Abstract
The mammalian embryonic gonad is a unique organ primordium in that it can adopt two different developmental fates - namely, differentiate as either a testis or an ovary - with dramatic consequences for an individual. While a molecular cascade culminating in testis development is well characterized, the ovarian pathways still remain enigmatic. The canonical Wnt/β-catenin signaling implements a conserved mechanism of regulating gene expression that is integral to development of all metazoans. In this review we summarize the recent evidence that suggests a central role for this signaling pathway in the development of the mammalian female.
Keywords: Wnt, β-catenin, canonical, ovary, Rspo1
Introduction
In most mammals sex is dictated by the presence of the Y chromosome at fertilization. Sry, the Y chromosome-linked testis-determining gene, initiates sex determination in males and leads to the formation of the testes rather than ovaries from a bipotential embryonic gonad. The genetic program guiding mammalian male development (with mice being the best understood species) has been the focus of intense investigation for over twenty years and is the subject of many excellent reviews (e.g., (Swain and Lovell-Badge, 1999; Capel, 2000; Wilhelm et al., 2007)).
Sry expression at ~10.5–11 days postcoitum (dpc) promptly triggers a number of profound cellular transformations in mouse embryonic testis; in contrast, the contemporaneous embryonic ovary does not seem to undergo any major morphological changes and appears dormant (reviewed in (Brennan and Capel, 2004)). The first cytologically detectable feature of the ovarian landscape is distinguishable only at 13.5dpc when gonocytes enter meiosis; however, even this female-specific process was considered to be independent of ovarian environment. The idea that ovarian differentiation requires its own set of genes and occurs shortly after (Eicher and Washburn, 1986) or even precedes (McElreavey et al., 1993) that of the testes had been put forward a number of years ago; however, the genes dedicated to early ovarian development have been identified only in the beginning of the 21st century. This limited knowledge of the genetic network operating in the embryonic ovary has precluded an instructive characterization of ovarian development until recently. Driven in large part by advances in microarray technology, the work performed by many laboratories has now uncovered a number of sexually dimorphic genes in the developing ovary and the functional relationship between these genes is beginning to emerge (e.g. (Menke and Page, 2002; Menke et al., 2003; Yao et al., 2004; Jorgensen and Gao, 2005; Nef et al., 2005; Bouma et al., 2007a). At this point the evidence that the ovary activates a complex genetic program and embarks on female development at about the same time the testis initiates the male pathway, is incontrovertible.
In both males and females sexual determination and differentiation are thought to be initiated by the sex-specific transcription factors in a limited number of somatic cells in the gonadal primordia to be later expanded and reinforced by extracellular signals with the cascade culminating in the development of the respective organs (Kim and Capel, 2006). This review attempts to integrate the recent results that identify the canonical β-catenin signaling pathway as a centerpiece of this controlling mechanism in ovarian development in mammals.
Wnt Signaling Pathways in Vertebrates
The Wnts comprise a large family (19 homologues in vertebrates) of secreted proteins that play key roles in diverse processes such as embryonic induction, generation of cell polarity and cell differentiation. The so-called 'canonical' or ‘β-catenin-dependent’ WNT signaling pathway is understood best (with many excellent reviews available, e.g. (Clevers, 2006; Gordon and Nusse, 2006; Huang and He, 2008)) (Fig.1). This signaling is initiated by a WNT ligand binding to Frizzled (Fz) and LRP5/6 co-receptors ultimately leading to β̃catenin protein stabilization and entry into the nucleus. Nuclear β-catenin binding to T-cell factor (TCF)/lymphoid enhancer factor (LEF) transcription factors leads to the activation of target gene expression. Importantly, in the absence of nuclear β-catenin, TCF/LEF proteins act as transcriptional repressors by recruiting Groucho proteins (reviewed in (Eastman and Grosschedl, 1999)). This ability to switch between repressor and activator states probably provides tight control over Wnt target gene expression. A ‘noncanonical’ (β-catenin independent) Wnt pathway was originally described in flies where Wnt signaling was shown to be required for the establishment of planar cell polarity (PCP), a way of tissue organization in which cells demonstrate a defined, clearly identifiable orientation relative to the plane in which they reside. A similar pathway controls cell migration during vertebrate development (e.g. body axis extension and neural tube closure) (reviewed in (Karner et al., 2006)).
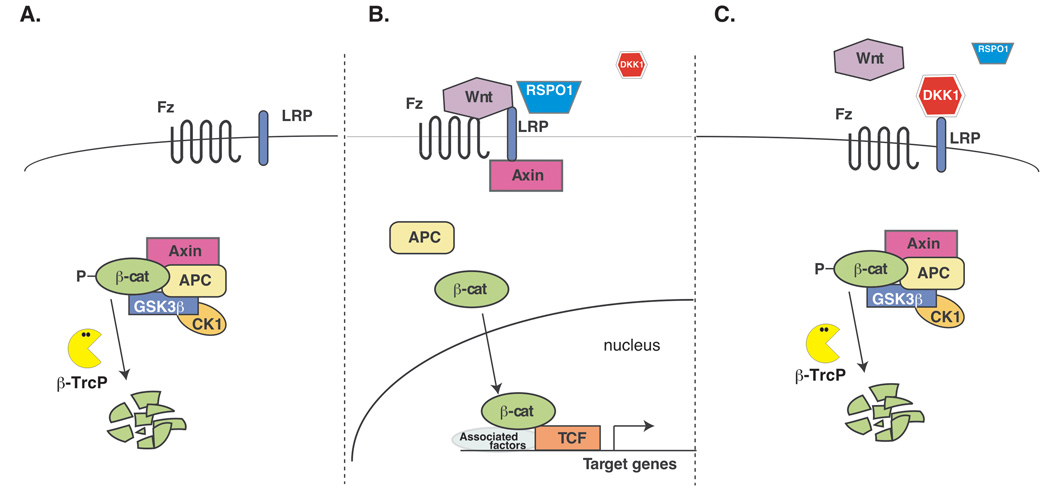
Fig. 1.
The canonical Wnt/β-catenin pathway (modified after (Benchabane and Ahmed, 2008)). (A). The default “off-state”. In the absence of a Wnt ligand, cytoplasmic β-catenin is constitutively engaged by a group of proteins termed “the destruction complex” containing the Axin and Adenomatous Polyposis Coli (APC) proteins. By recruiting the kinases, Casein Kinase 1α and GSK-3β, the destruction complex facilitates the phosphorylation of β-catenin. The phosphorylated β-catenin serves as a substrate for the β-Trcp E3 ubiquitin ligase that targets it for ubiquitination and proteasome-mediated degradation. (B). The “on-state”. The signaling is initiated by WNT ligand binding to Frizzled (Fz) and LRP5/6 co-receptors ultimately leading to the inhibition of the destruction complex and β̃catenin protein stabilization and entry into the nucleus. Nuclear β-catenin binding to T-cell factor (TCF)/lymphoid enhancer factor (LEF) transcription factors leads to the activation of target gene expression. The RSPO1 protein is thought to facilitate Wnt-Fz-LRP complex formation through fending off a secreted inhibitor, DKK1 (C). The regulated “off-state”. DKK1 binding to the LRP6 co-receptor blocks Wnt signal transduction by preventing formation of the Wnt-Fz-LRP6 signaling complex.
Another β-catenin independent Wnt pathway is Wnt/Ca2+ signaling, in which WNT–Fz co-receptors activate heterotrimeric G proteins and phospholipase C and trigger the release of intracellular calcium (Ca2+). It remains to be established, however, whether these pathways are overlapping or whether context-specific induction of separate noncanonical WNT pathways can occur. It was originally proposed that only a subset of WNTs (e.g. Wnt1 and Wnt3a) is capable of inducing a canonical cascade, while others (e.g. Wnt5a and Wnt4) act exclusively in a noncanonical fashion (Wong et al., 1994; Shimizu et al., 1997). The current thinking favors a different model whereby a WNT family member can act canonically or noncanonically depending on the available receptors. For example, canonical Wnt3a can activate Rho kinase and the PCP pathway (Kishida et al., 2004), while Wnt5a was recently shown to either act canonically in the presence of LRP5/Fz4 receptor complex or suppress the canonical pathway acting through a different receptor, ROR2 (Mikels and Nusse, 2006). ‘Noncanonical’ Wnt signal transduction and the cross-talk between the canonical and noncanonical Wnt pathways appear to be very complex; these mechanisms are just beginning to be understood (Cadigan and Liu, 2006; Kikuchi et al., 2007).
Wnt Signaling in the Developing Gonads
Although several Wnt family members are expressed in the developing mammalian gonads (Kimura et al., 2006; Cederroth et al., 2007) it is primarily Wnt4 that has been associated with sexual development. Initially, both XX and XY gonadal primordia express modest levels of Wnt4; however, this expression is promptly down-regulated in the male after sexual determination at 11.5dpc, while it is increased in the female (Vainio et al., 1999; Jeays-Ward et al., 2003).
The role for Wnt4 in sexual development has been examined both by a knockout and transgenesis experiments in mice. Wnt4-null females are masculinized as demonstrated by the absence of Müllerian ducts and the retention of Wolffian ducts; Wnt4 deficiency also results in a dramatic reduction in the number of developing oocytes. Further studies established that Wnt4 is required to repress steroidogenic and vascular endothelial cell migration into the developing XX gonad; absence of Wnt4 leads to both the ectopic steroid (e.g. testosterone) production and formation of a male-specific coelomic blood vessel (Jeays-Ward et al., 2003; Heikkila et al., 2005). Markers of early ovarian development, Follistatin (Fst) and Bmp2, are not expressed in the absence of Wnt4 (Yao et al., 2004).
The affect of the increased Wnt4 dosage in mice is relatively mild and is mostly confined to the abnormal development of the coelomic blood vessel in the male (Jeays-Ward et al., 2003; Jordan et al., 2003). In contrast, in four known XY human patients with duplications of chromosome 1p35 that includes the WNT4 locus the symptoms are much more severe and range from isolated cryptorchidism to severe genital ambiguity (Jordan et al., 2001; Jordan et al., 2003). This discrepancy may be accounted for by the fact that human duplication also encompasses an RSPO1 (see below) gene. In summary, although the mere presence (or absence) of Wnt4 expression appears to be insufficient by itself to program the differentiation of the somatic cells, WNT4 signaling controls several critical elements of the sexual development program. Despite these recent advances in understanding Wnt signaling in sexual differentiation, the specifics of Wnt4 signaling and its intracellular consequences in gonadal development remain enigmatic (for a recent review see (Bernard and Harley, 2007)).
Rspo1 and Ovarian Development
Coincidentally (or not), Rspo, another gene family with a role in female sexual development, also targets the β-catenin transduction pathway. A recently reported recessive mutation in the gene encoding R-SPONDIN1 (RSPO1) results in a complete female-to-male sex reversal associated with palmoplantar hyperkeratosis and a predisposition to skin cancer in human patients (Parma et al., 2006). The R-spondins (Rspos) have been clearly established as a novel family of soluble regulators of β-catenin signaling (Kim et al., 2006a). A founding member, Rspo1 is expressed in the coelomic epithelium of the bipotential urogenital ridge as early as 10.5dpc followed by a sex-specific increase in the somatic cells of the 12.5dpc XX gonad; by 14.5dpc, expression in XX gonads is fivefold higher than in XY gonads (Parma et al., 2006). The mechanism of the RSPO proteins’ action is currently not well understood. Some studies indicate that RSPO could interact directly with WNT proteins and/or engage a Fz/LRP receptor complex (Kazanskaya et al., 2004; Nam et al., 2006), while others argue that RSPOs may also act independently of WNTs, through a separate receptor/signaling pathway that leads to β̃catenin activation without utilizing the Fz/LRP complex (Kim et al., 2005; Kim et al., 2006a). A conciliatory model of RSPO action proposes that RSPO acts in the presence of both WNT and DKK ligands and functions to relieve the DKK1-imposed inhibition of the β-catenin pathway (Binnerts et al., 2007)
The initial report connecting RSPO1 gene mutation to sex reversal raised the expectations that RSPO1 could be a universal female-determining gene in mammals; not surprisingly, the results of the Rspo1 knockout in mice (Chassot et al., 2008; Tomizuka et al., 2008) were awaited with bated breath. Superficially, the result seems somewhat of a setback: the XX Rspo1−/− mice remain phenotypically female. A more thorough analysis demonstrated that XX gonads in the Rspo1 mutants underwent sex reversal, however, it was incomplete, and embryonic mutant XX gonads resemble ovaries rather than testes and contain oocytes. Masculinization in the absence of Rspo1 occurs only postnatally and the adult XX gonad takes on a testis appearance, with tubules devoid of germ cells.
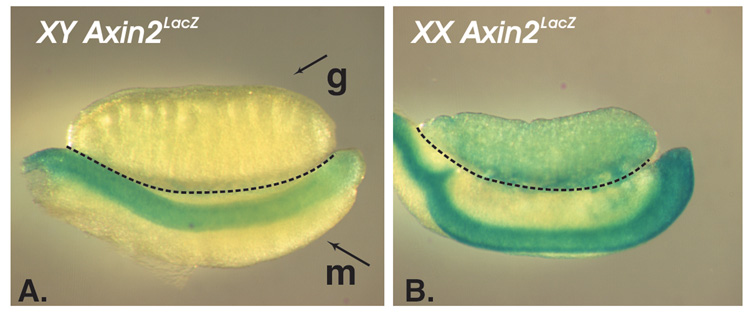
Although Rspo1 mutation does not result in sex reversal, these animals were instrumental for exposing the connection between RSPO proteins and the Wnt/β-catenin cascade in the ovary. The authors (Chassot et al., 2008) provide critical evidence that supports the role for the canonical β-catenin pathway in female differentiation; until this work the very existence of β-catenin signaling in the developing ovary was uncertain ((Bernard and Harley, 2007; Bernard et al., 2008)). This uncertainty may have, at least partially, arisen from the observation that the embryos carrying a TOPGAL reporter (the first Wnt/β-catenin reporter widely used for inspecting the sites of β-catenin signaling in mice; (DasGupta and Fuchs, 1999)) do not show a signal in the ovary (e.g., Manuylov et al., unpublished). Here the authors (Chassot et al., 2008) used a more sensitive reporter, Axin2LacZ that clearly demonstrates the presence of canonical signaling in the somatic cells of developing ovaries but not the testes ((Chassot et al., 2008) and Fig.2).
Fig. 2.
The canonical Wnt/β- catenin pathway is active in the embryonic ovaries but not the testes. The gonads from the embryos carrying an Axin2lacZ allele were stained for β-galactosidase (lacZ) expression. The samples (13.5dpc) are oriented with the anterior end towards the left; the gonad (g) and the mesonephros (a segmentally organized structure that serves as a base for the gonad and a duct system, m) are separated by the dotted line. The downstream target of the canonical β-catenin pathway, Axin2, is expressed in the ovary (B) and not the testis (A).
Exploring the RSPO1-Wnt4 connection is one of the mechanistically gratifying aspects of this work. The authors (Chassot et al., 2008) show that Rspo1 acts upstream of Wnt4 in the female differentiation cascade: in 12.5dpc XX Rspo1−/− gonads Wnt4 is dramatically down-regulated while in the XX Wnt4−/− gonads Rspo1 levels do not change. Interestingly, the female-specific increase of Wnt4 expression in the Rspo1− − gonads is not completely lost but only delayed and from 14.5dpc onwards Wnt4 expression is normal. Accordingly, in contrast to Wnt4 gene ablation, in the XX Rspo1 mutants there is no dramatic loss of germ cells during embryogenesis - although the two papers differ slightly on this account. Both groups underscore the lower number gonocytes entering meiosis in Rspo1−/− XX mutants attributing this to either changes in germ cell adhesion (Chassot et al., 2008) or increased apoptosis (Tomizuka et al., 2008). As meiotic cells are known to oppose testis differentiation (Adams and McLaren, 2002; Yao et al., 2003) it is possible that the lower number of meiotic gonocytes in the Rspo1 mutant is a contributing factor in the emergence of the masculinization later in development. The impaired ability of germ cells to enter meiosis is unlikely, however, to be the sole cause of the masculinization since even a complete block in meiosis does not lead to sex-reversal (Baltus et al., 2006).
The female gene expression program in XX Rspo1−/− gonads is compromised as manifested by a dramatic (10–15 times) decrease in the Fst gene expression; it will be interesting to distinguish whether this results from a transient loss of gonadal Wnt4 expression (that is upstream of Fst (Yao et al., 2004)) or is Wnt4-unrelated. The other known elements of the female program appear to be less perturbed: the Foxl2 gene expression is unchanged and the Bmp2 expression is stated as unaffected (Chassot et al., 2008) or reduced by 50% (Tomizuka et al., 2008). The authors also show a significant increase in the ectopic expression of steroidogenic enzymes; however, this, again, most likely results indirectly from a temporary loss of Wnt4 expression that leads to an influx of the steroidogenic cells from the mesonephros as has been reported (Vainio et al., 1999; Jeays-Ward et al., 2003).
In summary, although the critical role for Rspo1 in regulating mammalian female development is incontrovertible, the genetic underpinning of the Rspo1−/− phenotype (especially the mechanism of late sex reversal) remains to be understood. Whether Rspo1 is normally involved in the suppression of the male program in the XX gonad is also uncertain. The Rspo1 knockout does not include an early induction of Sox9 expression and appears to present the case for a partial suppression of the XX (female) program rather than for a sex reversal. Sox9 gene transcription and the nuclear import of its protein in the testis are potentiated by prostaglandin D2, a hormone synthesized by prostaglandin D synthase (Pgds) (Malki et al., 2005; Wilhelm et al., 2005); similarly, FGF9 is known to activate Sox9 expression (Kim et al., 2006b). Although a moderate increase in Pgds and Fgf9 gene expression is observed in the Rspo1−/− XX gonads by 14.5dpc, Sox9 expression appears to switch on only by 18.5dpc, hence the connection between these events may be indirect.
β-catenin stabilization leads to a male-to-female sex reversal
The current view of mammalian sex determination emphasizes the notion that the two alternative fates, female and male, arise as closely intertwined parities determined by antagonistic activities. For this to hold true one should be able to tip experimentally the balance in favor of either of the two possible developmental outcomes. While it was known for quite some time that activating Sry or Sox9 in the female will override the ovarian pathway and activate the male program in the XX gonad, it was not at all clear whether the female program, similarly, could be imposed to overpower the male one in the normal XY environment. In contrast to the Sox9 gene that is both necessary and sufficient for male differentiation (Vidal et al., 2001; Chaboissier et al., 2004; Sekido and Lovell-Badge, 2008) - an early activation of Sox9 may, in fact, be quintessential for complete female-to-male sex reversal - no single ‘female’ gene seemed to possess both of these qualifications.
A major advancement towards restoring the required symmetry came recently from Blanche Capel's lab that reported a male-to-female sex reversal upon stabilization of the β-catenin pathway (Maatouk et al., 2008). These authors reasoned that individually, the loss of Wnt4 or Rspo1 in mice results only in a partial XX sex-reversal. Similarly, over-expressing Wnt4 has not been successful to sex-reverse XY mice (Jeays-Ward et al., 2003; Jordan et al., 2003), suggesting that simply increasing the Wnt4 dosage is insufficient to override the male pathway. However, an XY patient carrying a chromosome duplication which serendipitously includes the WNT4 and RSPO1 genes exhibited sex-reversal (Jordan et al., 2001) making it tempting to speculate that the upregulation of both of these ligands in mice may be required to antagonize male development. Since both WNT4 and RSPO1 signaling converge (at least partially) on activating the canonical β-catenin pathway, the authors took a more direct approach: instead of trying to overexpress the ligands, they produced a constitutively active (undegradable) form of β-catenin (Harada et al., 1999) in the somatic cells of the developing ovary. In their report the authors convincingly show that the stabilization of β-catenin is sufficient to block the male pathway in the XY gonads (Maatouk et al., 2008). The stabilization of β-catenin led to a somatic cell fate switch and a loss of male-specific SOX9 and AMH expression while the genes normally found in the ovarian somatic cells (Foxl2, Bmp2, Wnt4 and Fst) were increased. Not surprisingly, testis cords did not form in these XY mutants (Maatouk et al., 2008).
The β-catenin protein could be stabilized (constitutively activated) by removing its N terminal domain; in their studies the authors relied on the recently developed Sf1Cre strain of mice (Bingham et al., 2006) to delete the exons that encode this part of the protein. Although the 'surgically' precise Sf1-Cre gene targeting approach allows one to activate β-catenin in a defined cell population, it is not a guarantee of a complete excision (and, hence, a robust gene activation) within the desired developmental window. This presents an especially pressing problem for a rapidly developing organ where the appearance of a cellular population with a conspicuous gene expression program is separated from the terminal differentiation of these cells only by a narrow 24-hour period. In other words, the amount of CRE enzyme produced from the Sf1 transgene has to be sufficient for ultimately generating enough stabilized β-catenin - in time to block the rapid expansion of the male program in the XY gonad.
Although sex-reversed, the XY females with stabilized β-catenin are not completely devoid of embryonic male characteristics, with one of the earliest male traits, a coelomic vessel, still being present. This incomplete block of the male program could be attributable to an early 'burst' of Sox9 expression that transiently appears in the XY gonads with stabilized β-catenin. The residual Sox9 expression could be caused by the delayed accumulation of ectopic β-catenin or could be β-catenin independent. To address this question the authors complemented their genetic approach with the pharmacological activation of the Wnt/β-catenin pathway by Lithium Cloride (LiCl) in cultured gonads. LiCl is known to block the GSK-3β enzyme, a major component of the β-catenin destruction complex (Doble and Woodgett, 2003).
The pharmacological approach allowed for the complete and early inhibition of the male program, including Sox9 expression and vessel formation. LiCl-mediated stabilization of β-catenin suppresses both of these 'remnants' of the male program, hence their interdependence remains unclear. It is of note, though, that the ectopic coelomic vessel forms in several XX mutants (e.g., Fst, Wnt4 (Yao et al., 2004) and Rspo1 (Chassot et al., 2008; Tomizuka et al., 2008) in the absence of any detectable Sox9 expression. Although the genetic and pharmacological approach appear to be in agreement, one has to keep the perspective that the pleiotropic nature of LiCl may be a contributing factor in the stronger block of the male program: lithium ions can target kinases other than GSK-3β (Davies et al., 2000); similarly, GSK-3β may have targets other than β-catenin (Orme et al., 2003).
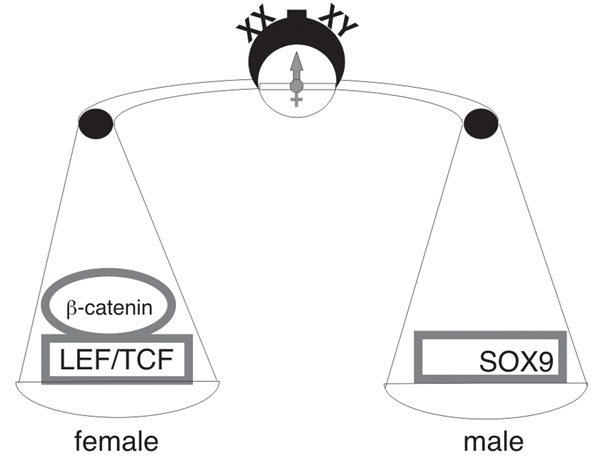
So what is the likely mechanism of β-catenin induced sex reversal? The authors (Maatouk et al., 2008) propose what seems to be a very compelling model: the stabilized β-catenin (if expressed sufficiently early) effectively preempts the male-promoting action of SOX9 (Fig.3). The opposing actions of these two proteins are well documented. Sox9 inhibits canonical Wnt/β-catenin signaling in the ovary (Chassot et al., 2008); SOX proteins are also known to interfere with β-catenin in other developmental settings (e.g., (Liu et al., 2007)). The heterodimerization of SOX9 and β-catenin proteins in chondrocyte differentiation leads to their mutual degradation (Akiyama et al., 2004; Hill et al., 2005). This β-catenin-SOX9 mutual “annihilation” may not necessarily be the only mechanism: for example, the partners of β-catenin, TCF/LEF, belong to the same HMG-domain superfamily as SOX proteins and this structural relationship hints that other scenarios (e.g. competing for a common binding partner or a DNA binding site) are also possible. Whatever the mechanism, this study finally restores the long anticipated symmetry between the two opposing pathways of sexual development by demonstrating that constitutive activation of 'the woman's part', the canonical β-catenin pathway, can completely shift the balance in favor of female development.
Fig. 3.
Sexual differentiation in mammals is driven by the opposing antagonistic activities of the transcriptional regulatory proteins: SOX9 in males and β-catenin/TCF/LEF in females.
Analysis of the Sexual Differentiation Phenotype in the Mutants with Somatic Cell Loss of β-catenin
Mammalian sex determination requires both GATA4 and FOG2 transcriptional regulators and relies on their direct physical interaction to assemble the functioning testis (Tevosian et al., 2002; Bouma et al., 2007b; Manuylov et al., 2007). A recent study now demonstrates that in gonadal development the GATA4/FOG2 transcription complex acts to control gene expression in the ovaries as well (Manuylov et al., 2008); the control of gonadal development by GATA4 and FOG2 proteins is reviewed elsewhere.
The connection between the GATA4-FOG2 transcriptional regulation and the Wnt/β-catenin pathway was revealed in a microarray experiment that profiled gene expression in Fog2-null or Gata4ki/ki mutant XX gonads at 12.5dpc. GATA4ki is a V217G mutation that specifically cripples the interaction between GATA4 and FOG proteins; the gonadal phenotypes of Fog2 null and Gata4ki/ki mutant embryos are essentially identical. Among others, the analysis of microarray data identified the Dkk1 gene as a target of GATA4/FOG2 repression in the developing ovary; the expression of Dkk1 was dramatically increased (~10 fold) in embryonic gonads with GATA4-FOG2 interaction loss (Manuylov et al., 2008). At present, a preponderance of data defines DKK1, a secreted protein, mainly within the context of the antagonism of canonical Wnt signaling (reviewed in (Niehrs, 2006; Kikuchi et al., 2007). DKK1, the founding member of the DKK family, is a potent Wnt-signaling inhibitor (Glinka et al., 1998), it binds to the LRP receptors (LRP5 or LRP6) with high affinity (Chen et al., 2008) and prevents interaction between the WNT ligands and the LRPs (Bafico et al., 2001; Mao et al., 2001; Semenov et al., 2001; Semenov et al., 2008). Dkks have not been found in invertebrates, but mice and humans have multiple Dkk genes with four main members identified in vertebrates (Dkk1-4) (Krupnik et al., 1999; Monaghan et al., 1999). The sensitivity to DKK1 inhibition overwhelmingly serves as one of the strongest proofs for canonical Wnt pathway activity in a tissue (e.g., (Liu et al., 2007)) and could be considered a trademark feature of canonical β-catenin signaling.
The observation of Axin2LacZ activity in the ovary (Fig.2), its loss in Rspo1 null genetic females, and the activation of the Dkk1 gene in the GATA4/FOG2 mutants strongly implicated a canonical β-catenin pathway in ovarian development; however, it did not constitute definitive proof. The most straightforward approach for demonstrating the contribution of canonical β-catenin signaling is a conditional excision of the β-catenin gene in the ovaries. Conditional deletion was accomplished by crossing Sf1Cre+/β-cateninflox/+ males with β-cateninflox/flox females to generate experimental animals with the Sf1Cre+/β-cateninflox/flox genotype. Loss of β-catenin has a dramatic effect on the ovarian gene expression program, with essential regulators of ovarian development - Fst, Wnt4 and Foxl2 - severely down-regulated (Manuylov et al., 2008). Still, the dimorphic gene expression program is not completely extinguished in β-catenin mutants; for example, a female-specific gene Sprr2d (Beverdam and Koopman, 2006) is expressed as normal in the β-catenin XX mutant gonad.
Fst expression is lost in the XX Wnt4 and Rspo1 null mutants while in the XY gonads with constitutively active β-catenin, Fst levels are increased. Constitutively active β-catenin is also sufficient for rescuing ovarian development in the Rspo1-null mice (Chassot et al., 2008); although Fst expression was not directly examined in the β-catenin rescued ovaries, the rescue suggests that the RSPO1 ligand likely engages β-catenin in regulating Fst expression. Moreover, regulation of a Fst promoter (Miyanaga and Shimasaki, 1993) by canonical β-catenin signaling has been previously reported in the cell culture model; mutation of the putative TCF binding site (CTTTGAT) at position −223 to −217 from the start of Fst transcription led to the abrogation of the WNT3A response (Willert et al., 2002). A conditional knockout of the β-catenin gene in the gonad results in a drastic reduction of Fst expression hence validating the essential requirement for canonical β-catenin signaling in Fst regulation in vivo (Manuylov et al., 2008).
β-catenin deletion in the ovary does not result in the activation of the alternative (male) pathway defined by the lack of expression of the male regulatory genes, Sox9, Mis/Amh and Dhh at 13.5dpc and 18.5dpc (Manuylov et al., 2008). Since the balance model (Fig.3) predicts that removing a weight ‘piece’ from one dish will produce the same effect as piling more ‘weight’ on the other, it comes somewhat as a surprise. One explanation for the observed lack of sex reversal stems from two general considerations/concerns for a conditional gene deletion experiment: the timing and the completeness of the Cre excision. In the case of the Sf1Cre-induced deletion of β̃catenin, the excision is, at the very least, extensive if not complete in the CRE-positive (Manuylov et al., 2008); timing, however, is a more difficult matter to address experimentally. The window of opportunity to tip the sex determination scales - all the way ‘down’ to the opposite sex - by removing rather than by piling up ‘weights’ could be extremely narrow. In the Sox9 gene deletion experiment (Chaboissier et al., 2004) the expression of female markers was only observed when the complete (and not conditionally targeted) XY Sox9 knockout gonads were examined by an organ culture approach that extended the viability of the samples beyond embryonic death at 11.5dpc. Analysis of the XY gonads with CRE-excised Sox9 also suggested that the male program was not terminated early enough; even when Sox9 expression was reduced to that of the wild-type ovaries, the expression of Mis and Sox8 was still higher than in the XX control (Chaboissier et al., 2004). Hence, if the authors (Chaboissier et al., 2004) were to rely exclusively on their conditional knockout they could have concluded that Sox9 deletion in the male does not result in the activation of the female program. Although a severe down-regulation of female-specific genes is observed in the β-catenin knockout (Manuylov et al., 2008), it is difficult to know whether an earlier excision would have additionally caused a sex reversal. Unfortunately, a similar organ culture “extension” experiment is not possible for β-catenin: its knock-out results in a defect in anterior-posterior axis formation and the mutants do not develop beyond 5.5dpc (Huelsken et al., 2000).
The delayed timing of a β-catenin excision can easily account for the absence of sex reversal; however, a function for β-catenin that is separate from its serving as a ‘weight’ on a sex determination ‘dish’ could be another contributing factor. While Sox9’s major (or only) gonadal function appears to be limited to the transcriptional control of sexual differentiation, β-catenin may play another role(s) in developing ovarian cells. A dramatic increase in the somatic cell death in the ovaries (but not the testis) with deleted β-catenin (Manuylov et al., 2008) undoubtedly decreases the number of progenitor cells that could otherwise have been available to undergo sex reversal and express male-specific markers.
Finally, our understanding of the interplay between the two opposing pathways is still incomplete and restricting the battle of the sexes to a duel between SOX9 and β-catenin (Fig.3) may prove premature. In the past, studies of early gonadal development in genetically modified mice relied mostly on in situ hybridizations (or immunofluorescence) to demonstrate absence or presence of expression for a particular gene/protein. Also, the conclusions with respect to sex reversal (especially in cases of embryonic lethality that precluded analysis of the adult phenotypes) were often based upon inspecting a limited number of genes for both programs; after all, our knowledge of female-specific gene expression in early development is very new. A thorough analysis in recent publications now incorporates qRT-PCRs and a more extensive marker roster to describe gonadal phenotypes; this body of work reveals a complex blend (rather than exclusive dominance) of female-specific and male-specific expression in gonads when the female program is perturbed. While it is almost certainly true that (early) Sox9 expression is required for the complete execution of the male program and testis cords formation, some male-specific gene expression occurs in the gonad in the absence of Sox9, especially if the opposing female program is compromised. Specifically, male-specific genes are activated in the Wnt4 (Vainio et al., 1999) and Rspo1 XX mutants (Chassot et al., 2008; Tomizuka et al., 2008) in the absence of detectable early Sox9 expression. Hence, it is not entirely surprising that in β-catenin XX mutants some genes normally associated with male development are up-regulated (e.g., Inha and Cyp11α1), while Sox9 is not (Manuylov et al., 2008). Similarly, some female-specific expression is retained in the β-catenin mutant (e.g., Sprr2d) indicating that, while severely compromised, the female program is not completely extinguished (Manuylov et al., 2008). It remains to be established whether these irregularities in gene expression reflect diversity in the somatic cell population in the early ovary (Yao et al., 2004; Kocer et al., 2008).
In summary, β-catenin is required for normal ovarian development. Importantly, testis differentiation in the absence of β-catenin proceeds apparently as normal (Manuylov et al., 2008); a recent work also confirms that Sertoli cell expression of β-catenin is dispensable for normal testis development (Chang et al., 2008).
Future Directions
These new exciting developments incontrovertibly point to a central role for the canonical β-catenin pathway in ovarian development; however, new questions understandably arise. Currently, our knowledge of this intricate pathway in its ovarian "adaptation" remains rudimentary and the relevant ligands (Wnts) and their receptors remain to be identified. Wnt4 has been connected to β-catenin function in the ovary (Mizusaki et al., 2003) and Wnt4 was proved capable to act in the canonical fashion (e.g. (Park et al., 2007)); however, in the absence of Wnt4, β-catenin signaling is only partially ablated (Chassot et al., 2008). RSPO1 is certainly essential for active canonical β-catenin signaling in the ovary; nevertheless, recent data suggests that RSPO proteins require (rather than replace) Wnt ligands (Kim et al., 2008). On the receiving side, identifying a relevant Fz receptor will likely prove itself difficult (given a large number of these receptors and their redundancy); however, a better characterization of the ovarian phenotype in Lrp6 mouse mutants is undoubtedly on the way. LRP6 null mutation was originally recovered in a screen for recessive lethal phenotypes in mice caused by gene trap insertions in cell-surface proteins (Pinson et al., 2000); the embryos homozygous for the insertion in Lrp6 die at birth with a variety of severe developmental abnormalities including a truncation of the axial skeleton, limb defects, microphthalmia and, most pertinently, a malformation of the female urogenital system.
As described above, β-catenin is not a DNA binding protein and normally relies on the TCF/LEF transcription factors to activate gene expression; some of the prospective gene targets for this complex in the ovary have been uncovered (see above) and more, undoubtedly, are on the way. While best understood, TCF/LEF are not the only known DNA binding protein partners for β-catenin, and other transcription factors (e.g., PITX2 or PROP1 (Kioussi et al., 2002; Olson et al., 2006; Amen et al., 2008)) can tether it to DNA; although these pathway require β-catenin they are not, strictly speaking, canonical. Interestingly, no gonadal abnormalities for TCF/LEF-deficient animals have been reported.
Another area of immediate interest is the input of the canonical β-catenin signaling pathway into the antagonism between the male and female developmental programs. Both Sox9 and Sry can interfere with β-catenin signaling, although the molecular mechanism of this inhibition remains to be understood (Bernard et al., 2008). Similarly, constitutively active ectopic β-catenin can down-regulate SOX9 protein expression in the XY gonad which makes it tempting to speculate that it may function in this same fashion in the normal female environment to oppose male development (Maatouk et al., 2008). Ultimately, provided the regulation of the β-catenin pathway in human ovarian development is conserved, this new knowledge should aid in understanding and potentially treating diseases such as gonadal dysgenesis or premature ovarian failure.
Acknowledgments
The authors would like to thank Hassina Benchabane and Yashi Ahmed for their helpful suggestions. The work is the authors’ laboratory was supported by a grant from the National Institutes of Health (NIH NICHD) to S.G.T.
Grant information: The NIH grant (HD42751)
Contributor Information
Sergei G. Tevosian, Email: sergei.g.tevosian@dartmouth.edu.
Nikolay L. Manuylov, Email: manuilov@dartmouth.edu.
References
- Adams IR, McLaren A. Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development. 2002;129:1155–1164. doi: 10.1242/dev.129.5.1155. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen M, Espinoza HM, Cox C, Liang X, Wang J, Link TM, Brennan RG, Martin JF, Amendt BA. Chromatin-associated HMG-17 is a major regulator of homeodomain transcription factor activity modulated by Wnt/β-catenin signaling. Nucleic Acids Res. 2008;36:462–476. doi: 10.1093/nar/gkm1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–1434. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- Benchabane H, Ahmed Y. In: The Adenomatous polyposis coli tumor suppressor and Wnt signaling in the regulation of apoptosis. Nathke I, McCartney B, editors. Austin, TX: Landes Bioscience; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard P, Fleming A, Lacombe A, Harley VR, Vilain E. Wnt4 inhibits β-catenin/TCF signalling by redirecting β-catenin to the cell membrane. Biol Cell. 2008;100:167–177. doi: 10.1042/BC20070072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard P, Harley VR. Wnt4 action in gonadal development and sex determination. Int J Biochem Cell Biol. 2007;39:31–43. doi: 10.1016/j.biocel.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Beverdam A, Koopman P. Expression profiling of purified mouse gonadal somatic cells during the critical time window of sex determination reveals novel candidate genes for human sexual dysgenesis syndromes. Hum Mol Genet. 2006;15:417–431. doi: 10.1093/hmg/ddi463. [DOI] [PubMed] [Google Scholar]
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas WE, 3rd, Dixon M, Hazell SA, Wagle M, Nie WS, Tomasevic N, Williams J, Zhan X, Levy MD, Funk WD, Abo A. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci U S A. 2007;104:14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma GJ, Affourtit JP, Bult CJ, Eicher EM. Transcriptional profile of mouse pre-granulosa and Sertoli cells isolated from early-differentiated fetal gonads. Gene Expr Patterns. 2007a;7:113–123. doi: 10.1016/j.modgep.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Bouma GJ, Washburn LL, Albrecht KH, Eicher EM. Correct dosage of Fog2 and Gata4 transcription factors is critical for fetal testis development in mice. Proc Natl Acad Sci U S A. 2007b;104:14994–14999. doi: 10.1073/pnas.0701677104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- Capel B. The battle of the sexes. Mech Dev. 2000;92:89–103. doi: 10.1016/s0925-4773(99)00327-5. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Pitetti JL, Papaioannou MD, Nef S. Genetic programs that regulate testicular and ovarian development. Mol Cell Endocrinol. 2007:265–266. 3–9. doi: 10.1016/j.mce.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- Chang H, Gao F, Guillou F, Taketo MM, Huff V, Behringer RR. Wt1 negatively regulates β-catenin signaling during testis development. Development. 2008;135:1875–1885. doi: 10.1242/dev.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC. Activation of β-catenin signalling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang K, Shao Y, Huang J, Li X, Shan J, Wu D, Zheng JJ. Structural insight into the mechanisms of Wnt signaling antagonism by Dkk. J Biol Chem. 2008;83:23364–23370. doi: 10.1074/jbc.M802375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman Q, Grosschedl R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr Opin Cell Biol. 1999;11:233–240. doi: 10.1016/s0955-0674(99)80031-3. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL. Genetic control of primary sex determination in mice. Annu Rev Genet. 1986;20:327–360. doi: 10.1146/annurev.ge.20.120186.001551. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila M, Prunskaite R, Naillat F, Itaranta P, Vuoristo J, Leppaluoto J, Peltoketo H, Vainio S. The partial female to male sex reversal in Wnt-4-deficient females involves induced expression of testosterone biosynthetic genes and testosterone production, and depends on androgen action. Endocrinology. 2005;146:4016–4023. doi: 10.1210/en.2005-0463. [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Huang H, He X. Wnt/β-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for β-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- Jordan BK, Mohammed M, Ching ST, Delot E, Chen XN, Dewing P, Swain A, Rao PN, Elejalde BR, Vilain E. Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet. 2001;68:1102–1109. doi: 10.1086/320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BK, Shen JH, Olaso R, Ingraham HA, Vilain E. Wnt4 overexpression disrupts normal testicular vasculature and inhibits testosterone synthesis by repressing steroidogenic factor 1/β-catenin synergy. Proc Natl Acad Sci U S A. 2003;100:10866–10871. doi: 10.1073/pnas.1834480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JS, Gao L. Irx3 is differentially up-regulated in female gonads during sex determination. Gene Expr Patterns. 2005;5:756–762. doi: 10.1016/j.modgep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Karner C, Wharton KA, Jr, Carroll TJ. Planar cell polarity and vertebrate organogenesis. Semin Cell Dev Biol. 2006;17:194–203. doi: 10.1016/j.semcdb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-Spondin2 is a secreted activator of Wnt/β-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004;7:525–534. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007;19:659–671. doi: 10.1016/j.cellsig.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- Kim KA, Wagle M, Tran K, Zhan X, Dixon MA, Liu S, Gros D, Korver W, Yonkovich S, Tomasevic N, Binnerts M, Abo A. R-spondin family members regulate the wnt pathway by a common mechanism. Mol Biol Cell. 2008;19:2588–2596. doi: 10.1091/mbc.E08-02-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, Abo A, Tomizuka K, Funk WD. R-Spondin proteins: a novel link to β-catenin activation. Cell Cycle. 2006a;5:23–26. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- Kim Y, Capel B. Balancing the bipotential gonad between alternative organ fates: a new perspective on an old problem. Dev Dyn. 2006;235:2292–2300. doi: 10.1002/dvdy.20894. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006b;4:e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Nakamura T, Murayama K, Umehara H, Yamano N, Watanabe S, Taketo MM, Nakano T. The stabilization of β-catenin leads to impaired primordial germ cell development via aberrant cell cycle progression. Dev Biol. 2006;300:545–553. doi: 10.1016/j.ydbio.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/β-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Kikuchi A. Wnt-3a and Dvl induce neurite retraction by activating Rho-associated\ kinase. Mol Cell Biol. 2004;24:4487–4501. doi: 10.1128/MCB.24.10.4487-4501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocer A, Pinheiro I, Pannetier M, Renault L, Parma P, Radi O, Kim KA, Camerino G, Pailhoux E. R-spondin1 and FOXL2 act into two distinct cellular types during goat ovarian differentiation. BMC Dev Biol. 2008;8:36. doi: 10.1186/1471-213X-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, Chang B, Duong T, Goodearl AD, Gearing DP, Sokol SY, McCarthy SA. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Asakura M, Inoue H, Nakamura T, Sano M, Niu Z, Chen M, Schwartz RJ, Schneider MD. Sox17 is essential for the specification of cardiac mesoderm in embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:3859–3864. doi: 10.1073/pnas.0609100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatouk DM, Dinapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of β-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008 doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malki S, Nef S, Notarnicola C, Thevenet L, Gasca S, Mejean C, Berta P, Poulat F, Boizet-Bonhoure B. Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. EMBO J. 2005;24:1798–1809. doi: 10.1038/sj.emboj.7600660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuylov NL, Fujiwara Y, Adameyko II, Poulat F, Tevosian SG. The regulation of Sox9 gene expression by the GATA4/FOG2 transcriptional complex in dominant XX sex reversal mouse models. Dev Biol. 2007;307:356–367. doi: 10.1016/j.ydbio.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuylov NL, Smagulova FO, Leach LL, Tevosian SG. Ovarian Development in Mice Requires the GATA4/FOG2 Transcriptional Complex. Development. 2008 doi: 10.1242/dev.024653. In press. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- McElreavey K, Vilain E, Abbas N, Herskowitz I, Fellous M. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc Natl Acad Sci U S A. 1993;90:3368–3372. doi: 10.1073/pnas.90.8.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- Menke DB, Page DC. Sexually dimorphic gene expression in the developing mouse gonad. Gene Expr Patterns. 2002;2:359–367. doi: 10.1016/s1567-133x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanaga K, Shimasaki S. Structural and functional characterization of the rat follistatin\ (activin-binding protein) gene promoter. Mol Cell Endocrinol. 1993;92:99–109. doi: 10.1016/0303-7207(93)90080-4. [DOI] [PubMed] [Google Scholar]
- Mizusaki H, Kawabe K, Mukai T, Ariyoshi E, Kasahara M, Yoshioka H, Swain A, Morohashi K. Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region\ on the X chromosome, gene 1) gene transcription is regulated by wnt4 in the female developing gonad. Mol Endocrinol. 2003;17:507–519. doi: 10.1210/me.2002-0362. [DOI] [PubMed] [Google Scholar]
- Monaghan AP, Kioschis P, Wu W, Zuniga A, Bock D, Poustka A, Delius H, Niehrs C. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech Dev. 1999;87:45–56. doi: 10.1016/s0925-4773(99)00138-0. [DOI] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled8 and LRP6 receptors and activate β-catenin-dependent gene expression. J Biol Chem. 2006;281:13247–13257. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, Parker KL, Vassalli JD. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG. Homeodomain-mediated β-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Orme MH, Giannini AL, Vivanco MD, Kypta RM. Glycogen synthase kinase-3 and Axin function in a β-catenin-independent pathway that regulates neurite outgrowth in neuroblastoma cells. Mol Cell Neurosci. 2003;24:673–686. doi: 10.1016/s1044-7431(03)00229-x. [DOI] [PubMed] [Google Scholar]
- Park JS, Valerius MT, McMahon AP. Wnt/β-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134:2533–2539. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Zhang X, He X. DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. J Biol Chem. 2008;283:21427–21432. doi: 10.1074/jbc.M800014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of β-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- Swain A, Lovell-Badge R. Mammalian sex determination: a molecular drama. Genes Dev. 1999;13:755–767. doi: 10.1101/gad.13.7.755. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development. 2002;129:4627–4634. doi: 10.1242/dev.129.19.4627. [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008;17:1278–1291. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, de RD, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, Bowles J, Mizusaki H, Koopman P. Sertoli cell differentiation is induced both cell-autonomously and through\ prostaglandin signaling during mammalian sex determination. Dev Biol. 2005;287:111–124. doi: 10.1016/j.ydbio.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GT, Gavin BJ, McMahon AP. Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol. 1994;14:6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, DiNapoli L, Capel B. Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads. Development. 2003;130:5895–5902. doi: 10.1242/dev.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]