymic progenitor receptivity in ZAP-70–deficient mice. Given the lack of a medulla in ZAP-70–deficient mice and the subsequent appropriate compartmentalization of medullary/cortical regions in the thymus after IT transplantation of progenitors, it will be important to study whether the thymic organ structure itself is a factor in the expression of CCL25/P-selectin.
The decrease in long-term thymopoiesis in patients with SCID who receive a transplant has been associated with an absence of conditioning before the stem cell transplantation.10,38,39 Moreover, Cavazzana-Calvo et al10 have shown that improved thymic output observed in patients with SCID who received prior conditioning correlated with multilineage engraftment. These clinical observations strongly support the premise that after the conventional intravenous administration of WT hematopoietic progenitor cells, long-term T-cell differentiation requires stem cell engraftment. The data presented here show that the IT injection of progenitors supersedes the requirement for stem cell BM engraftment, as it pertains to T-cell differentiation. Thus, under conditions in which recipients are not conditioned, this is the first demonstration that in situ progenitors can sustain long-term thymopoiesis, provided that they are directly injected into a thymus with available stromal niches. Modulating the administration of allogeneic hematopoietic stem cells by IT injection may therefore enhance the dynamics of T-cell reconstitution, providing the basis for an improved clinical approach for hematopoietic stem cell transplantation in patients requiring long-term thymocyte differentiation.
Acknowledgments
We thank all members of our laboratory for their scientific critique and support and thank Al Singer for his important insights and input. We thank Stephanie Cochonneau, Franck Court, and Julie Borgel for their support and expertise.
This work was supported by the National Institute of Allergy and Infectious Diseases (grant R01AI059349), the Association Française contre les Myopathies, the European Community (contract LSHC-CT-2005-018914 “ATTACK”), the Portuguese Foundation for Science and Technology (fellowship SFRH/BD/23 553/2005; R.V.), Inserm (C.J. and N.T.), Centre National de la Recherche Scientifique (CNRS; V.Z.), and by successive grants from the French Ministry of Health and ATTACK (O.A.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.V. participated in study design, performed significant numbers of experiments, and contributed to the writing of the manuscript; O.A. and C.J. designed and performed experiments and analyzed data; and V.S.Z. and N.T. were responsible for the overall study, designed the research, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Valérie S. Zimmermann, Institut de Génétique Moléculaire de Montpellier (IGMM), 1919 Route de Mende, 34293 Montpellier Cedex 5, France; e-mail: zimmermann@igmm.cnrs.fr; or Naomi Taylor, IGMM, 1919 Route de Mende, 34293 Montpellier Cedex 5, France; e-mail: taylor@igmm.cnrs.fr.
References
- 1.Spangrude GJ, Weissman IL. Mature T cells generated from single thymic clones are phenotypically and functionally heterogeneous. J Immunol. 1988;141(6):1877–1890. [PubMed] [Google Scholar]
- 2.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193(3):365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldschneider I, Komschlies KL, Greiner DL. Studies of thymocytopoiesis in rats and mice, I: kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. J Exp Med. 1986;163(1):1–17. doi: 10.1084/jem.163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scollay R, Smith J, Stauffer V. Dynamics of early T cells: prothymocyte migration and proliferation in the adult mouse thymus. Immunol Rev. 1986;91:129–157. doi: 10.1111/j.1600-065x.1986.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 5.Frey JR, Ernst B, Surh CD, Sprent J. Thymus-grafted SCID mice show transient thymopoiesis and limited depletion of V beta 11+ T cells. J Exp Med. 1992;175(4):1067–1071. doi: 10.1084/jem.175.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarzotti M, Patel DD, Li X, et al. T cell repertoire development in humans with SCID after nonablative allogeneic marrow transplantation. J Immunol. 2003;170(5):2711–2718. doi: 10.4049/jimmunol.170.5.2711. [DOI] [PubMed] [Google Scholar]
- 7.Patel DD, Gooding ME, Parrott RE, Curtis KM, Haynes BF, Buckley RH. Thymic function after hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 2000;342(18):1325–1332. doi: 10.1056/NEJM200005043421804. [DOI] [PubMed] [Google Scholar]
- 8.Antoine C, Muller S, Cant A, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968-99. Lancet. 2003;361(9357):553–560. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 9.Muul LM, Tuschong LM, Soenen SL, et al. Persistence and expression of the adenosine deaminase gene for 12 years and immune reaction to gene transfer components: long-term results of the first clinical gene therapy trial. Blood. 2003;101(7):2563–2569. doi: 10.1182/blood-2002-09-2800. [DOI] [PubMed] [Google Scholar]
- 10.Cavazzana-Calvo M, Carlier F, Le Deist F, et al. Long-term T-cell reconstitution after hematopoietic stem-cell transplantation in primary T-cell-immunodeficient patients is associated with myeloid chimerism and possibly the primary disease phenotype. Blood. 2007;109(10):4575–4581. doi: 10.1182/blood-2006-07-029090. [DOI] [PubMed] [Google Scholar]
- 11.Adjali O, Vicente RR, Ferrand C, et al. Intrathymic administration of hematopoietic progenitor cells enhances T cell reconstitution in ZAP-70 severe combined immunodeficiency. Proc Natl Acad Sci U S A. 2005;102(38):13586–13591. doi: 10.1073/pnas.0504268102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreau A, Vicente R, Dubreil L, et al. Efficient intrathymic gene transfer following in situ administration of a rAAV serotype 8 vector in mice and nonhuman primates. Mol Ther. 2009;17(3):472–479. doi: 10.1038/mt.2008.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148(6):1604–1612. [PubMed] [Google Scholar]
- 14.Allman D, Sambandam A, Kim S, et al. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4(2):168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 15.Perry SS, Wang H, Pierce LJ, Yang AM, Tsai S, Spangrude GJ. L-selectin defines a bone marrow analog to the thymic early T-lineage progenitor. Blood. 2004;103(8):2990–2996. doi: 10.1182/blood-2003-09-3030. [DOI] [PubMed] [Google Scholar]
- 16.Lai AY, Kondo M. Identification of a bone marrow precursor of the earliest thymocytes in adult mouse. Proc Natl Acad Sci U S A. 2007;104(15):6311–6316. doi: 10.1073/pnas.0609608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negishi I, Motoyama N, Nakayama K, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376(6539):435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 18.Wiest DL, Ashe JM, Howcroft TK, et al. A spontaneously arising mutation in the DLAARN motif of murine ZAP-70 abrogates kinase activity and arrests thymocyte development. Immunity. 1997;6(6):663–671. doi: 10.1016/s1074-7613(00)80442-2. [DOI] [PubMed] [Google Scholar]
- 19.Kadlecek TA, van Oers NS, Lefrancois L, et al. Differential requirements for ZAP-70 in TCR signaling and T cell development. J Immunol. 1998;161(9):4688–4694. [PubMed] [Google Scholar]
- 20.Fichtner AT, Anderson S, Mage MG, Sharrow SO, Thomas CA, III, Kung JT. Subpopulations of mouse Lyt-2+ T cells defined by the expression of an Ly-6-linked antigen, B4B2. J Immunol. 1987;138(7):2024–2033. [PubMed] [Google Scholar]
- 21.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83(1):64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 22.Prockop SE, Petrie HT. Regulation of thymus size by competition for stromal niches among early T cell progenitors. J Immunol. 2004;173(3):1604–1611. doi: 10.4049/jimmunol.173.3.1604. [DOI] [PubMed] [Google Scholar]
- 23.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91(5):661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 24.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 25.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98(25):14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168(6):2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE, Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178(4):2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- 28.Benz C, Bleul CC. A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J Exp Med. 2005;202(1):21–31. doi: 10.1084/jem.20050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hikosaka Y, Nitta T, Ohigashi I, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29(3):438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med. 2006;203(1):73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palacios EH, Weiss A. Distinct roles for Syk and ZAP-70 during early thymocyte development. J Exp Med. 2007;204(7):1703–1715. doi: 10.1084/jem.20070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.David-Fung ES, Butler R, Buzi G, et al. Transcription factor expression dynamics of early T-lymphocyte specification and commitment. Dev Biol. 2009;325(2):444–467. doi: 10.1016/j.ydbio.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacharya D, Bryder D, Rossi DJ, Weissman IL. Rapid lymphocyte reconstitution of unconditioned immunodeficient mice with non-self-renewing multipotent hematopoietic progenitors. Cell Cycle. 2006;5(11):1135–1139. doi: 10.4161/cc.5.11.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akala OO, Park IK, Qian D, Pihalja M, Becker MW, Clarke MF. Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature. 2008;453(7192):228–232. doi: 10.1038/nature06869. [DOI] [PubMed] [Google Scholar]
- 35.Benz C, Martins VC, Radtke F, Bleul CC. The stream of precursors that colonizes the thymus proceeds selectively through the early T lineage precursor stage of T cell development. J Exp Med. 2008;205(5):1187–1199. doi: 10.1084/jem.20072168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi FM, Corbel SY, Merzaban JS, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6(6):626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 37.Gossens K, Naus S, Corbel SY, et al. Thymic progenitor homing and lymphocyte homeostasis are linked via S1P-controlled expression of thymic P-selectin/CCL25. J Exp Med. 2009;206(4):761–778. doi: 10.1084/jem.20082502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedrich W, Honig M, Muller SM. Long-term follow-up in patients with severe combined immunodeficiency treated by bone marrow transplantation. Immunol Res. 2007;38(1–3):165–173. doi: 10.1007/s12026-007-0030-2. [DOI] [PubMed] [Google Scholar]
- 39.Mazzolari E, Forino C, Guerci S, et al. Long-term immune reconstitution and clinical outcome after stem cell transplantation for severe T-cell immunodeficiency. J Allergy Clin Immunol. 2007;120(4):892–899. doi: 10.1016/j.jaci.2007.08.007. [DOI] [PubMed] [Google Scholar]