Abstract
Antigen-specific memory T cells (Tms) are essential in the immune surveillance of residual and metastatic tumors. Activation of Tms requires designing vaccines based on tumor rejection antigens, which are often not available to cancer patients. Therefore, it is desirable to have a general applicable approach to activate Tms without extensive knowledge of tumor antigens. Here, we report that activation of antigen-specific Tms could be achieved by the administration of agonistic anti-CD137 monoclonal antibody without additional tumor vaccination, leading to the prevention of recurrence and metastases after surgical resection of primary tumors in mouse models. By reconstitution with CD137-deficient Tms, we demonstrate that expression of CD137 on antigen-specific Tms is only partially required for the effect of anti-CD137 antibody. Other host cells, including those from hematopoietic and nonhematopoietic origins, are also important because ablation of CD137 from these cells partially but significantly eliminates antitumor effect of anti-CD137 antibody. Our findings implicate a potential new approach to prevent recurrence and metastases in cancer patients.
Introduction
CD137 (4-1BB, TNFRSF9) glycoprotein is a member of the tumor necrosis factor receptor superfamily1 and binds to a high-affinity ligand (CD137L, 4-1BBL, TNFSF9) expressed on antigen-presenting cells such as dendritic cells, macrophages, and activated B cells.2 Expression of CD137 is found on various hematopoietic cells, including primed T cells, natural killer (NK) cells, neutrophils, monocytes, dendritic cells, and mast cells.3 CD137 on cells other than those of hematopoietic origin is rare, but there are reports indicating that endothelial and epithelial cells could be induced to express CD137 during inflammation.4 CD137 is shown to be an important costimulatory molecule for T-cell activation. Engagement of CD137 on T cells by natural ligand or agonist monoclonal antibody (mAb) enhances T-cell proliferation and provides protection to CD8 T cells from activation-induced cell death through nuclear factor κB–mediated activation and up-regulation of the antiapoptotic Bcl-2 family members Bcl-xL and Bfl-1.5 Stimulation of CD137 could also lead to activation of dendritic cells,6 NK cells,7 and macrophages8,9 in vitro. Numerous studies demonstrate that agonistic CD137 antibody costimulates T-cell responses and induces regression of established tumors in various animal models.10–13 Furthermore, the administration of anti-CD137 antibody could also prevent and break established tolerance of antigen-specific T cells in mouse models.14 Based on these findings, clinical trials of anti-CD137 mAb for the patients with advanced melanoma were recently initiated.15 In addition to costimulation of T-cell receptor (TCR)–mediated responses, our recent study shows that ligation of CD137 by CD137L or agonist antibody stimulates proliferation and functional maturation of Tms in the absence of major histocompatibility complex or TCR triggering. Interestingly, CD137-mediated proliferation of Tms does not require interleukin-15 (IL-15),16 indicating that CD137 transmits a unique growth and differentiation signal to Tms.
Naive CD8+ cytolytic T cells (CTLs) recognize aberrant antigens expressed by cancers, resulting in the proliferation and differentiation of naive CTLs into effector T cells. Once the inflammation is resolved and the antigens have been cleared, the majority of the effector T cells undergo apoptosis, and only a small fraction of these cells differentiates into long-lived Tms. Persistent exposure to antigen may impair the generation of Tms due to exhaustion, tolerance, or death.17 However, in cancer patients, Tms do not seem to be eliminated completely, even in patients in advanced stages. T-cell responses against tumor antigens could often be recalled in vitro by restimulation of antigen in both animal models18 and cancer patients.19 In addition, high frequency of tumor antigen–specific Tms could be found in the bone marrow of cancer patients.20 Tms, including central Tms in lymphoid organs and effector Tms in peripheral tissues, have superior ability to proliferate after secondary exposure to antigen and to quickly obtain effector function.21 The most straightforward approach to boost tumor antigen–specific Tms is antigen-based vaccination. However, the design of these vaccines often requires knowledge of tumor antigens, which may not be available for a given cancer. In addition, antigen-based vaccines may boost only monoclonal or oligoclonal T cells, which could lead to selection pressure for emergence of antigen-loss variants.22 Therefore, a strategy that is independent from antigen-based vaccines for activation of Tms is highly desirable.
In this study, we show that the administration of agonist CD137 mAb stimulates expansion of tumor antigen–specific Tms in mouse models with surgical resection of primary tumors. Importantly, anti-CD137 mAb could prevent recurrence and metastases of the same tumors independent of additional vaccination.
Methods
Mice, cell lines, and reagents
Female C57BL/6 (B6/Thy1.2), B6/Thy1.1, DBA/2, and BALB/c mice (6–10 weeks old) were purchased from the National Cancer Institute. OT-1/RAG−/− mice were purchased from Taconic Farms. CD137-deficient (KO) mice were generated as previously described.16 OT-1/Thy1.1 and OT-1/CD137KO mice were obtained by backcrossing corresponding parent strains. All mice were housed under specific pathogen–free conditions in the Johns Hopkins animal facility and all protocols were approved by the Institutional Animal Care and Use Committee. Parental B16 melanoma, B16-OVA (B16 expressing chicken ovalbumin), P815 mastocytoma, and 4T1 breast cancer were purchased from ATCC. B16-OVA and 4T1 lines were maintained in Iscove modified Dulbecco medium supplemented with 10% fetal bovine serum, 100 units/mL penicillin G, and 100 μg/mL streptomycin. P815 was maintained in RPMI-1640 supplemented with 10% fetal bovine serum, 100 units/mL penicillin G, 100 μg/mL streptomycin, 25mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and 55μM 2-mercaptoethanol.
Antibodies and flow cytometric analysis
Purified mAbs specific for mouse CD3, CD8, CD28, CD44, CD62L, interferon-γ (IFN-γ), Thy1.1, and Thy1.2 were purchased from BD Biosciences. SIINFEKL/H-2Kb-PE tetramer (OT-1 tetramer) was purchased from Beckman Coulter. The generation and purification of anti–mouse CD137 mAb (clone 2A) were previously described.23 Control rat immunoglobulin G (IgG) was purchased from Sigma-Aldrich. BD Cytofix/Cytoperm Kit (BD Biosciences) and Brefeldin A solution (eBioscience) were used for intracellular staining. Fluorescence was detected by FACSCalibur flow cytometry and analyzed with BD CellQuest (BD Biosciences) and FlowJo (TreeStar Inc).
Activation of T cells in vitro
Naive T cells were harvested from donor spleen and stimulated with immobilized anti-CD3 mAb and anti-CD28 mAb for 2 days. Then, activated T cells were incubated in RPMI-1640 medium containing 20 IU/mL recombinant IL-2 for an additional 3 days and used in adoptive transfer.
Animal studies and tumor models
Initially, all tumor lines were administered at their minimal tumorigenic dose subcutaneously into the shaved left flank of mice in 100 μL of Hanks balanced salt solution. Seven to 10 days later, tumor nodules were removed surgically under anesthesia with 2-2-2 tribromoethanol. After 1 month, operated mice were given 100 μg of control rat Ig or anti-CD137 mAb on days −9 and −7 before tumor rechallenge. On day 0, tumor was injected subcutaneously on the right flank or intravenously though the tail vein. Tumor size, presented as the average of 2 perpendicular diameters (millimeters), was measured at regular intervals.
The method for generation of Tms in vivo was previously described.16 Briefly, OT-1 T cells were activated in vitro and transferred into naive B6 mice. One month after transfer, mice were given 100 μg of control Ig or anti-CD137 mAb on days −9 and −7, and inoculated with B16-OVA subcutaneously or intravenously on day 0.
In the bone marrow transplantation model, C57BL/6 (Thy1.1) and CD137KO (Thy1.2) mice were irradiated (650 Gy) twice in the same day. Bone marrow cells were harvested from Thy1.1 mice, Thy1.2 mice, or CD137KO mice and transferred into irradiated Thy1.1 or CD137KO recipients. The percentage of bone marrow chimerism was evaluated at day 30 after bone marrow transplantation.
In vivo cytotoxicity assay
In vivo cytotoxicity assay was performed by transferring naive splenocytes from C57BL/6 mice, which were labeled with high-dose carboxyfluorescein succinimidyl ester (CFSEhi, 5μM) or low-dose (CFSElo, 0.5μM) CFSE. Only CFSEhi spleen cells were pulsed with 10μM OVA peptide (SIINFEKL). A total of 5 million cells, with an equal mix of CFSEhi- and CFSElo-labeled cells, were injected intravenously into the mice with OVA-specific Tms. The proportions of CFSEhi and CFSElo cells were measured in spleen cells by flow cytometry at 6 and 24 hours, and the cytotoxicity was calculated by the formula: % cytotoxicity = 100 − ([%CFSEhi/%CFSElo]/[%CFSEhi control/%CFSElo control] × 100).
ELISPOT assay
MultiScreen HTS Filter plates (Millipore) were used for enzyme-linked immune spot (ELISPOT) assays. Plates were coated with mAb against IFN-γ (clone AN-18; eBioscience) in phosphate-buffered saline (PBS), pH 7.4, overnight at 4°C. After extensive washing with PBS with 0.05% Tween 20 (Sigma-Aldrich), plates were blocked with RPMI-1640 medium with 10% fetal bovine serum. Spleen cells were harvested from the mice that were stimulated to generate OVA-specific Tms, and were treated with rat Ig or anti-CD137 mAb on day 0. The cell suspension (2 × 106/mL) was plated at 100 μL per well. OVA peptides were added to the cultures at a final concentration of 10 ng/mL or 100 ng/mL. Mitomycin C (Sigma-Aldrich)–treated B16-OVA cells were also added to the plate. As a positive control, phorbol myristate acetate and Ca-ionophore (Sigma-Aldrich) were added to control wells. Plates were incubated at 37°C, 5% CO2 for 48 hours. After incubation with cells, plates were washed with PBS-0.05% Tween 20. Biotinylated anti–IFN-γ antibody (clone R4-6A2; eBioscience) was added, and plates were incubated at room temperature for 2 hours. After washing, streptavidin–horseradish peroxidase conjugate (eBioscience) was added at room temperature for 1 hour. After washes with PBS-Tween 20, spots were developed with 3-amino-9-ethylcarbazole (AEC) substrate (Sigma-Aldrich) incubation for 15 to 20 minutes at room temperature. Plates were washed with distilled water and then dried before counting spot number.
Statistical analysis
Statistical significance at the 95% confidence interval was analyzed using the unpaired Student t test. Tumor growth kinetics were analyzed by Student t test and analysis of variance (ANOVA). Survival data were analyzed with log-rank (Mantel-Cox) test. All statistical data were calculated with Microsoft Excel (Microsoft Corporation) and Prism 5 (GraphPad Software Inc).
Results
Prevention of tumor recurrence and metastases by anti-CD137 mAb in the absence of antigen-based vaccine
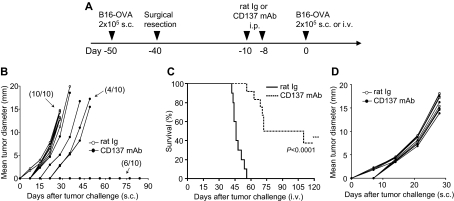
We showed previously that agonist anti-CD137 mAb (clone 2A) induced expansion of antigen-specific memory T cells without re-exposure to antigen.16 To determine whether this finding could be applied to prevent tumor recurrence, we first established a mouse model (Figure 1A) in which B16-OVA melanoma was implanted subcutaneously for 10 days to establish growth of tumors (3-5 mm in average mean diameters). Upon complete surgical removal of tumors, the mice rested for 30 days to facilitate the development of Tms. Anti-CD137 mAb was inoculated twice on 2 separate days to stimulate Tms. Eight days after antibody injection, the mice were challenged with a lethal dose of B16-OVA either subcutaneously (solid tumor model) or intravenously (disseminated tumor model) to mimic recurrence of tumors. As we reported previously,14 7 to 9 days after anti-CD137 antibody injection, expansion of memory T cells reached its peak.
Figure 1.
Effect of anti-CD137 mAb in the prevention of recurrence and metastases of murine B16-OVA melanoma. (A) A schematic of mouse models of melanoma recurrence and metastases and anti-CD137 mAb treatment protocol. (B) Effect of anti-CD137 mAb in the prevention of recurrence of B16-OVA tumor. Upon removal of primary tumors, mice were challenged with B16-OVA tumor cells subcutaneously, and the sizes of tumors were measured individually and recorded as the mean tumor diameter regularly. Results are 1 representative of 3 independent experiments. (C) Effect of anti-CD137 mAb in the prevention of metastases of B16-OVA tumor. Upon removal of primary tumors, mice were challenged with B16-OVA tumor cells intravenously, and the survival of mice was monitored daily up to 120 days. Results are 1 representative of 3 independent experiments. ***Significantly different from control mAb (rat Ig) treatment group, P < .001. (D) Presensitization of mice to primary tumors is required for the effect of anti-CD137 mAb. Mice were mock-treated by surgery, as in panel B but without primary tumor inoculation, and subsequently treated with anti-CD137 mAb. B16-OVA tumors were then inoculated, measured individually, and recorded as the mean tumor diameter regularly. Results are 1 representative of 3 independent experiments.
After administration of anti-CD137 mAb, we determined the outgrowth of subcutaneously inoculated tumors (Figure 1B) or the survival of mice with intravenously injected disseminated tumors (Figure 1C). Treatment by anti-CD137 mAb inhibited the growth of subcutaneous tumor in 60% (6 of 10) of mice in comparison with 0% (0 of 10) of rat IgG–treated control mice. Furthermore, treatment by anti-CD137 mAb also improved long-term survival (40%) of mice with disseminated tumors (up to 120 days), whereas all mice treated by control mAb died of tumors within 54 days. We also obtained similar results in P815 mastocytoma and 4T1 breast cancer models (supplemental Figure 1A-B, available on the Blood website; see the Supplemental Materials link at the top of the online article). The effect of anti-CD137 mAb requires pre-exposure to the tumor because the same treatment did not work in naive mice without preinoculation of B16-OVA tumors (Figure 1D), although T cells with memory phenotype expand significantly.16 Together, our results suggest that anti-CD137 mAb may prevent tumor recurrence through expansion of tumor antigen–specific memory T cells without additional tumor vaccination.
Antigen-specific memory T cells are required for the effect of anti-CD137 mAb to prevent tumor recurrence and metastases
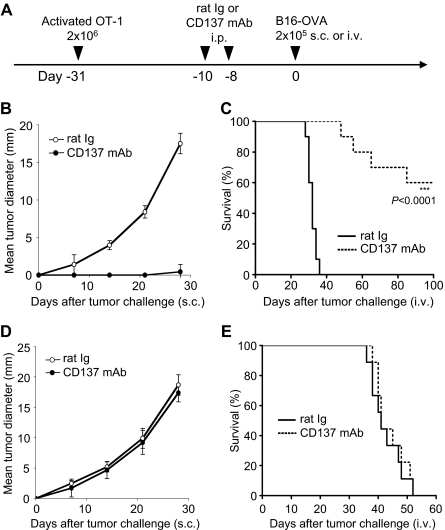
We further established a murine tumor model to facilitate analysis of memory T-cell responses to the tumor antigen. OT-1/CD8+ T cells were activated in vitro by chicken ovalbumin as a surrogate tumor antigen in the presence of antigen-presenting cells. Activated OT-1 T cells were then adoptively transferred into naive B6/Thy1.2 mice and allowed to rest for 30 days to develop Tms. After treatment by anti-CD137 mAb, the mice were challenged with B16-OVA tumors (Figure 2A). In this setting, memory or effector cells could be traced by OVA-specific tetramer for analysis. As shown in Figure 2B, pretreatment by anti-CD137 mAb, not control mAb, prevents outgrowth of the lethal dose of B16-OVA tumor cells in nearly all mice (P < .001, 2-way ANOVA). In addition, the same treatment also leads to survival of 60% more mice at 100 days after tumor challenge, whereas all control mice died of disseminated metastases within 40 days (Figure 2C). In this model, OT-1 Tms were required for the effect of anti-CD137 mAb to prevent outgrowth and metastases of B16-OVA tumor because anti-CD137 mAb treatment was not effective upon transfer of naive versus activated OT-1 T cells (Figure 2D-E). This result also shows that endogenous tumor-specific T cells are not sufficient to prevent tumor growth in this model. Our results thus indicate that activation of tumor antigen–specific memory T cells is required for the effect of anti-CD137 mAb.
Figure 2.
Effect of anti-CD137 mAb in the stimulation of Tms to prevent tumor outgrowth in the OT-1 adoptive transfer model. (A) A schematic of the treatment of a melanoma mouse model with Tms and anti-CD137 mAb. In vitro–activated OT-1 TCR-transgenic T cells were transferred into mice for the development of Tms. (B) Effect of anti-CD137 mAb in preventing B16-OVA tumor challenge. One month after transfer of activated OT-1 T cells, mice were treated with anti-CD137 mAb and subsequently challenged with B16-OVA tumor cells subcutaneously. The sizes of tumors were measured individually and recorded as the mean tumor diameter regularly. Results are 1 representative of 4 independent experiments. Each point is the mean ± SD of tumor diameters in a group of 5 mice. (C) Effect of anti-CD137 mAb in the prevention of metastases of B16-OVA tumor. One month after transfer of activated OT-1 T cells, mice were treated with anti-CD137 mAb and subsequently challenged with B16-OVA tumor cells intravenously. The survival of mice was monitored daily up to 100 days. Results are 1 representative of 3 independent experiments. ***Significantly different from control mAb (rat Ig) treatment group, P < .001. (D-E) Effect of anti-CD137 mAb fails to prevent tumor challenge after transfer of naive OT-1 cells. The procedure is the same as in panel A, but naive instead of activated OT-1 T cells were transferred. Tumor growth (D) and survival (E) of the mice were monitored as described in panels B-C. Results are 1 representative of 2 independent experiments.
Anti-CD137 mAb is sufficient to induce expansion of tumor antigen–specific memory T cells
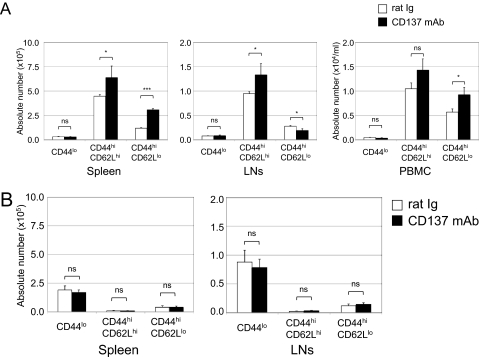
We next determined whether anti-CD137 mAb could increase the number of OT-1 T cells with memory phenotype in the absence of additional antigen stimulation. After transfer of activated or naive OT-1 T cells, the mice rested for 3 weeks and subsequently were treated with anti-CD137 mAb without tumor challenge. After 10 days of administering antibody, OT-1 T cells were gated by tetramer, and CD44 and CD62L, markers of Tms, were determined.
Absolute numbers of CD8+ OT-1 effector Tms (CD44hiCD62Llo) were increased significantly in the spleen and peripheral blood mononuclear cells. Interestingly, central Tms (CD44hiCD62Lhi) were also increased significantly in spleen and lymph nodes by anti-CD137 mAb treatment. On the other hand, we observed a small but significant decrease of CD8+ OT-1 effector Tms in tumor-draining lymph nodes. A small population of naive OT-1 T cells (CD44lo) did not change (Figure 3A), indicating that anti-CD137 mAb did not stimulate naive T cells in the absence of antigen. This observation is consistent with the finding that anti-CD137 mAb did not stimulate expansion of naive OT-1 T cells upon transfer (Figure 3B). In addition to OT-1 T cells, non–OT-1 endogenous CD8+ T cells also underwent expansion. Both endogenous central Tms (CD44hiCD62Lhi) and effector Tms (CD44hiCD62Llo) increased significantly, whereas endogenous naive CD8 T cells (CD44lo) did not (supplemental Figure 2). These results indicate that anti-CD137 mAb could stimulate polyclonal expansion of Tms.
Figure 3.
Flow cytometric analysis of mononuclear cells after anti-CD137 mAb treatment. Naive B6 mice (Thy1.2) were transferred with 107 purified activated (A) or naive (B) OT-1 T cells (CD8+ Thy1.1). As previously described, 3 weeks later they were treated with rat Ig or anti-CD137 mAb. At 10 days after antibody treatment, mononuclear cells were harvested from spleen, lymph nodes (axilla and inguinal), and peripheral blood. OT-1 T cells were identified by anti-Thy1.1 mAb. Absolute numbers of OT-1 T cells of spleen, lymph nodes, and peripheral blood were calculated. Subsets of effector Tms (CD44hiCD62Llo), central Tms (CD44hiCD62Lhi), and naive T cells (CD44lo) of OT-1 T cells were identified by the indicated specific mAbs. Results are 1 representative of at least 3 independent experiments. ns indicates not significant; *P < .05; ***P < .005.
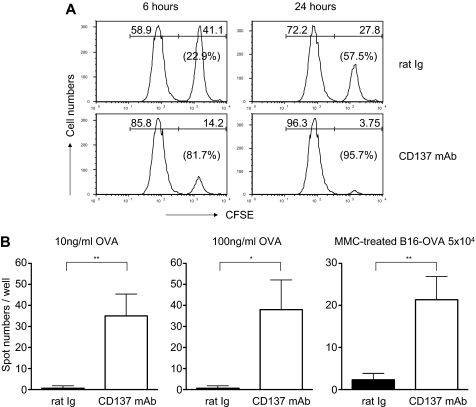
To determine whether the increased number of Tms is associated with functional activation, we performed an in vivo CTL assay. As described in “Animal studies and tumor models,” OT-1 Tms were generated in vivo as a source of effector cells. To prepare target cells, spleen cells were labeled with CFSE in high (right peak) or low (left peak) concentrations and subsequently pulsed with OVA or control peptide, respectively. Seven days after anti-CD137 mAb treatment, target cells were injected intravenously into the mice, and splenocytes were collected 6 and 24 hours later to analyze CFSE-labeled cells. As shown in Figure 4A, specific lysis was 22.9% at 6 hours and 57.5% at 24 hours in the mice treated with control antibody, indicating an activity of OT-1 memory T cells. However, anti-CD137 mAb–treated mice acquired much higher cytolytic activity, with a specific lysis of 81.7% at 6 hours and 95.7% at 24 hours, compared with that found in control antibody–treated mice.
Figure 4.
Treatment by anti-CD137 mAb promotes functions of memory OT-1 T cells in vivo. (A) In vivo CTL assay. Naive B6 spleen cells were labeled with CFSE at a high (5μM, CFSEhi, right peaks) or low dose (0.5μM, CFSElo, left peaks) and pulsed with OVA (SIINFEKL) or the control peptide, respectively, as target cells. Target cells at 1 × 107 were injected intravenously into the recipient mice 1 month after transfer of activated OT-1 cells and treatment with anti-CD137 mAb or control Ig. Splenocytes were collected from the recipient mice for detection of CFSE-labeled cells by flow cytometry at 6 and 24 hours. Percentage lysis of peptide-pulsed target cells was calculated from the ratio of CFSEhi/CFSElo target cells. (B) ELISPOT assays for IFN-γ–producing cells. ELISPOT assays were performed in triplicate, using spleen cells with OVA peptide or mitomycin C–treated B16-OVA tumor cells. The spots that represent IFN-γ–producing cells were counted, and the mean values ± SD are shown. Results represent 3 independent experiments. *P < .05; **P < .01.
In addition to cytolytic activity of recalled Tms, the spleen cells from anti-CD137 mAb– or rat Ig–treated mice were also harvested and examined for the frequency of IFN-γ–producing cells in an ELISPOT assay (Figure 4B). The spleen cells from anti-CD137 mAb–treated mice showed significantly more spots by stimulation with either OVA peptide (at either 10 or 100 ng/mL) or B16-OVA tumor cells. This observation is consistent with augmented Tm absolute numbers and cytolytic activity upon anti-CD137 mAb treatment.
In contrast to augmented frequency of antigen-specific Tms, there was no change in terms of IFN-γ production upon anti-CD137 mAb or rat Ig treatment on OT-1 cells. No significant difference was observed within the OT-1 cells in the percentage (supplemental Figure 3A) or intensity (supplemental Figure 3B) of intracellular IFN-γ staining with either treatment in the spleen and lymph nodes. Our results support that anti-CD137 mAb affects the quantity but not the quality of antigen-specific OT-1 Tms.
CD137 signaling to both transferred antigen-specific Tms and host cells is required for the maximal effect of anti-CD137 mAb
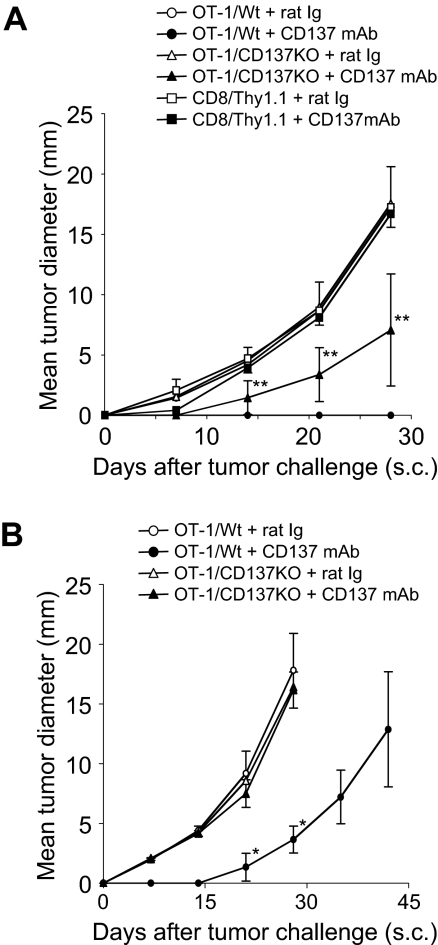
To determine whether CD137 expressed on memory T cells is required for the effect of anti-CD137 mAb, we first backcrossed CD137KO mice to OT-1 transgenic mice (OT-1/CD137KO) to obtain CD137-deficient OT-1 T cells. OT-1/CD137KO or wild phenotype OT-1 (OT-1/Wt) cells were activated in vitro as described in “Animal studies and tumor models.” There is no difference in proliferation and cytolytic activity of activated OT-1/CD137KO versus OT-1/Wt cells (Zhu et al16 and data not shown). Activated T cells were purified and transferred into naive B6 mice for the development of Tms as described in “Animal studies and tumor models.” Purified polyclonal Thy1.1+CD8+ T cells (Thy1.1/CD8) were also activated and transferred as an additional control. After treatment by anti-CD137 mAb, mice were challenged subcutaneously with B16-OVA tumor cells. As predicted, after control rat Ig treatment the mice with OT-1/Wt Tms rapidly developed tumors, whereas after anti-CD137 mAb treatment the mice with OT-1/Wt Tms controlled tumor growth (P < .001, 2-way ANOVA). On the other hand, the mice with OT-1/CD137KO Tms had a significantly weaker response against tumor after anti-CD137 mAb treatment in comparison with those with OT-1/Wt (P = .026, 2-way ANOVA), indicating that CD137 on Tms is required, at least partially, for the effect of anti-CD137 mAb. However, in the absence of CD137 on Tms, the mice treated with anti-CD137 mAb could also resist tumor growth partially compared with those treated with control Ig (P = .035, 2-way ANOVA), suggesting the host cells, in addition to OT-1 Tms, play a role. Presence of antigen-specific Tms is important because the transfer of purified polyclonal Thy1.1/CD8 T cells from naive mice did not prevent tumor outgrowth after anti-CD137 mAb injection (Figure 5A). These results suggest that CD137 on Tms is only partially required and other host cells also play a role for the effect of anti-CD137 mAb.
Figure 5.
CD137 expression on both OT-1 Tms and host cells is required for the effect of anti-CD137 mAb. (A) CD137 on OT-1 Tms is required for the effect of anti-CD137 mAb. Spleen cells were prepared from wild-type OT-1 (OT-1/Wt) or CD137-deficient OT-1 (OT-1/CD137KO) mice, and activated by anti-CD3/CD28 mAb in vitro for 2 days. In addition, spleen cells from Thy1.1 mice (CD8+Thy1.1) were also activated with the same procedure as controls. Activated cells were transferred into naive B6 mice for memory T-cell development. One month later, mice were treated with rat Ig or anti-CD137 mAb as indicated. One week after antibody treatment, B16-OVA was inoculated subcutaneously. Tumor sizes were measured regularly. Each point is the mean (± SD) tumor diameter in a group of 5 mice, and the result is a representative of 3 independent experiments. **P < .01 compared with the OT-1/Wt Tms treated with control rat Ig. Mice treated with OT-1/CD137KO Tms and anti-CD137 mAb had a significantly weaker response against tumor in comparison with those treated with OT-1/Wt Tms and anti-CD137 mAb (P = .026, 2-way ANOVA). (B) CD137 on host cells is required for the effect of anti-CD137 mAb. OT-1/Wt and OT-1/CD137KO were transferred into naive CD137KO mice and subsequently treated with rat Ig or anti-CD137 mAb. Each point indicates the mean tumor diameters in a group of 5 mice, and error bars show SD. Results are 1 representative of 3 independent experiments. *P < .05 compared with the OT-1/CD137KO treated with anti-CD137 mAb.
To validate this observation, we transferred activated OT-1/Wt or OT-1/CD137KO CD8+ T cells into CD137KO mice to evaluate the role of CD137 on T cells and host cells separately. As expected, whereas deletion of CD137 on host cells partially impedes the effect of anti-CD137 mAb, deletion of CD137 on both OT-1 T cells and host cells completely eliminated the effect of anti-CD137 mAb, similar to the treatment by control antibody (Figure 5B). Our results thus indicate an unexpected finding. In addition to Tms, host cells, which are largely antigen-nonspecific components, are also required for the effect of anti-CD137 mAb.
Both hematopoietic and nonhematopoietic host cells are required for the antirecurrence/metastases effect of anti-CD137 mAb
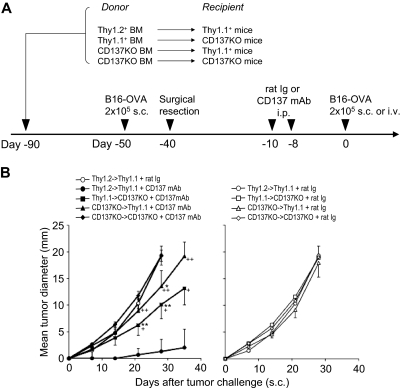
To further dissect the components of host cells that are required for the effect of anti-CD137 mAb, we used bone marrow chimera as recipients to determine the role of CD137 expression on hematopoietic versus nonhematopoietic cells. Mice were lethally irradiated and reconstituted with bone marrow from Wt (Thy1.2 or Thy1.1) or CD137KO mice. One month after bone marrow reconstitution, we confirmed chimerism in the mice by Thy1.1 and Thy1.2 markers. Hematopoietic cells from the donor comprised 90% to 97% of the peripheral blood mononuclear cells (data not shown). In the second group of chimeric mice in our protocol, we examined the effect of CD137 deficiency in the host, and in the third group of chimeric mice, we examined the effect of CD137 deficiency on hematopoietic cells (Figure 6A). Based on our tumor surgical resection protocol, chimeric mice were treated with anti-CD137 mAb or control rat Ig and challenged with tumor cells (Figure 6A). As shown in Figure 6B (left panel), lack of CD137 on either hematopoietic (CD137KO→Thy1.1 + anti-CD137 mAb; P = .003, 2-way ANOVA) or nonhematopoietic (Thy1.1→CD137KO + anti-CD137 mAb; P = .013, 2-way ANOVA) cells in chimeric mice significantly impaired the effect of anti-CD137 mAb, compared with nearly complete protection of tumor challenge in chimeric mice with wild-type bone marrow (Thy1.2→Thy1.1 + anti-CD137 mAb). As a control, treatments of chimeric mice with control antibody did not have an antirecurrence/metastases effect in any combination (Figure 6B right panel). Taken together, our results demonstrate that CD137 on antigen-specific Tms as well as host cells of both hematopoietic and nonhematopoietic origin are required for the antirecurrence/metastases effect of anti-CD137 mAb.
Figure 6.
Both hematopoietic and nonhematopoietic host cells are required for the antirecurrence/metastases effect of anti-CD137 mAb. (A) A schematic of the mouse model for melanoma recurrence/metastases after bone marrow (BM) reconstitution and anti-CD137 mAb treatment. At day −90, indicated recipient mice were irradiated with 650 Gy twice on the same day. BM cells were harvested from the indicated donor mice and transferred at 5 × 106/mouse into indicated recipient mice. The percentage of bone marrow chimerism was evaluated 30 days after BM transplantation. Chimeric mice were inoculated with 2 × 105 B16-OVA tumor cells subcutaneously at day −50. Ten days later, tumor nodules were surgically resected. Mice rested for 1 month to facilitate the development of memory T cells and were subsequently treated with anti-CD137 mAb twice as indicated. At day 0, mice were rechallenged with B16-OVA tumor cells at 2 × 105 subcutaneously or intravenously. (B) BM-reconstituted and tumor-resected mice were treated with control rat Ig or anti-CD137 mAb (left panel) or control rat Ig (right panel), and B16-OVA tumor cells were inoculated subcutaneously 1 week later. Each point indicates the mean tumor diameters in a group of 5 mice, and error bars show SD. *P < .05, **P < .01 compared with Thy1.2BM/Thy1.1 host treated with control rat Ig. +P < .05, ++P < .01 compared with Thy1.2BM/Thy1.1 host treated with anti-CD137 mAb.
Discussion
Although there is ample evidence that tumor antigen–specific memory T cells are present in cancer patients, the approach to boost them is limited, largely because of the lack of knowledge of tumor antigens in the given cancer patients. In addition, antigen-based vaccines often limit the number of antigens to be incorporated, which prevents a broad-spectrum activation of the available T-cell repertoire.24 Our study demonstrates that antigen-specific memory T cells could be activated in vivo by agonistic CD137 mAb, thus leading to the protection of tumor challenges. This method does not require knowledge of tumor antigens and is not dependent on tumor antigen–based vaccination. We also provide evidence that expansion of Tms is polyclonal in nature. Our finding may represent a new and promising approach to prevent recurrence of cancer in the clinic.
Previously, we showed that anti-CD137 agonistic mAb could induce proliferation and activate memory T cells in vivo.16 In this study, we further expand this finding and show that this approach could be applied to enhance immune responses in the mice with memory T cells against tumor antigens. Using a model antigen OVA, we show that antigen-specific memory T cells could be induced to divide and be increased in function, including increased cytolytic activity and IFN-γ secretion without re-exposure to tumor antigen. As a result of this activation, the mice are protected from rechallenge of the lethal dose of subcutaneously or disseminated tumor cells, models mimicking recurrence and metastases of cancers. Furthermore, the P815 and 4T1 surgical resection models showed that anti-CD137 mAb could potentially induce Tms to respond against endogenous tumor antigens, in addition to the ova antigen expressed on B16-OVA (supplemental Figure 1). The anti-CD137 mAb used in our experiments is of rat origin and has a half-life of approximately 7 days. Therefore, there is a small possibility that the mAb remains in the mice until tumor challenge (8-10 days) and activates naive T cells together with the antigen provided by tumor cells. This possibility, however, could be excluded by our experiment because transfer of naive OT-1 is completely insufficient to resist tumor growth after anti-CD137 mAb treatment (Figure 2D-E). Our results thus demonstrate that Tm is required for the effect.
CD137 stimulation is selective for memory but not naive T cells.16 In our experiments, in addition to OT-1 Tms, endogenous non–OT-1 T cells with memory phenotype could also increase in number, indicating that expansion of Tms by anti-CD137 mAb is polyclonal in nature (supplemental Figure 2). Although we observed a consistent increase of CD8+ T cells with central memory T-cell phenotype in spleen, lymph nodes, and blood, a small but significant decrease of CD8+ T cells with effector memory phenotype was noted. This observation is in contrast to the increase of such T cells in spleen and blood (Figure 3A). It is possible that a small phenotype modification may be responsible for this difference. We found that this effector memory T-cell population in lymph nodes expresses a low level of Ly-6C (H.N., unpublished observation, January 2008), which contributes to migration of these T cells, upon anti-CD137 mAb treatment. In adults, a significant portion of T cells has a Tm phenotype, and this treatment may therefore lead to an increase of T cells and nonspecific functional activation. It has been shown that repeated injections of anti-CD137 mAb enhance myelogenesis,25,26 which is potentially caused by Tm-released cytokines. However, these effects are different from the “cytokine storm” induced by anti-CD28 antibody27 and are relatively milder. Furthermore, recent results from phase 1 clinical trials using anti-CD137 mAb demonstrate a mild toxicity in cancer patients.15
It was believed that the major effect of anti-CD137 mAb was simply binding to CD137 on T cells and costimulating T cells. However, many immune cells are found to express or induce CD137 on the cell surface, including NK cells, NKT cells, dendritic cells (DCs), and tumor-associated endothelium.4,6,7,28 Previously, we reported CD137 is an important receptor for the modulation of DC function.6 In addition, CD137 could be induced on human umbilical vein endothelial cells and human aortic smooth muscle cells with tumor necrosis factor-α, IL-1, and IFN-γ, and CD137 promotes the development of plaque inflammation in the hypercholesterolemic mice model.29 Our results indicate that expression of CD137 on Tms is critical because transfer of CD137-deficient T cells significantly impedes the effect of anti-CD137 mAb. However, cells other than transferred Tms in the recipient mice also play an important role because the antitumor effect of anti-CD137 mAb is significantly impaired in CD137-deficient mice, even in the presence of CD137+ OT-1 Tms (Figure 5B). More surprisingly, further dissection of host components by bone marrow chimera shows the importance of host nonhematopoietic cells. It is possible that expression of CD137 on NK cells, DCs, and neutrophils is up-regulated during the growth of tumors, and this leads to activation of these cells, which contribute to tumor immunity. In addition, endothelium, smooth muscle cells, and even other stromal cells may also express CD137 at the tumor site and promote migration of macrophages and other antigen-presenting cells for more efficient antigen presentation. Therefore, our results underscore the important role of CD137 on non-T cells in a disease model. Future investigation will need to selectively ablate CD137 on each cell population, including those from hematopoietic and nonhematopoietic cells, using conditional knockout mice to elucidate the contribution of these cell subsets.
Although we present a clinically relevant model for the use of anti-CD137 mAb to prevent tumor recurrence and metastases after surgery, this method may also be suitable to use with other methods such as antigen-based vaccines to further enhance immunity to several broader antigens. Appropriate combination of anti-CD137 mAb with other methods thus may provide a promising approach not only to treat primary tumors but also to prevent recurrence and metastases in cancer patients.
Acknowledgments
We thank Jennifer Osborne for manuscript editing and Kyung H. Yi for careful reading.
This work was supported by National Institutes of Health grants CA-106861 and AI-072592.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.N. and L.C. designed experiments and wrote the paper; and H.N., Y.Z., L.L., and G.Z. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lieping Chen, Johns Hopkins Medicine, 2M07 David H. Koch Cancer Research Bldg, 1550 Orleans St, Baltimore, MD 21231; e-mail: lchen42@jhmi.edu.
References
- 1.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76(6):959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 2.Armitage RJ. Tumor necrosis factor receptor superfamily members and their ligands. Curr Opin Immunol. 1994;6(3):407–413. doi: 10.1016/0952-7915(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 3.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3(8):609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 4.Broll K, Richter G, Pauly S, Hofstaedter F, Schwarz H. CD137 expression in tumor vessel walls: high correlation with malignant tumors. Am J Clin Pathol. 2001;115(4):543–549. doi: 10.1309/e343-kmyx-w3y2-10ky. [DOI] [PubMed] [Google Scholar]
- 5.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169(9):4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox RA, Chapoval AI, Gorski KS, et al. Cutting edge: expression of functional CD137 receptor by dendritic cells. J Immunol. 2002;168(9):4262–4267. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 7.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190(2):167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 8.Kienzle G, von Kempis J. CD137 (ILA/4-1BB), expressed by primary human monocytes, induces monocyte activation and apoptosis of B lymphocytes. Int Immunol. 2000;12(1):73–82. doi: 10.1093/intimm/12.1.73. [DOI] [PubMed] [Google Scholar]
- 9.Langstein J, Michel J, Schwarz H. CD137 induces proliferation and endomitosis in monocytes. Blood. 1999;94(9):3161–3168. [PubMed] [Google Scholar]
- 10.Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3(6):682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 11.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–1096. [PubMed] [Google Scholar]
- 12.Tan JT, Whitmire JK, Murali-Krishna K, et al. 4-1BB costimulation is required for protective anti-viral immunity after peptide vaccination. J Immunol. 2000;164(5):2320–2325. doi: 10.4049/jimmunol.164.5.2320. [DOI] [PubMed] [Google Scholar]
- 13.Tamada K, Chen L. Renewed interest in cancer immunotherapy with the tumor necrosis factor superfamily molecules. Cancer Immunol Immunother. 2006;55(4):355–362. doi: 10.1007/s00262-005-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox RA, Tamada K, Flies DB, et al. Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood. 2004;103(1):177–184. doi: 10.1182/blood-2003-06-2184. [DOI] [PubMed] [Google Scholar]
- 15.Sznol M, Hodi FS, Margolin K, et al. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA) [abstract]. J Clin Oncol (Meeting Abstracts) 2008;26(15) suppl:3007. [Google Scholar]
- 16.Zhu Y, Zhu G, Luo L, Flies AS, Chen L. CD137 stimulation delivers an antigen-independent growth signal for T lymphocytes with memory phenotype. Blood. 2007;109(11):4882–4889. doi: 10.1182/blood-2006-10-043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mescher MF, Curtsinger JM, Agarwal P, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: a preliminary report. N Engl J Med. 1988;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz-Winnenthal FH, Volk C, Z'Graggen K, et al. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65(21):10079–10087. doi: 10.1158/0008-5472.CAN-05-1098. [DOI] [PubMed] [Google Scholar]
- 21.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 22.Kerkmann-Tucek A, Banat GA, Cochlovius B, Zoller M. Antigen loss variants of a murine renal cell carcinoma: implications for tumor vaccination. Int J Cancer. 1998;77(1):114–122. doi: 10.1002/(sici)1097-0215(19980703)77:1<114::aid-ijc18>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Wilcox RA, Flies DB, Zhu G, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109(5):651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda Y, Hida N, Niiya F, et al. Detection of peptide-specific CTL-precursors in peripheral blood lymphocytes of cancer patients. Br J Cancer. 2002;87(7):796–804. doi: 10.1038/sj.bjc.6600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foell J, Strahotin S, O'Neil SP, et al. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB x NZW F1 mice. J Clin Invest. 2003;111(10):1505–1518. doi: 10.1172/JCI17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu L, Strahotin S, Hewes B, et al. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J Immunol. 2007;178(7):4194–4213. doi: 10.4049/jimmunol.178.7.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corry DB, Lewis DE. Cytokine storm and an anti-CD28 monoclonal antibody. N Engl J Med. 2006;355(24):2592. author reply 2593–2594. [PubMed] [Google Scholar]
- 28.Schwarz H. Biological activities of reverse signal transduction through CD137 ligand. J Leukoc Biol. 2005;77(3):281–286. doi: 10.1189/jlb.0904558. [DOI] [PubMed] [Google Scholar]
- 29.Olofsson PS, Soderstrom LA, Wagsater D, et al. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation. 2008;117(10):1292–1301. doi: 10.1161/CIRCULATIONAHA.107.699173. [DOI] [PubMed] [Google Scholar]