Abstract
The incidence of the metabolic syndrome represents a spectrum of disorders that continue to increase across the industrialized world. Both genetic and environmental factors contribute to metabolic syndrome and recent evidence has emerged to suggest that alterations in circadian systems and sleep participate in the pathogenesis of the disease. In this review, we highlight studies at the intersection of clinical medicine and experimental genetics that pinpoint how perturbations of the internal clock system, and sleep, constitute risk factors for disorders including obesity, diabetes mellitus, cardiovascular disease, thrombosis and even inflammation. An exciting aspect of the field has been the integration of behavioural and physiological approaches, and the emerging insight into both neural and peripheral tissues in disease pathogenesis. Consideration of the cell and molecular links between disorders of circadian rhythms and sleep with metabolic syndrome has begun to open new opportunities for mechanism-based therapeutics.
Keywords: Clock, Circadian, Metabolic syndrome
I) Introduction: metabolic syndrome and obesity
The metabolic syndrome (MS) is comprised of several metabolic abnormalities, including central (intra-abdominal) obesity, dyslipidaemia, hyperglycaemia, and hypertension. This syndrome has become a major public-health challenge worldwide; an estimated 25–40% of individuals between the ages of 25 and 64 years of age have MS (San Antonio Heart Study)1–4. MS is further defined by the presence of other components, including elevated circulating levels of triglycerides, reduced levels of HDL-cholesterol, high blood pressure and impaired fasting glycaemia. Elevated circulating inflammatory and/or thrombotic markers [C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and plasminogen activator inhibitor type 1 (PAI-1)] or reduced levels of anti-inflammatory molecules such as adiponectin are further markers of MS2, 4.
Excess food intake and physical inactivity underlie the growing worldwide epidemic of obesity and MS, not only in industrialized nations but also in developing countries. In addition, mounting evidence from clinical epidemiological studies has led to the hypothesis that one of the major changes in the industrialized world that contributes to the pathogenesis of the MS involves the introduction of artificial light and work into the night-time, in addition to the pervasive rise in voluntary sleep curtailment 5. Indeed these common disorders of circadian behaviour and sleep are associated with increased hunger, decreased glucose and lipid metabolism, and broad changes in the hormonal signals involved in satiety 6. Recently, Sheer et al. demonstrated adverse cardiometabolic endpoints in human subjects who underwent forced misalignment of behavioural and circadian cycles, simulating the conditions of jet lag and shift work within a controlled clinical setting 7. Against this backdrop of human studies, advances in the field of experimental genetics have uncovered the fundamental molecular mechanism governing these 24 h circadian rhythms of physiology, revealing that all circadian processes are programmed by a conserved transcription-translation feedback loop that oscillates with a periodicity of 24 h 8. Remarkably, obesity and high-fat feeding also reciprocally affect the circadian system in mice, indicating that metabolism, circadian rhythms, and possibly sleep, are interconnected through complex behavioral and molecular pathways 9. Thus alterations in energy homeostasis associated with obesity may set in motion a “vicious cycle” of circadian disruption, in turn leading to exacerbation of the original metabolic disturbance.
II) Adverse effects of alterations in circadian rhythms: clinical evidence
The decrease in sleep duration in the US has occurred over the same time period as the increase in the prevalence of metabolic disease (for review, see 5). Numerous cross-sectional, as well as prospective clinical studies, have demonstrated that short-duration and poor-quality sleep predicts the development of type 2 diabetes and obesity after age, Body Mass Index (BMI) and various other confounding variables are taken into account 10–13. For instance, reduced sleep duration in children is associated with increased risk of being overweight 14. The gradual decline in the amount of time spent asleep and also the routine extension of normal activity during the night may disrupt synchrony between the periods of sleep/activity with alternating periods of feeding/fasting and energy storage/utilization. Indeed, the relationship between sleep restriction, weight gain and diabetes risk may involve at least in part alterations in glucose metabolism, stimulation of appetite, and decreased energy expenditure (for review, see 5). For example, healthy subjects who underwent six consecutive nights of sleep restricted to 4 h exhibited impaired insulin sensitivity following a glucose challenge 15, 16. Moreover, the induction of hunger may be partially related to reduced circulating levels of leptin (an adipose tissue-specific hormone which promotes satiety) and increased levels of the orexigenic hormone ghrelin (a peptide released primarily from the stomach) induced by sleep deprivation 17. Both hormones may also impact energy expenditure (for review 18). Curiously, individuals diagnosed with night eating syndrome appear to have greater propensity towards obesity 19. Diseases related to changes in time and/or quality-sleep duration are also associated with metabolic disorders. For example, sleep apnoea syndrome, a sleep disorder that is highly prevalent in metabolic disorders 20, was proposed to cause clock gene dysfunction 21, and effective treatments of sleep apnoea have been found to improve glucose metabolism and energy balance 11. In addition, the circadian oscillation of leptin was found to be disrupted in narcoleptic patients, which may predispose them to weight gain 22. Understanding the molecular pathophysiology of metabolic disorders in states of disrupted sleep remains a major challenge.
It has also long been recognized that serious adverse cardiovascular events, including myocardial infarction, sudden cardiac death, pulmonary embolism, limb ischemia, and aortic aneurysm rupture, all have pronounced circadian rhythmicity, reaching a peak during the morning 23. More recent evidence has accumulated to suggest that chronic circadian disruption may also increase susceptibility to such disorders. For example, shift work is associated with a 1.6 and 3.0-fold increased risk of cardiovascular disease for 45–55 years old men and women, respectively 24. Cardiovascular disease and hypertension are also associated with sleep loss: the risk of a fatal heart attack increases 45% in individuals who chronically sleep 5 h per night or less 25. Interestingly, the incidence of acute myocardial infarction was also significantly increased for the first 3 weekdays after the transition to daylight saving time in the spring 26. This observation underscores the deleterious effects of transitions involved in daylight saving time on the disruption of chronobiologic rhythms. Another adverse aspect of sleep perturbation is its impact on the human immune system 27–29. For instance, sleep deprivation dysregulates monocyte production of several pro-inflammatory cytokines, including IL-6 and TNF-α 30, 31. This point is of interest since obesity is recognized to involve a low-grade inflammatory state (for review, see 32). Conversely, the inflammatory process can induce sleep disturbances 27. Other metabolic disorders may be induced by a phase shift, such as altered postprandial lipid excursion, thereby providing a partial explanation for the increased occurrence of cardiovascular disease reported in shift workers 33.
Recently, Sheer et al. investigated the causal link between circadian misalignment and metabolic homeostasis using a controlled simulation of ‘shift-work’ in the clinical laboratory 7. In this study, 10 subjects underwent a progressive misalignment of behavioural and circadian cycles. Their behavioural cycle was extended to a 28h-day, under dim light, with 14h rest and fasting alternated with 14h of wakefulness, interspersed with four evenly spaced and isocaloric meals. When subjects ate and slept approximately 12 h out of phase from their habitual times, circadian desynchrony decreased leptin levels and resulted in hyperglycemia and hyperinsulinemia. In addition, their daily cortisol rhythm was reversed, arterial pressure elevated and sleep efficiency decreased. Interestingly, some of the subjects also exhibited postprandial glucose responses comparable to those of a prediabetic state 7. Thus, this study suggests that synchrony between behavioural and physiological rhythms is advantageous to maintain normal glucose metabolism in otherwise healthy persons 34. An important question for future clinical studies will be to determine the impact of short sleep and/or circadian misalignment on molecular clock function, especially within metabolic tissues.
In addition to environmental sleep disruption (e.g. shift work disorders), genetic polymorphisms in several clock genes have also been linked to sleep disorders 35–37. For instance, genetic variation in circadian clock genes has been associated with psychiatric diseases, such as bipolar disorders and schizophrenia 35, while many depressed patients, particularly bipolar patients, show delayed sleep phase 38, and depression is also a co-morbidity of obesity 39. Interestingly, polymorphisms in Clock and Bmal1, whose proteins form the core mammalian clock, have been linked to some features of the metabolic syndrome. In small sample populations, polymorphisms in the Clock gene have been correlated with predisposition to obesity 40, 41, and two Bmal1 haplotypes are associated with type 2 diabetes and hypertension 42. Polymorphisms within other clock core genes (i.e Per2 and Npas2) have also been associated with hypertension and high fasting blood glucose in studies of similar sample size 43. Interestingly, a rare variant in Nampt (Visfatin/Pbef1), which is involved in a negative clock feedback loop 44, 45, is associated with protection from obesity 46.
Recently, several genome-wide association studies led to the unexpected discovery that melatonin, a hormone implicated in seasonal and circadian rhythms, may be important in the regulation of mammalian glucose levels 47, 48. Indeed genetic variants of the melatonin 1B receptor gene (mtnr1b) increase type 2 diabetes risk 47, 48. In agreement, mtnr1b is expressed in pancreatic β-cells, and melatonin modulates glucose-stimulated insulin secretion 49. Interestingly, melatonin secretion is reported to be impaired in type 2 diabetic patients 50, and the melatonin profile relative to the feeding/ fasting cycle is reversed when individuals are subjected to forced desynchrony 7. Taken together, these recent findings raise the possibility that disruption of circadian systems, either directly at the level of altered clock gene expression, or indirectly through effects on melatonin, may contribute to human metabolic syndrome and cardiovascular complications.
III) Molecular and hierarchical organisation of the clock
A) The core molecular clock network
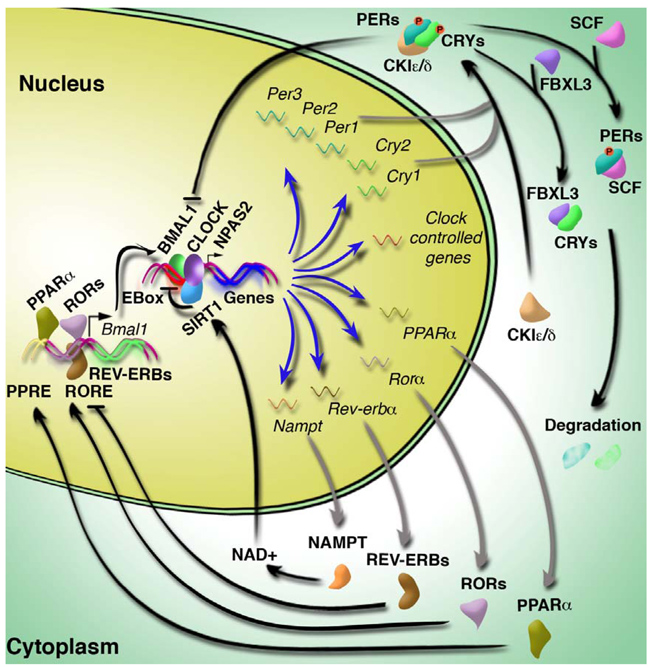
Forward genetics and positional cloning enabled identification of the first mammalian circadian clock gene and provided an entrée point into a molecular understanding of the clock mechanism 51, 52. The core molecular clock is composed of transcription/translation feedback loop that oscillates with 24 hr rhythmicity (Fig 1). The driving force is the positive limb of the clock comprised of the basic helix-loop-helix (bHLH)-PAS (Period-Arnt-Single-minded) transcription factors CLOCK (Circadian locomotor output cycles kaput), and its paralogue NPAS2 (neuronal PAS domain protein 2), and BMAL1/ARNTL (aryl-hydrocarbon receptor nuclear translocator-like). CLOCK or NPAS2 and BMAL1 heterodimerize and activate the rhythmic transcription of downstream target genes that contain E-box cis-regulatory enhancer sequences, including the Period (Per1, Per2, and Per3) and Cryptochrome (Cry1 and Cry2) genes 51, 53–56. Following translation, PER/CRY dimerize and translocate back to the nucleus where they directly inhibit the CLOCK/BMAL1 complex, effectively repressing their own transcription 57–59.
Fig 1. The core molecular clock components.
The mammalian circadian clock consists of a series of interlocking transcription/translation feedback loops. The positive limb of the clock is composed of the transcription factors CLOCK/NPAS2 and BMAL1, which heterodimerize and activate transcription of downstream clock target genes, including the period (Per1, 2, and 3) and cryptochrome (Cry1 and 2) genes, Rev-erba, Rora, and other clock-controlled genes. Upon translation, the PERs and CRYs heterodimerize, translocate back to the nucleus, and inhibit CLOCK/BMAL1. Multiple additional interlocking loops are shown and are described within the text.
Additional regulatory loops are interconnected with the core loop described above, providing multiple layers of control of the core circadian clock 60, 61. In addition to the Per and Cry genes, CLOCK/BMAL1 also activate transcription of the retinoic acid-related orphan nuclear receptors Rev-erbα̣and Rorα.62–65. REV-ERBα binds to the retinoic acid-related orphan receptor response element (RORE) in the Bmal1 promoter resulting in inhibition of transcription and this action is opposed by RORα̣ which activates the RORE 62–64, 66. In addition to the nuclear hormone receptor feedback loop, PAR-domain basic leucine zipper transcription factors (PAR bZIP), including DBP, TEF, HLF, and the cyclic adenosine 3',5'-monophosphate (cAMP) pathway (CREB-ATF-CREM) also feedback on the clock, acting through cognate D box and CREB elements respectively 60, 61, 67–70. Post-translational modification, including phosphorylation and ubiquitination, provide further regulation of the clock network. Casein kinase 1 epsilon and delta (CK1ε and CK1δ) phosphorylates PER and CRY, tagging them for polyubiquitylation by the E3 ubiquitin ligase complexesβ̣̃TrCP1 and FBXL3, respectively, ultimately leading to their degradation by the 26S proteosome 71–78. In addition to phosphorylation mediated by the caseine kinase family, in Drosophila and mammalian cells, a role for GSK3-β signalling has been established 68, 79. As a result of this degradation, CLOCK/BMAL1 is released from repression, activating the forward limb of the 24 hr cycle. Lastly, epigenetic regulation has emerged as an additional node in circadian systems (discussed further below), including the possibility that CLOCK participates directly in protein acetylation and chromatin modification 80.
Genetic mouse models have revealed key roles for each of the core clock genes in the generation and maintenance of circadian rhythms. Bmal1 knockout mice display a complete loss of circadian rhythmicity in constant darkness 53. Mice with a dominant-negative Clock mutation have an approximately 4 hr lengthening of their free-running period and become arrhythmic in constant darkness 52. Of note, Clock knockout mice have normal locomotor activity rhythms, due to developmental compensation by NPAS2 81, 82. Compensation can also be demonstrated within the negative limb of the clock, as both Per1/Per2 and Cry1/Cry2 knockout mice display a much more pronounced loss of circadian rhythmicity compared to their single mutant counterparts 56, 83–86. While the aforementioned studies have focused on overt locomotor activity rhythms, it remains uncertain as to whether compensation extends to functions of the clock within peripheral tissues. It is likely that future genetic studies will continue to identify additional regulators and modifying loops of the core clock mechanism.
B) Central clock organisation
Many metabolic functions occur at specific times of the day. Indeed, understanding the effects of molecular clock gene disruption on organismal physiology can be advanced through consideration of the molecular and hierarchical organization of the clock. The location of the master neural clock in mammals was originally discovered through classical lesioning studies within pacemaker neurons of the brain: the suprachiasmatic nucleus (SCN) of the hypothalamus (for more complete review, see 87, 88). The SCN controls physiological and behavioural circadian rhythms and coordinates peripheral clocks through hormonal and neural signals 87. The master role played by SCN was demonstrated by transplantation experiments in hamster. Neural grafts from the suprachiasmatic region restored circadian locomotor and feeding activity to arrhythmic animals whose own SCN had been ablated 89. The restored rhythms of the host always matched the rhythms of the donor, demonstrating the strong impact of this nucleus in circadian activity. However, despite the restoration of locomotor rhythmicity, melatonin and glucocorticoids remained arrhythmic, suggesting that neural connections must be critical for the generation of certain circadian rhythms 90. Interestingly, this master pacemaker has anatomic connections with several regions of the CNS involved in the control of appetite, energy expenditure regulation and behavioural activity, namely with the subparaventricular area, the arcuate nucleus and the lateral hypothalamic area 91.
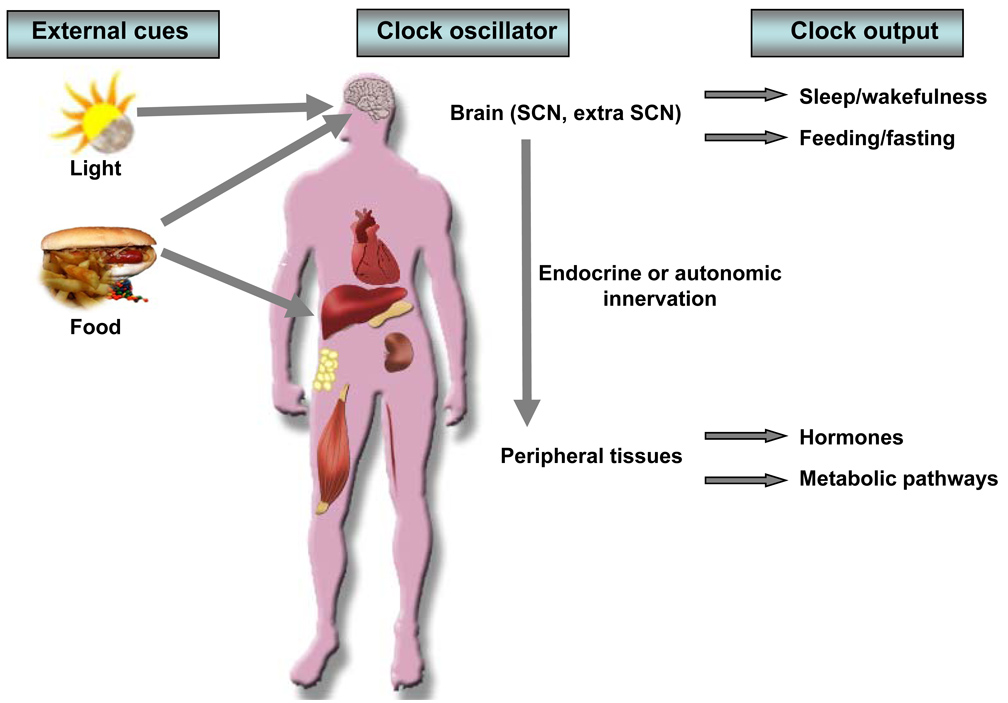
The SCN clock is entrained by light through the retinohypothalamic tract (Fig. 2). Photic input provides a dominant time-keeping signal (zeitgeiber), orienting the animal each day to geophysical time. Endogenous period length is not precisely 24 hr (humans are longer while mice are shorter), thus the daily entrainment to light is a critical mechanism to maintain organismal synchrony with the external environment (Fig 2). Perception of light occurs through activation of a population of directly light-sensitive ganglion cells within the eye, the melanopsin cells; these regulate both circadian rhythms and melatonin synthesis 92, 93. Direct output of the SCN, and the entrainment of the SCN axis to light, plays a key role in synchronizing endogenous hormonal rhythms including the glucocorticoid rhythm 94. In turn, light-induced entrainment of the glucocorticoid rhythm may maintain phase coherence of multiple cellular oscillators, such as those in fibroblasts and liver 95, 96
Fig. 2. Synchronization of internal biological rhythms by external cues.
Light is the predominant environmental cue that is received by the SCN; photic input is transmitted via the retinohypothalamic tract. In turn, the SCN maintains circadian synchrony of peripheral clocks, a process that involves transmission via both autonomic innervation and/or humoral signals. Circadian oscillators may also be entrained by food and hormones. Circadian synchrony and the entrainment process are reflected in the robustly rhythmic behavioral and physiological outputs such as feeding, sleep-wakefulness, hormone secretion, and metabolic homeostasis.
In addition to photic entrainment, food also entrains circadian processes in neural and peripheral cells (Fig. 2). Food restriction to the normal rest period in rodents also induces a burst of anticipatory activity, an effect that is altered in some experimental systems following lesioning of the dorsomedial nucleus 97–99. However there remains controversy concerning the involvement of circadian oscillators in food anticipatory activity (FAA) since the behavior persists in Bmal1 nullizygous mice 97, 100, 101. Interestingly, FAA appears to involve the melanocortin signalling pathway, since restriction failed to increase wakefulness before food presentation in melanocortin-3 receptor null mice 102. Rather than localization to a single nucleus of hypothalamus, the food entrainable oscillator may in fact involve a more dispersed network of cell groups 103. Further, the FAA may constitute a metabolic oscillator responsive to peripheral neural or circulating signals elicited by food ingestion 104, 105. An interesting question remains concerning whether macronutrient flux in the postprandial state may participate in establishing FAA (reviewed in 104, 105). A related question is whether nutrient signalling per se may affect core properties of the SCN pacemaker.
C) Peripheral clocks
Molecular analyses have revealed that the clock network is also widely expressed throughout nearly every tissue/ cell type in vertebrates 106, 107. Original studies by Schibler and colleagues demonstrated cell autonomous clock gene oscillation within fibroblasts ex vivo 108. Following this discovery, in addition to the master clock in the SCN, independent circadian oscillators have been found in a number of peripheral tissues in mammals. Gene expression profiling has shown that 3% to 20% of genes display a 24h rhythmic expression, and a large proportion of these genes have a role in metabolic processes (for review, see 8). The circadian rhythms of peripheral organs are also self-sustained, as demonstrated using a mouse line in which luciferase expression is driven from the endogenous Per1 or Per2 loci 106, 107. Variation in temporal gene expression was reported to play an important role in tissues implicated in glucose and lipid metabolism, such as fat, liver, cardiac and skeletal muscle 109–117. Many nuclear receptors expressed in liver and white and brown adipose tissues also display rhythmic patterns of expression 118–120. Therefore, the nuclear receptors may link clock genes to metabolism by integrating energy flux with varying physiological demands across the light-dark cycle. In this way, circadian patterns of metabolic gene expression may optimize the switch between daily anabolic and catabolic states corresponding with periods of feeding and fasting. For example, the cyclic expression of gastrointestinal tract enzymes may ensure that factors involved in nutrient absorption are expressed in anticipation of daily episodes of food ingestion 105. In addition, adipose enzymes involved in fatty acid storage peak coincident with feeding (for review, 121). Moreover, components of gluconeogenesis, glycolysis, and fatty acid metabolism cycle with a peak during the subjective night in mouse liver 112. Coordinating gene expression patterns according to the varying metabolic demands across the active and rest period is also important in muscle, where elaboration of aerobic and anaerobic enzymes varies during the sleep-wake cycle 111, 122. Thus, peripheral oscillators are self-sustained, cell autonomous and tissue-specific, yet a major question is: What are the mechanisms involved in maintaining synchrony within and between peripheral tissue clocks? A related question is whether misalignment of local circadian oscillation within and between peripheral tissues contributes to cardiovascular and metabolic pathologies?
To discern whether the rhythmic expression of genes in peripheral organs is driven by local (cell autonomous) oscillators or by circadian systemic signals, Schibler and colleagues have recently exploited the tetracycline-inducible system of Bujard, enabling conditional Rev-erbα overexpression within liver 123. In this model, REV-ERBα represses the transcription of the essential core clock gene Bmal1 in a doxycycline-dependent manner. Among 351 genes with rhythmic expression revealed in the doxycycline-fed mice, only 31 genes, including the core clock gene mPer2, oscillated robustly irrespective of whether the liver clock was running or not. These studies suggest that the rhythmicity of metabolic liver genes is driven by both cell-autonomous and non-autonomous signals 123.
Multiple signals related to feeding, and even fasting, may entrain peripheral clocks. Indeed, in in vitro experiments, a bewildering variety of stimuli can induce or reset circadian gene expression. These factors include chemical activators of protein kinase A (forskolin, butyryl cAMP), protein kinase C and/or MAP kinase (phorbol esters, FGF, endothelin) and glucocorticoids receptors (dexamethasone), and even glucose; dissecting how these signalling pathways converge on the clock remains an area of intensive investigation (reviewed in 108).
IV) Evidence for a molecular link between circadian and metabolism systems
The availability of genetic models of circadian disruption has provided new opportunities to dissect the interrelationship of circadian and metabolic systems. Early studies indicated the cellular redox status, represented by the nicotinamide adenine dinucleotide cofactors NAD(H) and NADP(H), regulate the transcriptional activity of CLOCK/BMAL1 and NPAS2/BMAL1 124. The reduced forms of these cofactors increase DNA binding, while the oxidized forms decrease binding, thus coupling activity of these core clock components with the metabolic state of the cell. Two recent studies have further linked the biology of NAD production with the core molecular clock 44, 125. The gene encoding the rate-limiting enzyme in NAD biosynthesis, nicotinamide phosphoribosyltransferase (NAMPT), displays circadian rhythmicity in peripheral tissues, including liver and WAT, and is under the direct control of CLOCK/BMAL1. Such rhythmicity translates to daily oscillations in NAD levels in liver. Both Nampt RNA and NAD levels are reduced in liver from ClockΔ19/Δ19 and Bmal1−/− mice, while increased in liver from mice deficient for both CRY1 and CRY2, suggesting that Nampt, and therefore NAD production, is a downstream target of CLOCK/BMAL1. Not only is NAD important in cellular redox reactions, but it also serves as a substrate for SIRT1, an NAD-dependent and nutrient responsive deacetylase, which has also recently been described as a novel regulator of circadian clock function 126, 127. Of note, the timing of the peak in NAMPT and NAD levels corresponds with the peak in SIRT1 activity. SIRT1 then physically associates with components of the positive limb of the core clock machinery (CLOCK and BMAL1) and is recruited to clock target genes. Genetic and pharmacologic manipulation of SIRT1 and the NAD biosynthesis pathway reveal that SIRT1 negatively regulates CLOCK and BMAL1. Together, these studies demonstrate the existence of a negative feedback loop whereby CLOCK/BMAL1 positively regulate both NAD production and SIRT1 activity, while in turn, SIRT1 negatively regulates the activity of CLOCK/BMAL1.
The existence of this pathway is particularly intriguing in light of the fact that NAMPT and SIRT1 are regulated not only by the clock, but also by the nutritional status of the organism. For example, Nampt is upregulated in response to glucose restriction in skeletal muscle in an AMPK-dependent manner 128, 129, and SIRT1 has been demonstrated in numerous tissues to be increased during fasting or caloric restriction 130–133. Thus, regulation of the clock by NAD and SIRT1 allows for coordination and fine-tuning of the core clock machinery with the daily cycles of fasting/feeding and rest/activity. Furthermore, NAD and SIRT1 are also involved in the regulation of a myriad of metabolic processes, including regulation of glucose-stimulated insulin secretion, adipocyte differentiation, and gluconeogenesis 134. Regulation of NAD and SIRT1 by the clock likely has a cascade of effects on downstream metabolic pathways, and it is tempting to speculate that the reduction in NAD and SIRT1 activity in the circadian mutant mice contributes to some of their metabolic phenotypes. It has also recently been demonstrated that NAMPT is secreted and is present in the circulation 135, though it is not yet known whether extracellular NAMPT is regulated in a circadian manner, thereby influencing downstream processes on a systemic level 136. Recent evidence has also implicated the other NAD-dependent-sirtuin family members (SIRT2–7) in a variety of metabolic processes 137; it will therefore be of great interest to determine whether any of these other sirtuins are also involved in the crosstalk between the core circadian clock and metabolism.
Additional key nutrient sensors that have been implicated in the cross-talk between circadian rhythms and metabolism are the nuclear receptor peroxisome proliferator-activated receptor-γ̣ (PPARγ) and the co-activator PGC1α (PPARγ̣ coactivator 1-α). PPARγ is rhythmically expressed and directly regulates Bmal1 transcription, and mice lacking PPARγ exhibit reduced rhythmicity of clock gene expression, blood pressure, and heart rate 138. It is interesting to note that SIRT1 promotes fat mobilization during fasting by binding to and repressing PPARγ 139. PGC1α also displays circadian oscillations in liver and skeletal muscle and upregulates the transcription of Bmal1 and Rev-erbα. Mice lacking PGC1α have abnormal diurnal locomotor activity rhythms, body temperature, and metabolic rate, along with altered expression of clock and metabolic genes 140. PGC1α̣ levels are elevated in response to cold exposure, starvation, and physical activity, and hence may also help coordinate the circadian clock with the nutritional status of the organism. Of note, SIRT1 also deacetylates and activates PGC1 133, indicating an additional mechanism linking molecular clock function and energy utilization. A more detailed understanding of the molecular links between the core molecular clock machinery and metabolism will be necessary in order to develop therapies targeting disease states involving disruption of both rhythms and metabolism, such as type 2 diabetes.
V) From circadian disruption to metabolic disease
A) What have we learned from the experimental models?
How might circadian misalignment impact the metabolic co-morbidities of obesity, diabetes, and cardiovascular disease? Several lines of evidence suggest that circadian dysregulation may exert a broad impact not only on glucose control, but also on inflammation, fibrinolysis, fluid balance, and vascular reactivity. A central node linking metabolic and circadian pathways involves the nuclear receptor superfamily, including those downstream of REV-ERBα and the RORs that modulate the core clock and diverse metabolic processes ranging from adipogenesis to inflammation and thrombosis (reviewed in 141). Experimental models have helped to demonstrate the impact of the clock network in metabolic gene expression and provide evidence that this clock disruption leads to metabolic abnormalities. Homozygous ClockΔ19 mutant mice, which express a loss of function mutation in Clock, have yielded new insight in this field 142. In addition to disruptions in sleep and circadian behaviour, these mice also develop hyperphagia early in life, with subsequent development of hyperlipidemia, hyperleptinemia, and hypoinsulinemic hyperglycemia, indicating that this animal exhibits features of the metabolic syndrome 142. The feeding rhythm in these mice is damped, with increased food intake during the day, and, in addition, these mice have significantly increased food intake overall. High-fat feeding studies revealed exaggerated weight gain of ClockΔ19 mutant mice, and DEXA scanning and fat pad weight both demonstrated significant increases in fat and lean mass relative to controls following high fat feeding 142. It is likely that the obese phenotype results, at least in part, from altered rhythms of neuropeptides in the hypothalamus, as ghrelin, CART and orexin are all expressed at constitutively low levels in the ClockΔ19 mutant mice 142. In addition, the anorectic neuropeptide POMC was decreased throughout the entire LD cycle in hypothalami of young ClockΔ19 mutant prior to the onset of weight gain and overt diabetes and is consistent with a deficit in the central homeostatic regulation of weight constancy. Since the original ClockΔ19 mutant was developed in a melatonin-deficient strain (C57BL/6J), Kennaway et al. evaluated the contribution of melatonin deficiency on glucose metabolism by crossing ClockΔ19 mutant mouse with the melatonin-producing CBA strain to produce the “ClockΔ19 + MEL” mouse 143. Interestingly, in this model, the restoration of melatonin did not rescue gene expression rhythms in liver or muscle 144. Such studies underscore the importance of strain background in the evaluation of metabolic phenotype. For example, when introgressed onto the ICR strain, the ClockΔ19 mutation results in malabsorption of lipid and thus resistance to diet-induced obesity, thus primary effects of the Clock mutation on energy balance and fuel homeostasis cannot be evaluated in the ICR strain 145. Disruption of other circadian clock genes also leads to metabolic alterations. For example, gene disruption in Bmal1 induces an abnormal metabolic phenotype characterised by impaired gluconeogenesis, hyperleptinemia, glucose intolerance and dyslipidemia 146–148. In addition, Per2 knock-out mice develop increased weight gain on high-fat diet (HFD) 149. Conversely, mice deficient in the circadian deadenylase nocturin remain lean and resistant to hepatic steatosis when fed a HFD despite equivalent caloric intake, similar metabolic rates, and reduced activity compared with control mice 150.
Although clock genes impact metabolic homeostasis, a reciprocal effect of metabolic disruption on circadian rhythms also exists, since diet-induced obesity per se alters circadian behavioral and molecular rhythms in C57BL/6J mice 9. Indeed, HFD also attenuates the amplitude of diurnal rhythms of feeding and locomotor activity, as high fat fed mice increase their food intake during their normal rest (light) period 9. Interestingly, genetically obese animals are resistant to weight gain when feeding is restricted to the active (dark) phase 151. In agreement with these observations, recent evidence demonstrated that circadian timing of food intake contributes to weight gain 152. Indeed, mice fed a HFD only during the 12-h light phase gain significantly more weight compared to isocalorically-fed mice provided food only during the 12-h dark phase 152. Further studies are necessary in order to understand how the timing of food intake impacts energy constancy. Interestingly, a recent study demonstrated that treatment with an antagonist of T-type calcium channel, which is involved in sleep-wake regulation, improved HFD-induced behavioural alterations, including both a decrease in inactive phase activity, core body temperature, feeding and adiposity 153. Taken together, these observations largely based upon animal studies, raise important questions concerning the impact of circadian misalignment and clock gene disruption on obesity and its metabolic complications, and suggest avenues for future investigation in human subjects.
B) Clock disruption in adipose tissue
Excess adipose tissue and altered body fat distribution, rather than adiposity per se, is an important risk factor for obesity-related disorders. Excess intra-abdominal fat rather than subcutaneous fat (central vs. peripheral obesity) is associated with MS and cardiovascular disease 3. However, the mechanisms responsible for this association, and its causality, remain uncertain. Emerging evidence from both cell-based and human studies suggests that expression of the circadian clock transcription network within adipose tissue may influence both adipogenesis and the relative distribution of subcutaneous versus visceral depots 121, 154, 155.
In adipose tissue, the clock machinery controls the expression of a large array of enzymes involved in lipid metabolism. Indeed, adenovirus-mediated expression of BMAL1 in 3T3-L1 adipocytes resulted in induction of several factors involved in lipogenesis, while BMAL1 deletion in adipose cell lines resulted in impaired adipogenesis 148. Furthermore, heme, the REV-ERBα/β natural ligand, has long been known to enhance adipocyte differentiation in vitro 156. Activation of SIRT1, which regulates the clock network, may increase insulin sensitivity and reduce the inflammatory response in adipocytes 157, 158, however it is unclear whether the effect is direct or not.
Experiments in mice have revealed that temporally restricted feeding causes a coordinated phase-shift in circadian expression of core clock genes and their downstream targets in adipose tissues 117. In addition, high fat diet also alters the cyclic expression and function of core clock genes and clock-controlled genes in adipose tissue, resulting in disrupted fuel utilization 9, 159. Of further interest, clock gene disruption targeted to the fat body in flies is sufficient to induce increased food consumption, decreased glycogen levels, and increased sensitivity to starvation 160. At least in flies, these findings suggest involvement of a peripheral tissue clock in neural energy homeostasis 160.
Several teams have recently started to examine the potential relationship between clock gene expression and metabolic syndrome parameters in humans. Expression levels of the core molecular clock genes in cultured visceral and subcutaneous fat explants obtained from morbidly obese subjects correlated with certain metabolic syndrome parameters, such as waist circumference, sagittal diameter and BMI 154, 155, 161. However, biopsies from human fat likely represent a heterogenous mixture of adipose cells in addition to macrophages, thus conclusions must be viewed with caution regarding the contribution of adipose tissue to the observed circadian patterns of gene expression. Interestingly, circadian rhythms of gene expression are sustained ex vivo in human fat explants 161, 162, including the rhythmic oscillation of genes involved in glucocorticoid turnover 162. In agreement, human adipose biopsies removed at different zeitgeber times reflect different levels of gene expression consistent with the observed circadian rhythmicity found in cell-culture studies 163. Further studies are necessary in order to gain more detailed insight into the relationship between temporal patterns of gene expression in adipose tissue and development of MS.
In addition to effects of circadian transcription on intracellular metabolic pathways, clock dysregulation in AT and/or misalignement with meal times may lead to inappropriate expression patterns of enzymes involved in lipid metabolism such as lipoprotein lipase 121. For example, misalignment between the fasting/feeding cycle and lipogenic and/or lipid catabolic gene expression pathways may perturb fatty acid flux and contribute to lipotoxicity. Indeed, circadian synchrony may play a distinct role not only within different tissue types (liver vs muscle), but also within distinct adipose depots (visceral vs subcutaneous) 161. It is further possible that differences of circadian gene expression patterns within visceral AT and subcutaneous AT depots may contribute to cell-autonomous differences in inflammatory, lipogenic and/or lipolytic pathways within these locales 164, 165. The limited storage-capacity of fat and/or increased lipolysis results in an overflow of fatty acids to ectopic sites such as liver, muscle, and islets (for review 166, 167). Interestingly, both have been proposed to be involved in the etiology of the MS 3, 168.
Another important function of adipose tissue is its secretion of numerous bioactive peptides or proteins, collectively named “adipokines”. These may play a central role in energy and vascular homeostasis, as well as immunity, and are fundamental to the pathogenesis of the MS (for review 169). As obesity-related inflammation is receiving increased attention for its potential role in the pathogenesis of MS, steatosis and cardiovascular disease, it may be opportune to consider the impact of circadian systems at the level of adipokine regulation. In mice, leptin exhibits rhythmic production across the light-dark cycle that appears to be dependent of the feeding rhythm 170. Interestingly, in obese humans, disruption of the 24 hr profiles of leptin and adiponectin was observed compared to healthy lean subjects 171, 172. These adipokines play major roles in fuel partitioning and insulin sensitivity but also regulate immunity 169, 173. Indeed, leptin was the first adipokine found to control energy balance 174. The metabolic effects of leptin are thought to primarily involve its actions within brain 175–177, while adiponectin function primarily within peripheral target tissues. Many of the metabolic effects of leptin and adiponectin involve activation of AMPK signaling in muscle/liver 178, 179. Following the discovery of leptin, a growing list of adipokines has been identified, some of which also exert pro-inflammatory roles. Since many adipokines are expressed in a circadian fashion in humans 163, it is tempting to speculate that regulation of adipokine oscillation may be important in metabolic homeostasis. In addition, certain adipokines may also interact with or modulate sleep. For instance, leptin is involved in sleep regulation as demonstrated by EEG monitoring of sleep in leptin deficient and leptin resistant mice 180, 181. IL-6 and TNF-α plasma levels, which are increased in obesity 182, 183 may also impair circadian clock gene oscillations and promote sleep 184, 185.
Circadian regulation may extend to effects within adipose tissue on endoplasmic reticulum (ER) stress, an important component of the inflammatory response in this tissue 186. Obesity results in conditions that increase demand on the ER in metabolic tissues including liver, adipocytes and pancreas, resulting in a persistent inflammatory state 187. For example, accumulation of reactive oxygen species, which are abundantly produced by both the ER and the mitochondria during conditions of stress are increased in metabolic organs in MS 186, 187. In adipose tissue, ER stress is involved in adipogenesis and adipokine oversecretion 188, 189. Interestingly, the endoplasmic reticulum chaperone protein BiP, a key protein involved in the ER stress response, is expressed in a circadian manner in flies 190. It has also been reported that clock genes may influence the production of reactive oxygen species 8, 191, 192. Thus disrupted synchrony of stress response gene expression may alter adipose function and thereby directly contribute to insulin resistance. ER stress may also be induced in brain following high fat feeding, thereby contributing to leptin resistance 193 and perhaps circadian and sleep disturbances (for review, 194).
In addition to the impact of white AT excess in MS pathogenesis, several independent groups recently demonstrated that brown adipose tissue is present and active in adult humans, and its presence and activity are inversely associated with adiposity and indexes of the metabolic syndrome 195–197. As numerous genes including nuclear receptors exert circadian expression profiles in BAT 116, 117, alterations of circadian oscillator genes in fat may have significant metabolic implications.
C) Clock disruption and impaired glucose tolerance
Disruption in the normal cyclic pattern of glucose tolerance is a hallmark of type 2 diabetes 198, and as such, understanding the circadian control of glucose metabolism is critical for delivering the best clinical diabetes management. Strong evidence from human studies demonstrates rhythmic variation in glucose tolerance and insulin action across the day 199–203. For example, oral glucose tolerance is impaired in the evening compared to the morning 204, 205, an effect which is believed to be due to a combination of both decreased insulin secretion and altered insulin sensitivity in the evening 201, 206–211. The ‘dawn phenomenon’ is also a well-described phenomenon where glucose levels are known to peak prior to the onset of the active period 212, 213. Furthermore, studies in rats have revealed that the SCN is critical for the maintenance of diurnal variations in glucose metabolism 208.
While these studies indicate a role for circadian systems in the control of glucose metabolism, the molecular mechanisms underlying these phenomena are not well understood. Recently, human genome-wide association studies and experimental mouse model systems have begun to provide clues as to the nature of the molecular links between rhythms and glucose metabolism. As described above, data from several independent groups has now demonstrated that genetic variants of the melatonin receptor may be involved in abnormal glucose homeostasis 47, 48 and that melatonin treatment of pancreatic β-cells inhibits glucose-induced insulin release 49. Further, mice that have a mutation in the Clock gene develop hypoinsulinemic hyperglycemia, and mice nullizygous for Bmal1 have impaired glucose tolerance 142, 146, 147, suggesting that a functional clock network is required for the maintenance of glucose homeostasis. The cellular etiology of impaired glucose tolerance in circadian mutant animals remains an important yet unresolved area of research.
Finally, it is interesting to speculate that the NAD biosynthetic enzyme NAMPT and SIRT1 may play an important role in the circadian control of glucose metabolism. As described above, NAMPT and SIRT1 are regulated by CLOCK/BMAL1 and constitute a negative feedback loop within the core circadian network. NAMPT and SIRT1 have been demonstrated to be involved in a myriad of metabolic functions, including regulation of gluconeogenesis in liver and glucose-stimulated insulin secretion in islets 44, 125. Indeed Nampt-deficient (Nampt +/−) mice showed impaired glucose tolerance due to a defect in glucose-stimulated insulin secretion, which was corrected by intraperitoneal administration of NMN 135, and mice overexpressing SIRT1 specifically in their β-cells displayed improved glucose tolerance and increased glucose-stimulated insulin secretion 214. As NAMPT and SIRT1 function are impaired in circadian mutant mice, these data suggest that circadian rhythms of NAMPT and SIRT1 may act in β-cells to regulate the daily cycles of insulin secretion and that NAD+ might function as an oscillating metabolite linking circadian and metabolic cycles.
D) Impact of circadian systems on cardiovascular function
Circadian variation in endogenous factors such as autonomic nervous system function, blood catecholamine concentrations, coagulability, heart rate, blood pressure regulation, and platelet aggregability have been suggested to explain the morning onset of myocardial infarction 23. Conversely, pressure overload-induced hypertrophy and diabetes mellitus result in alterations in the circadian clock within the heart 215, 216. In this regard, it was suggested that alterations in circadian control of cardiac fuel handling may contribute to myocardial contractile dysfunction (for review, see 217). The identification of genes that exhibit circadian regulation within large vessels of the mouse 218–221 has provided clues as to the impact of the circadian system within vasculature. Indeed, clock gene expression within vasculature has been shown to impact blood pressure and thrombo-occlusive response 220, 222, 223. Clock genes may influence the temporal incidence of clinical cardiovascular events by regulating the magnitude of the early morning rise in blood pressure 220. Indeed, the circadian variation in blood pressure and heart rate is disrupted in mice with deleted or mutated core clock genes, a phenomenon that may be partially explained by the altered diurnal variation in epinephrine and norepinephrine in these mice 220. Interestingly, the mutated mice also showed a reduced response to immobilization stress compared to wild-type mice. Thus, expression of the core clock within peripheral vasculature may modulate the capacity to respond to environmental stressors at different times of day 220. Effects of the clock system on blood pressure may also involve the modulation of aldosterone biosynthesis by Per1 224. Further, Anea and colleagues demonstrated that Bmal1-knockout and Clock mutant mice present a loss of vascular adaptation and predisposition to thrombosis 223, both hallmarks of endothelial dysfunction 225. The endothelial dysfunction in Bmal1-knockout mice has been related to defects in Akt and nitric oxide signaling 223. Interestingly, the defects in endothelium-dependent arterial relaxation of Clock mutant mice were normalized by entrainment to light, indicating that the vascular phenotype is not simply a consequence of Clock mutation or Bmal1 deficiency, but rather the result of behavioral disruption in these animals 223.
As noted above, NAD+ regulation has recently emerged as a major factor coupling circadian rhythms and metabolic signalling pathways. Since NAMPT-mediated NAD biosynthesis has also been shown to impact cardiomyocyte survival pathways, it will be important to ascertain whether dysregulation of NAD contributes to the adverse cardiovascular consequences of circadian disruption 226.
Cardiomyocytes must adapt rapidly to changes in circulating fatty acid, the primary fuel source for contraction. In this regard, PPAR signalling is important in the control of cardiac energy metabolism 227. Of note, it was also demonstrated that the circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids 228–230. To address the circadian function of vascular tissue and the role of PPARγ in the vascular clock, conditional deletion of PPARγ targeted to this tissue was performed 231. These mice developed abnormalities in blood pressure and heart rate in parallel with a reduction of diurnal variation in the sympathetic nerve activity 231. Furthermore, vascular PPARγ exhibits a robust cyclic expression, whose rhythmic phase may be reset by changes in feeding time as well as changes in the photoperiod 231. Thus, the temporal environment may be integrated within the heart by PPARγ 231. In agreement, PPARγ agonists were found to shift the circadian fluctuation of blood pressure in patients with type 2 diabetes, indicating that vasculoprotective actions of thiazolidinediones may in part involve effects on the clock transcription network 232.
Emerging clinical evidence has uncovered unique actions of the PPARα agonist fenofibrate in the circadian control of blood pressure and heart rate in diabetic subjects. In particular, fenofibrate exerted its most marked anti-hypertensive effects at night 233. In contrast, only modest decrease anti-hypertensive effects were detected in studies involving a single measurement during the day 234, 235. In addition, fenofibrate lowered heart rate throughout the 24-h period 233. Taking together, these data suggest an interaction between PPARα, blood pressure control and circadian rhythms in diabetes.
A comprehensive temporal map of the nuclear receptor transcriptome provides additional clues concerning the circadian control of cardiovascular physiology 120. For instance, RARα and RXRα interact with CLOCK and MOP4 resulting in repression of CLOCK/MOP4: BMAL1 activity in vascular cells 219. Indeed, the ligation of retinoic acid, the oxidized form of Vitamin A, to its receptors can phase shift Per2 mRNA rhythm in vivo and in smooth muscle cells in vitro 219. Whether additional nuclear hormone receptor agonists impact circadian regulation of vascular tone remains a question for future investigation.
Finally, the role of circadian rhythms in the time of onset of thrombotic events has been recognized for many years. Numerous coagulation/ fibrinolytic factors, such as protein C, anti-thrombin, Factor VII, protein S and fibrinogen, have been demonstrated to fluctuate in a circadian manner in humans. Among these factors, PAI-1, the most important physiological inhibitor of plasminogen activation, peaks in the early morning, explaining at least in part the occurrence of hypofibrinolysis and of pro-thrombotic state 236. Circadian control of PAI-1 gene expression by the REV-ERBα in liver may contribute to the circadian variation in fibrinolysis 237, an effect that may also involve interactions between the cycle-like factor (CLIF) and CLOCK 238. Thus further studies on the circadian gene control of fibrinolysis may shed new light on factors contributing to the prothrombotic state.
E) Circadian rhythms and hepatic function
Liver also plays a key role in the development of metabolic syndrome (for review, see 239). BMAL1 and CLOCK control gene expression of enzymes critical in liver and influence both glucose and lipid homeostasis (for review, see 240). A recent study reported that mice with a liver-specific deletion of Bmal1 exhibited hypoglycemia during fasting, indicating a role for the liver clock in maintaining euglycemia during rest 147. Some of the effects of BMAL1 and CLOCK in liver may involve direct regulation of phosphoenolpyruvate carboxykinase (Pepck) 146. In addition, circadian gene expression in hepatocytes is altered in mouse models of type 2 diabetes 241 and by high fat feeding 9, 242. Moreover, HFD induced a phase delay of components of the adiponectin signaling pathway 242. Alterations in circadian control of adiponectin signalling may reduce its protective effects 242 and thereby increase susceptibility to steatosis, a major risk factor in cardiovascular disease 243.
Hepatic clock gene expression also modulates both bile acid and apolipoprotein biosynthesis, raising the possibility that clock disruption may impact multiple components of hepatic lipid homeostasis 244. For example, several proteins involved in lipid metabolism (such as hepatic cytochrome P450 cholesterol 7 α-hydroxylase, HMG CoA reductase, or apolipoprotein AIV) show diurnal variation in both humans and rodents 105. Interestingly, Rev-erbα was recently found to play an important role in the control of bile acid metabolism via the regulation of the neutral bile acid synthesis pathway 245. In mouse liver, Rev-erbα expression levels are high during the late light phase, leading to the repression of both small heterodimer partner (SHP) and E4 promoter binding protein 4 (E4BP4) hepatic expression. Reduced levels of SHP and E4BP4 may counter the suppressive effects of bile acids on the cholesterol 7α-hydroxylase (CYP7A1) gene transcription, thereby contributing to the circadian regulation of bile acid and cholesterol homeostasis 245. In addition, Rev-erbα also controls the daily expression of genes involved in cholesterol and lipid homeostasis through circadian modulation of SREBP signalling 246.
F) Clock dysfunction in the immune system
In addition to effects on lipogenesis, lipid catabolism and thrombosis, the circadian system may also promote inflammatory pathways that contribute to the development of cardiovascular disease. At the molecular level, the circadian transcription factor Rev-erbα, which is expressed in cells from the immune system such as macrophages and other cell types, may impact the inflammatory response 141 (also see for review, see 247). Intriguingly, REV-ERBα increases the TNF-α-induced NF-κB response, whereas RORα impedes it 141. As rhythmic mRNA expression of the clock genes is dampened in peripheral leucocytes of patients with type 2 diabetes, this impairment might be involved in its pathogenesis 248.
VI) Conclusion
Both inter- and intra-organ desynchrony may be involved in the pathogenesis of cardiometabolic disease due to effects in brain and multiple metabolic tissues including heart, liver, fat, muscle, pancreas and gut. In this context, strategies to improve alignment between the cycles of sleep/wakefulness and feeding/fasting may ameliorate physiological processes including appetitive behavior, carbohydrate and lipid metabolism, inflammation, thrombosis and sodium handling. Efforts to dissect the molecular mediators that coordinate circadian, metabolic and cardiovascular systems may ultimately lead to both improved therapeutics and preventive interventions.
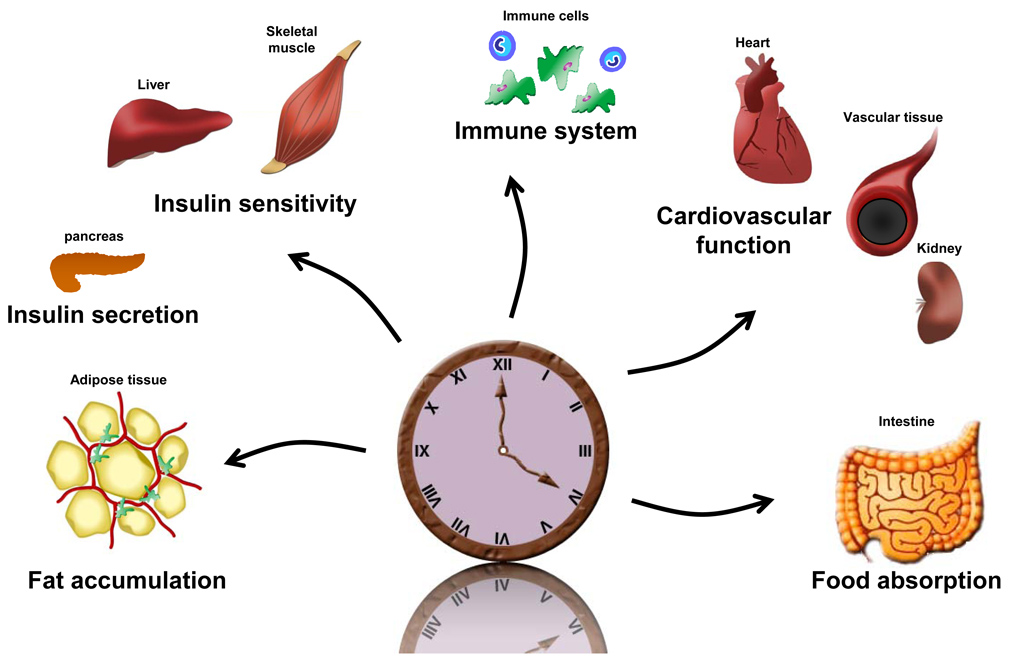
Fig. 3. Peripheral clock output.
The core clock machinery has been identified in most peripheral tissues. In addition, rhythmic gene expression appears to be regulated in a tissue-specific manner, enabling each tissue to appropriately calibrate local physiological processes within the appropriate overall temporal schedule. However, circadian disruption either within or amongst individual tissues may lead to organ dysfunction. Indeed, recent studies suggest that peripheral clock alteration is involved in body weight gain as well as abnormalities in glucose homeostasis and blood pressure regulation, thereby contributing to the development of the metabolic syndrome. These alterations may be initiated by disruptions in circadian behavioral and/or environmental factors such as high-fat diet. Circadian and physiological systems are interconnected through reciprocal feedback loops within each tissue locale.
Acknowledgements
We thank members of the Bass, Takahashi, Turek and Allada laboratories for helpful discussions.
Sources of Funding
Work was supported by grants from Alfediam to E.M.; NIDDK (T32 DK007169) to K.M.R.; NIH (PO1 AG011412 and R01HL097817-01), ADA, Chicago Biomedical Consortium Searle Funds, and JDRF to J.B., and the University of Chicago DRTC (P60 DK020595).
Glossary
Non-standard Abbreviations and Acronyms
- ATF
Activating transcription factor
- ARNTL
Aryl-hydrocarbon receptor nuclear translocator-like
- BMAL1
Brain and muscle aryl-hydrocarbon receptor nuclear translocator-like1
- BMI
Body mass index
- bHLH-PAS
basic helix-loop-helix-Period-Arnt-Single-minded
- bZIP
basic leucine zipper transcription factor
- cAMP
cyclic adenosine 3',5'-monophosphate
- CART
Cocaine- and amphetamine-regulated transcript
- CK1
Casein kinase 1
- CLIF
Cycle-like factor
- CLOCK
Circadian locomotor output cycles kaput
- CREB
cAMP response element binding protein
- CREM
cAMP-responsive element modulator
- CRP
C-reactive protein
- Cry
Cryptochrome
- DBP
D-site binding protein
- ER
Endoplasmic reticulum
- FAA
Food anticipatory activity
- FBXL3
F-box and leucine-rich repeat protein 3
- HLF
Hepatic leukemia factor
- IL-6
Interleukin-6
- LD
Light Dark cycle
- MS
Metabolic syndrome
- NAD
Nicotinamide adenine dinucleotide
- NAMPT
Nicotinamide phosphoribosyltransferase
- NF-κB
Nuclear factor-κB
- NPAS2
Neuronal PAS domain protein 2
- PAI-1
Plasminogen activator inhibitor type 1
- Pbef
Pre-B-cell colony enhancing factor
- Pepck
Phosphoenolpyruvate carboxykinase
- Per
Period
- PGC1
PPARγ̣ coactivator 1
- PPARγ
Peroxisome proliferator-activated receptor-γ
- ROR
Retinoic acid-related orphan receptor
- SCN
Suprachiasmatic nucleus
- SIRT1
Sirtuin 1
- SHP
Small heterodimer partner
- SREBP
Sterol regulatory element-binding protein
- TEF
Thyrotroph embryonic factor
- TNF-α
Tumor necrosis factor-α
- β̣̃TrCP
β-transducin repeat containing protein
Glossary
- Core Molecular Clock
molecular machinery of the clock within all cells. The circadian gene network in mammals is controlled by a network of autoregulatory transcription-translation feedback loops.
- Circadian rhythm
biochemical, physiological or behavioral processes that persist under constant conditions with a period length of ~24 hours
- Clock
a central mechanism controlling circadian rhythms
- Clock-controlled gene
a gene whose expression is rhythmically regulated by a clock
- Entraining agent or external cues or zeitgeber or inputs
extrinsic stimuli able to reset the rhythms (i.e. daylight or food).
- Oscillator
a system of components that produces a circadian rhythm
- Outputs
circadian rhythmicity of most physiological and behavioral functions, such as feeding, sleep-wakefulness, hormone secretion, and metabolic homeostasis.
- Period
duration of one complete cycle in a rhythmic variation
Footnotes
Disclosures
J.B. is a member of the scientific advisory board of a cofounder of ReSet Therapeutics Inc.. J.B. is also an advisor and receives support from Amylin Pharmaceuticals.
References
- 1.Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program - Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30:8–13. doi: 10.2337/dc06-1414. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 3.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R. Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care. 2007;30:1647–1652. doi: 10.2337/dc07-9921. [DOI] [PubMed] [Google Scholar]
- 4.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 5.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27:282–283. doi: 10.2337/diacare.27.1.282. [DOI] [PubMed] [Google Scholar]
- 11.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Kido T, Nogawa K. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity (Silver Spring) 2008;16:1887–1893. doi: 10.1038/oby.2008.298. [DOI] [PubMed] [Google Scholar]
- 13.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 14.Lumeng JC, Somashekar D, Appugliese D, Kaciroti N, Corwyn RF, Bradley RH. Shorter sleep duration is associated with increased risk for being overweight at ages 9 to 12 years. Pediatrics. 2007;120:1020–1029. doi: 10.1542/peds.2006-3295. [DOI] [PubMed] [Google Scholar]
- 15.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 17.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramsey KM, Bass J. Lean gene and the clock machine. Proc Natl Acad Sci U S A. 2007;104:9553–9554. doi: 10.1073/pnas.0703516104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stunkard AJ, Allison KC, Geliebter A, Lundgren JD, Gluck ME, O'Reardon JP. Development of criteria for a diagnosis: lessons from the night eating syndrome. Compr Psychiatry. 2009;50:391–399. doi: 10.1016/j.comppsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Sousa AG, Cercato C, Mancini MC, Halpern A. Obesity and obstructive sleep apnea-hypopnea syndrome. Obes Rev. 2008;9:340–354. doi: 10.1111/j.1467-789X.2008.00478.x. [DOI] [PubMed] [Google Scholar]
- 21.Burioka N, Koyanagi S, Endo M, Takata M, Fukuoka Y, Miyata M, Takeda K, Chikumi H, Ohdo S, Shimizu E. Clock gene dysfunction in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2008;32:105–112. doi: 10.1183/09031936.00138207. [DOI] [PubMed] [Google Scholar]
- 22.Kok SW, Meinders AE, Overeem S, Lammers GJ, Roelfsema F, Frolich M, Pijl H. Reduction of plasma leptin levels and loss of its circadian rhythmicity in hypocretin (orexin)-deficient narcoleptic humans. J Clin Endocrinol Metab. 2002;87:805–809. doi: 10.1210/jcem.87.2.8246. [DOI] [PubMed] [Google Scholar]
- 23.Oishi K. Plasminogen activator inhibitor-1 and the circadian clock in metabolic disorders. Clin Exp Hypertens. 2009;31:208–219. doi: 10.1080/10641960902822468. [DOI] [PubMed] [Google Scholar]
- 24.Knutson KL, Curiel TJ, Salazar L, Disis ML. Immunologic principles and immunotherapeutic approaches in ovarian cancer. Hematol Oncol Clin North Am. 2003;17:1051–1073. doi: 10.1016/s0889-8588(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 25.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 26.Janszky I, Ljung R. Shifts to and from daylight saving time and incidence of myocardial infarction. N Engl J Med. 2008;359:1966–1968. doi: 10.1056/NEJMc0807104. [DOI] [PubMed] [Google Scholar]
- 27.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licinio J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 29.Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin HM, Vela-Bueno A, Kales A, Chrousos GP. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51:887–892. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- 30.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14:3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64:631–636. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- 32.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro DC, Hampton SM, Morgan L, Deacon S, Arendt J. Altered postprandial hormone and metabolic responses in a simulated shift work environment. J Endocrinol. 1998;158:305–310. doi: 10.1677/joe.0.1580305. [DOI] [PubMed] [Google Scholar]
- 34.Ramsey KM, Bass J. Obeying the clock yields benefits for metabolism. Proc Natl Acad Sci U S A. 2009;106:4069–4070. doi: 10.1073/pnas.0901304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuninkova L, Brown SA. Peripheral circadian oscillators: interesting mechanisms and powerful tools. Ann N Y Acad Sci. 2008;1129:358–370. doi: 10.1196/annals.1417.005. [DOI] [PubMed] [Google Scholar]
- 36.He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL, Jr, Rossner MJ, Nishino S, Fu YH. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009;325:866–870. doi: 10.1126/science.1174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ptacek LJ, Jones CR, Fu YH. Novel insights from genetic and molecular characterization of the human clock. Cold Spring Harb Symp Quant Biol. 2007;72:273–277. doi: 10.1101/sqb.2007.72.017. [DOI] [PubMed] [Google Scholar]
- 38.Wirz-Justice A. Diurnal variation of depressive symptoms. Dialogues Clin Neurosci. 2008;10:337–343. doi: 10.31887/DCNS.2008.10.3/awjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pulkki-Raback L, Elovainio M, Kivimaki M, Mattsson N, Raitakari OT, Puttonen S, Marniemi J, Viikari JS, Keltikangas-Jarvinen L. Depressive symptoms and the metabolic syndrome in childhood and adulthood: a prospective cohort study. Health Psychol. 2009;28:108–116. doi: 10.1037/a0012646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 41.Sookoian S, Gemma C, Gianotti TF, Burgueno A, Castano G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 42.Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Englund A, Kovanen L, Saarikoski ST, Haukka J, Reunanen A, Aromaa A, Lonnqvist J, Partonen T. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms. 2009;7:5. doi: 10.1186/1740-3391-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blakemore AI, Meyre D, Delplanque J, Vatin V, Lecoeur C, Marre M, Tichet J, Balkau B, Froguel P, Walley AJ. A Rare Variant in the Visfatin Gene (NAMPT/PBEF1) Is Associated With Protection From Obesity. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.75. [DOI] [PubMed] [Google Scholar]
- 47.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chevre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, Weill J, Heude B, Tauber M, Lemaire K, Schuit F, Elliott P, Jorgensen T, Charpentier G, Hadjadj S, Cauchi S, Vaxillaire M, Sladek R, Visvikis-Siest S, Balkau B, Levy-Marchal C, Pattou F, Meyre D, Blakemore AI, Jarvelin MR, Walley AJ, Hansen T, Dina C, Pedersen O, Froguel P. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 48.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, Bergman RN, Bochud M, Bonnycastle LL, Buchanan TA, Cao A, Cervino A, Coin L, Collins FS, Crisponi L, de Geus EJ, Dehghan A, Deloukas P, Doney AS, Elliott P, Freimer N, Gateva V, Herder C, Hofman A, Hughes TE, Hunt S, Illig T, Inouye M, Isomaa B, Johnson T, Kong A, Krestyaninova M, Kuusisto J, Laakso M, Lim N, Lindblad U, Lindgren CM, McCann OT, Mohlke KL, Morris AD, Naitza S, Orru M, Palmer CN, Pouta A, Randall J, Rathmann W, Saramies J, Scheet P, Scott LJ, Scuteri A, Sharp S, Sijbrands E, Smit JH, Song K, Steinthorsdottir V, Stringham HM, Tuomi T, Tuomilehto J, Uitterlinden AG, Voight BF, Waterworth D, Wichmann HE, Willemsen G, Witteman JC, Yuan X, Zhao JH, Zeggini E, Schlessinger D, Sandhu M, Boomsma DI, Uda M, Spector TD, Penninx BW, Altshuler D, Vollenweider P, Jarvelin MR, Lakatta E, Waeber G, Fox CS, Peltonen L, Groop LC, Mooser V, Cupples LA, Thorsteinsdottir U, Boehnke M, Barroso I, Van Duijn C, Dupuis J, Watanabe RM, Stefansson K, McCarthy MI, Wareham NJ, Meigs JB, Abecasis GR. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radziuk J, Pye S. Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Suprachiasmatic deficit or limit cycle behaviour? Diabetologia. 2006;49:1619–1628. doi: 10.1007/s00125-006-0273-9. [DOI] [PubMed] [Google Scholar]
- 51.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 55.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 56.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 57.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 58.Sato TK, Yamada RG, Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, Welsh DK, Kay SA, Ueda HR, Hogenesch JB. Feedback repression is required for mammalian circadian clock function. Nat Genet. 2006;38:312–319. doi: 10.1038/ng1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shearman LP, Jin X, Lee C, Reppert SM, Weaver DR. Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Mol Cell Biol. 2000;20:6269–6275. doi: 10.1128/mcb.20.17.6269-6275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baggs JE, Price TS, DiTacchio L, Panda S, Fitzgerald GA, Hogenesch JB. Network features of the mammalian circadian clock. PLoS Biol. 2009;7:e52. doi: 10.1371/journal.pbio.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ueda HR. Systems biology of mammalian circadian clocks. Cold Spring Harb Symp Quant Biol. 2007;72:365–380. doi: 10.1101/sqb.2007.72.047. [DOI] [PubMed] [Google Scholar]
- 62.Akashi M, Takumi T. The orphan nuclear receptor RORalpha regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol. 2005;12:441–448. doi: 10.1038/nsmb925. [DOI] [PubMed] [Google Scholar]
- 63.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 64.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 65.Triqueneaux G, Thenot S, Kakizawa T, Antoch MP, Safi R, Takahashi JS, Delaunay F, Laudet V. The orphan receptor Rev-erbalpha gene is a target of the circadian clock pacemaker. J Mol Endocrinol. 2004;33:585–608. doi: 10.1677/jme.1.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guillaumond F, Dardente H, Giguere V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 67.Gachon F, Olela FF, Schaad O, Descombes P, Schibler U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab. 2006;4:25–36. doi: 10.1016/j.cmet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 68.Maldonado R, Smadja C, Mazzucchelli C, Sassone-Corsi P. Altered emotional and locomotor responses in mice deficient in the transcription factor CREM. Proc Natl Acad Sci U S A. 1999;96:14094–14099. doi: 10.1073/pnas.96.24.14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- 71.Akashi M, Tsuchiya Y, Yoshino T, Nishida E. Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in cultured cells. Mol Cell Biol. 2002;22:1693–1703. doi: 10.1128/MCB.22.6.1693-1703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eide EJ, Vielhaber EL, Hinz WA, Virshup DM. The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iepsilon. J Biol Chem. 2002;277:17248–17254. doi: 10.1074/jbc.M111466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eide EJ, Woolf MF, Kang H, Woolf P, Hurst W, Camacho F, Vielhaber EL, Giovanni A, Virshup DM. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol Cell Biol. 2005;25:2795–2807. doi: 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Godinho SI, Maywood ES, Shaw L, Tucci V, Barnard AR, Busino L, Pagano M, Kendall R, Quwailid MM, Romero MR, O'Neill J, Chesham JE, Brooker D, Lalanne Z, Hastings MH, Nolan PM. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316:897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- 75.Reischl S, Vanselow K, Westermark PO, Thierfelder N, Maier B, Herzel H, Kramer A. Beta-TrCP1-mediated degradation of PERIOD2 is essential for circadian dynamics. J Biol Rhythms. 2007;22:375–386. doi: 10.1177/0748730407303926. [DOI] [PubMed] [Google Scholar]
- 76.Shirogane T, Jin J, Ang XL, Harper JW. SCFbeta-TRCP controls clock-dependent transcription via casein kinase 1-dependent degradation of the mammalian period-1 (Per1) protein. J Biol Chem. 2005;280:26863–26872. doi: 10.1074/jbc.M502862200. [DOI] [PubMed] [Google Scholar]
- 77.Siepka SM, Yoo SH, Park J, Song W, Kumar V, Hu Y, Lee C, Takahashi JS. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011–1023. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, Saigoh K, Ptacek LJ, Fu YH. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 79.Hirota T, Lewis WG, Liu AC, Lee JW, Schultz PG, Kay SA. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc Natl Acad Sci U S A. 2008;105:20746–20751. doi: 10.1073/pnas.0811410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 81.DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeBruyne JP, Weaver DR, Reppert SM. Peripheral circadian oscillators require CLOCK. Curr Biol. 2007;17:R538–R539. doi: 10.1016/j.cub.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 83.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 84.Cermakian N, Monaco L, Pando MP, Dierich A, Sassone-Corsi P. Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. EMBO J. 2001;20:3967–3974. doi: 10.1093/emboj/20.15.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, Buijs R, Bootsma D, Hoeijmakers JH, Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 86.Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, Takahashi JS, Sancar A. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci U S A. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okamura H. Suprachiasmatic nucleus clock time in the mammalian circadian system. Cold Spring Harb Symp Quant Biol. 2007;72:551–556. doi: 10.1101/sqb.2007.72.033. [DOI] [PubMed] [Google Scholar]
- 88.Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 89.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 90.Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140:207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- 91.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 92.Foster RG, Hankins MW, Peirson SN. Light, photoreceptors, and circadian clocks. Methods Mol Biol. 2007;362:3–28. doi: 10.1007/978-1-59745-257-1_1. [DOI] [PubMed] [Google Scholar]
- 93.Sancar A. Regulation of the mammalian circadian clock by cryptochrome. J Biol Chem. 2004;279:34079–34082. doi: 10.1074/jbc.R400016200. [DOI] [PubMed] [Google Scholar]
- 94.Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 95.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 96.Brown SA, Fleury-Olela F, Nagoshi E, Hauser C, Juge C, Meier CA, Chicheportiche R, Dayer JM, Albrecht U, Schibler U. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 2005;3:e338. doi: 10.1371/journal.pbio.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 99.Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci U S A. 2006;103:12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1527–R1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- 101.Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci U S A. 2009;106:6808–6813. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, Gimble JM, Tschop MH, Butler AA. The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci. 2008;28:12946–12955. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davidson AJ. Search for the feeding-entrainable circadian oscillator: a complex proposition. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1524–R1526. doi: 10.1152/ajpregu.00073.2006. [DOI] [PubMed] [Google Scholar]
- 104.Stephan FK. The "other" circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- 105.Hussain MM, Pan X. Clock genes, intestinal transport and plasma lipid homeostasis. Trends Endocrinol Metab. 2009;20:177–185. doi: 10.1016/j.tem.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 107.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 109.Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- 110.Kita Y, Shiozawa M, Jin W, Majewski RR, Besharse JC, Greene AS, Jacob HJ. Implications of circadian gene expression in kidney, liver and the effects of fasting on pharmacogenomic studies. Pharmacogenetics. 2002;12:55–65. doi: 10.1097/00008571-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 111.McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics. 2007;31:86–95. doi: 10.1152/physiolgenomics.00066.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 113.Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O'Neill JS, Wong GK, Chesham J, Odell M, Lilley KS, Kyriacou CP, Hastings MH. Circadian orchestration of the hepatic proteome. Curr Biol. 2006;16:1107–1115. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 114.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 115.Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- 116.Yang H, Lavu S, Sinclair DA. Nampt/PBEF/Visfatin: a regulator of mammalian health and longevity? Exp Gerontol. 2006;41:718–726. doi: 10.1016/j.exger.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
- 118.Alenghat T, Meyers K, Mullican SE, Leitner K, Adeniji-Adele A, Avila J, Bucan M, Ahima RS, Kaestner KH, Lazar MA. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature. 2008;456:997–1000. doi: 10.1038/nature07541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Teboul M, Guillaumond F, Grechez-Cassiau A, Delaunay F. The nuclear hormone receptor family round the clock. Mol Endocrinol. 2008;22:2573–2582. doi: 10.1210/me.2007-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 121.Gimble JM, Floyd ZE. Fat Circadian Biology. J Appl Physiol. 2009 doi: 10.1152/japplphysiol.00090.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A. 2007;104:3342–3347. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 125.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai SI, Bass J. Circadian Clock Feedback Cycle Through NAMPT-Mediated NAD+ Biosynthesis. Science. 2009 doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 127.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD(+) metabolism and SIRT1 activity. Nature. 2009 doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- 131.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 132.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 133.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 134.Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 135.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Imai SI. The NAD World: A New Systemic Regulatory Network for Metabolism and Aging-Sirt1, Systemic NAD Biosynthesis, and Their Importance. Cell Biochem Biophys. 2009;15:20–28. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the 'magnificent seven', function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 138.Wang J, Lazar MA. Bifunctional role of Rev-erbalpha in adipocyte differentiation. Mol Cell Biol. 2008;28:2213–2220. doi: 10.1128/MCB.01608-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 141.Duez H, Staels B. The nuclear receptors Rev-erbs and RORs integrate circadian rhythms and metabolism. Diab Vasc Dis Res. 2008;5:82–88. doi: 10.3132/dvdr.2008.0014. [DOI] [PubMed] [Google Scholar]
- 142.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kennaway DJ, Voultsios A, Varcoe TJ, Moyer RW. Melatonin and activity rhythm responses to light pulses in mice with the Clock mutation. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1231–R1240. doi: 10.1152/ajpregu.00697.2002. [DOI] [PubMed] [Google Scholar]
- 144.Kennaway DJ, Owens JA, Voultsios A, Boden MJ, Varcoe TJ. Metabolic homeostasis in mice with disrupted Clock gene expression in peripheral tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1528–R1537. doi: 10.1152/ajpregu.00018.2007. [DOI] [PubMed] [Google Scholar]
- 145.Oishi K, Atsumi G, Sugiyama S, Kodomari I, Kasamatsu M, Machida K, Ishida N. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett. 2006;580:127–130. doi: 10.1016/j.febslet.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 146.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci U S A. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150:2153–2160. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Green CB, Douris N, Kojima S, Strayer CA, Fogerty J, Lourim D, Keller SR, Besharse JC. Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc Natl Acad Sci U S A. 2007;104:9888–9893. doi: 10.1073/pnas.0702448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Masaki T, Chiba S, Yasuda T, Noguchi H, Kakuma T, Watanabe T, Sakata T, Yoshimatsu H. Involvement of hypothalamic histamine H1 receptor in the regulation of feeding rhythm and obesity. Diabetes. 2004;53:2250–2260. doi: 10.2337/diabetes.53.9.2250. [DOI] [PubMed] [Google Scholar]
- 152.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian Timing of Food Intake Contributes to Weight Gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Uebele VN, Gotter AL, Nuss CE, Kraus RL, Doran SM, Garson SL, Reiss DR, Li Y, Barrow JC, Reger TS, Yang ZQ, Ballard JE, Tang C, Metzger JM, Wang SP, Koblan KS, Renger JJ. Antagonism of T-type calcium channels inhibits high-fat diet-induced weight gain in mice. J Clin Invest. 2009;119:1659–1667. doi: 10.1172/JCI36954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gomez-Abellan P, Hernandez-Morante JJ, Lujan JA, Madrid JA, Garaulet M. Clock genes are implicated in the human metabolic syndrome. Int J Obes (Lond) 2008;32:121–128. doi: 10.1038/sj.ijo.0803689. [DOI] [PubMed] [Google Scholar]
- 155.Wu X, Xie H, Yu G, Hebert T, Goh BC, Smith SR, Gimble JM. Expression profile of mRNAs encoding core circadian regulatory proteins in human subcutaneous adipose tissue: correlation with age and body mass index. Int J Obes (Lond) 2009;33:971–977. doi: 10.1038/ijo.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen JJ, London IM. Hemin enhances the differentiation of mouse 3T3 cells to adipocytes. Cell. 1981;26:117–122. doi: 10.1016/0092-8674(81)90039-8. [DOI] [PubMed] [Google Scholar]
- 157.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol. 2009 doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 158.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146:5631–5636. doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 160.Xu K, Zheng X, Sehgal A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 2008;8:289–300. doi: 10.1016/j.cmet.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gomez-Santos C, Gomez-Abellan P, Madrid JA, Hernandez-Morante JJ, Lujan JA, Ordovas JM, Garaulet M. Circadian rhythm of clock genes in human adipose explants. Obesity (Silver Spring) 2009;17:1481–1485. doi: 10.1038/oby.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hernandez-Morante JJ, Gomez-Santos C, Milagro F, Campion J, Martinez JA, Zamora S, Garaulet M. Expression of cortisol metabolism-related genes shows circadian rhythmic patterns in human adipose tissue. Int J Obes (Lond) 2009;33:473–480. doi: 10.1038/ijo.2009.4. [DOI] [PubMed] [Google Scholar]
- 163.Loboda A, Kraft WK, Fine B, Joseph J, Nebozhyn M, Zhang C, He Y, Yang X, Wright C, Morris M, Chalikonda I, Ferguson M, Emilsson V, Leonardson A, Lamb J, Dai H, Schadt E, Greenberg HE, Lum PY. Diurnal variation of the human adipose transcriptome and the link to metabolic disease. BMC Med Genomics. 2009;2:7. doi: 10.1186/1755-8794-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hocking SL, Chisholm DJ, James DE. Studies of regional adipose transplantation reveal a unique and beneficial interaction between subcutaneous adipose tissue and the intra-abdominal compartment. Diabetologia. 2008;51:900–902. doi: 10.1007/s00125-008-0969-0. [DOI] [PubMed] [Google Scholar]
- 165.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hsu IR, Kim SP, Kabir M, Bergman RN. Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr. 2007;86:s867–s871. doi: 10.1093/ajcn/86.3.867S. [DOI] [PubMed] [Google Scholar]
- 167.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Harris RB, Leibel RL. Location, location, location. Cell Metab. 2008;7:359–361. doi: 10.1016/j.cmet.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 169.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2009 doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 170.Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101:1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mingrone G, Manco M, Granato L, Calvani M, Scarfone A, Mora EV, Greco AV, Vidal H, Castagneto M, Ferrannini E. Leptin pulsatility in formerly obese women. FASEB J. 2005;19:1380–1382. doi: 10.1096/fj.04-3453fje. [DOI] [PubMed] [Google Scholar]
- 172.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A. 2004;101:10434–10439. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 174.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 175.Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 177.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 178.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 180.Laposky AD, Bradley MA, Williams DL, Bass J, Turek FW. Sleep-wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2059–R2066. doi: 10.1152/ajpregu.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered Sleep Regulation in Leptin Deficient Mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R894–R903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- 182.Bastard JP, Lagathu C, Caron M, Capeau J. Point-counterpoint: Interleukin-6 does/does not have a beneficial role in insulin sensitivity and glucose homeostasis. J Appl Physiol. 2007;102:821–822. doi: 10.1152/japplphysiol.01353.2006. author reply 825. [DOI] [PubMed] [Google Scholar]
- 183.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83:2907–2910. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 184.Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci U S A. 2007;104:12843–12848. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vanitallie TB. Sleep and energy balance: Interactive homeostatic systems. Metabolism. 2006;55:S30–S35. doi: 10.1016/j.metabol.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 186.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond) 2008;32 Suppl 7:S52–S54. doi: 10.1038/ijo.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 189.Sha H, He Y, Chen H, Wang C, Zenno A, Shi H, Yang X, Zhang X, Qi L. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009;9:556–564. doi: 10.1016/j.cmet.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 191.Kondratov RV, Gorbacheva VY, Antoch MP. The role of mammalian circadian proteins in normal physiology and genotoxic stress responses. Curr Top Dev Biol. 2007;78:173–216. doi: 10.1016/S0070-2153(06)78005-X. [DOI] [PubMed] [Google Scholar]
- 192.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 194.Scharf MT, Naidoo N, Zimmerman JE, Pack AI. The energy hypothesis of sleep revisited. Prog Neurobiol. 2008;86:264–280. doi: 10.1016/j.pneurobio.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 197.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 198.Holterhus PM, Odendahl R, Oesingmann S, Lepler R, Wagner V, Hiort O, Holl R. Classification of distinct baseline insulin infusion patterns in children and adolescents with type 1 diabetes on continuous subcutaneous insulin infusion therapy. Diabetes Care. 2007;30:568–573. doi: 10.2337/dc06-2105. [DOI] [PubMed] [Google Scholar]
- 199.Aparicio NJ, Puchulu FE, Gagliardino JJ, Ruiz M, Llorens JM, Ruiz J, Lamas A, De Miguel R. Circadian variation of the blood glucose, plasma insulin and human growth hormone levels in response to an oral glucose load in normal subjects. Diabetes. 1974;23:132–137. doi: 10.2337/diab.23.2.132. [DOI] [PubMed] [Google Scholar]
- 200.Bowen AJ, Reeves RL. Diurnal variation in glucose tolerance. Arch Intern Med. 1967;119:261–264. [PubMed] [Google Scholar]
- 201.Carroll KF, Nestel PJ. Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes. 1973;22:333–348. doi: 10.2337/diab.22.5.333. [DOI] [PubMed] [Google Scholar]
- 202.Jarrett RJ, Keen H. Diurnal variation of oral glucose tolerance: a possible pointer to the evolution of diabetes mellitus. Br Med J. 1969;2:341–344. doi: 10.1136/bmj.2.5653.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Roberts HJ. Afternoon Glucose Tolerance Testing: A Key to the Pathogenesis, Early Diagnosis and Prognosis of Diabetogenic Hyperinsulinism. J Am Geriatr Soc. 1964;12:423–472. doi: 10.1111/j.1532-5415.1964.tb05730.x. [DOI] [PubMed] [Google Scholar]
- 204.Shapiro ET, Tillil H, Polonsky KS, Fang VS, Rubenstein AH, Van Cauter E. Oscillations in insulin secretion during constant glucose infusion in normal man: Relationship to changes in plasma glucose. J. Clin. Endocrinol. Metab. 1988;67:307–314. doi: 10.1210/jcem-67-2-307. [DOI] [PubMed] [Google Scholar]
- 205.Van Cauter E, Desir D, Decoster C, Fery F, Balasse EO. Nocturnal decrease in glucose tolerance during constant glucose infusion. J Clin Endocrinol Metab. 1989;69:604–611. doi: 10.1210/jcem-69-3-604. [DOI] [PubMed] [Google Scholar]
- 206.Baker IA, Jarrett RJ. Diurnal variation in the blood-sugar and plasma-insulin response to tolbutamide. Lancet. 1972;2:945–947. doi: 10.1016/s0140-6736(72)92471-3. [DOI] [PubMed] [Google Scholar]
- 207.Boden G, Ruiz J, Urbain JL, Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol. 1996;271:E246–E252. doi: 10.1152/ajpendo.1996.271.2.E246. [DOI] [PubMed] [Google Scholar]
- 208.la Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes. 2001;50:1237–1243. doi: 10.2337/diabetes.50.6.1237. [DOI] [PubMed] [Google Scholar]
- 209.Lee A, Ader M, Bray GA, Bergman RN. Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects. Diabetes. 1992;41:742–749. doi: 10.2337/diab.41.6.750. [DOI] [PubMed] [Google Scholar]
- 210.Melani F, Verrillo A, Marasco M, Rivellese A, Osorio J, Bertolini MG. Diurnal variation in blood sugar and serum insulin in response to glucose and/or glucagon in healthy subjects. Horm Metab Res. 1976;8:85–88. doi: 10.1055/s-0028-1095597. [DOI] [PubMed] [Google Scholar]
- 211.Verrillo A, De Teresa A, Martino C, Di Chiara G, Pinto M, Verrillo L, Torello F, Gattoni A. Differential roles of splanchnic and peripheral tissues in determining diurnal fluctuation of glucose tolerance. Am J Physiol. 1989;257:E459–E465. doi: 10.1152/ajpendo.1989.257.4.E459. [DOI] [PubMed] [Google Scholar]
- 212.Arslanian S, Ohki Y, Becker DJ, Drash AL. Demonstration of a dawn phenomenon in normal adolescents. Horm Res. 1990;34:27–32. doi: 10.1159/000181791. [DOI] [PubMed] [Google Scholar]
- 213.Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Calcinaro F, Lolli C, Campbell P, Brunetti P, Gerich JE. Demonstration of a dawn phenomenon in normal human volunteers. Diabetes. 1984;33:1150–1153. doi: 10.2337/diab.33.12.1150. [DOI] [PubMed] [Google Scholar]
- 214.Moynihan KA, Imai S. Sirt1 as a key regulator orchestrating the response to caloric restriction. Drug Discovery Today - Disease Mechanisms. 2006;3:11–17. [Google Scholar]
- 215.Young ME, Razeghi P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res. 2001;88:1142–1150. doi: 10.1161/hh1101.091190. [DOI] [PubMed] [Google Scholar]
- 216.Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H. Alterations of the circadian clock in the heart by streptozotocin-induced diabetes. J Mol Cell Cardiol. 2002;34:223–231. doi: 10.1006/jmcc.2001.1504. [DOI] [PubMed] [Google Scholar]
- 217.Bray MS, Young ME. Diurnal variations in myocardial metabolism. Cardiovasc Res. 2008;79:228–237. doi: 10.1093/cvr/cvn054. [DOI] [PubMed] [Google Scholar]
- 218.Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB, FitzGerald GA. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation. 2005;112:2716–2724. doi: 10.1161/CIRCULATIONAHA.105.568626. [DOI] [PubMed] [Google Scholar]
- 219.McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 220.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Curtis AM, Seo SB, Westgate EJ, Rudic RD, Smyth EM, Chakravarti D, FitzGerald GA, McNamara P. Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J Biol Chem. 2004;279:7091–7097. doi: 10.1074/jbc.M311973200. [DOI] [PubMed] [Google Scholar]
- 222.Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, Bradfield CA, FitzGerald GA. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 2008;117:2087–2095. doi: 10.1161/CIRCULATIONAHA.107.739227. [DOI] [PubMed] [Google Scholar]
- 223.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest. 2009;119:2423–2434. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 226.Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bray MS, Young ME. Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obes Rev. 2007;8:169–181. doi: 10.1111/j.1467-789X.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 229.Durgan DJ, Trexler NA, Egbejimi O, McElfresh TA, Suk HY, Petterson LE, Shaw CA, Hardin PE, Bray MS, Chandler MP, Chow CW, Young ME. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281:24254–24269. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- 230.Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Physiol Heart Circ Physiol. 2006;290:H1–H16. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]
- 231.Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE, Yang T. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 2008;8:482–491. doi: 10.1016/j.cmet.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Anan F, Masaki T, Fukunaga N, Teshima Y, Iwao T, Kaneda K, Umeno Y, Okada K, Wakasugi K, Yonemochi H, Eshima N, Saikawa T, Yoshimatsu H. Pioglitazone shift circadian rhythm of blood pressure from non-dipper to dipper type in type 2 diabetes mellitus. Eur J Clin Invest. 2007;37:709–714. doi: 10.1111/j.1365-2362.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- 233.Chew GT, Watts GF, Davis TM, Stuckey BG, Beilin LJ, Thompson PL, Burke V, Currie PJ. Hemodynamic effects of fenofibrate and coenzyme Q10 in type 2 diabetic subjects with left ventricular diastolic dysfunction. Diabetes Care. 2008;31:1502–1509. doi: 10.2337/dc08-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 235.Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001;357:905–910. [PubMed] [Google Scholar]
- 236.Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3:1879–1883. doi: 10.1111/j.1538-7836.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- 237.Wang J, Yin L, Lazar MA. The orphan nuclear receptor Rev-erb alpha regulates circadian expression of plasminogen activator inhibitor type 1. J Biol Chem. 2006;281:33842–33848. doi: 10.1074/jbc.M607873200. [DOI] [PubMed] [Google Scholar]
- 238.Maemura K, de la Monte SM, Chin MT, Layne MD, Hsieh CM, Yet SF, Perrella MA, Lee ME. CLIF, a novel cycle-like factor, regulates the circadian oscillation of plasminogen activator inhibitor-1 gene expression. J Biol Chem. 2000;275:36847–36851. doi: 10.1074/jbc.C000629200. [DOI] [PubMed] [Google Scholar]
- 239.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 240.Marcheva B, Ramsey KM, Affinati A, Bass J. Clock Genes and Metabolic Disease. J Appl Physiol. 2009 doi: 10.1152/japplphysiol.00698.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ando H, Oshima Y, Yanagihara H, Hayashi Y, Takamura T, Kaneko S, Fujimura A. Profile of rhythmic gene expression in the livers of obese diabetic KK-A(y) mice. Biochem Biophys Res Commun. 2006;346:1297–1302. doi: 10.1016/j.bbrc.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 242.Barnea M, Madar Z, Froy O. High-fat diet delays and fasting advances the circadian expression of adiponectin signaling components in mouse liver. Endocrinology. 2009;150:161–168. doi: 10.1210/en.2008-0944. [DOI] [PubMed] [Google Scholar]
- 243.Kamada Y, Takehara T, Hayashi N. Adipocytokines and liver disease. J Gastroenterol. 2008;43:811–822. doi: 10.1007/s00535-008-2213-6. [DOI] [PubMed] [Google Scholar]
- 244.Galman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology. 2005;129:1445–1453. doi: 10.1053/j.gastro.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 245.Duez H, van der Veen JN, Duhem C, Pourcet B, Touvier T, Fontaine C, Derudas B, Bauge E, Havinga R, Bloks VW, Wolters H, van der Sluijs FH, Vennstrom B, Kuipers F, Staels B. Regulation of bile acid synthesis by the nuclear receptor Rev-erbalpha. Gastroenterology. 2008;135:689–698. doi: 10.1053/j.gastro.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 246.Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Sasso GL, Moschetta A, Schibler U. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7:e1000181. doi: 10.1371/journal.pbio.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Albrecht U, Bordon A, Schmutz I, Ripperger J. The multiple facets of Per2. Cold Spring Harb Symp Quant Biol. 2007;72:95–104. doi: 10.1101/sqb.2007.72.001. [DOI] [PubMed] [Google Scholar]
- 248.Ando H, Takamura T, Matsuzawa-Nagata N, Shima KR, Eto T, Misu H, Shiramoto M, Tsuru T, Irie S, Fujimura A, Kaneko S. Clock gene expression in peripheral leucocytes of patients with type 2 diabetes. Diabetologia. 2009;52:329–335. doi: 10.1007/s00125-008-1194-6. [DOI] [PubMed] [Google Scholar]