Abstract
Objective
High velocity, low amplitude (HVLA) manipulation is an effective treatment for low back pain (LBP); however, the corresponding mechanisms are undetermined. Hypoalgesia is associated with HVLA manipulation and suggests specific mechanisms of action. An audible pop (AP) is also associated with HVLA manipulation; however, the influence of the AP on the hypoalgesia associated with HVLA manipulation is not established. The purpose of the current study was to observe the influence of the AP on hypoalgesia associated with HVLA manipulation.
Methods
The current study represents a secondary analysis of 40 participants. All participants underwent thermal pain sensitivity testing to their leg and low back using protocols specific to Aδ fiber mediated pain and temporal summation. Next, participants received HVLA manipulation to their low back and the examiner recorded whether or not an AP was perceived. Finally, participants underwent immediate follow up thermal pain sensitivity testing using the same protocols. Separate repeated measure ANOVAs were used to observe changes in pain sensitivity prior to and immediately following HVLA manipulation.
Results
Hypoalgesia of Aδ fiber mediated pain was observed in the low back following HVLA (p< 0.05) and this was independent of whether an AP was perceived (p> 0.05). Hypoalgesia of temporal summation was observed in the lower extremity following HVLA (p< 0.05) and this was independent of whether an AP was perceived (p= 0.08). However, a moderate effect size for temporal summation was observed favoring participants in whom an AP was perceived.
Conclusion
The current study suggests hypoalgesia is associated with HVLA manipulation and occurs independently of a perceived AP. Inhibition of lower extremity temporal summation may be larger in individuals in whom an AP is perceived, but further study is necessary to confirm this finding.
Introduction
High velocity, low amplitude (HVLA) thrust manipulation is suggested as an effective intervention in the treatment of low back pain.1-4 An audible “pop” (AP) is characteristic of HVLA and may distinguish these interventions from other forms of manual therapy such as massage and mobilization. While the AP is associated with HVLA manipulation, a consensus is currently lacking as to the clinical relevance. For example, manuscripts have been published suggesting biomechanically efficient ways to achieve the AP,5, 6 research studies have used the AP as an indication of a successfully applied HVLA manipulation,7, 8 and sham HVLA manipulation techniques have been designed based on an avoidance of the AP.9, 10 In contrast, clinical studies11-13 and a literature review14 have reported a lack of association between the AP and clinical outcomes.
Hypoalgesia, or a decrease in pain perception in response to an unchanging stimulus, is associated with HVLA.15-18 For example, HVLA corresponds to an increase in pain pressure threshold15 and a decrease in pain perception to unchanging thermal stimuli.16 Hypoalgesia following HVLA is hypothesized to indicate potential neurophysiological mechanisms of action.19, 20
Central sensitization is an exaggerated pain response characterized by allodynia (pain perception to a previously non- painful stimulus) and hyperalgesia (heightened pain severity in response to a previously painful stimulus). HVLA is hypothesized to affect musculoskeletal pain through the alteration of changes associated with central sensitization19 and immediate hypoalgesia may be a clinical indicator of such an effect. Temporal summation is a clinical measure of central sensitization. Specifically, temporal summation is an increase in pain perception to an unchanging repetitive, painful stimulus applied at a frequency of ≤ 3 seconds and is observed in both healthy individuals and, to a greater extent, in those experiencing pain conditions.21, 22 We have previously observed hypoalgesia of temporal summation following HVLA manipulation to the lumbar spine which was not observed in comparison groups riding a stationary bike or performing lumbar extension range of motion exercises.16 In contrast, hypoalgesia for A-delta fiber mediated pain perception did not differ between the 3 groups. 16 Subsequently, HVLA manipulation may produce a hypoalgesic response which differs from other common rehabilitation interventions due to a specific effect on temporal summation.
The influence of the AP on immediate hypoalgesia corresponding to HVLA manipulation is not clear. Hypoalgesia represents a potential neurophysiological mechanism behind the clinical effectiveness of HVLA manipulation. An association between hypoalgesia and an AP would suggest a greater neurophysiological response corresponding to the AP with a implications for a potential mechanism of HVLA manipulation. Therefore, the purpose of the present study was to assess the role of the AP in HVLA manipulation associated thermal pain sensitivity to both A-delta fiber mediated pain perception and temporal summation following HVLA manipulation. We hypothesized that the AP would not be associated with greater hypoalgesia to thermal pain perception, similar to the findings of prior studies related to clinical pain.11-14
Methods
The current study represented a planned secondary analysis. The protocol and results of the primary study are provided in detail elsewhere.23 Briefly, in the primary study, we studied the association between expectation and changes in thermal pain sensitivity associated with HVLA manipulation in 60 healthy participants. The purpose of the primary study was to examine the influence of expectation on thermal pain sensitivity outcomes. Including the AP in our primary analysis would have required a larger sample size to ensure adequate power and we had no specific hypotheses about the interaction between the AP and expectation. Additionally, we would have had less control over balance of the groups due to the unpredictability of achieving an AP. Subsequently, we tracked the AP in the primary study for the express purpose of performing a secondary analysis. In the primary study, a negative treatment effect was observed in one of our intervention groups. Therefore, the current analysis only included participants in the two remaining intervention groups in whom a treatment effect was not observed (n = 40).
Participants
The University of Florida Institutional Review Board approved the current study. A sample of convenience was recruited from the University of Florida Health Science Center community by flyer and word of mouth. Potential participants were screened for appropriateness by a study representative and those wishing to participate signed an informed consent form. Inclusion criterion was age eighteen to sixty and exclusion criteria were non-English speaking, systemic medical conditions (e.g. diabetes, hypertension), current use of psychiatric medication, pregnancy, regular use of prescription medication for management of pain, presently experiencing low back pain, or history of surgery to the low back.
Measures
Demographic Questionnaire
Participants completed a demographic questionnaire regarding age, sex, race, education, and prior history of low back pain.
Psychological Questionnaires
Psychological questionnaires specific to pain catastrophizing, fear of pain, and anxiety were included due to a known influences on experimental pain. 24-27 We chose to evaluate for baseline group differences in these factors to account for potential confounders that could affect reporting of thermal pain sensitivity.
Pain Catastrophizing Scale (PCS)
The PCS consists of 13 items specific to individual coping styles with pain which are each quantified with a five point ordinal scale. Higher scores indicate greater levels of catastrophizing. The score may be taken as a whole or as individual factors of rumination, helplessness, and magnification. Prior studies have validated the factor structure and found good internal consistency reliability and validity of the PCS. 25, 28-30
Fear of Pain Questionnaire-III (FPQ-III)
The FPQ-III 31 consists of 30 items, each scored on a 5-point adjectival scale, which measures fear of normally painful situations. Higher scores indicate greater pain related fear. The FPQ has demonstrated sound psychometric properties in both experimental and clinical pain studies.26, 31, 32
Anxiety Visual Analog Scale (VAS)
Anxiety was measured through a 10 cm VAS. Participants were asked to indicate along the VAS anchored with none and most severe anxiety imaginable the amount of anxiety they were currently feeling regarding the experimental pain task they were about to experience. VASs have been used to measure anxiety in other studies and have demonstrated sound psychometric properties 33-36.
Thermal Pain Sensitivity
Thermal pain sensitivity testing was performed using the Medoc Neurosensory Analyzer (TSA-2001, Ramat Yishai, Israel) with hand- held peltier- element- based stimulator. We included previously established protocols biased towards Aδ fiber mediated pathways and temporal summations. 21, 37 Participants first underwent a practice session in order to familiarize themselves with the pain testing protocol immediately followed by the full QST.
Numeric Rating Scale (NRS)
NRSs were used as a measure of evoked thermal pain sensitivity. Participants were asked to quantify any evoked pain using a NRS anchored by “0” (no pain at all) and “100” (worst imaginable pain). The NRS is frequently used as a measure of both clinical and experimental pain and has demonstrated sound psychometric properties in previous studies 38-41.
Aδ fiber mediated pain (first pain)
Aδ fiber mediated pain was assessed in the non- dominant posterior calf and adjacent to the non-dominant posterior superior iliac spine through the application of heat pulses of 3 seconds duration. The baseline temperature of the thermode was 35°C and rose at a rate of 10°C/sec to 47 or 49°C. All participants received pulses of both 47 and 49°C in a random order. The research assistant recorded NRS ratings of pain intensity for each heat pulse. Participants were asked to rate their “first” pain intensity felt, believed to be primarily mediated by input from A-delta fibers. 21, 37 Participants underwent the protocol two times at each anatomic site waiting 60 seconds between trials. Pain sensitivity scores in response to both the 47 and 49°C heat pulses were pooled and the average NRS rating of the combined temperatures in both the low back and the lower extremity served as the measure of pain sensitivity at each location.
Temporal summation
Temporal summation was assessed in the non- dominant plantar surface of the foot and the non-dominant posterior superior iliac spine following a previously established protocol.21, 37 A train of ten consecutive heat pulses of less than one second duration at an inter- stimulus frequently of .33 Hz (temporal summation) was applied with a baseline temperature of 35°C and peak temperature of 51°C. Participants were asked to rate their delayed (second) pain using a NRS and the average of the first 5 pulses served as the measure of pain perception.
HVLA Intervention
The HVLA technique has been shown to be effective in the treatment of low back pain in participants meeting a clinical prediction rule. 7, 8 Similar to the protocol used in a prior study,16 we performed the technique (Fig 1) two times on each side, regardless, of whether an AP was perceived. Immediately following the HVLA manipulation, the researcher marked on an intake form whether or not an AP had occurred during any of the thrusts. The determination of an AP having occurred was based upon the researcher's perception of either having felt or heard an AP during the application of the technique. This method of identifying an AP has been reported as valid.42 Following the application of the HVLA manipulation, the same quantitative sensory testing protocol was performed.
Figure 1. High velocity, low amplitude thrust technique used in this study.
Reprinted from Cibulka MT. The treatment of the sacroiliac joint component to low back pain: a case report. Phys Ther. 1992;72:917–922, with the permission of the American Physical Therapy Association. This material is copyrighted, and any further reproduction or distribution is prohibited.
Statistical Analysis
Descriptive statistics were generated for continuous and categorical measures. Univariate ANOVA was used to assess differences in continuous variables of demographic, psychological, and baseline thermal testing measures between participants in whom an AP was perceived and those in whom an AP was not perceived. Chi- square analysis was used to assess differences in categorical demographic variables between participants in whom an AP was perceived and those in whom an AP was not perceived. We assessed baseline measures to account for differences in potential confounding variables between individuals in whom an AP was perceived and those in whom one was not.
In order to assess the influence of the AP on pain perception following HVLA manipulation, we performed separate 2 × 2 repeated measure ANOVAs for each of the pain protocols (Aδ and temporal summation) at each anatomic location (lower extremity and low back). AP status (perceived or not perceived) served as the between subject factor and time of thermal pain sensitivity assessment (pre to post HVLA) as the within subject factor. Alpha levels were set at 0.05 and all analysis was performed using the SPSS statistical package version 16.0 (SPSS Inc, Chicago, IL).
Results
Forty participants were included in this analysis. Baseline demographic, thermal pain threshold and psychological variables did not differ by participants on AP status, with the exception of those in whom an AP was perceived having less education (Table 1).
Table 1.
Baseline measures of demographics, thermal pain threshold, and psychological variables comparing participants in whom an audible pop (AP) was perceived and not perceived.
| AP | No- AP | Total Sample | p | |
|---|---|---|---|---|
| Sex: Male: | 5 | 4 | 9 | |
| Female: | 13 | 18 | 31 | 0.47 |
| Age (years (sd)) | 22.17 (1.72) | 23.73 (3.37) | 23.03 (2.83) | 0.08 |
| Race: Caucasian: | 14 | 18 | 32 | |
| African American: | 2 | 0 | 2 | |
| Other: | 2 | 4 | 6 | 0.34 |
| Education (years (sd)) | 15.67 (1.14) | 16.67 (1.46) | 16.21 (1.40) | 0.02 |
| History of LBP: Yes: | 5 | 3 | 8 | |
| No: | 12 | 17 | 29 | 0.52 |
| Threshold Pain Temperature (sd) | 43.41 (2.53) | 42.13 (5.17) | 42.70 (4.20) | 0.35 |
| Threshold Pain Rating (NRS (sd)) | 15.1 (13.6) | 18.1 (19.0) | 16.8 (16.6) | 0.57 |
| Psychological Questionnaires | ||||
| PCS (sd) | 16.44 (8.49) | 14.05 (10.08) | 16.22 (9.29) | 0.90 |
| FPQ (sd) | 78.11 (19.07) | 75.50 (15.66) | 76.68 (17.10) | 0.64 |
| Anxiety VAS (sd) | 19.11 (19.12) | 25.52 (21.93) | 22.56 (20.67) | 0.34 |
Key: LBP= low back pain; NRS= numeric rating scale from 0= no pain at all to 100= the worst pain imaginable; PCS= Pain Catastrophizing Scale; FPQ= Fear of Pain Questionnaire; VAS= visual analog scale from 0= none at all to 100= worst imaginable
Aδ fiber mediated pain perception (Table 2, Fig 2)
Table 2.
Summary of comparison of within group and between group changes in Aδ fiber mediated pain sensitivity.
| Pre | Post | Mean Difference | 95% CI | Effect Size (Cohen's d) | |
|---|---|---|---|---|---|
| WITHIN GROUP COMPARISONS | |||||
| Low Back | |||||
| AP | 35.7(20.4) | 30.1(17.0) | 5.6(11.0) | 0.1 – 11.1* | 0.30 |
| No AP | 36.5(20.4) | 29.9(22.3) | 6.6(12.3) | 1.1 – 12.1* | 0.31 |
| Lower Extremity | |||||
| AP | 31.1(19.4) | 28.3(17.0) | 2.8(11.7) | -1.4 – 7.1 | 0.15 |
| No AP | 31.7(22.1) | 32.2(26.1) | -0.5(12.0) | -5.8 – 4.8 | -0.02 |
| BETWEEN GROUP COMPARISONS (Change Scores) | |||||
| Low Back | |||||
| AP × No AP | -1.0(11.3) | -6.5 – 8.6 | -0.09 | ||
| Lower Extremity | |||||
| AP × No AP | 3.3(10.3) | -10.1 – 3.5 | 0.32 | ||
Key: AP= audible pop, No AP= no audible pop; Pain sensitivity assessed with 101 point numeric rating scale anchored with 0= no pain and 100= worst pain sensation imaginable. All findings are presented in mean (standard deviation).
= significant at p< 0.05.
Figure 2. Pain perception to Aδ fiber mediated pain.
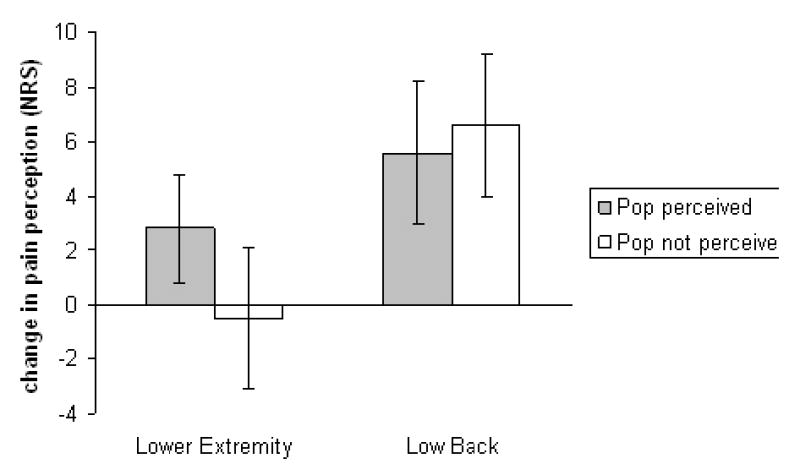
Key: Pre to post high velocity, low amplitude thrust manipulation change in self report of pain to standard thermal stimuli. Positive numbers on the Y axis indicate hypoalgesia while negative numbers indicate hyperalgesia. NRS= numeric rating scale anchored with 0= no pain at all and 100= worst pain imaginable. Error bars indicate one standard error of the mean.
Lower extremity
Neither a significant group (AP perceived versus AP not perceived) by time (pre to post HVLA) interaction (F(1,38)= 0.97, p= 0.33, partial η2= 0.03) nor a main treatment effect for time (F(1,38)= 0.50, p= 0.48, partial η2= 0.01) were observed in the lower extremity of participants for Aδ fiber mediated pain perception.
Low back
No significant group (AP perceived versus AP not perceived) by time (pre to post HVLA) interaction was observed in the low back (F(1,38)= 0.07, p= 0.79, partial η2< 0.01) for Aδ fiber mediated pain perception. Conversely, a significant main treatment effect for time was present (F(1,38)= 10.67, p< 0.01, partial η2= 0.22). These findings corresponded to a mean hypoalgesia of 6.2, Cohen's d= 0.30.
Temporal Summation (Table 3, Fig 3)
Table 3.
Summary of comparison of within group and between group changes in temporal summation.
| Pre | Post | Mean Difference | 95% CI | Effect Size (Cohen's d) | |
|---|---|---|---|---|---|
| WITHIN GROUP COMPARISONS | |||||
| Low Back | |||||
| AP | 47.9(24.0) | 44.1(22.1) | 3.7(9.5) | -1.0 – 8.5 | 0.16 |
| No AP | 51.0(27.2) | 48.8(25.9) | 2.2(13.8) | -3.9 – 8.4 | 0.08 |
| Lower Extremity | |||||
| AP | 35.3(23.6) | 25.8(19.4) | 9.5(11.7) | 3.6 – 15.3* | 0.44 |
| No AP | 33.8(26.4) | 30.4(26.9) | 3.4(9.3) | -0.7 – 7.5 | 0.13 |
| BETWEEN GROUP COMPARISONS (Change Scores) | |||||
| Low Back | |||||
| AP × No AP | 1.5(11.8) | -9.2 – 6.3 | 0.13 | ||
| Lower Extremity | |||||
| AP × No AP | 6.1(10.2) | -12.8 – 0.6 | 0.60 | ||
Key: AP= audible pop, No AP= no audible pop; Pain sensitivity assessed with 101 point numeric rating scale anchored with 0= no pain and 100= worst pain sensation imaginable. All findings are presented in mean (standard deviation).
= significant at p< 0.05.
Figure 3. Change in temporal summation.
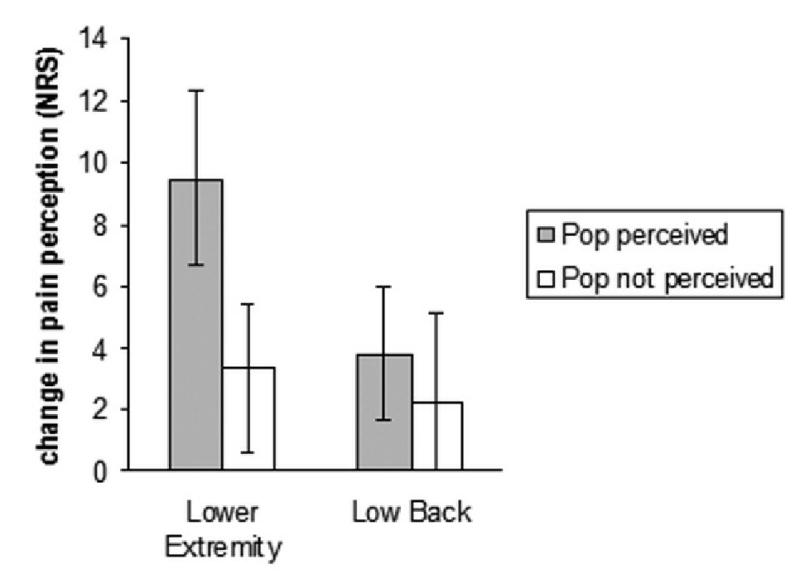
Key: Pre to post high velocity, low amplitude thrust manipulation change in self report of pain to standard thermal stimuli. Positive numbers on the Y axis indicate hypoalgesia. NRS= numeric rating scale anchored with 0= no pain at all and 100= worst pain imaginable. Error bars indicate one standard error of the mean.
Lower extremity
A significant group (AP perceived versus AP not perceived) by time (pre to post HVLA) interaction was neared (F(1,38)= 3.35, p= 0.08, partial η2= 0.08) favoring the participants in whom an AP was perceived. A significant main effect for time was observed suggesting hyopalgesia to temporal summation occurred regardless of whether or not an AP was perceived (F(1,38)= 15.12, p< 0.01, partial η2= 0.29). This finding corresponded to a mean hypoalgesia of 6.2, Cohen's d= 0.26.
Low back
Neither a group (AP perceived versus AP not perceived) by time (pre to post HVLA) interaction (F(1,38)= 0.15, p= 0.70, partial η2< 0.01) nor a main treatment effect for time were observed in the low back for the temporal summation pain protocol (F(1,38)= 2.43, p= 0.13, partial η2= 0.06).
Discussion
Central sensitization is characterized by allodynia and hyperalgesia. Clinical signs of central sensitization have been observed in individuals experiencing LBP. 43-45 For example, generalized hyperalgesia44 and lower pain thresholds and greater cortical activation in response to evoked pain45 have been observed in individuals with chronic LBP in comparison to healthy controls. Central sensitization is theorized as instrumental in the progression of acute pain to chronic pain and in the maintenance of chronic pain.46-48 Subsequently, interventions effective in altering central sensitization may be particularly beneficial in the treatment of LBP. Similar to prior studies, 15-17 we observed hypoalgesia associated with HVLA manipulation suggestive of a mechanism of action upon central sensitization of pain; however, this finding was independent of whether or not an AP was recorded by the practitioner. These findings are similar to prior studies observing the lack of an association between the AP and the clinical effectiveness11-13 or mechanisms of HVLA manipulation.49 Our findings suggest that HVLA manipulation is associated with hypoalgesia to Aδ fiber mediated pain and temporal summation regardless of whether an AP is perceived.
Interestingly, a trend of moderate magnitude was observed suggesting greater hypoalgesia to temporal summation in the lower extremity in individuals in whom an AP was perceived. Prior mechanistic studies have not consistently supported a relationship between neurophysiological effects and the AP. For example, Herzog et al49 observed EMG responses associated with high velocity spinal manipulative treatments that occurred independently of an AP. Conversely, Teodorczyk-Injeyan et al9 observed a reduction of proinflammatory cytokine secretion in participants receiving HVLA manipulation with an AP in comparison to those without an AP. In a separate study, Teodorczyk-Injeyan et al10 observed Staphylococcal protein A induced secretion of immunoregulatory cytokine interleukin 2 increased significantly following HVLA manipulation regardless of whether an AP was perceived. However, a trend was observed over 2 hours for a reduction of this finding in participants in whom and AP was not observed in comparison to those in whom AP was observed. Similar to Teodorczyk-Injeyan et al, 9, 10 our findings suggest neurophysiological effects corresponding with HVLA manipulation may be of a greater magnitude when associated with an AP. We have previously observed greater hypoalgesia to temporal summation in healthy participants receiving HVLA manipulation in comparison to those performing lumbar extension exercises or riding a stationary bike.16 These findings suggest a mechanism of action related to hypoalgesia of temporal summation at the dorsal horn of the spinal cord and specific to HVLA manipulation. The present study suggests this specific potential mechanism of HVLA manipulation may be magnified in individuals in whom an AP is perceived.
Clinically, HVLA manipulation is frequently applied with a biomechanical emphasis. For example, a misaligned or hypomobile segment is identified through careful examination and then a specific HVLA is applied to correct the noted fault. A number of inconsistencies are related to this method of clinical practice. Specifically, the biomechanical examination is unreliable,50, 51 techniques are not specific to a targeted vertebral level,52-54and lasting positional changes are not obvious following the interventions.55 Despite these inconsistencies, HVLA manipulation is an effective intervention for certain types of musculoskeletal pain suggesting a mechanism of action not specific to a biomechanical effect. Neurophysiological mechanisms are suggested as pertinent in the mechanisms behind the clinical effectiveness of HVLA manipulation and may provide a more reasonable explanation than a strictly biomechanical effect. Our findings suggest that a neurophysiological mechanism, hypoalgesia is associated with HVLA manipulation and that this potential mechanism may be enhanced when HVLA manipulation is accompanied by an AP.
Limitations
The present study included only participants who were pain free and experience evoked pain of a relatively brief duration. We are uncertain if similar findings would have occurred in participants experiencing LBP since response to the AP may be different in a clinical sample of individual experiencing LBP. We used protocols that are currently considered to discriminate between A-delta fiber mediated pain processes and temporal summation, but do not directly measure these processes in human subjects. Therefore, our thermal pain sensitivity measures are not definitively directly linked to the development of chronic pain syndromes. Furthermore, expectation has been observed as influential in the outcomes associated with manual therapy.23, 56 We did not assess individual expectation specific to the AP and future studies should consider this factor. We did not ask individual participants whether they perceived an AP and a prior study11 using both researcher and participant perception reported higher rates of an AP occurrence than we observed in the present study. Subsequently, a greater number of participants may have experienced an AP undetected by the study representative. Finally, our relatively low p value and moderate effect size for hypoalgesia to temporal summation in the lower extremity favoring participants in whom an AP was perceived suggests we may have been underpowered to observe these differences. A subsequent power analysis indicated we would have required 35 participants in each group in order to observe significant group differences at p< 0.05 with β> 0.80 at a similar effect size. Subsequently, future studies should attempt to replicate our findings in a larger sample.
Conclusion
Hypoalgesia to thermal pain was observed in pain free participants regardless of whether or not an AP was perceived. A trend was observed for a greater magnitude of hypoalgesia to lower extremity temporal summation in participants in whom an AP was perceived, but future research is necessary to confirm this finding.
Acknowledgments
The authors thank Valdora Martelli, Lauren Gates, and Josh Barabas who assisted with the data collection for this study.
Funding Sources: The project was supported by Grant Number R-21 AT002796-01 from the National Institutes of Health – National Center for Complimentary and Alternative Medicine (SZG, MDB, MER). JEB received support from the National Institutes of Health T-32 Neural Plasticity Research Training Fellowship (T32HD043730).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No conflicts of interest were reported for this study.
- An audible pop is associated with HVLA manipulation; however, the clinical and mechanistic relevance of the pop is not known.
- We observed HVLA manipulation related hypoalgesia to thermal pain sensitivity and this finding was not dependent upon the presence of a pop.
Contributor Information
Joel E Bialosky, Email: bialosky@phhp.ufl.edu.
Mark D Bishop, Email: bish@phhp.ufl.edu.
Michael E Robinson, Email: merobin@ufl.edu.
Steven Z George, Email: szgeorge@phhp.ufl.edu.
References
- 1.Bronfort G, Haas M, Evans RL, Bouter LM. Efficacy of spinal manipulation and mobilization for low back pain and neck pain: a systematic review and best evidence synthesis. Spine J. 2004 May;4(3):335–356. doi: 10.1016/j.spinee.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Koes BW, Assendelft WJ, van der Heijden GJ, Bouter LM. Spinal manipulation for low back pain. An updated systematic review of randomized clinical trials. Spine. 1996 December 15;21(24):2860–2871. doi: 10.1097/00007632-199612150-00013. [DOI] [PubMed] [Google Scholar]
- 3.van TM, Becker A, Bekkering T, et al. Chapter 3. European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J. 2006 March;15 2:S169–S191. doi: 10.1007/s00586-006-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007 October 2;147(7):478–491. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 5.Evans DW, Breen AC. A biomechanical model for mechanically efficient cavitation production during spinal manipulation: prethrust position and the neutral zone. J Manipulative Physiol Ther. 2006 January;29(1):72–82. doi: 10.1016/j.jmpt.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons P, Tehan P. Patient positioning and spinal locking for lumbar spine rotation manipulation. Man Ther. 2001 August;6(3):130–138. doi: 10.1054/math.2001.0404. [DOI] [PubMed] [Google Scholar]
- 7.Childs JD, Fritz JM, Flynn TW, et al. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Ann Intern Med. 2004 December 21;141(12):920–928. doi: 10.7326/0003-4819-141-12-200412210-00008. [DOI] [PubMed] [Google Scholar]
- 8.Flynn T, Fritz J, Whitman J, et al. A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with spinal manipulation. Spine. 2002 December 15;27(24):2835–2843. doi: 10.1097/00007632-200212150-00021. [DOI] [PubMed] [Google Scholar]
- 9.Teodorczyk-Injeyan JA, Injeyan HS, Ruegg R. Spinal manipulative therapy reduces inflammatory cytokines but not substance P production in normal subjects. J Manipulative Physiol Ther. 2006 January;29(1):14–21. doi: 10.1016/j.jmpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Teodorczyk-Injeyan JA, Injeyan HS, McGregor M, Harris GM, Ruegg R. Enhancement of in vitro interleukin-2 production in normal subjects following a single spinal manipulative treatment. Chiropr Osteopat. 2008;16:5. doi: 10.1186/1746-1340-16-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn TW, Childs JD, Fritz JM. The audible pop from high-velocity thrust manipulation and outcome in individuals with low back pain. J Manipulative Physiol Ther. 2006 January;29(1):40–45. doi: 10.1016/j.jmpt.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Flynn TW, Fritz JM, Wainner RS, Whitman JM. The audible pop is not necessary for successful spinal high-velocity thrust manipulation in individuals with low back pain. Arch Phys Med Rehabil. 2003 July;84(7):1057–1060. doi: 10.1016/s0003-9993(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 13.Cleland JA, Flynn TW, Childs JD, Eberhart S. The audible pop from thoracic spine thrust manipulation and its relation to short-term outcomes in patients with neck pain. J Man Manip Ther. 2007;15(3):143–154. doi: 10.1179/106698107790819828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reggars JW. The therapeutic benefit of the audible release associated with spinal manipulative therapy. A critical review of the literature. Australas Chiropr Osteopathy. 1998 July;7(2):80–85. [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-de-las-Penas C, onso-Blanco C, Cleland JA, Rodriguez-Blanco C, burquerque-Sendin F. Changes in pressure pain thresholds over C5-C6 zygapophyseal joint after a cervicothoracic junction manipulation in healthy subjects. J Manipulative Physiol Ther. 2008 June;31(5):332–337. doi: 10.1016/j.jmpt.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 16.George SZ, Bishop MD, Bialosky JE, Zeppieri G, Jr, Robinson ME. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskelet Disord. 2006;7:68. doi: 10.1186/1471-2474-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Carnero J, Fernandez-de-las-Penas C, Cleland JA. Immediate hypoalgesic and motor effects after a single cervical spine manipulation in subjects with lateral epicondylalgia. J Manipulative Physiol Ther. 2008 November;31(9):675–681. doi: 10.1016/j.jmpt.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Saez M, Fernandez-de-las-Penas C, Blanco CR, Martinez-Segura R, Garcia-Leon R. Changes in pressure pain sensitivity in latent myofascial trigger points in the upper trapezius muscle after a cervical spine manipulation in pain-free subjects. J Manipulative Physiol Ther. 2007 October;30(8):578–583. doi: 10.1016/j.jmpt.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Boal RW, Gillette RG. Central neuronal plasticity, low back pain and spinal manipulative therapy. J Manipulative Physiol Ther. 2004 June;27(5):314–326. doi: 10.1016/j.jmpt.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Wright A. Hypoalgesia post-manipulative therapy: a review of a potential neurophysiological mechanism. Man Ther. 1995 November;1(1):11–16. doi: 10.1054/math.1995.0244. [DOI] [PubMed] [Google Scholar]
- 21.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001 March;91(12):165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 22.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007 November;8(11):893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bialosky JE, Bishop MD, Robinson ME, Barabas JA, George SZ. The influence of expectation on spinal manipulation induced hypoalgesia: an experimental study in normal subjects. BMC Musculoskelet Disord. 2008 February 11;9(1):19. doi: 10.1186/1471-2474-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George SZ, Dannecker EA, Robinson ME. Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor predicts tolerance or blood pressure reactivity: An experimental investigation in pain-free individuals. Eur J Pain. 2005 August 8; doi: 10.1016/j.ejpain.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000 August;23(4):351–365. doi: 10.1023/a:1005548801037. [DOI] [PubMed] [Google Scholar]
- 26.Osman A, Breitenstein JL, Barrios FX, Gutierrez PM, Kopper BA. The Fear of Pain Questionnaire-III: further reliability and validity with nonclinical samples. J Behav Med. 2002 April;25(2):155–173. doi: 10.1023/a:1014884704974. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt NB, Cook JH. Effects of anxiety sensitivity on anxiety and pain during a cold pressor challenge in patients with panic disorder. Behav Res Ther. 1999 April;37(4):313–323. doi: 10.1016/s0005-7967(98)00139-9. [DOI] [PubMed] [Google Scholar]
- 28.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997 December;20(6):589–605. doi: 10.1023/a:1025570508954. [DOI] [PubMed] [Google Scholar]
- 29.Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002 April;96(3):319–324. doi: 10.1016/S0304-3959(01)00463-8. [DOI] [PubMed] [Google Scholar]
- 30.D'Eon JL, Harris CA, Ellis JA. Testing factorial validity and gender invariance of the pain catastrophizing scale. J Behav Med. 2004 August;27(4):361–372. doi: 10.1023/b:jobm.0000042410.34535.64. [DOI] [PubMed] [Google Scholar]
- 31.McNeil DW, Rainwater AJ., III Development of the Fear of Pain Questionnaire--III. J Behav Med. 1998 August;21(4):389–410. doi: 10.1023/a:1018782831217. [DOI] [PubMed] [Google Scholar]
- 32.Roelofs J, Peters ML, Deutz J, Spijker C, Vlaeyen JW. The Fear of Pain Questionnaire (FPQ): further psychometric examination in a non-clinical sample. Pain. 2005 August;116(3):339–346. doi: 10.1016/j.pain.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Tamiya N, Araki S, Ohi G, et al. Assessment of pain, depression, and anxiety by visual analogue scale in Japanese women with rheumatoid arthritis. Scand J Caring Sci. 2002 June;16(2):137–141. doi: 10.1046/j.1471-6712.2002.00067.x. [DOI] [PubMed] [Google Scholar]
- 34.Boker A, Brownell L, Donen N. The Amsterdam preoperative anxiety and information scale provides a simple and reliable measure of preoperative anxiety. Can J Anaesth. 2002 October;49(8):792–798. doi: 10.1007/BF03017410. [DOI] [PubMed] [Google Scholar]
- 35.Millar K, Jelicic M, Bonke B, Asbury AJ. Assessment of preoperative anxiety: comparison of measures in patients awaiting surgery for breast cancer. Br J Anaesth. 1995 February;74(2):180–183. doi: 10.1093/bja/74.2.180. [DOI] [PubMed] [Google Scholar]
- 36.Davey HM, Barratt AL, Butow PN, Deeks JJ. A one-item question with a Likert or Visual Analog Scale adequately measured current anxiety. J Clin Epidemiol. 2007 April;60(4):356–360. doi: 10.1016/j.jclinepi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002 September;99(12):49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 38.Bolton JE, Wilkinson RC. Responsiveness of pain scales: a comparison of three pain intensity measures in chiropractic patients. J Manipulative Physiol Ther. 1998 January;21(1):1–7. [PubMed] [Google Scholar]
- 39.DeLoach LJ, Higgins MS, Caplan AB, Stiff JL. The visual analog scale in the immediate postoperative period: intrasubject variability and correlation with a numeric scale. Anesth Analg. 1998 January;86(1):102–106. doi: 10.1097/00000539-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure. Pain Pract. 2003 December;3(4):310–316. doi: 10.1111/j.1530-7085.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 41.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986 October;27(1):117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 42.Herzog W, Zhang YT, Conway PJ, Kawchuk GN. Cavitation sounds during spinal manipulative treatments. J Manipulative Physiol Ther. 1993 October;16(8):523–526. [PubMed] [Google Scholar]
- 43.Diers M, Koeppe C, Diesch E, et al. Central processing of acute muscle pain in chronic low back pain patients: an EEG mapping study. J Clin Neurophysiol. 2007 February;24(1):76–83. doi: 10.1097/01.wnp.0000241093.00844.0e. [DOI] [PubMed] [Google Scholar]
- 44.O'Neill S, Manniche C, Graven-Nielsen T, rendt-Nielsen L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur J Pain. 2007 May;11(4):415–420. doi: 10.1016/j.ejpain.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004 February;50(2):613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 46.Rygh LJ, Svendsen F, Fiska A, Haugan F, Hole K, Tjolsen A. Long-term potentiation in spinal nociceptive systems--how acute pain may become chronic. Psychoneuroendocrinology. 2005 November;30(10):959–964. doi: 10.1016/j.psyneuen.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Winkelstein BA. Mechanisms of central sensitization, neuroimmunology & injury biomechanics in persistent pain: implications for musculoskeletal disorders. J Electromyogr Kinesiol. 2004 February;14(1):87–93. doi: 10.1016/j.jelekin.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 48.Staud R, Domingo M. Evidence for abnormal pain processing in fibromyalgia syndrome. Pain Med. 2001 September;2(3):208–215. doi: 10.1046/j.1526-4637.2001.01030.x. [DOI] [PubMed] [Google Scholar]
- 49.Herzog W, Conway PJ, Zhang YT, Gal J, Guimaraes AC. Reflex responses associated with manipulative treatments on the thoracic spine: a pilot study. J Manipulative Physiol Ther. 1995 May;18(4):233–236. [PubMed] [Google Scholar]
- 50.Seffinger MA, Najm WI, Mishra SI, et al. Reliability of spinal palpation for diagnosis of back and neck pain: a systematic review of the literature. Spine. 2004 October 1;29(19):E413–E425. doi: 10.1097/01.brs.0000141178.98157.8e. [DOI] [PubMed] [Google Scholar]
- 51.Troyanovich SJ, Harrison DD, Harrison DE. Motion palpation: it's time to accept the evidence. J Manipulative Physiol Ther. 1998 October;21(8):568–571. [PubMed] [Google Scholar]
- 52.Beffa R, Mathews R. Does the adjustment cavitate the targeted joint? An investigation into the location of cavitation sounds. J Manipulative Physiol Ther. 2004 February;27(2):e2. doi: 10.1016/j.jmpt.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Herzog W, Kats M, Symons B. The effective forces transmitted by high-speed, low-amplitude thoracic manipulation. Spine. 2001 October 1;26(19):2105–2110. doi: 10.1097/00007632-200110010-00012. [DOI] [PubMed] [Google Scholar]
- 54.Ross JK, Bereznick DE, McGill SM. Determining cavitation location during lumbar and thoracic spinal manipulation: is spinal manipulation accurate and specific? Spine. 2004 July 1;29(13):1452–1457. doi: 10.1097/01.brs.0000129024.95630.57. [DOI] [PubMed] [Google Scholar]
- 55.Tullberg T, Blomberg S, Branth B, Johnsson R. Manipulation does not alter the position of the sacroiliac joint. A roentgen stereophotogrammetric analysis. Spine. 1998 May 15;23(10):1124–1128. doi: 10.1097/00007632-199805150-00010. [DOI] [PubMed] [Google Scholar]
- 56.Kalauokalani D, Cherkin DC, Sherman KJ, Koepsell TD, Deyo RA. Lessons from a trial of acupuncture and massage for low back pain: patient expectations and treatment effects. Spine. 2001 July 1;26(13):1418–1424. doi: 10.1097/00007632-200107010-00005. [DOI] [PubMed] [Google Scholar]


