Abstract
Objectives. Our community–academic partnership employed community-based participatory research to develop and pilot a simple, peer-led intervention to promote weight loss, which can prevent diabetes and eliminate racial/ethnic disparities in incident diabetes among overweight adults with prediabetes.
Methods. We recruited overweight adults at community sites, performed oral glucose tolerance testing to identify persons with blood glucose levels in the prediabetes range, and randomized eligible people to a peer-led lifestyle intervention group or delayed intervention in 1 year. Outcomes, including weight, blood pressure, and health behaviors, were measured at baseline and 3, 6, and 12 months.
Results. More than half of those tested (56%, or 99 of 178) had prediabetes and enrolled in the study. Participants were predominantly Spanish-speaking, low-income, undereducated women. The intervention group lost significantly more weight than the control group and maintained weight loss at 12 months (7.2 versus 2.4 pounds; P < .01). One fourth (24 of 99) of participants progressed to diabetes.
Conclusions. In underserved minority communities, prediabetes prevalence may be higher than previously reported. Low-cost, community-based interventions can succeed in encouraging weight loss to prevent diabetes.
Despite compelling evidence that diabetes is escalating in the United States and that promoting weight loss can mitigate its rise, implementation of effective, sustainable diabetes prevention interventions has been slow and sporadic.1–4 More than 1 in 8 American adults have diabetes.5 Blacks and Hispanics are hit hardest by this epidemic: diabetes mortality for these groups is nearly double that of Whites.6–8 Half of Hispanic and nearly half of Black children born in the first decade of this century will develop diabetes if adequate preventive measures are not taken.9
To date, the most effective diabetes prevention strategy entails identifying people with prediabetes and implementing lifestyle changes leading to modest weight loss.10–13 Prediabetes is defined as impaired fasting glucose (100–125 mg/dL) or impaired glucose tolerance (140–199 mg/dL) after a 75-g glucose load.14 Thirty percent of Americans have prediabetes, more than double the prevalence of diabetes.5 People with prediabetes have an annual 10% progression to diabetes, and up to 70% develop diabetes.9,10 They also have an increased risk of cardiovascular disease (250%) and all-cause mortality (50%).15
Weight loss prevents or delays diabetes among persons with prediabetes: a 33% to 68% reduction in incident diabetes has been observed with 5% to 10% weight loss.10,12,13,15–19 Blacks and Hispanics benefit more from lifestyle interventions than do Whites, and disparities in incident diabetes between these groups are eliminated with weight loss.10 However, most successful interventions are resource intensive and are rarely sustainable or scalable for the enormous population at risk, particularly in communities of color, which are hardest hit by diabetes. With few exceptions,20 diabetes prevention studies in real-world settings are small, nonrandomized, resource intensive, clinically based, and unable to generate sustainable weight loss.21–28 These results starkly illustrate the need for new approaches to stem the diabetes epidemic.
In 2005, community and academic partners in East Harlem in New York City, New York, came together to write a grant to address local health disparities. Once funded, a Community Action Board, comprising 20 leaders, activists, and residents (15 of the 20 members were residents), conducted local assessments and chose to focus on diabetes prevention. Their choice was made in part because the population of East Harlem is the poorest and most obese in Manhattan, and its adults have the highest diabetes prevalence and mortality rates in the city.29,30 As a pastor on our board said, “I feel like the people in our community are walking toward a cliff, and we need to join together, put our arms around them and pull them back.”
The board used a community-based participatory research (CBPR) approach to develop and pilot a randomized controlled trial to measure the effectiveness of a peer-led lifestyle intervention (Project HEED, or Help Educate to Eliminate Diabetes) in promoting weight loss among overweight adults with prediabetes in East Harlem. Here we describe the implementation of Project HEED's pilot, its results, and participants' perspectives on their experiences in the trial.
METHODS
The board formed 5 subcommittees to develop a community-driven, culturally appropriate, scientifically sound diabetes prevention intervention to benefit East Harlem residents. The Community Engagement Subcommittee developed and implemented a social marketing campaign to promote diabetes prevention, reviewed recruitment materials, developed the participant incentive strategy, and built partnerships with local leaders and activists.
The Evaluation and Policy Subcommittee developed data collection tools and procedures. They reviewed existing surveys and the board's conceptual model, which depicted factors that influence diabetes development, and chose validated scales that were supplemented with board-developed questions to assess knowledge, attitudes, and behaviors related to diabetes prevention.31–34 Validated food frequency questionnaires and some board-developed questions assessed diet,35–38 and the Global Physical Activity Questionnaire assessed physical activity.39 To enhance responses, we supplemented the survey questions with food models and pictures of leisure-time activities. The survey, targeted to a fourth-grade reading level, was translated into Spanish and back-translated before the pilot intervention in the community began. The survey used the 2000 Census definitions of race and ethnicity.40 The subcommittee also chose to conduct interviews and focus groups with participants after the intervention.
The Intervention Subcommittee reviewed existing health education programs that have a theoretical background and show promising results.31,41–43 The group developed criteria for the intervention: be culturally sensitive; empower, educate, and motivate participants to eat healthy and be more active; inform participants about prediabetes and diabetes prevention; give control to community members; and be sustainable in community settings. The subcommittee chose to modify Healthy Eating Active Lifestyles, a derivative of the Chronic Disease Self-Management Program,44–46 a peer-led education program developed by Harlem residents and local weight loss experts, with promising pilot results.31
Project HEED's curriculum followed self-efficacy theory47,48; contained simple, actionable messages; was easily taught by lay leaders; and focused on enhancing self-efficacy to make lifestyle changes. It was presented in a workshop consisting of eight 1.5-hour sessions over 10 weeks. Topics included diabetes prevention, finding and affording healthy foods, label reading, fun physical activity, planning a healthy plate, making traditional foods healthy, and portion control. We reviewed the curriculum with scientific and peer education experts, tested it with English (n = 6) and Spanish (n = 12) speakers, and revised accordingly.
The Latino Education Subcommittee reviewed all study materials to ensure that the content was appropriate and accurate for the spectrum of Spanish speakers in East Harlem. The Clinician Education Subcommittee developed a tool kit to educate primary care clinicians about prediabetes and the study. The tool kits contained educational materials, a laminated card illustrating fasting and postprandial prediabetes and diabetes glucose levels, and a form to refer patients to the study. These kits were mailed to more than 350 local clinicians.
Recruitment and Implementation
The board developed several recruitment strategies that members of the board and study personnel implemented at community sites and events, such as churches, social service agencies, senior centers, and health fairs. The most successful recruitment (accounting for 68% of participants) took place when community leaders championed the study and spearheaded recruitment at their organizations.49
Recruitment occurred in 2 phases between May and July 2007. In phase 1, we screened for eligibility. Individuals were eligible if they were aged 18 years or older, resided in East Harlem, spoke English or Spanish, were overweight (measured body mass index [BMI; defined as weight in kilograms divided by height in meters squared] ≥ 25 kg/m2), were not currently pregnant, did not have diabetes, did not use glucose-altering medications, and were able to participate in a group session. Individuals meeting these criteria gave written informed consent and were asked to return while fasting for an oral glucose tolerance test on another morning.
In phase 2, we used finger sticks to obtain fasting glucose levels measured with Accu-chek glucometers (Roche, Nutley, NJ) that were calibrated daily. Participants with nondiabetes glucose levels (< 126 mg/dL) drank a 75-g glucose load and had a finger stick 2 hours later. Trained staff measured weight (without shoes, in the morning while fasting) with a Siltec PS500L scale (Precision Weighing Balances, Bradford, MA). Blood pressure (in the nonprimary arm) and waist circumference (1 inch above the umbilicus) were measured twice and the readings averaged. The staff also administered the survey, which lasted approximately 30 minutes. Participants with normal glucose levels were informed that they were ineligible for the study and given information on weight loss. Those with diabetes-level glucose readings were referred to local health care providers for follow-up. The remainder, who had glucose levels in the prediabetes range, had venous blood drawn for hemoglobin A1c (HbA1c) and serum cholesterol levels. The board requested an additional tube of blood to be drawn from participants who consented, to be banked for future research, which would be contingent on the board approving proposals presented by researchers.
Participants were randomized to intervention or delayed intervention (in 1 year) by blocked randomization (block size = 4) by recruitment site. Intervention participants attended the workshop at community sites, often where recruitment occurred, between July 2007 and February 2008. Both groups received brief verbal and written information about prediabetes and results of all their screening tests, with a copy to take home that they could also share with their clinicians. The team repeated all measurements at 3, 6, and 12 months after enrollment. Participants received a $50 gift card and lunch at each follow-up.
Data Analysis and Follow-up
In this intention-to-treat analysis with weight as our primary outcome, we used a last-observation-carry-forward strategy to impute missing weights at follow-up. We compared participants' self-reported demographic characteristics at baseline and performed bivariate comparisons with t tests, χ2 tests, and analysis of variance. We assessed changes in participants' weights and behaviors between baseline and 12 months with paired t tests. We used SAS version 9.1.3 (SAS Institute Inc, Cary, NC) and defined statistical significance at .05.
We invited 93 control and intervention participants (6 withdrew at 12 months) to share their thoughts and experiences about the study in focus groups and interviews. Participants were separated by trial arm and asked about their reasons for participating and their reactions to recruiting, screening, and the intervention itself. The board wrote an interview guide, which was followed by experienced moderators. Audiotapes were transcribed and, when appropriate, translated. A community coinvestigator and a board member developed themes, coded groups, and compared results to calculate interrater reliability.
RESULTS
Over 3 months, we approached 555 people for preconsent screening, obtained consent from 249 (45% of those approached), and performed 178 oral glucose tolerance tests (71% of those who consented). More than half (58%; n = 103) of participants had prediabetes-level glucose readings. Only a minority (29%) had normal glucose levels, and 13% had diabetes-range levels (Figure A, available as an online supplement to this article at www.ajph.org). Participants with normal glucose levels were typically younger (P < .01), less overweight (P < .05), and less likely to report a family history of diabetes (P = .05) than were participants with elevated blood sugars.
There were no statistically significant differences between the intervention (n = 50) and control (n = 49) participants at baseline in demographic characteristics, anthropometric measures, or behaviors, except that intervention participants drank significantly more juice (Table 1). Participants had a mean age of 48 years (range = 25–84 years), were predominantly female (85%), Hispanic (89%), Spanish speaking (77%), unemployed (70%), uninsured (49%), low income (62% were below the poverty level50), and undereducated (58% had not graduated from high school). Many reported hypertension (31%), hyperlipidemia (25%), food insufficiency (25%), depressive symptoms (49%), and a family history of diabetes (43%). All participants were overweight (BMI ≥ 25 kg/m2), with 56% obese (BMI = 30–39 kg/m2) and 6% morbidly obese (BMI ≥ 40 kg/m2). Their mean HbA1c level was 5.6 (5.5–6.0 is considered prediabetes range).51,52
TABLE 1.
Baseline Characteristics of Enrolled Participants: Project HEED, East Harlem, New York City, May 2007–August 2008
| Total (N = 99), % or Mean (SD) | Control Group (n = 49), % or Mean (SD) | Intervention Group (n = 50), % or Mean (SD) | Pa | |
| Age, y | 48 (16.5) | 50 (18) | 46 (15) | .28 |
| Female | 85 | 84 | 86 | .75 |
| Race/ethnicity | ||||
| Black | 9 | 6 | 12 | .39 |
| Hispanic | 89 | 92 | 86 | .39 |
| Spanish speaking only | 77 | 82 | 72 | .34 |
| Education < high school | 58 | 61 | 54 | .72 |
| Unemployed | 70 | 73 | 66 | .08 |
| Uninsured | 49 | 49 | 50 | .58 |
| Difficulty accessing medical care | 28 | 27 | 30 | .47 |
| Food insufficiency | 25 | 18 | 32 | .17 |
| Yearly household income, $ | .94 | |||
| < 15 000 | 62 | 43 | 48 | |
| 15 000–30 000 | 26 | 18 | 20 | |
| > 30 000 | 12 | 12 | 6 | |
| Weight, lb | 168.0 (34) | 162.0 (27.0) | 174.0 (39.0) | .08 |
| Height, in | 60.6 (3.4) | 60.1 (3.3) | 61.1 (3.5) | .13 |
| BMI, kg/m2 | ||||
| Total | 31.5 (4.8) | 31.0 (5.0) | 32.0 (4.0) | .46 |
| Overweight, 25–29.9 | 38 | 49 | 26 | |
| Obese, 30.0–39.9 | 56 | 45 | 68 | |
| Morbidly obese, ≥ 40.0 | 6 | 6 | 6 | |
| Waist circumference, in | 40.0 (4.0) | 39.0 (4.0) | 40.0 (4.0) | .17 |
| Blood pressure, mmHg | ||||
| Systolic | 115 (20) | 119 (25) | 112 (13) | .09 |
| Diastolic | 71 (9) | 73 (10) | 70 (7) | .17 |
| LDL cholesterol, mg/dL | 105 (33) | 103 (33) | 109 (32) | .31 |
| Glucose, mg/dL | ||||
| Fasting | 103 (9.6) | 102 (9.5) | 104 (9.6) | .32 |
| At 2 h | 105 (23.3) | 155 (23.3) | 152 (23.3) | .51 |
| Isolated impaired fastingb | 23.2 | 28.6 | 18.0 | |
| Isolated impaired tolerancec | 36.4 | 36.7 | 36.0 | .37d |
| Isolated impaired fasting and tolerance | 40.4 | 34.7 | 46.0 | |
| Hemoglobin A1c | 5.6 (0.3) | 5.6 (0.2) | 5.6 (0.3) | .76 |
| Physical activity, h/wk | ||||
| Total | 27 (29) | 26 (28) | 28 (32) | .68 |
| Leisure | 3.9 (9.5) | 3.9 (10.2) | 4.0 (8.93) | .94 |
| Walking/cycling | 11.7 (13.4) | 11.6 (10.9) | 11.7 (15.7) | .99 |
| Food intake, servings/d | ||||
| Fat | 2.5 (0.4) | 2.4 (0.3) | 2.5 (0.3) | .28 |
| Juice | 0.7 (1.3) | 0.5 (0.6) | 1.0 (1.8) | .04 |
| Fruit | 0.8 (1.0) | 0.9 (1.2) | 0.8 (0.9) | .53 |
| Lettuce salad | 0.4 (0.6) | 0.4 (0.6) | 0.4 (0.6) | .89 |
| Soda | 0.54 (1.33) | 0.31 (0.78) | 0.77 (1.69) | .09 |
| Diet soda | 0.13 (0.70) | 0.04 (0.22) | 0.22 (0.96) | .20 |
Note. BMI = body mass index; HEED = help educate to eliminate diabetes; LDL = low-density lipoprotein.
Derived from the t test except where indicated.
Prediabetes defined as 100–125 mg/dL.
Prediabetes defined as 140–199 mg/dL after a 75-g glucose load.
Derived from the χ2 test.
The study had some attrition: 83 participants returned at 3 months, 79 at 6 months, and 72 at 12 months (37 control, 35 intervention). Four participants became ineligible because of pregnancy. The 23 participants lost to follow-up at 12 months did not differ from those who returned for the final check-up in age, gender, weight, BMI, or family history of diabetes. Reasons for attrition included relocation, family responsibilities, and doctors telling participants that their elevated blood sugar did not need attention.
Intervention
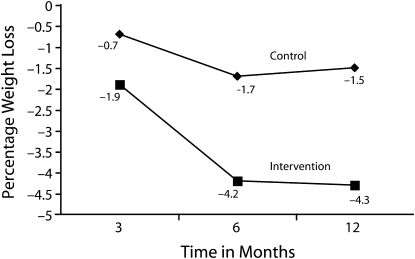
The intervention group lost significantly more weight than the control group (the latter had nonsignificant weight loss). The majority of the weight loss occurred during the first 6 months. At 12 months, intervention participants had lost on average 7.2 pounds, or 4.3% of their baseline weight; members of the control group had lost an average of 2.4 pounds, or 1.5% of their baseline weight (P = .01; Figure 1). After adjustment for loss to follow-up by our last-observation-carry-forward strategy, intervention participants lost 5.5 pounds (3.3%) and control participants, 2.3 pounds (1.4%; P < .05). Sixteen intervention participants (34%) lost at least 5% of their baseline weight in 12 months; only 6 control participants (14%; P = .03) achieved this. Waist circumference decreased significantly. We observed no changes in blood pressure or in low-density lipoprotein cholesterol or glucose levels (Table 2).
FIGURE 1.
Weight change among intervention and control groups: Project HEED, East Harlem, New York City, May 2007–August 2008.
Note. P < .05.
TABLE 2.
Changes in Biological Measures, Exercise, and Diet at 12 Months: Project HEED, East Harlem, New York City, May 2007–August 2008
| Control Group (n = 37), Mean (SD) | Intervention Group (n = 35), Mean (SD) | Pa | |
| Weight, lb | −2.4 (8.1) | −7.2 (7.3) | .01 |
| Waist circumference, in | 0.1 (3.4) | −1.3 (2.6) | .05 |
| Blood pressure, mmHg | |||
| Systolic | −7 (17) | −1 (13) | .13 |
| Diastolic | −4 (8) | −2 (9) | .31 |
| LDL cholesterol, mg/dL | 4 (29) | −1 (35) | .42 |
| Glucose, mg/dL | |||
| Fasting | 11 (11) | 10 (13) | .83 |
| At 2 h | 11 (37) | 3 (34) | .26 |
| % Hemoglobin A1c | −0.3 (0.2) | −0.3 (0.2) | .13 |
| Leisure-time physical activity, h/wk | −1.1 (3.5) | −1.5 (5.4) | .72 |
| Food intake, servings/d | |||
| Fat | 0.1 (0.4) | 0.0 (0.4) | .32 |
| Juice | −0.2 (0.5) | 0.8 (0.6) | .05 |
| Fruit | −0.2 (1.0) | −0.1 (0.9) | .43 |
| Lettuce salad | −0.1 (0.6) | −0.5 (1.9) | .24 |
| Soda | −0.07 (0.53) | −0.62 (1.71) | .07 |
| Diet soda | −0.16 (0.35) | −0.18 (0.39) | .84 |
Note. HEED = help educate to eliminate diabetes; LDL = low-density lipoprotein.
Derived from the t test.
Although intervention participants achieved significant and sustained weight loss, they reported very limited behavior changes. Self-reported physical activity did not differ between the 2 groups. Intervention participants reported eating more green salad (P = .05) and drinking fewer sugary beverages (regular soda, juice, and sweetened drinks; P < .01); control group diet did not change (Table 2). Fat and fast-food intake, label reading, binge eating, television watching, self-efficacy to prevent diabetes, and perceived importance of losing weight were unchanged in both groups. However, fewer intervention participants reported at 12 months that they had to travel outside their neighborhood to find healthy foods (P = .02).
Over the study period, 24 participants (24%) had follow-up glucose readings consistent with a diagnosis of diabetes. The incidence rate of diabetes was the same in both groups (intervention, 0.36 cases per person-year; control, 0.33). Although participants with diabetes-range glucose levels did not differ from other participants in BMI and family history of diabetes, they tended to be older (54 versus 46 years; P = .06).
Focus Groups and Interviews
We interviewed 16 intervention participants (10 in a Spanish-language focus group, 6 in open-ended interviews conducted in English) and 20 control participants (14 in Spanish and 6 in English groups). These 36 respondents (39% of the 93 invited to participate) did not differ from the total study group by age, education, or marital, employment, or insurance status. Significantly more of the focus group participants were foreign born (P < .01). Interrater reliability ranged from 92% to 100%. The data collected shed light on key study findings, including high recruitment rates and perceived benefits of the intervention.
The most common reasons cited for participating were concern about personal health or the health of family or friends with diabetes; being influenced to join by a familiar, trusted person or organization; and wanting to help their community by participating in a research study about diabetes. Intervention and control participants stated that receiving elevated glucose results motivated them and their families to begin to make changes in their diet and activity. A Spanish-speaking woman said, “I don't do it for the money but for my health… . With the checkups, you're finding out what's good for you… . This is free … sometimes we don't have this chance.” Control participants even formed a walking group while waiting to take the workshop.
A majority of those who attended the workshop stated that group support helped them to make small changes to lose weight. One said, “I'm very grateful for this program… . I lost 22 pounds.” Intervention participants stated that they learned simple steps to bring exercise into their daily routine, adapt traditional foods to make them healthier, and control portions. One man said, “I still drink soda, but before I had three sodas, not now, I drink half a soda can and add ice.” They also related how their lifestyle changes had affected other family members positively. A Latina commented, “My husband … says it's healthy—no, it's not healthy, and it does you a lot of harm. So I remove all the fat … and say no, I don't have to eat so much.” Respondents reported a sense of empowerment and increased confidence that they could accomplish their goals step by step. One Black woman said, “I have learned how to eat, do exercises, I go to the park to run… . I promised … and I think I can get there.”
DISCUSSION
Community and academic partners used a CBPR approach to develop, pilot, and evaluate a community-led, community-based, diabetes prevention lifestyle intervention among overweight adults with prediabetes in East Harlem. Over 3 months, the partnership screened 178 overweight adults for prediabetes through formal oral glucose tolerance testing at community locations, found that nearly three quarters had elevated glucose levels, and recruited many of them into a randomized trial. Six months after an 8-session peer-led weight-loss workshop, intervention participants had lost significantly more weight than had control participants, and they maintained their weight loss for an additional 6 months, despite not being exposed to reinforcement activities. We observed no significant changes in other physiological measures in this small pilot, but our results suggest that weight loss among people with prediabetes, the most effective means of diabetes prevention, may be achievable through low-cost, peer-led programs.
Although we found some changes in self-reported diet (decreased sugary drink and increased salad consumption), participants reported no changes in fat intake or physical activity. Our primary outcome, weight, was objective and was significantly affected. It is difficult to hypothesize mechanisms for weight loss in community settings that do not involve more significant changes in caloric intake or expenditure than those we found. Measuring diet and exercise with brief scales is subjective, difficult, and often unreliable. It will be important for the field to continue to develop more sensitive measures of weight-related behaviors.
Limitations
We recruited a vulnerable cohort mostly composed of low-income, undereducated, medically underserved, recent Hispanic immigrants, a population typically difficult to engage in research. However, our study had limitations. This pilot had too small a sample size to explore more variables. Contamination of intervention to control participants cannot be ruled out, because we randomly assigned participants from the same community to the trial's 2 arms. However, intervention influence on control participants would have biased the study to the null hypothesis, and we found significant weight loss only in the intervention group. Because weight loss is convincingly linked to diabetes prevention,9–13 we chose it as the primary outcome, rather than development of diabetes, which would require a larger sample size and a longer follow-up period. Larger studies should be conducted to determine whether weight loss and diabetes prevention are as tightly linked in community settings as they have been in more traditional clinical trials.
Only 29% of the individuals we tested had normal glucose levels; the remainder had either prediabetes- or diabetes-range levels. We obtained these results from formal oral glucose tolerance testing, the gold standard for identifying prediabetes.53 However, we did not repeat testing on a separate day, as is often recommended, because this approach was deemed infeasible and burdensome to community members. Our glucometers may have given higher glucose readings than venous samples, although HbA1c levels were in the prediabetes range.51,52 It may be that the population we reached is at an unusually high risk for prediabetes and diabetes: fully 24% of our participants developed diabetes-range glucose levels within 1 year of study enrollment, although published progression rates are closer to 10% annually.9,10 Rigorous, community-based testing programs are clearly feasible and may help identify those at highest risk for diabetes and motivate them to action, especially if simple, effective interventions are made available to them.
The study also apparently affected the control group positively. Our qualitative findings revealed that control participants benefited from just knowing they had prediabetes, because they lost a mean 2 pounds at 1 year, by contrast with the average adult, who gains 1 pound annually.54–56 Future research should explore whether informing people that they have a high risk of developing diabetes (prediabetes glucose levels) and giving them some simple messages about prevention is a useful tool to motivate weight loss. Individuals with poor access to care and few skills for negotiating the health system may be more interested in being tested for diabetes and more receptive to educational interventions, although our small sample size precluded such an analysis.
Our pilot was also not powered to detect changes in either diet or physical activity as measured by questionnaire. Differences in physical activity between the study groups may have been diminished by participants' overestimating their activity level and underestimating their caloric intake at baseline and by control participants' initiative in increasing activity independently, as they revealed in poststudy focus groups. Interestingly, intervention—but not control—participants reported finding increased local availability of healthy foods at the 1-year follow-up. Perhaps the intervention altered their perception of what healthy food is, or it inspired them to find local stores that carried healthier items.
Conclusions
Suggestions from peer leaders and participants to improve the workshop's efficacy in stimulating lifestyle changes included further cultural tailoring, more visual aids depicting appropriate portion sizes, and refresher classes. Future workshops will incorporate this feedback, and future surveys will include new items, which together may lead to changes in reported diet and physical activity.
The CBPR approach meant that community partners were involved at every step in this research: writing a grant to address health disparities; choosing to focus on diabetes; developing the intervention, study design, and instruments for evaluation; leading recruitment; and actively partnering in analyses. This made the study simultaneously rigorous and relevant, novel and practical, and potentially sustainable beyond the funded demonstration of its effectiveness. We met our recruitment goal in just 3 months and had a waiting list of interested community members, which may signify that community partners not only engendered trust and comfort in the research but also developed a program that resonated with and attracted their friends and neighbors more effectively than one developed by academics alone.
A community-driven approach to diabetes prevention in a high-risk community of color may be quite feasible and effective. Because efficacy trials resulting in weight loss among overweight adults with prediabetes eliminated racial and ethnic disparities in incident diabetes,10 this type of program may also help narrow disparities in diabetes rates in the future. Peer educators, particularly those who teach in group settings, are a less expensive and more readily available resource than are health professionals. This intervention can be readily adopted by local organizations and serve as a model for other communities hard hit by the diabetes epidemic. It may also realize the promise of CBPR, harnessing local expertise and assets to conduct research and translate findings into actions of direct benefit to communities.57
Acknowledgments
This study was supported by the National Center on Minority Health and Health Disparities (grant 1R24 MD001691-03) and the New York State Department of Health Diabetes Prevention and Control Program (grants C020123 and C021751).
The authors thank all members of the Community Action Board of the East Harlem Partnership for Diabetes Prevention, chaired by Cesar Vasquez, for their vision, creativity, and leadership; community partners and organizations who worked with us; Project HEED participants; the community outreach teams led by Guedy Arniella and Barbara Brenner; Kate Lorig at the Stanford Patient Education Research Center, and Judith Goldfinger for guidance in curriculum development; Kim Gans, Judy Wylie-Rosett, and Derek Leroith for developing study instruments and providing scientific oversight; and project staff and peer educators including Duna Amara, Judit Dieguez, Anika Martin, Carlo Canepa, Kenneth Fernandez, Carlton Bailey, Ellen Plumb, for recruiting participants, collecting qualitative data, and conducting workshops.
Human Participant Protection
This study was approved by the Mount Sinai School of Medicine's institutional review board.
References
- 1.Smedley BD, Stith A, Nelson AR, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2002 [PubMed] [Google Scholar]
- 2.US Dept of Health and Human Services Healthy People 2010. Available at: http://www.healthypeople.gov. Accessed April 8, 2009
- 3.US Dept of Health and Human Services National Healthcare Disparities Report, 2004. Washington, DC: Agency for Healthcare Research and Quality; 2004. Publication 05-0014 [Google Scholar]
- 4.US Dept of Health and Human Services National Healthcare Disparities Report, 2003. Washington, DC: Agency for Healthcare Research and Quality; 2003 [Google Scholar]
- 5.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32(2):287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the US population: National Health and Nutrition Survey 1999–2002. Diabetes Care. 2006;29(6):1263–1268 [DOI] [PubMed] [Google Scholar]
- 7.Harris MI. Racial and ethnic differences in health care access and health outcomes for adults with type 2 diabetes. Diabetes Care. 2001;24(3):454–459 [DOI] [PubMed] [Google Scholar]
- 8.Lanting LC, Lamberts SWJ, Joung IMA, Bootsman AH, Mackenbach JP. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients. Diabetes Care. 2005;28(9):2280–2288 [DOI] [PubMed] [Google Scholar]
- 9.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350 [DOI] [PubMed] [Google Scholar]
- 10.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franz MJ. The evidence is in: lifestyle interventions can prevent diabetes. Am J Lifestyle Med. 2007;1(2):113–121 [Google Scholar]
- 13.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544 [DOI] [PubMed] [Google Scholar]
- 14.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167 [DOI] [PubMed] [Google Scholar]
- 15.Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance. The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116(2):151–157 [DOI] [PubMed] [Google Scholar]
- 16.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753–759 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Nutrition Recommendations and Interventions for Diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2007;30(Suppl 1):S48–S65 [DOI] [PubMed] [Google Scholar]
- 18.Biuso TJ, Butterworth S, Linder A. A conceptual framework for targeting prediabetes with lifestyle, clinical, and behavioral management interventions. Dis Manag. 2007;10(1):6–15 [DOI] [PubMed] [Google Scholar]
- 19.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005;142(1):56–66 [DOI] [PubMed] [Google Scholar]
- 20.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med. 2008;35(4):357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boltri JM, Davis-Smith YM, Seale JP, Shellenberger S, Okosun IS, Cornelius ME. Diabetes prevention in a faith-based setting: results of translational research. J Public Health Manag Pract. 2008;14(1):29–32 [DOI] [PubMed] [Google Scholar]
- 22.Pagoto SL, Kantor L, Bodenlos JS, Gitkind M, Ma Y. Translating the diabetes prevention program into a hospital-based weight loss program. Health Psychol. 2008;27(1 Suppl):S91–S98 [DOI] [PubMed] [Google Scholar]
- 23.Whittermore R, Melkus G, Wagner J, Dziura J, Northrup V, Grey M. Translating the Diabetes Prevention Program to primary care: a pilot study. Nurs Res. 2009;58(1):2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an urban medically underserved community: a nonrandomized prospective intervention study. Diabetes Care. 2008;31(4):684–689 [DOI] [PubMed] [Google Scholar]
- 25.Cramer JS, Sibley RF, Bartlett DP, Kahn LS, Loffredo L. An adaptation of the Diabetes Prevention Program for use with high-risk, minority patients with type 2 diabetes. Diabetes Educ. 2007;33(3):503–508 [DOI] [PubMed] [Google Scholar]
- 26.Amundson HA, Butcher MK, Gohdes D, et al. Translating the Diabetes Prevention Program into practice in the general community: findings from the Montana Cardiovascular Disease and Diabetes Prevention Program. Diabetes Educ. 2009;35(2):209–223 [DOI] [PubMed] [Google Scholar]
- 27.McBride PE, Einerson JA, Grant C, et al. Putting the Diabetes Prevention Program into practice: a program for weight loss and cardiovascular risk reduction for patients with metabolic syndrome or type 2 diabetes mellitus. J Nutr Health Aging. 2008;12(10):745S–749S [DOI] [PubMed] [Google Scholar]
- 28.Aldana S, Barlow M, Smith R, et al. A worksite diabetes prevention program: two-year impact on health. AAOHN. 2006;54(9):389–395 [DOI] [PubMed] [Google Scholar]
- 29.Olson EC, Van Wye G, Kerker B, Thorpe L, Frieden TR. Take Care East Harlem. 2nd ed New York, NY: New York City Dept of Health and Mental Hygiene; 2006. NYC Community Health Profiles [Google Scholar]
- 30.Kim M, Berger D, Matte T. Diabetes in New York City: Public Health Burden and Disparities. New York: New York City Dept of Health and Mental Hygiene; 2006 [Google Scholar]
- 31.Goldfinger JZ, Arniella G, Wylie-Rosett J, Horowitz CR. Project HEAL: peer-led education leads to weight loss in Harlem. J Health Care Poor Underserved. 2008;19(1):180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–1791 [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292 [DOI] [PubMed] [Google Scholar]
- 34.Walker EA, Caban A, Schechter CB, et al. Measuring comparative risk perceptions in an urban minority population: the risk perception survey for diabetes. Diabetes Educ. 2007;33(1):103–111 [DOI] [PubMed] [Google Scholar]
- 35.Shannon J, Kristal AR, Curry SJ, Beresford SA. Application of a behavioral approach to measuring dietary change: the fat- and fiber-related diet behavior questionnaire. Cancer Epidemiol Biomarkers Prev. 1997;6(5):355–361 [PubMed] [Google Scholar]
- 36.Thompson FE, Kipnis V, Subar AF, et al. Evaluation of 2 brief instruments and a food-frequency questionnaire to estimate daily number of servings of fruit and vegetables. Am J Clin Nutr. 2000;71(6):1503–1510 [DOI] [PubMed] [Google Scholar]
- 37.Thompson FE, Subar AF, Smith AF, et al. Fruit and vegetable assessment: performance of 2 new short instruments and a food frequency questionnaire. J Am Diet Assoc. 2002;102(12):1764–1772 [DOI] [PubMed] [Google Scholar]
- 38.Spoon MP, Devereux PG, Benedict JA, et al. Usefulness of the food habits questionnaire in a worksite setting. J Nutr Educ Behav. 2002;34(5):268–272 [DOI] [PubMed] [Google Scholar]
- 39.Armstrong T, Bull F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J Public Health. 2006;14(2):66–70 [Google Scholar]
- 40.US Census Bureau Questions and answers for Census 2000 data on race. March 14, 2001. Available at: http://www.census.gov/Press-Release/www/2001/raceqandas.html. Accessed December 29, 2009
- 41.DiClemente RJ, Wingood GM. A randomized-controlled trial of an HIV sexual risk-reduction intervention for young African-American women. JAMA. 1995;274(16):1271–1276 [PubMed] [Google Scholar]
- 42.The CDC. AIDS Community Demonstration Projects Research Group. Community-level HIV intervention in 5 cities: final outcome data from the CDC AIDS Community Demonstration Projects. Am J Public Health. 1999;89(3):336–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ. 2007;33(1):69–76 [DOI] [PubMed] [Google Scholar]
- 44.Lorig KR, Ritter P, Stewart AL, et al. Chronic Disease Self-Management Program: 2-year health status and health care utilization outcomes. Med Care. 2001;39(11):1217–1223 [DOI] [PubMed] [Google Scholar]
- 45.Lorig K, Ritter PL, Villa F, Piette JD. Spanish diabetes self-management with and without automated telephone reinforcement: two randomized trials. Diabetes Care. 2008;31(3):408–414 [DOI] [PubMed] [Google Scholar]
- 46.Lorig KR, Hurwicz ML, Sobel D, Hobbs M, Ritter PL. A national dissemination of an evidence-based self-management program: a process evaluation study. Patient Educ Couns. 2005;59(1):69–79 [DOI] [PubMed] [Google Scholar]
- 47.Bandura A. Self-efficacy mechanism in physiological activation and health-promoting behavior. : Madden JI, Matthysee S, Barchas J, Adaptation, Learning, and Affect. New York, NY: Raven Press; 1991:229–269 [Google Scholar]
- 48.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37(1):5–14 [DOI] [PubMed] [Google Scholar]
- 49.Horowitz C, Brenner BL, Lachapelle S, Amara DA, Arniella G. Effective recruitment of minority populations through community-led strategies. Am J Prev Med. 2009;37(6 Suppl 1):S195–S200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Department of Health and Human Services Federal Register. 2007;72(15):3147–3148 [Google Scholar]
- 51.Geberhiwot T, Haddon A, Labib M. HbA1c predicts the likelihood of having impaired glucose tolerance in high-risk patients with normal fasting plasma glucose. Ann Clin Biochem. 2005;42(3):193–195 [DOI] [PubMed] [Google Scholar]
- 52.American Diabetes Association Standards of medical care in diabetes—2009. Diabetes Care. 2009;32(Suppl 1):S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.American Diabetes Association Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(Suppl 1):S12–S54 [DOI] [PubMed] [Google Scholar]
- 54.Williamson DF. Descriptive epidemiology of body weight and weight change in U.S. adults. Ann Intern Med. 1993;119(7):646–649 [DOI] [PubMed] [Google Scholar]
- 55.Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med. 1990;150(3):665–672 [PubMed] [Google Scholar]
- 56.Roberts SB, Williamson DF. Causes of adult weight gain. J Nutr. 2002;132(12):3824S–3825S [DOI] [PubMed] [Google Scholar]
- 57.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202 [DOI] [PubMed] [Google Scholar]