Abstract
Na4Si4 and Na4Ge4 are ideal chemical precursors for inorganic clathrate structures, clusters, and nanocrystals. The monoclinic Zintl phases, Na4Si4 and Na4Ge4, contain isolated homo-tetrahedranide [Si4]4− and [Ge4]4− clusters surrounded by alkali metal cations. In this study, a simple scalable route has been applied to prepare Zintl phases of composition Na4Si4 and Na4Ge4 using the reaction between NaH and Si or Ge at low temperature (420 °C for Na4Si4 and 270 °C for Na4Ge4). The method was also applied to K4Ge4, using KH and Ge as raw materials, to show the versatility of this approach. The influence of specific reaction conditions on the purity of these Zintl phases has been studied by controlling five factors: the method of reagent mixing (manual or ball milled), the stoichiometry between raw materials, the reaction temperature, the heating time and the gas flow rate. Moderate ball-milling and excess NaH or KH facilitate the formation of pure Na4Si4, Na4Ge4 or K4Ge4 at 420 °C (Na4Si4) or 270 °C (both M4Ge4 compounds, M = Na, K). TG/DSC analysis of the reaction of NaH and Ge indicates that ball milling reduces the temperature for reaction and confirms the formation temperature. This method provides large quantities of high quality Na4Si4 and Na4Ge4 without the need for specialized laboratory equipment, such as Schlenk lines, niobium/tantalum containers, or an arc welder, thereby expanding the accessibility and chemical utility of these phases by making them more convenient to prepare. This new synthetic method may also be extended to lithium-containing Zintl phases (LiH is commercially available) as well as to alkali metal-tetrel Zintl compounds of other compositions, e.g. K4Ge9.
Introduction
Zintl phases, containing main group polyanions counterbalanced by electropositive cations, have been of great interest in the chemistry and materials community because of their rich variety in structure type and also because of their synthetic versatility. Metal silicides and germanides, in particular, have shown great promise as starting materials for main group metalloid clusters,1 group 14 nanoparticles exhibiting a range of sizes and surface terminations,2–7 and various porous frameworks, 8–10 and these materials afford applications ranging from electronics to photonics.11 Also, thermal decomposition of the alkali metal silicides and germanides, under vacuum with ammonium salts or in an inert atmosphere, provides clathrate-type metal silicides and germanides.12–15 group 14 clathrate phases are attractive for superconductivity,16 thermoelectricity,13 hydrogen storage,17–20 and as wide band gap semiconductor materials.21
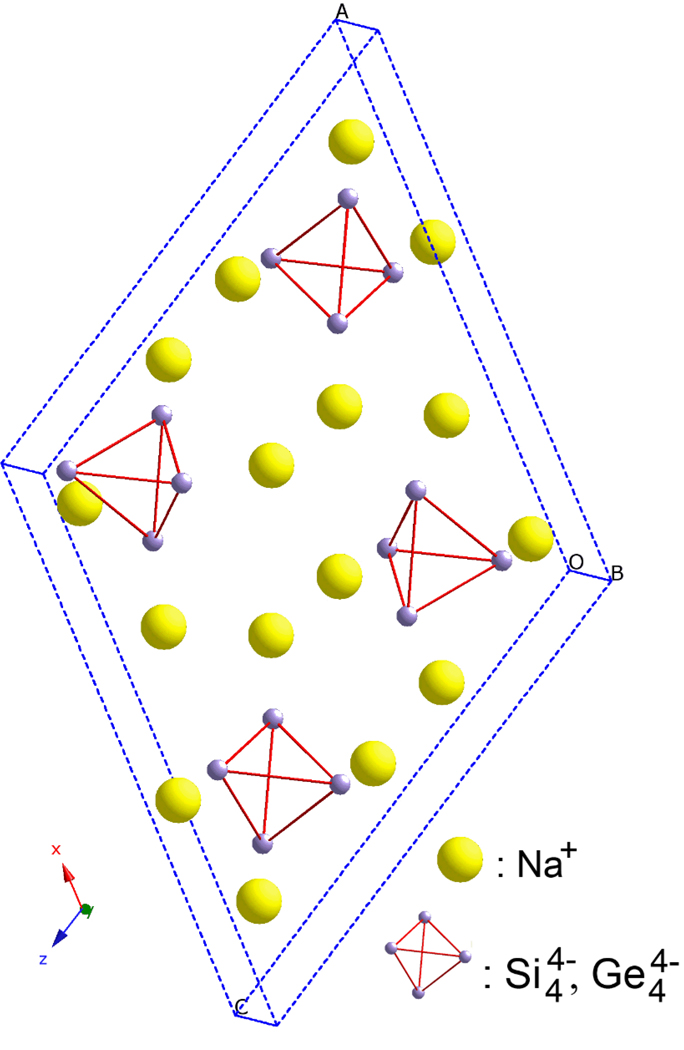
The monoclinic Zintl phases, Na4Si4 and Na4Ge4, contain isolated homo-tetrahedranide clusters, [Si4]4− or [Ge4]4−, each surrounded by four alkali metal cations (Figure 1).22 The polyanions can be understood within the expanded definition of the Zintl concept, which includes electron-deficient polyanionic main group structures in addition to traditional valence precise members. The [Tt4]4− anions can therefore be classified as nido-trigonal bipyramids, isostructural with the P4 unit in white phosphorus. Each alkali metal donates one electron to a Tt4 unit (Tt = tetrel = Si, Ge, Sn, Pb), leading to 4- charged, group 14 polyanions, and each alkali metal cation sits over one face of the [Tt4]4− anion to which it donates. To date, many heavy metal M4Tt4 phases have been prepared (M = Na, K, Rb, Cs, Tt = Si, Ge, Sn, Pb), and all members of this family contain the tetrel in isolated tetrahedral clusters.23, 24 However, LiSi, whose structure has a unique 3-D framework of three-coordinate Si atoms arranged into interpenetrating sheets, does not contain isolated clusters and can only be prepared at high pressure. 25
Figure 1.
A view of the structure of the monoclinic Zintl phases, Na4Si4 and Na4Ge4.
Traditionally, Na4Si4 and Na4Ge4 are prepared by stoichiometric reaction of the elements sealed in inert metal containers, such as niobium or tantalum tubing, at temperatures of approximately 650–750 °C.4, 26 Reaction times are generally on the order of several days to one week when a phase pure material is needed. Because of formation of the stable phases NbSi2 and NbGe2,12 more costly Ta containers are necessary to make high purity materials. Also, an arc welder is required to seal elements in metal tubing, and then the inert metal containers need to be jacketed in fused silica or in steel pressure vessels under vacuum to prevent oxidation of the metal and to prevent the diffusion of gases through the metal container at reaction temperatures. This method requires metal tubing, which is not readily reusable. In addition, this method does not consistently yield pure material as elemental sodium around the closure site of the metal tubing can result in a poorly welded reaction vessel. Even small amounts of sodium pieces in the tubing crimp causes problems with sealing the reaction vessel, and the situation is more pronounced when potassium is used, rather than sodium, to make the potassium tetrelides. Given the large number of applications of Na4Si4 and Na4Ge4 for the synthesis of new materials, there have been several reported attempts to find simpler routes to these phases. One involves the heating of sodium and silicon together in high-boiling, specialty mineral oils in a 4-neck metal flask under inert atmosphere.27 Another utilizes sodium and silica gel,28, 29 which provides Na4Si4 with lower purity (~ 80 %) and requires the subsequent removal of residual oxide impurities.6 Therefore, an efficient route for the preparation of large amounts of high quality alkali metal-group 14 Zintl phase precursors without the use of expensive metal containers and other specialized equipment is very important for further development of these types of materials.
Here, we report a new method for preparing large amounts of Zintl phase materials utilizing commercially available alkali metal hydrides and tetrel elements. We have shown that Na4Ge4 and Na4Si4-xMnx prepared by this method can provide germanium nanoparticles30 and manganese doped silicon nanoparticles.31 This report provides detailed synthetic parameters for these alkali metal/tetrel precursors. This new preparation can be performed at low cost with common laboratory equipment, using a standard tube furnace and commercially available alumina boats at low temperatures (420 °C for Na4Si4 and 270 °C for Na4Ge4). Using this method, we can decrease the formation temperature of Na4Si4 by 230–330 °C and of Na4Ge4 by 380–480 °C compared to the conventional metal-melt method.4, 26 Furthermore, the optimized synthesis takes much less time than other preparations, involves simple handling of powdered reagents, and can be easily scaled to produce gram quantities. Since this route avoids use of niobium or tantalum containers and thus prevents reaction of silicon or germanium with niobium or tantalum, it yields sodium silicides or germanides with high purity. NaH powder has been shown to be a good reducing agent,32 and excess NaH decomposes at low temperatures to Na and H2, which is helpful in removing the surface oxide present on silicon or germanium powder. NaH is an advantageous starting material relative to elemental sodium, because of its simpler handling and also because of its ability to provide a high purity product when the reaction is performed under flowing gas or dynamic vacuum. This synthetic method may be considered as a self-cleaning process – newly-formed Na, decomposed from excess NaH, reacts with surface oxide on silicon or germanium powder. Additionally, H2 is produced as a volatile side product of the reaction; and it may aid in the cleaning of the desired product by reacting with oxide on the surface of silicon or germanium, providing volatiles that can be removed with an Ar flow and transported to the cold zone outside the product container. This new synthetic method may be extended to lithium-containing Zintl phases as well as to alkali metal-tetrel Zintl compounds of other compositions, e.g. Na8Si46, and K4Ge9.
Results and Discussion
Optimized Parameters
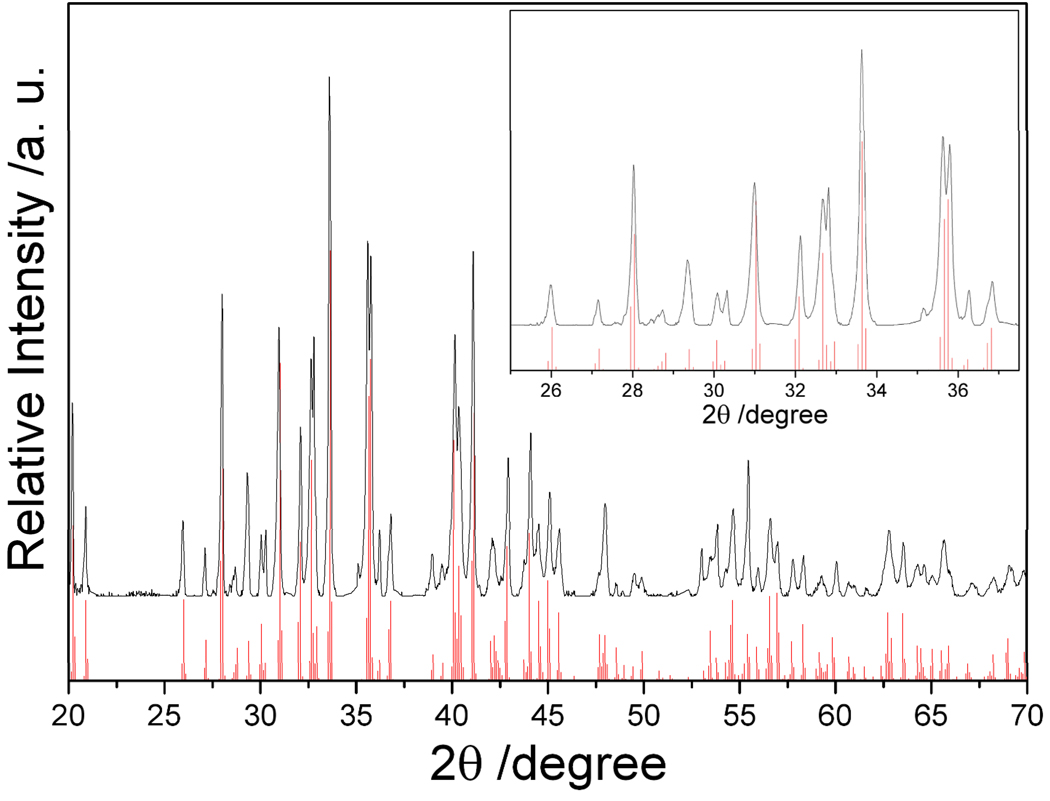
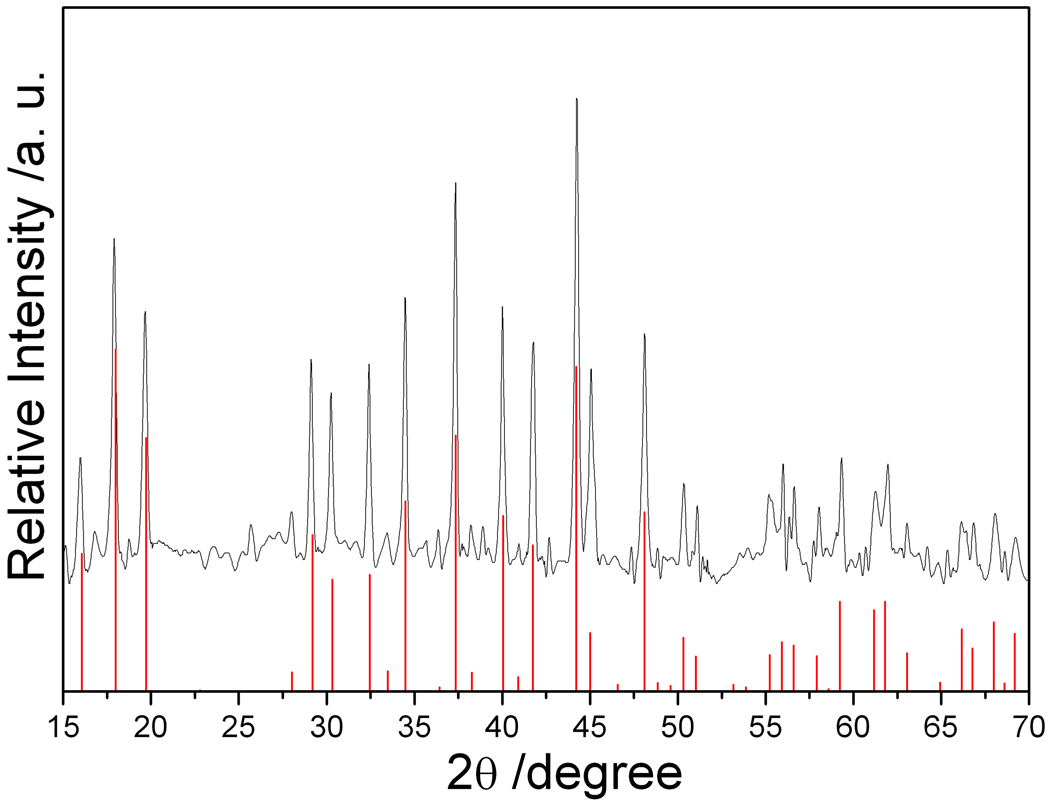
The X-ray diffraction (XRD) pattern of the as-prepared Na4Si4, obtained by heating the reagents for 48 hours at 420 °C under flowing argon atmosphere, is shown in Figure 2. It is consistent with the calculated Na4Si4 powder pattern. The calculated intensities and peak positions in Figure 2 are derived from single-crystal XRD data.22 There are no unindexed peaks that could be attributed to Na, NaH, Si or any other impurities. This result compares well with the product obtained from the published high temperature method, where stoichiometric amounts of Na and Si in a sealed Nb tube are heated at 650 °C for 3 days.4, 6, 7 Lattice parameters determined by Rietveld refinement of the XRD pattern are a = 12.1567(1) Å, b = 6.5487(6) Å, c = 11.1593(2) Å, and β = 118.9248(1) °, consistent with the published lattice parameters of Na4Si4 (a = 12.1536(5) Å, b = 6.5452(5) Å, c = 11.1323(6) Å, and β = 118.9(1) °).26
Figure 2.
XRD patterns of the products Na4Si4 from optimized reactions of NaH with Si. The inset is an enlarged view of the XRD pattern in the range of 25– 38 °. The red lines indicate the calculated X-ray diffraction peak positions for Na4Si4.
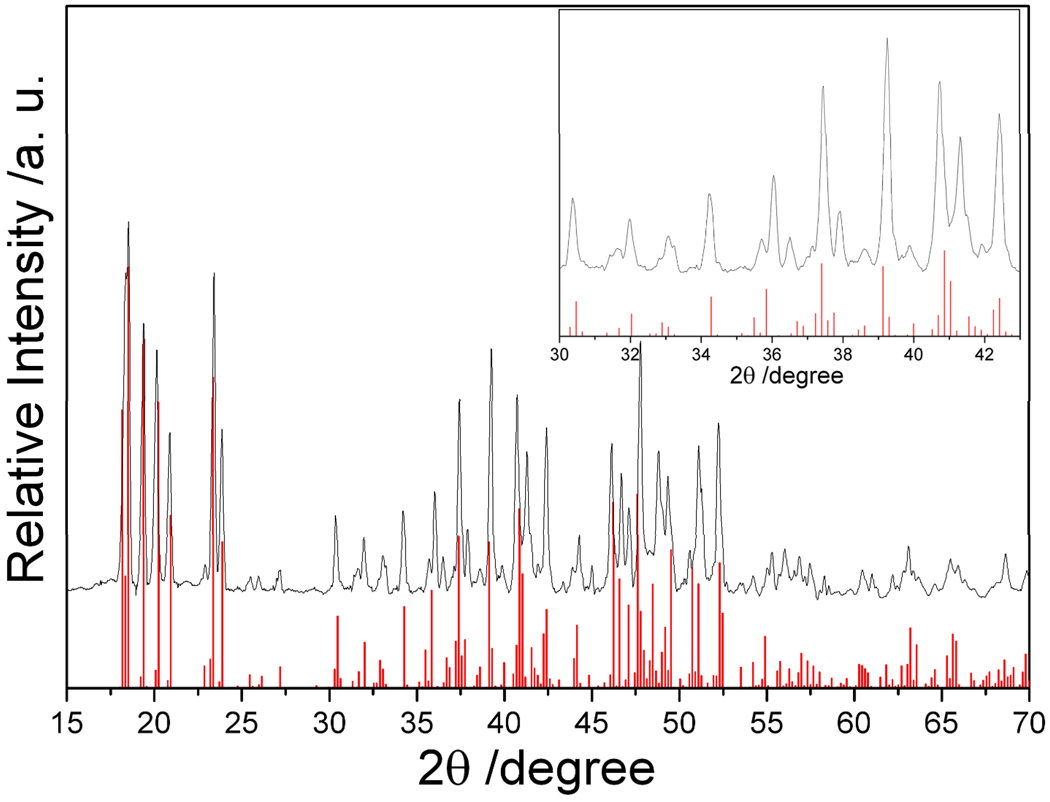
The XRD pattern in Figure 3 is that of the as-prepared product Na4Ge4 obtained from heating a ball-milled mixture of NaH and Ge for 3 hours at 270 °C under flowing argon. All the peaks are indexed to Na4Ge4 and there is no evidence for NaH, Ge, Na or other impurities. This result agrees with the product obtained from the published high temperature method.3 The lines in Figure 3 belong to the XRD pattern of pure Na4Ge4 calculated from single crystal data.33 Lattice parameters obtained from the powder XRD data of the as-prepared product are a = 12.296(8) Å, b = 6.703(5) Å, c = 11.419(6) Å, and β = 120.09(5) °, consistent with the published parameters of Na4Ge4 (a = 12.33 Å, b = 6.70 Å, c = 11.42 Å, and β = 119.9 °).22, 34 For both reactions, the final products are pure phases, Na4Si4 or Na4Ge4, confirming the success of this method.
Figure 3.
XRD patterns of the products Na4Ge4 from optimized reactions of NaH with Ge. The inset is an enlarged view of the XRD pattern in the range of 30– 43 °. The red lines indicate the calculated X-ray diffraction peak positions for Na4Ge4.
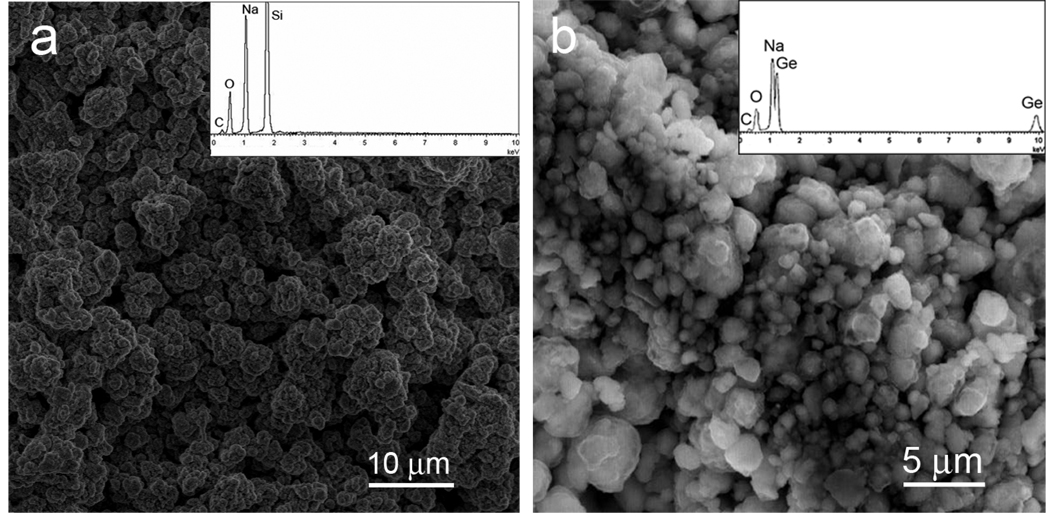
Figure 4 shows SEM images of the resulting Na4Si4 (Fig. 4a) and Na4Ge4 (Fig. 4b) products, indicating that they are micron sized particles with diameters of 1.5 – 4 µm. EDX spectra of the products obtained from optimized reactions of NaH with Si or Ge are shown as insets. Na and Si are detected with an atomic ratio of Na:Si of 1:1.02, consistent with the stoichiometric formula of Na4Si4. Na and Ge are detected in an atomic ratio of Na:Ge of 1:1.05, in good agreement with the stoichiometric formula of Na4Ge4. The observed carbon (C) signal comes from the carbon tape (support), while the oxygen (O) signal most likely originates from oxidation of the sample surface, which may occur during transfer from the glove box to the SEM. No other peaks can be found in the EDX spectrum, indicating that the as-prepared products are pure Na4Si4 and Na4Ge4.
Figure 4.
SEM images of (a) Na4Si4 and (b) Na4Ge4. The insets show the EDX spectra of the corresponding compounds.
We describe this as a solid-state reaction from the viewpoint of the state of the raw materials and the lack of any extraneous solvent, even though the present reaction methodology relies on the in situ formation of intermediate liquid Na metal from solid NaH powder and thus it is not strictly a traditional solid-state reaction. The molten reagent aids in overcoming diffusion-limited formation of the products. However, quantitative reaction of solid tetrel with molten alkali metal is expected to proceed more rapidly and at lower temperatures if an intimately ground mixture of the reagents is used. Although the powdered reagents may appear to be well-mixed macroscopically by grinding in a mortar, they are quite inhomogeneous on the microscopic scale. Ball-milling can provide more homogeneous mixing and activate the surface of reagents. Our method takes advantage of homogeneous mixing of solid-state reagents and the formation of the Na intermediate to prepare the Zintl phases Na4Si4 and Na4Ge4. Therefore, besides adjusting the molar ratio between raw materials, the reaction temperature and time, the gas flow rate, along with mechanical mixing of the reagents was investigated.
Ball-milling
It has been reported that when materials are ground in a high-energy ball mill, not only are their physical properties changed (including particle size and crystallinity) but their chemical properties are also changed by the mechanochemical reaction.35 An XRD study of the ball-milled mixture suggested that no Na4Si4 or Na4Ge4 forms during the ball-milling procedure. Without ball-milling, pure Na4Si4 and Na4Ge4 were not obtained. When the reagents were mixed by grinding in a mortar and then heated at 420 °C for 72 hours for Na4Si4 or at 270 °C for 3 hours for Na4Ge4, the XRD patterns of the products showed strong diffraction peaks from Na4Si4, unreacted Si, and Na or Na4Ge4, unreacted NaH, and Ge. Obviously, ball-milling provides more intimately mixed starting materials, thereby facilitating the reaction.
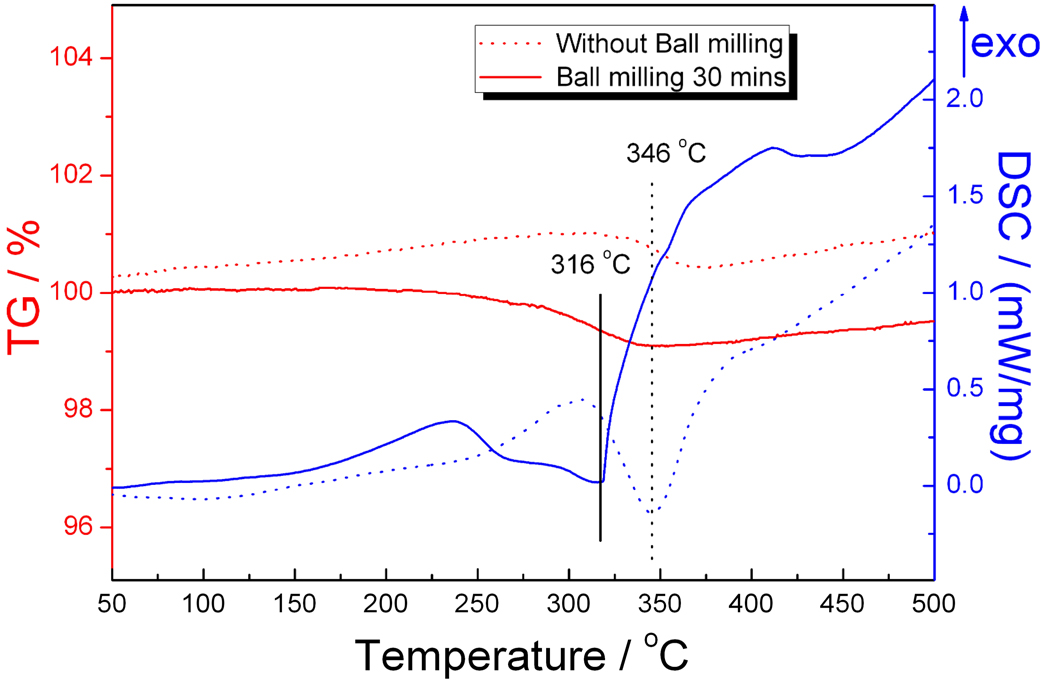
The effect of ball-milling can additionally be investigated by TG/DSC measurements. Figure 5 provides TG/DSC curves for the starting reagents NaH and Ge, reacted either with or without prior ball-milling. Comparing the solid curves with the dotted curves, we note that the initial reaction event between manually mixed (dotted curves) NaH and Ge is exothermic, with a maximum at 307°C, which is followed by an endothermic minimum at 346 °C. For the ball-milled reagents (solid curves), the maximum in the exotherm and the minimum in the endotherm occur at 235 °C and 316 °C, respectively, 72 °C and 30 °C lower than for the mixture heated without prior ball-milling. Between 250 °C and 350 °C, a weight loss of ca. 1 wt % is attributed to the hydrogen released during the reaction. Similarly, a decrease in the initial reaction temperature is observed in the case of NaH and Si. After ball milling the mixture of NaH and Si, the endothermic peak occurs 21 °C lower than that of the manually mixed reagents. The initial exotherm suggests that there could be a chemical reaction between NaH and Si or Ge to form Na-Si-H or Na-Ge-H phases, analogous to the previous works that report the formation of Li-Si-H and Li-Ge-H.20 The endotherm and weight loss are consistent with subsequent dehydrogenation to form Na4Si4 or Na4Ge4. However, this speculation needs further confirmation, since we did not explore the intermediate phase formation during this study
Figure 5.
The TG-DSC curves of mixtures of NaH and Ge (no ball-milling, dotted curves; ball-milling for 30 minutes, solid curves). The minima of the endotherms are indicated. The curves were obtained under flowing Ar (flow rate was 20 mL/min) with a 10 K/min ramp from 25 °C to 500 °C.
Stoichiometry
For both reactions, an optimized ratio of starting reagents is critical for phase purity. For Na4Si4, a significant excess of NaH (90 % excess) was required to give a pure phase product according to the powder XRD pattern. There was still unreacted Si in the product when the ratio of NaH to Si was decreased to 1.8:1 as evidenced by the diffraction peak from Si seen in the diffraction pattern. When the ratio of NaH to Si was 1.9:1, the final product was pure Na4Si4. For Na4Ge4, when the ratio of NaH to Ge was 1:1, the diffraction pattern was consistent with the final products being Na4Ge4 and unreacted Ge. Increasing the amount to an excess of 10 mol % NaH, there were still several diffraction peaks attributed to unreacted Ge. When the NaH content exceeded 20 % excess over the stoichiometric amount required to react with Ge, all the diffraction peaks could be indexed to Na4Ge4.
In both the Si and Ge cases, excess NaH is necessary in our reactions to form the pure phases. The large excess in the case of Si may be required to remove all the oxide from the surface. It was found that a white solid deposited on the cold end of the glass tube during reaction and this product was identified by powder XRD as a mixture of sodium hydroxide and disodium oxide. Presumably, the combination of Na, H2, and H2O, resulting from the reaction of hydrogen with the oxide surface of Si, can form volatiles that are transported and deposited in the form of sodium hydroxide and disodium oxide at the cooler end of the tube. The required excess of NaH may also depend upon the heating rate and the pressure, as well as the flow rate of the argon gas used in the reaction. However, heating rate and pressure were not investigated. These results suggest that optimization of the relative stoichiometry may also depend on the source and purity of silicon powder. In any case, there is no evidence for the presence of unreacted Na or NaH in the Na4Si4 or Na4Ge4 products by powder X-ray diffraction, suggesting that, if they are present, they are either present in a very small amount (below the detection limit of powder XRD) or are amorphous.
Reaction temperature, time, and gas flow rate
The effects of reaction temperature, heating time, and gas flow rate were explored in this study. A series of controlled experiments were carried out at different temperatures while keeping the other parameters constant. In the case of Na4Si4, if the reaction temperature was lower than 420 °C, there was still unreacted Si in the products. For Na4Ge4, if the reaction temperature was below 270 °C, there was residual Ge in the samples, even for reaction times prolonged to 12 hours.
The reaction time was found to be important for both reactions (NaH + Si, NaH + Ge). As expected due to the passivating oxide surface, formation of pure Na4Si4 requires a longer reaction time. When the reaction time was shorter than the optimized reaction time (even 36 hours), final products were mixtures of Na4Si4 and residual Si. For Na4Ge4, when increasing the reaction time from 0.5 hour to 1 hour, the intensity of the diffraction peaks assigned to Ge decreased concomitant with an increase in intensity of those assigned to Na4Ge4. When the reaction time reached 3 hours, only diffraction peaks assigned to pure Na4Ge4 appeared in the product, indicating the formation of pure Na4Ge4.
The effect of the gas flow rate was also investigated. For both reactions (NaH + Si, NaH + Ge), the optimal gas flow rate is 30 mL/min. If the gas flow rate was lower than 10 mL/min, unreacted Na could be found in the product (determined by XRD); therefore, excess Na was not removed by the flowing gas during the reaction. If the gas flow was higher than 50 mL/min, the starting material Si did not completely react with the Na metal, as unreacted Si could be found in the product.
We also extended this method to the synthesis of K4Ge4. The XRD pattern of Figure 6 is that of the as-prepared product K4Ge4 obtained from heating a ball-milled mixture of KH and Ge for 3 hours at 270 °C under flowing argon at 30 mL/min. All the main peaks are indexed to K4Ge4 and there is no evidence for KH, Ge, K or other impurities. This result agrees with the product obtained from the published high temperature method.23, 36 The optimized parameters for the synthesis of Na4Si4, Na4Ge4, and K4Ge4 are shown in Table 1.
Figure 6.
XRD pattern of the product K4Ge4 from the reaction of KH with Ge. The red lines indicate the calculated X-ray diffraction peak positions for K4Ge4.
Table 1.
The optimized parameters for the synthesis of Zintl phases Na4Si4, Na4Ge4, and K4Ge4.
| Ball-milled Starting Materials |
Stoichiometry (molar ratio) |
Temperature (°C) |
Time (hour) |
Gas Flow Rate (mL/min) |
Products |
|---|---|---|---|---|---|
| NaH : Si | 1.9 : 1 | 420 | 48 | 30 | Na4Si4 |
| NaH : Ge | 1.2 : 1 | 270 | 3 | 30 | Na4Ge4 |
| KH : Ge | 1.2 : 1 | 270 | 3 | 30 | K4Ge4 |
Conclusions
A facile and scalable preparation method for the Zintl phases Na4Si4 and Na4Ge4 has been developed. The synthesis employs the reaction between NaH and Si or Ge under mild conditions. Ball-milling and excess NaH are necessary conditions for the formation of pure Na4Si4 and Na4Ge4 by this method. In addition, the extension of this method, utilizing KH and Ge, to K4Ge4 was also demonstrated. These compounds are straightforward to prepare by this method, and the method provides easy access to precursors for the syntheses of group 14 nanoparticles, novel cluster-containing complexes, and new clathrate-type framework materials. The method provides a convenient avenue for doping and formation of more complex alkali-metal tetrel phases, and thereby complex group 14 nanoparticles, clusters, and clathrate framework materials. A similar method may be effective in the fabrication of other Zintl phase salts, such as K4Si4, alkali metal-tetrel phases of other compositions (e.g., Na8Si46, and K4Ge9), and more complex multicomponent Zintl phases, such as new ternary alkali metal hydrides.
Experimental
Chemicals
Sodium hydride powder (NaH, 95 %), silicon powder (Si, 99 %), and germanium powder (Ge, ≥ 99 %) were purchased from Aldrich and were used without further treatment. Potassium hydride (KH, 30 wt % dispersion in mineral oil) was purchased from Aldrich and dried before use. All manipulations were carried out under dry N2 or Ar gas, in either a glove box or a tube furnace, using standard anaerobic and anhydrous techniques. CAUTION: sodium hydride and potassium hydride powder are reactive to oxygen and moisture and must be handled under inert atmosphere with care.
Synthesis of sodium silicide (Na4Si4), sodium germanide (Na4Ge4) and potassium germanide (K4Ge4)
A high-energy Spex 8000M mill with a tungsten carbide milling vial and two tungsten carbide balls (diameter of ~ 1 cm) was used to ball-mill mixtures of NaH or KH and Si or Ge powders with an appropriate molar ratio (mNaH:mSi = 1.9:1, mNaH:mGe = 1.2:1 and mKH:mGe = 1.2:1). Mixing the starting materials and transferring mixtures to the milling vial were operations carried out in a glove box filled with N2. Before it was removed from the glove box, the milling vial was additionally sealed with plastic film that helps reduce oxygen diffusion during ball-milling. The reagents were then intimately mixed in the mill for 30 minutes, and the milling vial was subsequently returned to the glove box for further manipulation.
The pre-milled mixture of NaH (91.2 mg, 3.8 mmol) and Si (56 mg, 2 mmol) was placed into a 1 mL alumina crucible with an alumina cover (a 1.5 mL boat serves as a cover) in a silica glass (or pyrex) tube with stopcocks at both ends. The silica tube was seated in a horizontal tube furnace, placed under a stream of Ar flowing at 30 mL/min, and heated to 420 °C using a 180 °C/hour ramp. The reaction vessel was allowed to dwell at this temperature for 48 hours before the furnace was shut off manually. For the synthesis of Na4Ge4 and K4Ge4, the procedures were similar as that of Na4Si4. However, a smaller excess of NaH (57.6 mg, 2.4 mmol) or KH (96.3 mg, 2.4 mmol) was reacted with Ge (145.2 mg, 2 mmol) and the reaction mixture was heated at 270 °C (ramp rate = 180 °C/hour), rather than 420 °C, for only 3 hours. In all cases, after cooling to room temperature, the black products were transferred in an air-free manner to the glove box for further characterization.
When the Na4Si4 powder was exposed to air without any disturbance, it oxidized slowly and no obvious color change was observed. However, when a fresh surface was exposed or the powder is exposed to water, the product bursts into flame, concomitant with a color change from black/dark grey to pale grey. Compared to Na4Si4, the Na4Ge4 and K4Ge4 powders reacted more violently when exposed to air or water, and flames were observed upon exposure to water. CAUTION: Na4Si4, Na4Ge4 and K4Ge4 powders are highly reactive to moisture and must be handled under inert atmosphere with care.
Characterization
Powder X-ray diffraction (XRD) data were collected using an air sensitive holder on an INEL CPS 120 diffractometer (for Na4Ge4) with Co Kα radiation (λ = 1.78897 Å) or on a Bruker D8 diffractometer (for Na4Si4 and K4Ge4) operating at 40 kV and 40 mA with Cu Kα radiation (λ = 1.54184 Å). Lattice parameters were calculated by Rietveld refinement using JADE 6.1.37 X-ray powder diffraction patterns of Na4Si4 and Na4Ge4 were calculated using the program CrystalDiffract.38 Morphology and chemical composition were analyzed by a Hitachi S-800T scanning electron microscope (SEM) and an Oxford INCA energy-dispersive X-ray (EDX) spectrometer, with an accelerating voltage of 20 kV. SEM and EDX samples were prepared by standard techniques in which grain dispersions were supported on double-sided carbon tape on specimen holders. The specimen holders were kept in a N2-filled jar in a glove box and quickly transferred to the SEM instrument. The time of exposure to air was less than 5 seconds. Simultaneous thermogravimetric analysis (TG) and differential scanning calorimetry (DSC) were carried out with a Netzsch 409 Thermal Analyzer under Ar flowing at 20 mL/min with a 10 K/min ramp from 25 °C to 500 °C. TG/DSC samples of approximately 25 mg were placed in alumina crucibles with lids inside the glove box and transferred under inert atmosphere to the instrument.
Acknowledgments
We thank the National Science Foundation (grant DMR-0600742) and Nanohmics, Inc. for funding.
References
- 1.Hull MW, Urginov A, Petrov I, Sevov SC. Inorg. Chem. 2007;46:2704. doi: 10.1021/ic0623314. [DOI] [PubMed] [Google Scholar]
- 2.Bley RA, Kauzlarich SM. J. Am. Chem. Soc. 1996;118:12461. [Google Scholar]
- 3.Taylor BR, Kauzlarich SM, Delgado GR, Lee HWH. Chem. Mater. 1999;11:2493. [Google Scholar]
- 4.Mayeri D, Phillips BL, Augustine MP, Kauzlarich SM. Chem. Mater. 2001;13:765. [Google Scholar]
- 5.Pettigrew KA, Liu Q, Power PP, Kauzlarich SM. Chem. Mater. 2003;15:4005. [Google Scholar]
- 6.Zhang XM, Neiner D, Wang SZ, Louie AY, Kauzlarich SM. Nanotechnolugy. 2007;18 doi: 10.1088/0957-4484/18/9/095601. 095601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neiner D, Chiu HW, Kauzlarich SM. J. Am. Chem. Soc. 2006;128:11016. doi: 10.1021/ja064177q. [DOI] [PubMed] [Google Scholar]
- 8.Armatas GS, Kanatzidis MG. Nature. 2006;441:1122. doi: 10.1038/nature04833. [DOI] [PubMed] [Google Scholar]
- 9.Armatas GS, Kanatzidis MG. Science. 2006;313:817. doi: 10.1126/science.1130101. [DOI] [PubMed] [Google Scholar]
- 10.Sun D, Riley AE, Cadby AJ, Richman EK, Korlann SD, Tolbert SH. Nature. 2006;441:1126. doi: 10.1038/nature04891. [DOI] [PubMed] [Google Scholar]
- 11.Fauchet PM. Mater. Today. 2005 January;:26. [Google Scholar]
- 12.Bobev S, Sevov SC. J. Solid State Chem. 2000;153:92. [Google Scholar]
- 13.Beekman M, Nolas GS. Physica B-: Condensed Matter. 2006;383:111. [Google Scholar]
- 14.Bohme B, Guloy A, Tang ZJ, Schnelle W, Burkhardt U, Baitinger M, Grin Y. J. Am. Chem. Soc. 2007;129:5348. doi: 10.1021/ja0705691. [DOI] [PubMed] [Google Scholar]
- 15.Horie HO, Kikudome T, Teramura K, Yamanaka S. J. Solid State Chem. 2009;182:129. [Google Scholar]
- 16.Fukuoka H, Kiyoto J, Yamanaka S. Inorg. Chem. 2003;42:2933. doi: 10.1021/ic020676q. [DOI] [PubMed] [Google Scholar]
- 17.Neiner D, Okamoto NL, Condron CL, Ramasse QM, Yu P, Browning ND, Kauzlarich SM. J. Am. Chem. Soc. 2007;129:13857. doi: 10.1021/ja0724700. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Hartman MR, Udovic TJ, Rush JJ, Zhou W, Bowman RC, Vajo JJ. Acta Crystallogr. Sect. B:-Struct. Sci. 2007;63:63. doi: 10.1107/S0108768106046465. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Zhou W, Udovic TJ, Rush JJ. Chem. Mater. 2007;19:329. [Google Scholar]
- 20.Wu H, Zhou W, Udovic TJ, Rush JJ, Yildirim T, Hartman MR, Bowman RC, Vajo JJ. Phy. Rev. B. 2007;76:224301. [Google Scholar]
- 21.Gryko J, McMillan PF, Marzke RF, Ramachandran GK, Patton D, Deb SK, Sankey OF. Phys. Rev. B. 2000;62:R7707. [Google Scholar]
- 22.Witte J, von Schnering HG. Z. Anorg. Allg. Chem. 1964;327:260. [Google Scholar]
- 23.von Schnering HG, Llanos J, Chang JH, Peters K, Peters EM, Nesper R. Zeitschrift Fur Kristallographie-New Crystal Structures. 2005;220:324. [Google Scholar]
- 24.von Schnering HG, Schwarz M, Chang JH, Peters K, Peters EM, Nesper R. Z. Kristallogr. - New Cryst. Struct. 2005;220:525. [Google Scholar]
- 25.Stearns LA, Gryko J, Diefenbacher J, Ramachandran GK, McMillan PF. J. Solid State Chem. 2003;173:251. [Google Scholar]
- 26.Goebel T, Prots Y, Haarmann F. Z. Kristallogr.-New Cryst. Struct. 2008;223:187. [Google Scholar]
- 27.Bach RO, Gillespie AS., Jr. USA Pat. 1971 3563730. [Google Scholar]
- 28.Shatnawi M, Paglia G, Dye JL, Cram KC, Lefenfeld M, Billinge SJL. J. Am. Chem. Soc. 2007;129:1386. doi: 10.1021/ja067140e. [DOI] [PubMed] [Google Scholar]
- 29.Dye JL, Cram KD, Urbin SA, Redko MY, Jackson JE, Lefenfeld M. J. Am. Chem. Soc. 2005;127:9338. doi: 10.1021/ja051786+. [DOI] [PubMed] [Google Scholar]
- 30.Ma XC, Wu FY, Kauzarich SM. J. Solid State Chem. 2008;181:1628. [Google Scholar]
- 31.Tu CQ, Ma XC, Pantazis P, Kauzlarich SM, Louie AY. 2009 Submitted. [Google Scholar]
- 32.Fan YH, Li WN, Zou YL, Liao SJ, Xu J. J. Nanopart. Res. 2006;8:935. [Google Scholar]
- 33.Schäfer R, Klemm W. Z. Anorg. Allg. Chem. 1961;312:214. [Google Scholar]
- 34.Tegze M, Hafner J. Phys. Rev. B. 1989;40:9841. doi: 10.1103/physrevb.40.9841. [DOI] [PubMed] [Google Scholar]
- 35.Beyer MK, Clausen-Schaumann H. Chem. Rev. 2005;105:2921. doi: 10.1021/cr030697h. [DOI] [PubMed] [Google Scholar]
- 36.Busmann E. Naturwissenschaften. 1960;47:82. [Google Scholar]
- 37.MDI JADE 6.1. Livermore, CA: Materials Data Inc.; [Google Scholar]
- 38.CrystalDiffract. Oxfordshire, U. K.: Crystal Maker Software Limited; 2006. [Google Scholar]