Abstract
One of the reasons multiple sclerosis (MS) has been considered a T-cell mediated autoimmune disease is that a similar experimental disease can be induced in certain rodents and primates by immunization with myelin antigens, leading to T-cell-mediated inflammatory demyelination in the CNS. In addition, most if not all pharmacological treatments available for MS are biologically active on T cells. In this article we review the principles of T-cell-based immunotherapies and the specific actions of current and novel treatments on T-cell functions, when these are known. For both licensed and innovative agents, we also discuss biological actions on other immune cell types. Finally, we offer a brief perspective on expected changes in the use of MS immunotherapies in the near future.
Keywords: disease-modifying treatment, experimental autoimmune encephalomyelitis, immune system, immunotherapy, multiple sclerosis, T cell
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating disease of the CNS [1,2] that usually begins in young adulthood. It is characterized by lesions within the CNS and demyelination is a key feature of these lesions. There is also evidence of axonal transection or axonal degeneration. Genetic and environmental factors confer susceptibility to MS, as shown by epidemiological studies. These factors include gender, race, exposure to infections (particularly during childhood and adolescence), and geographical variables such as latitude and sun exposure [2]. The clinical symptoms vary depending on the parts of the CNS affected – they include focal neurological deficits such as motor weakness or paralysis, sensory loss, loss or blurring of vision and cognitive deficits [1].
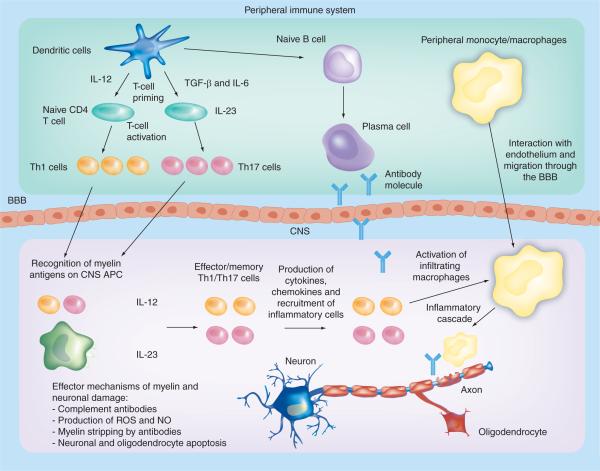
The etiology of MS remains unknown; however, data from MS lesions, genetic susceptibility, the use of immunotherapies and extensive studies on animal models of MS suggest that it is an immune-mediated disease. Autoreactive T cells are thought to initiate an autoimmune response directed against components of CNS myelin (Figure 1). The main targets of the autoimmune reactions are thought to be myelin basic protein (MBP), proteolipid protein (PLP) and myelin oligodendrocyte glycoprotein (MOG) [3]. There is also thought to be a role for antibody-mediated responses to myelin antigens in disease pathogenesis. Although primary demyelination is considered a key feature of MS, extensive axonal pathology is also present. Postmortem analysis of brains of MS patients demonstrated axonal transection in both active and chronic lesions, the frequency of which was related to the degree of inflammation [4]. Furthermore, loss of parental cell bodies in the cortex has also been observed [5]. Experimental autoimmune encephalomyelitis (EAE), an animal model of MS induced by immunization with myelin components in adjuvant substances, shows comparable neuronal pathology, which renders this model suitable for both neuro- and immunopathological studies of autoimmune inflammatory demyelination.
Figure 1. Pathogenesis of experimental autoimmune encephalomyelitis and multiple sclerosis.
In the peripheral immune system, APCs (mainly DCs but also B cells and macrophages) produce IL-12, TGF-β and IL-6, and prime naive CD4+ T cells during activation by interaction of the T-cell receptor with cognate antigens presented by MHC class II complexes. Activated Th1 and Th17 cells cross the BBB into the CNS. Resident APCs interact with activated myelin-specific Th1 and Th17 cells, which become reactivated (Th2 cells can also be primed by APCs). Inflammatory cytokines and chemokines are produced and an inflammatory cascade is initiated. Infiltrating macrophages become activated and these, alongside antibodies, attack the myelin sheath on neurons.
APC: Antigen-presenting cell; BBB: Blood-brain barrier; NO: Nitric oxide; ROS: Reactive oxygen species; Th: T helper.
In this article, we will review the known roles of T cells in initiating and orchestrating autoimmune responses in EAE and MS, and define those that have been targeted for immunotherapy (Table 1).
Table 1.
Current and potential T-cell immunotherapies for multiple sclerosis.
| Treatments currently used | Mechanism/targets | Comments |
|---|---|---|
|
IFN-β: type I interferon with immunoregulatory and antiviral properties |
Targets T cells, antigen-presenting cells, and cells of the blood-brain barrier; induces the production of IL-10; other unknown mechanisms |
Most commonly used treatment in RRMS |
|
| ||
|
GA: random copolymer of alanine, lysine, glutamic acid and tyrosine |
GA can bind to HLA class II molecules including HLA DR2, and thereby inhibits the activation of myelin basic protein-reactive T cells |
Used as a treatment for RRMS |
|
| ||
|
Anti-adhesion molecule antibodies: directed against very late antigen-4 and other adhesion molecules (e.g., natalizumab) |
Blocks activated leukocytes from entering the CNS | Most potent licensed disease-modifying treatment for RRMS; concerns for immunosuppression in rare cases |
|
| ||
|
Immunosuppressants: used to generally suppress immune system reactions (e.g., azathioprine and mitoxantrone) |
Azathioprine is a purine-synthesis inhibitor, inhibiting the proliferation of cells, especially leukocytes Mitoxantrone is a stronger immunosuppressive drug that inhibits type II topisomerase |
Azathioprine has been used as an oral treatment for MS; Mitoxantrone is given intravenous and is used for more aggressive disease (RR or SP with relapses); There is caution regarding side effects, as the immune system is not functioning to its full potential |
| Treatments tested/under investigation | Mechanism/targets | Comments |
|---|---|---|
|
Tolerizing agents: the administration of tolerogenic forms of myelin to induce antigen-specific inhibition of disease |
Apoptosis of antigen specific T cells, anergy of T cells to antigens induction of antigen-specific cells that negatively regulate autoimmune cells |
Three types of administration intravenous, intranasal and oral; potential for individually tailored antigen-specific treatment |
|
| ||
|
Altered peptide ligands: MHC binding antigenic peptide modified by substitution of one or more amino acids to change the MHC or T-cell receptor binding characteristics |
Tolerance can be induced by mechanisms including partial activation of T cells and antagonism at the T-cell activation level |
Need to target specific MHC/HLA types to reduce potential side effects, including exacerbation of disease |
|
| ||
| Cytokine-based therapies: | ||
|
a) Anti-cytokine monoclonal antibodies Neutralization of cytokines relevant to disease (e.g., anti-IL-12 [ustekinumab]), anti-IL-23 anti-IL-17 |
Blocking of cytokines that are considered crucial in disease pathogenesis |
Limited efficacy to date |
|
b) lnduction of regulatory cytokines Those that suppress the differentiation of Th17 cells and/or promote the development of Tregs |
Cytokines that negatively regulate Th17 differentiation could have therapeutic potential (e.g., IL-27) |
New targets likely to appear as knowledge progresses |
|
| ||
|
RA: the oxidized form of vitamin A; immunomodulation |
RA induces the production of TGF-β that can convert naive T cells into Tregs. RA has also been found to inhibit IL-17 production |
Good characterization of pharmacokinetics in human therapy |
|
| ||
|
Tregs: naturally occurring Tregs can inhibit autoimmune diseases by suppressing the function of effector T cells |
Methods to expand populations of Tregs could provide the rapeutic potential by suppressing the function of effector Th1 and Th17 cells |
Cell-based therapy would be individualized |
|
| ||
|
Immunoregulators: used to generally suppress immune system reactions (e.g., fingolimod) |
Immunoregulators can reduce the number of Th cells in the CNS. Fingolimod reduces Th1 cytokines in CNS by antagonising sphingosine 1-phosphate receptor-1 on T cells, which in turn inhibits lymphocyte migration |
There is caution regarding side effects, as the immune system is not functioning to its full potential |
GA: Glatiramer acetate; MS: Multiple sclerosis; RA: Retinoic acid; RR: Relapsing-remitting; SP: Secondary progressive; Tregs: Regulatory T cells.
T-helper cells in MS & EAE
T-helper 1/T-helper 2
Until recent years, pathological processes in MS and EAE were thought to be initiated by myelin-reactive T helper (Th)1 cells. However, abundant evidence has implicated IL-17-producing Th17 cells as the main cells involved in the pathogenesis of autoimmune demyelination in EAE and MS (Figure 2) [6–10]. Originally it was thought that CD4+ effector T cells were divided into two lineages: Th1 and Th2. Th1 are induced by IL-12 and produce IFN-γ, while Th2 cells secrete IL-4, IL-5 and IL-13. Th1 cells can mediate proinflammatory or cell-mediated immune responses, whereas Th2 cells mainly promote certain types of humoral immunity [11]. The Th1/Th2 paradigm in the mouse was subsequently extended to human T cells [12].
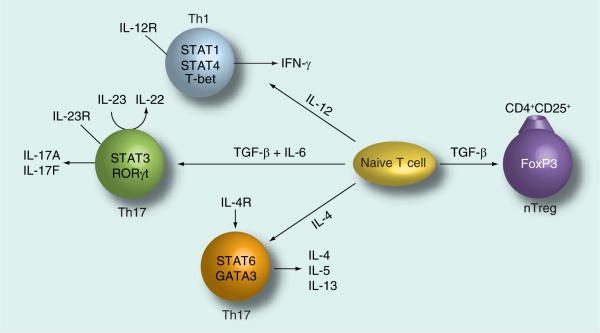
Figure 2. T-cell differentiation.
Naive T cells differentiate into four different subsets of effector cells depending on the cytokines present during initial activation. First, IL-12 induces a Th1 differentiation program via activation of the transcription factors, STAT1, STAT4 and T-bet resulting in IFN-γ production. Second, IL-4 induces Th2 differentiation via the transcription factors, STAT6 and GATA3 resulting in the production of IL-4, IL-5 and IL-13 by Th2 effector cells. Third, in the presence of IL-6, TGF-β drives Th17 differentiation via activation of the transcription factors RORγt and STAT3. IL-23 also supports the Th17 lineage of effector T cells, which produce IL-17A and IL-17F. However, when naive T cells are exposed to TGF-β only (without IL-6), the transcription factor FoxP3 is activated, resulting in the differentiation of regulatory T cells that produce IL-10.
Th: T helper; Treg: Regulatory T cell; nTreg: Naturally occurring Treg.
IL-12 is a heterodimeric cytokine formed by a large (p40) subunit and a small (p35) subunit. When it was discovered that IL-23 shares the p40 subunit with IL-12 [13], many questions were raised as to which cytokine was the key cytokine involved in disease. IL-12(p40)-deficient mice had been shown to be resistant to EAE [14]. However, it became clear after the discovery of IL-23 [13] that the deletion of IL-12(p40) had eliminated the expression of IL-12(p40p35) but also IL-23(p40p19) [15]. We and others then demonstrated that IL-12 was redundant in EAE susceptibility, as IL-12(p35)-knockout mice were in fact susceptible to disease [6,8]. In 2003, Cua and colleagues directly showed that IL-23 rather than IL-12 is crucial for autoimmune inflammation by using IL-23-knockout mice. The p19 subunit of IL-23 was knocked out and these mice were resistant to EAE [16]. IL-12 was in fact found to suppress EAE when administered to mice during the early phase of EAE induction. The induction of IFN-γ mediated this effect [17]. IL-23 was found to induce the production of IL-17 from both effector and memory CD4+ T cells, whereas IL-12 had only marginal effects on IL-17 production [18].
Th17/regulatory T cells
Langrish and colleagues clearly defined the role of IL-17-producing Th cells, which are driven by IL-23, in an adoptive transfer model of EAE. They cultured PLP-primed CD4+ T cells in vitro in the presence of IL-23. These cells that produce IL-17 were passively transferred into recipient mice, which developed severe EAE [10]. Komiyana and colleagues found that IL-17-deficient mice develop attenuated EAE [9]. After initial identification of the Th17 lineage, research has focused on Th17 regulation [19–25]. Th17 polarization has been shown to be supported by a number of cytokines such as IL-1γ, IL-23, IL-18, IL-22 and TNF-α. In addition, a surprising role of TGF-β in the presence of IL-6 has been elucidated. TGF-β induces naive T cells to produce IL-21, which then acts as a positive autocrine loop to upregulate the Th17-specific transcription factor RORγt [23,24]. Along with the inducers of Th17 polarization, a number of negative regulators have also been identified. IL-27, a new member of the IL-12/IL-23 family of heterodimeric cytokines, is a potent negative regulator of Th17 differentiation [25,26]. IL-2 is also a negative regulator, as this cytokine is important for the generation and survival of natural regulatory T cells (Tregs) expressing the transcription factor Foxp3 [27]. CD4+CD25hiFoxp3+ Tregs represent a naturally occurring immunoregulatory population of T cells. In contrast to effector T cells, Tregs protect against tissue damage and inhibit autoimmune disease [28]. Therefore, autoimmune responses appear to be regulated by a balance between effector cells and Tregs.
Role of T cells in EAE & MS
The important role of T cells in autoimmune inflammatory demyelination was established based on several lines of evidence; inflammatory lesions in the CNS of patients with MS were found to contain both CD4+ and CD8+ T cells [29]; adoptive transfer of immune cells, in particular Lyt 1+ 2− cells (later defined as CD4+ Th cells), from mice with EAE could transfer disease to healthy recipients [30,31]; depletion of CD4+ Th cells from mice with actively induced EAE led to disease suppression [30,32]; most therapies used in MS are in fact immunotherapies directed at T cells [33]; and genes that are associated with MS susceptibility are involved in antigen presentation to T cells or recognition of cytokine signals by T cells [34–36].
It should be noted that there are differences in the importance of different subsets of T cells between MS and EAE. EAE is mediated by CD4+ T cells. However, although CD4+ T cells dominate the perivascular regions of the inflammatory focus in EAE induced by MBP and PLP, MS lesions contain more CD8+ than CD4+ T cells. CD4+ T cells in MS lesions have been shown to have either pathogenic or neuroprotective functions dependent on the cytokines that are produced. Babbe and colleagues demonstrated that active MS lesions have a predominance of CD8+ T cells [37]. Upon further investigation it was found that expansion of the CD8+ T-cell repertoire was more antigen-driven than the CD4+ T-cell repertoire. Over-representation of CD8+ T cells was observed in cerebrospinal fluid of MS patients, and T cells were found to be stable over several months. Analysis of the TCR Vβ gene expression pattern indicated that in some patients, CD8+ T cells were clonally expanded [38].
Owing to the clear key role of T cells in MS and EAE, there has been a vast amount of research in the past two decades in an effort to elucidate new methods for T-cell immunotherapy. Some of the following immunotherapies are currently used, some have not been successful, and others warrant further study.
Principles of T-cell-based immunotherapies
Induction of tolerance
T cells have the ability to react to a variety of antigens, both self and nonself. Therefore, there are many mechanisms that exist naturally to eliminate potentially selfreactive responses – this is known as natural tolerance. The main mechanism for eliminating potential auto-reactive T cells occurs in the thymus and is known as central tolerance. Some potentially autoreactive T cells escape central tolerance and, therefore, peripheral tolerance mechanisms also exist. Despite these mechanisms, some selfreactive T cells may `escape' and be present in the repertoire, and their activation may lead to autoimmune diseases.
Studies on therapeutic tolerance have attempted to induce and amplify potent physiological mechanisms of tolerance in order to eliminate or neutralize selfreactive T cells and prevent or treat autoimmune diseases.
Experimental autoimmune encephalomyelitis can be induced via active immunization with CNS tissue or myelin peptides, such as MBP, PLP and MOG in complete Freund's adjuvant, or via adoptive transfer of CD4+ T cells. Several animal models of EAE have been developed over the last few decades. They include murine models such as the C57BL/6 mouse, which exhibits a monophasic or a chronic, sustained form of EAE induced by immunization with MOG35–55 (an immunodominant peptide of MOG) in complete Freund's adjuvant, and the SJL/J mouse, a relapsing model of EAE induced by immunization with PLP139–151 (an immunodominant peptide of PLP).
Antigen-specific inhibition of EAE can be achieved by administration of tolerogenic forms of myelin antigens. Tolerogenic antigens can be administered intravenously (iv.), intranasally or orally. Different administration routes may lead to different forms of tolerance induction [39]: clonal deletion – the elimination of antigen-specific cells by apoptosis; clonal anergy – the induction of functional hyporesponsiveness to antigens; and active suppression – the induction of an antigen-specific population of cells. This antigen-specific population negatively regulates the antigen-specific autoreactive cells. This type of suppression occurs in immune deviation where there is conversion from one Th phenotype (e.g., Th1) to another Th phenotype (e.g., Th2). The Th2 phenotype can then suppress disease by antagonising the Th1 phenotype, which is considered pathogenic [40].
Intravenous tolerance
Tolerance induction in naive T cells
High doses of iv.-injected soluble antigen can induce T-cell anergy or apoptosis. Liblau and colleagues demonstrated tolerance induced by both thymic and peripheral apoptosis and also T-cell-receptor desensitization in mice injected with high doses of antigen [41]. Kearney and colleagues demonstrated that the route of administration of antigen could determine the level of tolerance achieved and showed that iv.-injected antigen was much more effective than subcutaneous injection [42]. Tolerance could further be improved by chemically cross-linking peptide antigen to splenocytes with 1-ethyl-3-carbodiamide, instead of using soluble antigen. This method was found to inhibit spinal cord homogenate-induced chronic relapsing EAE in SJL mice [43–45]. In another model of chronic relapsing EAE using Biozzi ABH mice [46], disease severity was also found to be reduced by iv. injection of myelin antigen. However, if the treatment is not initiated until late stage of disease, relapses are inhibited but disease progression is not prevented [47].
The effect of time of iv. administration of peptide to induce tolerance was also studied in Lewis rats. MBP was administered at different times both before and after active immunization of rats to induce EAE. The treatment of rats with iv. MBP before immunization was most successful 14 days prior to immunization, whereas after immunization the most effective iv. administration was at onset of disease. Disease was suppressed in a dose-dependent manner at these time points. In adoptive transfer experiments, it was found that recipient mice must be treated with iv. MBP after, but not before, the transfer of MBP-reactive EAE effector cells [48]. The tolerization of Lewis rats after iv. MBP administration was found to be controlled at least partly by a T-cell control mechanism in the spleen and the role of Tregs was speculated [49].
More recently, we have focused on the role of antigen-presenting cells (APCs) in iv. tolerance. We studied the interaction between APC and CD4+ T cells in iv. tolerance. Surprisingly, we found that APCs that had been purified from MOG iv.-treated mice were immunostimulatory [50]. Following this we examined the function of distinct subsets of APCs with particular focus on dendritic cells (DCs). We found that the population of CD11b+CD11c+ DCs are expanded in the spleen and CNS after iv. injection of MOG peptide to induce tolerance. These DCs exhibit immunoregulatory activity by increasing IL-10 and TGF-β, and inducing the generation of CD4+CD25+ Tregs [51]. Recently, we have assessed whether Th17 cells are involved in the tolerance induced in mice after iv. MOG administration. We found a decrease in JAK/STAT-1,4, ERK1/2 and NF-κBp65 phosphorylation in iv.-tolerized mice. A reduction in IL-17 was also observed. We transferred CD11b+ splenocytes of tolerized mice into actively induced EAE mice and found that these splenocytes delayed disease onset and reduced severity of disease [52].
Tolerance induction in memory autoreactive T cells
T cells that escape natural tolerance may become selfreactive memory T cells if they become activated. Such selfreactive T cells play major roles in the development of autoimmune diseases and because of this, much effort has been placed on designing strategies to tolerize these cells. As memory T cells do not require costimulation to the same extent as naive T cells they are more difficult to tolerize [53]. Verbeek and colleagues studied the effectiveness of iv. injection of soluble antigen in eliminating memory T-cell response against αβ-crystallin, which is a candidate autoantigen in MS. They found a long-lasting specific tolerance induced by iv. administration [54].
Intranasal tolerance
The second route for inducing tolerance is intranasal administration. Bai and colleagues found that intranasal administration of MBP prevents relapses in the DA rat model of EAE where the rats were immunized with complete spinal cord homogenate. Prevention of relapses was associated with activation of regulatory cells expressing IL-4 and TGF-β [55]. Burkhart and colleagues studied intranasal administration of MBP in a mouse model of EAE. They found that intranasal tolerance was dependent on IL-10. They demonstrated this by using an IL-10-neutralizing antibody in vivo that restored susceptibility to EAE [56].
Oral tolerance
The third route for inducing tolerance is orally. Three different mechanisms of tolerance can be induced orally depending on the amount of oral antigen administered. Active suppression is favored by low doses, whereas clonal anergy or deletion is favored by higher doses of oral antigen. In the 1980s, the oral administration of antigen was found to suppress EAE [57,58] and, therefore, trials were initiated in patients. Weiner and colleagues found that the treatment of relapsing-remitting (RR) patients with bovine myelin containing MBP and PLP reduced the frequency of MBP-reactive T cells compared to controls. There was a tendency for the treated group to have less exacerbations and this was particularly evident in the subgroup of DR2-males [59]. It was later uncovered that oral antigen induces Th2 and Th3 (TGF-β-producing) T cells, CD4+CD25- and latency-associated peptide+ (LAP) T cells [60]. This group then found that oral administration of CD3-specific monoclonal antibody suppresses EAE. The suppression was observed before the induction of disease and also at the peak of disease, and was associated with the induction of such CD4+CD25-LAP+ Tregs. These cells were found to be dependent on TGF-β for their suppressive function both in vitro and in vivo [61].
Use of altered peptide ligands
The trimolecular complex of T-cell antigen recognition involves an immunodominant antigenic peptide bound to a MHC (human histocompatibility antigens [HLA] in humans) molecule recognized by a T-cell receptor (TCR). An altered peptide ligand (APL) is the MHC-binding antigenic peptide modified by substitution of one or more amino acids to change the MHC or TCR binding characteristics. This can induce tolerance to the original peptide through several mechanisms, including partial activation of T cells [62,63] and antagonism at the T-cell activation level [64].
Brocke and colleagues used an altered peptide ligand of MBP to treat EAE. They induced EAE with a T-cell clone that is specific for MBP p87–99. They tolerized the clone in vivo with an analog of MBP p87–99 (phenylalanine at residue 96 was replaced by alanine) and found that paralysis was reversed, inflammatory infiltrates regressed and brain T-cell infiltrates were depleted [65]. Interestingly, it was also found that the simple administration of the native MBP peptide was equally effective, indicating that a `tolerizing' injection before the `immunizing' one was sufficient to prevent disease [65].
The generation of TCR transgenic (Tg) mice specific for MBP provided a system of extreme importance for evaluating the mechanisms of disease development and regulation in EAE. Goverman and colleagues developed mice that were Tg for either TCR-α or TCR-β chains of a T-cell clone specific for MBP [66]. T-cell responses to peptides were observed in double-Tg mice and some mice developed spontaneous EAE [66,67]. Further studies by Lafaille and colleagues demonstrated that spontaneous EAE could be significantly enhanced by crossing the TCR-αβ Tg mice with recombination-activating gene-deficient mice that cannot rearrange endogenous TCR genes [68]. These results paved the way for studies using TCR in protection against EAE as they suggested that endogenous rearrangement using APLs may induce a population of Tregs that could prevent EAE. Offner and colleagues studied the immune regulation directed at TCR determinants using single TCR Tg mice expressing a TCR-β chain specific for MBP. They found that vaccination with the same target TCR protein amplifies TCR-specific regulation and protects against EAE [69].
`Humanized mice' that were Tg for HLA class II (human) molecules were also generated. These mice allowed for the testing of T-cell determinants that were vital in disease. Vandenbark and colleagues generated a HLD-DR2+ Tg mouse that lacked all MHC (murine) class II genes. These mice suffered from severe EAE after immunization with MOG35–55 peptide. They used these mice to test the efficacy of a novel recombinant TCR ligand in inducing tolerance. They found that this peptide could induce sustained tolerance in MOG-peptide induced EAE [70]. More recently, the human MBP84–102 epitope has been genetically linked to the HLA-DR2 by two heterodimeric receptors where a TCR-ζ signaling domain is either present or absent. The aim of this study was to use chimeric receptors to target therapeutic T cells against selfreactive T cells. The MBP-specific T cells were attacked by the ζ-modified cytotoxic T lymphocyte (CTL) and this CTL was found to block EAE [71].
The studies of Tg mice expanded to different regions of HLA-DR in addition to DR2. Ito and colleagues developed HLA-DR4-IE and HLA-DRB1*0401-IE β chimeric Tg mice and found these mice to be susceptible to EAE [72]. Muraro and colleagues studied HLA-DRB-restricted MBP111–129 as MBP111–129 is the immunodominant epitope in humans carrying HLA-DRB1*0401 [73]. They used HLA-DRB1*0401 Tg mice to study the pathogenic potential of HLA-DRB1*0401-restricted MBP111–129 and found that this was indeed pathogenic [73,74]. Glatiramer acetate (GA) is a therapy used in MS that binds HLA tightly and can displace MBP [75] (see `Immunotherapies used in MS' below).
The use of TCR peptides was not only being studied in EAE, but also for the treatment of MS [76]. The hypothesis behind these experiments was as follows: as both α- and β-TCR chains are expressed in abundance, these unassembled TCR chains, or peptides thereof, could be presented on the surface of T cells in association with self-MHC molecules. This would provide MHC-restricted targets that could combine specifically with the TCR of Tregs (which were thought to be Th2 cells). Vaccination of a peptide highly similar to an autoreactive TCR fragment would enhance the regulatory Th2 priming and recognition that would control the Th1-mediated inflammation. This procedure was tested in patients and was found to be well tolerated and also showed modest signs of clinical improvement [77].
However, there were some major concerns raised about the use of APLs for immunotherapy, as a study by Bielekova and colleagues demonstrated that APLs could actually exacerbate MS. They used an MBP APL in a Phase II clinical trial and found that three patients developed exacerbations, two of which were shown to be linked to the APL [78]. This study highlighted the level of caution needed when transferring a therapy that has been successful in animal models into humans.
Anti-cytokine monoclonal antibodies Anti-IL-12
IL-12 is a Th1 cytokine that was previously considered to be the main cytokine involved in disease pathogenesis in EAE and possibly in MS. As a result of this, much effort was placed into designing ways to decrease IL-12 production. Leonard and colleagues induced relapsing EAE in SJL/J mice by adoptive transfer of PLP peptide-specific T cells and found that administration of sheep-polyclonal anti-IL-12 antibody prevented the transient mild course of demyelinating neurological disease [79]. The treatment had to be prolonged for a dramatic reduction in disease severity to be observed. Constantinescu and colleagues also studied the effect of anti-IL-12 in relapsing EAE and found that when the antibody is administered after recovery from initial attack, spontaneous relapses are prevented [80]. Ichikawa and colleagues studied the effect of anti-IL-12 antibody in chronic relapsing EAE induced with MOG peptide. They observed suppression of disease clinically and histologically. Spleen cells of mice treated with anti-IL-12 antibody had a reduced proliferative response to MOG peptide, and IFN-γ production was also decreased [81].
The effect of the IL-12/23p40-neutralizing monoclonal antibody (ustekinumab) was recently tested in RRMS patients to assess the drug's safety and efficacy. This Phase II trial included 249 patients with RRMS. Patients were followed for 37 weeks and ustekinumab was generally well tolerated. However, there was no significant reduction in disease after ustekinumab treatment as assessed by the number of gadolinium-enhancing T1-weighted lesions [82].
Anti-IL-23
IL-23 has been found to be crucial in driving a pathogenic T-cell response, and results in the expansion and maintenance of Th17 cells. Therefore, it was vital that the effects of neutralizing IL-23 be examined. As IL-23 shares the p40 subunit with IL-12, an antibody specific to the p19 subunit of IL-23 was required. Chen and colleagues developed anti-IL-23p19 and tested the efficacy in EAE. Treatment with the antibody resulted in reduced IL-17, IFN-γ, IL-10, IL-6 and TNF in the CNS. Epitope spreading of PLP was prevented and this resulted in prevention of disease relapse [7].
Anti-IL-17
As mentioned above, recent data have shown that IL-17 is the main cytokine involved in EAE and not IL-12. Hofstetter and colleagues studied the pathogenic role of IL-17 in EAE and they also tested the effect of neutralization of IL-17 on disease severity. They neutralized IL-17 with IL-17-receptor-Fc-protein, which was found to slightly reduce clinical symptoms. Neutralization with an IL-17 monoclonal antibody was also tested and found to reduce disease severity, but not to the degree that was expected. It was hypothesized that other factors can compensate for the lack of IL-17. This view was confirmed by reduced EAE severity rather than complete resistance to disease in IL-17−/− mice [83].
Translational pitfalls
One has to be extremely careful when transferring knowledge from animal models to humans. Therapies that are successful in animal models may be unsuccessful and possibly dangerous in humans. An example of this can be seen from the `cytokine storm' incident of 2006. TGN1412, which is an anti-CD28 monoclonal antibody, was administered to six healthy young male volunteers in Phase I of a clinical trial. All six volunteers developed a systemic inflammatory response and became critically ill, with renal failure, pulmonary infiltrates and lung injury. The volunteers were transferred to intensive care where they received dialysis and anti-IL-2 receptor antagonist antibody. Two of the patients required organ support for 8 and 16 days. Even though all patients survived, this episode highlights the need for the extreme care to be taken when administering successful animal-model treatments to humans [84].
Therapies used in MS
The following therapies are used or under investigation for the treatment of MS. Many of the exact mechanisms of action are unknown; therefore, we are unsure if these are T-cell therapies. However, all exert some effect on T cells and are thus discussed here.
IFN-β
IFN-β is the most commonly used treatment for RRMS patients, although the mechanisms of action are still poorly understood. There are three commercially available IFN-β formulations, two forms of IFN-β1a (produced in eukaryotic cells, fully glycosylated, for subcutaneous or intramuscular injection) and one form of IFN-β1b (produced in prokaryotic cells, not glycosylated, for subcutaneous injection). IFN-β has been shown to be a Th1 and more recently a Th17 inducer, therefore it is a paradox that it can treat disease. It does, however, reduce T-cell activation by inhibiting the upregulation of HLA class II and thereby counteracting IFN-γ effects [85,86]. IFN-β is also thought to affect costimulatory molecule interaction such as CD40–CD40L [87] and also B7–CD28 [88]. Immune deviation can also be a result of IFN-β treatment where IL-10, a regulatory cytokine, is induced [89,90]. Another effect of IFN-β is on the blood–brain barrier. T-cell adhesion to the endothelium is thought to be interfered with, and also an increase in soluble vascular cell adhesion molecule (sVCAM) has been observed [91,92]. This sVCAM has been correlated with a reduction in disease severity as measured by gadolinium-enhancing lesions [91]. IFN-βs are biologically active on other immune-cell types, including APCs. The relevance of these actions to MS immunotherapy are being investigated [93,94].
Glatiramer acetate
Glatiramer acetate is a random copolymer of alanine, lysine, glutamic acid and tyrosine that is used in the treatment of RRMS. It was first studied in EAE and later was approved for use in MS patients. The effect of GA on T-cell clones was examined and it was found that there is preferential inhibition of self-reactive T-cell clones after treatment with GA. GA was cross-recognized by myelin basic protein-reactive T cells [95]. GA can bind to HLA class II molecules, including HLA-DR2, and thereby inhibit the activation of MBP-reactive T cells [75,96]. In human T-cell lines, it was found to induce proliferation without prior exposure to polymer; however, in MS patients that received daily injections of GA, this response was not present. GA was found to induce a Th2 rather than a Th1 response, which is usually present in MS patients [97]. GA not only has an effect on T cells but also has effects on APCs. GA was found to bind to MHC molecules on APCs and therefore competes with MBP binding to HLA [75]. GA has an inhibitory effect on monocyte reactivity. Activation of monocytes after TLR stimulation was considerably reduced after GA treatment [98]. GA activates type II monocytes that secrete IL-10 and TGF-β, and reduces the production of IL-12 and TNF. These type II monocytes were found to differentiate Th2 cells and Tregs [99]. In summary, the mechanism of action of GA is at least partly T-cell mediated, although other studies indicate that APCs are also involved. Neuroprotective mechanisms have also been proposed [100].
Natalizumab
The development of natalizumab arose from studies in EAE models where antibodies against α4b1 integrin were found to prevent disease. This was associated with a prevention of leukocytes from accumulating in the CNS [101]. Natalizumab was later developed based on this principle, and it is an antibody against very late activating antigen. It blocks the endothelial interaction, thereby blocking entry of leukocytes into the CNS, and has been found to be a successful treatment for MS. CSF leuckocyte counts of CD4+ and CD8+ T cells, CD19+ B cells and CD138+ plasma cells have all been found to be lower in MS patients treated with natalizumab compared with controls [102]. Natalizumab has also recently been approved for therapy in Crohn's disease. In spite of the very large number of MS patients treated worldwide, there is still some concern regarding safety, as rare cases of progressive multi-focal leukoencephalopathy (PML) have been reported. PML is a severe opportunistic infection of the brain that appears to arise owing to the lack of immune surveillance after natalizumab treatment, usually but not exclusively, when patients have also received additional immunotherapies [103].
Immunosuppressive agents
Immunosuppressive agents are drugs that inhibit or prevent activity of the immune system. However, because they dampen immune responses and are often nonspecific, there is concern over side effects such as being unable to resist infections. Azathioprine inhibits purine synthesis and cell proliferation, in particular that of leukocytes. It has been used as an oral treatment for MS where it has been found to reduce the relapse rate and be effective on MRI parameters of inflammation [104]. There was some concern over the risk of cancer after azathioprine treatment; however, the doses used to treat MS patients are quite low and overall safety data have been reassuring [105,106]. Mitoxantrone is a stronger immunosuppressive drug that inhibits Type II topoisomerase. It disrupts DNA synthesis and DNA in both healthy and cancer cells. As a Type II topoisomerase it inhibits the proliferation of T and B cells and macrophages [107]. Mitoxantrone is administered intravenously and is used for more aggressive disease (RR or secondary progressive with relapses) [108]. There are some concerns for myelosuppressive effects and cardiac toxicity and, therefore, there is a limit on the dose a patient can receive in a lifetime [109].
Cladribine
Cladribine is a lympholytic drug that is used in the treatment of chronic progressive MS patients and is generally well tolerated [110]. Cladribine targets lymphoctyes and can kill activated myelin-reactive T cells [111]. Cladribine also affects blast cells as well as mature cells. The efficacy of cladribine had been debated as it was found not to affect disability as measured by the expanded disability status score (EDSS) [112]. It was proposed for use in long-term patients but not as a first-line treatment of MS [113]. However, a recent trial for the use of cladribine in RR patients has shown that after a 2-year period, the EDSS of patients treated with cladribine remained stable and the need for steroid treatment was reduced. Cladribine administration was associated with lower lymphocyte counts [114]. Therefore, this therapy is showing more potential for the treatment of MS than originally anticipated.
Fingolimod
Fingolimod (FTY720) was developed as an immunomodulator for use in organ transplantation [115]. It was later tested for its efficacy in EAE. It was found to protect Lewis rats from EAE and this was associated with a reduction in Th1 cytokines in the CNS [116]. FTY720 antagonises sphingosine 1-phosphate receptor-1 on T cells and inhibits the S1P/SIP1-dependent lymphocyte migration from the lymph node. It is under investigation as an oral therapy for MS. FTY720 reduces the number of naive T cells and central memory T cells in the blood, but does not affect effector T cells [117]. FTY720 limits the efflux of immune cells from the lymph nodes, which reduces peripheral lymphocyte counts and the recirculation of lymphyocytes to the CNS [118ȓ120]. A recent study of efficacy and safety has shown that FTY720 is well tolerated and reduces relapse rate and disease activity as measured by MRI [121].
Daclizumab
Daclizumab is a monoclonal antibody against CD25 (anti-IL2-Rα). IL-2Rα is upregulated on activated or abnormal T cells such as those in autoimmune diseases, and thus it was chosen as a target for therapy in MS [122]. Daclizumab has been well tolerated and has been found to be successful as both an add-on treatment to IFN-β and a standalone treatment [123,124]. The anticipated mode of action of daclizumab was that it would block the proliferation of activated autoreactive T cells; however, studies have found that this may not be the most important effect of treatment. Consistently, a striking expansion of a subset of natural killer (NK) cells has been observed. These NK cells are CD16–CD56bright and have immunoregulatory as well as antiviral and antitumor properties. The expansion of these CD56bright NK cells is correlated with a reduction in contrast-enhancing MRI lesions [125].
Alemtuzumab
Alemtuzumab is a monoclonal antibody against CD52, which is a cell-surface glycoprotein expressed on T and B cells, eosinophils and monocytes. It is expressed on mature lymphocytes, but not on lymphoid progenitor cells, which makes it a good candidate for therapy. It is used in the treatment of chronic lymphocytic leukaemia and has been found to impressively reduce relapses in RRMS patients and also to significantly reduce the rate of sustained neurological disability [126]. The mode of action of alemtuzumab is to deplete lymphocytes. CD4+ T cells are depleted for approximately 60 months, and monocytes and B cells are depleted for 3 months [127]. Because of this lymphocyte depletion there are concerns regarding the adverse effects. It is associated with a risk of secondary antibody-mediated diseases such as Graves disease [128]. A recent study has demonstrated that secondary autoimmunity after treatment with alemtuzumab occurs in patients that have genetically higher levels of IL-21 prior to treatment that leads to higher levels of T-cell apoptosis and cell cycling [129]. However, as the efficacy of alemtuzumab is impressive, there are currently two Phase III trials underway to determine the safety of the drug.
Teriflunomide
Teriflunomide is the active metabolite of leflunomide, a disease-modifying agent used in the treatment of rheumatoid arthritis. It is a pyrimidine-synthesis inhibitor with selective immunosuppressive and immunomodulatory properties [130]. It can inhibit T and B lymphocyte proliferation [131]. Teriflunomide has been successful in treating EAE and is now in Phase III clinical trials as an oral treatment for MS.
Retinoic acid
Retinoic acid (RA) has been implicated as a suppressive agent in EAE for many years. Massacesi and colleagues found that EAE can be prevented in the Lewis rat by two forms of RA [132]. It came to light that RA induces the production of TGF-β [133]. More light has now been shed on this topic since it was discovered that TGF-β can convert naive T cells into Tregs, which can suppress autoimmune diseases. Thus, if RA can induce TGF-β, this can then reduce disease severity by the induction of Tregs. It is very interesting to note that TGF-β in the presence of IL-6 can result in the differentiation of naive T cells into Th17 cells. The role of retinoic acid was studied with regards to which differentiation pathway TGF-β would lead T cells into. Mucida and colleagues found that retinoic acid regulates the TGF-β response and can drive the differentiation of Tregs and also inhibit the production of IL-17 [134].
Potential targets
Regulatory T cells
CD4+CD25+Foxp3+ Tregs occur naturally in the thymus and control effector T-cell responses in the periphery. TGF-β can act on naive T cells to induce Foxp3 and generate induced Tregs that suppress effector T-cell immune responses. However, in the presence of TGF-β and IL-6 there is a predominant generation of Th17 cells [19]. This suggests that not only is there an antagonistic relationship between Th17 and Tregs, there is also a dichotomy in their generation. During a steady state in the immune system in the absence of inflammation, TGF-β induces the differentiation of Foxp3+ Tregs. However, once inflammation is initiated, IL-6 is produced by the activated innate immune system and this suppresses the generation of TGF-β-induced Tregs and generates Th17 cells [135]. There is a Treg population present in the CNS during EAE. These Tregs are functional in suppressing MOG-specific responses of naive T cells, but they fail to inhibit antigen-specific effector T cells that have been isolated from the target organ at the acute disease phase [136]. Kohm and colleagues adoptively transferred Tregs into EAE mice and found suppression of disease [137]. Viglietta and colleagues found a significant decrease in function of Tregs from peripheral blood of MS patients compared with healthy controls [138]. However, recent studies have paradoxically shown that human Tregs produce IL-17, and these IL-17-producing Tregs can still retain their suppressive function [139]. Tregs offer therapeutic potential for MS, however further study is warranted before their therapeutic potential can be reached.
IL-21
IL-21 belongs to the IL-2 family of cytokines and is critical in the differentiation of Th17 cells. In the absence of IL-6, IL-21 has been found to cooperate with TGF-β to induce the differentiation of Th17 cells [23]. IL-6-induced expression of IL-21 leads to further IL-21 induction by an autocrine loop, which also induces the IL-23 receptor (IL-23R) in naive CD4+ T cells [140]. IL-23 function is at least partly dependent on IL-21, which renders Th cells susceptible to IL-23 signals. IL-21 effects are dependent on the transcription factor STAT3, which is required for the differentiation of Th17 cells in vivo [140,141]. IL-23 induces the orphan nuclear receptor RORγt. RORγt in turn synergizes with STAT3 to promote the expression of IL-17 [142].
Interestingly, IL-21 also appears to mediate secondary autoimmunity in MS patients after treatment with alemtuzumab [129]. A monoclonal antibody against IL-21 could offer therapeutic potential in MS.
IL-22
IL-22 is a member of the IL-10 family of cytokines. However, unlike IL-10, it exhibits pro-inflammatory properties. IL-22 is secreted by Th17 cells and promotes antimicrobial defenses [21]. The IL-22R is a heterodimeric receptor complex that is composed of the IL-22R1 and IL-10R2 chains [143]. IL-22 does not itself directly regulate the function of CD4+ T cells as IL-22R is not expressed on these cells [144]. In vitro, the secretion of large quantities of IL-22 by Th17 cells requires IL-23, therefore it is thought that it is produced by terminally differentiated Th17 cells [145]. Becher and colleagues generated IL-22-knockout mice and found that they were fully susceptible to EAE, which indicates that IL-22 is not crucial to the development of EAE [146]. Nevertheless, further study is needed to elucidate its exact role in autoimmune diseases.
Negative regulation of Th17 differentiation: IL-27
IL-27 is a heterodimeric cytokine that is a member of the IL-12/IL-23 superfamily. IL-27 signals through WSX-1, and mice lacking this receptor develop increased pathological inflammation during Th1 and Th2 responses, indicating an anti-inflammatory role of IL-27. IL-27 has also been found to suppress Th17 responses [26]. This suppression is STAT1 dependent. Batten and colleagues demonstrated that mice lacking the IL-27 receptor are hyper-sensitive to actively induced EAE and show stronger Th17 responses [25]. For the purposes of immunotherapy it is critical to determine the phase of EAE at which IL-27 suppresses disease. We examined the effect of exogenous administration of IL-27 and found that over a 7-day period of subcutaneous osmotic pump administration, disease was suppressed, indicating a therapeutic potential of this cytokine. We also studied an adoptive transfer model of EAE and showed that Th17 effector responses are suppressed after IL-27 treatment, and the potential to transfer disease is reduced [147]. We and others later found that suppression of EAE by IL-27 was dependent on IL-10 [26,148,149]. The exact role of IL-27 in MS needs to be elucidated; however, given its role in EAE, it may be therapeutically relevant in MS.
Th9 cells
Until recent years, the lineages of Th cells were divided into Th1 and Th2. Th17 was the next addition and now it seems that there may be yet another Th lineage: Th9, independently described last year by both Veldhoen and colleagues and Dardalhon and colleagues [150,151]. They demonstrated that TGF-β can divert Th2 cells to secrete IL-9. The differentiation of Th9 cells can inhibit Foxp3 expression, thereby suppressing the generation of Tregs [150]. IL-9-producing T cells also produce IL-10. However, these IL-10-producing cells have lost their immunosuppressive function and may even be involved in tissue inflammation. Therefore, the Th9 subset is a new potential target for autoimmune diseases, including EAE and MS.
Conclusion
In the past two decades, significant advancement has been made in the treatment of MS. However, therapies are still only partially effective. We believe that as our understanding of the pathophysiology of MS continues to develop this will pave the way for future drug development directed at these discoveries. We must note that extreme caution has to be taken when transferring a successful treatment in EAE into humans. This can be highlighted from previous failed clinical trials that were successful in EAE. An open-label trial in 16 patients was performed on a monoclonal antibody, anti-CD3. The anti-CD3 infusions were associated with significant toxicity where patients developed severe T-cell cytopenia and many adverse effects, including hypotension and nausea. An increase in IFN-γ and TNF-α levels was observed and, although there were no new MRI lesions, disease worsened [152]. Another example of failed translational immunotherapy can be seen in the trial of anti-CD4 monoclonal antibody. The Phase I trial results of this antibody were promising, as there was no major toxicity, and a long lasting reduction in CD4 T cells was observed [153,154]. However in the Phase II clinical trial there was no effect on lesions as measured by gadolinium-enhanced MRI, and EDSS was also not affected [155]. MS is a disease where many different components of the immune system interact and the importance of each component also depends on a number of variables including genetic and environmental factors. Therefore, using a therapy directed at one of these components obviously has its drawbacks. We are constantly gaining a greater insight into the pathogenesis of both MS and EAE and, therefore, we should be optimistic that new therapeutic opportunities will arise.
Future perspective
Considerable effort is going into the development of oral immunotherapies, an innovation that most MS patients would welcome. As treatment options increase, we anticipate that in the next 5–10 years it will be possible to increasingly tailor immunotherapies to the characteristics of individual patients. Variables such as age, gender, disease duration, severity as measured clinically but also radiologically, previous responses to other treatments, risk/benefit ratios and, of course, patient goals and preferences will be increasingly used to make therapeutic decisions. Administration of immunotherapies at earlier stages of disease, when they are more likely to be effective, is also expected to increase. There is also agreement among experts that combination therapy will be given more frequently, again taking into account the above mentioned factors and, importantly, specific interactions and synergy between different drugs. The fact that MS is more prevalent in affluent countries makes it likely that availability of treatments will be extended to larger percentages of patients affected by this debilitating disease.
Executive summary.
-
▪
CD4+ T helper (Th) type 1 (Th1) and Th17 cells are thought to initiate and coordinate immune responses that lead to tissue damage in experimental autoimmune encephalitis and multiple sclerosis (MS). By contrast, regulatory CD4+ T cells, and to some extent Th2 cells, are considered anti-inflammatory and protective. However, the immunological scenario of immune-mediated CNS damage is more complex and the contribution of these and novel T-cell subsets continues to be clarified.
-
▪
CD8+ T cells participate in the pathogenesis of autoimmune inflammatory demyelination and are found in higher numbers than CD4+ T cells in MS lesions. In the spinal fluid of patients with MS, memory CD8+ T cells have been reported to be selectively enriched, suggesting a role for these cells in the pathogenesis of the disease.
-
▪
Induction of immune tolerance to myelin antigens such as proteolipid protein, myelin oligodendrocyte glycoprotein and myelin basic protein is an important strategy for MS immunotherapy. Tolerance can be induced via different routes, including intravenous, intranasal and oral administration of antigen, aiming at the deletion of autoreactive T-cell clones, the induction of clonal anergy (unresponsiveness to antigen), or the expansion of antigen-specific T cells secreting anti-inflammatory cytokines.
-
▪
Anti-cytokine antibodies offer the opportunity to neutralize proinflammatory cytokines. Theoretically they are an important tool, but in clinical trials these antibodies have not been particularly successful thus far. Monoclonal antibodies aimed at blocking the interaction of activated T cells with the endothelium (anti-VLA4) or at depleting CD4+ T cells as well as other immune cells (anti-CD52) appear much more effective in suppressing inflammatory activity in the CNS.
-
▪
The unexpected development of a cytokine storm and systemic inflammatory response syndrome in a group of healthy volunteers treated with an anti-CD28 antibody underscores the difficulty in predicting the biological effects of antibodies proven to be safe and effective in preclinical animal models. This is relevant to T-cell directed therapies (the above mentioned antibody was a humanized superagonist anti-CD28 monoclonal antibody that stimulates and expands T cells), but also to antibody therapies directed at other immune cell populations.
-
▪
Both established and innovative T-cell therapies used in MS very often have additional effects on other immune cell populations, including B cells and different types of antigen-presenting cells. In some cases these effects are known (e.g., anti-CD52 depletes CD4+ and CD8+ T cells, as well as monocytes and macrophages), whereas in others cases the effects are less clear or are under investigation.
Footnotes
Financial & competing interests disclosure The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N. Engl. J. Med. 2000;343(13):938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 3.Grigoriadis N, Hadjigeorgiou GM. Virus-mediated autoimmunity in multiple Sclerosis. J. Autoimmune Dis. 2006;3(1) doi: 10.1186/1740-2557-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998;338(5):278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 5.Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 2001;50(3):389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 6.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest. 2002;110(4):493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Langrish CL, McKenzie B, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 2006;116(5):1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gran B, Zhang G-X, Yu S, et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J. Immunol. 2002;169(12):7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 9.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177(1):566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 10.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 12.Romagnani S, Parronchi P, D'Elios MM, et al. An update on human Th1 and Th2 cells. Int. Arch. Allergy Immunol. 1997;113(1–3):153–156. doi: 10.1159/000237532. [DOI] [PubMed] [Google Scholar]
- 13.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 14.Segal BM, Shevach EM. IL-12 unmasks latent autoimmune disease in resistant mice. J. Exp. Med. 1996;184(2):771–775. doi: 10.1084/jem.184.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segal BM, Dwyer BK, Shevach EM. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J. Exp. Med. 1998;187(4):537–546. doi: 10.1084/jem.187.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 17.Gran B, Chu N, Zhang GX, et al. Early administration of IL-12 suppresses EAE through induction of interferon-γ. J. Neuroimmunol. 2004;156(1–2):123–131. doi: 10.1016/j.jneuroim.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal S, Ghilardi N, Xie M-H, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278(3):1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006 doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 20.Gutcher I, Urich E, Wolter K, Prinz M, Becher B. Interleukin 18-independent engagement of interleukin 18 receptor-α is required for autoimmune inflammation. Nat. Immunol. 2006;7(9):946–953. doi: 10.1038/ni1377. [DOI] [PubMed] [Google Scholar]
- 21.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203(10):2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGF-b in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]; ■■ The first report to show that TGF-β in the presence of IL-6 induces T-helper (Th)17 differentiation.
- 23.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ [23] and [24] show that IL-21 cooperates with TGF-β to induce Th17 differentiation.
- 24.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]; ■ [23] and [24] show that IL-21 cooperates with TGF-β to induce Th17 differentiation.
- 25.Batten M, Li J, Yi S, et al. Interleukin-27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 2006;7(9):929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 26.Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin-27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7(9):937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 27.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Ann. Rev. Immunol. 2004;22(1):531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 29.Traugott U, Reinherz EL, Raine CS. Multiple sclerosis. Distribution of T cells, T-cell subsets and Ia-positive macrophages in lesions of different ages. J. Neuroimmunol. 1983;4(3):201–221. doi: 10.1016/0165-5728(83)90036-x. [DOI] [PubMed] [Google Scholar]
- 30.Brostoff SW, Mason DW. Experimental allergic encephalomyelitis: successful treatment in vivo with a monoclonal antibody that recognizes T helper cells. J. Immunol. 1984;133(4):1938–1942. [PubMed] [Google Scholar]
- 31.Pettinelli CB, McFarlin DE. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2-T lymphocytes. J. Immunol. 1981;127(4):1420–1423. [PubMed] [Google Scholar]
- 32.Waldor MK, Sriram S, Hardy R, et al. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985;227(4685):415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- 33.Hemmer B, Hartung HP. Toward the development of rational therapies in multiple sclerosis: what is on the horizon? Ann. Neurol. 2007;62(4):314–326. doi: 10.1002/ana.21289. [DOI] [PubMed] [Google Scholar]
- 34.Compston A. The genetics of multiple sclerosis. J. Neurovirol. 2000;6(Suppl 2):S5–S9. [PubMed] [Google Scholar]
- 35.Hafler DA, Compston A, Sawcer S, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 2007;357(9):851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 36.Haines JL, Ter-Minassian M, Bazyk A, et al. A complete genomic screen for multiple sclerosis underscores a role for the major histocompatability complex. The Multiple Sclerosis Genetics Group. Nat. Genet. 1996;13(4):469–471. doi: 10.1038/ng0896-469. [DOI] [PubMed] [Google Scholar]
- 37.Babbe H, Roers A, Waisman A, et al. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 2000;192(3):393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobsen M, Cepok S, Quak E, et al. Oligoclonal expansion of memory CD8+ T cells in cerebrospinal fluid from multiple sclerosis patients. Brain. 2002;125(3):538–550. doi: 10.1093/brain/awf059. [DOI] [PubMed] [Google Scholar]
- 39.Miller A, Hafler DA, Weiner HL. Tolerance and suppressor mechanisms in experimental autoimmune encephalomyelitis: implications for immunotherapy of human autoimmune diseases. FASEB J. 1991;5(11):2560–2566. doi: 10.1096/fasebj.5.11.1868980. [DOI] [PubMed] [Google Scholar]
- 40.Racke MK, Bonomo A, Scott DE, et al. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J. Exp. Med. 1994;180(5):1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liblau RS, Tisch R, Shokat K, et al. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc. Natl Acad. Sci. USA. 1996;93(7):3031–3036. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction. in vivo. Immunity. 1994;1(4):327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 43.Miller A, Zhang ZJ, Sobel RA, al-Sabbagh A, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. VI. Suppression of adoptively transferred disease and differential effects of oral vs. intravenous tolerization. J. Neuroimmunol. 1993;46(1–2):73–82. doi: 10.1016/0165-5728(93)90235-q. [DOI] [PubMed] [Google Scholar]
- 44.Miller SD, McRae BL, Vanderlugt CL, et al. Evolution of the T-cell repertoire during the course of experimental immune-mediated demyelinating diseases. Immunol. Rev. 1995;144:225–244. doi: 10.1111/j.1600-065x.1995.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 45.Tan JK, O'Neill HC. Maturation requirements for dendritic cells in T-cell stimulation leading to tolerance versus immunity. J. Leukoc. Biol. 2005;78(2):319–324. doi: 10.1189/jlb.1104664. [DOI] [PubMed] [Google Scholar]
- 46.Baker D, O'Neill JK, Gschmeissner SE, et al. Induction of chronic relapsing experimental allergic encephalomyelitis in Biozzi mice. J. Neuroimmunol. 1990;28(3):261–270. doi: 10.1016/0165-5728(90)90019-j. [DOI] [PubMed] [Google Scholar]
- 47.Pryce G, O'Neill JK, Croxford JL, et al. Autoimmune tolerance eliminates relapses but fails to halt progression in a model of multiple sclerosis. J. Neuroimmunol. 2005;165(1–2):41–52. doi: 10.1016/j.jneuroim.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Hilliard B, Ventura ES, Rostami A. Effect of timing of intravenous administration of myelin basic protein on the induction of tolerance in experimental allergic encephalomyelitis. Mult. Scler. 1999;5(1):2–9. doi: 10.1191/135245899701564308. [DOI] [PubMed] [Google Scholar]
- 49.Hilliard BA, Kamoun M, Ventura E, Rostami A. Mechanisms of suppression of experimental autoimmune encephalomyelitis by intravenous administration of myelin basic protein: role of regulatory spleen cells. Exp. Mol. Pathol. 2000;68(1):29–37. doi: 10.1006/exmp.1999.2290. [DOI] [PubMed] [Google Scholar]
- 50.Zhang GX, Yu S, Li Y, et al. A paradoxical role of APCs in the induction of intravenous tolerance in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005;161(1–2):101–112. doi: 10.1016/j.jneuroim.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Zhang GX, Chen Y, et al. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J. Immunol. 2008;181(4):2483–2493. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang Z, Li H, Fitzgerald DC, Zhang GX, Rostami A. MOG(35-55) i.v suppresses experimental autoimmune encephalomyelitis partially through modulation of Th17 and JAK/STAT pathways. Eur. J. Immunol. 2009;39(3):789–799. doi: 10.1002/eji.200838427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J. Immunol. 2000;164(1):265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 54.Verbeek R, van der Mark K, Wawrousek EF, Plomp AC, van Noort JM. Tolerization of an established αβ-crystallin-reactive T-cell response by intravenous antigen. Immunology. 2007;121(3):416–426. doi: 10.1111/j.1365-2567.2007.02592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai XF, Shi FD, Xiao BG, et al. Nasal administration of myelin basic protein prevents relapsing experimental autoimmune encephalomyelitis in DA rats by activating regulatory cells expressing IL-4 and TGF-β mRNA. J. Neuroimmunol. 1997;80(1–2):65–75. doi: 10.1016/s0165-5728(97)00133-1. [DOI] [PubMed] [Google Scholar]
- 56.Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int. Immunol. 1999;11(10):1625–1634. doi: 10.1093/intimm/11.10.1625. [DOI] [PubMed] [Google Scholar]
- 57.Bitar DM, Whitacre CC. Suppression of experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. Cell Immunol. 1988;112(2):364–370. doi: 10.1016/0008-8749(88)90305-x. [DOI] [PubMed] [Google Scholar]
- 58.Higgins PJ, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein and its fragments. J. Immunol. 1988;140(2):440–445. [PubMed] [Google Scholar]
- 59.Weiner HL, Mackin GA, Matsui M, et al. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993;259(5099):1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 60.Faria AM, Weiner HL. Oral tolerance. Immunol. Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ochi H, Abraham M, Ishikawa H, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+CD25−LAP+ T cells. Nat. Med. 2006;12(6):627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]; ■ Immune tolerance was induced by administration of oral CD3-specific antibody. Tolerance correlated to the expansion of a CD4+CD25−LAP+ population of regulatory T cells.
- 62.Evavold BD, Allen PM. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991;252(5010):1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- 63.Racioppi L, Ronchese F, Matis LA, Germain RN. Peptide-major histocompatibility complex class II complexes with mixed agonist/antagonist properties provide evidence for ligand-related differences in T cell receptor-dependent intracellular signaling. J. Exp. Med. 1993;177(4):1047–1060. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Magistris MT, Alexander J, Coggeshall M, et al. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992;68(4):625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 65.Brocke S, Gijbels K, Allegretta M, et al. Treatment of experimental encephalomyelitis with a peptide analogue of myelin basic protein. Nature. 1996;379(6563):343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 66.Goverman J, Woods A, Larson L, et al. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72(4):551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 67.Brabb T, Goldrath AW, von Dassow P, et al. Triggers of autoimmune disease in a murine TCR-transgenic model for multiple sclerosis. J. Immunol. 1997;159(1):497–507. [PubMed] [Google Scholar]
- 68.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78(3):399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 69.Offner H, Adlard K, Bebo BF, Jr, et al. Vaccination with BV8S2 protein amplifies TCR-specific regulation and protection against experimental autoimmune encephalomyelitis in TCR BV8S2 transgenic mice. J. Immunol. 1998;161(5):2178–2186. [PubMed] [Google Scholar]
- 70.Vandenbark AA, Rich C, Mooney J, et al. Recombinant TCR ligand induces tolerance to myelin oligodendrocyte glycoprotein 35–55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in HLA-DR2 transgenic mice. J. Immunol. 2003;171(1):127–133. doi: 10.4049/jimmunol.171.1.127. [DOI] [PubMed] [Google Scholar]
- 71.Moisini I, Nguyen P, Fugger L, Geiger TL. Redirecting therapeutic T cells against myelin-specific T lymphocytes using a humanized myelin basic protein-HLA-DR2-ζ chimeric receptor. J. Immunol. 2008;180(5):3601–3611. doi: 10.4049/jimmunol.180.5.3601. [DOI] [PubMed] [Google Scholar]
- 72.Ito K, Bian HJ, Molina M, et al. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J. Exp. Med. 1996;183(6):2635–2644. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muraro PA, Vergelli M, Kalbus M, et al. Immunodominance of a low-affinity major histocompatibility complex-binding myelin basic protein epitope (residues 111–129) in HLA-DR4 (B1*0401) subjects is associated with a restricted T cell receptor repertoire. J. Clin. Invest. 1997;100(2):339–349. doi: 10.1172/JCI119539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huh J, Yao K, Quigley L, et al. Limited repertoire of HLA-DRB1*0401-restricted MBP111-129-specific T cells in HLA-DRB1*0401 Tg mice and their pathogenic potential. J. Neuroimmunol. 2004;151(1–2):94–102. doi: 10.1016/j.jneuroim.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 75.Fridkis-Hareli M, Teitelbaum D, Gurevich E, et al. Direct binding of myelin basic protein and synthetic copolymer 1 to class II major histocompatibility complex molecules on living antigen-presenting cells – specificity and promiscuity. Proc. Natl Acad. Sci. USA. 1994;91(11):4872–4876. doi: 10.1073/pnas.91.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vandenbark AA, Chou YK, Whitham R, et al. Treatment of multiple sclerosis with T-cell receptor peptides: results of a double-blind pilot trial. Nat. Med. 1996;2(10):1109–1115. doi: 10.1038/nm1096-1109. [DOI] [PubMed] [Google Scholar]
- 77.Vandenbark AA, Morgan E, Bartholomew R, et al. TCR peptide therapy in human autoimmune diseases. Neurochem. Res. 2001;26(6):713–730. doi: 10.1023/a:1010951706830. [DOI] [PubMed] [Google Scholar]
- 78.Bielekova B, Goodwin B, Richert N, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a Phase II clinical trial with an altered peptide ligand. Nat. Med. 2000;6(10):1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 79.Leonard JP, Waldburger KE, Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin-12. J. Exp. Med. 1995;181(1):381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Constantinescu CS, Wysocka M, Hilliard B, et al. Antibodies against IL-12 prevent superantigen-induced andspontaneous relapses of experimental autoimmune encephalomyelitis. J. Immunol. 1998;161(9):5097–5104. [PubMed] [Google Scholar]
- 81.Ichikawa M, Koh CS, Inoue A, et al. Anti-IL-12 antibody prevents the development and progression of multiple sclerosis-like relapsing-remitting demyelinating disease in NOD mice induced with myelin oligodendrocyte glycoprotein peptide. J. Neuroimmunol. 2000;102(1):56–66. doi: 10.1016/s0165-5728(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 82.Segal BM, Constantinescu CS, Raychaudhuri A, et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a Phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7(9):796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]; ■ Neutralization of IL-12 and IL-23 was tested. The negative result may have been due to the inclusion of patients with advanced disease.
- 83.Hofstetter HH, Ibrahim SM, Koczan D, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237(2):123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 84.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a Phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355(10):1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]; ■■ Highlights the need for extreme caution in the translation of therapies that are considered safe and effective in animal models to use in human subjects.
- 85.Jiang H, Milo R, Swoveland P, et al. Interferon β-1b reduces interferon γ-induced antigen-presenting capacity of human glial and B cells. J. Neuroimmunol. 1995;61(1):17–25. doi: 10.1016/0165-5728(95)00072-a. [DOI] [PubMed] [Google Scholar]
- 86.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with γ-interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37(7):1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 87.Teleshova N, Bao W, Kivisakk P, et al. Elevated CD40 ligand expressing blood T-cell levels in multiple sclerosis are reversed by interferon-β treatment. Scand. J. Immunol. 2000;51(3):312–320. doi: 10.1046/j.1365-3083.2000.00688.x. [DOI] [PubMed] [Google Scholar]
- 88.Genc K, Dona DL, Reder AT. Increased CD80+ B cells in active multiple sclerosis and reversal by interferon β-1b therapy. J. Clin. Invest. 1997;99(11):2664–2671. doi: 10.1172/JCI119455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dhib-Jalbut S. Mechanisms of interferon-β action in multiple sclerosis. Mult. Scler. 1997;3(6):397–401. doi: 10.1177/135245859700300609. [DOI] [PubMed] [Google Scholar]
- 90.Rep MH, Schrijver HM, van Lopik T, et al. Interferon (IFN)-β treatment enhances CD95 and interleukin 10 expression but reduces interferon-γ producing T cells in MS patients. J. Neuroimmunol. 1999;96(1):92–100. doi: 10.1016/s0165-5728(98)00271-9. [DOI] [PubMed] [Google Scholar]
- 91.Calabresi PA, Tranquill LR, Dambrosia JM, et al. Increases in soluble VCAM-1 correlate with a decrease in MRI lesions in multiple sclerosis treated with interferon β-1b. Ann. Neurol. 1997;41(5):669–674. doi: 10.1002/ana.410410517. [DOI] [PubMed] [Google Scholar]
- 92.Dhib-Jalbut S, Jiang H, Williams GJ. The effect of interferon β-1b on lymphocyte-endothelial cell adhesion. J. Neuroimmunol. 1996;71(1–2):215–222. doi: 10.1016/s0165-5728(96)00156-7. [DOI] [PubMed] [Google Scholar]
- 93.Schreiner B, Mitsdoerffer M, Kieseier BC, et al. Interferon-β enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J. Neuroimmunol. 2004;155(1–2):172–182. doi: 10.1016/j.jneuroim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 94.Shapiro S, Galboiz Y, Lahat N, Kinarty A, Miller A. The `immunological-synapse' at its APC side in relapsing and secondary-progressive multiple sclerosis: modulation by interferon-β. J. Neuroimmunol. 2003;144(1–2):116–124. doi: 10.1016/j.jneuroim.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 95.Gran B, Tranquill LR, Chen M, et al. Mechanisms of immunomodulation by glatiramer acetate. Neurology. 2000;55(11):1704–1714. doi: 10.1212/wnl.55.11.1704. [DOI] [PubMed] [Google Scholar]
- 96.Neuhaus O, Farina C, Wekerle H, Hohlfeld R. Mechanisms of action of glatiramer acetate in multiple sclerosis. Neurology. 2001;56(6):702–708. doi: 10.1212/wnl.56.6.702. [DOI] [PubMed] [Google Scholar]
- 97.Duda PW, Schmied MC, Cook SL, Krieger JI, Hafler DA. Glatiramer acetate (Copaxone®) induces degenerate, Th2-polarized immune responses in patients with multiple sclerosis. J. Clin. Invest. 2000;105(7):967–976. doi: 10.1172/JCI8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weber MS, Starck M, Wagenpfeil S, et al. Multiple sclerosis: glatiramer acetate inhibits monocyte reactivity in vitro and in vivo. Brain. 2004;127(Pt 6):1370–1378. doi: 10.1093/brain/awh163. [DOI] [PubMed] [Google Scholar]
- 99.Weber MS, Prod'homme T, Youssef S, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat. Med. 2007;13(8):935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 100.Maier K, Kuhnert AV, Taheri N, et al. Effects of glatiramer acetate and interferon-β on neurodegeneration in a model of multiple sclerosis:a comparative study. Am. J. Pathol. 2006;169(4):1353–1364. doi: 10.2353/ajpath.2006.060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yednock TA, Cannon C, Fritz LC, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature. 1992;356(6364):63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 102.Stuve O, Marra CM, Jerome KR, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann. Neurol. 2006;59(5):743–747. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- 103.Stuve O. The effects of natalizumab on the innate and adaptive immune system in the central nervous system. J. Neurol. Sci. 2008;274(1–2):39–41. doi: 10.1016/j.jns.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 104.Massacesi L, Parigi A, Barilaro A, et al. Efficacy of azathioprine on multiple sclerosis new brain lesions evaluated using magnetic resonance imaging. Arch. Neurol. 2005;62(12):1843–1847. doi: 10.1001/archneur.62.12.1843. [DOI] [PubMed] [Google Scholar]
- 105.Amato MP, Pracucci G, Ponziani G, et al. Long-term safety of azathioprine therapy in multiple sclerosis. Neurology. 1993;43(4):831–833. doi: 10.1212/wnl.43.4.831. [DOI] [PubMed] [Google Scholar]
- 106.Casetta I, Iuliano G, Filippini G. Azathioprine for multiple sclerosis. Cochrane Database Syst. Rev. 2007;(4):CD003982. doi: 10.1002/14651858.CD003982.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fox EJ. Mechanism of action of mitoxantrone. Neurology. 2004;63(12 Suppl 6):S15–S18. doi: 10.1212/wnl.63.12_suppl_6.s15. [DOI] [PubMed] [Google Scholar]
- 108.Martinelli Boneschi F, Rovaris M, Capra R, Comi G. Mitoxantrone for multiple sclerosis. Cochrane Database Syst. Rev. 2005;(4):CD002127. doi: 10.1002/14651858.CD002127.pub2. [DOI] [PubMed] [Google Scholar]
- 109.Fox EJ. Management of worsening multiple sclerosis with mitoxantrone: a review. Clin. Ther. 2006;28(4):461–474. doi: 10.1016/j.clinthera.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 110.Sipe JC, Romine JS, Koziol JA, et al. Cladribine in treatment of chronic progressive multiple sclerosis. Lancet. 1994;344(8914):9–13. doi: 10.1016/s0140-6736(94)91046-4. [DOI] [PubMed] [Google Scholar]
- 111.Sipe JC, Romine JS, Koziol JA, et al. Development of cladribine treatment in multiple sclerosis. Mult. Scler. 1996;1(6):343–347. doi: 10.1177/135245859600100612. [DOI] [PubMed] [Google Scholar]
- 112.Rice GP, Filippi M, Comi G. Cladribine and progressive MS: clinical and MRI outcomes of a multicenter controlled trial. Cladribine MRI Study Group. Neurology. 2000;54(5):1145–1155. doi: 10.1212/wnl.54.5.1145. [DOI] [PubMed] [Google Scholar]
- 113.Brousil JA, Roberts RJ, Schlein AL. Cladribine: an investigational immunomodulatory agent for multiple sclerosis. Ann. Pharmacother. 2006;40(10):1814–1821. doi: 10.1345/aph.1H037. [DOI] [PubMed] [Google Scholar]
- 114.Stelmasiak Z, Solski J, Nowicki J, et al. Effect of parenteral cladribine on relapse rates in patients with relapsing forms of multiple sclerosis: results of a 2-year, double-blind, placebo-controlled, crossover study. Mult. Scler. 2009;15(6):767–770. doi: 10.1177/1352458509103610. [DOI] [PubMed] [Google Scholar]
- 115.Budde K, Schmouder RL, Brunkhorst R, et al. First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. J. Am. Soc. Nephrol. 2002;13(4):1073–1083. doi: 10.1681/ASN.V1341073. [DOI] [PubMed] [Google Scholar]
- 116.Fujino M, Funeshima N, Kitazawa Y, et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J. Pharmacol. Exp. Ther. 2003;305(1):70–77. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- 117.Mehling M, Brinkmann V, Antel J, et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71(16):1261–1267. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- 118.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am. J. Transplant. 2004;4(7):1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 119.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 120.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 121.O'Connor P, Comi G, Montalban X, et al. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a Phase II extension study. Neurology. 2009;72(1):73–79. doi: 10.1212/01.wnl.0000338569.32367.3d. [DOI] [PubMed] [Google Scholar]
- 122.Waldmann TA. Anti-Tac (daclizumab, Zenapax®) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: a 25-year personal odyssey. J. Clin. Immunol. 2007;27(1):1–18. doi: 10.1007/s10875-006-9060-0. [DOI] [PubMed] [Google Scholar]
- 123.Bielekova B, Richert N, Howard T, et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon β. Proc. Natl Acad. Sci. USA. 2004;101(23):8705–8708. doi: 10.1073/pnas.0402653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rose JW, Watt HE, White AT, Carlson NG. Treatment of multiple sclerosis with an anti-interleukin-2 receptor monoclonal antibody. Ann. Neurol. 2004;56(6):864–867. doi: 10.1002/ana.20287. [DOI] [PubMed] [Google Scholar]
- 125.Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2Ra-targeted therapy (daclizumab) in multiple sclerosis. Proc. Natl Acad. Sci. USA. 2006;103(15):5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126 ■■.Coles AJ, Compston DA, Selmaj KW, et al. Alemtuzumab vs. interferon β-1a in early multiple sclerosis. N. Engl. J. Med. 2008;359(17):1786–1801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]; Alemtuzumab is a monoclonal antibody against CD52. It is considered the most effective experimental therapy for patients with early relapsing–remitting multiple sclerosis. Careful monitoring is required for potential immune-mediated side effects.
- 127.Cox AL, Thompson SA, Jones JL, et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur. J. Immunol. 2005;35(11):3332–3342. doi: 10.1002/eji.200535075. [DOI] [PubMed] [Google Scholar]
- 128.Coles AJ, Wing M, Smith S, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet. 1999;354(9191):1691–1695. doi: 10.1016/S0140-6736(99)02429-0. [DOI] [PubMed] [Google Scholar]
- 129.Jones JL, Phuah CL, Cox AL, et al. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H) J. Clin. Invest. 2009 doi: 10.1172/JCI37878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Herrmann ML, Schleyerbach R, Kirschbaum BJ. Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology. 2000;47(2–3):273–289. doi: 10.1016/s0162-3109(00)00191-0. [DOI] [PubMed] [Google Scholar]
- 131.Korn T, Magnus T, Toyka K, Jung S. Modulation of effector cell functions in experimental autoimmune encephalomyelitis by leflunomide – mechanisms independent of pyrimidine depletion. J. Leukoc. Biol. 2004;76(5):950–960. doi: 10.1189/jlb.0504308. [DOI] [PubMed] [Google Scholar]
- 132.Massacesi L, Abbamondi AL, Sarlo F, Amaducci L. The control of experimental allergic encephalomyelitis with retinoic acid. Further studies. Riv. Neurol. 1987;57(3):166–169. [PubMed] [Google Scholar]
- 133.Roberts AB, Sporn MB. Mechanistic interrelationships between two superfamilies: the steroid/retinoid receptors and transforming growth factor-β. Cancer Surv. 1992;14:205–220. [PubMed] [Google Scholar]
- 134.Mucida D, Park Y, Kim G, et al. Reciprocal Th17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 135.Bettelli E, Oukka M, Kuchroo VK. Th-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8(4):345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 136.Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 2007;13(4):423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 2002;169(9):4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 138.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199(7):971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beriou G, Costantino CM, Ashley CW, et al. IL-17 producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113(18):4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou L, Ivanov, II, Spolski R, et al. IL-6 programs Th-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 141.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J. Biol. Chem. 2007;282(48):34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huber M, Brustle A, Reinhard K, et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc. Natl Acad. Sci. USA. 2008;105(52):20846–20851. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wolk K, Sabat R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 2006;17(5):367–380. doi: 10.1016/j.cytogfr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 144.Chung Y, Yang X, Chang SH, et al. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16(11):902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 145.Zheng Y, Valdez PA, Danilenko DM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 146.Kreymborg K, Etzensperger R, Dumoutier L, et al. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 2007;179(12):8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- 147.Fitzgerald DC, Ciric B, Touil T, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 2007;179(5):3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 148.Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007;8(12):1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 149 ■.Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 2007;8(12):1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]; IL-27 is a negative regulator of Th17. It suppresses autoimmune inflammation by secreting IL-10.
- 150 ■.Dardalhon V, Awasthi A, Kwon H, et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nat. Immunol. 2008;9(12):1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]; [150] and [151] introduced a new lineage of T helper cells designated as Th9. These effector cells can inhibit the generation of regulatory T cells by suppressing Foxp3.
- 151 ■.Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-β `reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008;9(12):1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]; [150] and [151] introduced a new lineage of T helper cells designated as Th9. These effector cells can inhibit the generation of Treg cells by suppressing Foxp3.
- 152.Weinshenker BG, Bass B, Karlik S, Ebers GC, Rice GP. An open trial of OKT3 in patients with multiple sclerosis. Neurology. 1991;41(7):1047–1052. doi: 10.1212/wnl.41.7.1047. [DOI] [PubMed] [Google Scholar]
- 153.Lindsey JW, Hodgkinson S, Mehta R, et al. Repeated treatment with chimeric anti-CD4 antibody in multiple sclerosis. Ann. Neurol. 1994;36(2):183–189. doi: 10.1002/ana.410360210. [DOI] [PubMed] [Google Scholar]
- 154.Lindsey JW, Hodgkinson S, Mehta R, et al. Phase 1 clinical trial of chimeric monoclonal anti-CD4 antibody in multiple sclerosis. Neurology. 1994;44(3 Pt 1):413–419. doi: 10.1212/wnl.44.3_part_1.413. [DOI] [PubMed] [Google Scholar]
- 155.van Oosten BW, Lai M, Barkhof F, et al. A phase II trial of anti-CD4 antibodies in the treatment of multiple sclerosis. Mult. Scler. 1996;1(6):339–342. doi: 10.1177/135245859600100611. [DOI] [PubMed] [Google Scholar]