Abstract
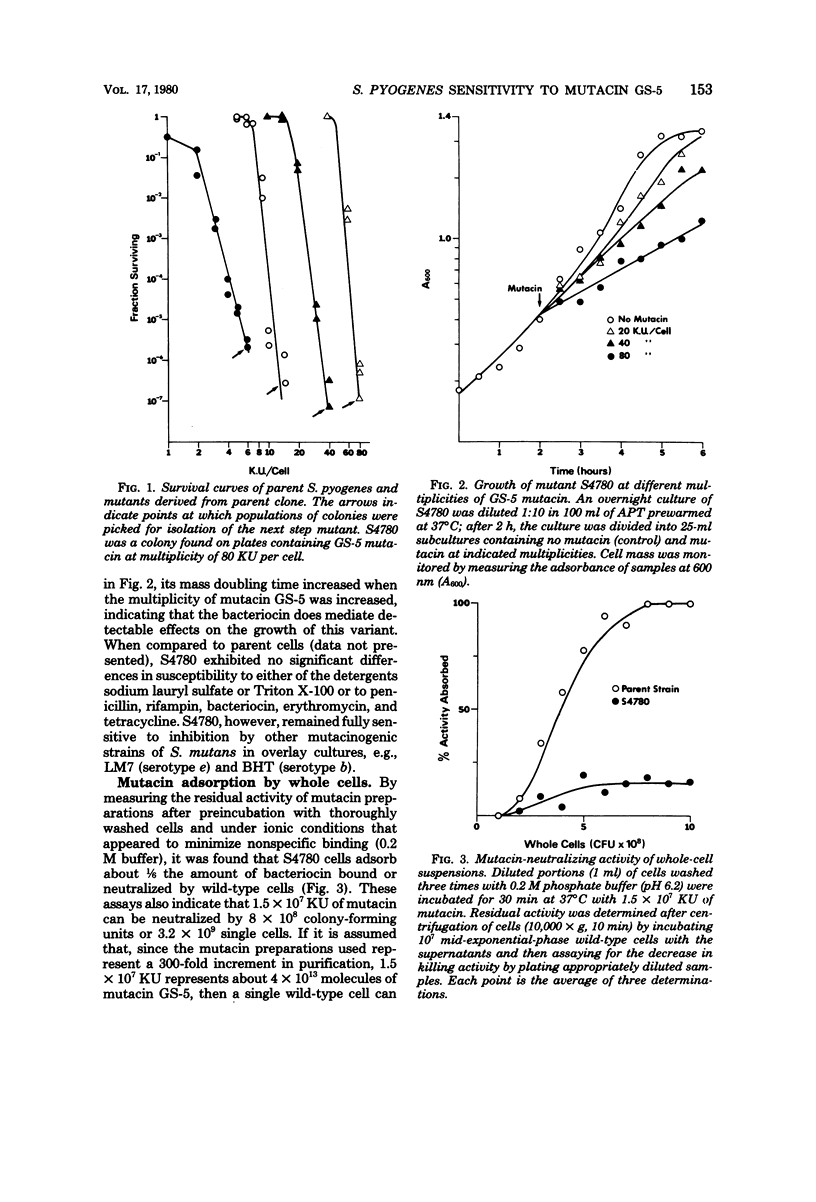
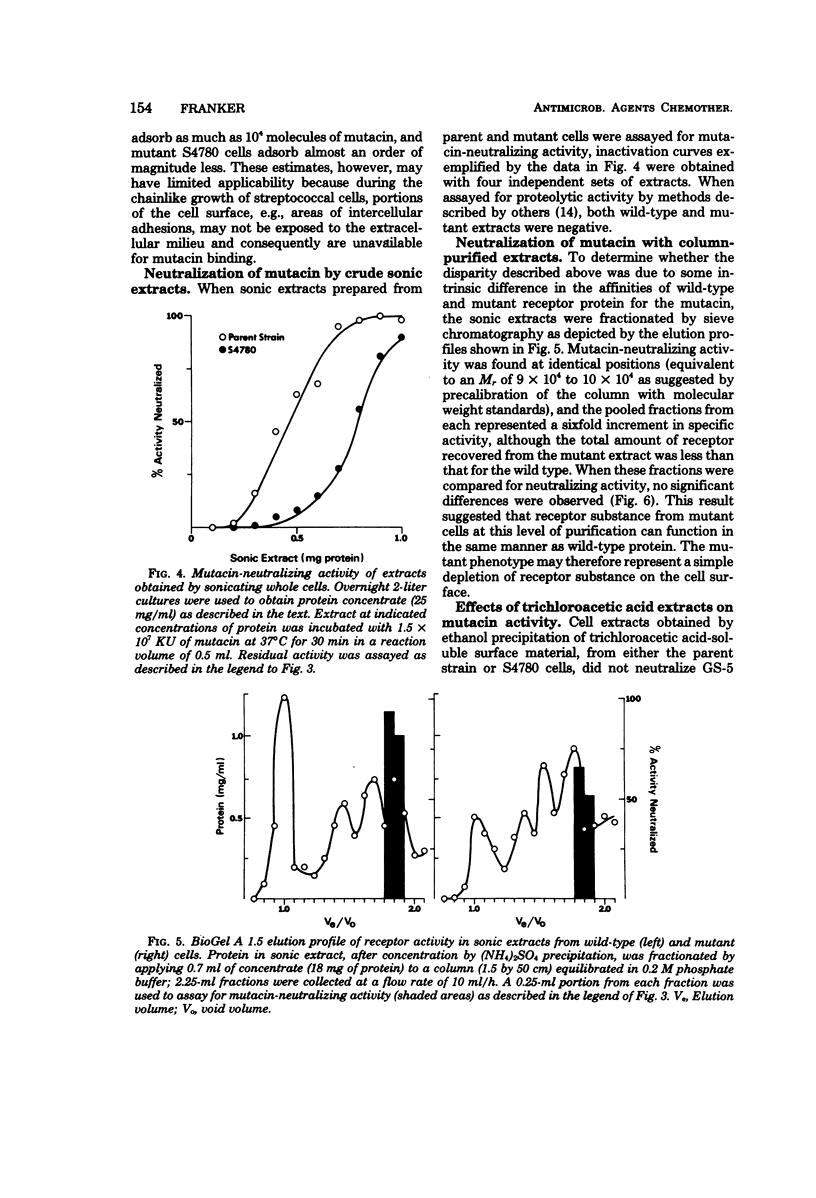
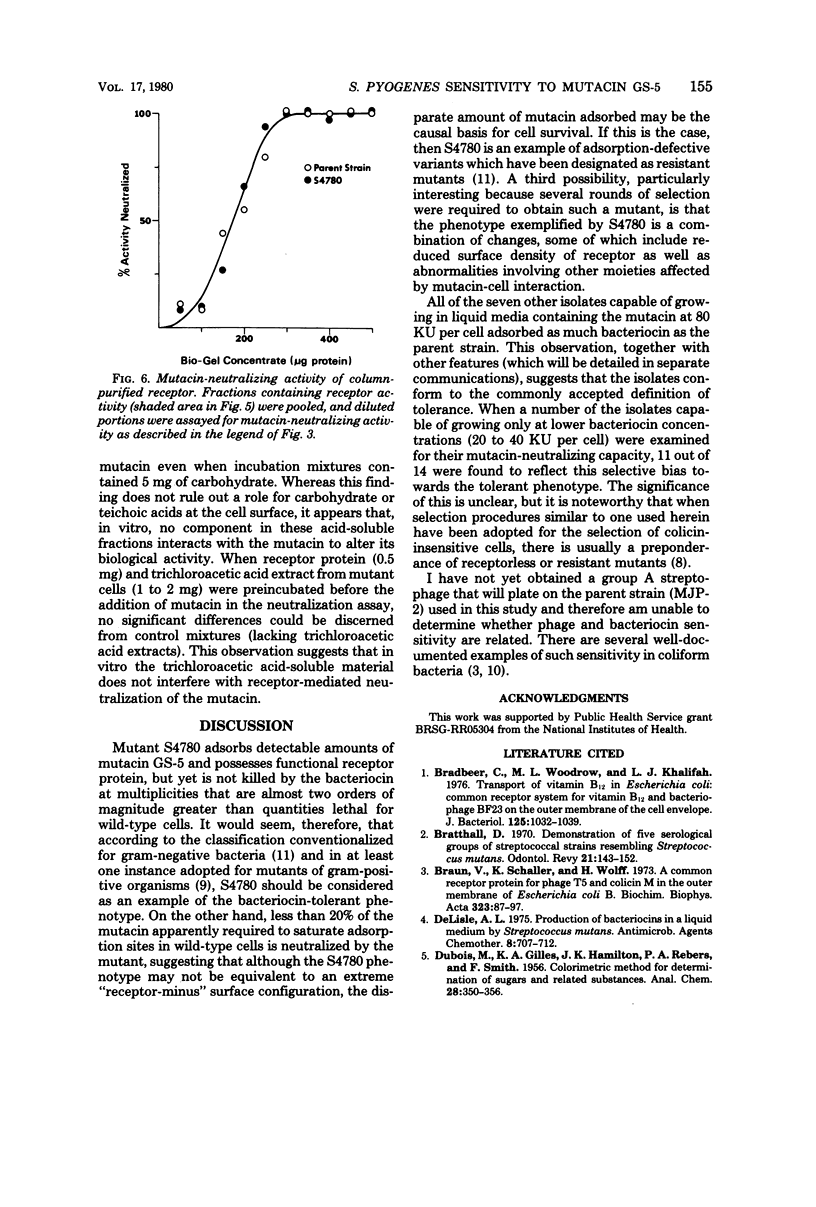
By means of a stepwise selection procedure, mutants capable of growing in the presence of relatively high multiplicities of a bacteriocin from Streptococcus mutans GS-5 were obtained from a sensitivie strain of Streptococcus pyogenes. Mutacin-neutralizing activity of cell extracts containing receptor protein was examined in one variant that adsorbed 1/6 the amount of bacteriocin adsorbed by the parent strain under conditions equivalent to "saturation." Partially purified receptor protein from both parent and mutant cells neutralized an equivalent amount of bacteriocin on a weight-to-weight basis, indicating that in vitro there was no significant difference in affinity for the mutacin between the respective receptor fractions. Cell extracts from the mutant, solubilized by treatment with trichloroacetic acid, neither neutralized mutacin activity nor interfered with receptor protein-mediated mutacin neutralization in vitro. The mutant phenotype may thus represent a cell surface density of receptor protein which results in the adsorption of sublethal amounts of mutacin. The mutant retained its sensitivity to other mutacins, e.g., those produced by strains LM-7 and BHT of S. mutans, and did not differ from wild-type cells with respect to either detergent sensitivity (sodium lauryl sulfate and Triton X-100) or to inhibition by penicillin, rifampin, bacitracin, erythromycin, and tetracycline.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbeer C., Woodrow M. L., Khalifah L. I. Transport of vitamin B12 in Escherichia coli: common receptor system for vitamin B12 and bacteriophage BF23 on the outer membrane of the cell envelope. J Bacteriol. 1976 Mar;125(3):1032–1039. doi: 10.1128/jb.125.3.1032-1039.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Braun V., Schaller K., Wolff H. A common receptor protein for phage T5 and colicin M in the outer membrane of Escherichia coli B. Biochim Biophys Acta. 1973 Sep 27;323(1):87–97. doi: 10.1016/0005-2736(73)90433-1. [DOI] [PubMed] [Google Scholar]
- Delisle A. L. Production of bacteriocins in a liquid medium by Streptococcus mutans. Antimicrob Agents Chemother. 1975 Dec;8(6):707–712. doi: 10.1128/aac.8.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins. Annu Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- Hamada S., Ooshima T. Inhibitory spectrum of a bacteriocinlike substance (mutacin) produced by some strains of Streptococcus mutans. J Dent Res. 1975 Jan-Feb;54(1):140–145. doi: 10.1177/00220345750540010801. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Effects of colicin A and staphylococcin 1580 on amino acid uptake into membrane vesicles of Escherichia coli and staphylococcus aureus. Biochim Biophys Acta. 1973 Jul 18;311(4):483–495. doi: 10.1016/0005-2736(73)90124-7. [DOI] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega B., Oldenziel-Werner W. J., Klaasen-Boor P., Rezee A., Glas J., de Graaf F. K. Purification and characterization of cloacin DF13 receptor from Enterobacter cloacae and its interaction with cloacin DF13 in vitro. J Bacteriol. 1979 Apr;138(1):7–16. doi: 10.1128/jb.138.1.7-16.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D., Slade H. D. Production and properties of an extracellular bacteriocin from Streptococcus mutans bacteriocidal for group A and other streptococci. Infect Immun. 1975 Dec;12(6):1375–1385. doi: 10.1128/iai.12.6.1375-1385.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Slade H. D. Isolation and characterization of a Streptococcus mutans bacteriocin inhibitor from Streptococcus pyogenes. Infect Immun. 1978 May;20(2):578–580. doi: 10.1128/iai.20.2.578-580.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


