Abstract
Study Design
To characterize age-related changes in the matrix of human intervertebral disc specimens, human specimens from the 3rd to the 8th decade of life were collected and analyzed for collagen and proteoglycan composition.
Objectives
To identify age-related changes in the concentration of matrix macromolecules (collagen and proteoglycans, including the small leucine-rich proteoglycans biglycan, decorin, fibromodulin and lumican) in human anulus fibrosus and nucleus pulposus.
Summary of Background Data
Intervertebral disc degeneration is associated with changes in the concentration and fragmentation of matrix molecules. Deciphering age-related matrix alterations may help us to better understand the regulatory mechanisms underlying intervertebral disc degeneration.
Methods
Forty-six whole intervertebral discs were obtained from the thoracolumbar spines (T11-L5) of humans aged between 32 and 80 years. All specimens were classified as Thompson Grade 1 or 2 according to MRI criteria. Specimens were separated into (i) outer- and (ii) inner anulus fibrosus as well as (iii) nucleus pulposus. DNA, collagen and proteoglycan contents were measured using chemical assays while small non-aggregating proteoglycan levels were analyzed by comparative Western blotting.
Results
Total proteoglycan and collagen contents in both the anulus fibrosus and nucleus pulposus consistently decreased with aging. The concentrations of small non-aggregating proteoglycans varied. In the outer anulus, decorin levels decreased while biglycan and fibromodulin levels increased with age. In the inner anulus and nucleus, biglycan demonstrated a significant increase with aging. These changes differed in most cases from those previously reported for degenerating disc tissues.
Conclusions
Collagen and proteoglycans appeared to undergo specific age-related changes in the human intervertebral disc. While the total contents of these two families of molecules decreased during aging, individual species of small non-aggregating proteoglycans showed species-specific age-related changes. Interestingly, the level of biglycan rose and remained elevated in all three compartments of the disc with aging. The functional significance of these alterations is yet to be determined.
Keywords: aging, intervertebral disc, proteoglycan, collagen, decorin, biglycan, fibromodulin, lumican
Introduction
The intervertebral disc (IVD) is a highly organized matrix that serves as an articulating structure between the vertebral bodies allowing for complex spinal motion.1 It also functions as a load-bearing unit with two distinct compartments: the nucleus pulposus (NP) and the anulus fibrosus (AF).2 Each component has very distinct biomechanical and biochemical properties. The NP, rich in proteoglycans (PGs), acts as an internal semi-fluid mass, whereas the AF, rich in collagens, acts as a laminar fibrous container.3,4
Biochemical analysis of the extracellular matrix of IVD tissues has helped identify its two major macromolecular components as PGs and collagens. 5–7 The PGs in the IVD consist of two classes: large aggregating (aggrecan and versican) and small interstitial (biglycan, decorin, fibromodulin and lumican) PGs (also called SLRPs or small leucine-rich PGs).8–15 The large, negatively-charged PGs are responsible for most of the water-retaining properties of the disc tissues, which they exert via their associated glycosaminoglycan (GAG) substituents.5–7 Aggrecan, a large molecular weight PG whose protein core is substituted with numerous GAG side-chains, provides osmotic pressure important for resistance to compression and water flow.16 Versican, another large PG with a few GAG substituents, also is present in the IVD, especially in the AF, however its functional role is uncertain.17
Members of the small PG family interact with different collagen molecules, growth factors and other components of the ECM. These small PGs are thought to play important roles in the assembly and repair of the ECM after injury. 11–14,18,19 Decorin demonstrates an anatomic preponderance towards the AF, while biglycan is found at a higher concentration in the NP.20–24 Fibromodulin is predominantly found in the AF25,26 and lumican is present at comparable levels in both AF and NP in adult human IVD25,26.
IVD degeneration is associated with a loss of matrix molecules resulting in an alteration of the biochemical and biomechanical properties of the tissue.9,21,27,28 During IVD degeneration, proteolytic fragmentation of large and small PGs may induce or inhibit essential inflammatory response pathways altering the ability of the ECM to maintain its structural integrity.16,26,28–30 Ultimately, gross ECM changes ensue including increased lamellar disorganization and fissuring resulting in macroscopic alterations of the functional spine unit.1,31
Aging is considered to have a significant but yet undetermined role in symptomatic IVD degeneration. Age-related changes in ECM molecules may influence matrix quality, potentially contributing to IVD degeneration. Matrix accumulation and/or degradation due to aging may alter cellular reparative pathways. Since IVD degeneration is driven partially by aging, it is not easy to separate changes that are a consequence of aging itself from those that develop because of tissue degeneration and the accompanying repair process. Shedding light on age-related matrix alterations may help improve our understanding of the regulatory mechanisms involved during IVD degeneration. The purpose of this study was to quantify age-related changes in a select group of ECM molecules (collagen and PGs) normally present in the AF and NP of human IVDs, using normal IVD samples from various age groups.
Materials and Methods
Disc samples
Thoracolumbar spinal segments (T11-L5, male/female, age range 32–80) were received from the Gift of Hope Organ and Tissue Donor Network after obtaining the informed consent of the donor’s immediate family and with the approval of the Human Subject Institutional Review Board. It should be noted none of the samples were derived from individuals who experienced any spinal trauma. Lumbar spine segments were received within 24 hours of death and were processed immediately. The total of 46 IVD specimens were collected.
IVDs were graded using the Thompson’s magnetic resonance image (MRI) grading system.32 The spinal columns were imaged in a 5-inch solenoid coil with a 1.5T MR unit (Signa 1.5T, General Electric, Milwaukee, WI). T2-weighted sagittal (TR/TE 2000/80 msec, 2 NEX, 8×8 cm FOV, 512×256 matrix) and proton density axial images (TR/TE 2000/33 msec, 2 NEX, 8×8 cm FOV, 512×256 matrix) were obtained from 3-mm thick consecutive slices. T2-weighted sagittal images were used to grade disc degeneration (grade 1: normal to grade 5: advanced degeneration). There was good inter-observer agreement on grading the degenerative changes of the disc (calculated kappa values for perfect agreement: 0.77).
Only specimens classified as Thompson Grade 1 or 2 according to MR evaluation were analyzed. The number of specimens used in this study, the age-range and grade distribution, are listed in Table 1 and Table 2. IVDs were dissected and assigned to one of three groups: inner AF (i-AF), outer AF (o-AF), and NP. The intermediate tissue between the inner AF and NP was discarded. Each AF and NP sample was weighed, freeze-dried, re-weighed and an aliquot of each sample processed for DNA, proteoglycan and collagen content determinations. A subset of samples was used for protein extraction as listed in Table 2.
Table 1.
Intervertebral disc specimens used for collagen and proteoglycan content determinations (Distribution according to age demographics, average±SD)
| Age group | Thompson Grade |
Average Age |
Number of Specimens |
|---|---|---|---|
| 31–40 | 1.8±0.5 | 32.0±0.0 | 4 |
| 41–50 | 2.0±0.0 | 46.7±0.5 | 6 |
| 51–60 | 2.0±0.0 | 53.2±2.0 | 9 |
| 61–70 | 1.8±0.4 | 68.3±1.9 | 11 |
| 71–80 | 1.9±0.3 | 75.4±2.9 | 16 |
Table 2.
Intervertebral disc specimens used for Western blot hybridization (Distribution according to age demographics, average±SD)
| Age group | Thompson Grade |
Average Age |
Number of Specimens |
|---|---|---|---|
| 41–50 | 2 | 46.7±0.5 | 6 |
| 51–60 | 2 | 53.7±2.1 | 6 |
| 61–70 | 2 | 68.0±1.5 | 6 |
| 71–80 | 2 | 76.3±0.8 | 6 |
DNA assay
PicoGreen DNA assay was performed on each dried sample in triplicate according to the manufacturer’s instructions (Molecular Probes, Eugene, OR) after digesting the tissue aliquots with papain overnight 33. Fluorescence was measured in a spectrofluorometer (emission 415nm, excitation 365nm) and DNA contents were calculated using a standard curve calibrated using calf thymus DNA.
Total proteoglycan content determination
Total GAG content, relative to the total PG content, of each sample was determined by the DMMB (Dimethylmethylene blue) assay in triplicate. 33 A serial dilution of purified bovine nasal cartilage aggrecan was used as a standard. The absorbencies were measured at 530 nm and 595 nm and the values calculated by using the Kineticalc Program on the Bio-Tek Microplate reader (Denkendorf, Germany). Total PG content was normalized to DNA content of the same sample.
Total collagen content determination
Hydroxyproline content of the samples was determined by an HPLC-based assay.34 Collagen content was then calculated and total collagen content was normalized to DNA content of the same sample.
Protein extraction
Weighed aliquots of freeze-dried AF and NP samples were extracted with 1:100 mg/µl of 4 M guanidine hydrochloride in 0.05 M sodium acetate (Na-acetate), pH 6.8, containing proteinase inhibitors at 4°C for 24 h, as previously reported1,31. The extracts were then dialyzed against 0.1 M Tris, 0.05 M Na-acetate, pH 6.5 and digested with a mixture of proteinase-free chondroitinase ABC, keratanase and keratanase II (Seikagaku America, Falmouth, MA) to remove GAG side chains from the protein cores of the PGs before electrophoretic separation1,31.
Determination of small PG protein levels
Core proteins of the small PGs in tissue extracts were semi-quantified using comparative Western blotting1,10,27,28,31 AF and NP samples, normalized to tissue dry weight, were separated by 12% SDS-PAGE under reducing conditions and then transferred onto nitrocellulose membranes (BioRad, Hercules, CA) at 30 V overnight. Transfer membranes were immunostained with purified rabbit antibodies to small PGs.10,27,28 Binding of the first antibody was detected with peroxidase-labeled goat anti-rabbit IgGs (Accurate Chemical Co., Westbury, NY) and visualized by the enhanced chemi-luminescence method (Amersham, Arlington Heights, IL). Relative proportions of the matrix components were determined by densitometric scanning of the signals (BioRad Laboratories, Hercules, CA) and integration of the peaks. Internal standards were used in all gels to normalize the densitometric readings among the blots. Each sample was analyzed at least in duplicates and, for each decade of age, the average density for each antibody-stained band was used to calculate the average signal.
Statistical analyses
Statistical analyses were performed by one-way ANOVA with Fisher’s PLSD test as a post hoc test. The effects of age by decades were analyzed separately for each experiment. For the whole sample set, grade of degeneration versus gender was determined by ANOVA. As noted in a previous study by our group, gender does not appear to play a role in influencing the biochemical parameters measured in this study.28 Additionally, the Spearman rank correlation analysis was performed to calculate the significance of the changes of protein contents as a function of age decade. Significance in all cases was set at p< 0.05.
Results
Changes in DNA, Collagen and PG Contents with Age
DNA contents in the o-AF and i-AF as well as the NP were very similar (0.8 µg/mg tissue dry weight). DNA content of the 51–70 year age group was slightly but significantly higher than in the other age groups (Figure 1).
Figure 1.
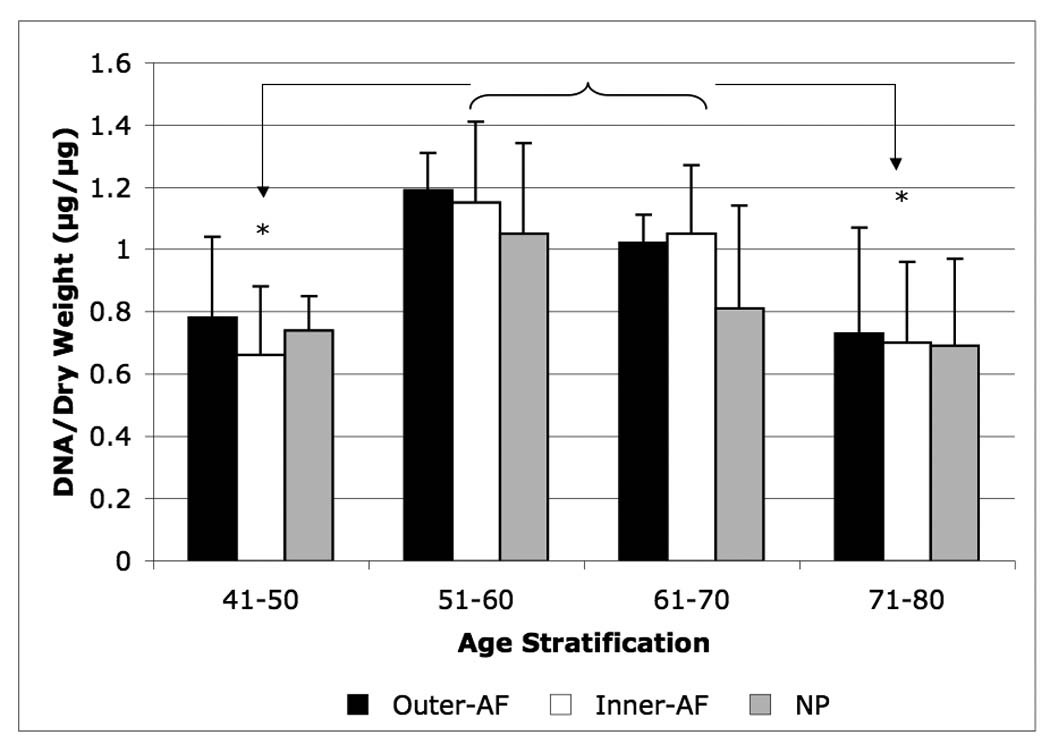
Age-related changes in DNA content of the outer-AF (black bars), inner-AF (white bars) and NP (gray bars) of human intervertebral discs. Data normalized to tissue dry weight in each case. (* p<0.01)
Collagen content, normalized to DNA, was higher in both o-AF and i-AF (600 µg / µg DNA) than in NP (250 µg/ µg DNA) in the youngest age group. Collagen content decreased with aging in both the AF and NP: this may be related in part to the high DNA content in the 51–70 year age range. Collagen concentration was not significantly lowered in the 71–80 year range (p <0.05) (Figure 2).
Figure 2.
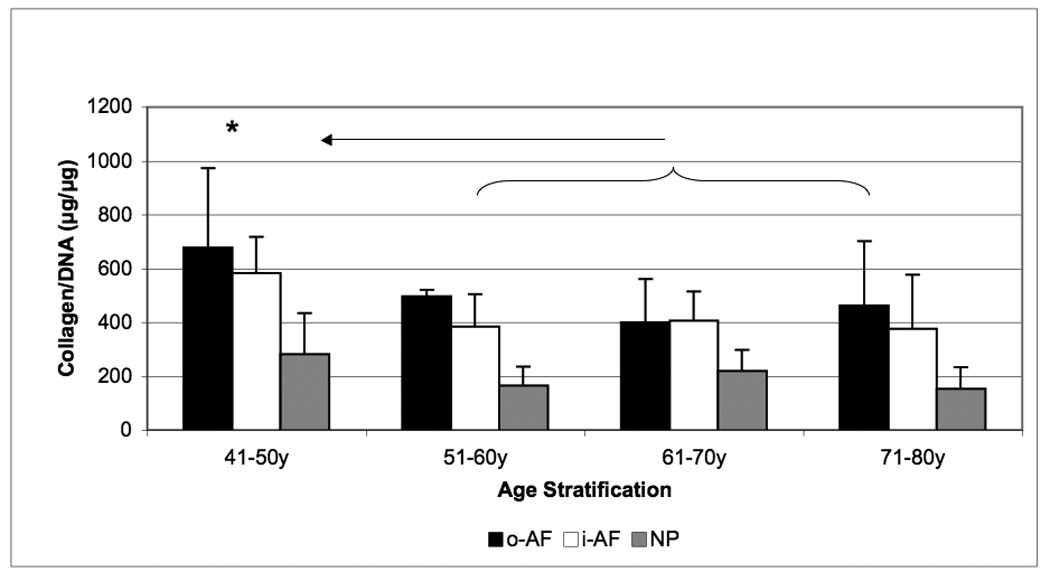
Age-related changes in collagen content of the outer-AF (black bars), inner-AF (white bars) and NP (gray bars) of human intervertebral discs. Data normalized to DNA content in each case. (* p<0.05)
Total PG content was highest in the NP and the i-AF (500 µg/µg DNA) and showed a decrease in the o-AF. Similar to collagen content, a decrease in total PG content as a function of age was observed, however, no further significant change was observed in the 71–80 year range (p < 0.05) (Figure 3).
Figure 3.
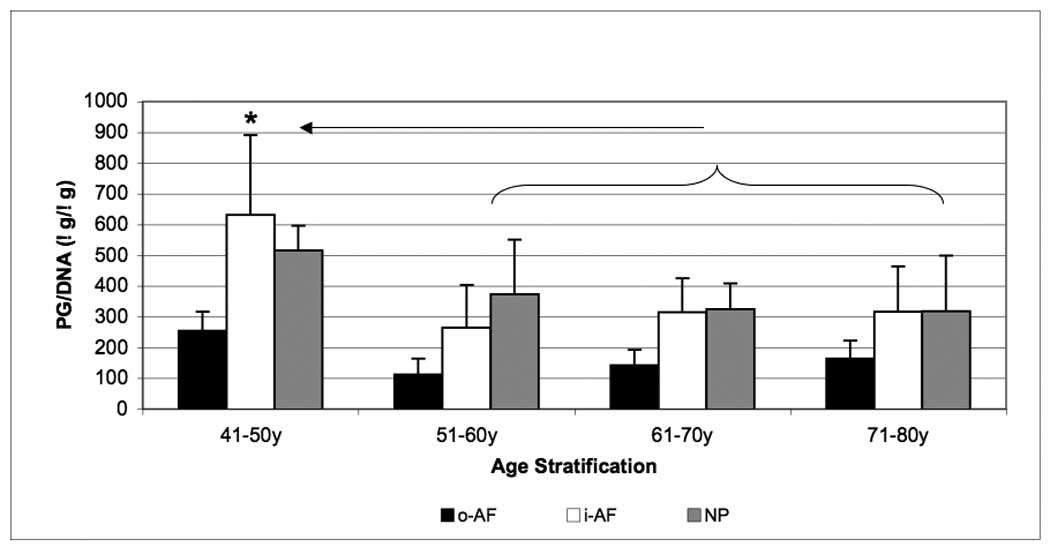
Age-related changes in proteoglycan content of the outer-AF (black bars), inner-AF (white bars) and NP (gray bars) of human intervertebral discs. Data normalized to DNA content in each case. (* p<0.05)
Changes in Small Proteoglycan Content with Age
Outer-AF
Among the four small PGs, decorin, lumican, and fibromodulin exhibited age-related changes in o-AF. Both decorin and lumican contents reached its peak concentrations in the 61–70 year age-range. A statistically significant decrease in concentration for both decorin and lumican occurred during the 71–80 year range (p < 0.05). Decorin exhibited approximately a 60% decrease in average density in the 71–80 year group as compared to the 61–70 year group (Figure 4), whereas an 80% decrease in average density was observed in the case of lumican (Figure 5).
Figure 4.
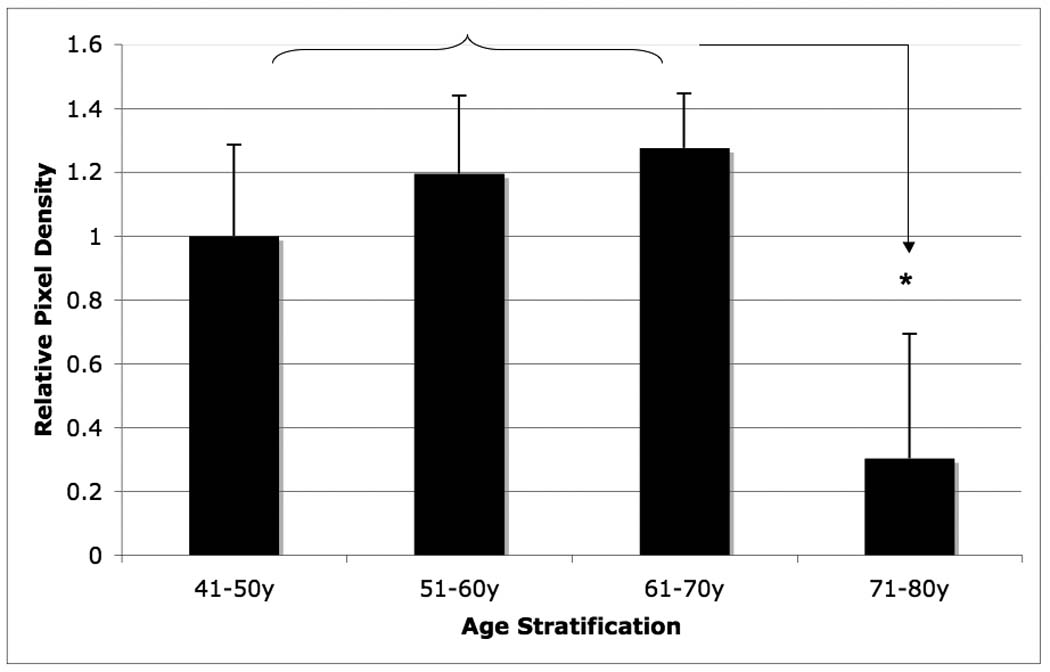
Age-related changes in relative lumican content of the outer-AF of human intervertebral disc. Data normalized to tissue dry weight in each case. (* p<0.05)
Figure 5.
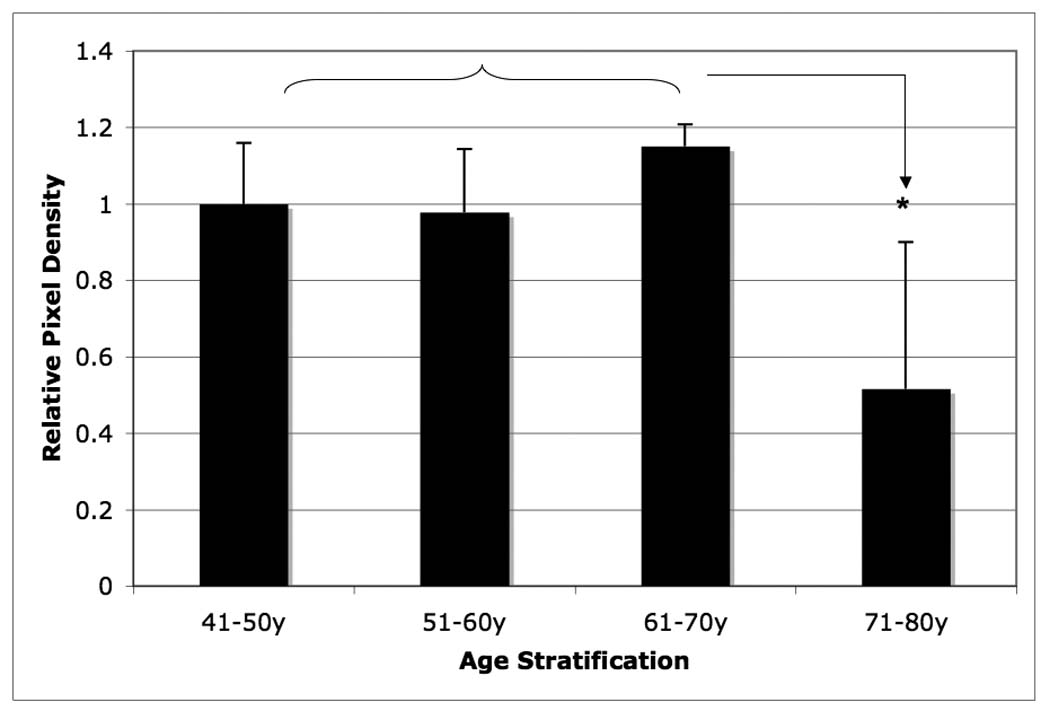
Age-related changes in relative decorin content of the outer-AF of human intervertebral disc. Data normalized to tissue dry weight in each case. (* p<0.05)
Fibromodulin exhibited a concentration variation with aging dissimilar to that of decorin and lumican. The fibromodulin content increased as a function of age with its peak occurring at 71–80 years of age (p < 0.05). It should be noted that the lower molecular weight, degraded fibromodulin showed minimal variation with aging, thus the total concentration increased due to the increasing production of intact fibromodulin with an overall increase in concentration of approximately 45% (Figure 6). Although, biglycan also showed a trend to accumulate in older samples, this did not prove to be significant.
Figure 6.
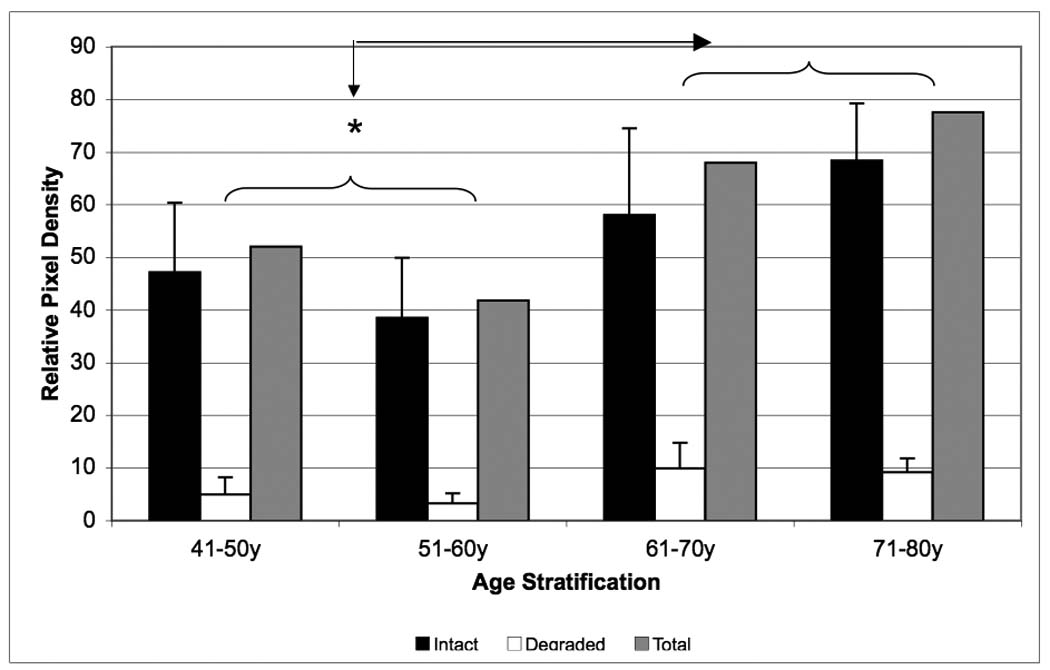
Age related changes in relative fibromodulin content of the outer-AF of human intervertebral disc. The intact core protein (black bars), the degraded portion (white bars) and the total fibromodulin (gray bars) contents were compared by specific antibody signal on Western blots. Data normalized to tissue dry weight in each case. (* p<0.05)
Inner-AF
In the i-AF, biglycan demonstrated an overall increasing concentration with aging due to an increase in the intact component (p < 0.05). Although decorin demonstrated a decreasing and fibromodulin an increasing trend in contents, these changes did not prove to be significant (p=0.12). All populations of biglycan (intact, degraded and total) reached their peak values in the 71–80 year range (Figure 7). It should be noted that biglycan exhibited a nearly three-fold increase in total concentration with increasing age of donors.
Figure 7.
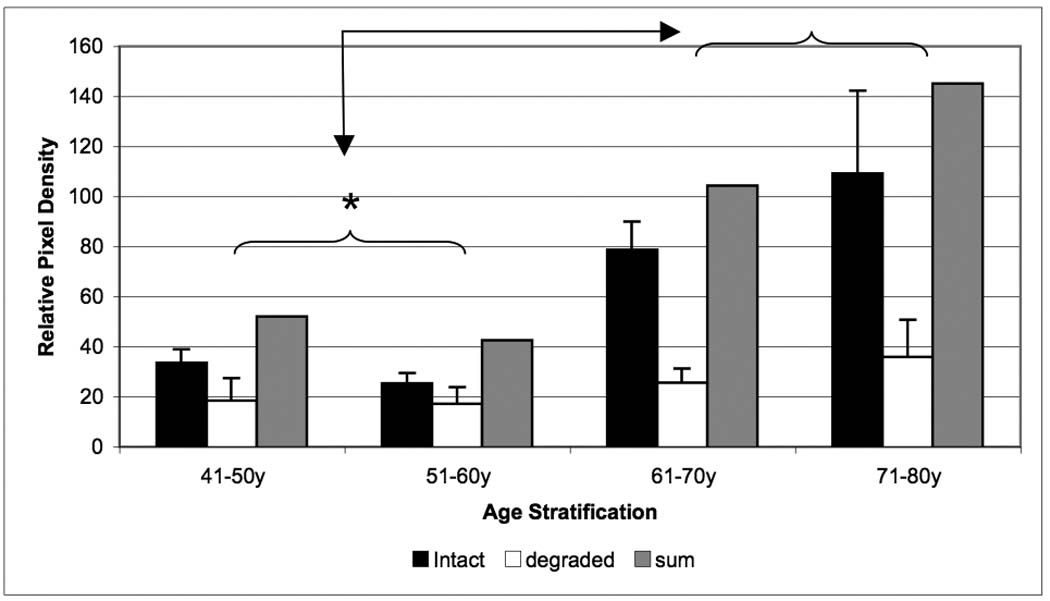
Age related changes in relative biglycan content of the inner- F of human intervertebral disc. The intact core protein (black bars), the degraded portion (white bars) and the total biglycan (gray bars) contents were compared by specific antibody signal on Western blots. Data normalized to tissue dry weight in each case. (* p<0.05)
NP
Biglycan in the NP demonstrated a similar variation in concentration as found in the i-AF. The total and intact biglycan content increased as a function of age with its peak concentration occurring in the 71–80 year range (p < 0.05). The degraded component of biglycan showed a decreasing trend in concentration with aging, though this trend was not statistically significant (p=0.12) (Figure 8). Although decorin levels decreased with aging this was not significantly different from the 41–50-y old group (p=0.20). Since almost no fibromodulin was detectable in NP, it was difficult to draw conclusions about its change with aging.
Figure 8.
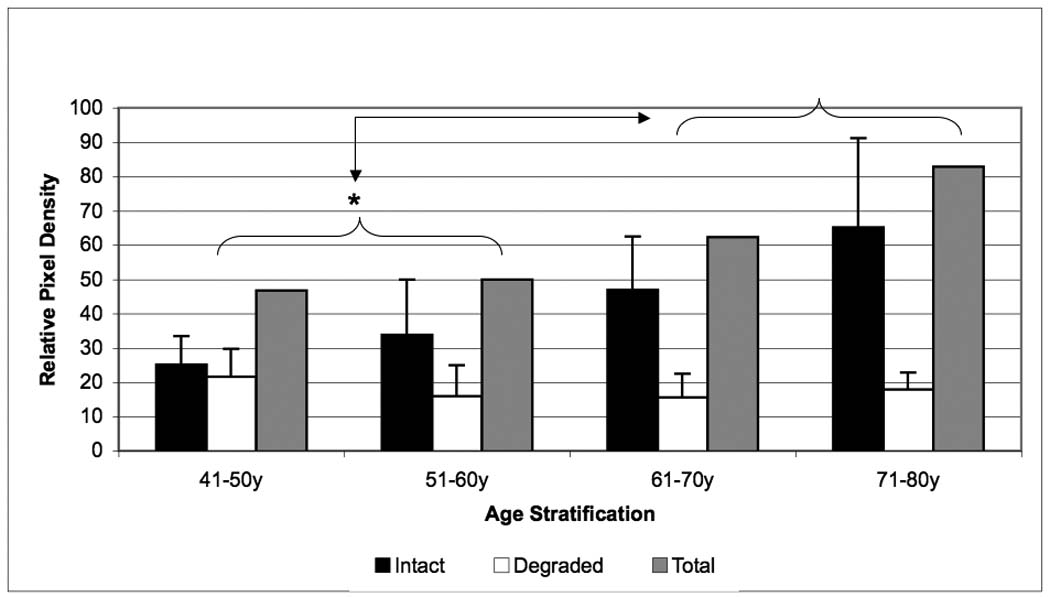
Age related changes in relative biglycan content of the NP of human intervertebral disc. The intact core protein (black bars), the degraded portion (white bars) and the total biglycan (gray bars) contents were compared by specific antibody signal on Western blots. Data normalized to tissue dry weight in each case.
Discussion
In this study, normal IVD specimens showing no signs of extensive degeneration were used from donors ranging 32–80 year of age. Although this gives a unique importance to this work, it also presents itself as a hardship in collecting these specimens. Since IVD degeneration starts as early as the 3rd decade of life, it is increasingly difficult to find specimens from middle aged to old individuals without signs of IVD degeneration. Consequently, in this study a relatively small number of specimens were used. Small sample sizes were noted in the lower age groups: this reflects principally the fact that it is difficult to obtain IVD specimens from youngest age group (31–40 year old) because of the relatively low rate of mortality in this age group (Table 1 and 2). Consequently, we have not included this younger age –group in our figures. It is worth noting that only Thompson grades 1 and 2 discs were analyzed: this was done to keep to a minimum metabolic changes associated with progressive disc degeneration rather than with age.28
It has become increasingly apparent that the IVD must be analyzed as three separate components (o-AF, i-AF, and NP).8,28 The AF is richer in collagen fibrils, whereas the NP is richer in proteoglycans.9–11,18 One must therefore surmise that each region of the intervertebral disc may exhibit different age-related changes. The volume of human NP obtained is often small and thus, a limited number of biochemical analyses can be performed. Nevertheless, to truly understand the complex biochemical composition of the IVD, the AF (outer and inner) and the NP must be analyzed independently.
Tissue dehydration is known to be associated with age-related degeneration of the IVD.8,21 Though variation in IVD degeneration was controlled for by using only those discs that exhibited Thompson grades 1 and 2 MR findings, an additional level of stringency was added by using tissue dry-weight for normalizing protein extraction. Data comparison to others who report their findings per tissue wet-weight is therefore difficult.
Several authors have reported that PG and collagen concentrations in the IVD tissue vary markedly with aging.5,14,16,21,26,29,32 In this study we demonstrated that as a general measure of metabolic function, both the overall PG and collagen concentration appear to decrease with aging, particularly in the AF and NP. There was a noticeable difference between the under-50 and the over-50-year old groups regarding both collagen and PG content of the IVD. There was a decline in contents of these molecules in early aging, but no significant change after 50-years of age was found in any of the compartments of the IVD. This decrease is suggestive of an age-related generalized decrease in cellular biochemical activity. While it is hard to generalize the significance of the decrease, it is suggestive of a decreasing cellular response and extra-cellular matrix production that may have a significant role in how tissue injury is managed as a consequence of aging.
It is even more interesting when we compare these data on age-related changes of PGs (controlled for degenerative changes) with those that we generated for the comparison of matrix PGs as a function of IVD degeneration (controlled for aging; samples represented more of the 50–60-year old population)5,14,16,21,26,29,32. There was an increase in the PG content of AF in grades 1–4 samples and a drop in content at grade 5. Thus, aging and degeneration have different effects on PG content in AF. In the same study, we found a decline in PG content in NP as a function of degeneration, which is more similar to the changes detected in aging. We hypothesized, that this phenomenon is indicative of the ability of the AF cells to respond to tissue degeneration with upregulating repair processes, while the NP cells are unable to doing so. 5,14,16,21,26,28,29,32
This study provides invaluable age-related information with regards to the metabolic activity of the small PG family in the AF and NP. Small PGs bind to collagens, growth factors and other matrix components thereby regulating the assembly of ECM and influencing repair processes after injury.9,13,17,19,27 The changes in small PG content in the IVD may thus have important metabolic consequences. For example, an increased quantity of small PGs in the ECM of the AF may trigger molecular events that alter the composition and/or functional properties of the matrix. It is currently believed that these small PGs help regulate collagen fibril formation and are capable of interacting with several ECM molecules, such as growth factors, thrombospondin, fibronectin, and cell surface receptors to help regulate repair that follows tissue injury.9,13,17,19,27 Indeed, the heterogeneity of collagen fibril size detected in aging tissue25, the increased cell senescence 33 and decreased response to growth factors in older tissues 33 may be explained by one or the other effects of the small PGs.
Lumican concentrations in the outer AF appear to reach a peak concentration during the 8th decade of life and thereafter demonstrate an abrupt decrease in its production. It has been suggested that there is a coordinated role of fibromodulin and lumican during collagen fibrillogenesis.25–27,35 In the case of the fibromodulin-null mouse, collagen fibers are thinner then in its wild-type counterpart.25 At the same time, the closely related lumican is present in larger amounts, apparently due to a lack of replacement of this molecule during tissue development by fibromodulin that binds to the same binding site on the surface of collagen.25
Fibromodulin contents in the o-AF and i-AF appear to increase with aging while the degraded component remains relatively constant. Fibromodulin has been implicated as a factor in the degenerative cascade.6,10,13,17,25–27 The fragmentation and release of fibromodulin elicits an inflammatory response.25 Sjoberg et al. demonstrated that fibromodulin tightly binds C1q activating the complement cascade and initiating a local inflammatory response that may be a factor in causing pain and irritation of the nerve roots.36 The fibromodulin content showed a similar trend in degenerating AF tissues, although after an increase in grades 1–4, there was a sudden loss of fibromodulin from the grade 5 tissues.5,14,16,21,26,29,32 This similarity in aging and tissue degeneration suggests a similar role for fibromodulin in responding to changes implemented by either aging or tissue damage. This role of fibromodulin should be further investigated in the future.
Decorin concentrations appear to be relatively constant until the 71–80 year range. At this point, there is an abrupt decrease in its content, similar to the response seen with lumican. There also is a trend for decorin to decrease in concentration in both i-AF and NP. This loss of decorin may also cause problems with collagen fibrillogenesis. While decorin content declines with aging, it demonstrates an increase in tissue degeneration.18 Thus, we can assume that decorin is a part of the repair process that follows tissue damage, and its loss in aging may be a trigger for age-related tissue degeneration.
Biglycan, another member of the small PG family, has an unknown role in the regulation of the extracellular matrix.13,14,17,20 Biglycan has been found in higher concentrations with aging in both NP and AF.18 Interestingly, unlike the other proteoglycans, the total and intact biglycan concentrations (in the i-AF and NP) increased with aging whereas the degraded concentration remained relatively constant. The increased production of biglycan as a function of aging goes parallel with increased biglycan production in AF as a function of tissue degeneration5,14,16,21,26,29,32. On the contrary, biglycan is not changed significantly in degenerating NP. This increased presence of biglycan appears to be a trend in both aging and degeneration that needs to be analyzed in greater detail.
In summary, the data presented here suggest that collagen as well as PGs (large and small) undergo age-related metabolic variations. There appears to be an overall trend towards decreased cellular metabolic activity as manifested by decreasing concentrations of collagen and total PG. However, several small PGs show accumulation rather than loss from the ECM. Interestingly, fibromodulin (in o-AF) and biglycan (in i-AF and NP) appear to be significantly up-regulated with aging. The functional significance of these changes is to be determined. Nevertheless, understanding the quantitative changes of these PGs associated with aging is an important step towards defining their roles in regulating ECM production, particularly in tissue repair in response to IVD degeneration.
Key points.
Large proteoglycans play a major role in water retention within the intervertebral disc, while small proteoglycans regulate formation of the extracellular matrix.
During aging, proteoglycan and collagen protein levels decrease, while some of the small proteoglycans show different patterns of changes in the inner and outer anulus fibrosus and nucleus pulposus.
The concentration of biglycan increases in all three compartments of the disc with aging, while decorin content tends to decline.
These changes are clearly distinguishable from those present in degenerating disc tissues.
The decrease in total contents of collagens and proteoglycans may increase the susceptibility of the IVD to degeneration, but accompanying changes in the regulatory small proteoglycan molecules may counterbalance some of these age-related changes.
Acknowledgements
The authors are grateful to Peter Roughley, Ph.D., Shriners Hospital, Montreal, Canada, for providing the antibodies to the small proteoglycans. The collaboration with the Gift of Hope Organ and Tissue Network of Illinois and the technical help of A. Margulis, M.D. in tissue collection are greatly appreciated. We also thank Ms. Mary-Ellen Lenz, Mr. David Gerard and Mr. Daniel Petryla for technical help.
Statement: This work was supported by a grant from the National Institutes of Health (2PO1 AR-48152).
References
- 1.Gruber HE, Hanley EN., Jr Ultrastructure of the human intervertebral disc during aging and degeneration: comparison of surgical and control specimens. Spine. 2002;27:798–805. doi: 10.1097/00007632-200204150-00004. [DOI] [PubMed] [Google Scholar]
- 2.Niosi CA, Oxland TR. Degenerative mechanics of the lumbar spine. Spine J. 2004;4:202S–208S. doi: 10.1016/j.spinee.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 3.An HS, Thonar EJ, Masuda K. Biological repair of intervertebral disc. Spine. 2003;28:S86–S92. doi: 10.1097/01.BRS.0000076904.99434.40. [DOI] [PubMed] [Google Scholar]
- 4.Oegema TR., Jr Biochemistry of the intervertebral disc. Clin Sports Med. 1993;12:419–439. [PubMed] [Google Scholar]
- 5.Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24:12–21. doi: 10.1002/art.1780240103. [DOI] [PubMed] [Google Scholar]
- 6.Melching LI, Cs-Szabo G, Roughley PJ. Analysis of proteoglycan messages in human articular cartilage by a competitive PCR technique. Matrix Biol. 1997;16:1–11. doi: 10.1016/s0945-053x(97)90111-6. [DOI] [PubMed] [Google Scholar]
- 7.Melrose J, Ghosh P, Taylor TK, et al. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J Orthop Res. 1992;10:665–676. doi: 10.1002/jor.1100100509. [DOI] [PubMed] [Google Scholar]
- 8.Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckwalter JA, Roughley PJ, Rosenberg LC. Age-related changes in cartilage proteoglycans: quantitative electron microscopic studies. Microsc Res Tech. 1994;28:398–408. doi: 10.1002/jemt.1070280506. [DOI] [PubMed] [Google Scholar]
- 10.Cs-Szabo G, Roughley PJ, Plaas AH, et al. Large and small proteoglycans of osteoarthritic and rheumatoid articular cartilage. Arthritis Rheum. 1995;38:660–668. doi: 10.1002/art.1780380514. [DOI] [PubMed] [Google Scholar]
- 11.Gruber HE, Fisher EC, Jr, Desai B, et al. Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 12.Heinegard D, Aspberg A, Franzen A. Glycosylated matrix proteins. New York: Wiley-Liss; 2002. [Google Scholar]
- 13.Hildebrand A, Romaris M, Rasmussen LM, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inkinen RI, Lammi MJ, Lehmonen S, et al. Relative increase of biglycan and decorin and altered chondroitin sulfate epitopes in the degenerating human intervertebral disc. J Rheumatol. 1998;25:506–514. [PubMed] [Google Scholar]
- 15.Kang JD, Stefanovic-Racic M, McIntyre LA, et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Sztrolovics R, Alini M, Roughley PJ, et al. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326(Pt 1):235–241. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melrose J, Ghosh P, Taylor TK. A comparative analysis of the differential spatial and temporal distributions of the large (aggrecan, versican) and small (decorin, biglycan, fibromodulin) proteoglycans of the intervertebral disc. J Anat. 2001;198:3–15. doi: 10.1046/j.1469-7580.2001.19810003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotz W, Barnert S, Bertagnoli R, et al. Immunohistochemical localization of the small proteoglycans decorin and biglycan in human intervertebral discs. Cell Tissue Res. 1997;289:185–190. doi: 10.1007/s004410050864. [DOI] [PubMed] [Google Scholar]
- 19.Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274:18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- 20.Patel S, Santra M, McQuillan DJ, et al. Decorin activates the epidermal growth factor receptor and elevates cytosolic Ca2+ in A431 carcinoma cells. J Biol Chem. 1998;273:3121–3124. doi: 10.1074/jbc.273.6.3121. [DOI] [PubMed] [Google Scholar]
- 21.Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987;5:198–205. doi: 10.1002/jor.1100050206. [DOI] [PubMed] [Google Scholar]
- 22.Schollmeier G, Lahr-Eigen R, Lewandrowski KU. Observations on fiber-forming collagens in the anulus fibrosus. Spine. 2000;25:2736–2741. doi: 10.1097/00007632-200011010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Svensson L, Aszodi A, Heinegard D, et al. Cartilage oligomeric matrix protein-deficient mice have normal skeletal development. Mol Cell Biol. 2002;22:4366–4371. doi: 10.1128/MCB.22.12.4366-4371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danielson KG, Baribault H, Holmes DF, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svensson L, Aszodi A, Reinholt FP, et al. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- 26.Sztrolovics R, Alini M, Mort JS, et al. Age-related changes in fibromodulin and lumican in human intervertebral discs. Spine. 1999;24:1765–1771. doi: 10.1097/00007632-199909010-00003. [DOI] [PubMed] [Google Scholar]
- 27.Cs-Szabo G, Melching LI, Roughley PJ, et al. Changes in messenger RNA and protein levels of proteoglycans and link protein in human osteoarthritic cartilage samples. Arthritis Rheum. 1997;40:1037–1045. doi: 10.1002/art.1780400607. [DOI] [PubMed] [Google Scholar]
- 28.Cs-Szabo G, Ragasa-Sanjuan D, Turumella V. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine. 2002;27:2212–2219. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 29.Takaishi H, Nemoto O, Shiota M, et al. Type-II collagen gene expression is transiently upregulated in experimentally induced degeneration of rabbit intervertebral disc. J Orthop Res. 1997;15:528–538. doi: 10.1002/jor.1100150408. [DOI] [PubMed] [Google Scholar]
- 30.Vogel KG, Trotter JA. The effect of proteoglycans on the morphology of collagen fibrils formed in vitro. Coll Relat Res. 1987;7:105–114. doi: 10.1016/s0174-173x(87)80002-x. [DOI] [PubMed] [Google Scholar]
- 31.Kim KS, Yoon ST, Li J, et al. Disc degeneration in the rabbit: a biochemical and radiological comparison between four disc injury models. Spine. 2005;30:33–37. doi: 10.1097/01.brs.0000149191.02304.9b. [DOI] [PubMed] [Google Scholar]
- 32.Thompson JP, Pearce RH, Schechter MT, et al. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Petit B, Masuda K, D'Souza AL, et al. Characterization of crosslinked collagens synthesized by mature articular chondrocytes cultured in alginate beads: comparison of two distinct matrix compartments. Exp Cell Res. 1996;225:151–161. doi: 10.1006/excr.1996.0166. [DOI] [PubMed] [Google Scholar]
- 34.Petit B, Masuda K, D'Souza AL, et al. Characterization of crosslinked collagens synthesized by mature articular chondrocytes cultured in alginate beads: comparison of two distinct matrix compartments. Exp Cell Res. 1996;225:151–161. doi: 10.1006/excr.1996.0166. [DOI] [PubMed] [Google Scholar]
- 35.Chakravarti S, Magnuson T, Lass JH, et al. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sjoberg A, Onnerfjord P, Morgelin M. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J Biol Chem. 2005;280:301–308. doi: 10.1074/jbc.M504828200. [DOI] [PubMed] [Google Scholar]


