Abstract
Objectives. We investigated whether there were socioeconomic and racial/ethnic disparities in recent reported declines in overall US breast cancer incidence rates attributed to post-2002 declines in hormone therapy use following publication of the Women's Health Initiative study.
Methods. We analyzed 1992–2005 US breast cancer incidence data from the US Surveillance, Epidemiology and End Result (SEER) 13 Registries Database, stratified by race/ethnicity, county income level, age, and estrogen receptor (ER) status.
Results. As we hypothesized, between 1992 and 2005, the temporal pattern of rising and then falling US breast cancer incidence rates occurred only among White non-Hispanic women who lived in high-income counties, were aged 50 years and older, and had ER-positive tumors. No such trends were evident—regardless of county income level, ER status, or age—among Black non-Hispanic, Asian/Pacific Islander, Hispanic, or—where numbers were sufficient to conduct meaningful analyses—American Indian/Alaska Native women.
Conclusions. The recent decline in US breast cancer incidence was not equally beneficial to all women, but instead mirrored the social patterning of hormone therapy use. Joint information on socioeconomic resources and race/ethnicity is vital for correctly understanding disease distribution, including that of breast cancer.
Since 2006, 14 population-based studies—8 American,1–8 5 European,9–13 and 1 Australian14—have documented unanticipated annual declines in breast cancer incidence of around 10%, especially among women aged 50 years and older with estrogen receptor (ER)-positive tumors. All of these investigations have attributed these declines to the dramatic reduction in use of hormone therapy15–18 following the publication, in July 2002, of the results of the Women's Health Initiative (WHI) study.19 Like the 1998 Heart and Estrogen/Progestin Replacement Study (HERS)20 and its 2002 follow-up,21 the WHI found that, contrary to expectations, hormone therapy did not decrease—and may in fact have increased—risk of cardiovascular disease, and it also confirmed that long-term use of combined estrogen plus progestin increased risk of breast cancer.
To date, however, scant research has examined whether recent reported declines in breast cancer incidence varied not only by age and ER status but also—like hormone therapy use16,18,20–26—by race/ethnicity (examined in only 3 US studies4–6) and socioeconomic position (not examined in any studies, in the United States or any other country). Within the United States, data indicate that, until 2002, use of hormone therapy was most common among more affluent, healthier, and predominantly White women (i.e., women with access to medical care, who could afford hormone therapy and who did not have contraindications against its use).16,18,20–24 Socioeconomic gradients in hormone therapy use have also been reported in 2 countries with national health systems—Britain25 and Sweden26—suggesting that access to medical care alone is not the full reason for the higher use by more affluent women.
We accordingly designed our study to test the hypothesis that the recent reported declines in US breast cancer incidence varied not only by age and ER status but also by race/ethnicity and socioeconomic position. Our study base consisted of county-level incidence data from the US Surveillance, Epidemiology and End Result (SEER) 13 Registries Database,27 which in 2000 covered 14% of the total US population.28 The time period spanned the years 1992 to 2005, thereby encompassing the initial recommendations by the Food and Drug Administration and American College of Physicians (in 1991 and 1992, respectively) for use of hormone therapy to prevent cardiovascular disease17 as well as the 1998 HERS20 and 2002 WHI study results19 and HERS follow-up results.21 Our prediction was that the sharpest post-WHI declines in breast cancer incidence would occur among White non-Hispanic women living in high-income counties, especially those aged 50 to 69 years who had ER-positive tumors.
METHODS
The study base consisted of all women with primary invasive breast cancer included in the catchment area of the public access SEER 13 Registries Database27 from January 1, 1992 through December 31, 2005, and the population from which they arose. Population denominator data (stratified by age, gender, and race/ethnicity), which were obtained from the SEER data Web site,29 consisted of US census county population estimates for the year 2000 and annual US Census intercensal county population estimates for all other years.30
The cases in the SEER 13 Registries Database are associated with census-derived population denominator data, at the county level, for the following 4 US census “racial” groups: White, Black, American Indian/Alaska Native, and Asian/Pacific Islander, each of which can additionally be stratified by Hispanic ethnicity.27 As of 2000, the percentage of the US population included in the SEER 13 Registries Database was 11.8% for Whites, 11.9% for Blacks, 21.0% for American Indians/Alaska Natives, 35.0% for Asians, 54.4% for Native Hawaiians/Pacific Islanders, and 21.8% for Hispanics.28 Because all patient data in SEER registries are obtained from medical charts, it is unknown whether breast cancer patients' racial/ethnic data were based on self-report or observer report.31 In our analyses, we employed the following 5 racial/ethnic groups: White non-Hispanic, Black non-Hispanic, American Indian/Alaska Native non-Hispanic, Asian/Pacific Islander non-Hispanic, and Hispanic.
The ER status data from SEER were likewise based on information abstracted from medical charts. For our analyses, we employed 3 categories: (1) ER positive, (2) ER negative, and (3) ER unknown (no test done; test ordered, but results not in the chart; unknown or no information about whether a test was ordered or done); we also incorporated borderline cases (< 1% of total) into the “ER unknown” category. We included the ER unknown category in our analysis because our previous work indicated that in the United States, the frequency of unknown ER status was much higher among economically deprived women than among more affluent women, and among women of color than among White women, with the latter racial/ethnic disparities accounted for by racial/ethnic inequities in socioeconomic position.32
We obtained data on the breast cancer patients' age at diagnosis from the SEER data. No data were available from the cancer registry database, however, on breast cancer patients' use of hormone therapy.
County Economic Resources
We obtained the 1999 county median household income data for all US counties from the SEER 2000 county attribute variables, which were obtained from Census SF3 data (Table P53).33 We assigned counties to quintiles of US county median household income, on the basis of the full distribution of US counties weighted by county population size, given the enormous variation in the size of county populations.34 Noting that in 1999 the US median household income was $41 944,35 we grouped the county income quintile data into 2 categories: “high income,” consisting of the top 3 county median household income quintiles (range = $40 000–$82 930), and “low income,” encompassing the bottom 2 county median household income quintiles (range = $9330–$39 999).
Statistical Analyses
For each year (1992–2005), we first computed the yearly annual age-standardized incidence rate (per 100 000) and its 95% confidence interval, stratified by county income quintile (high income vs low income), using the direct method, standardized to the year 2000 standard million.36 We calculated these rates for (1) all women, overall and stratified by race/ethnicity, and (2) all women and within each racial/ethnic group, additionally stratified by age (less than 50, 50–69, and 70 years and older) and ER status. We then depicted the observed trends by plotting these age-standardized incidence rates by year, using a 3-year moving average to provide stability to the estimates.37
To test our hypotheses regarding temporal trends in breast cancer incidence rates, we used the SEER*Stat Joinpoint 3.0 software,38 which is designed to fit joinpoint regression models. In these models, also called segmented line regression models, line segments are joined at points called “joinpoints.” When fit on the log scale, the slope of the line segments are interpretable as the annual percentage change in the rate, and the joinpoints denote statistically significant changes (P < .05) in the time trend.39,40 Of note, Joinpoint 3.0 does not require prespecification of a fixed joinpoint and instead treats the number and location of the joinpoints as unknown. The algorithm employs a grid-search method to determine the likely number and location of joinpoints, and conducts permutation tests to calculate their statistical significance.39 This approach allows for detection of joinpoints across the full time period. We consequently conducted the joinpoint regressions for each county income quintile, both for all women and stratified by race/ethnicity, and then additionally stratified by age and by ER status.
RESULTS
Table 1 presents data on the 350 075 women diagnosed with primary invasive breast cancer between 1992 and 2005 who were included in the SEER 13 Registries Database. Overall, 75% of these women were White non-Hispanic, and the rest were evenly divided among Black non-Hispanic, Asian/Pacific Islander non-Hispanic, and Hispanic women (range = 7%–9%), with only 1% identified as American Indian/Alaskan Native non-Hispanic. Nearly half were aged 50 to 69 years at diagnosis, with one third aged 70 and older; slightly over three fifths were ER positive, and equal proportions (just under one fifth) were ER negative and ER status unknown. As an indication that the population in the catchment area of the SEER 13 Registries Database resided in counties more affluent than those of the United States as a whole, only 10.5% of the cases resided in the bottom 2 US county income quintiles and 38% lived in the top US county income quintile.
TABLE 1.
Characteristics of Women With Primary Invasive Breast Cancer: US Surveillance, Epidemiology and End Result (SEER) 13 Registries Database, 1992–2005
| No. of Cases (%) | Person-Years | |
| Total | 350 075 | 535 811 082 |
| Race/ethnicity | ||
| White, non-Hispanic | 263 320 (75.2) | 313 121 603 |
| Black, non-Hispanic | 30 368 (8.7) | 57 478 375 |
| American Indian/Alaskan Native, non-Hispanic | 1 859 (0.5) | 6 267 482 |
| Asian/Pacific Islander, non-Hispanic | 25 967 (7.4) | 55 888 512 |
| Hispanic | 27 021 (7.7) | 103 055 110 |
| Age, y | ||
| < 50 | 81 946 (23.4) | 401 736 049 |
| 50–69 | 152 614 (43.6) | 91 481 819 |
| ≥ 70 | 115 515 (33.0) | 42 593 214 |
| County median household income quintile,a $ | ||
| 9 330–34 819 | 14 081 (4.1) | 11 971 039 |
| 34 820–39 999 | 22 235 (6.4) | 15 339 425 |
| 40 000–43 819 | 106 469 (30.6) | 91 560 916 |
| 43 820–51 579 | 73 976 (21.3) | 58 146 615 |
| 51 580–82 930 | 130 877 (37.6) | 94 144 351 |
| Estrogen receptor (ER) status | ||
| ER unknown | 64 951 (18.6) | |
| ER positive | 219 382 (62.7) | |
| ER negative | 64 355 (18.4) | |
| ER borderline | 1 387 (0.4) | |
Note. The 13 SEER registries in this database comprise 5 US states (Connecticut, Hawaii, Iowa, New Mexico, Utah); 6 cities and their corresponding counties (Atlanta, GA; Detroit, MI; Los Angeles, CA; San Francisco–Oakland, CA; San Jose–Monterey, CA; Seattle–Puget Sound, WA); and 2 additional areas or populations (rural Georgia; Alaska Native Tumor Registry).
Quintiles 1 and 2 were low-income households; quintiles 3–5 were high-income households.
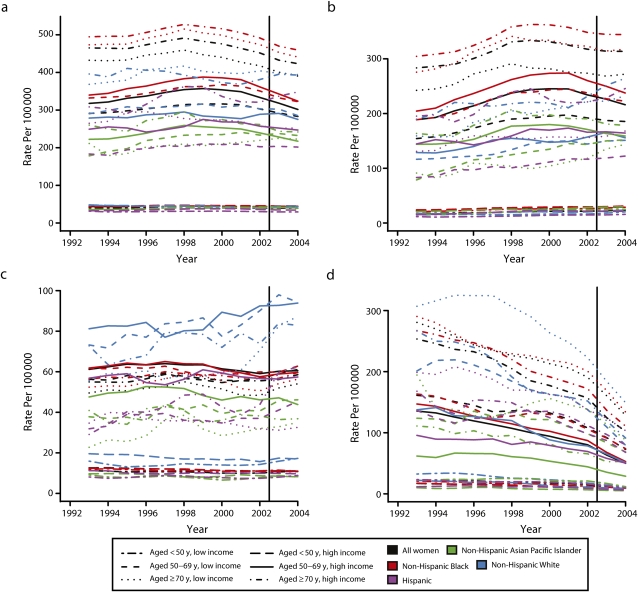
Figure 1 depicts the temporal patterning of the moving 3-year average age-standardized breast cancer incidence rates (per 100 000), stratified by county income level (high vs low), for 3 age groups: younger than 50, 50 to 69, and 70 years and older. Each figure separately portrays the trends for all women combined and for White non-Hispanic, Black non-Hispanic, Asian/Pacific Islander non-Hispanic, and Hispanic women; we do not provide a figure for the American Indian/Alaskan Native women because small numbers precluded calculating stable estimates of their incidence rates, but we do include their data in the joinpoint analyses. Figure 1a provides the results for all cases (regardless of ER status), Figure 1b for ER-positive cases, Figure 1c for ER-negative cases, and Figure 1d for cases with unknown ER status. The vertical line denotes July 2002, when the WHI results were published. As expected, Figure 1a shows that among women aged 50 years and older, incidence rates were highest among women in high-income counties, a pattern than held for all racial/ethnic groups except Black non-Hispanic women aged 70 years and older, for whom there was no discernable difference.
FIGURE 1.
Age-standardized breast cancer incidence rates (per 100 000; for all women and by race/ethnicity), stratified by county income level and age, for (a) all cases, (b) estrogen receptor (ER)-positive cases, (c) ER-negative cases, and (d) ER status unknown cases: US Surveillance, Epidemiology and End Result (SEER) 13 Registries Database, 1992–2005.
Note. Rates are based on 3-year rolling averages, standardized to the year 2000 standard million.
As also shown in Figure 1a, the groups exhibiting the strongest pattern of a rising and then falling breast cancer incidence rate were (1) all women combined, aged 50 to 69 or 70 years and older, in both high- and low-income counties; (2) White non-Hispanic women, also aged 50 to 69 or 70 years and older, in both high- and low-income counties, and (3) Asian/Pacific Islander women, aged 50 to 69 years, in high-income counties. These temporal trends were evident only among women with ER-positive tumors (Figure 1b), and not among those with ER-negative tumors (Figure 1c). Contrary to our prediction, however, the graphed data suggested that the inflection point marking the start of the decline in breast cancer incidence rates preceded 2002.
Figure 1d, in turn, shows the markedly declining incidence rate of ER-unknown tumors among women aged 50 years and older, in all racial/ethnic groups and at both county income levels. The relevance of these data are that any observed decline in ER-positive incidence rates over time would have to exceed the counterbalancing trend toward increasing incidence rates due to reassignment of cases who otherwise would have been ER unknown.32
The joinpoint analyses presented in supplemental Table 1 (overall and stratified by race/ethnicity and age, available as a supplement to the online version of this article at http://www.ajph.org) and in both Table 2 (additionally stratified by ER status for women age 50 and older for ER-positive and ER-negative tumors) and supplemental Table 2 (additionally stratified by ER status for women younger than 50 years; it also includes women aged 50 and older with ER-unknown tumors; available online at http://www.ajph.org) formally tested for the changes in slope depicted in Figures 1a–d and also quantified the annual percentage change in rates. Among all women (all racial/ethnic groups and all ages combined), the age-standardized breast cancer rates rose from 1992 to 1999 among women in both low- and high-income counties by 1.0% per year (95% confidence interval [CI] = 0.1, 1.9) and 1.4% per year (95% CI = 0.6, 2.3), respectively, after which rates declined by 2.0% per year (95% CI = −3.0, −1.0) and 2.5% per year (95% CI = −3.4, −1.6), respectively (see supplemental Table 1).
TABLE 2.
Joinpoint Analyses Showing APC in Breast Cancer Incidence Among Women Aged 50 Years and Older With Known ER Status, by County Median Household Income, Race/Ethnicity, and Age at Diagnosis: US SEER 13 Registries Database, 1992–2005
| County Median Household Income | Segment 1, APC (95% CI) | Segment 2 |
|||
| ER Status | Race/Ethnicity | Joinpoint (95% CI) | APC (95% CI) | ||
| Aged 50–69 y | |||||
| ER positive | All women | Low | 4.1 (2.1, 6.2) | 1999 (1996, 2002) | −0.7 (−2.8, 1.5) |
| ER positive | All women | High | 4.9 (3.0, 6.9) | 1999 (1998, 2002) | −2.5 (−4.3, −0.5) |
| ER positive | White, non-Hispanic | Low | 4.6 (2.3, 6.9) | 1999 (1996, 2002) | −1.5 (−4.0, 1.1) |
| ER positive | White, non-Hispanic | High | 4.6 (2.9, 6.3) | 2000 (1998, 2002) | −3.7 (−6.5, −0.9) |
| ER positive | Black, non-Hispanic | Low | 2.5 (1.5, 3.5) | ||
| ER positive | Black, non-Hispanic | High | 1.6 (0.1, 3.0) | ||
| ER positive | API, non-Hispanic | Low | 5.1 (3.4, 6.8) | ||
| ER positive | API, non-Hispanic | High | 4.6 (2.0, 7.3) | 1999 (1997, 2002) | −2.3 (−4.8, 0.3) |
| ER positive | Hispanic | Low | 3.6 (2.4, 4.8) | ||
| ER positive | Hispanic | High | 1.5 (0.4, 2.7) | ||
| ER negative | All women | Low | 0.7 (−0.1, 1.5) | ||
| ER negative | All women | High | −0.2 (−0.8, 0.3) | ||
| ER negative | White, non-Hispanic | Low | 0.1 (−0.7, 1.0) | ||
| ER negative | White, non-Hispanic | High | −0.4 (−1.1, 0.3) | ||
| ER negative | Black, non-Hispanic | Low | 2.1 (0.3, 3.9) | ||
| ER negative | Black, non-Hispanic | High | 1.2 (0.0, 2.5) | ||
| ER negative | API, non-Hispanic | Low | 1.9 (−0.5, 4.3) | ||
| ER negative | API, non-Hispanic | High | −0.9 (−2.2, 0.3) | ||
| ER negative | Hispanic | Low | 3.0 (1.0, 5.0) | ||
| ER negative | Hispanic | High | 0.2 (−1.1, 1.5) | ||
| Aged ≥ 70 y | |||||
| ER positivea | All women | Low | −2.8 (−18.1, 15.4) | 1994 (1994, 2000) | 6.8 (−9.4, 25.8) |
| ER positivea | All women | High | 1.4 (0.1, 2.7) | 1996 (1994, 1997) | 5.1 (1.3, 9.1) |
| ER positive | White, non-Hispanic | Low | 4.0 (1.2, 6.8) | 1998 (1996, 2001) | −0.8 (−2.9, 1.2) |
| ER positivea | White, non-Hispanic | High | 1.5 (0.2, 2.9) | 1996 (1994, 1997) | 5.5 (1.5, 9.7) |
| ER positive | Black, non-Hispanic | Low | 3.3 (2.1, 4.6) | ||
| ER positive | Black, non-Hispanic | High | 2.4 (1.0, 3.8) | ||
| ER positive | API, non-Hispanic | Low | 3.8 (1.5, 6.5) | ||
| ER positive | API, non-Hispanic | High | 0.7 (−0.8, 2.3) | ||
| ER positive | Hispanic | Low | 1.8 (0.6, 3.1) | ||
| ER positive | Hispanic | High | 1.9 (0.4, 3.5) | ||
| ER negative | All women | Low | 0.5 (−0.5, 1.6) | ||
| ER negative | All women | High | 0.5 (−0.2, 1.2) | ||
| ER negative | White, non-Hispanic | Low | 0.5 (−0.6, 1.2) | ||
| ER negative | White, non-Hispanic | High | 0.4 (−0.4, 1.2) | ||
| ER negative | Black, non-Hispanic | Low | 3.8 (1.9, 5.8) | ||
| ER negative | Black, non-Hispanic | High | 1.9 (−0.3, 4.2) | ||
| ER negative | API, non-Hispanic | Low | 2.2 (−2.0, 6.5) | ||
| ER negative | API, non-Hispanic | High | −0.9 (−3.2, 1.5) | ||
| ER negative | Hispanic | Low | −0.9 (−3.3, 1.6) | ||
| ER negative | Hispanic | High | 6.0 (2.4, 9.7) | ||
Note. API = Asian/Pacific Islander; APC = annual percentage change; ER = estrogen receptor; SEER = Surveillance, Epidemiology and End Result; CI = confidence interval.
Additional segments: joinpoint (95% CI) and APC (95% CI): age ≥ 70: (1) all women, low-income county, ER+: segment 3 joinpoint = 1997 (1997, 2003), APC = −0.8 (−2.4, 0.0.9); (2) all women, high-income county, ER+: segment 3 joinpoint = 1999 (1997, 2000), APC = −2.8 (−4.6, −1.0), and segment 4 joinpoint = 2003 (2000, 2003), APC = 2.6 (−1.1, 6.4); (3) White non-Hispanic, high-income county, ER+: segment 3 joinpoint = 1999 (1997, 2000), APC = −2.6 (−4.5, −0.8), and segment 4 joinpoint = 2003 (2000, 2003), APC = 3.0 (−0.9, 7.2).
These trends were driven chiefly by the rise and fall of breast cancer incidence among the White non-Hispanic women, among whom the declining rates were most pronounced among women aged 50 to 69 years in high-income counties (annual percentage change [APC] = −4.6; 95% CI = −6.4, −2.7), followed by women aged 50 to 69 years in low-income counties (APC = −4.0; 95% CI = −6.5, −1.5) and by those aged 70 years and older in both high- and low-income counties (APC between −2.6 and −2.8). By contrast, no changes in breast cancer incidence rates of any other racial/ethnic group were detected, except among the Asian/Pacific Islander women aged 50 to 69 years living in the high-income counties, whose rates likewise began to decline in 1999 (95% CI = 1997, 2001; APC = −3.4% [95% CI = −5.4, −1.3]; see supplemental Table 1).
Results additionally stratified by ER status demonstrated that only the ER-positive incidence rates among women aged 50 years and older showed a temporal pattern of rising and then falling between 1992 and 2005 (Table 2), with this pattern evident only among White non-Hispanic women with ER-positive tumors who lived in high-income counties. Among this group, whose experience drove the observed national reduction in breast cancer incidence, the decline commenced in 1999, with the post-1999 annual percentage change for women aged 50 to 69 and 70 years and older, respectively, equaling −3.7 (95% CI = −6.5, −0.9) and −2.6 (95% CI = −4.5, −0.8). No secular trends in ER-negative incidence rates were observed for any group of women, and the ongoing decline in the incidence of ER-unknown cases occurring among all groups of women notably accelerated after 2000.
DISCUSSION
Our results, based on the SEER 13 Registry Database, indicate that between 1992 and 2005, US age-standardized breast cancer incidence rates rose between 1992 and 1999 and then declined, with the fall in rates driven exclusively by trends among White non-Hispanic women aged 50 years and older who both had ER-positive tumors and resided in high-income counties. No such trends were evident—regardless of county income level, ER status, or age—among the Black non-Hispanic women, the Hispanic women, or—where numbers were sufficient to conduct meaningful analyses—among the American Indian/Alaskan Native women. These findings are in accord with our a priori hypothesis that the social patterning of US breast cancer incidence declines would mirror those of US hormone therapy use, with the sharpest declines occurring among White non-Hispanic women aged 50 to 69 years who lived in high-income counties and had ER-positive tumors. Contrary to our expectation, however, the decline in breast cancer incidence rates that we observed commenced in 1999, preceding the July 2002 publication of the WHI results.
Consideration of both study limitations and strengths lends credence to our findings. First, the lack of socioeconomic data in US cancer registry records,41,42 combined with the aggregation of the SEER 13 Registry Database to the county level,27 meant that we were constrained to conduct county-level analyses, using only county-level income data. Despite well-known problems affecting analyses using only group-level (“ecologic”) data,43 research nevertheless indicates that similar patterns of socioeconomic inequities in health, including in cancer incidence, have been detected through use of socioeconomic measures at the county, census tract, household, and individual level.37,44–46 Second, offsetting concerns that results could be biased by racial/ethnic misclassification, a recent large multisite SEER validation study, based on
self-reported data among 13,538 cancer patients diagnosed between 1973–2001 in the SEER-National Longitudinal Mortality Study linked database,
reported that the
overall agreement was excellent on race (κ = 0.90, 95% CI = 0.88, 0.91)” and “moderate to substantial on Hispanic ethnicity (κ = 0.61, 95% CI = 0.58, 0.64).31(p177)
Although the SEER results31 suggest that misclassification of race/ethnicity among Hispanics could lead to underestimates of incidence rates for them, it is unlikely that such misclassification would be linked to hormone therapy use, and so would be unlikely to bias results. Finally, although it would have been ideal to have had access to a large, longitudinal, representative US study cohort with detailed data—for both the women in the study and the population from which they arose—on lifetime socioeconomic position, race/ethnicity, nativity, hormone therapy use and other breast cancer risk factors (e.g., reproductive history),24,47 to our knowledge no such database exists. This limitation is offset by the fact that our study had access to records spanning the period 1992 to 2005 for over 350 000 cases and the catchment populations for 13 large US cancer registries, thereby enabling us to look meaningfully at patterns by age, socioeconomic position, race/ethnicity, and ER status.
Adding additional plausibility to our findings and their interpretation, literature addressing the observed recent declines in US, European, and Australian breast cancer incidence rates has discussed at length why evidence indicates that these secular trends are not explained by declines in breast cancer detection or changes in other risk factors (e.g., body mass index), and they likewise have not offered any other additional competing hypotheses or evidence to explain these declines.1–14,48–50 Concomitantly, US data provide strong evidence that before the WHI, hormone therapy use was substantially lower among women with fewer versus more socioeconomic resources, and also among US Black, Latina, and Asian women compared with White women.23,24,51,52 Moreover, research on the role of steroids—including hormone therapy—as breast cancer tumor promoters renders biologically plausible a short lag time between cessation of hormone therapy exposure and a decline in risk of developing a detectable incident breast cancer.3,53,54 It is not our purpose to repeat these discussions here. Instead, we would like to emphasize 3 new contributions that our analyses provide.
First, our inclusion of socioeconomic data, in conjunction with race/ethnicity, age, and ER status, revealed that the pattern of a recent rise and fall in US breast cancer incidence has been restricted solely to women who at time of diagnosis resided in a high-income county, were White, were aged 50 years and older, and had ER-positive tumors. These findings bolster the hypothesis that declines in hormone therapy use led to declines in breast cancer incidence among the sociodemographic groups of women most likely to be prescribed and to use hormone therapy, since no other hypotheses have been advanced that would explain the sociodemographic and tumor-type specificity of the incidence patterns we report. Additionally, the results underscore the insufficiency of conventional US racialized approaches to analyzing cancer and other health data in relation only to race/ethnicity55,56; instead, joint information on socioeconomic resources and race/ethnicity is vital for correctly understanding disease distribution, including that of cancer.34,37,41,55,56
Second, by also including data on incidence rates of tumors with unknown ER status, our study newly indicates that estimates of declines in the incidence of ER-positive breast cancer based only on observed data are likely to be underestimates. This is because the observed, and especially older, ER-positive rates fail to include those cases with unknown ER status that would otherwise have been characterized as ER positive had the data been available. Indeed, on the basis of our previous research investigating the impact of imputing missing ER status on racial/ethnic and socioeconomic disparities in risk of ER-positive and ER-negative tumors, it is conceivable that rates of ER-positive breast cancer among White non-Hispanic women living in affluent areas in the early to mid-1990s (when a higher proportion of cases had ER status unknown) could have been underestimated by as much as 15%.32 The net implication is that the actual secular decline in breast cancer incidence among these women is larger than has been reported on the basis of the observed data.
Third, our results indicate that the lesser access to hormone therapy among women subjected to socioeconomic deprivation and among women of color, initially considered a problem before the WHI results were reported,23,24,51,52 may in fact have spared them iatrogenic increases in their breast cancer incidence rates. The magnitude of increases and declines in breast cancer incidence observed among women in those sectors of society who were most likely to have been exposed to hormone therapy in turn underscores the dangers of inadequately understood pharmacological manipulation of complex hormonal systems22,57–59—a caution that ought be kept in mind when considering past and present proposals to prevent breast cancer by administering regimens of powerful hormones to healthy young women.60–63
Finally, one additional question highlighted by our results concerns why the decline in US breast cancer incidence rates—including those among women aged 50 to 69 years with ER-positive tumors who lived in high-income counties—began in 1999, before publication of the WHI results in 2002. Of note, our findings are in accord with those of other studies reporting that use of hormone therapy in the United States peaked in 1999 and 200016,17,21,48,64 and that US breast cancer incidence rates began to decline starting in the period 1999 to 2001,4,7,8,49 albeit with the rate of decline accelerating after the WHI study.3,6–8 Together, these findings lend credence to the hypothesis that the 1998 HERS results played more of a role than previously appreciated in reducing physicians' willingness to prescribe—and women's willingness to use—hormone therapy.4,16,17 Better understanding of why hormone therapy use peaked between 1999 and 2001, not only in the United States15,17,64 but also the United Kingdom,13 Norway,11 and Australia,14 would be useful in preventing future iatrogenic illness; any such analysis will likely necessitate a longer-term historical perspective attuned to societal determinants of health.22,34,57,65,66
Acknowledgments
This project was funded by the National Institutes of Health (grant 5R03CA132131).
Human Participant Protection
Use of the de-identified public access county-level cancer registry data and US county census population data analyzed in this study was approved as exempt by the Harvard School of Public Health Human Subjects Committee (HSC protocol P14605-101).
References
- 1.Clarke CA, Glaser SL, Uratsu CS, Selby JV, Kushi LH, Herrinton JL. Recent declines in hormone therapy utilization and breast cancer incidence: clinical and population-based evidence. J Clin Oncol. 2006;24(33):e49–e50 [DOI] [PubMed] [Google Scholar]
- 2.Ravdin PM, Cronin KA, Howlader N, Chlebowski RT, Berry DA. A sharp decrease in breast cancer incidence in the United States in 2003. Presented at: 29th annual San Antonio Breast Cancer Symposium; December 14–17, 2006; San Antonio, TX: Available at: http://www.sabcs.org. Accessed February 8, 2007 [Google Scholar]
- 3.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356(16):1670–1674 [DOI] [PubMed] [Google Scholar]
- 4.Clarke CA, Glaser SL. Declines in breast cancer after the WHI: apparent impact of hormone therapy. Cancer Causes Control. 2007;18(8):847–852 [DOI] [PubMed] [Google Scholar]
- 5.Hausauer AK, Keegan THM, Chang ET, Clarke CA. Recent breast cancer trends among Asian/Pacific Islander, Hispanic, and African-American women in the US: changes by tumor subtype. Breast Cancer Res. 2007;9(6):R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart SL, Sabatino SA, Foster SL, Richardson LC. Decline in breast cancer incidence—United States, 1999–2003. MMWR Morb Mortal Wkly Rep. 2007;56(22):549–553 [PubMed] [Google Scholar]
- 7.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among US women. Breast Cancer Res. 2007;9(3):R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass G, Lacey JV, Jr, Carreon JD, Hoover RN. Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening, mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99(15):1152–1161 [DOI] [PubMed] [Google Scholar]
- 9.Bouchardy C, Morabia A, Verkooijen HM, Fioretta G, Wespi Y, Schäfer P. Remarkable change in age-specific breast cancer incidence in the Swiss canton of Geneva and its possible relation with the use of hormone replacement therapy. BMC Cancer. 2006;6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katalinic A, Rawal R. Decline in breast cancer incidence after decrease in utilization of hormone replacement therapy. Breast Cancer Res Treat. 2008;107(3):427–430 [DOI] [PubMed] [Google Scholar]
- 11.Kumle M. Declining breast cancer incidence and decreased HRT use. Lancet. 2008;372(9639):608–610 [DOI] [PubMed] [Google Scholar]
- 12.Verkooijen HM, Koot VCM, Fioretta G, et al. Hormone replacement therapy, mammography screening and changing age-specific incidence rates of breast cancer: an ecological study comparing two European populations. Breast Cancer Res Treat. 2008;107(3):389–395 [DOI] [PubMed] [Google Scholar]
- 13.Parkin DM. Is the recent fall in incidence of post-menopausal breast cancer in UK related to changes in use of hormone replacement therapy? Eur J Cancer. 2009;45(9):1649–1653 [DOI] [PubMed] [Google Scholar]
- 14.Canfell K, Banks E, Moa AM, Beral V. Decrease in breast cancer incidence following a rapid fall in use of hormone therapy in Australia. Med J Aust. 2008;188(11):641–644 [DOI] [PubMed] [Google Scholar]
- 15.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53 [DOI] [PubMed] [Google Scholar]
- 16.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140(3):184–188 [DOI] [PubMed] [Google Scholar]
- 17.Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Prev Med. 2005;118(12 suppl. 2):64S–73S [DOI] [PubMed] [Google Scholar]
- 18.Wei F, Miglioretti DL, Connelly MT, et al. Changes in women's use of hormones after the Women's Health Initiative estrogen and progestin trial by race, education, and income. J Natl Cancer Inst Monograph. 2005;35:106–112 [DOI] [PubMed] [Google Scholar]
- 19.Rossouw RE, Anderson GL, Prentice RL, et al. Risk and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333 [DOI] [PubMed] [Google Scholar]
- 20.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–613 [DOI] [PubMed] [Google Scholar]
- 21.Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy. Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288(1):49–57 [DOI] [PubMed] [Google Scholar]
- 22.Krieger N, Löwy I, Aronowitz R, et al. Hormone replacement therapy, cancer, controversies, and women's health: historical, epidemiological, biological, clinical and advocacy perspectives. J Epidemiol Community Health. 2005;59(9):740–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman-Koss D, Crespo CJ, Bellantoni MF, Andersen RE. The relationship of race/ethnicity and social class to hormone replacement therapy: results from the Third National Health and Nutrition Examination Survey 1988–1994. Menopause. 2002;9(4):264–272 [DOI] [PubMed] [Google Scholar]
- 24.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–448 [DOI] [PubMed] [Google Scholar]
- 25.Lawlor DA, Davey Smith G, Ebrahim S. Socioeconomic position and hormone replacement therapy use: explaining the discrepancy in evidence from observation and randomized controlled trials. Am J Public Health. 2004;94(12):2149–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persson I, Bergkvist L, Lindgren C, Yuen J. Hormone replacement therapy and major risk factors for reproductive cancers, osteoporosis, and cardiovascular diseases: evidence of confounding by exposure characteristics. J Clin Epidemiol. 1997;50(5):611–618 [DOI] [PubMed] [Google Scholar]
- 27.Surveillance Epidemiology and End Results (SEER) Program Limited-Use Data (1973–2005), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on the November 2007 submission. Available at: http://seer.cancer.gov/data/index.html. Accessed August 3, 2009
- 28.Surveillance Epidemiology and End Results (SEER) Program Number of persons by race and Hispanic ethnicity for SEER participants (2000 census data). Available at: http://seer.cancer.gov/registries/data.html. Accessed August 3, 2009
- 29.Surveillance Epidemiology and End Results (SEER) Program SEER*Stat Database: Populations—Total US (1969–2005) <Single Ages to 85+, Katrina/Rita Adjustment>—Linked To County Attributes—Total US 1969–2005 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released February 2008. Available at: http://seer.cancer.gov/data/index.html. Accessed August 3, 2009
- 30.US Census Bureau Population estimates. Available at: http://www.census.gov/popest/estimates.php. Accessed August 3, 2009
- 31.Clegg LX, Reichman ME, Hankey BF, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18(2):177–187 [DOI] [PubMed] [Google Scholar]
- 32.Krieger N, Chen JT, Ware JH, Kaddour A. Race/ethnicity and breast cancer estrogen receptor status: impact of class, missing data, & modeling assumptions. Cancer Causes Control. 2008;19(10):1305–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surveillance Epidemiology and End Results (SEER) Program County attributes. Available at: http://seer.cancer.gov/seerstat/variables/countyattribs/index.html#ca2000. Accessed August 3, 2009
- 34.Krieger N, Rehkopf DH, Chen JT, Waterman PD, Marcelli E, Kennedy M. The fall and rise of US inequities in premature mortality: 1960–2002. PLoS Med. 2008;5(2):e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.US Census Bureau American fact finder. Available at: http://factfinder.census.gov/home/saff/main.html?_lang=en. Accessed August 3, 2009.
- 36.Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998;37(3). [PubMed] [Google Scholar]
- 37.Singh GK, Miller BA, Hankey BF, Edwards BK. Area Socioeconomic Variations in US Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975–1999. Bethesda, MD: National Cancer Institute; 2003. NIH publication 03-5417. NCI Cancer Surveillance Monograph Series No. 4 [Google Scholar]
- 38.Surveillance Research Program National Cancer Institute SEER*Stat software, version 3.0. Available at: http://seer.cancer.gov/seerstat/index.html. Accessed August 3, 2009
- 39.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351 [DOI] [PubMed] [Google Scholar]
- 40.Kim H-J, Fay MP, Yu B, Barrett MJ, Feuer EJ. Comparability of segmented line regression models. Biometrics. 2004;60(4):1005–1014 [DOI] [PubMed] [Google Scholar]
- 41.Krieger N, Chen JT, Ebel G. Can we monitor socioeconomic inequalities in health? A survey of US Health Departments' data collection and reporting practices. Public Health Rep. 1997;112(6):481–491 [PMC free article] [PubMed] [Google Scholar]
- 42.Krieger N, Chen JT, Waterman PD, Soobader M-J, Subramanian SV, Carson R. Geocoding and monitoring US socioeconomic inequalities in mortality and cancer incidence: does choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156(5):471–482 [DOI] [PubMed] [Google Scholar]
- 43.Subramanian SV, Jones K, Kaddour A, Krieger N. Revisiting Robinson: the perils of individualistic and ecologic fallacy. Int J Epidemiol. 2009;38(2):342–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rehkopf DH, Haughton L, Chen JT, Waterman PD, Subramanian SV, Krieger N. Monitoring socioeconomic disparities in death: comparing individual-level education and area-based socioeconomic measures. Am J Public Health. 2006;96(12):2135–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Public Health. 1996;17:47–67 [DOI] [PubMed] [Google Scholar]
- 48.Chlebowski RT, Kuller LH, Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer incidence by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56(3):168–183 [DOI] [PubMed] [Google Scholar]
- 50.Roberts H. Reduced use of hormones and the drop in breast cancer [editorial]. BMJ. 2009;338:b2116. [DOI] [PubMed] [Google Scholar]
- 51.Stafford RS, Saglam D, Causino N, Blumenthal D. The declining impact of race and insurance status on hormone replacement therapy. Menopause. 1998;5(3):140–144 [PubMed] [Google Scholar]
- 52.Brett KM, Madans JH. Difference in use of postmenopausal hormone replacement therapy by Black and White women. Menopause. 1997;4(2):66–70 [Google Scholar]
- 53.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–282 [DOI] [PubMed] [Google Scholar]
- 54.Cordera F, Jordan VC. Steroid receptors and their role in the biology and control of breast cancer growth. Semin Oncol. 2006;33(6):631–641 [DOI] [PubMed] [Google Scholar]
- 55.Krieger N, Emmons K, Williams D. Defining, investigating, and addressing cancer inequities: critical issues. : Koh H, Toward the Elimination of Cancer Disparities: A Clinical and Public Health Perspective. New York, NY: Springer; 2009:3–28 [Google Scholar]
- 56.Trans-HHS Cancer Health Disparities Progress Review Group Making Cancer Health Disparities History. Submitted to the Secretary, US Department of Health and Human Services, January 2004. Available at: http://www.hhs.gov/chdprg. Accessed February 8, 2007
- 57.Krieger N. Hormone therapy and the rise and perhaps fall of US breast cancer incidence rates: critical reflections. Int J Epidemiol. 2008;37(3):627–637 [DOI] [PubMed] [Google Scholar]
- 58.Seaman B. The Greatest Experiment Ever Performed on Women: Exploding the Estrogen Myth. New York, NY: Hyperion; 2003 [Google Scholar]
- 59.Petitti D. Commentary: hormone replacement therapy and coronary heart disease: four lessons. Int J Epidemiol. 2004;33(3):461–463 [DOI] [PubMed] [Google Scholar]
- 60.Henderson BE, Ross RK, Pike MC. Hormonal chemoprevention of cancer in women. Science. 1993;259(5095):633–638 [DOI] [PubMed] [Google Scholar]
- 61.Pike MC, Spicer DV. Hormonal contraception and chemoprevention of female cancers. Endocr Relat Cancer. 2000;7(2):73–83 [DOI] [PubMed] [Google Scholar]
- 62.de Waard F, Thijssen JH. Hormonal aspects in the causation of human breast cancer: epidemiological hypotheses reviewed, with special reference to nutritional status and first pregnancy. J Steroid Biochem Mol Biol. 2005;97(5):451–458 [DOI] [PubMed] [Google Scholar]
- 63.Tsubura A, Uehara N, Matsuoka Y, Yoshizawa K, Yuri T. Estrogen and progesterone treatment mimicking pregnancy for protection from breast cancer. In Vivo. 2008;22(2):191–201 [PubMed] [Google Scholar]
- 64.Wysowski DK, Governale LA. Use of menopausal hormones in the United States, 1992 through June, 2003. Pharmacoepidemiol Drug Saf. 2005;14(3):171–176 [DOI] [PubMed] [Google Scholar]
- 65.Kunitz S. The Health of Populations: General Theories and Particular Realities. Oxford, England: Oxford University Press; 2006 [Google Scholar]
- 66.Krieger N. Ways of asking and ways of living: reflections on the 50th anniversary of Morris' ever-useful Uses of Epidemiology. Int J Epidemiol. 2007;36(6):1173–1180 [DOI] [PubMed] [Google Scholar]