Abstract
Background
In giant cell arteritis (GCA), vasculitic damage of the aorta and its branches is combined with a syndrome of intense systemic inflammation. Therapeutically, glucocorticoids (GC) remain the golden standard as they promptly and effectively suppress acute manifestations; however, they fail to eradicate vessel wall infiltrates. The effects of glucocorticoids on the systemic and vascular components of GCA are not understood.
Methods and Results
The immunoprofile of untreated and GC-treated GCA was examined in peripheral blood and temporal artery biopsies with protein quantification assays, flow cytometry, quantitative real-time PCR, and immunohistochemistry. Plasma IFN-γ and IL-17 and frequencies of IFN-γ-producing and IL-17-producing T cells were markedly elevated prior to therapy. GC treatment suppressed the Th17 but not the Th1 arm in the blood and the vascular lesions. Analysis of monocytes/macrophages in the circulation and in temporal arteries revealed GC-mediated suppression of Th17-promoting cytokines (IL-1β, IL-6, and IL-23), but sparing of Th1-promoting cytokines (IL-12). In human artery-SCID mouse chimeras, in which patient-derived T cells cause inflammation of engrafted human temporal arteries, glucocorticoids were similarly selective in inhibiting Th17 cells and leaving Th1 cells unaffected.
Conclusions
Two pathogenic pathways mediated by Th17 and Th1 cells contribute to the systemic and vascular manifestations of GCA. IL-17-producing Th17 cells are sensitive to GC-mediated suppression, but IFN-γ-producing Th1 responses persist in treated patients. Targeting steroid-resistant Th1 responses will be necessary to resolve chronic smoldering vasculitis. Monitoring Th17 and Th1 frequencies can aid in assessing disease activity in GCA.
Keywords: giant cell arteritis, glucocorticoids, Th1, Th17
Introduction
Giant cell arteritis (GCA) is a systemic vasculitis with two disease components: vessel wall inflammation inducing arterial stenosis/occlusion and a systemic inflammation leading to polymyalgias, anemia, failure-to-thrive, and malaise 1. Prototypic vessel wall inflammation preferentially affects the upper extremity and extracranial branches of the aorta causing blindness, stroke, aortic arch syndrome, aortic aneurysm, or dissection 1, 2.
Glucocorticoids (GC) remain the golden standard of therapy; attempts to introduce steroid-sparing agents, including anti-TNF blockers have not been successful 3, 4. Methotrexate may have minor benefits when given over prolonged periods 5. In a double-blind placebo controlled study employing GC pulse therapy, patients pulsed at diagnosis had less disease flares and discontinued therapy earlier than patients without initial pulse therapy. However, benefits of pulse GC were delayed until 12-18 months into treatment 6. Acetylsalicylic acid has a role as adjuvant therapy, partially by inhibiting inflammation and partially through anti-platelet effects 7, 8. Steroid withdrawal after more than 2 years of therapy is often accompanied by recurrence of inflammatory markers, and vascular inflammation persists despite GC treatment 9-11. In essence, in spite of their prompt, impressive therapeutic effects, GC fail to abrogate vasculitis. Improved disease management and avoidance of severe GC side effects will require a better understanding of underlying immune abnormalities.
Both the innate and adaptive immune system contribute to GCA pathogenesis 12. Granulomatous inflammatory infiltrates composed of CD4 T cells, activated macrophages, and multinucleated giant cells induce intimal hyperplasia and luminal compromise 13. In cell-depletion studies, dendritic cells (DC) have emerged as the predominant antigen-presenting cell 14, 15. DC are now recognized as an indigenous cell population in human macrovessels where they have important surveillance functions and sense pathogen-derived motifs 16, 17.
The nature of T cells driving GCA and how they are affected by immunosuppression are unresolved. The finding of identical T cells in the right and left temporal artery has strongly supported antigen as an instigator 18. Functional selection and mechanisms-of-action of wall-infiltrating T cells remain to be elucidated. IFN-γ is a dominant cytokine in the arteries whereas Th2-derived cytokines are consistently absent 19, 20. Whether IL-17-producing Th17 cells have a role in GCA is also unknown.
GCA is a treatable yet incurable disease. Here, we have made use of the therapeutic effects of GC to decipher which immunopathways maintain systemic and vascular inflammation. By studying patients before and after initiation of GC, immune abnormalities responsive to this immunosuppressive treatment could be dissected from those resistant to GC. GC inhibit the production of cytokines that promote and sustain CD4 T-cell differentiation into Th17 cells. Conversely, cytokines regulating the differentiation of Th1 cells and Th1 responses persist even in chronically treated patients. Differential susceptibility of Th1 and Th17 cells to steroid-mediated suppression provides important pathogenic clues and encourages the development of therapeutic strategies that can disrupt both arms of vasculitic T-cell responses.
Methods
Study cohorts
Demographic and clinical characteristics of patients and controls enrolled into this study are listed in Supplemental Table 1. Eleven patients donated samples for studies before therapy was initiated and were reexamined on steroid therapy. In addition, 15 patients were tested only prior to therapy initiation and 23 patients were recruited while they were already on therapy. Thirty-four healthy volunteers aged 61 to 80 years old served as controls. The age distribution of patients and controls was not significantly different (Supplemental Table 1). Prior or current diagnosis of cancer, autoimmune disease other than GCA or chronic infection was an exclusion criterion. Emory University and the Mayo Clinic Review Boards approved the protocol and all donors gave informed consent.
Glucocorticoid Treatment
Patients with a positive temporal artery biopsy started on prednisone 60 mg daily. Patients were monitored monthly for clinical signs of disease, and GC was tapered by 10% every two weeks. Adjustment of GC therapy was left to the decision of the treating physician and was based on clinical and laboratory findings.
Reagents and Antibodies
Reagents and antibodies are listed in Supplemental Table 2.
Cell Preparation, Culture, and Flow Cytometry
Peripheral blood mononuclear cells (PBMC) were isolated from healthy donors and GCA patients. After 4 hour stimulation with phorbol 12-myristate 13-acetate (PMA), and ionomycin in the presence of brefeldin A, cells were stained with anti-CD3 (PE), anti-CD4 (PerCP), anti-IFN-γ (FITC), and anti-IL-17 (APC) antibodies, or alternatively stained with anti-CD3 (FITC) and anti-Foxp3 (PE). Flow cytometry was performed on a LSR II (BD Biosciences), and data analyzed with FlowJo 7.2.2 software.
Naïve CD4 T cells and CD14+ monocytes were purified as described 16 and co-cultured at a 2:1 ratio for 4 days with LPS, anti-CD3, and anti-IL-1β, anti-IL-6, or anti-IL-23p19 neutralizing antibodies. Relevant antibody isotype served as control. For enrichment of Th17 cells, CD45RO+ memory CD4 T cells were isolated from PBMC and co-cultured with CD14+ monocytes (2:1) for seven days with LPS (1 μg/ml), anti-CD3 (1 μg/ml), anti-human IFN-γ (2 μg/ml) and anti-human IL-4 (2 μg/ml) antibodies. Accumulation of Th17 and Th1 cells in such T cell lines was monitored by flow cytometry.
Quantitative Real-Time PCR
Total RNA was isolated from human tissues and cells. IL-17, IFN-γ, Foxp3, IL-1β, IL-6, IL-23p19, IL-12p35, IL-12p40, MMP-9 or β-actin gene transcripts were quantified by real-time PCR and adjusted to 2×105 copies of the house-keeping gene beta-actin as described 10, 16, 17. Primer sequences and PCR conditions are listed in Supplemental Table 3.
ELISArray
Plasma cytokine levels (IL-1β, IL-6, IL-12, IL-17, IFN-γ) were analyzed with Human Autoimmune Response Multi-Analyte ELISArray kits (SABiosciences Corp. Frederick, MD). Plasma samples (1:2 dilutions) were incubated in 96-well plates precoated with individual cytokine capture antibodies for 2 hours. After washing, biotin-conjugated cytokine detection antibodies were added for 30 minutes. Bound antibodies were detected with Avidin-HRP and development solution. Color reactions were quantified at OD 450.
Immunohistochemical Staining
Sections of paraffin-embedded arteries were processed and stained with anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD15, anti-IL-17, or anti-IFN-γ antibodies as described 16, 21-24. Purified mouse or rabbit IgG control antibodies were used instead of the primary antibody as a control.
Human Temporal Artery-SCID Mouse Chimeras
Human temporal artery-SCID mouse chimeras were generated as previously described 16-17. Mice implanted with artery tissues from the same donor were assigned to either of two treatment arms and a control arm. Day 7 following implantation, mice were injected intraperitoneally with LPS (3 μg/mouse) or PBS (control arm). On day 8, T cell lines enriched for Th17 cells (107 cells/mouse) were adoptively transferred into the chimeras intravenously. On day 9, chimeras were treated with dexamethasone (4 mg/kg, Sigma-Aldrich) as described 11. 15 days following implantation, arterial grafts were harvested for RNA extraction or OCT embedded for immunostaining. The protocol was approved by the Emory University Institutional Animal Care and Use Committee.
Data Analysis
A detailed description of the statistical tools and study cohorts is given in Supplemental Statistical Methods and in Supplemental Table 4. Analysis of data in Fig.1 required the development of a customized approach as some patients were tested only before treatment, some only while on treatment and some patients were tested twice, prior to initiation of GC and while on GC. Analysis of data sets in Fig 2, 3, 4 and 5 involved one-sample t or Wilcoxon test, two-sample t or Wilcoxon test or Behrens-Fisher-Welch testing, as appropriate. Friedman rank sum test was applied to compare results from antibody blocking assays with anti-IL1β, anti-IL-6, and anti-IL-23. p-value of <0.05 was considered significant. Data are presented as mean ± standard error (SEM).
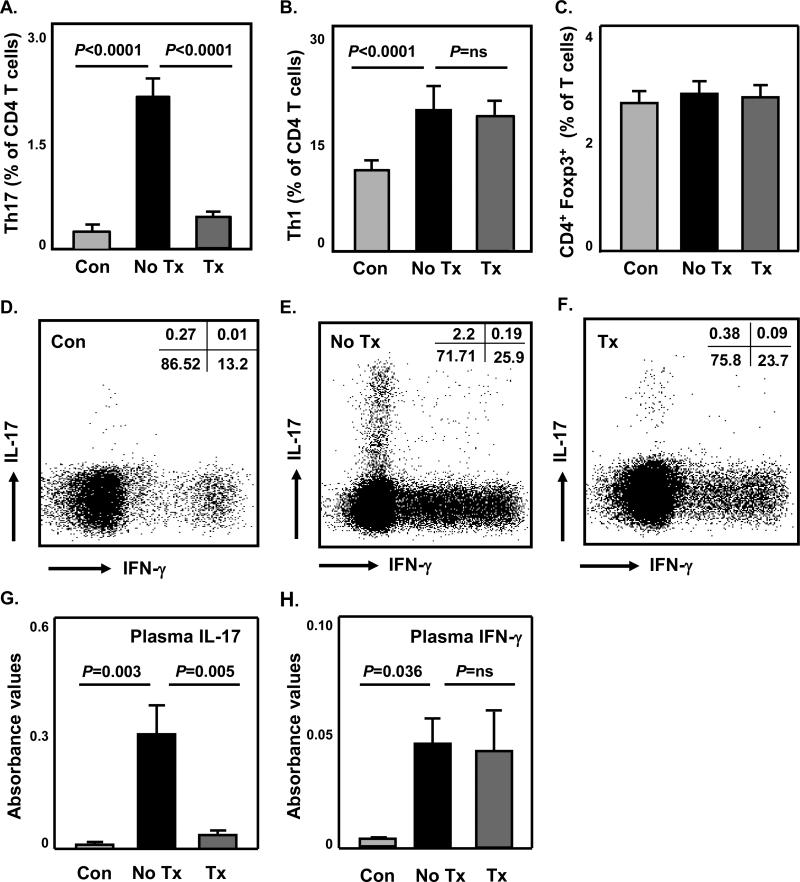
Figure 1. Circulating Th1 and Th17 cells are markedly expanded in GCA and differentially suppressed by Glucocorticoids.
PBMC were isolated from untreated patients (No Tx, n=26) and GC-treated patients (Tx, n=34) or age-matched healthy controls (Con, n=34). Cells were stimulated with PMA/ionomycin in the presence of brefeldin A for 4 hours, stained with PE anti-CD3, PerCP anti-CD4, FITC anti-IFN-γ, and APC anti-IL-17 antibodies. Alternatively, PBMC were stained with FITC anti-CD3, PerCP anti-CD4, PE anti-Foxp3 antibodies, and analyzed by flow cytometry. Frequencies of IL-17+ Th17 (A) and IFN-γ+ Th1 cells (B) amongst CD4 T cells, and Foxp3+ CD4 Treg (C) amongst CD3 T cells are presented. Representative cytometric dot plots of Th17 and Th1 cells from a healthy individual (D), a GCA patient before therapy (E), and a GCA patient on treatment (F) are shown. Plasma concentrations of IL-17 (G) and IFN-γ (H) protein were measured in six GCA patients before and after initiation of GC therapy and six age-matched healthy controls with ELISArray kits. Results are presented as absorbance value at 450nm. ns: not significant.
Figure 2. Steroid sensitivity of tissue IL-17 and steroid resistance of tissue IFN-γ in temporal artery lesions.
Total RNA was isolated from temporal arteries harvested before initiation of GC therapy (No Tx) (n=8) and from a second-side biopsy 3-9 months after initiation of therapy (Tx). Arteries without GCA served as controls (Con) (n=8). Transcripts of IL-17 (A), IFN-γ (B), and Foxp3 (C) mRNA in the tissues were quantified by real-time PCR. Paraffin-embedded tissue sections of temporal arteries were stained with anti-CD3 (E) or anti-IL-17 (F-H) antibodies; (D) IL-17+ cells in arteries harvested before (G) and on treatment (H) were quantified in 10 randomly selected high power fields. (I) IFN-γ staining showed IFN-γ+ cells in the inner adventitia of a temporal artery on treatment. The images presented are representative of six biopsy samples. (J) Purified rabbit IgG served as isotype control antibody. Magnification 200× in (E), (F) and (J); 400× in (H) and (I); 600× in (G).
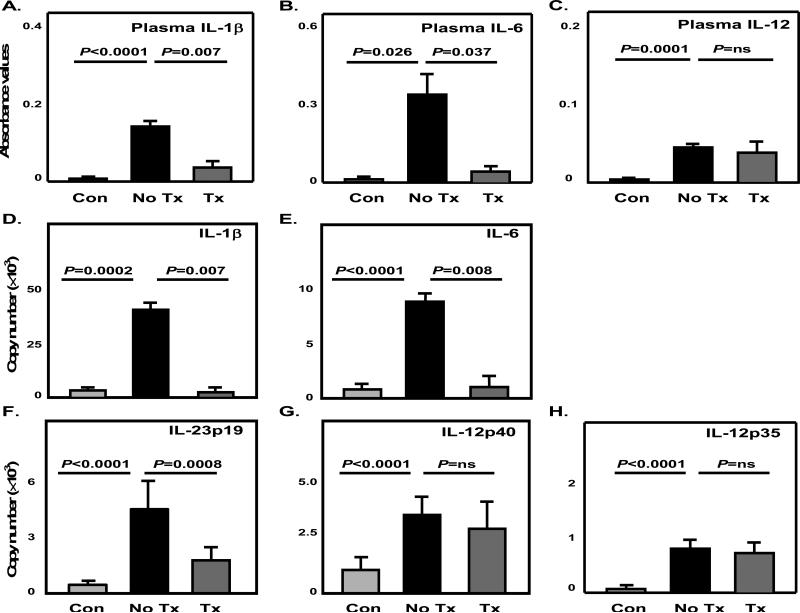
Figure 3. Glucocorticoids suppress Th17-promoting cytokines and spare Th1-promoting cytokines in the circulation.
The proinflammatory cytokines IL-1β (A), IL-6 (B) and IL-12 (C) were measured in plasma samples of GCA patients before (No Tx) and after initiation of GC therapy (Tx) (n=6), and age-matched controls (Con) (n=6) by ELISArrays. Data are presented as absorbance values at 450nm. (D-H) CD14+ monocytes were purified from PBMC of GCA patients prior to steroid therapy (n=8) and after steroid therapy (n=8), or GCA negative donors (n=8). Transcripts of IL-1β (D), IL-6 (E), IL-23p19 (F), IL-12p40 (G) and IL-12p35 (H) in cell extracts were quantified by qRT-PCR.
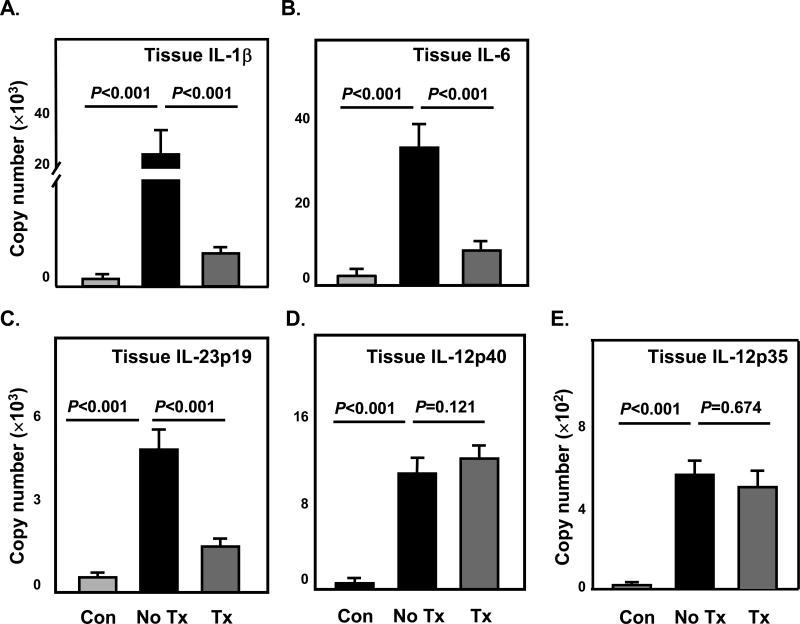
Figure 4. Effects of Glucocorticoids on the expression of Th17-promoting and Th1-promoting cytokines in inflamed temporal arteries.
RNA was extracted from temporal artery biopsies collected as described in Figure 2 prior to steroid therapy (first side) (No Tx) and after steroid therapy (second side) (Tx). Arteries without GCA served as controls (Con). Transcripts of IL-1β (A), IL-6 (B), IL-23p19 (C), IL-12p40 (D) and IL-12p35 (E) were quantified by qRT-PCR.
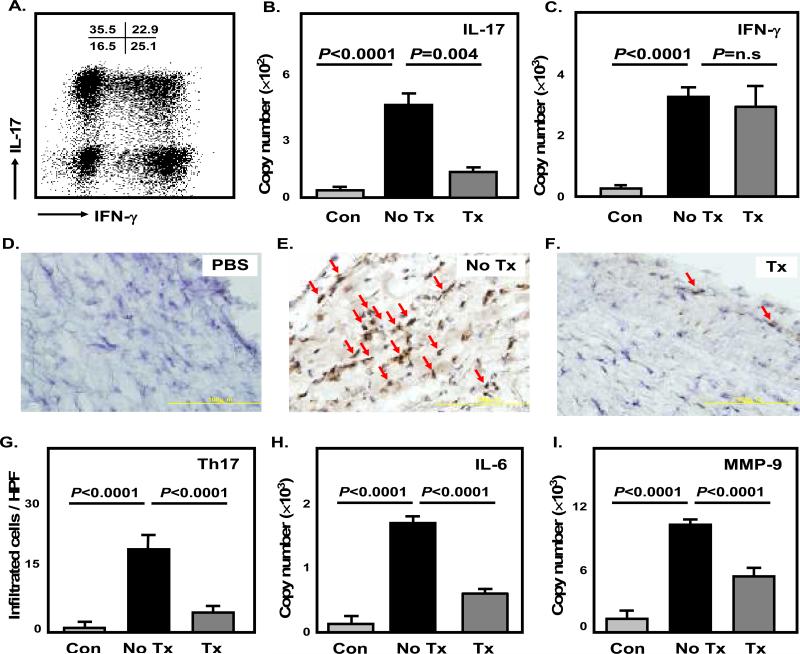
Figure 5. Dexamethasone prevents Th17- but not Th1-mediated vessel wall inflammation in human artery-SCID chimeras.
Normal human arteries were engrafted into SCID mice. One week later mice were injected with LPS or saline as control (Con). 24 hours later, T-cell lines established from the blood of GCA patients were adoptively transferred (107 cells/mouse). Chimeras were treated with either dexamethasone (4 mg/kg) (Tx) or saline (No Tx), and temporal arteries were explanted. T-cell lines from the GCA patients had similar proportions of Th17, Th1, and Th17/Th1 cells; a representative cytometric dot plot is shown (A). Tissue extracts from explanted arteries were analyzed for IL-17 (B) and IFN-γ (C) by qRT-PCR. Data are presented as mean ± SEM from three independent experiments. Tissue sections from explanted arteries were stained with anti-IL-17 antibodies (D-F). IL-17+ T cells (dark brown) were quantified in a minimum of 12 high-powered fields for each arterial cross-section (G). Transcript levels of IL-6 (H) and MMP-9 (I) in the implants were measured by qRT-PCR as well. Magnification 600× in (D), (E) and (F).
The authors have full access to and take full responsibility for the integrity of the data. All authors have read and agreed to the manuscript as written.
Results
Circulating Th1 and Th17 cells are markedly expanded in GCA, and are differentially affected by GC therapy
To identify functionally relevant T-cell subsets in GCA, frequencies of IL-17-producing Th17, and IFN-γ-producing Th1 and Foxp3+ regulatory CD4 T cells were compared in patients with untreated GCA (n=26) and age-matched healthy controls (n=34). In GCA patients, frequencies of circulating Th17 cells amongst CD4 T cells were 8-fold expanded, ranging from 1.1% to 5.3% and averaging 2.2% (Figure 1A). In healthy controls, only 0.03% to 0.59% of T cells produced IL-17 (Figure 1A). Th1 cells accounted for 20.6% of CD4 T cells in GCA patients in contrast to only 11.8% in controls (Figure 1B). The patients also carried a population of IL-17+IFN-γ+ double-positive cells (Figure 1E), which were essentially lacking in controls (Figure 1D). Frequencies of CD4 Foxp3+ Treg were indistinguishable in patients and controls (Figure 1C and Supplemental Figure 1). In essence, untreated GCA was associated with a marked expansion of IFN-γ and IL-17 producing T-cell subsets.
To explore the effect of GC therapy on cytokine-producing T-cell subsets, frequencies of circulating Th1 and Th17 cells were measured in steroid-treated patients (n=34) who were on an average dose of 16.7 mg of prednisone daily. All patients initially received prednisone at 60 mg/day with continuous dose reduction by 10% every two weeks, unless the patients had recurrence of vasculitic symptoms. GC therapy markedly suppressed circulating Th17 cells, normalizing the average frequencies to 0.46% of CD4 T cells (Figure 1A). In treated patients, Th17 frequencies were indistinguishable from those in control donors (range 0.11% - 0.89%) (p=0.101). GC also ablated the IL-17+IFN-γ+ double positive cells (Figure 1F). However, GC essentially left unaffected frequencies of Th1 cells (Figure 1B, F), with a mean of 20.2% and 18.4% before and after therapy, respectively. Also, frequencies of Foxp3+ Treg were unaltered by GC therapy (Figure 1C and Supplemental Figure 1). In essence, in steroid-treated patients, Th17 frequencies returned to normal whereas the expansion of Th1 cells persisted.
Measurement of circulating IL-17 and IFN-γ protein confirmed elevation of both inflammatory cytokines prior to GC therapy and effective suppression of IL-17 but not IFN-γ in treated patients (Figure 1G, H).
To assess the time frame of GC-mediated suppression of Th17 cells and understand whether Th17 cells rebound once steroid doses were tapered, 11 patients were examined prior to the initiation of prednisone therapy and reexamined at different time points while on steroids (Table 1; Suppl. Table 5). Treatment for 10 weeks was sufficient to bring Th17 frequencies into the normal range. With decreasing steroid doses, the suppressive effect was maintained and was still observed after treating for nine months. There was no evidence that tapering steroids below 10 mg daily resulted in a rebound of Th17 cells.
Table 1.
| Patients | Median (%) | Range (%) | Mean (%) | Standard deviation | One-sample t-test |
|---|---|---|---|---|---|
| Untreated n=11 | 2.2 | 1.6 - 4.3 | 2.42 | 0.8035 | |
| Treated n=11 | 0.42 | 0.18 – 0.65 | 0.43 | 0.1421 | p < 0.0001 |
GC therapy selectively suppresses IL-17-producing cells and spares IFN-γ-secreting cells in the vasculitic lesions
Temporal artery samples with typical changes of GCA were collected from 8 untreated patients and the second side biopsy was obtained after 3-9 months of GC therapy. All second side biopsies examined in this study were read positive for vasculitis by histomorphology. Temporal artery specimens free of GCA served as controls. Extracts from the tissue samples were analyzed for the expression of IL-17, IFN-γ, and Foxp3-specific sequences by RT-PCR, and IL-17- and IFN-γ-producing cells were identified by immunohistochemical staining of tissue sections. The control samples were essentially negative for IL-17, IFN-γ and Foxp3 transcripts, and no cells staining positive for IL-17 or IFN-γ were detected. The samples from untreated patients expressed high transcript levels for IL-17, IFN-γ and Foxp3 (Figure 2A-C). GC therapy resulted in marked suppression of IL-17 production (Figure 2A). In contrast, IFN-γ and Foxp3 expression was indistinguishable between untreated and treated arteries (Figure 2B, C and Supplemental 1C).
In the temporal arteries of untreated GCA patients, immunostaining for CD3 localized tissue-infiltrating T cells in all wall layers, with preferential clustering in the medial smooth muscle layer (Figure 2E). IL-17-producing cells were abundant (Figure 2F) and followed the distribution pattern of CD3+ T cells (Figure 2F, G). IL-17+ cells were also found around adventitial vasa vasorum and diffusely spread through the hyperplastic intima. Median numbers of IL-17-producing cells reached 36 per high-powered field (Figure 2D). Almost all lymphocytic cells in the vessel wall infiltrates are CD4+ with only very few CD8 T cells represented, assigning IL-17 production mostly to CD4 T cells (Supplemental Figure 2). IFN-γ-producing cells were represented amongst all wall-infiltrating T cells, but displayed a preference for the adventitial layer. In arteries harvested from treated patients, the infiltrates continued to include abundant IFN-γ-producing cells (Figure 2I), mainly so in the inner adventitia. However, only few cells stained positive for IL-17 and the intensity of staining was reduced (Figure 2H), suggesting reduced production by individual cells.
GC inhibit Th17-promoting cytokines in peripheral monocytes and in the vasculitic lesions
T-cell differentiation into either Th1 or Th17 cells is directed by the cytokine environment that is shaped by antigen-presenting cells, such as DC and monocytes/macrophages. IL-1β, IL-6, and IL-23 contribute to Th17 differentiation; IL-12 supports the development of Th1 cells 25. To quantify the contribution of different cytokines to the expansion of Th17 cells in GCA, naïve CD4 T cells were co-cultured with autologous CD14+ monocytes in the presence of neutralizing antibodies against IL-1β, IL-6, or IL-23. Monocytes from GCA patients effectively drove Th17 and Th1 differentiation (Supplemental Figure 3). Th17 differentiation was suppressed by anti-IL-1β, anti-IL-6 and anti-IL-23, implicating all three cytokines in the process. Blocking IL-1β or IL-6 had no effect on the development of Th1 cells. Removing IL-23 activity, however, enhanced the outgrowth of Th1 cells markedly. The data assigned a critical role to IL-1β, IL-6, and IL-23 in promoting the shift towards Th17 cells in GCA.
To understand whether GC functions by targeting Th1- and Th17-promoting cytokines, we analyzed protein levels in the plasma and gene expression in circulating monocytes and temporal artery tissue samples. Protein concentrations of circulating IL-1β, IL-6, and IL-12 were elevated significantly in untreated patients (Figure 3A-C). In response to therapy, IL-1β and IL-6 were markedly reduced (Figure 3A, B), but IL-12 remained unaffected (Figure 3C). In circulating monocytes transcript levels for IL-1β, IL-6, IL-12p35, IL-12p40 and IL-23p19 were all increased in GCA patients compared to healthy individuals (Figure 3D-H). Upon GC therapy, IL-1β and IL-6 gene expression in GCA monocytes were completely inhibited (Figure 3D, E). IL-23p19 gene expression was suppressed by 52% in CD14+ monocytes from treated patients (Figure 3F). The treatment did not affect IL-12 gene expression (Figure 3G, H). A similar pattern emerged for the tissue expression of Th17- and Th1-promoting cytokines. In untreated cases IL-1β, IL-6, IL-23p19, IL-12p40, and IL-12p35 gene expression levels were markedly upregulated (Figure 4A-E). GC treatment resulted in suppression of IL-1β, IL-6, and IL-23p19 transcription (Figure 4A-C), but production of IL-12p35 and IL-12p40 transcripts was maintained in the arteries (Figure 4D, E).
GC selectively inhibits recruitment and survival of Th17 cells in vessel wall inflammation in human artery-SCID mouse chimeras
To verify the differential effects of corticosteroid treatment on the accumulation of T-cell effectors in vasculitic lesions, T-cell lines generated from GCA patients were adoptively transferred into artery-SCID chimeras engrafted with human temporal arteries (Figure 5). Expanded T-cell lines contained a high frequency of Th17 cells as well as Th17/Th1 cells, confirming a bias towards the induction of the Th17 lineage (Figure 5A). Under similar conditions, T-cell cultures from healthy controls yielded less than 2% Th17 cells (data not shown). Following injection of the patient-derived T-cell lines, Th17 as well as Th1 cells accumulated in the arteries as evidenced by the levels of IL-17 and IFN-γ specific sequences (Figure 5B, C). Treatment with dexamethasone (4 mg/kg) inhibited accumulation of Th17 mRNA but did not affect production of IFN-γ. Immunohistochemical analysis confirmed dense infiltrates of IL-17+ cells in the arteries from untreated chimeras (Figure 5E, G). Conversely, after corticosteroid therapy, the density of IL-17+ cells in the vessel wall was markedly diminished (Figure 5F, G). Reduction in the frequency of wall-infiltrating IL-17+ cells was associated with significantly lower levels of IL-6 sequences (Figure 5H). Also, MMP-9 transcript levels, a marker for tissue-destructive macrophages, declined, suggesting that preventing the infiltration/survival of IL-17 cells in the infiltrates may have tissue-protective effects (Figure 5I). In essence, glucocorticoids selectively regulate the composition of the inflammatory T-cell infiltrate; Th17 cells are highly sensitive to the inhibitory effects whereas the recruitment and enrichment of Th1 cells and their cytokine-producing functions are unaffected.
Discussion
Glucocorticoids are highly effective in treating the constitutional symptoms of GCA, including myalgias, malaise, fever, anorexia and headaches. However, most patients require therapy for years to avoid disease flares suggesting only partial responsiveness to this immunosuppressive approach. Here, we report that GC act by selectively suppressing Th17 responses in the vasculitic lesions and in the periphery whereas Th1 responses are spared. The anti-inflammatory actions of GC result from modulation of monocytes /macrophages; Th17-promoting cytokines decline markedly, the Th1-promoting IL-12 continues to be produced. In essence, GC effectively treat the acute manifestations of GCA which appear to be closely associated with excessive production of Th17-promoting cytokines by innate immune cells and IL-17 by T cells. GC fail to abrogate vasculitis, as Th1-driving cytokines and Th1 cells are refractory to GC-mediated suppression. To reset immune abnormalities in GCA, GC must be combined with additional therapies.
GCA is considered a prototypic steroid-responsive disease. Patients have fast and profound improvement of constitutional symptoms, such as malaise, anorexia, fevers, and myalgias. Steroids are believed to be vision protective, and headaches and scalp tenderness often respond promptly 26-28. However, vascular lesions persist and disease flares are frequent upon tapering of GC.
The current study has made use of comparing abnormalities in the innate and adaptive immune system pre and post therapy. T cells and macrophages are critical players in the vasculitic process sustaining the granulomatous inflammation in the arteries 22, 29. Highly activated macrophages not only occupy the wall infiltrates; they also circulate in the blood 30-32. High levels of IL-6 have been associated with disease activity and therapeutic response 11, 33. Besides IL-6, circulating IL-1β and IL-12 proteins were elevated in untreated patients (Figure 3A-C). The cytokine production pattern found in the vasculitic lesions is essentially identical to that detected in circulating monocytes. On the side of the adaptive immune system, GCA patients have marked expansions of Th1 and Th17 cells. Th1 and Th17 cells are now considered to drive two distinct inflammatory pathways 34-36. Only one of these pathways in GCA is amendable to GC-mediated suppression, in both patients and mouse chimeras. GCA patients utilize IL-1β, IL-6, and IL-23 to induce Th17 cells, and these cytokines are effectively suppressed by GC. From these data it appears that the patients’ immune defects originate in the innate immune system where excessive amounts of IL-1β, IL-6, and IL-23 are produced. Multiple cell populations, including DC and monocytes and macrophages, are the main sources of IL-1β, IL-6, and IL-23. The current study assigns a top position to these cells in the pathogenic events leading to GCA, supporting the disease model in which vessel-wall residing DC have checkpoint function and are the first to respond to disease-inducing danger signals 16, 17.
Th17 cells have been linked to a number of autoimmune diseases 37, cardiac allograft rejection, and vasculopathy 38. How IL-17 facilitates vascular tissue damage is less well understood, but IL-17 receptors are expressed on fibroblasts, endothelial cells, and vascular smooth muscle cells, all instrumental in the arterial wall structure (data not shown). With 8-fold enrichment of Th17 cells in the blood IL-17 may even have a role in the typical myalgias and muscle stiffness associated with GCA.
A model system in which vessel wall inflammation can be induced in normal human arteries allowed analysis of some of the mechanisms related to Th1 and Th17-mediated disease. T cell lines from GCA patients were tested for their inflammation-inducing capacity. T cell subsets analysis revealed a strong bias towards the enrichment of Th17 and Th17/Th1 cells. In normal donors Th17 cells account for only a minor fraction of effector T cells. Conversely, T cell lines expanded from GCA patients fell into three equally proportioned subsets: Th1 cells, Th17 cells and Th1/Th17 cells. Whether the expansion of Th17 cells is solely a consequence of disturbed innate immune function, producing excess amounts of Th17-promoting cytokines, or whether the patients also have restructuring of the T-cell repertoire needs to be addressed in further studies.
Corticosteroids essentially depleted Th17 cells from the lesions, either by inhibiting recruitment or by jeopardizing survival in the vessel wall environment. Recent data suggest that tissue invasion of human arteries is a feature of CCR6+ T cells 16. The CCR6 receptor is typically found on Th17 cells (data not shown). CCL20, the ligand for CCR6, has been implicated in facilitating selective recruitment of T cells causing panarteritis as opposed to a periarteritic architecture of the vascular infiltrates 16. After dexamethasone treatment, Th1 cells continued to infiltrate the wall recapitulating findings in the arteries from the treated patients.
The current study has multiple implications for our understanding of GCA pathogenesis and management. The data suggest that at least two arms of the adaptive immune system contribute to GCA. Th17 immunity seems to be more important for acute manifestations, both systemically and in the blood vessels; Th1 immunity is associated with chronically persistent vascular lesions. From a mechanistic point of view the ability to suppress IL-1β, IL-6, and IL-23 while IL-12 production is essentially unaffected suggests that two different types of antigen-presenting cells or two different danger signals may promote T-cell differentiation in GCA. It is important to realize, however, that the approach taken in this study, analyzing the different blood and tissues cytokines as dependent parameters does not entirely reflect the complex and interrelated biology of the disease, yet this is the only approach currently available.
The potency of GC to normalize proinflammatory Th17 cells is consistent with the clinical experience that this therapy is prompt and effective. In most patients, constitutional symptoms (myalgias, fever, malaise, anorexia) and signs of vascular inflammation (headaches, temporal tenderness) are markedly improved. However, vessel wall infiltrates, and thus continuous wall damage, can persist over extended periods. The resistance of circulating and tissue Th1 cells indicates partial response, even on high doses of GC 9, 10. Current data support the concept that many of the acute disease manifestations are mediated by IL-17 but further work is necessary to separate the distinct biologic and clinical consequences of Th1- and Th17-dependent disease components. A recent double-blinded study exploring the therapeutic benefits of pulse GC demonstrated that patients who received high doses pulse steroids at the beginning of therapy had less disease flares and could discontinue therapy earlier 6. Thus, inhibiting the Th17 arm of immunity may have long-lasting effects. One possible explanation is that IL-17 functions mainly as an inflammatory amplifier. Advancing from partial to more comprehensive disease control in GCA would require combining GC therapy with Th1-targeted interventions. The most effective approach may be to inhibit Th1-promoting cytokines, in particular IL-12. The tight correlation between blood and tissue T cell subsets opens the door for blood-based biomarkers that can be used to closely follow the extent of T-cell and monocyte abnormalities and to appropriately tailor suppression of the multiple pathways driving this vasculitis.
Short Commentary.
Giant cell arteritis, an inflammatory vasculopathy of the aorta and its branches, causes blindness, stroke and aortic arch syndrome combined with a syndrome of intense systemic inflammation. Pathognomic findings are granulomatous vessel wall infiltrates of activated T cells and macrophages in a temporal artery biopsy. Corticosteroids are highly effective in suppressing the systemic manifestations of disease. In the current study patients with GCA were studied before and after corticosteroid treatment to identify which immunopathways mediate acute and chronic vasculitis and how therapy affects the immune-inflammatory abnormalities underlying GCA. In temporal artery biopsies and in the blood of untreated patients, two distinct types of effector T cells were expanded; IL-17 producing Th17 cells and IFN-γ producing Th1 cells. Corticosteroids effectively suppressed Th17 cells but left Th1 cells unaffected. Corticosteroids functioned by inhibiting macrophages that produce Th17-promoting cytokines, including IL-1β, IL-6, and IL-23. Th1-promoting macrophages escaped from corticosteroid suppression, chronically sustaining one arm of vasculitic T cell responses. In essence, the systemic and vascular inflammation of GCA involves abnormally activated innate and adaptive immune responses with both Th17 and Th1 cells contributing to disease. Corticosteroids disrupt abnormal Th17 responses but fail to induce disease remission as Th1 cells are refractory and continue to support vascular inflammation. Thus, successful management of GCA requires combination therapy targeting several arms of the immune system. The identified immune abnormalities emerge as potential biomarkers to optimize monitoring of disease activity in large vessel vasculitis.
Supplementary Material
Acknowledgments
The authors thank all patients for participating in this study, and Linda Arneson for editorial assistance.
Sources of funding
This work was funded in part from the National Institutes of Health (PO1 HL 058000, U19 AI 57266, R01 AR 042527, R01 AG 015043, ULI RR025744, R01 EY 11916) and the Vasculitis Foundation.
Footnotes
Disclosures
none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003;139:505–515. doi: 10.7326/0003-4819-139-6-200309160-00015. [DOI] [PubMed] [Google Scholar]
- 2.Evans JM, O'Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med. 1995;122:502–507. doi: 10.7326/0003-4819-122-7-199504010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman GS, Cid MC, Rendt-Zagar KE, Merkel PA, Weyand CM, Stone JH, Salvarani C, Xu W, Visvanathan S, Rahman MU. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med. 2007;146:621–630. doi: 10.7326/0003-4819-146-9-200705010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Salvarani C, Macchioni P, Manzini C, Paolazzi G, Trotta A, Manganelli P, Cimmino M, Gerli R, Catanoso MG, Boiardi L, Cantini F, Klersy C, Hunder GG. Infliximab plus prednisone or placebo plus prednisone for the initial treatment of polymyalgia rheumatica: a randomized trial. Ann Intern Med. 2007;146:631–639. doi: 10.7326/0003-4819-146-9-200705010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Mahr AD, Jover JA, Spiera RF, Hernandez-Garcia C, Fernandez-Gutierrez B, Lavalley MP, Merkel PA. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum. 2007;56:2789–2797. doi: 10.1002/art.22754. [DOI] [PubMed] [Google Scholar]
- 6.Mazlumzadeh M, Hunder GG, Easley KA, Calamia KT, Matteson EL, Griffing WL, Younge BR, Weyand CM, Goronzy JJ. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum. 2006;54:3310–3318. doi: 10.1002/art.22163. [DOI] [PubMed] [Google Scholar]
- 7.Stone JH. Antiplatelet versus anticoagulant therapy in patients with giant cell arteritis: which is best? Nat Clin Pract Rheumatol. 2007;3:136–137. doi: 10.1038/ncprheum0431. [DOI] [PubMed] [Google Scholar]
- 8.Weyand CM, Kaiser M, Yang H, Younge B, Goronzy JJ. Therapeutic effects of acetylsalicylic acid in giant cell arteritis. Arthritis Rheum. 2002;46:457–466. doi: 10.1002/art.10071. [DOI] [PubMed] [Google Scholar]
- 9.Achkar AA, Lie JT, Hunder GG, O'Fallon WM, Gabriel SE. How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis? Ann Intern Med. 1994;120:987–992. doi: 10.7326/0003-4819-120-12-199406150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Brack A, Rittner HL, Younge BR, Kaltschmidt C, Weyand CM, Goronzy JJ. Glucocorticoid-mediated repression of cytokine gene transcription in human arteritis-SCID chimeras. J Clin Invest. 1997;99:2842–2850. doi: 10.1172/JCI119477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weyand CM, Fulbright JW, Hunder GG, Evans JM, Goronzy JJ. Treatment of giant cell arteritis: interleukin-6 as a biologic marker of disease activity. Arthritis Rheum. 2000;43:1041–1048. doi: 10.1002/1529-0131(200005)43:5<1041::AID-ANR12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349:160–169. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 13.Weyand CM, Younge BR, Goronzy JJ. T cells in arteritis and atherosclerosis. Curr Opin Lipidol. 2008;19:469–477. doi: 10.1097/mol.0b013e32830bfdc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krupa WM, Dewan M, Jeon MS, Kurtin PJ, Younge BR, Goronzy JJ, Weyand CM. Trapping of misdirected dendritic cells in the granulomatous lesions of giant cell arteritis. Am J Pathol. 2002;161:1815–1823. doi: 10.1016/S0002-9440(10)64458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma-Krupa W, Jeon MS, Spoerl S, Tedder TF, Goronzy JJ, Weyand CM. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–183. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng J, Ma-Krupa W, Gewirtz AT, Younge BR, Goronzy JJ, Weyand CM. Toll-like receptors 4 and 5 induce distinct types of vasculitis. Circ Res. 2009;104:488–495. doi: 10.1161/CIRCRESAHA.108.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weyand CM, Schonberger J, Oppitz U, Hunder NN, Hicok KC, Goronzy JJ. Distinct vascular lesions in giant cell arteritis share identical T cell clonotypes. J Exp Med. 1994;179:951–960. doi: 10.1084/jem.179.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weyand CM, Hicok KC, Hunder GG, Goronzy JJ. Tissue cytokine patterns in patients with polymyalgia rheumatica and giant cell arteritis. Ann Intern Med. 1994;121:484–491. doi: 10.7326/0003-4819-121-7-199410010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Weyand CM, Tetzlaff N, Bjornsson J, Brack A, Younge B, Goronzy JJ. Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum. 1997;40:19–26. doi: 10.1002/art.1780400105. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser M, Younge B, Bjornsson J, Goronzy JJ, Weyand CM. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155:765–774. doi: 10.1016/S0002-9440(10)65175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner AD, Bjornsson J, Bartley GB, Goronzy JJ, Weyand CM. Interferon-gamma-producing T cells in giant cell vasculitis represent a minority of tissue-infiltrating cells and are located distant from the site of pathology. Am J Pathol. 1996;148:1925–1933. [PMC free article] [PubMed] [Google Scholar]
- 23.Nordborg C, Larsson K, Nordborg E. Stereological study of neovascularization in temporal arteritis. J Rheumatol. 2006;33:2020–2025. [PubMed] [Google Scholar]
- 24.Rodriguez-Pla A, Bosch-Gil JA, Rossello-Urgell J, Huguet-Redecilla P, Stone JH, Vilardell-Tarres M. Metalloproteinase-2 and -9 in giant cell arteritis: involvement in vascular remodeling. Circulation. 2005;112:264–269. doi: 10.1161/CIRCULATIONAHA.104.520114. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, O'Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol Res. 2008;41:87–102. doi: 10.1007/s12026-007-8014-9. [DOI] [PubMed] [Google Scholar]
- 26.Cantini F, Niccoli L, Nannini C, Bertoni M, Salvarani C. Diagnosis and treatment of giant cell arteritis. Drugs Aging. 2008;25:281–297. doi: 10.2165/00002512-200825040-00002. [DOI] [PubMed] [Google Scholar]
- 27.Langford CA. Vasculitis in the geriatric population. Rheum Dis Clin North Am. 2007;33:177–195. doi: 10.1016/j.rdc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Seo P, Stone JH. Large-vessel vasculitis. Arthritis Rheum. 2004;51:128–139. doi: 10.1002/art.20083. [DOI] [PubMed] [Google Scholar]
- 29.Weyand CM, Goronzy JJ. Arterial wall injury in giant cell arteritis. Arthritis Rheum. 1999;42:844–853. doi: 10.1002/1529-0131(199905)42:5<844::AID-ANR2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 30.Rittner HL, Hafner V, Klimiuk PA, Szweda LI, Goronzy JJ, Weyand CM. Aldose reductase functions as a detoxification system for lipid peroxidation products in vasculitis. J Clin Invest. 1999;103:1007–1013. doi: 10.1172/JCI4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner AD, Goronzy JJ, Weyand CM. Functional profile of tissue-infiltrating and circulating CD68+ cells in giant cell arteritis. Evidence for two components of the disease. J Clin Invest. 1994;94:1134–1140. doi: 10.1172/JCI117428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weyand CM, Wagner AD, Bjornsson J, Goronzy JJ. Correlation of the topographical arrangement and the functional pattern of tissue-infiltrating macrophages in giant cell arteritis. J Clin Invest. 1996;98:1642–1649. doi: 10.1172/JCI118959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roche NE, Fulbright JW, Wagner AD, Hunder GG, Goronzy JJ, Weyand CM. Correlation of interleukin-6 production and disease activity in polymyalgia rheumatica and giant cell arteritis. Arthritis Rheum. 1993;36:1286–1294. doi: 10.1002/art.1780360913. [DOI] [PubMed] [Google Scholar]
- 34.Boniface K, Blom B, Liu YJ, de Waal Malefyt R. From interleukin-23 to T-helper 17 cells: human T-helper cell differentiation revisited. Immunol Rev. 2008;226:132–146. doi: 10.1111/j.1600-065X.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 36.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D'Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH, Sayegh MH, Ansari MJ. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.