Abstract
Prevention of cardiovascular disease through nutritional supplements is growing in popularity throughout the world. Multiple epidemiologic studies found that moderate consumption of alcohol, particularly red wine, lowers mortality rates from coronary heart diseases (CHD). Chronic inflammation and atherosclerosis associated with CHD culminate in aberrant intravascular expression of tissue factor (TF), which triggers blood coagulation leading to thrombosis, a major cause for heart attack. We showed earlier that two red wine phenolics, resveratrol and quercetin, suppressed TF induction in endothelial cells. In the present study, we investigated efficacy of seven resveratrol derivatives, which were shown to be effective in regulating cancer cell growth in vitro at much lower concentrations than the parent compound resveratrol, in inhibiting TF induction in peripheral blood mononuclear cells (PBMCs). We also tested possible synergistic effects of resveratrol and quercetin with the other major red wine phenolics in suppression of lipopolysaccharide-induced TF expression in human PBMCs. We found that several resveratrol derivatives were 2- to 10-fold more efficient than resveratrol in inhibiting TF induction. Our study found no evidence for synergism among red wine polyphenolics. These data suggest that structural alterations of resveratrol can be effective in producing potent antithrombotic agents that will have therapeutic potential in the improvement of cardiovascular health and prevention of CHD. Among major red wine phenolics, quercetin appears to be the predominant suppressor of TF induction.
Keywords: Tissue factor, Coronary heart diseases, Red wine phenolics, Resveratrol, Quercetin
Introduction
Overwhelming epidemiological evidence suggests that moderate consumption of alcoholic beverages, particularly red wine, may lower mortality rates from coronary heart diseases [1–6]. Cardiovascular benefits associated with moderate wine consumption have been thought to stem, at least partly, from antioxidant [7–9] and anti-platelet activities [10–12] of wine phenolics, particularly resveratrol. Recent studies published by us [13,14] and others [15] showed that resveratrol and quercetin suppressed tissue factor induction in vascular cells. Resveratrol and other wine phenolics were also shown to inhibit synthesis of proatherogenic eicosanoids [12], reduce inflammation [16–18] and suppress skin carcinogenesis [19,20]. Several of the red wine polyphenols, notably catechin, quercetin and resveratrol, promote nitric oxide production in vascular endothelium [21]. In yeast, resveratrol mimics calorie restriction by stimulating Sir2 (sirtuin 2, a histonedeacytelase), increasing DNA stability and extending lifespan by 70% [22]. In most of the studies described above, relatively high concentrations of resveratrol (10 to 100 µM range) were required to modulate various cellular effects. In order to improve the efficacy of the resveratrol, with the aim of discovering new lead compounds with the clinical treatment potential, a series of cis- and trans-stilbene-based resveratrols were synthesized [23–25]. Of the compounds tested for the anti-proliferative and apoptotic activity on HL60 promyelocytic leukemia cells, cis-3, 4′ , 5-trimethoxy-3′ -aminostilbene (7b) and cis-3, 4′, 5-trimethoxy-3′- hydroxystilbene (11b) were effective at the nanomolar concentrations [23]. Resveratrol derivative, R3 (cis-Z-3, 5, 4′ -trimethoxystilbene) was shown to be 100-fold more active than resveratrol in causing cell cycle arrest in colon cancer cells [25].
Tissue factor (TF), a cellular receptor of coagulation factor VIIa, is a primary initiator of blood coagulation cascade [26]. In health, TF is highly expressed on cells of vascular adventitia but not on the endothelium and other cells in circulating blood [27,28]. Several studies demonstrated that bacterial/viral infections and certain pathophysiological stimuli induce TF expression in monocytes and endothelial cells [29]. This explains the association of certain infectious diseases with hematological complications such as thromboembolism, and septic shock [30,31]. Recent studies suggest that exposure of TF to circulating blood upon rupture of atherosclerotic plaque plays an important role in the pathogenesis of thrombus formation at rupture site, resulting in acute coronary events and myocardial infarction [32–35]. In addition, TF-FVIIa and other activated coagulation proteases activate protease-activated receptors (PARs), which trigger a plethora of signaling events leading to functional alterations that are relevant to pathogenesis of atherosclerosis and coronary heart diseases (CHD) [36–39]. Therefore, we hypothesized earlier that the red wine phenolics provide protection against atherosclerosis and CHD by suppressing the aberrant expression of TF, and showed that the wine phenolics, resveratrol and quercetin, suppressed TF induction on endothelial cells and mononuclear cells [13,14]. However, a 10 to 100 µM concentration of resveratrol or quercetin was required to suppress TF induction. In vivo pharmacokinetics data suggest that accumulation of such concentrations of resveratrol and quercetin is unlikely after moderate consumption of red wine [40–42].
In the present study, we have investigated potential synergistic effects of various phenolic compounds present in wine in suppressing bacterial lipopolysaccharide (LPS)-induced TF expression in human peripheral blood mononuclear cells (PBMCs). Further, we have tested for the first time, whether chemically modified derivatives of resveratrol, which were shown to be highly effective in inhibiting cancer cell proliferation than resveratrol [23,25], could inhibit LPS-induced TF expression in peripheral blood mononuclear cells at much lower doses than resveratrol.
Materials and methods
Materials
Wine phenolics (resveratrol, quercetin, catechin, epicatechin, and rutin), LPS and Histopaque 1077 were obtained from Sigma (St. Louis, MO). Resveratrol derivatives were synthesized as described earlier [23,25]. Recombinant factor VIIa was a kind gift from Novo Nordisk (Copenhagen, Denmark). n-Octyl-B-d-glucopyranoside was from Calbiochem (La Jolla, CA). Chromogenic substrate Chromozym X, was purchased from Roche Diagnostics (Indianapolis, IN). RPMI 1640 culture medium and FBS were obtained from Invitrogen (Carlsbad, CA). Factor X and factor Xa were purchased from Enzyme Research Laboratories (South Bend, IN) or from Haematological Technologies (Essex Junction, VT).
Isolation of peripheral blood mononuclear cells (PBMC)
Thirty milliliters of venous blood was drawn from healthy volunteers, after the informed consent and the institutional review board approval, into a plastic syringe with a 19-gauge needle and immediately added to a plastic tube containing heparin (10 U/ml final concentration). PBMCs were isolated by applying blood, which was diluted with an equal volume of sterile saline, layered on top of Histopaque 1077 (1.5 ml of Histopaque for 1 ml of blood) in a 50 ml conical tube, followed by density gradient centrifugation at 650 × g for 30 min at room temperature. The white PBMC band in the interface layer was collected and washed by mixing with 4 volumes of sterile saline and centrifuging at 400 × g for 10 min. The washing step was repeated two more times by resuspending the cell pellet in 10 ml of saline. The final cell pellet was suspended in RPMI medium containing 10% serum at a density of 3 × 106 cells/ml.
Induction and measurement of TF activity
Induction of TF and measurement of TF procoagulant activity were performed essentially as described earlier [13,14]. Briefly, 1 ml of PBMC was aliquoted into round bottom polypropylene tubes. The PBMC were preincubated with wine phenolics for 2 h, and then stimulated with 10 ng/ml of LPS for 6 h in a tissue culture incubator to induce TF expression. During the incubation, cells were gently shaken every 30 min to keep them in suspension. However, most of the PBMCs in LPS treatment tend to attach to the bottom of the tubes. After the incubation, the cells were sedimented by centrifugation at 400 × g, and were washed once with 1 ml of buffer A (10 mM Hepes, 0.15 M NaCl, 4 mM KCl, 11 mM glucose, pH 7.5). The cell pellets were suspended in 250 µl of buffer A containing 15 mM n-octyl-B-d-glucopyranoside, freeze-thawed twice, and sonicated for 30 s. Aliquots of cell lysates were incubated in a 96-well microplate with a reagent mixture containing factor VIIa (10 nM), factor X (175 nM) and CaCl2 (5 mM, all concentrations are final concentrations, total volume, 50 µl) for 15 min. Factor Xa generated in the reaction mixture was measured in a chromogenic assay as described earlier [13]. Serial dilutions of relipidated human TF were used (substituted in place of cell lysates) to construct a standard curve.
Cell survival assay
Cell survival was determined using a tetrazolium based colorimetric assay [43]. The assay is dependent on the reduction of tetrazolium salt 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT), which results in formation of a blue formazan product, by various mitochondrial dehydrogenase enzymes of viable cells. Briefly, 100 µl aliquots of cells were transferred to wells of 96-well cell culture plate, and treated with phenolic compounds and LPS as described for TF induction. Following the treatment, 10 µL of 5 mg/ml MTT was added for each well and allowed to incubate for 4 h. At the end of 4 h, the medium was removed, and 100 µL of acid isopropanol (0.04 N HCl in isopropanol) was added to each well. The color intensity was measured using the microplate reader (Molecular Dynamics) as differences in the absorbance between 563 nm and 650 nm wave-lengths. To construct a standard curve, varying number of cells (25,000 to 1500) were placed in a microplate well and subjected to MTT assay without any treatment.
Statistical analysis
Statistical analysis was performed using Graph Pad Prism software. The data are expressed as means ± S.E.M. The p values are calculated using Student’s t-test and values <0.05 is considered statistically significant.
Results
Effect of combined treatments of resveratrol and quercetin on LPS-induced TF expression in human PBMC
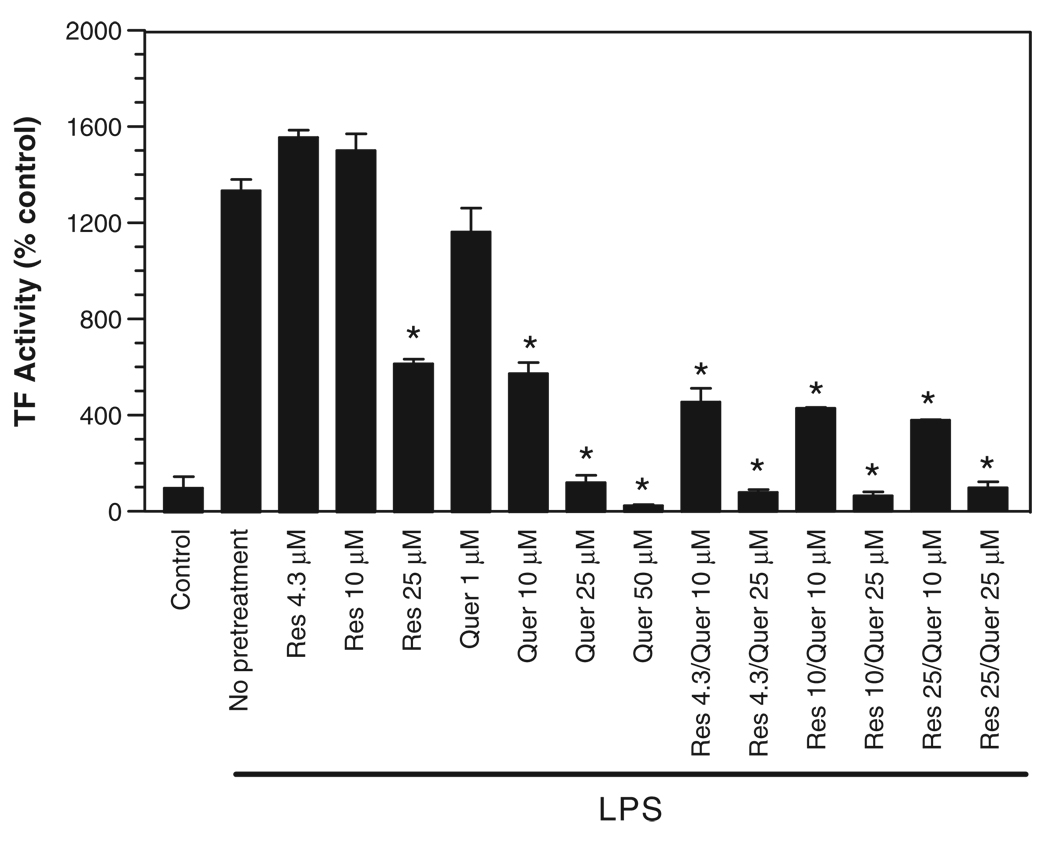
To test whether resveratrol and quercetin (two phenolic compounds that were shown to inhibit TF expression [13,14]) exhibit synergism, PBMC were pretreated with varying concentrations of resveratrol, quercetin alone or in combination of the two, followed by a 6 h incubation with 10 ng/ml LPS to induce tissue factor expression. As evident from Fig. 1, treatment of PBMCs with LPS increased TF activity about 5- to 15-fold over the control unstimulated cells. Resveratrol, when used at an approximate concentration present in red wines [44], i.e., 4.3 µM, failed to suppress the induction of TF expression. As expected, a higher concentration of resveratrol (25 µM) inhibited the TF induction by more than 50%. The dose–response analysis of quercetin showed that it is effective at concentrations as low as 1 µM; a 10 µM concentration inhibited TF induction by 50%, indicating that quercetin is 2- to 3-fold more effective than resveratrol in suppressing LPS-induced TF expression in PBMC. Next, we examined the effect of combined treatments of resveratrol and quercetin on TF induction. As shown in Fig. 1, treating the cells with both resveratrol and quercetin yielded an overlapping effect, i.e., the inhibition was increased very little over that was obtained with quercetin alone. For example, treating PBMCs with 10 µM quercetin and 4.3 µM resveratrol suppressed the TF induction to the same extent as 10 µM quercetin alone; increasing the concentration of resveratrol up to 25 µM reduced the TF induction by 30% further than that obtained with quercetin alone. These data indicate no synergism between quercetin and resveratrol. If there was a synergism between the two compounds, we expect a far greater TF inhibition with the combined treatment over the inhibition obtained with the individual phenolics or a simple addition of their inhibitory effects.
Figure 1.
Effect of resveratrol and quercetin, alone or in combination, on LPS-induced TF expression. Freshly isolated PBMC were pretreated for 2 h with various concentrations of resveratrol or quercetin or in combination, followed by LPS (10 ng/ml) for 6 h. At the end of LPS treatment, TF activity in PBMC was determined as described in methods. Asterisk denotes a statistically significant (p <0.01) suppression in TF expression in PBMC pretreated with phenolics vs. control vehicle before adding LPS (n = 4, mean ± S.E.M.).
Quercetin is the principal inhibitor of TF induction in the cocktail of red wine polyphenols
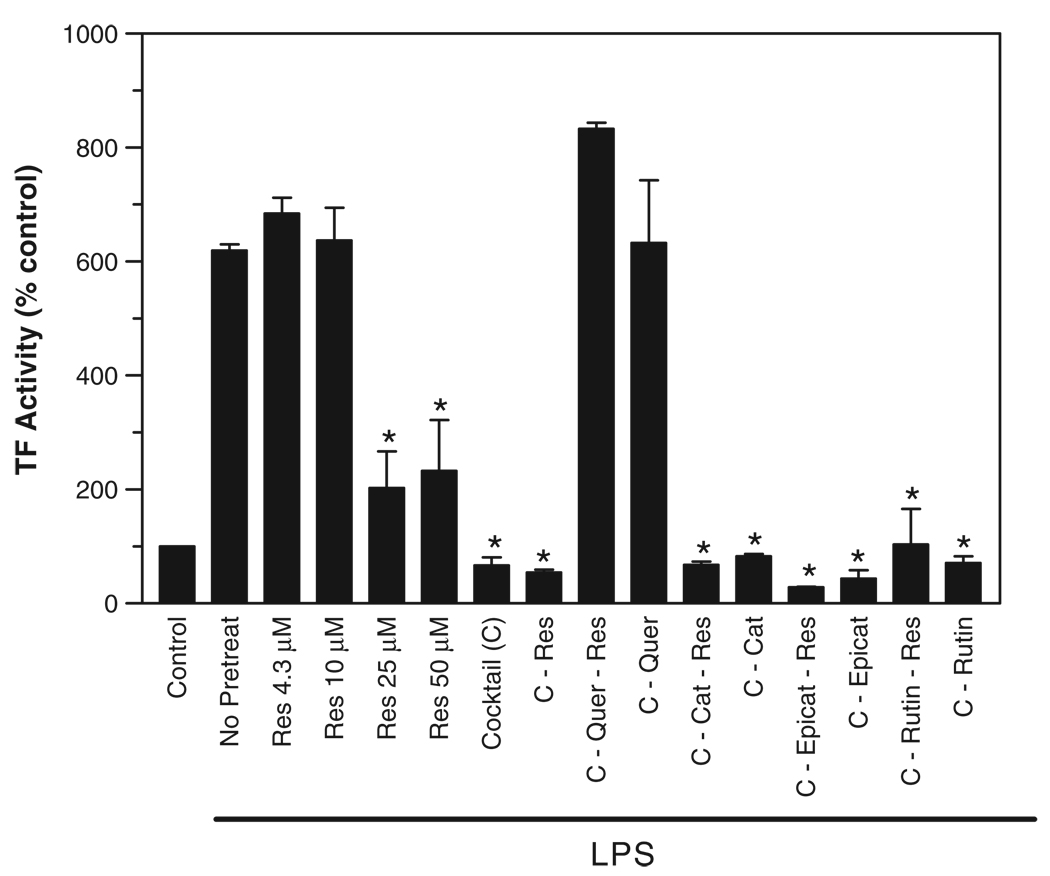
To test synergism among wine phenolics, we pretreated PBMCs with a cocktail containing five major phenolics at approximate concentrations present in most common red wines [44,45] or with a cocktail lacking one or more of the constituents. The cocktail–a mixture of rutin, catechin, epicatechin, resveratrol, and quercetin–inhibited the TF induction completely (Fig. 2). Removal of quercetin from the cocktail reversed its suppressive effect. In contrast, removal of resveratrol or other wine phenolics from the cocktail did not diminish the inhibitory effect of the cocktail. These data suggest that quercetin, and not resveratrol, is the principal active ingredient among red wine phenolics to inhibit TF induction in monocytes. These data also suggest that there was no apparent synergism between resveratrol and other wine polyphenols; otherwise, we would have seen some suppressive effect with the cocktail lacking quercetin.
Figure 2.
Removal of quercetin but not resveratrol from the cocktail of red wine polyphenolic compounds reversed the inhibitory effect of the cocktail on LPS-induced TF expression. PBMC were pretreated for 2 h with cocktail containing major constituents of wine phenolics (C) or the cocktail lacking resveratrol, quercetin, or resveratrol and one of the other wine phenolic compounds. PBMC were treated with LPS (10 ng/ml) for 6 h to induce TF expression. The cocktail contains the following compounds, resveratrol, 4.3 µM; quercetin, 55 µM; catechin, 300 µM; epicatechin, 197 µM; rutin, 898 µM. Asterisk denotes a statistically significant difference (p <0.01) in TF expression between LPS stimulated PBMC and PBMC treated with each cocktail before TF induction (n = 4, mean ± S.E.M.).
Effect of novel resveratrol derivatives on LPS-induced TF expression in mononuclear cells
A number of cis- and trans-stilbene-based resveratrol derivatives were synthesized with aim of improving the effectiveness of resveratrol in modulating various cellular activities [23–25]. In the present study, we investigated the ability of some of the analogues (Fig. 3) to inhibit TF induction in PBMC. A methyl derivative of trans resveratrol, i.e., 3,5,4′ -trimethoxystilbene (TMS), had a slightly increased inhibitory activity compared to resveratrol. A 10 µM concentration of resveratrol suppressed TF induction by about 20% or less, whereas TMS inhibited the induction by about 40%. Switching the trans conformation of TMS to cis (denoted as R3) markedly increased the inhibitory potency of the compound. R3 was 10-times more effective than resveratrol and 5-fold more effective than quercetin in inhibiting TF induction (Fig. 4). Other derivatives of resveratrol also exhibited a higher inhibitory activity over resveratrol. However, in contrast to differences observed between trans- and cis-isomers of the methylated resveratrol (TMS vs. R3), other analogues exhibited no significant differences in their inhibitory activities between the isomers. The cis-3,5-dimethoxy derivative of rhapontigenin (trans3,3′ ,5-trihydroxy-4′ -methoxystilbene) and its amino derivative (11b and 7b, respectively), and their trans-isomers (12b and 8b, respectively), inhibited TF activity with a similar potency. Both of the isomers were 5-times more effective than resveratrol in inhibiting LPS-induced TF induction in PBMCs. It is interesting to note that, in contrast to 12b (which contains 3′ hydroxy group and a 4′ methoxy group), its isomer 12C (3′ -methoxy group and a 4′ hydroxy group) did not gain inhibitory activity over the parent compound resveratrol. These data suggest that the methoxy function at the C-3′ position could play a critical role in determining the inhibitory activity of resveratrol derivatives.
Figure 3.
Chemical structures of resveratrol derivatives used in the present study.
Figure 4.
Effect of resveratrol derivatives on LPS-induced TF expression. PBMC were treated with varying concentrations of resveratrol or its derivatives (in concentrations as indicated in the figure) for 2 h, and then stimulated with LPS (10 ng/ml) to induce TF expression. At the end of 6 h LPS treatment, PBMC were harvested to measure TF activity. Asterisk denotes a statistically significant difference (p <0.02) in expression of TF between PBMC stimulated with LPS alone and the phenolic compound pretreatment before the stimulation with LPS (n = 6, mean ± S.E.M.).
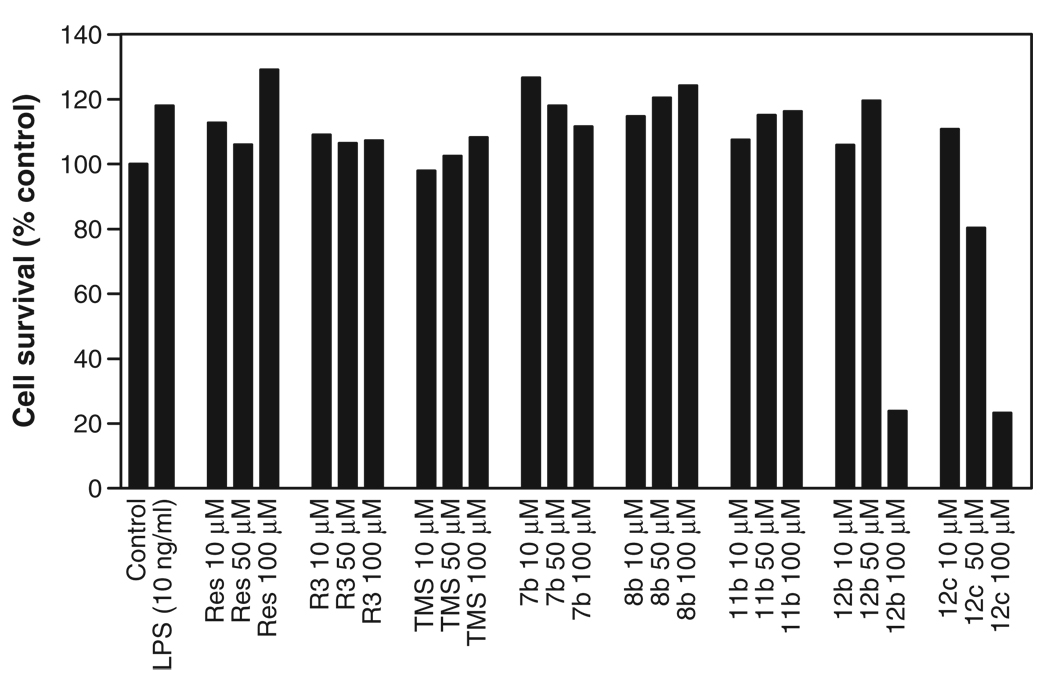
Effect of resveratrol derivatives on viability of PBMC
Next, we investigated the effect of resveratrol and its derivatives on cell viability since some of the above derivatives, upon prolonged incubation period (48 h), were shown to be cytotoxic [25] and induce apoptosis [23]. Under our experimental conditions, none of the derivatives were found to be cytotoxic at 10 µM concentrations for the duration of the experiments. Even at high concentrations (up to 100 µM), resveratrol, TMS, R3, 7b, 8b and 11b had no effect on the cell viability (Fig. 5). However, both 12b and 12c, when used at 100 µM concentration, markedly reduced the cell viability. The data indicate that the reduced TF expression found in PBMC treated with resveratrol derivatives did not stem from reduced cell viability.
Figure 5.
Effect of resveratrol and its derivatives on cell viability. PBMC were treated with varying concentrations of resveratrol or its derivatives for a total of 8 h, and the cell viability was measured in MTT assay. The data presented in the graph is a representative experiment done in triplicate.
Discussion
Aberrant expression of TF contributes to pathogenesis of atherosclerosis and CHD [30,35,46]. Resveratrol and quercetin of red wine were shown to down-regulate TF expression in vascular cells [13–15]. However, high micromolar concentrations of resveratrol and quercetin were required to modulate TF expression and other cellular activities (see Introduction). This plus poor absorption of these compounds in vivo [40–42] raise valid doubts about the importance of the individual polyphenolic constituents of red wine in providing the cardio-protective effect. However, earlier studies suggested that synergism could exist between wine phenolics in inhibiting platelet function [47] and in promoting antioxidation [48]. These data and the inability of individual wine phenolics to effectively modulate various cellular activities raise the possibility that overall cardiovascular protection afforded by a moderate consumption of red wine could perhaps come from combined and synergistic effects of various polyphenolic constituents of red wine or metabolic modification of polyphenols in digestive system to make them more potent in modulating cellular activities. The data presented in the manuscript show no evidence for the synergism between resveratrol and quercetin, the two compounds that were shown to inhibit TF induction in vascular cells. We also investigated potential synergism among other wine phenolics by deleting individual polyphenolic components from the cocktail of major red wine polyphenolics. Here, it may be important to point out that the cocktail contains five major polyphenolics (catechin, epicatechin, rutin, quercetin, and resveratrol) at concentrations that are present in most of the common red wines [21]. Since the cocktail contained quercetin at 55 µM concentration and quercetin alone at this concentration effectively inhibits TF induction, it was not surprising to see the cocktail completely suppressed LPS-induced TF expression in PBMC. Consistent with this, the deletion of quercetin from the cocktail fully reversed the inhibitory effect. In contrast, removal of resveratrol or other components had no effect. The failure of the cocktail lacking quercetin to inhibit TF induction indicates that other wine phenolics have no effect on TF induction. These data also suggest that there was no synergism between resveratrol and other wine phenolics.
It is interesting to note that quercetin appeared to be more effective than resveratrol in inhibiting TF induction in mononuclear cells. These data is in contrast to our earlier studies on endothelial cells, where we found quercetin had a similar or slightly lower inhibitory activity than resveratrol. A potential reason for this contradiction could be due to differences in levels of specific cellular receptors for quercetin and resveratrol and/or metabolism of the compounds between the two cell types. It is conceivable that stimulant used to induce TF could also influence the suppressive effects of these compounds. In the earlier study, we used IL-1β to stimulate TF induction in endothelial cells, whereas LPS was used to stimulate TF induction in mononuclear cells in the present study. In this context, it may be pertinent to point out that resveratrol and quercetin suppressed TF induction to varied extents in mononuclear cells and endothelial cells when stimulated with different agonists [15]. Overall the present data, combined with the above reports, indicate that quercetin, which is present at 10-fold higher molar concentration than resveratrol, would be the principal active ingredient in red wine that is capable of suppressing TF induction in vascular cells.
Although the precise mechanism by which resveratrol and quercetin suppress TF induction in vascular cells remains to be elucidated, they appear to down-regulate TF expression at the mRNA levels by inhibiting the transcription of TF gene [13–15]. NF-κB pathway plays a pivotal role in the activation of TF gene in monocytes and endothelial cells [49]. In earlier studies, we found that resveratrol suppressed the transactivation potential of p65 and the LPS-induced phosphorylation of p65 without impairing c-Rel/p65 binding to DNA [13,50]. In contrast, Di Santo et al. [15] showed that both resveratrol and quercetin down-regulated TF expression by inhibiting nuclear translocation of c-Rel/p65. Not withstanding these differences, based on most of the published data, it is clear that both resveratrol and quercetin down-regulate NF-κB pathway. This overlapping inhibitory mechanism may explain why we found no synergism between the inhibitory flavonoids.
In earlier studies, we investigated the effect of various natural and commercially available stilbene analogues in suppressing TF induction in endothelial cells with the aim of discovering new compounds that would be more effective than resveratrol. Although a number of the analogues inhibited TF induction, none of them was more effective than resveratrol [14]. However, recent studies show that chemical structural alterations of the stilbene motif of resveratrol could yield extremely effective analogues [23,25]. A cis-form of methyl derivative of resveratrol (R3) was shown to be a 100-fold more potent than resveratrol in inhibiting cell proliferation by arresting cell cycle at the G2/M phase transition in Caco-2 cells [25]. Similarly, resveratrol analogues bearing the 3,5-dimethoxy motif at the A phenyl ring with amino, methoxy, and hydroxyl moieties at the 3′ - and 4′ -positions (7b, 8b, 11b, 12b, and 12c) were found to be more effective than resveratrol as apoptosis-inducing agents [23]. Particularly, introduction of either a hydroxy (11b) or an amino group (7b) at C-3′ position and methoxy groups at C-3, C-4′ , and C-5 produced extremely effective agents that could inhibit cell proliferation at nanomolar concentration range (IC50, 50 nM).
We found that a number of the above derivatives suppressed the TF induction in PBMC more potently than resveratrol. For example, 25 µM concentration of resveratrol inhibited the TF induction by about 60% whereas a similar inhibition was attained with 2µM of R3 compound. Other derivatives, except 12c, were found to be 2- to 5-fold more effective than resveratrol. Compared to quercetin, R3 compound was 5 times more effective whereas other derivatives of resveratrol exhibited similar or 2-fold higher inhibitory activity. In line with the earlier study [25], the present study shows cis-conformation was more effective than trans-conformation (R3 vs. TMS). It is also interesting to note that a switch in the methoxy and hydroxyl moieties as in compounds 12b vs. 12c influenced the inhibitory activity. These data clearly illustrate that it is possible to synthesize effective inhibitory agents for suppressing aberrant expression of TF by modifying the structure of stilbene motif of resveratrol. Since quercetin appears to be more potent than resveratrol in inhibiting TF expression in mononuclear cells, it would be interesting to test whether quercetin derivatives would have much higher inhibitory activity than the derivatives of resveratrol. Unavailability of chemically synthesized quercetin derivatives prevented us from investigating this possibility.
It is important to note that although R3 and other derivatives of resveratrol exerted a higher inhibitory activity than resveratrol in suppressing TF induction, still low micromolar concentrations of these compounds were required to suppress the TF induction. This differs from the earlier reports in which R3, 7b, 11b, and 12b were shown to inhibit cell proliferation of Caco-2 cells (R3) or HL-60 (7b, 11b, and 12b) at 0.4, 0.03, 0.03, and 0.7 µM, respectively. At present, it is unclear whether differences in their potencies to modulate various cellular activities stem from differences in cellular pathways for specific function or differences in cell types employed in these studies. Overall, the present data clearly illustrate the feasibility of developing effective antithrombotic drugs to suppress aberrant expression of TF by chemical engineering of resveratrol.
Acknowledgements
This work was funded by a grant from American Heart Association (Texas Affiliate) grant number 0355121Y.
Contributor Information
Gurjeet Kaur, Email: Gurjeet.kaur@uthct.edu.
Marinella Roberti, Email: mrobi@alma.unibo.it.
Francis Raul, Email: francis.raul@ircad.u-strasbg.fr.
Usha R. Pendurthi, Email: Usha.Pendurthi@uthct.edu.
References
- 1.Rimm EB, Giovannucci EL, Willet WC, Colditz GA, Aschrio A, Rosner B, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 2.Rimm EB, Klatsky A, Grobbee D, Stampfer MJ. Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer wine or spirits. BMJ. 1996;312:731–737. doi: 10.1136/bmj.312.7033.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szmitko PE, Verma S. Cardiology patient pages. Red wine and your heart. Circulation. 2005;111:e10–e11. doi: 10.1161/01.CIR.0000151608.29217.62. [DOI] [PubMed] [Google Scholar]
- 4.Jackson R, Scragg R, Beaglehole R. Alcohol consumption and risk of coronary heart disease. BMJ. 1991;303:211–216. doi: 10.1136/bmj.303.6796.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renaud S, De Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1993;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 6.Constant J. Alcohol, ischemic heart disease, and the French paradox. Coron Artery Dis. 1997;8:645–659. doi: 10.1097/00019501-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Frankel EN, Kanner J, German JB, Parks E, Kinsella JE. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341:454–457. doi: 10.1016/0140-6736(93)90206-v. [DOI] [PubMed] [Google Scholar]
- 8.Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 9.Aviram M, Fuhrman B. Wine flavonoids protect against LDL oxidation and atherosclerosis. Ann N Y Acad Sci. 2002;957:146–161. doi: 10.1111/j.1749-6632.2002.tb02913.x. [DOI] [PubMed] [Google Scholar]
- 10.Renaud SC, Beswick AD, Fehily AM, Sharp DS, Elwood PC. Alcohol and platelet aggregation: the Caerphilly Prospective Heart Disease Study. Am J Clin Nutr. 1992;55:1012–1017. doi: 10.1093/ajcn/55.5.1012. [DOI] [PubMed] [Google Scholar]
- 11.Bertelli AAE, Giovannini L, Giannessi D, Migliori M, Bernini W, Fregoni M, et al. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React. 1995;17:1–3. [PubMed] [Google Scholar]
- 12.Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis implications for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 13.Pendurthi UR, Williams JT, Rao LVM. Resveratrol, a polyphenolic compound found in wine, inhibits tissue factor expression in vascular cells. A possible mechanism for the cardiovascular benefits associated with moderate consumption of wine. Arterioscler Thromb Vasc Biol. 1999;19:419–426. doi: 10.1161/01.atv.19.2.419. [DOI] [PubMed] [Google Scholar]
- 14.Pendurthi UR, Rao LVM. Effect of wine phenolics and stilbene analogues on tissue factor expression in endothelial cells. Thromb Res. 2002;106:205–211. doi: 10.1016/s0049-3848(02)00143-3. [DOI] [PubMed] [Google Scholar]
- 15.Di Santo A, Mezzetti A, Napoleone E, Di Tommaso R, Donati MB, de Gaetano G, et al. Resveratrol and quercetin down-regulate tissue factor expression by human stimulated vascular cells. J Thromb Haemost. 2003;1:1089–1095. doi: 10.1046/j.1538-7836.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- 16.Tsai S-H, Lin-Shiau S-Y, Lin J-K. Suppression of nitric oxide synthase and the down-regulation of the activation of NFκB in macrophages by resveratrol. Br J Pharmacol. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertelli AA, Baccalini R, Battaglia E, Falchi M, Ferrero ME. Resveratrol inhibits TNF alpha-induced endothelial cell activation. Therapie. 2001;56:613–616. [PubMed] [Google Scholar]
- 18.Donnelly LE, Newton R, Kennedy GE, Fenwick PS, Leung RH, Ito K, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004;287:L774–L783. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- 19.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CWW, et al. Cancer chemopreventive activity of resveratrol a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 20.Jang M, Pezzuto JM. Effects of resveratrol on 12-O-tetrdecanoylphorbol-13-acetate-induced oxidative events and gene expression in mouse skin. Cancer Lett. 1998;134:81–89. doi: 10.1016/s0304-3835(98)00250-x. [DOI] [PubMed] [Google Scholar]
- 21.Soleas GJ, Diamandis EP, Goldberg DM. Wine as biological fluid: history, production, and role in disease prevention. J Clin Lab Anal. 1997;11:287–313. doi: 10.1002/(SICI)1098-2825(1997)11:5<287::AID-JCLA6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 23.Roberti M, Pizzirani D, Simoni D, Rondanin R, Baruchello R, Bonora C, et al. Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J Med Chem. 2003;46:3546–3554. doi: 10.1021/jm030785u. [DOI] [PubMed] [Google Scholar]
- 24.Stivala LA, Savio M, Carafoli F, Perucca P, Bianchi L, Maga G, et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J Biol Chem. 2001;276:22586–22594. doi: 10.1074/jbc.M101846200. [DOI] [PubMed] [Google Scholar]
- 25.Schneider Y, Chabert P, Stutzmann J, Coelho D, Fougerousse A, Gosse F, et al. Resveratrol analog (Z)-3,5,4′ -trimethoxystilbene is a potent anti-mitotic drug inhibiting tubulin polymerization. Int J Cancer. 2003;107:189–196. doi: 10.1002/ijc.11344. [DOI] [PubMed] [Google Scholar]
- 26.Rapaport SI, Rao LVM. The tissue factor pathway: how it has become a “prima ballerina”. Thromb Haemost. 1995;74:7–17. [PubMed] [Google Scholar]
- 27.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues: implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 28.Fleck RA, Rao LVM, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor anti-body. Thromb Res. 1990;59:421–437. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 29.Camerer E, Kolsto AB, Prydz H. Cell biology of tissue factor, the principal initiator of blood coagulation. Thromb Res. 1996;81:1–41. doi: 10.1016/0049-3848(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 30.Semeraro N, Colucci M. Tissue factor in health and disease. Thromb Haemost. 1997;78:759–764. [PubMed] [Google Scholar]
- 31.Eilertsen KE, Osterud B. Tissue factor: (patho)physiology and cellular biology. Blood Coagul Fibrinolysis. 2004;15:521–538. doi: 10.1097/00001721-200410000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Ardissino D, Merlini PA, Arlens R, Coppola R, Bramucci E, Lucreziotti S, et al. Tissue factor in human coronary atherosclerotic plaques. Clin Chim Acta. 2000;291:235–240. doi: 10.1016/s0009-8981(99)00231-4. [DOI] [PubMed] [Google Scholar]
- 33.Ardissino D, Merlini PA, Bauer KA, Bramucci E, Ferrario M, Coppola R, et al. Thrombogenic potential of human coronary atherosclerotic plaques. Blood. 2001;98:2726–2729. doi: 10.1182/blood.v98.9.2726. [DOI] [PubMed] [Google Scholar]
- 34.Marmur JD, Thiruvikraman SV, Fyfe BS, Guha A, Sharma SK, Ambrose JA, et al. Identification of active tissue factor in human coronary atheroma. Circulation. 1996;94:1226–1232. doi: 10.1161/01.cir.94.6.1226. [DOI] [PubMed] [Google Scholar]
- 35.Taubman MB, Fallon JT, Schecter AD, Giesen P, Mendlowitz M, Fyfe BS, et al. Tissue factor in the pathogenesis of atherosclerosis. Thromb Haemost. 1997;78:200–204. [PubMed] [Google Scholar]
- 36.Ruf W, Mueller BM. Tissue factor signaling. Thromb Haemost. 1999;82:175–182. [PubMed] [Google Scholar]
- 37.Rao LVM, Pendurthi UR. Tissue factor–factor VIIa signaling. Arterioscler Thromb Vasc Biol. 2005;25:47–56. doi: 10.1161/01.ATV.0000151624.45775.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coughlin SR. Protease-activated receptors in vascular biology. Thromb Haemost. 2001;86:298–307. [PubMed] [Google Scholar]
- 39.Ruf W. Protease-activated receptor signaling in the regulation of inflammation. Crit Care Med. 2004;32:S287–S292. doi: 10.1097/01.ccm.0000126364.46191.12. [DOI] [PubMed] [Google Scholar]
- 40.Bertelli A, Bertelli AAE, Gozzini A, Giovannini L. Plasma and tissue resveratrol concentrations and pharmacological activity. Drugs Exp Clin Resx. 1998;24:133–138. [PubMed] [Google Scholar]
- 41.Soleas G, Angelini M, Grass L, Diamandis EP, Goldberg DM. Absorption of trans-resveratrol in rats. Methods Enzymol. 2001;335:145–154. doi: 10.1016/s0076-6879(01)35239-4. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 43.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg DM, Karumanchiri A, Yan J, Soleas G, Ng E, Waterhouse AL, et al. A global survey of trans-resveratrol concentrations in commercial wines. Am J Enol Vitic. 1995;46:159–165. [Google Scholar]
- 45.Tedesco I, Russo M, Russo P, Iacomino G, Russo GL, Carraturo A, et al. Antioxidant effect of red wine polyphenols on red blood cells. J Nutr Biochem. 2000;11:114–119. doi: 10.1016/s0955-2863(99)00080-7. [DOI] [PubMed] [Google Scholar]
- 46.Eilertsen KE, Osterud B. Tissue factor: (patho)physiology and cellular biology. Blood Coagul Fibrinolysis. 2004;15:521–538. doi: 10.1097/00001721-200410000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Pedrielli P, Skibsted LH. Antioxidant synergy and regeneration effect of quercetin, (−)-epicatechin, and (+)-catechin on alpha-tocopherol in homogeneous solutions of peroxidating methyl linoleate. J Agric Food Chem. 2002;50:7138–7144. doi: 10.1021/jf020437l. [DOI] [PubMed] [Google Scholar]
- 48.Pignatelli P, Pulcinelli FM, Celestini A, Lenti L, Ghiselli A, Gazzaniga PP, et al. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am J Clin Nutr. 2000;72:1150–1155. doi: 10.1093/ajcn/72.5.1150. [DOI] [PubMed] [Google Scholar]
- 49.Mackman N. Regulation of the tissue factor gene. Thromb Haemost. 1997;78:747–754. [PubMed] [Google Scholar]
- 50.Pendurthi UR, Meng F, Mackman N, Rao LVM. Mechanism of resveratrol-mediated suppression of tissue factor gene expression. Thromb Haemost. 2002;87:155–162. [PubMed] [Google Scholar]