Abstract
The transition of reticulocytes into erythrocytes is accompanied by extensive changes in the structure and properties of the plasma membrane. These changes include an increase in shear resistance, loss of surface area, and acquisition of a biconcave shape. The processes by which these changes are effected have remained largely undefined. Here we examine how the expression of 30 distinct membrane proteins and their interactions change during murine reticulocyte maturation. We show that tubulin and cytosolic actin are lost, whereas the membrane content of myosin, tropomyosin, intercellular adhesion molecule-4, glucose transporter-4, Na-K-ATPase, sodium/hydrogen exchanger 1, glycophorin A, CD47, Duffy, and Kell is reduced. The degradation of tubulin and actin is, at least in part, through the ubiquitin-proteasome degradation pathway. In regard to the protein-protein interactions, the formation of membrane-associated spectrin tetramers from dimers is unperturbed, whereas the interactions responsible for the formation of the membrane-skeletal junctions are weaker in reticulocytes, as is the attachment of transmembrane proteins to these structures. This weakness, in part, results from the elevated phosphorylation of 4.1R in reticulocytes, which leads to a decrease in shear resistance by reducing its interaction with spectrin and actin. These observations begin to unravel the mechanistic basis of crucial changes accompanying reticulocyte maturation.
Introduction
The red cell membrane is a composite structure, comprising a membrane skeleton protein lattice and a lipid bilayer to which the membrane skeleton is attached by way of interactions with trans-bilayer proteins. The major components of the membrane skeleton are α- and β-spectrin, predominantly in the form of α2β2 tetramers, ankyrin, F-actin in the form of short filaments (protofilaments), protein 4.1R, adducin, dematin, tropomyosin, tropomodulin, and proteins 4.2 and p55.1–4 The spectrin tetramers are formed by head-to-head association of 2 αβ heterodimers, their distal ends attached to the network junctions, which are separated by approximately 70 nm. A total of 6 spectrin molecules, on average, enter into ternary complexes with actin subunits and 4.1R in each junction. Phosphorylation of 4.1R by an endogenous protein kinase C (PKC) can modify the strength of the ternary complex.5 The resistance of the membrane to mechanical stresses and membrane cohesion depends on the spectrin tetramers, on the integrity of the junctions, and on their attachment to the bilayer by way of an ankyrin-based macromolecule complex and a 4.1R-based macromolecule complex.
More than 50 transmembrane proteins have been identified in the red cell, of which more than half carry blood group antigens.6 Certain transmembrane proteins function as transporters or pumps. These include the water transporter, aquaporin 1, the glucose transporters (GLUT1 and GLUT4), the sodium/hydrogen exchanger 1 (NHE1), and Na-K-ATPase. Some transmembrane components, in particular CD44, Lutheran, and intercellular adhesion molecule-4 (ICAM-4), are adhesion proteins involved in interactions with other blood cells and endothelial cells. The evidence is that the transmembrane proteins are partitioned between 2 distinct macromolecular complexes, one centered on ankyrin,7 the other on 4.1R.8 A subpopulation of the band 3, presumably with its associated transmembrane proteins, is linked to spectrin through the interaction of the cytoplasmic band 3 domain with ankyrin, to which the Rh-associated glycoprotein (RhAG) also binds.9,10 A more recent study reveals that band 3 also can be attached to the junctions by adducin.11 If either type of bilayer-membrane-skeleton linkage is disturbed, lipid loss with diminution of the membrane surface area ensues.
Reticulocyte maturation is the final step of terminal erythroid differentiation. During this process, changes in both protein content and membrane organization occur. Specifically, it is known that membrane vesiculation leads to approximately 20% loss of surface area12,13 and that membranes of young reticulocytes are mechanically much less stable than those of mature cells.14,15 These differences in membrane properties clearly imply major reorganization of membrane and skeletal components accompanying the maturation of multilobular reticulocytes into discoid mature erythrocytes.16 However, little is known about the specific molecular changes. The observations described herein provide insights into some of the molecular changes that account for the differences in membrane properties between reticulocytes and erythrocytes.
Methods
Reagents
A Cholesterol Quantification Kit was purchased from BioVision. Isopropyl β-D-1-thiogalactopyranoside, α-thioglycerol, dimethyl sulfoxide (DMSO), bovine serum albumin (BSA), Percoll, cellulose, and protease inhibitor cocktail were from Sigma-Aldrich. Gels for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and other electrophoresis reagents were from Bio-Rad. SuperSignal West Pico chemiluminescent substrate kit and GelCode Blue were from Pierce. Proteasome inhibitor MG132 was from Calbiochem. Iscove modified Dulbecco medium was from GIBCO. Fetal bovine serum, penicillin, and streptomycin were from the ATCC. Retic-COUNT Reagent (Thiazole Orange) was from BD Biosciences.
Antibodies
Anti-tropomyosin antibody was from Developmental Studies Hybridoma Bank. Anti-myosin (nonmuscle) and anti-actin antibodies were from Sigma-Aldrich. Anti-tubulin and anti-glyceraldehyde 3-phosphate dehydrogenase antibodies were from Abcam. Anti–glycophorin A (GPA) and anti-NHE1 antibodies were from BD Biosciences. Anti-CD71 antibody was from Invitrogen, and anti–Na-K-ATPase antibody was from Upstate. Alexa 488–conjugated anti–rabbit and anti–mouse immunoglobulin G (IgG) were from Invitrogen. Fluorescein isothiocyanate (FITC)–conjugated GPA and FITC-conjugated CD47 were from eBioscience. Horseradish peroxidase–conjugated anti–mouse, anti–rat, and anti–rabbit IgG were from Jackson ImmunoResearch Laboratories. All other antibodies were generated in our laboratory.
Antibodies against mouse transmembrane proteins glycophorin C (GPC), band 3, Rh, RhAG, XK, Kell, Duffy, ICAM-4, and CD47 were generated in rabbits as previously described.8 Antibodies against proteins 4.2, 4.9, adducin, tropomodulin, p55, GLUT4, phospho-4.1R 312S, phospho-4.1R 331S, and phospho-adducin 726S were raised in rabbits by the use of as antigens synthetic peptides chosen from the sequences of mouse proteins (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The antigens for rabbit antibodies against α-spectrin and β-spectrin were recombinant repeat 6 of α-spectrin and repeat 12 of β-spectrin, respectively. Antigens for rabbit antibodies against ankyrin R and 4.1R were full-length protein purified from human erythrocytes. The antibodies were affinity purified by the use of specific peptides immobilized to Sulfolink Coupling Gel (Pierce) or the corresponding proteins coupled to Affi-Gel10 (Bio-Rad).
Antibody specificities
The commercially available antibodies against tubulin, actin, myosin, glyceraldehyde 3-phosphate dehydrogenase, CD71, Na-K-ATPase alpha, NHE1, and GPA were monospecific in our hands and in Western blot analysis detected only the protein of appropriate molecular weight. The specificity of our antibodies against 4.1R, GPC, band 3, Rh, RhAG, Kell, XK, Duffy, and ICAM-4 has been previously published.8 The specificity of the antibodies against α-spectrin, adducin, ankyrin R, and p55 was examined by Western blotting by the use of the red cells from α-spectrin knockout mice, α-adducin knockout mice, nb/nb mice, and 4.1R knockout mice as negative controls (supplemental Figure 1A). Supplemental Figure 1B shows the full-length gels of mouse red cells stained with each of the antibodies for which we do not have appropriate knockout mice available: β-spectrin, proteins 4.2, 4.9, tropomodulin, CD47, and GLUT4. These antibodies detected only the protein of the appropriate molecular weight, demonstrating their specificities.
Mice
C57BL/6 mice were maintained at the animal facility of the New York Blood Center under specific pathogen-free conditions according to institutional guidelines. Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee. Three-month-old male mice were used for all of the experiments.
Induction of reticulocytosis
Reticulocytosis was induced by bleeding according to the protocol previously described by Joiner et al17 with slight modifications. On days 1 and 3, animals were injected intraperitoneally with 2 mL of normal saline, followed by removal of approximately 700 μL of blood by retro-orbital puncture. On day 5, blood samples were obtained by cardiac puncture for isolation of reticulocytes. With the use of this protocol, reticulocyte percentages reached 35% to 50% by day 5.
Separation of reticulocytes and mature red blood cells
Reticulocytes and erythrocytes were separated by centrifugation on a 2-step gradient of isotonic Percoll.18 The density of the upper layer (2 mL) of the gradient was 1.058 mg/mL, whereas that of the lower layer (3 mL) was 1.096 mg/mL. Approximately 1 mL of whole blood was layered on top of these gradients and centrifuged at 250g for 30 minutes at room temperature. Upon centrifugation, reticulocytes were enriched at the interface of the 2 layers, whereas erythrocytes accumulated at the bottom of the tube. The isolated reticulocytes were dual stained by the use of Retic COUNT Reagent and anti-CD71 and analyzed on a Becton Dickinson FACSCanto (Becton Dickinson). Reticulocyte purity was greater than 90%, and approximately 90% of these reticulocytes were RNA high/CD71 high, demonstrating that they are young reticulocytes. Because myosin and tropomyosin are minor components of erythrocytes and are abundant in white cells and platelets, to check expression of myosin and tropomyosin, we depleted isolated cell populations of contaminating white cells and platelets by the use of cellulose columns.19
Preparation of ghosts for Western blot analysis
For comparison of protein expression levels, cells were first washed 3 times with phosphate-buffered saline (PBS) and subsequently washed 3 times with 35 volumes of ice-cold 5K5T lysis buffer (5mM KCl; 5mM Tris, pH 7.4; 0.1mM diisopropyl fluorophosphate; 2mM MgCl2). For examination of protein phosphorylation, ghosts were prepared in the presence of a phosphatase inhibitor, calyculin A, at a concentration of 0.02μM. As a positive control for protein phosphorylation, erythrocytes were treated with 0.1μM phorbol 12-myristate 13-acetate (PMA) for 90 minutes at 37°C. The cholesterol content of the ghosts was measured by use of the Cholesterol Quantification Kit according to the manufacturer's instructions. Aliquots of reticulocyte and erythrocyte ghosts, matched for cholesterol content, were separated by 10% SDS-PAGE.
Flow cytometric assay
A total of 106 purified reticulocytes or erythrocytes were incubated for 20 minutes on ice with appropriate concentrations of antibodies specific for the extracellular domains of transmembrane proteins. For GPA and CD47, because the antibodies were directly conjugated with FITC, secondary antibody staining was not needed. For GPC, band 3, Kell, and Duffy, the cells were subsequently washed twice with PBS-BSA buffer (PBS supplemented with 0.1% BSA) and incubated with AlexaFluor488-conjugated anti–mouse IgG (for Kell) or anti–rabbit IgG (for GPC, band 3, and Duffy) for 20 minutes on ice. Samples were analyzed on a Becton Dickinson FACSCanto. Unstained cells (for GPA and CD47 staining) or preimmune IgGs were used as negative controls. The mean fluorescence intensity was used as a measure of the level of protein expression.
Incorporation of N-terminal β-spectrin fragment into ghosts
GST-tagged N-terminal β-spectrin fragment 1-301 was purified as previously described.20 Freshly isolated reticulocytes and erythrocytes were washed 3 times with Tris-buffered isotonic saline (0.12M potassium chloride; 10mM Tris, pH 7.4) and lysed with 35 volumes of ice-cold hypotonic buffer (5mM Tris; 5mM potassium chloride, pH 7.4; 2mM MgCl2). The isolated ghosts were resealed with various concentrations of the fragment by incubation for 40 minutes at 37°C under isotonic conditions.
Extraction and analysis of spectrin from ghosts
Spectrin was extracted from ghosts as previously described.21 In brief, the ghosts were washed first with isotonic buffer, followed by hypotonic buffer. Extraction was accomplished by suspension of the ghosts in 5 volumes of 0.25mM sodium phosphate, pH 7.4, overnight at 4°C. The spectrin was collected by centrifugation (21 000g) and examined with the use of a 5% native gel. The gel was run in Tris-glycine buffer, pH 8.3, at 4°C for 6 hours and stained with GelCode Blue.
Cell culture
Reticulocytes (3 × 107 cells/mL) were cultured for 48 hours in Iscove modified Dulbecco medium, 30% heat-inactivated fetal bovine serum, 1% deionized bovine albumin, 100 units/mL penicillin G, 100 μg/mL streptomycin, and 0.1mM α-thioglycerol in the presence of the proteasome inhibitor MG132 (10μM) or DMSO (0.05% vol/vol), as control.
Detergent extraction of membrane proteins
Reticulocyte or erythrocyte ghosts (50 μL of packed ghosts) were suspended in 100 μL of extraction buffer (PBS, pH 7.4, in the presence of 1% Triton X-100 for band 3, GPA, GPC, XK, Kell, and RhAG or 0.05% Triton X-100 for CD47, Rh, and Duffy) and incubated on ice for 30 minutes, followed by centrifugation at 21 000g with the use of a Benchtop centrifuge for 30 minutes at 4°C to collect the pellet. The pellet was resuspended in 120 μL of sample buffer and boiled for 10 minutes, and 10 μL of the sample was run on 10% SDS-PAGE followed by Western blotting with the use of antibodies specific for various proteins as previously described.8 The preparation of control samples followed the same procedure with the use of buffer without Triton X-100.
Triton shell preparation
To examine the incorporation of peptide into the membrane skeletons, Triton shells were prepared from reticulocyte and erythrocyte ghosts resealed with the β-spectrin fragment 1-301. Then, 50 μL of the resealed ghosts was washed 3 time times with PBS. The pellet was resuspended in 500 μL of extraction buffer (10mM Tris-HCl, pH 7.0; 0.8M NaCl; 1% Triton X-100; 0.02μM calyculin A; 1mM phenylmethylsulphonyl fluoride) and incubated on ice for 30 minutes, followed by centrifugation at 14 000 rpm for 30 minutes at 4°C to collect the pellet. The resulting Triton shells were suspended in 120 μL of sample buffer and boiled for 10 minutes, and 5 μL of the sample was run on 10% SDS-PAGE. The gels were stained with GelCode Blue and were evaluated by densitometry with ImageJ software.
Statistical analysis
The statistical difference between the groups was determined by analysis of variance and Tukey studentized range test. Differences among groups were considered statistically different at P less than .05.
Results
Cytoskeletal protein components in reticulocytes and erythrocytes
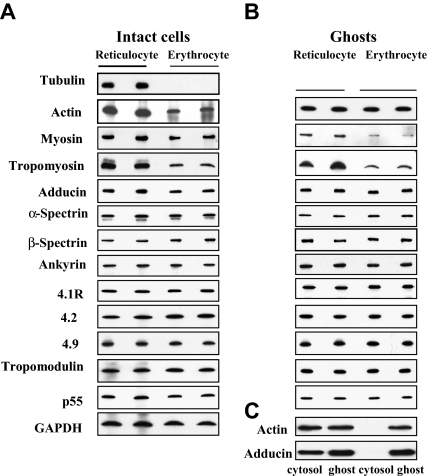
To explain the differences between the properties of reticulocyte and erythrocyte membranes, we first compared the cytoskeletal protein composition of purified reticulocytes (> 90%) with that of erythrocytes. Figure 1A, which refers to intact cells, shows that with reticulocyte maturation tubulin is lost; actin, tropomyosin, and myosin are greatly decreased; and adducin is slightly decreased. The cell content of spectrin, ankyrin, 4.1R, 4.2, p55, tropomodulin, and 4.9, by contrast, is not changed. Figure 1B shows the contents of these proteins in isolated membranes, which enables the quantitation of the extent to which the various skeletal proteins are associated with the membrane. The decrease of tropomyosin and myosin is still apparent in erythrocyte membranes.
Figure 1.
Immunoblots of cytoskeletal proteins of reticulocytes and erythrocytes. (A) Intact cells: Western blots of SDS-PAGEs of total cellular protein prepared from reticulocytes and erythrocytes probed with antibodies against the indicated proteins. Note the absence of tubulin and the decreased expression of tropomyosin, myosin, actin and adducin in erythrocytes. (B) Ghosts: Western blots of SDS-PAGEs of ghosts prepared from reticulocytes and erythrocytes probed with antibodies against the indicated proteins. Because ghosts of both reticulocytes and erythrocytes do not contain any tubulin, there is no corresponding panel for tubulin in panel B. Note the decreased expression of tropomyosin and myosin in the erythrocyte membranes but the similar level of actin and adducin. (C) Western blots of SDS-PAGEs of the cytosol and the membrane were probed with anti-actin or anti-adducin antibody. Note actin and adducin are present in both the cytosol and the membrane fraction of reticulocytes but are only present in the membrane of erythrocytes.
A quantitative analysis of the data is summarized in Table 1. Interestingly, actin and adducin are present at the same level, suggesting that the plasma membrane-associated actin and adducin are similar in reticulocytes and erythrocytes, whereas the cytoplasmic portion of actin and adducin in reticulocytes is lost during maturation. The presence of actin and adducin in the cytoplasm of reticulocytes but not erythrocytes is indeed apparent in Figure 1C. Thus, there are selective changes in cytoskeletal protein levels during reticulocyte maturation. It should be noted that for these analyses, cells and membranes with equivalent amounts of cholesterol were loaded onto the gels and that the cholesterol content of erythrocytes was approximately 20% less than that of reticulocytes (∼ 0.17 μg/106 erythrocytes and their membranes, ∼ 0.2 μg/106 reticulocytes and their membranes).
Table 1.
Comparison of protein expression in reticulocytes and erythrocytes
| Cytoskeletal proteins | Erythrocyte percentage of reticulocyte (intact cells, n) | Erythrocyte percentage of reticulocyte (ghosts, n) | Transmembrane proteins | Erythrocyte percentage of reticulocyte (ghosts, n) |
|---|---|---|---|---|
| Actin | 0.58 ± 0.01* (4) | 0.98 ± 0.06 (3) | CD71 | 0.14 ± 0.12* (3) |
| Myosin | 0.54 ± 0.16† (3) | 0.47 ± 0.02* (3) | GLUT4 | 0.21 ± 0.15* (3) |
| Tropomyosin | 0.30 ± 0.18‡ (3) | 0.40 ± 0.04* (3) | Na+-K+ ATPase α | 0.26 ± 0.01* (3) |
| Adducin | 0.74 ± 0.09‡ (4) | 1.03 ± 0.13 (3) | LW | 0.11 ± 0.08* (3) |
| α-Spectrin | 0.95 ± 0.09 (4) | 0.93 ± 0.07 (3) | NHE1 | 0.53 ± 0.31‡ (4) |
| β-Spectrin | 1.02 ± 0.12 (4) | 1.01 ± 0.15 (3) | Duffy | 0.71 ± 0.12‡ (4) |
| Ankyrin | 0.93 ± 0.11 (4) | 0.91 ± 0.13 (3) | Kell | 0.38 ± 0.19* (4) |
| 4.1R | 0.98 ± 0.00 (4) | 0.97 ± 0.15 (3) | GPA | 0.61 ± 0.19† (3) |
| 4.2 | 1.03 ± 0.24 (3) | 1.08 ± 0.16 (3) | CD47 | 0.70 ± 0.04* (4) |
| 4.9 (Dematin) | 0.91 ± 0.09 (4) | 0.87 ± 0.13 (3) | Band3 | 1.12 ± 0.09‡ (3) |
| Tropomodulin | 1.04 ± 0.05 (4) | 1.09 ± 0.06 (3) | Rh | 1.26 ± 0.05* (4) |
| p55 | 0.87 ± 0.10 (3) | 0.92 ± 0.14 (3) | RhAG | 1.26 ± 0.11* (4) |
| XK | 1.35 ± 0.20† (4) | |||
| GPC | 1.31 ± 0.20‡ (4) |
Note that equivalent amounts of cholesterol were loaded.
GPA indicates glycophorin A; GPC, glycophorin C; NHE1, sodium/hydrogen exchanger 1; and RhAG, Rh-associated glycoprotein.
P ≤ .005.
P ≤ .01.
P ≤ .05.
Tubulin and actin are degraded via the ubiquitin-proteosome pathway in reticulocytes
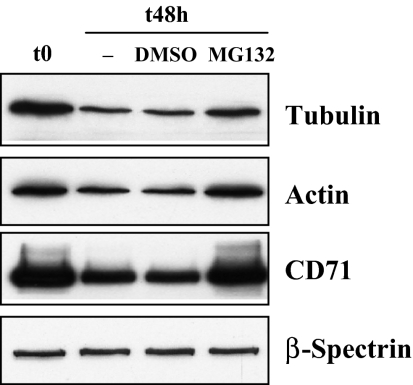
Cytosolic proteins are predominantly degraded by the ubiquitin-proteosome pathway, as was, in fact, first demonstrated in rat reticulocytes.22 To determine whether tubulin and cytosolic actin, which are lost during reticulocyte maturation, are degraded by this means, we cultured reticulocytes in vitro for 48 hours in the absence or presence of the proteosome inhibitor MG132. As shown in Figure 2, both tubulin and actin are significantly decreased in the absence of the inhibitor, but when the inhibitor is present, the degradation of both proteins is inhibited. CD71 and spectrin served as the positive and negative internal controls. We conclude that tubulin and actin are indeed eliminated from the reticulocyte cytosol, at least in part, by ubiquitin-proteosome-dependent proteolysis. Because the loss of tubulin is only partially prevented in the presence of MG132, other mechanisms also may contribute to their loss.
Figure 2.
Inhibition of tubulin and actin degradation by proteosome inhibitor MG132. Mouse reticulocytes were cultured for 48 hours in the presence of the proteasome inhibitor MG132 (10μM) or an equivalent volume of the solvent, DMSO. Western blots of SDS-PAGEs of total cellular protein were probed with antibodies against tubulin, actin, CD71 and β-spectrin. CD71 serves as a positive control and β-spectrin as a negative control.
Weaker junctional complex in reticulocytes
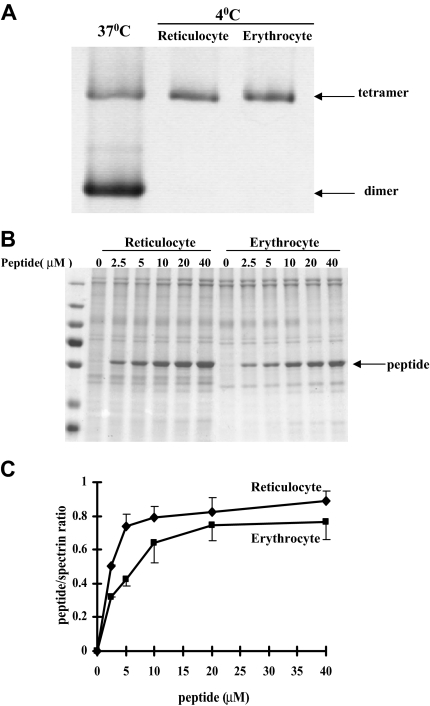
It is known that reticulocytes are mechanically less stable than erythrocytes.14,15 It is also well established that the stability of the red cell membrane is in large part determined by 2 major interactions, those between the 2 dimer components of spectrin tetramers and those between the spectrin-actin-4.1R components of the ternary complex. We accordingly examined the status of the spectrin dimer-tetramer equilibrium in reticulocytes. Figure 3A shows that, as in erythrocytes, the spectrin in reticulocytes is almost entirely tetrameric. To test whether the ternary junctional complex is more labile in reticulocytes than in erythrocytes, we compared the incorporation into the membranes of a spectrin fragment20 corresponding to the region of the β-chain involved in ternary complex formation. The incorporation is indeed clearly greater in reticulocytes (Figure 3B-C). Thus, a more labile junctional complex contributes, in part, to the lower stability of the reticulocyte membrane.
Figure 3.
Protein-protein associations in reticulocytes and erythrocytes. (A) Spectrin dimer-tetramer equilibrium in reticulocytes and mature red blood cells. Extracted spectrin was analyzed by nondenaturing gel electrophoresis. Note spectrin from both the reticulocytes and the erythrocytes is tetrameric. In panels B and C, incorporation of a β-spectrin fragment into reticulocyte and erythrocyte membranes. The Triton shells were prepared from reticulocyte or erythrocyte ghosts resealed with increasing concentrations of β-spectrin fragment 1-301. (B) Proteins retained in the Triton shells were analyzed by SDS-PAGE. (C) Quantitative analysis revealed greater incorporation of the peptide into the cytoskeleton of reticulocytes compared with that observed in erythrocytes.
Increased phosphorylation of 4.1R in reticulocytes
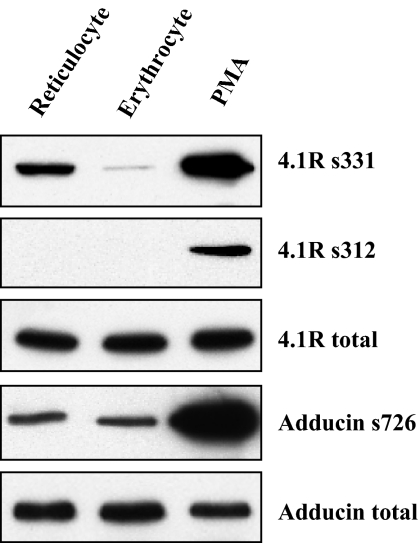
We and others5,23 have shown that 4.1R is a substrate for PKC in erythrocytes and can be phosphorylated at Ser312 and Ser331. We have also shown that such phosphorylation weakens the association of 4.1R with spectrin and actin, which in turn results in reduced membrane stability. This raises the possibility that the impaired junction stability in the reticulocytes may be a consequence of increased phosphorylation of 4.1R. Figure 4 shows that this is indeed the case because sequence-specific antibodies that recognize phosphorylated residue Ser331 in 4.1R reveal markedly increased phosphorylation of 4.1R in reticulocytes. By contrast, phosphorylation levels of adducin in reticulocytes and erythrocytes are indistinguishable. Thus, constitutive phosphorylation of 4.1R at residue Ser331 in reticulocytes, can at least in part, account for the relative instability of the junctional complexes in reticulocytes compared with erythrocytes.
Figure 4.
Phosphorylation of 4.1R and adducin in reticulocytes and erythrocytes. Reticulocyte and erythrocyte ghosts were prepared in the presence of the phosphatase inhibitor, calyculin A. Western blots of SDS-PAGEs were probed with phospho-residue specific antibodies as indicated. PMA-treated erythrocyte ghosts were used as a positive control. Note the markedly increased phosphorylation of 4.1R at residue 331 serine (4.1R s331) in reticulocytes.
Integral membrane proteins in reticulocytes and erythrocytes
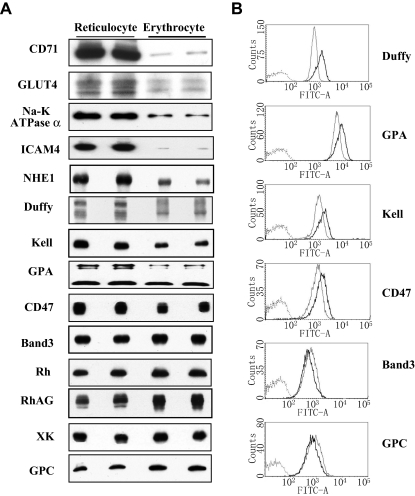
Although it is to be expected that the maturation of reticulocytes should be accompanied by changes in protein composition of the plasma membrane, only the loss of the transferrin receptor has previously been substantiated.24 However, functional studies25,26 suggested that the glucose transporter and Na/K-ATPase also might be lost. Figure 5A shows the relative cellular content of 20 integral membranes in reticulocytes and mature cells. It reveals that not only the transferrin receptor (CD71), but also GLUT4, Na/K-ATPase, and the adhesion molecule ICAM-4 are largely eliminated during maturation. Five other integral proteins, GPA, CD47, Duffy, NHE1, and Kell, also are decreased. However, band 3, Rh, RhAG, GPC, XK, are increased after maturation. All these experiments were each performed at least 3 times, and quantitative analysis of the data is summarized in Table 1.
Figure 5.
Expression of membrane proteins of reticulocytes and erythrocytes. (A) Immunoblots: Western blots of SDS-PAGEs of total cellular protein prepared from reticulocytes and erythrocytes probed with antibodies against the indicated proteins. Note the dramatic decrease of CD71, GLUT4, Na-K-ATPase α, ICAM4, and NHE1; decrease to a lesser extent of Duffy, Kell, GPA, and CD47; and increase of band 3, Rh, RhAG, XK, and GPC in erythrocytes compared with reticulocytes. (B) Flow cytometry: the ordinate measures the number of cells displaying the fluorescent intensity given by the abscissa. Dark lines indicate reticulocytes; light lines, erythrocytes. Note the decreased expression of Duffy, GPA, Kell, and CD47 and the increased expression of band 3 and GPC in erythrocytes compared with reticulocytes.
We further examined the surface exposure of band 3, GPC, GPA, Kell, Duffy, and CD47 on intact cells by flow cytometry. Consistent with Western blot analysis, band 3 and GPC are elevated in mature cells, whereas GPA, Kell, Duffy, and CD47 are reduced (Figure 5B; Table 2). It should be noted that densitometric analysis of the Western blot demonstrated that the expression of band 3 is greater in erythrocytes than that in reticulocytes (Table 1). Flow cytometric analysis also revealed a similar trend. However, the observed differences by flow cytometry did not reach statistical significance (P = .2). It is likely that this is finding is attributable to the fact that band 3 antibody used for analysis was a rabbit polyclonal antibody to the extracellular domain of band 3 and gave a larger variation in fluorescence intensity in comparison to monoclonal antibodies used in our analysis.
Table 2.
Expression of blood group antigen carrying proteins as assessed by flow cytometry
| Protein | Mean fluorescence |
Erythrocyte percentage of reticulocyte | P | |
|---|---|---|---|---|
| Reticulocyte (n) | Erythrocyte (n) | |||
| Duffy | 3425 ± 688 (8) | 2269 ± 360 (8) | 66 | < .001 |
| GPA | 16 960 ± 2550 (9) | 12 696 ± 1459 (9) | 74 | < .001 |
| Kell | 4324 ± 476 (6) | 2860 ± 532 (6) | 66 | < .001 |
| CD47 | 3225 ± 261 (6) | 1861 ± 165 (6) | 58 | < .001 |
| Band3 | 1834 ± 470 (8) | 2101 ± 315 (8) | 115 | .2 |
| GPC | 1929 ± 158 (8) | 2188 ± 188 (8) | 113 | .01 |
GPA indicates glycophorin A; and GPC, glycophorin C.
Linkage of integral membrane proteins to the membrane skeleton
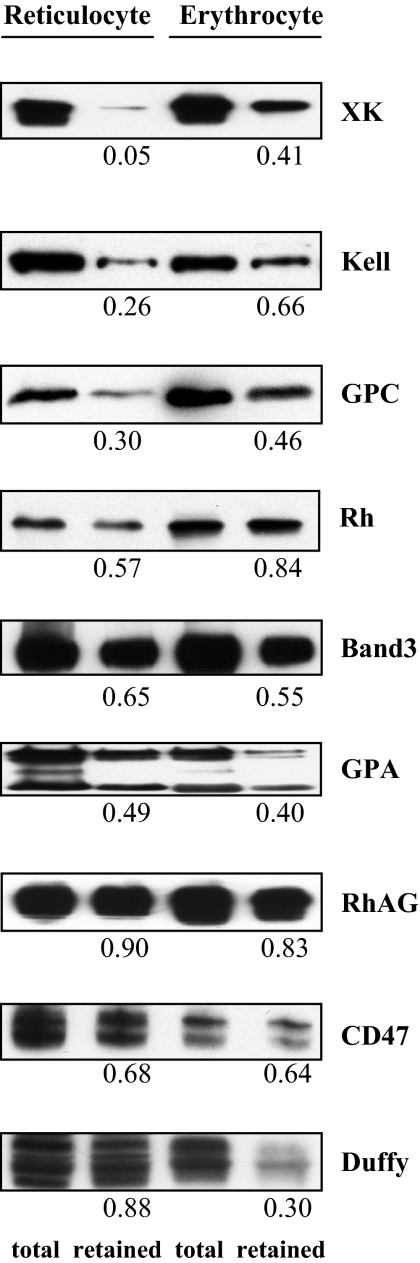
A feature of reticulocyte maturation is loss of approximately 20% of the membrane surface area by vesiculation. It is known that the cohesion between the lipid bilayer and the membrane skeleton determines the propensity to vesiculate and also that the cohesive energy is a function of the linkage between the integral and membrane-skeletal proteins.27 To obtain insights into the potential contribution of decreased membrane cohesion to reticulocyte membrane vesiculation, we examined the extent of these attachments by detergent extraction. Figure 6 shows the representative results of these experiments; it reveals that 3 components of the 4.1R-protein complex, GPC, XK, and Kell, were more readily extracted by nonionic detergent from reticulocyte membranes than from erythrocyte membranes (70%-90% from reticulocytes and 40%-50% from erythrocytes). Surprisingly, Duffy, another component of the 4.1R complex, was extracted more readily from mature red blood cells than from reticulocytes. However, band 3, and its associated proteins, GPA, Rh, and RhAG, were extracted to the same extent from both reticulocytes and erythrocytes.
Figure 6.
Detergent extractability of membrane proteins from reticulocytes and erythrocytes. Membrane proteins were detergent extracted and proteins retained with membrane skeletons were analyzed by SDS-PAGE and probed with antibodies as indicated. The numbers indicate the fraction retained in the pellet compared with the total protein. Note the decreased retention of XK, Kell, and GPC but the increased retention of Duffy in reticulocytes.
Discussion
Erythroid differentiation occurs within erythroblastic islands, composed of erythroblasts at various stages of maturation, surrounding a central macrophage.28 After nuclear extrusion, the immature reticulocytes continue to adhere to central macrophages before their egress into the circulation. Various adhesion molecules, expressed on the surface of erythroblasts and immature reticulocytes, mediate cell-cell and cell-extracellular matrix attachments within the erythroid niche. During steady-state erythropoiesis, reticulocytes remain and mature in the bone marrow for 24 to 48 hours and then circulate as recognizable reticulocytes for an additional 24 hours. During this extended period of reticulocyte maturation, marked changes in both the membrane and cytoplasmic content occur. The cell evolves from an adherent, immature reticulocyte with cytoplasmic organelles to a nonadherent erythrocyte devoid of organelles. Moreover, immature reticulocyte membranes undergo active endocytosis and exocytosis, which does not occur in normal, mature erythrocytes.29
The morphologic differences between circulating reticulocytes and erythrocytes are well known. The reticulocyte has a greater surface area by some 20%, has a stomatocytic profile, and contains vesicles, apparently adhering to the inner surface of the membrane, described as “pits.”16 It was also established14 that reticulocytes are much more rapidly fragmented under shear stress and also are less deformable than the mature cell. Although these numerous dissimilarities between reticulocytes and erythrocytes have been recognized for many years, the mechanistic basis of the changes accompanying reticulocyte maturation is incompletely understood.
A major finding of the current report is the identification of a biochemical basis for some of the differences in membrane stability observed in reticulocytes and erythrocytes. Red cell membrane deformability and mechanical stability are separable attributes.30 Studies on abnormal erythrocytes have identified 2 determinants of impaired mechanical stability: (1) mutant spectrin with an anomalously low capacity to self-associate, resulting in an increased proportion of spectrin dimers on the membrane and therefore discontinuities in the membrane skeletal network4; and (2) unstable network junctions, caused for instance, by a deficiency of 4.1R.31 Our finding that reticulocyte spectrin is almost entirely tetrameric, as in erythrocytes, eliminates the first of these 2 explanations. Importantly, we have clearly demonstrated that the network junctions are more labile in the reticulocyte than in the mature cell, as evidenced by elevated incorporation of the actin- and 4.1R-binding spectrin fragment into the membrane. It will be of interest to determine whether erythroblasts are similar to reticulocytes because in the bone marrow they are not subjected to high shearing forces and therefore do not require stress resistant membranes.
Our results further show that elevated phosphorylation of 4.1R, in part, contributes to the increased liability of the network junctions. The weakened shear resistance of the reticulocyte can be simulated in mature cells by increased phosphorylation, engendered by the PKC enhancer, PMA. An additional effect of elevated 4.1R phosphorylation in the reticulocyte is the more facile extraction of GPC, Kell, and XK. All these proteins bind to 4.1R and must therefore be located at the network junctions: all these interactions are suppressed by phosphorylation of the 4.1R.
Another significant change occurring during reticulocyte maturation is the reduction in surface area caused by the loss of membrane lipid. Studies of hereditary spherocytic erythrocytes suggest that loss of surface area is the result of loss of cohesion between the lipid bilayer and the membrane skeleton.27 Because cohesion between the lipid bilayer and the membrane skeleton is regulated by the association of integral, transbilayer proteins with cytoskeletal proteins, our current finding that several integral proteins are loosely linked to the membrane skeleton implies that a weaker linkage between transbilayer and membrane skeletal proteins can contribute to vesiculation in reticulocytes. The previous finding15 that lipid tethers are more easily drawn out of the reticulocyte membrane than out of the erythrocyte membrane validates a lower energy of cohesion between the protein network and the lipid bilayer in the reticulocytes.
The finding of significant amounts of tubulin and actin in reticulocytes is a reflection of an important role for these proteins during terminal erythroid differentiation in terms of cell division and cell motility. Because these functions are not required in erythrocytes, it is not surprising that these proteins are lost during reticulocyte maturation. Degradation of unfolded and misfolded proteins after ubiquination by the proteosome system has been well established. Our finding that remnant amounts of both tubulin and actin are degraded by the proteosome during reticulocte maturation is novel.
Our current data shed new mechanistic insights into the long-recognized dramatic changes in cell shape and membrane mechanical function that accompany the transformation of reticulocytes into erythrocytes during the course of 2 to 3 days of maturation. Because many inherited and acquired anemias are characterized by an increased number of circulating reticulocytes with distinctly different membrane composition and membrane properties, the present findings may well have implications to the pathophysiology of these disorders.
Acknowledgments
This work was supported in part by National Institutes of Health grants DK26263, DK32094, HL31579, and DK56267.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.L. and X.G. performed research and analyzed the data; and N.M., J.A.C., and X.A. designed experiments, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiuli An, Red Cell Physiology Laboratory, 310 E 67th St, New York, NY 10065; e-mail: xan@nybloodcenter.org.
References
- 1.Bennett V. The spectrin-actin junction of erythrocyte membrane skeletons. Biochim Biophys Acta. 1989;988(1):107–121. doi: 10.1016/0304-4157(89)90006-3. [DOI] [PubMed] [Google Scholar]
- 2.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81(3):1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 3.Mohandas N, An X. New insights into function of red cell membrane proteins and their interaction with spectrin-based membrane skeleton. Transfus Clin Biol. 2006;13(1-2):29–30. doi: 10.1016/j.tracli.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112(10):3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manno S, Takakuwa Y, Mohandas N. Modulation of erythrocyte membrane mechanical function by protein 4.1 phosphorylation. J Biol Chem. 2005;280(9):7581–7587. doi: 10.1074/jbc.M410650200. [DOI] [PubMed] [Google Scholar]
- 6.Reid ME, Mohandas N. Red blood cell blood group antigens: structure and function. Semin Hematol. 2004;41(2):93–117. doi: 10.1053/j.seminhematol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Bruce LJ, Beckmann R, Ribeiro ML, et al. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood. 2003;101(10):4180–4188. doi: 10.1182/blood-2002-09-2824. [DOI] [PubMed] [Google Scholar]
- 8.Salomao M, Zhang X, Yang Y, et al. Protein 4.1R-dependent multiprotein complex: new insights into the structural organization of the red blood cell membrane. Proc Natl Acad Sci U S A. 2008;105(23):8026–8031. doi: 10.1073/pnas.0803225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett V. Proteins involved in membrane–cytoskeleton association in human erythrocytes: spectrin, ankyrin, and band 3. Methods Enzymol. 1983;96(1):313–324. doi: 10.1016/s0076-6879(83)96029-9. [DOI] [PubMed] [Google Scholar]
- 10.Nicolas V, Le Van Kim C, Gane P, et al. Rh-RhAG/ankyrin-R, a new interaction site between the membrane bilayer and the red cell skeleton, is impaired by Rh(null)-associated mutation. J Biol Chem. 2003;278(28):25526–25533. doi: 10.1074/jbc.M302816200. [DOI] [PubMed] [Google Scholar]
- 11.Anong WA, Franco T, Chu H, et al. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood. 2009;114(9):1904–1912. doi: 10.1182/blood-2009-02-203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waugh RE, McKenney JB, Bauserman RG, Brooks DM, Valeri CR, Snyder LM. Surface area and volume changes during maturation of reticulocytes in the circulation of the baboon. J Lab Clin Med. 1997;129(5):527–535. doi: 10.1016/s0022-2143(97)90007-x. [DOI] [PubMed] [Google Scholar]
- 13.Come SE, Shohet SB, Robinson SH. Surface remodelling of reticulocytes produced in response to erythroid stress. Nat New Biol. 1972;236(66):157–158. doi: 10.1038/newbio236157a0. [DOI] [PubMed] [Google Scholar]
- 14.Chasis JA, Prenant M, Leung A, Mohandas N. Membrane assembly and remodeling during reticulocyte maturation. Blood. 1989;74(3):1112–1120. [PubMed] [Google Scholar]
- 15.Waugh RE, Mantalaris A, Bauserman RG, Hwang WC, Wu JH. Membrane instability in late-stage erythropoiesis. Blood. 2001;97(6):1869–1875. doi: 10.1182/blood.v97.6.1869. [DOI] [PubMed] [Google Scholar]
- 16.Mel HC, Prenant M, Mohandas N. Reticulocyte motility and form: studies on maturation and classification. Blood. 1977;49(6):1001–1009. [PubMed] [Google Scholar]
- 17.Joiner CH, Franco RS, Jiang M, Franco MS, Barker JE, Lux SE. Increased cation permeability in mutant mouse red blood cells with defective membrane skeletons. Blood. 1995;86(11):4307–4314. [PubMed] [Google Scholar]
- 18.Noble NA, Xu QP, Ward JH. Reticulocytes. I. Isolation and in vitro maturation of synchronized populations. Blood. 1989;74(1):475–481. [PubMed] [Google Scholar]
- 19.Beutler E, West C, Blume KG. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976;88(2):328–333. [PubMed] [Google Scholar]
- 20.An X, Salomao M, Guo X, Gratzer W, Mohandas N. Tropomyosin modulates erythrocyte membrane stability. Blood. 2007;109(3):1284–1288. doi: 10.1182/blood-2006-07-036954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An X, Lecomte MC, Chasis JA, Mohandas N, Gratzer W. Shear-response of the spectrin dimer-tetramer equilibrium in the red blood cell membrane. J Biol Chem. 2002;277(35):31796–31800. doi: 10.1074/jbc.M204567200. [DOI] [PubMed] [Google Scholar]
- 22.Hershko A, Ciechanover A, Rose IA. Resolution of the ATP-dependent proteolytic system from reticulocytes: a component that interacts with ATP. Proc Natl Acad Sci U S A. 1979;76(7):3107–3110. doi: 10.1073/pnas.76.7.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danilov YN, Fennell R, Ling E, Cohen CM. Selective modulation of band 4.1 binding to erythrocyte membranes by protein kinase C. J Biol Chem. 1990;265(5):2556–2562. [PubMed] [Google Scholar]
- 24.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone RM, Mathew A, Setchenska MS, Grdisa M, White MK. Loss of glucose transport in developing avian red cells. Eur J Cell Biol. 1998;75(1):66–77. doi: 10.1016/S0171-9335(98)80048-4. [DOI] [PubMed] [Google Scholar]
- 26.Mairbäurl H, Schulz S, Hoffman JF. Cation transport and cell volume changes in maturing rat reticulocytes. Am J Physiol Cell Physiol. 2000;279(5):C1621–C1630. doi: 10.1152/ajpcell.2000.279.5.C1621. [DOI] [PubMed] [Google Scholar]
- 27.Butler J, Mohandas N, Waugh RE. Integral protein linkage and the bilayer-skeletal separation energy in red blood cells. Biophys J. 2008;95(4):1826–1836. doi: 10.1529/biophysj.108.129163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112(3):470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanc L, Barres C, Bette-Bobillo P, Vidal M. Reticulocyte-secreted exosomes bind natural IgM antibodies: involvement of a ROS-activatable endosomal phospholipase iPLA2. Blood. 2007;110(9):3407–3416. doi: 10.1182/blood-2007-04-085845. [DOI] [PubMed] [Google Scholar]
- 30.Chasis JA, Mohandas N. Erythrocyte membrane deformability and stability: two distinct membrane properties that are independently regulated by skeletal protein associations. J Cell Biol. 1986;103(2):343–350. doi: 10.1083/jcb.103.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchernia G, Mohandas N, Shohet S. Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability. J Clin Invest. 1981;68(2):454–460. doi: 10.1172/JCI110275. [DOI] [PMC free article] [PubMed] [Google Scholar]