Abstract
Despite striking differences in climate, soils, and evolutionary history among diverse biomes ranging from tropical and temperate forests to alpine tundra and desert, we found similar interspecific relationships among leaf structure and function and plant growth in all biomes. Our results thus demonstrate convergent evolution and global generality in plant functioning, despite the enormous diversity of plant species and biomes. For 280 plant species from two global data sets, we found that potential carbon gain (photosynthesis) and carbon loss (respiration) increase in similar proportion with decreasing leaf life-span, increasing leaf nitrogen concentration, and increasing leaf surface area-to-mass ratio. Productivity of individual plants and of leaves in vegetation canopies also changes in constant proportion to leaf life-span and surface area-to-mass ratio. These global plant functional relationships have significant implications for global scale modeling of vegetation–atmosphere CO2 exchange.
Enormous interspecific variation in plant traits has been observed, with growth rate, photosynthesis, respiration, leaf life-span, leaf nitrogen, and specific leaf area (SLA) (a measure of leaf surface area per unit mass) varying 10- to 100-fold among species (1–5). Such variation is thought to represent adaptations to the range of environmental conditions that occur in habitats as divergent as alpine tundra, desert, and tropical rain forest. However, if combinations of plant functional characteristics recur repeatedly in distantly related taxa across a broad geographic range, it would indicate that nature’s selective forces constrain the collective traits that exist in any species (5, 6). Here, we test the hypothesis (5, 6) that variation in plant traits is highly constrained, with all plant species having traits that fall at some point on a universal tradeoff surface. To test this hypothesis, we quantified net photosynthesis, other leaf traits, and their interrelationships in the field for 111 species of terrestrial higher plants from six biomes that vary widely in climate (Fig. 1). Leaf trait data were collected for 111 tree, shrub, and herbaceous C3 species (10–43 per region) in the following biomes: lowland tropical rain forest in Venezuela, subtropical coastal plain forest in South Carolina; montane cool temperate forest in North Carolina; desert/shrubland in New Mexico; cold temperate forest, bog, and prairie in Wisconsin; and alpine tundra/subalpine forest in Colorado. These regions differ tremendously in climate, with mean annual temperature varying from −3 to 26°C, mean annual precipitation from 220 to 3500 mm, and elevation from 4 to 3500 m. Hence, the study areas span a broad range of vegetation types and almost the entire range of climates in which terrestrial plants grow (Fig. 1).
Figure 1.
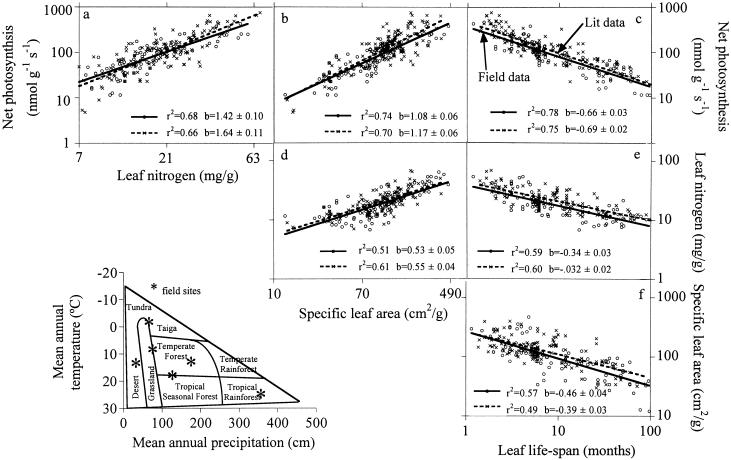
(a–f) Relations (of the form Y = aXb) among mass-based photosynthetic capacity, SLA, and leaf nitrogen concentration of young mature leaves and their expected life-span, fitted by type I regression (of log y = a + b∗log X) for species in two data sets (—o—, our field data for 111 species from six biomes; —x—, the global literature data set). The coefficients of determination (r2) and the power function (scaling) exponents (b ± 1 SE) are shown in each panel (all P < 0.001). (Inset) Diagram of global biome distribution in relation to annual temperature and precipitation (12) and location of our six field sites on that matrix.
Study species were chosen to span the range of leaf traits common in each biome (Table 1) based on a priori knowledge of the sites and their species. For young, mature leaves of each species, light-saturated net photosynthetic capacity (Amax) and leaf diffusive conductance were measured in the field (7–9) under ambient CO2 concentrations and SLA (projected leaf area per dry mass), dark respiration (at 25°C), and N concentration of those leaves were assessed, on both mass and area bases. Average leaf life-span (month = 30.4 days) was assessed for each species in the field, from long term monitoring of numerous leaves and/or phenological observation. Field data on leaf, plant, and ecosystem characteristics also were compiled from the literature for species from several continents and an equally broad array of biomes. Log (base 10) transformations of the data were required to normalize the data distribution, linearize the regression functions, and stabilize the error term variances. In addition to necessary statistical reasons, leaf trait relationships were quantified using power functions of the form log y = a + b log(x) because of their common application in biological scaling studies (5, 10, 11). Thus, the dependent variable changes as y ∝ xb. The slope (and SE) of these regressions is equivalent to the scaling exponent (and SE). Because of the large number of multiple pairwise tests of regions and the large number of leaf trait pairs, a conservative cutoff (α = 0.01) for significance was used to minimize the possibility of type I errors. Type I vs. II regression does not markedly alter the results of this study although the scaling exponent changes (11). We used multiple regression to determine if these leaf traits are consistently, quantitatively related among species, i.e., if each plant species is constrained to having a set of leaf traits that fall at some point on a multidimensional field.
Table 1.
Summary of mean leaf trait relationships (of the function Y = aXb, fitted using equations of the form log y = a + b*log X) for six diverse biomes (tropical rain forest, subtropical forest, cool temperate forest, cold temperate forest/prairie, desert, and alpine tundra/subalpine forest) represented in the field data set (all relationships P < 0.001)
| Leaf Property, Y | Mean
|
r2 (mean) | |
|---|---|---|---|
| Trait, X | Scaling slope, b(±SE) | ||
| Net photosynthesis (mass-basis) | Leaf lifespan | −0.74 ± 0.05 | 0.88 |
| Net photosynthesis (area-basis)* | Leaf lifespan | −0.29 ± 0.03 | 0.52 |
| Dark respiration | Leaf lifespan | −0.58 ± 0.05 | 0.79 |
| Leaf nitrogen concentration | Leaf lifespan | −0.32 ± 0.03 | 0.62 |
| Specific leaf area | Leaf lifespan | −0.49 ± 0.04 | 0.79 |
| Net photosynthesis (mass-basis) | SLA | 1.31 ± 0.10 | 0.88 |
| Dark respiration | SLA | 1.02 ± 0.11 | 0.80 |
| Leaf nitrogen concentration | SLA | 0.61 ± 0.07 | 0.67 |
| Leaf nitrogen content* | SLA | −0.42 ± 0.06 | 0.51 |
| Net photosynthesis (mass-basis) | Leaf nitrogen concentration | 1.73 ± 0.17 | 0.77 |
| Dark respiration | Leaf nitrogen concentration | 1.36 ± 0.11 | 0.71 |
| Net photosynthesis (mass-basis) | Dark respiration | 1.08 ± 0.09 | 0.79 |
| Units, abbreviations, and mean minimum (±SE) and maximum (±SE) for leaf traits among biomes | |||
|---|---|---|---|
| Mimimum ± SE | Maximum ± SE | ||
| Mass-based net photosynthesis (Amax) | nmol g−1s−1 | 21 ± 4 | 289 ± 64 |
| Area-based net photosynthesis | μmol m−2s−1 | 3.7 ± 0.3 | 14.6 ± 1.2 |
| Mass-based dark respiration | nmol g−1s−1 | 4.0 ± 0.3 | 35.2 ± 7.2 |
| Leaf nitrogen concentration (leaf N) | mg/g | 8.7 ± 0.6 | 40.9 ± 5.7 |
| Leaf nitrogen content | g/m2 | 1.2 ± 0.2 | 4.4 ± 1.1 |
| SLA | cm2/g | 35 ± 6 | 267 ± 62 |
| Leaf lifespan | months | 2.5 ± 0.6 | 66.2 ± 10.6 |
Relationships only shown if significant at five or six sites. The slopes did not differ significantly among biomes. The mean (±1 SE) of the slopes and of the coefficient of determination (r2) are shown (N = 6).
N = 5; data for site where relationship was not significant are not included.
In all six biomes and for all data pooled, mass-based Amax, dark respiration, SLA, and leaf N of young mature leaves consistently declined with increasing leaf life-span (as the approximate −0.7, −0.6, −0.5, and −0.3 power of leaf life-span, respectively) whereas Amax and dark respiration increased with leaf N (as ≈1.5 power) or SLA (as ≈1.2 power) (Table 1 and Fig. 1). In five of six biomes and for all data pooled, area-based net photosynthesis declined with leaf life-span, and leaf N content declined with SLA (Table 1). Otherwise, all other area-based relationships (e.g., photosynthesis–N, respiration–SLA, etc.) were not consistently significant among biomes. All further reference to leaf traits in this paper is on a mass basis, unless stated otherwise.
For the 12 relationships in Table 1, the slopes (b) were not significantly different from one biome to another (analysis of covariance, separate slopes analysis), indicating that the power function exponents relating one variable to another are similar across a broad climate and vegetation range. The possible existence of modest, but potentially important, differences among biomes in the slopes of these relationships cannot be ruled out, and sampling of a larger number of sites and a larger number of species per site would enable detection of finer differences among biomes. These data nonetheless suggest that there is a single unique relationship among each pair of leaf traits that holds generally for species within and among all biomes. Thus, there is strong and consistent interspecific variation in leaf form, function, chemistry, and longevity across diverse terrestrial ecosystems for species that have evolved separately in dramatically different climates, providing strong evidence for functional convergence.
In contrast to the similitude among slopes, the intercepts (a) of these relationships were often significantly different among biomes (analysis of covariance, same slopes analysis), but the proportion of total variation accounted for by biome was usually small. Intercept differences among biomes for all leaf pair relations in Table 1 usually were related to mean biome differences in SLA, which occur across a broad moisture gradient (P.B.R. and colleagues, unpublished work). These intercept differences would affect the absolute values of predicted leaf traits (such as photosynthesis) and hence are important. It is important to note, though, that all significant biome differences disappear when leaf traits are predicted from pairs of other leaf traits (see below).
When pooling data for the 111 species in the six biomes, we found that the relationships among pairs of leaf traits were remarkably similar to those for 170 species in a global literature data set (Fig. 1 a–f), i.e., the scaling exponents, intercepts, and closeness of fit matched well. Given the large sample size for each of these data sets and the diverse nature of the species and biomes included in each, these data provide strong evidence for the overall global generality of leaf trait relationships.
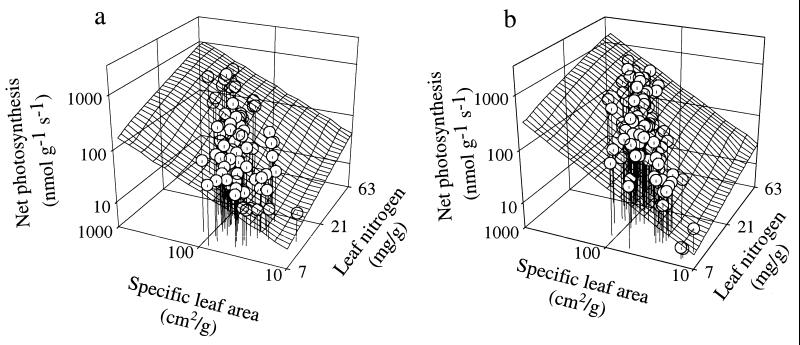
In our six-region study, “biome” was not a significant effect when biome and two or more leaf traits were included in multiple regression analyses. Thus, multiple leaf trait relationships are similar regardless of biome or climate regime. For example, predicting Amax from the combination of SLA and leaf N (Fig. 2a) effectively captures leaf structure and chemistry, not climate or biome, as the underlying universal sources of variation in Amax (8). A similar response surface occurs in the literature data (Fig. 2b) supporting the generality of these functional relationships. For our field data set, the best predictor of Amax was a model with SLA, leaf N, and leaf life-span (all P < 0.001, r2 = 0.90). We tested the generality of this model by using it to predict Amax for the species in the independent “literature” data set. The model worked well (r2 = 0.82, and the slope was not significantly different from 1:1), suggesting that there is functional interdependence among leaf photosynthesis, structure, N, and life-span that is independent of ecosystem or biome type.
Figure 2.
(a and b) Relations (all variables were log10-based) between Amax vs. leaf N and SLA using multiple regression of Amax as a function of leaf N (P < 0.001) and SLA (P < 0.001). (a) Field data from six biomes: log10 (Amax) = −0.46 + 0.77∗log10 (N) + 0.71∗log10 (SLA); r2 = 0.85, n = 104, P < 0.001. (b) Literature data: log10 (Amax) = −0.76 + 0.88∗log10 (N) + 0.82∗log10 (SLA); r2 = 0.8, n = 109, P < 0.001.
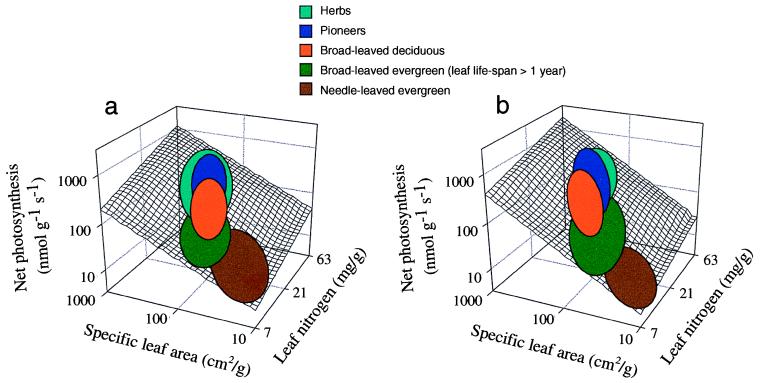
Of equal interest, nested within the global leaf trait relationships, different plant functional groups maintained constant differences in leaf traits across global gradients in climate and vegetation type (Fig. 3). Regardless of ecosystem or biome, needle-leaved evergreen conifers, broad-leaved woody evergreen species (with leaf life-span >1 year), broad-leaved woody deciduous species, pioneer tree species (evergreen or deciduous, with leaf life-span <1 year), and herbaceous species occupied consistently different positions on the multiple leaf trait (SLA, N, Amax) tradeoff surface.
Figure 3.
(a and b) Location of major plant functional groups (crossing biomes) on the three-dimensional leaf trait response surface for field data (a) and literature data (b). Major groups considered were: herbs (herbaceous species in all tundra, grassland, and forested ecosystems), pioneers (pioneer trees in boreal, temperate, and tropical forests), broad-leaved deciduous (mostly mid- to late successional temperate and tropical woody species that are deciduous; plus tropical broad-leaved evergreen tree species with leaf life-span <11 months), broad-leaved evergreen (woody broad-leaved species with leaf life-span >1 year, usually common to resource-poor environments), and needle-leaved evergreen (includes all evergreen coniferous species).
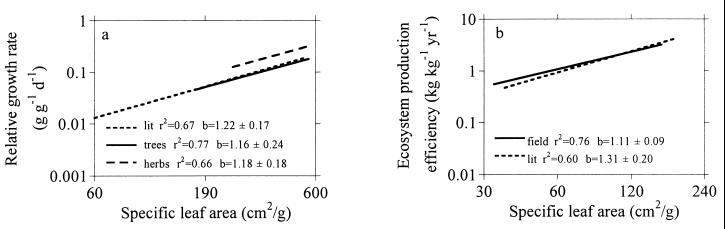
Variation in leaf traits among species also was related strongly to whole plant and ecosystem properties. Species of high SLA and Amax and short leaf life-span have high relative growth rates, and maximum relative growth rate generally scales well with these leaf traits (5, 13–16). These relationships are likely a consequence of the growth strategies of rapid vs. slow-growing plant species (5, 13, 17, 18). In three independent data sets, the slope of the relative growth rate–SLA relationship was similar (Fig. 4). SLA is important to growth because it leads to both a high leaf area displayed per unit mass invested (and hence efficient light capture) and high photosynthetic capacity (1–5, 13–16). At a stand scale, forest canopy foliage mass or area per unit ground area scales positively with leaf life-span and negatively with SLA (5, 20–22). In contrast, ecosystem production efficiency (annual productivity per unit canopy foliage mass or area) scales negatively with leaf life-span and positively with SLA in two independent data sets (Fig. 4). Thus, the maximum instantaneous productivity of a leaf (Table 1 and Fig. 1b), the potential growth rate of young whole plants, and the achieved annual aboveground production of a forest per unit canopy mass all scale positively with SLA with scaling exponents ≈1 (1.1–1.3). These results indicate that SLA and other leaf traits are important factors that contribute to the regulation of plant photosynthesis, growth, and productivity at scales from leaf to ecosystem. These traits may also be important in understanding species differences in growth responsiveness to increasing atmospheric CO2 concentration (23, 24). There are many other examples of functionally based leaf relationships similar to those shown here that are associated with important ecological patterns and processes. These include scaling of leaf traits with antiherbivore defense (17), sensitivity to ozone pollution stress (25), and species successional affinities (9, 15, 16, 26).
Figure 4.
(A) Relations (all P < 0.001) between relative growth rate of young plants and their SLA fitted by type I regression (of log Y = a + b∗log X) for species in three data sets [a literature data set of 26 woody and herbaceous species, a 24-species herbaceous data set (14), and a 9-tree species data set (19)]. log10 (Y) = 1.05 + 1.22∗log10 (SLA), r2 = 0.67, log10 (Y) = −0.73 + 1.18∗log10 (SLA), r2 = 0.66, and log10 (Y) = −0.92 + 1.16∗log10 (SLA), r2 = 0.77, respectively, for the three data sets. (B) Relations (P < 0.0001) between ecosystem production efficiency (annual aboveground net primary production/live canopy foliage dry mass) and mean canopy SLA fitted by regression (of log Y = a + b∗log X) for species in two data sets [a literature data set of 32 forest stands from a broad region (22) and a separate 48-forest stand data set from central North America (40)]. log10 (Y) = 2.33 + 1.31∗log10 (SLA), r2 = 0.60, n = 32; log10 (Y) = −1.95 + 1.11∗log10 (SLA), r2 = 0.76, n = 48, respectively.
Collectively, our data strongly suggest that there are fundamental repeatable global patterns of variation among leaf structure, longevity, metabolism, and chemistry. What selection pressures would lead to such common “solutions” among phylogenetically different groups? We hypothesize that there are interrelated constraints set by biophysics and natural selection that involve compromises in leaf structure and function because of limits on the multiple services that carbon and N can perform in a leaf. There are no species with thin, short-lived leaves and low Amax. This appears to be a poor life history strategy because low instantaneous productivity plus short duration ensures low total productivity over the leaf life-span, perhaps making it impossible to pay back construction and maintenance costs and in any case having no apparent advantages (27–29).
On the other extreme, why are there no thick, dense, and/or long-lived leaves with high mass-based leaf N, Amax, and dark respiration rates, which seems like a potentially advantageous strategy? Because of the general dependence of Amax and dark respiration on N-rich photosynthetic enzymes, pigments, and other compounds (4, 5, 13), a high allocation to leaf N can potentially provide high net CO2 exchange rates, but several constraints limit this potential for thick, dense, long-lived leaves. First, in thick and/or dense leaves, within-leaf shading can limit the amount of light reaching the chloroplasts (30), within-leaf diffusion limitations can limit the rate at which CO2 reaches the site of photosynthesis (31), and N may be allocated differently than in thin leaves; all of which may limit the effective photosynthetic use of high N concentrations (8). This would select against the combination of high N and thick, dense leaves. Second, leaves with high N are attractive nutritionally and thus subject to higher rates of herbivory, which limits their realized longevity and may select against the combination of high N and a long (genetic) potential life-span (5, 17, 18, 22). Third, species that grow fast and have high Amax would be at a disadvantage both energetically and competitively by maintaining long-lived foliage because this would impose a less than optimal allocation of resources at different positions along a shoot and limit the rate at which a stem can grow taller, which is important for light-demanding pioneer species in rapidly ascending canopies (32, 33). In contrast, slow-growing plants tend to regenerate in low light, low nutrient, or otherwise stressed environments (1–5) and may not benefit from the leaf traits that enable a high growth potential. Survival and/or the duration and efficiency of nutrient use may be more important than maximum growth potential in such microhabitats, and achieved growth may not mirror maximum growth potential in any case (9, 15, 16, 26–28). For such species, long-lived, thick leaves with low N, low Amax, and low dark respiration rates would be collectively selected (1–5).
The evidence presented in this paper for common leaf trait relationships in widely disparate biomes has important implications. It indicates global scale convergent evolution and provides a quantitative basis for evaluating inter- and intra-ecosystem species differences and for comparing among ecosystems and biomes. The discovery and use of general leaf trait relationships also should enable the development of accurate general models of vegetation productivity, distribution, and dynamics at levels of scale and for regions previously impossible (3, 34–38). Because these leaf traits are relatively easy to measure in the field and may potentially be estimated from remotely sensed data (39), collectively they provide a useful foundation for the development of scaling-based ecosystem or regional models (3, 34–38). Models based on regional-scale relationships of this kind predicted well both daily and seasonal patterns of forest CO2 flux in comparison with eddy covariance measures (36, 37). Thus, evidence of globally valid, functionally based leaf trait relationships represents an important step in making ecology a more quantitative and predictive science.
ABBREVIATIONS
- Amax
net photosynthetic capacity
- SLA
specific leaf area
Footnotes
A commentary on this article begins on page 13378.
References
- 1.Grime J P, Hunt R. J Ecol. 1975;63:393–422. [Google Scholar]
- 2.Chapin F S., III Annu Rev Ecol Syst. 1980;11:233–260. [Google Scholar]
- 3.Schulze E-D, Kelliher F M, Körner C, Lloyd J, Leuning R. Annu Rev Ecol Syst. 1994;25:629–660. [Google Scholar]
- 4.Field C, Mooney H A. In: On the Economy of Plant Form and Function. Givnish T, editor. Cambridge, U.K.: Cambridge Univ. Press; 1986. pp. 25–55. [Google Scholar]
- 5.Reich P B, Walters M B, Ellsworth D S. Ecol Monogr. 1992;62:365–392. [Google Scholar]
- 6.Reich P B. Funct Ecol. 1993;7:721–725. [Google Scholar]
- 7.Reich P B, Uhl C, Walters M B, Ellsworth D S. Oecologia. 1991;86:16–24. doi: 10.1007/BF00317383. [DOI] [PubMed] [Google Scholar]
- 8.Reich P B, Walters M B. Oecologia. 1994;97:73–81. doi: 10.1007/BF00317910. [DOI] [PubMed] [Google Scholar]
- 9.Reich P B, Ellsworth D S, Uhl C. Funct Ecol. 1995;9:65–76. [Google Scholar]
- 10.Peters R H. The Ecological Implications of Body Size. Cambridge, U.K.: Cambridge Univ. Press; 1983. [Google Scholar]
- 11.Niklas K. Plant Allometry. Chicago: Univ. of Chicago Press; 1992. [Google Scholar]
- 12.Whittaker R H. Communities and Ecosystems. 2nd Ed. New York: Macmillan; 1975. pp. 1–385. [Google Scholar]
- 13.Lambers H, Poorter P. In: Advances in Ecological Research. Begon M, Fitter A H, editors. London: Academic; 1992. pp. 187–261. [Google Scholar]
- 14.Poorter H, Remkes C. Oecologia. 1990;83:553–559. doi: 10.1007/BF00317209. [DOI] [PubMed] [Google Scholar]
- 15.Walters M B, Reich P B. Ecology. 1996;77:841–853. [Google Scholar]
- 16.Reich P B. In: Variation in Growth Rate and Productivity of Higher Plants. Lambers H, Poorter H, Van Vuuren M, editors. the Hague, the Netherlands: SPB Academic Publishing; 1998. , in press. [Google Scholar]
- 17.Coley P D, Bryant J P, Chapin F S., III Science. 1985;230:895–899. doi: 10.1126/science.230.4728.895. [DOI] [PubMed] [Google Scholar]
- 18.Coley P B. Oecologia. 1988;74:531–536. doi: 10.1007/BF00380050. [DOI] [PubMed] [Google Scholar]
- 19.Reich, P. B., Tjoelker, M. G., Walters, M. B., Vanderklein, D. & Buschena, C. (1997) Funct. Ecol., in press.
- 20.Pierce L L, Running S W, Walker J. Ecol Appl. 1994;4:313–321. [Google Scholar]
- 21.Gower S T, Reich P B, Son Y. Tree Physiol. 1993;12:327–345. doi: 10.1093/treephys/12.4.327. [DOI] [PubMed] [Google Scholar]
- 22.Reich P B, Koike T, Gower S T, Schoettle A W. In: Ecophysiology of Coniferous Forests. Smith W K, Hinckley T M, editors. San Diego: Academic; 1994. pp. 225–254. [Google Scholar]
- 23.Luo Y, Field C B, Mooney H A. Plant Cell Environ. 1994;17:1195–1204. [Google Scholar]
- 24.Roumet C, Roy J. New Phytol. 1996;134:615–621. doi: 10.1111/j.1469-8137.1996.tb04926.x. [DOI] [PubMed] [Google Scholar]
- 25.Reich P B, Amundson G R. Science. 1985;230:566–570. doi: 10.1126/science.230.4725.566. [DOI] [PubMed] [Google Scholar]
- 26.Bazzaz F A. Annu Rev Ecol Syst. 1979;10:351–371. [Google Scholar]
- 27.Kikuzawa K. Am Nat. 1991;138:1250–1263. [Google Scholar]
- 28.Aerts R. Trends Ecol Evol. 1995;10:402–407. doi: 10.1016/s0169-5347(00)89156-9. [DOI] [PubMed] [Google Scholar]
- 29.Williams L, Field C B, Mooney H A. Am Nat. 1989;133:198–211. [Google Scholar]
- 30.Terashima I, Hirosaka K. Plant Cell Environ. 1995;18:1111–1128. [Google Scholar]
- 31.Parkhurst D F. New Phytol. 1994;126:449–479. doi: 10.1111/j.1469-8137.1994.tb04244.x. [DOI] [PubMed] [Google Scholar]
- 32.Field C. Oecologia. 1983;56:341–347. doi: 10.1007/BF00379710. [DOI] [PubMed] [Google Scholar]
- 33.Ackerly D. In: Tropical Forest Plant Ecophysiology. Mulkey S S, Chazdon R L, Smith A P, editors. New York: Chapman & Hall; 1996. pp. 619–668. [Google Scholar]
- 34.Running S W, Loveland T R, Pierce L L. Ambio. 1994;23:77–81. [Google Scholar]
- 35.Running S W, Hunt E R. In: Scaling Physiological Processes: Leaf to Globe. Ehleringer J R, Field C B, editors. San Diego: Academic; 1993. pp. 141–158. [Google Scholar]
- 36.Lloyd J, Grace J, Miranda A C, Meir P, Wong S C. Plant Cell Environ. 1995;18:1129–1145. [Google Scholar]
- 37.Aber J D, Reich P B, Goulden M L. Oecologia. 1996;106:267–275. doi: 10.1007/BF00328606. [DOI] [PubMed] [Google Scholar]
- 38.DeFries R S, Field C B, Fung I, Justice C O, Los S. J Geophys Res. 1995;100:D10. , 20867–20882. [Google Scholar]
- 39.Wessman C A, Aber J D, Peterson D L, Melillo J M. Nature (London) 1988;335:154–156. [Google Scholar]
- 40.Reich P B, Grigal D F, Aber J D, Gower S T. Ecology. 1997;78:335–347. [Google Scholar]