Abstract
Prior studies have demonstrated P2X receptor expression in the majority of nodose neurons. Immunoreactivity for P2X receptors has also been seen in putative gastric mechanoreceptors, the intraganglionic laminar endings. We therefore hypothesized that deletion of P2X3 receptors will blunt responses to gastric distension in vagal sensory neurons. Using wildtype and P2X3−/− mice, we examined responses to purinergic agonists in retrogradely labelled gastric sensory neurons with patch-clamp techniques. Activation of gastro-oesophageal neurons by fluid distension was studied with intracellular electrodes. Distension-evoked ATP release into the gastric lumen was determined with the luciferase assay and intake and gastric emptying of a solid meal was assessed. ATP triggered inward currents in 80% of gastric nodose neurons. In P2X3−/− mice, the peak current density was lower compared to controls. Ten of 14 controls but none of 30 neurons from P2X3−/− mice responded to α,β-metATP. Gastro-oesophageal sensory neurons of P2X3−/− mice showed a blunted response to fluid distension of oesophagus and stomach. This difference was not explained by differences in distension-evoked ATP release, which did not differ between knockout mice and controls. Food intake during a 3-h period was lower in P2X3−/− mice. Gastric emptying of a solid meal was slightly faster in knockout mice after 1.5 h, but did not differ between groups at 3 h. Our data support a role of purinergic signalling in gastric vagal afferents. Considering the role of vagal input in sensations of fullness or nausea, P2X receptors may be interesting treatment targets for dyspeptic symptoms.
Keywords: mechanosensation, purinergic transmission, vagus, visceral sensation
INTRODUCTION
Several decades ago, Burnstock1 proposed that ATP and its metabolites may function as transmitters in the autonomic nervous system. While initially met with skepticism, purinergic signalling has now been accepted as playing an important role in the central and peripheral nervous system. Based on pharmacological properties and molecular characteristics, two distinct classes of purinergic receptors with preferential response to ATP as well as other single nucleotides have been identified: the family of ligand-gated P2X receptors and the G protein-coupled P2Y receptors.2 Experiments with knockout mice have highlighted the physiological importance of P2X receptors in visceral function. The bladder epithelium releases ATP in response to mechanical stimulation, which activates primary afferent neurons and triggers reflex micturition. Deletion of either the P2X3 or the P2X2 receptor results in a decrease in micturition frequency and bladder distension. 3–6 Purinergic signalling also contributes to the transduction of chemical signals. Elimination of P2X3 as well as P2X2 abolishes the activation of gustatory nerves by bitter and sweet substances.7
The majority of nodose neurons responds to purinergic agonist.8–10 Thus, it is possible that epithelial or other cells may release ATP and activate vagal afferents. Consistent with a potential role in nerve-epithelial/stromal cell interactions in gastric vagal sensory pathways, P2X receptor immunoreactivity has been identified in peripheral terminals of nodose neurons located in close proximity to epithelial structures.11 Within the proximal gastrointestinal tract, specialized vagal endings involved in mechanosensation, the intraganglionic laminar endings, have been shown to express P2X receptors.12–14 Detailed electrophysiological and pharmacological characterizations suggest that most of these vagal sensory neurons express heteromeric channels composed of P2X2 and P2X3 receptors. 8,10,15 Based on these results, we hypothesized that ATP release from epithelial and/or other cells in proximity to nerve terminals activates purinergic receptors and contributes vagal mechanosensation in the proximal gastrointestinal tract.
METHODS
Animals
All experiments were performed in mice 4–12 weeks of age, housed in a 12-h light and dark cycle with free access to water and food at the certified animal care facility of the University of Pittsburgh. Animal handling adhered to the Guide for the Care and Use of Laboratory Animals (National Research Council); all procedures were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. P2X3 knockout mice were provided by our collaborator Dr G.F. Gebhart through an agreement with Roche Biosciences (Palo Alta, CA, USA) and bred on a C57bl/6 background.5 C57bl/6 wildtype mice were used as controls (The Jackson Laboratory, Bar Harbor, ME, USA). Homozygous deletion of P2X3 receptors was confirmed by PCR for each animal used in the studies (data not shown).
Retrograde cell labelling of gastric sensory neurons
Anaesthesia was induced by inhalation of 5% isoflurane and maintained by inhalation of 2% isoflurane. The stomach was exposed through a midline incision and the retrograde label Alexa Fluor 488 cholera toxin subunit B conjugate (CTB, 2 mg mL−1 in sterile saline; Invitrogen, Carlsbad, CA, USA; SKU# C-34775) was injected to two sites within the ventral and dorsal wall of the distal stomach (4 μL per site). Considering the course of vagal fibres within the gastric wall,16 injections were placed close to the greater curvature to minimize labelling passing fibres. The wound was sutured and mice were allowed to recover. Nodose ganglia were harvested 5–7 days later and dissociated for electrophysiological recordings. Only CTB-labelled (i.e. gastric) neurons were studied.
Cell dissociation and culture
Mice were euthanized and both nodose ganglia removed and incubated for 40 min in D-MEM (Gibco; Invitrogen, Grand Island, NY, USA) supplemented with 1% penicillin/streptomycin and containing 2 mg mL−1 dispase and 1 mg mL−1 collagenase IV (Worthington Biochemical, Lakewood, NJ, USA) at 37 °C and 5% CO2. D-MEM culture medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and 625 μmol L−1 glutamine, was added and the tissue triturated. Cells were collected by 10 min centrifugation at 280 g, washed, resuspended, plated on poly-D-lysine-coated coverslips (BD Biosciences, San Jose, CA, USA) and incubated at 37 °C, 5% CO2 for up to 2 days before electrophysiological studies.
Patch clamp recordings
Patch clamp physiology
Cells were transferred to a recording chamber and superfused continuously (12 mL min−1) with external solution containing in mmol L−1: 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES and 10 glucose (305 mOsm). The pH was adjusted to 7.4 with NaOH. Neurons that innervate the stomach were identified by content of DiI using a rhodamine filter (excitation wavelength ~546 nm and barrier filter at 580 nm). Fire-polished micropipettes with tip resistances of 2–4 MΩ were used for voltage-clamp recordings. The pipette was filled with internal solution containing in mmol L−1: 130 KCl, 4 NaCl, 10 HEPES, 10 EGTA, 0.2 CaCl2, 0.5 NaGTP and 2 MgGTP. The pH was adjusted to 7.25 using KOH (290 mOsm). After establishing the whole cell configuration, the voltage was initially clamped at −70 mV using an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA), digitized at 10 kHz (Digidata 1320A; Axon Instruments), and controlled by CLAMPEX software (pClamp 10.2; Axon Instruments). In voltage-clamp experiments, cells were held at −70 mV. Cell capacitance was adjusted and noted by reading the value on the Axopatch 200B amplifier. To assure stable conditions, recordings were started 2 min after gaining whole cell access. Agonists were applied using a superfusion system with the inflow pipette placed in close proximity (100 μm) to the cell (Warner Instruments, Hamden, CT, USA). A washout period of 3 min was allowed between agonist applications. All experiments were performed at room temperature (21–23 °C).
In vitro characterization of gastro-oesophageal afferents
Animals were euthanized using an overdose of isoflurane and then transcardially perfused with carboxygenated, ice-cold artificial cerebrospinal fluid (aCSF: 127 mmol L−1 NaCl, 1.9 mmol L−1 KCl, 1.2 mmol L−1 KH2PO4, 1.3 mmol L−1 MgSO4, 2.4 mmol L−1 CaCl2, 26.0 mmol L−1 NaHCO3, 10.0 mmol L−1 D-glucose). The oesophagus, stomach, both vagal nerves and nodose ganglia were prepared as described recently.17 Briefly, after mobilizing duodenum, stomach, spleen, liver and mediastinal structures including oesophagus to the thoracic inlet, the neck, head, mediastinal organs and proximal gastrointestinal tract were removed en block and placed in a dish, where the dissection was continued. Once the preparation was reduced to oesophagus, stomach and vagal chain, it was moved into a recording dish, where oesophagus and stomach were intubated for luminal perfusion and pressure application, and the temperature was slowly raised to 33 °C. The nodose ganglia were secured with pins. A suction electrode was placed 4–5 mm distal of the nodose ganglion on the vagal nerve for electrical stimulation as described previously.
Recordings were made from individual nodose neurons using microelectrodes with tip resistances >100 MΩ filled with 1 mol L−1 potassium acetate. Experiments were performed using an Axoclamp 2B amplifier; data were digitized (CED Micro 1401; Cambridge Electronic Design, Cambridge, UK) with a sampling rate of 44 kHz and stored on a personal computer using SPIKE2 software (SPIKE2 software Cambridge Electronic Design) for offline analysis. Cells generating an action potential in response to electrical stimulation of the vagus were included in the study if their resting membrane potential exceeded −35 mV and if they showed a distinct overshoot above 0 mV during the action potential.17,18 The stomach and oesophagus were probed with a blunt glass rod (2 mm diameter) to identify gastro-oesophageal afferents and localize their receptive fields. This was followed by an ascending series of distensions with peak pressures of 10, 20, 30 and 40 cmH2O using the surrounding aCSF. Each distension trial lasted for 20 s and was followed by deflating the stomach for 30 s. After the initial distension series, the stomach was continuously perfused with aCSF (pH 7) for 3 min, followed by a 3-min perfusion with aCSF buffered to pH 4 with HCl. Baseline activity prior to and after the 3-min perfusion with acidic aCSF was determined and averaged in 10-s interval bins.
Measurement of gastric compliance
The stomach and distal oesophagus were removed, cleaned of luminal contents and placed into an organ bath as described above. The proximal oesophagus was occluded and the pyloric channel intubated with PE50 tubing with a pressure transducer placed in series. The stomach was then stepwise distended in 100 μL increments to a total volume of 1000 μL. At each step, pressures were allowed to equilibrate for 20 s.
Measurement of luminal ATP release
For measurement of luminal ATP release during distension, the stomach and oesophagus were removed on block as described above and placed in a dish that was constantly perfused with carboxygenated aCSF. The oesophagus was intubated with PE50 tubing and connected to a manometer for graded pressure applications between 10 and 40 cmH2O. The outflow was intubated by PE150 tubing with a dead space of <50 μL and occluded during distension trials. Tissue was allowed to equilibrate for 1 h prior to ATP release measurements.
Baseline luminal ATP release was obtained by opening the outflow and collecting the effluent for 3 min during continuous perfusion with an inflow pressure of 10 cmH2O. Individual distension pressures of 10–40 cmH2O were kept for 1 min and were separated by 3-min intervals with continuing perfusion as described above. After completion of stepwise distension, the stomach was continuously perfused for 3 min with acidic solution (pH 4.0) followed by a collection of effluent during a second 3-min interval. All samples were kept on ice and ATP content was assayed within 3 h of completion of the experiment. The ATP content of each sample was determined by utilizing an ATP bioluminescent assay kit (Sigma, St. Louis, MO, USA) that utilizes a luceferin–luciferase reaction. Luciferin bioluminescence was measured using a GloMax 20/20 luminometer (Promega, Madison, WI, USA), a standard curve was determined (detection limit was 10 fmol ATP/sample) and each sample analysed in triplicate.
Intake and gastric emptying of a solid meal
In separate groups of animals, intake and emptying of a solid meal was measured as described recently.19 After an overnight fast, mice were allowed free access to preweighed chow for 2 h, followed by removed of food. Animals were euthanized 1.5 and 3 h later, the stomach was removed and emptied. Emptying was calculated by dividing the amount of food retained in the stomach by the total food intake.
Data analysis
The main endpoints for experiments in isolated neurons were the fraction of cells responding to purinergic agonists and the peak current density triggered by the agonist. Time to peak and kinetics of current decay were calculated as described previously.10
For sharp electrode experiments, the resting membrane potential, conduction velocity, spike amplitude, action potential duration at 50% of peak amplitude, amplitude and duration of the afterhyperpolarization was determined as described previously.18 For gastro-oesophageal afferents, we determined resting activity, responses to mechanical stimulation and luminal acidification. To generate stimulus response functions, the mean action potential frequency during the 20-s stimulation period was plotted against the distension pressure. The time course of responses was analysed by measuring spike frequency in 1-s bins. As the kinetics of responses may differ between groups, we also compared the time-dependent changes during distension to different pressures. Changes in resting membrane potential during stimulation were expressed as a decrease in potential prior to the onset of an action potential before, and at the end of, the stimulation period.
Statistical analysis
All data are given as mean ± standard error of the mean. Results were analysed using the Mann–Whitney rank sum test, a two-way ANOVA followed by Holm–Sidak methods for multiple group comparisons, or Fisher exact test where appropriate. A value of P < 0.05 was considered statistically significant.
RESULTS
Responses to purinergic agonists in gastric nodose neurons in P2X3−/− mice
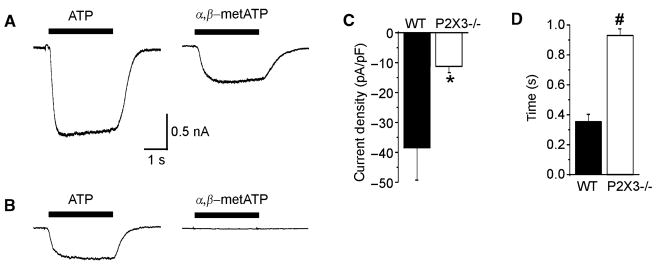
In wildtype animals, 30 μmol L−1 ATP triggered a rapidly activating inward current with a mean delay to peak of 0.35 ± 0.05 s in 12/15 gastric nodose neurons (Fig. 1). This current had a slow decay in all investigated cells. Using 30 μmol L−1 α,β-metATP, a more selective agonist of P2X3 receptors, we similarly saw responses in most gastric nodose neurons of wildtype mice (10/14). The peak current density induced by α,β-metATP was significantly lower compared to the non-selective agonist ATP (ATP: −38.5 ± 10.7 pA/pF; α,β-metATP: 16.4 ± 6.4 pA/pF; P < 0.05; Fig. 1) and the time to peak was significantly increased (ATP: 0.35 ± 0.05 s; α,β-metATP: 0.52 ± 0.04; P < 0.05).
Figure 1.
Stimulation of dissociated gastric sensory neurons with ATP and α,β-metATP. (A) Current responses during superfusion with 30 μmol L−1 of ATP (left) and 30 μmol L−1 of α,β-metATP (right) from a wildtype animal. (B) The corresponding responses from a gastric neuron of a P2X3−/− mouse. The peak current density (C) and time to peak (D) in response to 30 μmol L−1 of ATP are summarized for wildtype (black bars; n = 15) and P2X3−/− mice (white bars; n = 22). *P < 0.05; P < 0.01.
In P2X3−/− mice, 22/30 gastric nodose neurons responded to ATP. However, the peak current density was significantly lower (P2X3−/−: 11.3 ± 2.0 pA/pF; WT: −38.5 ± 10.7 pA/pF; P < 0.05; Fig. 1) and the time to peak significantly slower compared to wildtype controls (P2X3−/−: 0.93 ± 0.04; WT: 0.35 ± 0.05 s; P < 0.0001). None of the 30 gastric neurons studied responded to α,β-metATP.
Properties of nodose neurons in P2X3−/− mice
Stable recordings could be obtained from 134 nodose neurons from P2X3−/− mice, which were activated by the vagal stimulation through the suction electrode. All neurons were identified as C-fibres with the conduction velocity falling below 1 m s−1. Compared to wildtype controls (n = 278), we did not see differences in resting membrane potential, conduction velocity, action potential amplitude or duration, and the duration or amplitude of afterhyperpolarizations (Table 1). Thirty-two of the investigated cells were positively identified as gastro-oesophageal afferent neurons as they responded to mechanical probing of stomach (n = 21) or oesophagus (n = 11). Consistent with our previously reported findings,17,18 neurons showed little baseline activity (1.2 ± 0.2 Hz). As shown in Table 2, basic properties did not differ between P2X3−/− mice and controls.
Table 1.
Basic properties of nodose neurons
| Variable | Wildtype (n = 278) | P2X3−/− (n = 134) |
|---|---|---|
| Vm (mV) | −49.8 ± 0.5 | −49.9 ± 0.7 |
| CV (m s−1) | 0.48 ± 0.01 | 0.45 ± 0.02 |
| AP amplitude (mV) | 73.7 ± 0.7 | 77.9 ± 1.6 |
| AP duration (ms) | 2.0 ± 0.1 | 1.7 ± 0.5 |
| AHP amplitude (mV) | 16.0 ± 0.5 | 16.9 ± 0.8 |
| AHP duration (ms) | 18.9 ± 0.8 | 16.1 ± 0.9 |
Table 2.
Basic properties of gastro-oesophageal nodose neurons
| Variable | Wildtype (n = 37) | P2X3−/− (n = 32) |
|---|---|---|
| Vm (mV) | −50.32 ± 1.8 | −52.4 ± 1.7 |
| CV (m s−1) | 0.45 ± 0.02 | 0.44 ± 0.01 |
| AP amplitude (mV) | 69.7 ± 1.9 | 70.2 ± 3.1 |
| AP duration (ms) | 2.4 ± 0.17 | 2.1 ± 0.2 |
| AHP amplitude (mV) | 13.4 ± 1.5 | 14.6 ± 1.8 |
| AHP duration (ms) | 20.8 ± 3.4 | 19.0 ± 4.3 |
| Resting activity (Hz) | 1.4 ± 0.2 | 1.2 ± 0.2 |
| Location | ||
| Oesophagus | 7 | 11 |
| Stomach | 30 | 21 |
Activation of nodose neurons by gastric stimulation in P2X3−/− mice
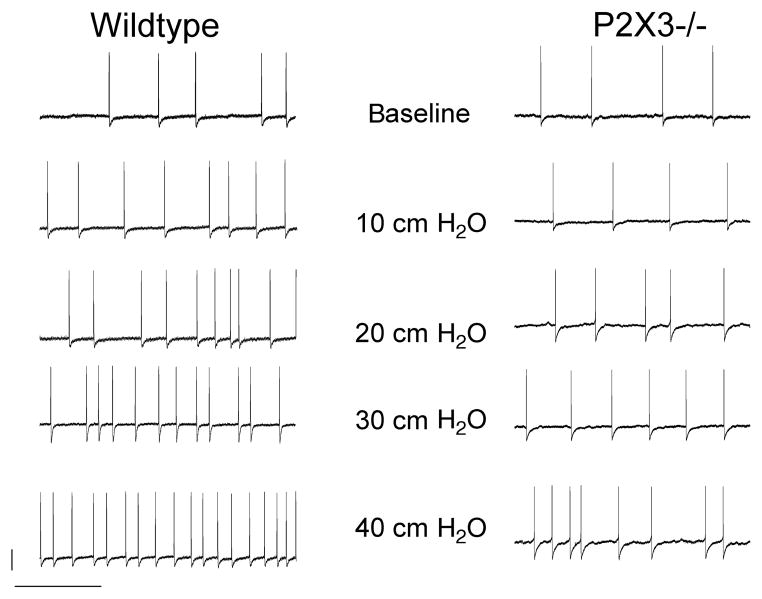
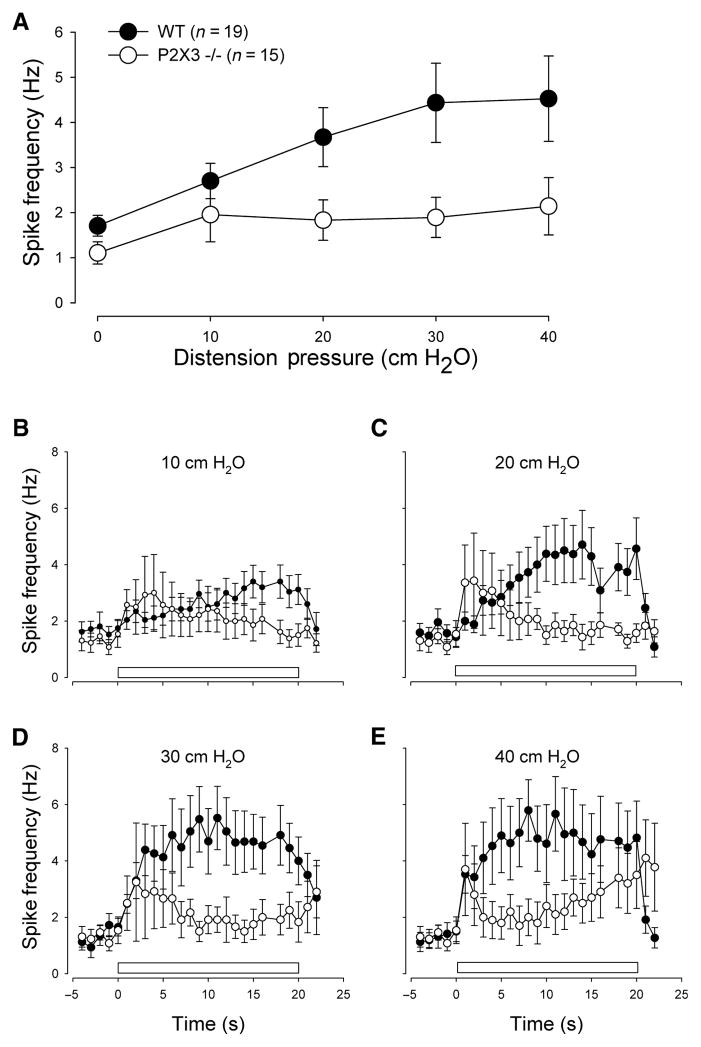
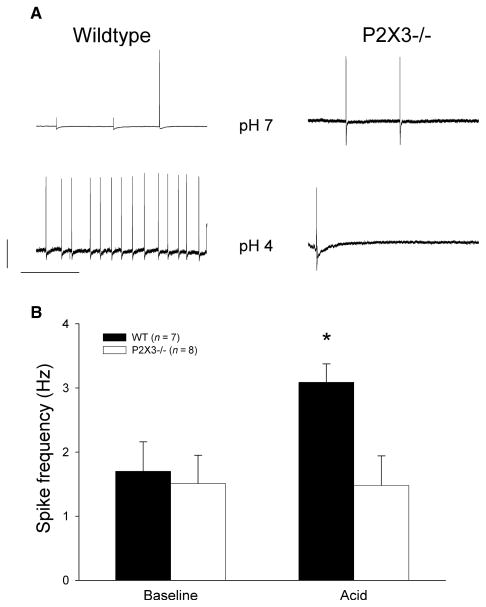
Wildtype and knockout mice were matched for age and sex (wildtype: six of 13 mice were female, age: 4.7 ± 0.8 weeks; P2X3−/− mice: four of 10 mice were female, age: 5.9 ± 1.2 weeks). Luminal distension triggered an increase in action potential firing that showed a slow adaptation during the 20-s stimulation interval at higher stimulus intensities. The average response frequency significantly rose with the distending pressure. Compared to wildtype controls, responses to mechanical stimulation were significantly blunted (Figs 2 and 3). We perfused the lumen with solutions at pH 7 and pH 4 for 3 min to determine whether chemical stimuli differentially affected responses in gastro-oesophageal sensory neurons of wildtype and P2X3−/− mice. While there was no difference during the control perfusion, spike frequency significantly increased in controls but not knockout mice (Fig. 4).
Figure 2.
Representative voltage tracings demonstrate the effects of gastro-oesophageal distension on action potential firing. The upper tracings demonstrate little baseline activity for wildtype (left) and P2X3−/− mice (right). Stepwise distension led to an increase in action potential frequency as shown in the lower tracings. Data were obtained 10 s after initiation of the stimulus. The calibration bars represent 20 mV and 1 s respectively.
Figure 3.
Response to gastric distension. The upper panel (A) shows the average spike frequency in gastric nodose neurons during stepwise fluid distension, measured during a 20-s interval in wildtype (filled circles) and P2X3−/− mice (open circles). Differences between the groups were significant (F = 6.2; P < 0.02). The lower panels show a more detailed time course for the distension pressures of (B) 10 cmH2O (F = 10.2; P < 0.01), (C) 20 cmH2O (F = 35.5; P < 0.01), (D) 30 cmH2O (F = 49.8; P < 0.001) and (E) 40 cmH2O (F = 31.8; P < 0.001). The bar represents the time of luminal pressure application.
Figure 4.
Effect of luminal acidification. (A) Representative voltage tracings with action potential firing after luminal perfusion at pH 7 (upper tracings) and pH 4 (lower tracings) for wildtype (left) and P2X3−/− mice (right). The calibration bars represent 20 mV and 1 s respectively. The data are summarized in the bar graph. (B) The average spike frequency at baseline (left side) and 3 min after decreasing the pH to 4 in wildtype animals (black bars) and P2X3−/− mice (white bars). While acidification triggered a significant increase in controls (P < 0.05), spike frequency did not change after exposure to pH 4 knockout mice.
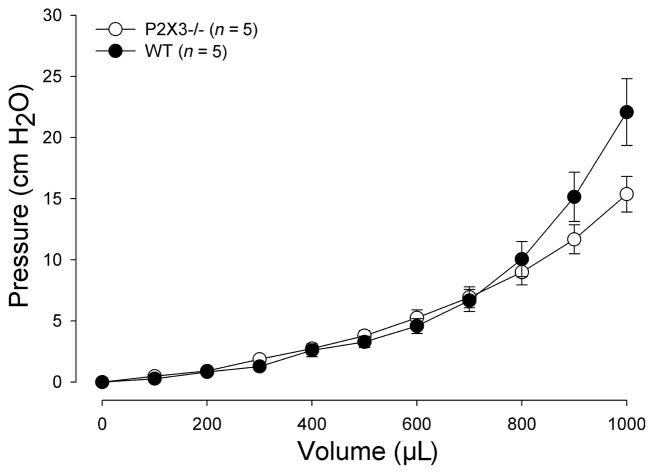
Gastric compliance
The volume–pressure relationship was nonlinear with only minimal pressure increases up to 400 μL. At higher volumes, there was a pressure rise, which tended to be more significant in P2X3−/− mice compared to controls. A post hoc analysis showed significant pressure differences at 900 and 1000 μL (Fig. 4). In complementary experiments, we distended the stomach to 40 cmH2O and measured the retrieved fluid, which did not differ between the two groups (WT: 1171 ± 104 μL; P2X3−/−: 1218 ± 125 μL; P = 0.77).
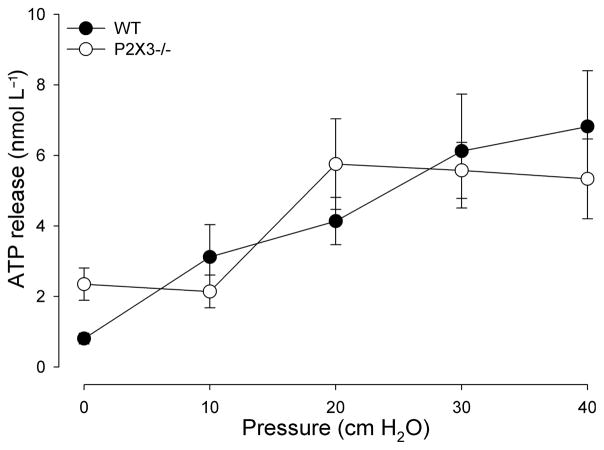
Luminal ATP release during gastro-oesophageal stimulation
Differences between the two groups of animals could be due to altered ATP release in response to stimulation. Therefore, we measured ATP concentrations released into the gastro-oesophageal lumen during distention. In control animals, the constant flow of aCSF at 10 cmH2O with an open outflow resulted in only minimal ATP release (0.7 ± 0.1 nmol L−1 ATP; Fig. 5). ATP concentrations within the effluent increased in response to the ascending series of distention stimuli. In P2X3−/− mice, ATP release into the lumen of the gastro-oesophageal tract in response to distension was not different (Fig. 6). No significant differences in ATP release were detected in response to chemical stimulation with acidic aCSF pH4.
Figure 5.
Gastric compliance is higher in P2X3−/− mice. The stomach was stepwise distended to a maximal volume of 1000 μL. Compared to wildtype mice (filled circles), P2X3−/− (open circles) mice tended to have a slightly higher compliance (F = 3.58; P = 0.062). A post hoc analysis showed significant differences between the groups at 900 and 1000 μL distending volume (P < 0.05).
Figure 6.
Mechanical stimulation induces release of ATP into the lumen of the stomach. The plot shows the mean release of ATP in nmol L−1 per sample in response to mechanical stimulation with an ascending series of distension stimuli from 10 to 40 cmH2O. Increases in pressure resulted in increased release of ATP in both wildtype controls (WT; circles; n = 8) and P2X3−/− animals (open circles; n = 6). Pressure significantly affected ATP release (F = 5.51; P < 0.01). There was no significant difference between WT and P2X3−/− mice (F = 0.39; P = 0.53).
Food intake and gastric emptying in P2X3−/− mice
Gastric filling provides satiation signals and contributes to the regulation of gastric function. Therefore, we screened eating patterns and gastric emptying of a solid meal, looking at two time points after a 2-h feeding period. Food intake over the 2-h time period was significantly lower in P2X3−/− mice (1.13 ± 0.09 g; n = 18) compared to controls (1.55 ± 0.08 g; n = 20; P < 0.01). As prior reports had demonstrated a slower growth rate,15 we controlled for body weight, which was higher in knockout animals (P2X3−/−: 27 ± 0.5 g vs WT: 24 ± 0.5 g; P < 0.01). While gastric contents emptied slightly slower in P2X3−/− mice during the initial 1.5 h (50.3 ± 3.1% compared to 67.4 ± 6.9% for WT; P < 0.05), there was no significant difference between the groups at the later time point (retention at 3 h: P2X3−/−: 75.0 ± 5.0%; WT: 80.9 ± 3.7%; ns).
DISCUSSION
Our results support the importance of purinergic signalling by showing blunted activation of gastric vagal afferents in P2X3−/− animals. These differences are due to lack of P2X3 receptors as ATP release was not altered and as we did not observe significant differences in the baseline properties of nodose neurons. Our findings are consistent with other studies demonstrating that ATP and its metabolites function as an important signal that is released by mechanical or chemical stimuli and activates primary sensory neurons. 3,5–7,20,21
Purinergic signalling and nodose neurons
Prior investigations have demonstrated that more than 80% of nodose neurons respond to purinergic agonists with inward currents due to activation of P2X receptors. 9,10,22 Consistent with the presence of P2X receptors in the peripheral terminals of vagal afferents, antibodies raised specifically against either P2X3 or P2X2 strongly labelled intraganglionic laminar endings within the proximal gastrointestinal tract in normal animals, but not after vagotomy.12,13 Thus, our functional data fall in line with these investigations, showing purinergic responses in mouse nodose neurons projecting to the stomach.
Responses of gastric sensory neurons to purinergic stimulation were complex with fast and slow components mediated by different receptor subunit compositions. 15,22 Our data indicate the presence of more than one purinergic receptor, as application of ATP but not α,β-metATP, a selective agonist of homomeric P2X1, P2X3, or heteromeric P2X2/3 receptor ion channels, triggered inward currents in gastric sensory neurons obtained from P2X3−/− mice. Detailed electrophysiological, pharmacological and immunohistochemical experiments have demonstrated the presence of homomeric P2X2 and heteromeric P2X2/3 receptors in nodose ganglia, which account for the differential responses to the two different agonists.22 While P2Y receptors have also been shown to be present in nodose neurons,23,24 it is unlikely that they contribute significantly to the current responses as genetic deletion of both P2X2 and P2X3 virtually abolished ATP-induced inward currents.15
The relatively high fraction of ATP responsive neurons may be due to our experimental approach. In mice, the nodose ganglion and the more rostrally located jugular ganglion are fused to a single structure, not allowing a macroscopic separation. In larger species, such as the guinea pig, the ganglia are distinct and have different properties and differential projections to the aerodigestive tract. The jugular ganglion, which is derived from the neural crest, largely projects to the extrapulmonary airways and proximal oesophagus, while the placode-derived nodose ganglion projects to the lung, distal oesophagus, stomach and intestine.25,26 Most peptidergic neurons are clustered in the rostral ganglion and do not respond to purinergic agonists.25,27 By focusing our investigations on neurons that were retrogradely labelled from the stomach, by definition we biased our results towards ATP-responsive neurons. However, our findings are in line with previous reports on the properties of mouse nodose neurons.15,22
Purinergic signalling and visceral sensation
The potential role of extracellular ATP as a signalling molecule was initially recognized in visceral structures but focused on efferent functions.28,29 Subsequent studies showed that ATP also functions as a fast signal for synaptic transmission, can activate a subset of sensory neurons, and may contribute to nociception due to ATP release from injured cells.30,31 However, ATP release from epithelial cells or other structures has since been recognized as playing a physiological role in the transduction of mechanical and chemical stimuli. Distension of the urinary bladder, colon and, as shown in this series of experiments, stomach triggers ATP release into the lumen that correlates with stimulus intensity.3,6,21,32 Mucosal application of ATP stimulated P2X receptor in myenteric neurons of the guinea-pig ileum, indicating that ATP release may contribute to signal transduction within the enteric nervous system.33 Intra-arterial injection of ATP but not α,β-metATP directly activates mesenteric bundle afferents in the rat small intestine, which probably contain extrinsic afferents projecting to the central nervous system and centrifugal fibres, projecting form the enteric neurons to the prevertebral ganglia.34 Serosal application of ATP or α,β-metATP activated colonic afferents and enhanced responses to distension; conversely, purinergic antagonists blunted the activation of primary afferents during colonic distension.21 In mouse bladder, luminal administration of α,β-metATP directly activates extrinsic afferents and sensitizes them to subsequent mechanical stimulation.35 Consistent with a role of P2X receptors in the physiology of visceral sensation, P2X3 knockout mice show impaired micturition reflexes and significantly blunted responses to gastric stimulation as demonstrated in the experiments described above.5,6 Considering the already known inhibition of colonic mechanosensation in the presence of purinergic antagonists,21 we chose an experimental approach relying on genetic deletion rather than pharmacologic inhibition of purinergic signalling. Our data thus provide further support to the importance of ATP as a signalling molecule within the gastrointestinal tract and extend the results to vagal afferents within the proximal gut. Complementary studies with P2Y receptor knockout mice and/or pharmacologic tools will provide further insight into the complex role of purinergic signalling in visceral sensory function.
While we measured the luminal ATP concentration in response to distension, we did not examine the effects of luminal ATP to gastro-oesophageal sensory neurons. Prior studies have demonstrated that mucosal administration of ATP or α,β-metATP does not activate colorectal or oesophageal afferents, which is likely due to the poor penetration of these charged molecules through the epithelial layer.21,36 We saw ATP increases within the lumen of the gut; yet, we see the increase in luminal ATP concentrations more as a surrogate marker for ATP release by physiological stimuli rather than interpreting the data as proof for a direct role of the epithelium in signal transduction. The epithelium may certainly be one of the sources of ATP analogous to the known importance of specialized epithelium cells (enteroendocrine cells) in the release of serotonin and peptides. Interestingly, vagal fibres projecting to the distal oesophagus responded to purinergic agonists. In contrast to our data, pharmacological block of P2X receptors did not alter responses to stretch recorded from the guinea-pig oesophagus.37 We performed experiments with an intact organ preparation while Zagorodnyuk et al. stripped the mucosa, indirectly suggesting that analogous to the urinary bladder, the gastro-oesophageal mucosa plays an important role in the signalling process. However, differences in experimental approach and species do not allow definitive conclusions about the source(s) of ATP release in gastrointestinal mechanosensation.
The changes in gastric mechanosensation are confounded by a change in compliance, which may contribute to the blunted activation of primary afferent neurons. Even more striking differences in the biomechanical properties have been described for the urinary bladder, where even very low distension volumes triggered significantly lower intraluminal pressure increases.6 The more subtle differences in gastric compliance argue against the higher compliance as the primary mechanism underlying the altered sensory function. The mechanisms of this change in compliance are unclear. We did not observe obvious changes in gastric size or volume with peak distension pressures. Thus, it is unlikely that chronic distension explains these findings as was suggested for the urinary bladder.6
Compared to controls, P2X3−/− mice also showed no lasting response to luminal acidification, suggesting a potential role of purinergic signalling in chemosensation as has been shown for taste buds in the oral cavity.7 However, the luminal perfusion with acidic solution leads to a complex response to the mechanical (i.e. flow and pressure) and chemical (i.e. change in pH) stimulus. We therefore only examined firing properties prior to and after the perfusion with control or acidic solutions. Considering the transient current responses seen in isolated vagal neurons,38 a more detailed assessment of response kinetics using a different approach is needed before we can truly assess the relevance of purinergic signalling in gastro-oesophageal acid sensation. Our experiments did not directly address the mechanisms by which acid activates gastro-oesophageal afferents. Analogous to the proposed mechanisms contributing to changes in mechanosensation, we propose that acid indirectly affects sensory neurons through release of ATP from non-neuronal cells in the vicinity of nerve terminals.
Purinergic signalling and gastrointestinal pathophysiology
Gastric filling functions as a satiety signalling. Thus, impaired mechanosensation may alter eating behaviour and/or the regulation of gastric function. Prior studies did not demonstrate obvious abnormalities within the gastrointestinal tract. However, P2X3−/− mice had a significantly lower body weight than wildtype controls.15 Based on these reports, we controlled for body weight in our experiments. Interestingly, we could not confirm the previously published weight differences. However, we cannot rule out developmental differences, as we did not monitor weight gain in our small number of knockout mice. We therefore examined intake and emptying of a solid meal. The observed decrease in food intake in P2X3−/− mice cannot be easily explained by blunted mechanosensation and impaired satiety signals, which should lead to changes in the opposite direction. More detailed assessment of feeding patterns with solids and liquids are needed to determine the underlying changes in feeding behaviour. The relatively subtle differences in gastric emptying are consistent with recent observation showing minor changes in peristaltic reflex activity but no significant difference in small intestinal transit of P2X3−/− mice.39 The lack of more significant alterations motility may be due to the redundancy of sensory mechanisms with purinergic signalling and the involvement of other ion channels regulating normal gastrointestinal function.17,40,41
P2X receptors have attracted significant attention as they have been implicated to be involved in nociception and neuropathic pain in spinal sensory neurons and may thus be targets for novel pharmacological interventions.42–44 We have previously shown an increase in the amplitude of purinergic currents after induction of gastric ulcers.10 Similarly, experimental colitis during the neonatal development increased current responses to P2X agonists even weeks after the initial insult and was associated with persistent hypersensitivity.45 This hypersensitivity can be blunted or abolished by purinergic antagonists. 45,46
Immunoreactivity for P2X3 receptors is increased in mucosa of patients with inflammatory bowel disease. Thus, changes in purinergic signalling may contribute to symptoms in human disease as well.47 Considering the high prevalence of acute and chronic visceral pain and the limited efficacy of currently available treatments, purinergic receptors may be an interesting treatment target for visceral pain syndromes. Our investigations are distinct from most of the previously performed studies on purinergic signalling in visceral sensation as we focused on vagal afferent pathways. While the vagal pathways may not play a primary role in nociception, they certainly contribute to the mediation of other sensations such as nausea, fullness or bloating. Targeting P2X receptors may thus provide a novel strategy to alleviate these common and difficult to treat problems.
Acknowledgments
Supported in part by a grant from the National Institutes of Health (NS19912).
References
- 1.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–81. [PubMed] [Google Scholar]
- 2.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 3.Birder LA, Barrick SR, Roppolo JR, et al. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423–9. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- 4.Birder LA, Ruan HZ, Chopra B, et al. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–91. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- 5.Cockayne DA, Hamilton SG, Zhu QM, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–5. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 6.Vlaskovska M, Kasakov L, Rong W, et al. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–7. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finger TE, Danilova V, Barrows J, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–9. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 8.Thomas S, Virginio C, North RA, et al. The antagonist trinitrophenyl-ATP reveals co-existence of distinct P2X receptor channels in rat nodose neurones. J Physiol (Lond) 1998;509:411–7. doi: 10.1111/j.1469-7793.1998.411bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khakh B, Humphrey P, Surprenant A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurones. J Physiol (Lond) 1995;484:385–95. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dang K, Bielefeldt K, Lamb K, et al. Gastric ulcers evoke hyperexcitability and enhance P2X receptor function in rat gastric sensory neurons. J Neurophysiol. 2005;93:3112–9. doi: 10.1152/jn.01127.2004. [DOI] [PubMed] [Google Scholar]
- 11.Brouns I, Adriaensen D, Burnstock G, et al. Intraepithelial vagal sensory nerve terminals in rat pulmonary neuroepithelial bodies express P2X3 receptors. Am J Respir Cell Mol Biol. 2000;23:52–61. doi: 10.1165/ajrcmb.23.1.3936. [DOI] [PubMed] [Google Scholar]
- 12.Castelucci P, Robbins HL, Furness JB. P2X(2) purine receptor immunoreactivity of intraganglionic laminar endings in the mouse gastrointestinal tract. Cell Tissue Res. 2003;312:167–74. doi: 10.1007/s00441-003-0715-3. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZJ, Neuhuber WL. Intraganglionic laminar endings in the rat esophagus contain purinergic P2X2 and P2X3 receptor immunoreactivity. Anat Embryol. 2003;207:363–71. doi: 10.1007/s00429-003-0351-4. [DOI] [PubMed] [Google Scholar]
- 14.Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 15.Cockayne DA, Dunn PM, Zhong Y, et al. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol (Lond) 2005;567:621–39. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol. 2000;421:302–24. [PubMed] [Google Scholar]
- 17.Bielefeldt K, Davis BM. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G130–8. doi: 10.1152/ajpgi.00388.2007. [DOI] [PubMed] [Google Scholar]
- 18.Bielefeldt K, Zhong F, Koerber HR, et al. Phenotypic characterization of gastric sensory neurons in mice. Am J Physiol Gastrointest Liver Physiol. 2006;291:G987–97. doi: 10.1152/ajpgi.00080.2006. [DOI] [PubMed] [Google Scholar]
- 19.Liu L-S, Winston JH, Shenoy MM, et al. A rat model of chronic gastric sensorimotor dysfunction resulting from transient neonatal gastric irritation. Gastroenterology. 2008;134:2070–9. doi: 10.1053/j.gastro.2008.02.093. [DOI] [PubMed] [Google Scholar]
- 20.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–8. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 21.Wynn G, Rong W, Xiang Z, et al. Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology. 2003;125:1398–409. doi: 10.1016/j.gastro.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Zhong Y, Dunn PM, Bardini M, et al. Changes in P2X receptor responses of sensory neurons from P2X3-deficient mice. Eur J Neurosci. 2001;14:1784–92. doi: 10.1046/j.0953-816x.2001.01805.x. [DOI] [PubMed] [Google Scholar]
- 23.Fong AY, Krstew EV, Barden J, et al. Immunoreactive localisation of P2Y1 receptors within the rat and human nodose ganglia and rat brainstem: comparison with [[alpha]33P]deoxyadenosine 5′-triphosphate autoradiography. Neuroscience. 2002;113:809–23. doi: 10.1016/s0306-4522(02)00237-3. [DOI] [PubMed] [Google Scholar]
- 24.Hoesch RE, Yienger K, Weinreich D, et al. Coexistence of functional IP3 and ryanodine receptors in vagal sensory neurons and their activation by ATP. J Neurophysiol. 2002;88:1212–9. doi: 10.1152/jn.2002.88.3.1212. [DOI] [PubMed] [Google Scholar]
- 25.Undem BJ, Chuaychoo B, Lee M-G, et al. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol (Lond) 2004;556:905–17. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhuo H, Ichikawa H, Helke CJ. Neurochemistry of the nodose ganglion. Prog Neurobiol. 1997;52:79–107. doi: 10.1016/s0301-0082(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 27.Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol (Lond) 2005;563:831–42. doi: 10.1113/jphysiol.2004.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasakov L, Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982;86:291–4. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- 29.Burnstock G, Cocks T, Kasakov L, et al. Direct evidence for ATP release from non-adrenergic, non-cholinergic (“purinergic”) nerves in the guinea-pig taenia coli and bladder. Eur J Pharmacol. 1978;49:145–9. doi: 10.1016/0014-2999(78)90070-5. [DOI] [PubMed] [Google Scholar]
- 30.Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–5. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- 31.Valera S, Hussy N, Evans RJ, et al. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994;371:516–9. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 32.Knight GE, Bodin P, De Groat WC, et al. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol. 2002;282:F281–8. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- 33.Bertrand PP, Bornstein JC. ATP as a putative sensory mediator: activation of intrinsic sensory neurons of the myenteric plexus via P2X receptors. J Neurosci. 2002;22:4767–75. doi: 10.1523/JNEUROSCI.22-12-04767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkup AJ, Booth CE, Chessell IP, et al. Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetised rat. J Physiol (Lond) 1999;520:551–63. doi: 10.1111/j.1469-7793.1999.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rong W, Spyer KM, Burnstock G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol (Lond) 2002;541:591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page AJ, O’donnell TA, Blackshaw LA. P2X purinoceptor-induced sensitization of ferret vagal mechanoreceptors in oesophageal inflammation. J Physiol (Lond) 2000;523:403–11. doi: 10.1111/j.1469-7793.2000.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zagorodnyuk VP, Chen BN, Costa M, et al. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol (Lond) 2003;553:575–87. doi: 10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugiura T, Dang K, Lamb K, et al. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25:2617–27. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bian X, Ren J, Devries M, et al. Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J Physiol. 2003;551:309–22. doi: 10.1113/jphysiol.2003.044172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Page AJ, Brierley SM, Martin CM, et al. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127:1739–47. doi: 10.1053/j.gastro.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 41.Page AJ, Brierley SM, Martin CM, et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–15. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barclay J, Patel S, Dorn G, et al. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J Neurosci. 2002;22:8139–47. doi: 10.1523/JNEUROSCI.22-18-08139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol (Lond) 2004;554:301–8. doi: 10.1113/jphysiol.2003.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souslova V, Cesare P, Ding Y, et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–7. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- 45.Xu G-Y, Shenoy M, Winston JH, et al. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230–7. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- 46.Wynn G, Ma B, Ruan HZ, et al. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G647–57. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- 47.Yiangou Y, Facer P, Baecker PA, et al. ATP-gated ion channel P2X3 is increased in human inflammatory bowel disease. Neurogastroenterol Motil. 2001;13:365–9. doi: 10.1046/j.1365-2982.2001.00276.x. [DOI] [PubMed] [Google Scholar]